Traditional and Recent Alternatives for Controlling Bacterial Foodborne Pathogens in Fresh Horticultural Commodities—A Review
Abstract
1. Introduction
1.1. Quality Parameters
1.2. Occurrence of Foodborne Pathogens in Horticultural Commodities
2. Control of Bacterial Foodborne Pathogens in Horticultural Commodities During Postharvest
2.1. Chemical Control
2.1.1. Chlorination
2.1.2. Oxidizers
2.2. Non-Chemical Control
2.3. Biological Control Strategies
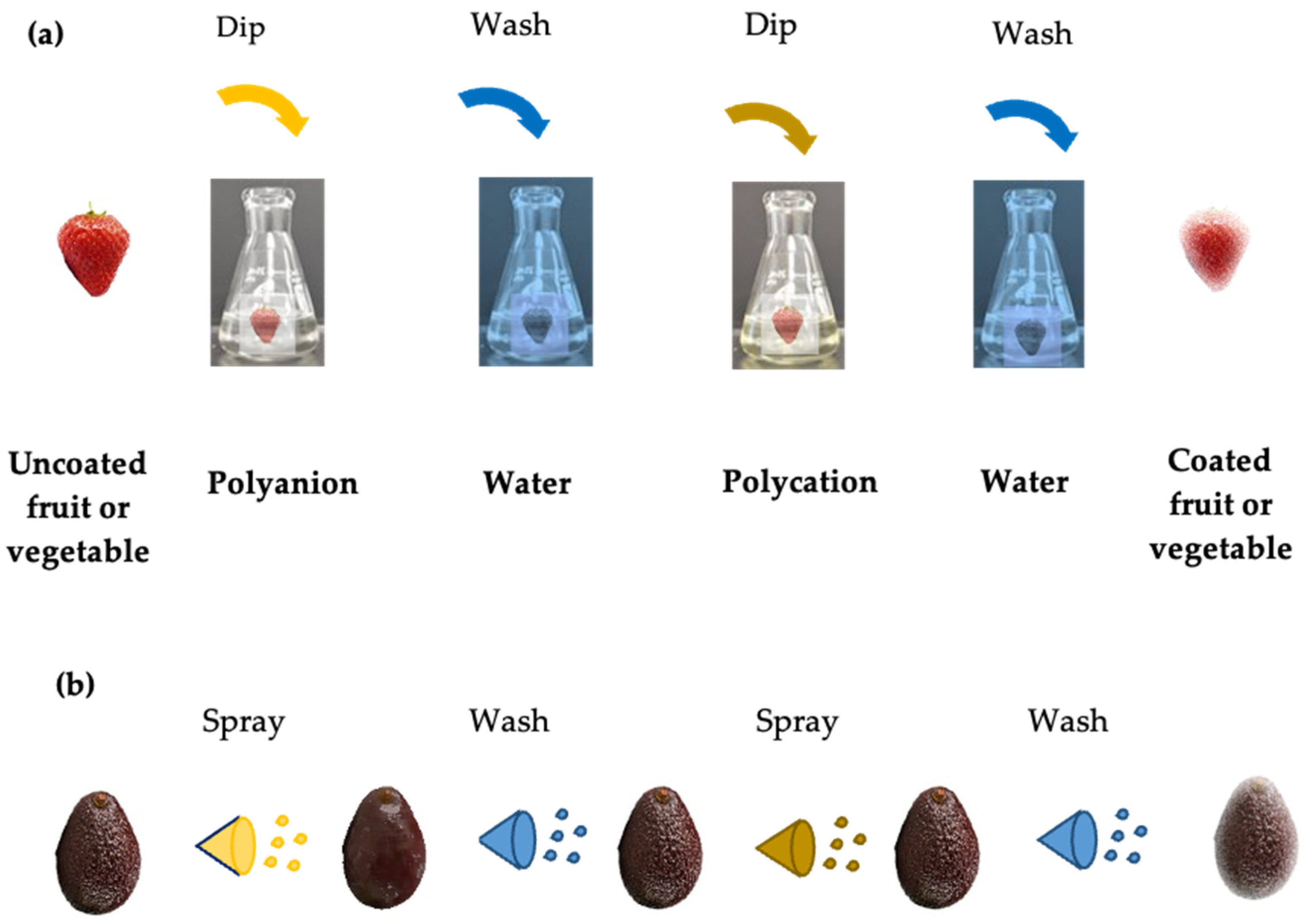
3. Coatings for Reducing Foodborne Pathogens
3.1. Functions and Materials for Coating Formulations
3.2. Coating Effects on Fruit and Vegetable Storage Quality and Presence of Foodborne Pathogens
3.3. Nanotechnology-Based Coatings: Regulatory and Safety Concerns
4. Development of New Materials and Effect on Foodborne Pathogens
5. Overall Mechanisms of Action of the Postharvest Technologies on Foodborne Pathogens
6. Conclusions and Outlook
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Heard, G.M. Microbiology of fresh-cut produce. In Fresh-Cut Fruits and Vegetables. Science, Technology, and Market; Lamikanra, O., Ed.; CRC Press: London, UK, 2002; pp. 187–248. [Google Scholar]
- FAO (Food and Agriculture Organization). Microbiological Hazards in Fresh Leafy Vegetables and Herbs: Meeting Report; Microbiological Risk Assessments. Series 14; FAO: Rome, Italy, 2008; p. 154. Available online: https://www.who.int/publications/i/item/9789241563789 (accessed on 11 February 2025).
- Avendaño, R.B.D.; Schwentesius, R.R.; Lugo, M.S. La inocuidad alimentaria en la exportación de hortalizas mexicanas a Estados Unidos. Comer. Exter. 2007, 57, 6–18. [Google Scholar]
- Lynch, M.F.; Tauxe, R.V.; Hedberg, C.W. The growing burden of foodborne outbreaks due to contaminated fresh produce: Risks and opportunities. Epidemiol. Infect. 2009, 137, 3017–3315. [Google Scholar] [CrossRef]
- Berger, C.N.; Samir, V.; Sodha, S.V.; Shaw, R.K.; Griffin, P.M.; Pink, D.; Hand, P.; Frankel, G. Fresh fruit and vegetables as vehicles for the transmission of human pathogens. Environ. Microbiol. 2010, 12, 2385–2397. [Google Scholar] [CrossRef]
- WHO (Word Health Organization). Food Safety. 2024. Available online: https://www.who.int/news-room/fact-sheets/detail/food-safety (accessed on 16 March 2025).
- GAO. U.S. Government Accountability Office Food Safety: Status of Foodborne Illness in the U.S. GAO-25-107606 Q&A Report to Congressional Addressees February 3, 2025. Available online: https://www.gao.gov/assets/gao-25-107606.pdf (accessed on 16 March 2025).
- Sarno, E.; Pezzutto, D.; Rossi, M.; Liebana, E.; Rizzi, V. A review of significant European foodborne outbreaks in the last decade. J. Food Prot. 2021, 84, 2059–2070. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Pires, S.M.; Liu, Z.; Ma, X.; Liang, J.; Jiang, Y.; Chen, J.; Liang, J.; Wang, S.; Wang, L.; et al. Surveillance of foodborne disease outbreaks in China, 2003–2017. Food Control 2020, 118, 107359. [Google Scholar] [CrossRef]
- Faour-Klingbeil, D.; Todd, E.C. Prevention and control of foodborne diseases in Middle-East North African countries: Review of National control systems. Int. J. Environ. Res. Public Health 2019, 17, 70. [Google Scholar] [CrossRef]
- Hashemi, M.; Salayani, M.; Afshari, A.; Kafil, H.S.; Noori, S. The global burden of viral food-borne diseases: A systematic review. Curr. Pharm. Biotechnol. 2023, 24, 1657–1672. [Google Scholar] [CrossRef]
- Marques, C.S.; Sousa, S.; Castro, A.; Ferreira, V.; Teixeira, P.; da Costa, J.M.C. Protozoa as the “underdogs” for microbiological quality evaluation of fresh vegetables. Appl. Sci. 2022, 12, 7145. [Google Scholar] [CrossRef]
- Khan, W.; Rafiq, N.; Nawaz, M.A.; Kabir, M.; Farooqie, Z.U.R.; Romman, M.; Parvez, R.; Alfarraj, S.; Noor, A.; Ujjan, A.A. Parasitic contamination of fresh vegetables sold in open markets: A public health threat. Braz. J. Biol. 2022, 82, e242614. [Google Scholar] [CrossRef]
- Siwila, J. The triple food-borne protozoan parasites: Cryptosporidium spp., Giardia duodenalis, Cyclospora cayetanensis—Hope in transmission reduction. Curr. Clin. Microbiol. Rep. 2023, 10, 99–107. [Google Scholar] [CrossRef]
- Mercanoglu, T.B.; Halkman, A.K. Do leafy green vegetables and their ready-to-eat [RTE] salads carry a risk of foodborne pathogens? Anaerobe 2011, 17, 286–287. [Google Scholar] [CrossRef]
- Aytac, S.T.; Ben, U.; Cengiz, C.; Taban, C.M. Evaluation of Salmonella and Listeria monocytogenes contamination on leafy green vegetables. J. Food Agric. Environ. 2010, 8, 275–279. [Google Scholar]
- Santillana, F.S.M.; Frank, J.F. Challenges in the control of foodborne pathogens in low-water activity foods and spices. In The Microbiological Safety of Low Water Activity Foods and Spices, Food Microbiology and Food Safety; Gurtler, J.B., Doyle, M.P., Kornacki, J.L., Eds.; Springer Science+Business Media: New York, NY, USA, 2014; pp. 15–34. [Google Scholar]
- Edelstein, M.; Sundborger, C.; Hergens, M.P.; Ivarsson, S.; Dryselius, R.; Insulander, M.; Jemberg, C.; Huntin, Y.; Wallensten, A. Barriers to trace-back in a salad-associated EHEC outbreak, Sweden, June 2013. PLoS Curr. 2014, 6. [Google Scholar] [CrossRef] [PubMed]
- Public Health Agency of Canada. E. coli O157:H7 Illnesses in the Maritimes and Ontario. 2013. Available online: https://www.canada.ca/en/public-health/services/food-safety/public-health-notice/2013/public-health-notice-e-coli-o157-h7-illnesses-maritimes-ontario.html (accessed on 5 February 2025).
- CDC (Centers for Disease Control and Prevention). Multistate Outbreak of Salmonella sanitpaul Infections Linked to Imported Cucumbers. 2013. Available online: https://archive.cdc.gov/www_cdc_gov/salmonella/saintpaul-04-13/advice-consumers.html?utm_source=chatgpt.com (accessed on 5 February 2025).
- Madrid, D. Lettuce Was the Likely Cause of an E. coli Outbreak That Sickened 94 People Eating at a Southwest Valley Federico’s Mexican Food Restaurant. 26 November 2013. Available online: https://www.foodsafetynews.com/2013/11/final-report-94-sick-in-federicos-e-coli-outbreak-lettuce-implicated/ (accessed on 5 February 2025).
- Vestrheim, D.F.; Lange, H.; Nygård, K.; Borgen, K.; Wester, A.L.; Kvarme, M.L.; Vold, L. Are ready to eat salads ready to eat? an outbreak of Salmonella Coeln linked to imported, mixed, pre-washed and bagged Salad, Norway, November 2013. Epidemiol. Infect. 2016, 144, 1756–1760. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Oh, S.S.; Oh, K.H.; Park, J.H.; Jang, E.J.; Chung, G.T.; Yoo, C.K.; Bae, G.R.; Cho, S.H. An outbreak of foodborne illness caused by Enteroaggregative Escherichia coli in a high school in South Korea. Jpn J. Infect. Dis. 2015, 68, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Vasala, M.; Hallanvuo, S.; Ruuska, P.; Suokas, R.; Siitonen, A.; Hakala, M. High frequency of reactive arthritis in adults after Yersinia pseudotuberculosis O:1 outbreak caused by contaminated grated carrots. Ann. Rheum. Dis. 2014, 73, 1793–1796. [Google Scholar] [CrossRef]
- MacDonald, E.; Einöder-Moreno, M.; Borgen, K.; Brandal, L.T.; Diab, L.; Fossli, Ø.; Guzman, B.; Hassan, A.A.; Johannessen, G.S.; Johansen, E.J.; et al. National outbreak of Yersinia enterocolitica infections in military and civilian populations associated with consumption of mixed salad, Norway, 2014. Eurosurveillance 2016, 21. [Google Scholar] [CrossRef]
- Angelo, K.M.; Chu, A.; Anand, M.; Nguyen, T.A.; Bottichio, L.; Wise, M.; Williams, I.; Seelman, S.; Bell, R.; Fatica, M.; et al. Outbreal of Salmonella Newport infections linked to cucumbers-United States 2014. Morb. Mortal. Wkly. Rep. 2015, 64, 144–147. [Google Scholar]
- Taylor, D.L. Tainted Celery Linked to Gonzalez Farm the Californian. 13 November 2014. Available online: https://www.thecalifornian.com/story/news/local/2014/11/13/tainted-celery-linked-gonzales-farm/19005599/ (accessed on 5 February 2025).
- Mossong, J.; Decruyenaere, F.; Moris, G.; Ragimbeau, C.; Olinger, C.; Johler, S.; Perrin, M.; Hau, P.; Weicherding, P. Investigation of a Staphylococcal food poisoning outbreak combining case control, traditional typing and whole genome sequencing methods, Luxembourg, June 2014. Eurosurveillance 2015, 20, 30059. [Google Scholar] [CrossRef]
- Public Health Agency of Canada. Outbreak of E. coli Infections with Possible Link to Leafy Greens. 2015. Available online: https://www.canada.ca/en/public-health/services/public-health-notices/2015/public-health-notice-update-outbreak-e-coli-infections-possible-link-leafy-greens.html (accessed on 5 February 2025).
- CDC (Centers for Disease Control and Prevention). Multistate Outbreak of Salmonella poona Infections Linked to Imported Cucumbers. 2015. Available online: https://archive.cdc.gov/#/details?q=Salmonella%20poona&start=0&rows=10&url=https://www.cdc.gov/salmonella/poona-09-15/index.html (accessed on 5 February 2025).
- CBS News. Our Fruits Safe: Rock Melon Farmers. 4 August 2016. Available online: https://www.sbs.com.au/news/our-fruit-is-safe-rockmelon-farmers (accessed on 5 February 2025).
- Thals, K. Lettuce Back on the Menu After Nationwide Scare. 8 February 2016. Available online: https://thenewdaily.com.au/news/national/2016/02/08/lettuce-is-back-on-the-menu-after-nation-wide-scare/ (accessed on 5 February 2025).
- CBC News. More E. coli Infections Linked to Contaminated Romaine Lettuce, 1 Case Fatal. CBC’S Journalist Standards and Practices. 2017. Available online: https://www.cbc.ca/news/canada/montreal/e-coli-romaine-lettuce-1.4461558 (accessed on 5 February 2025).
- Astill, G.M.; Kuchler, F.; Todd, J.E.; Page, E.T. Shiga toxin-producing Escherichia coli (STEC) O157:H7 and romaine lettuce: Source labeling, prevention, and business. Am. J. Public Health 2020, 110, 322–328. [Google Scholar] [CrossRef]
- Public Health Agency of Canada. Outbreak of E. coli Infections Linked to Romaine Lettuce. 2019. Available online: https://www.canada.ca/en/public-health/services/public-health-notices/2018/outbreak-ecoli-infections-linked-romaine-lettuce.html (accessed on 5 February 2025).
- ECDC (European Centre for Disease Prevention and Control). Rapid Risk Assessment: Multy-Country Outbreak of Listeria monocytogenes Serogroup Ivb, Multi-Locus Sequence Type 6, Infections Linked to Frozen Corn and Possibbly to Other Frozen Vegetables-Fist Update. 2018. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/2018_ECDC-EFSA_ROA_Update1_UI-444_Listeria.pdf (accessed on 5 February 2025).
- Whitworth, J. Snack Mix Recall Expanded as Patient Count Increase in Salmonella Outbreake. 20 March 2019. Available online: https://www.foodsafetynews.com/2019/03/snack-mix-recall-expanded-as-patient-count-increases-in-salmonella-outbreak/ (accessed on 5 February 2025).
- Lange, J. Alfalfa Sprouts Cause Salmonella Outbreak in New Zeland. 12 May 2019. Available online: https://www.foodsafetynews.com/2019/05/alfalfa-sprouts-linked-to-salmonella-outbreak-in-new-zealand/#:~:text=Almost%2070%20people%20have%20fallen,at%20the%20end%20of%20March (accessed on 5 February 2025).
- Whitworth, J. Tomatoes Linked to Salmonella Outbreak in Sweden 71 Infected. 10 October 2019. Available online: https://www.foodsafetynews.com/2019/10/tomatoes-linked-to-salmonella-outbreak-in-sweden-71-infected/ (accessed on 5 February 2025).
- CDC (Centers for Disease Control and Prevention). Outbreak of Salmonella Infections Linked to Cavi Brand Whole, Fresh Papayas. 2019. Available online: https://archive.cdc.gov/#/details?url=https://www.cdc.gov/salmonella/uganda-06-19/index.html (accessed on 5 February 2025).
- CDC (Centers for Disease Control and Prevention). Outbreak of Salmonella Infections Linked to Pre-Cut Melons. 2019. Available online: https://archive.cdc.gov/#/details?url=https://www.cdc.gov/salmonella/carrau-04-19/index.html (accessed on 5 February 2025).
- Espenhain, L.; Riess, M.; Müller, L.; Colombe, S.; Ethelberg, S.; Litrup, E.; Jemberg, C.; Kühlmann, S.; Lindblad, A.; Kühn, N.; et al. Cross-Border outbreak of Yersinia enterocolitica O3 associated with imported fresh spinach, Sweden and Denmark, March 2019. Eurosurveillance 2019, 24, 1900368. [Google Scholar] [CrossRef]
- CDC (Centers for Disease Control and Prevention). Outbreak of Salmonella Infections Linked to Cut Fruit. 2020. Available online: https://archive.cdc.gov/www_cdc_gov/salmonella/javiana-12-19/index.html (accessed on 5 February 2025).
- CDC. Salmonella Outbreak Linked to BrightFarms Packaged Salad Greens. 2021. Available online: https://archive.cdc.gov/www_cdc_gov/salmonella/typhimurium-07-21/details.html (accessed on 5 February 2025).
- CDC. Salmonella Outbreak Linked to Onions. 2021. Available online: https://archive.cdc.gov/www_cdc_gov/salmonella/oranienburg-09-21/index.html (accessed on 5 February 2025).
- CDC. Salmonella Outbreak Linked to Alfalfa Sprouts. 2022. Available online: https://www.cdc.gov/salmonella/outbreaks/typhimurium-12-22/index.html (accessed on 5 February 2025).
- CDC. Salmonella Outbreak Linked to Fresh Diced Onions. (December 2023). 2023. Available online: https://www.cdc.gov/salmonella/outbreaks/onions-10-23/index.html (accessed on 5 February 2025).
- CDC. Outbreak of Salmonella: Cantaloupes (November 2023). 2024. Available online: https://www.fda.gov/food/outbreaks-foodborne-illness/outbreak-investigation-salmonella-cantaloupes-november-2023 (accessed on 5 February 2025).
- FDA. Outbreak Investigation on Salmonella: Organic Basil (April 2024). 2024. Available online: https://www.fda.gov/food/outbreaks-foodborne-illness/outbreak-investigation-salmonella-organic-basil-april-2024 (accessed on 5 February 2025).
- FDA. Outbreak Investigation of E. coli O157:H7: Onions (October 2024). 2024. Available online: https://www.fda.gov/food/outbreaks-foodborne-illness/outbreak-investigation-e-coli-o157h7-onions-october-2024 (accessed on 5 February 2025).
- FDA. Outbreak Investigation of E. coli O121:H19: Organic Carrots (November 2024). 2024. Available online: https://www.fda.gov/food/outbreaks-foodborne-illness/outbreak-investigation-e-coli-o121h19-organic-carrots-november-2024 (accessed on 5 February 2025).
- Kang, J.H. Understanding inactivation of Listeria monocytogenes and Escherichia coli O157:H7 inoculated on romaine lettuce by emulsified thyme essential oil. Food Microbiol. 2022, 105, 104013. [Google Scholar] [CrossRef] [PubMed]
- Alreshoodi, F.M.; Alsuliman, B.; Alotaibi, N.M.; Althobaiti, A.; Mukhtar, L.E.; Alsaleh, S.; Alajlan, A.A.; Alakeel, S.I.; Alshabrmi, F.M.; Sarwar, T.; et al. Impact of various washing protocols on the mitigation of Escherichia coli contamination in raw salad vegetables. Microorganisms 2024, 12, 20103. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Lavalle, L.; Valero, A.; Cejudo-Gómez, M.; Carrasco, E. Assessment of the efficacy of decontamination treatments against Salmonella enterica subsp. enterica serovar Thompson on strawberries at different storage conditions. Postharvest Biol. Technol. 2024, 212, 112907. [Google Scholar]
- Fu, Y.; Deering, A.J.; Bhunia, A.K.; Yao, Y. Biofilm of Escherichia coli O157:H7 on cantaloupe surface is resistant to lauroyl arginate ethyl and sodium hypochlorite. Int. J. Food Microbiol. 2017, 260, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Akther, S.; Shamiul Alam, S.M.; Ahiduzzaman, M.; Nahidul Islam, M.; Shofiul Azam, M. Individual and combined effects of electrolyzed water and ultrasound treatment on microbial decontamination and shelf life extension of fruits and vegetables: A review of potential mechanisms. J. Food Process Preserv. 2022, 46, e16765. [Google Scholar] [CrossRef]
- Calderon, J. Acido hipocloroso (HOCl) “una nueva alternativa en antisepsia y desinfección desarrollada en Colombia. Laboratorio Actual. 2010, 42, 27–31. [Google Scholar]
- Ukuku, D. Effect of sanitizing treatments on removal of bacteria from cantaloupe surface and re-contamination with Salmonella. J. Food Microbiol. 2006, 23, 286–293. [Google Scholar] [CrossRef]
- Pangloli, P.; Hung, Y.C. Reducing microbiological safety risk on blueberries through innovative washing technologies. Food Control 2013, 32, 621–625. [Google Scholar] [CrossRef]
- Rajkowski, K.; Ashurst, K. Use of 1% Peroxyacetic acid sanitizer in an air-mixing wash basin to remove bacterial pathogens from seeds. Foodborne Pathog. Dis. 2009, 6, 1041–1046. [Google Scholar] [CrossRef]
- Mendoza, M.; Cantor, F. Efecto del Uso de Ácido Acético, Cítrico e Hipoclorito de Calcio Para Control de Escherichia coli (ATCC25922) en Lechuga (Lactuca sativa L.) y Chile Dulce (Capsicum annum L.). Bachelor’s Thesis, Universidad Zamorano, Tegucigalpa, Honduras, 2012; p. 27. [Google Scholar]
- Stearns, R.; Freshour, A.; Shen, C. Literature review for applying peroxyacetic acid and/or hydrogen peroxide to control foodborne pathogens on food products. J. Agric. Food Res. 2022, 10, 100442. [Google Scholar] [CrossRef]
- Feng, Y.; Suo, K.; Zhang, Y.; Yang, Z.; Zhou, C.; Shi, L.; Chen, W.; Wang, J.; Wang, C.; Zheng, Y. Ultrasound synergistic slightly acidic electrolyzed water treatment of grapes: Impacts on microbial loads, wettability, and postharvest storage quality. Ultrason. Sonochem. 2024, 103, 10675. [Google Scholar] [CrossRef]
- Marín, A.; Tudela, J.A.; Garrido, Y.; Albolafio, S.; Hernández, N.; Andújar, S.; Allende, A.; Gil, M. Chlorinated wash water and pH regulators affect chlorine gas emission and disinfection by-products. Innov. Food Sci. Emerg. Technol. 2020, 66, 102533. [Google Scholar] [CrossRef]
- Donato, F.; Zani, C. Chronic exposure to organochlorine compounds and health effects in adults: Diabetes and thyroid diseases. Ann. Ig. 2010, 22, 185–198. [Google Scholar] [PubMed]
- Abadias, M.; Alegre, I.; Usall, J.; Torres, R.; Viñas, I. Evaluation of alternative sanitizers to chlorine disinfection for reducing foodborne pathogens in fresh-cut apple. Postharvest Biol. Technol. 2011, 59, 289–297. [Google Scholar] [CrossRef]
- Bataller, M.; Cruz, S.; Fernández, I.; García, M.; Veliz, E.; Ramos, Y.; Menéndez, S. Ozone application for postharvest disinfection of tomatoes. Ozone Sci. Eng. 2010, 32, 361–371. [Google Scholar]
- Mahapatra, A.; Muthukumarappan, K.; Julson, J. Applications of ozone, bacteriocins and irradiation in food processing: A review. Crit. Rev. Food Sci. 2013, 45, 447–461. [Google Scholar] [CrossRef]
- Sommers, C.; Sites, J.; Musgrove, M. Ultraviolet light (254 nm) inactivation of pathogens on foods and stainless steel surfaces. J. Food Saf. 2010, 30, 470–479. [Google Scholar] [CrossRef]
- Roopesh, M.; Lu, X.; Sablani, S.; Annapure, U. Inactivation of Escherichia coli population on fruit surfaces using ultraviolet-C light: Influence of fruit surface characteristics. Food Bioprocess Technol. 2013, 6, 2959–2973. [Google Scholar]
- Poubol, J.; Phiriyangkul, P.; Boonyaritthongchai, P. Combination of chitosan coating and ultraviolet-c irradiation for reducing Escherichia coli and Salmonella sp. on asparagus spears. Int. J. Food Eng. 2015, 1, 50–54. [Google Scholar] [CrossRef]
- Kim, C.; Hung, Y. Inactivation of E. coli O157:H7 on blueberries by electrolyzed water, ultraviolet light, and ozone. J. Food Sci. 2012, 77, M206–M211. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Thakur, R. Postharvest applications of cold plasma treatment for improving food safety and sustainability outcomes for fresh horticultural produce. Posharv. Biol. Technol. 2024, 209, 112694. [Google Scholar] [CrossRef]
- Qian, J.; Ma, L.; Yan, W.; Zhuang, H.; Huang, M.; Zhang, J.; Wang, J. Inactivation kinetics and cell envelope damages of foodborne pathogens Listeria monocytogenes and Salmonella enteritidis treated with cold plasma. Food Microbiol. 2022, 101, 103891. [Google Scholar] [CrossRef] [PubMed]
- Jin-Young, H.; Sang-Hyun, P.; Dong-Hyun, K. Effects of plasma bubble-activated water on the inactivation against foodborne pathogens on tomatoes and its wash water. Food Control 2023, 144, 109381. [Google Scholar]
- Jin-Young, H.; Won-Jae, S.; Sangheum, E.; Seong, K.; Dong-Hyun, K. Antimicrobial efficacy of cold plasma treatment against food-borne pathogens on various foods. J. Phys. D Appl. Phys. 2020, 53, 204003. [Google Scholar]
- Song, Y.; Fan, X. Cold plasma enhances the efficacy of aerosolized hydrogen peroxide in reducing populations of Salmonella Typhimurium and Listeria innocua on grape tomatoes, apples, cantaloupe and romaine lettuce. Food Microbiol. 2020, 87, 103391. [Google Scholar] [CrossRef]
- Grzegorzewska, M.; Badełek, E.; Szczech, M.; Kosson, R.; Wrzodak, A.; Kowalska, B.; Colelli, G.; Szwejda-Grzybowska, J.; Maciorowski, R. The effect of hot water treatment on the storage ability improvement of fresh-cut Chinese cabbage. Sci. Hortic. 2022, 291, 110551. [Google Scholar] [CrossRef]
- Chen, L.; Liu, Q.; Zhao, X.; Zhang, H.; Pang, X.; Yang, H. Inactivation efficacies of lactic acid and mild heat treatments against Escherichia coli strains in organic broccoli sprouts. Food Control 2022, 133, 108577. [Google Scholar] [CrossRef]
- Delaquis, P.; Austin, J. The effect of heat treatments on the fate of foodborne pathogens in horticultural produce. Stewart Postharvest Rev. 2007, 3, 1–5. [Google Scholar]
- Lanchero, O.; Velandia, G.; Fischer, G.; Varela, C.; García, H. Comportamiento de la uchuva (Physalis peruviana L.) en poscosecha bajo condiciones de atmósfera modificada activa. Tecnol. Agropecu. 2007, 8, 61–68. [Google Scholar] [CrossRef]
- Ospina, S.; Cartagena, J. La atmosfera modificada: Una alternativa para la conservación de los alimentos. Rev. Lasallista Investig. 2008, 5, 112–123. [Google Scholar]
- Das, E.; Gürakan, C.; Bayindirli, A. Effect of controlled atmosphere storage, modified atmosphere packaging and gaseous ozone treatment on the survival of Salmonella enteritidis on cherry tomatoes. Food Microbiol. 2006, 23, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Sun-Young, L.; Seung-Youb, B. Effect of chemical sanitizer combined with modified atmosphere packaging on inhibiting Escherichia coli O157:H7 in commercial spinach. Food Microbiol. 2008, 25, 582–587. [Google Scholar]
- Keskinen, L.; Burke, A.; Annous, B. Efficacy of chlorine, acidic electrolyzed water and aqueous chlorine dioxide solutions to decontaminate Escherichia coli O157:H7 from lettuce leaves. Int. J. Food Microbiol. 2009, 132, 134–140. [Google Scholar] [CrossRef]
- Tomas-Callejas, A.; Lopez-Galvez, F.; Sbodio, A.; Artés, F.; Artés-Hernandez, F.; Suslow, T. Chlorine dioxide and chlorine effectiveness to prevent Escherichia coli O157:H7 and Salmonella cross-contamination on fresh-cut Red Chard. Food Control 2012, 23, 325–332. [Google Scholar] [CrossRef]
- Su-jin, K.; Woo-Suk, B. Efficacy of sodium hypochlorite against E. coli on various leafy green and stem vegetables. J. Food Hyg. Saf. 2023, 38, 31–36. [Google Scholar]
- Hopkins, D.; Parisi, M.; Dawson, P.; Northcutt, J.K. Surface decontamination of fresh, whole peaches (Prunus persica) using sodium hypochlorite or acidified electrolyzed water solutions. Int. J. Fruit Sci. 2020, 21, 1–11. [Google Scholar] [CrossRef]
- Bachelli, M.; Amaral, R.; Benedetti, B. Alternative sanitization methods for minimally processed lettuce in comparison to sodium hypochlorite. Braz. J. Microbiol. 2013, 44, 673–678. [Google Scholar] [CrossRef]
- Dong, L.; Li, Y. Fate of Salmonella typhimurium and Listeria monocytogenes on whole papaya during storage and antimicrobial efficiency of aqueous chlorine dioxide generated with HCl, malic acid or lactic acid on whole papaya. Foods 2021, 10, 1871. [Google Scholar] [CrossRef]
- Amaral, R.; Bachelli, M.; Zerbinati, M.; Benedetti, B. Effectiveness of different concentrations of ozonated water in the sanitization of fresh-cut green pepper. Agric. Eng. Int. 2012, 14, 131–135. [Google Scholar]
- Phakawan, J.; Tepsorn, R. Antimicrobial potential of gaseous ozone against Salmonella Thyphimurium and Escherichia coli O157:H7 contaminated on Bird Eye Chili (Capsicum frutescens L.). Int. J. Agric. Technol. 2024, 20, 697–710. [Google Scholar]
- Har, K.; Perera, C. Efficacy of sanitizers on three types of tropical fruits having different skin characteristics. J. Food Process. Beverages 2013, 1, 4. [Google Scholar]
- Zhou, Z.; Zuber, S.; Cantergiani, F.; Butot, S.; Li, D.; Stroheker, T.; Devlieghere, F.; Lima, A.; Piantini, U.; Uyttendaele, M. Inactivation of viruses and bacteria on strawberries using a levulinic acid plus sodium dodecyl sulfate based sanitizer, taking sensorial and chemical food safety aspects into account. Int. J. Food Microbiol. 2017, 257, 176–182. [Google Scholar] [CrossRef]
- Ells, T.C.; Fan, L.; LeBlanc, D.I.; Forney, C.F.; Bezanson, G.S. Impact of heat sanitation of fresh whole cantaloupe on fruit quality and volatile metabolism. Acta Hortic. 2014, 1079, 145–151. [Google Scholar]
- Mukhopadhyay, S.; Ukuku, D.; Juneja, V.; Fan, X. Effects of UV-C treatment on inactivation of Salmonella enterica and Escherichia coli O157:H7 on grape tomato surface and stem scars, microbial loads, and quality. Food Control 2014, 44, 110–117. [Google Scholar] [CrossRef]
- Martinez-Hernandez, G.B.; Huertas, J.P.; Navarro-Rico, J.; Gomez, P.; Artes, F.; Palop, A.; Artes-Hernandez, F. Inactivation kinetics of foodborne pathogens by UV-C radiation and its subsequent growth in fresh-cut kailan-hybrid broccoli. Food Microbiol. 2015, 46, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Yan, R.; Gurtler, J.B.; Mattheis, J.P.; Fan, X. Efecto de la eliminación de tricomas y la radiación UV-C en las poblaciones de E. coli O157:H7 y la calidad del melocotón. HortScience 2020, 55, 1626–1631. [Google Scholar] [CrossRef]
- Adam, A.; Yadav, B.; Prasad, A.; Gautam, B.; Tsui, Y.; Roopesh, M.S. Salmonella inactivation and rapid cooling of fresh cut apples by plasma integrated low-pressure cooling. Food Res. Int. 2021, 14, 110464. [Google Scholar] [CrossRef]
- Jin, T.Z.; Yu, Y.; Gurtler, J.B. Effects of pulsed electric field processing on microbial survival, quality change and nutritional characteristics of blueberries. LWT-Food Sci. Technol. 2017, 77, 517–524. [Google Scholar] [CrossRef]
- Kowalska, B.; Wrzodak, A. Application potential of lactic acid bacteria in horticultural production. Sustainability 2025, 17, 1385. [Google Scholar] [CrossRef]
- Duan, C.; Meng, X.; Meng, J.; Khan, M.I.H.; Dai, L.; Khan, A.; An, X.; Zhang, J.; Huq, T.; Ni, Y. Chitosan as a preservative for fruits and vegetables: A review on chemistry and antimicrobial properties. J. Bioresour. Bioprod. 2019, 4, 11–21. [Google Scholar] [CrossRef]
- Gurtler, J.B.; Garner, C.M. A review of essential oils as antimicrobials in foods with special emphasis on fresh produce. J. Food Prot. 2022, 85, 1300–1319. [Google Scholar] [CrossRef] [PubMed]
- Namiota, M.; Bonikowski, R. The current state of knowledge about essential oil fumigation for quality of crops during postharvest. Int. J. Mol. Sci. 2021, 22, 13351. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Qin, D.; Li, H.; Zhang, T.; Han, Y.; Huang, Y.; He, D.; Wu, K.; Chai, X.; Chen, C. Study of antimicrobial activity and mechanism of vapor-phase cinnamaldehyde for killing Escherichia coli based on fumigation method. Front. Nutr. 2022, 9, 1040152. [Google Scholar] [CrossRef]
- Lee, G.; Kim, Y.; Kim, H.; Beuchatc, L.R.; Jee-Hoon, R. Antimicrobial activities of gaseous essential oils against Listeria monocytogenes on a laboratory medium and radish sprouts. Int. J. Food Microbiol. 2018, 265, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Jurado, F.; Navarro-Cruz, A.R.; Ochoa-Velasco, C.E.; López-Malo, E.P.; Avila-Sosa, R. Essential oils in vapor phase as alternative antimicrobials: A review. Crit. Rev. Food Sci. Nutr. 2019, 60, 1641–1650. [Google Scholar] [CrossRef]
- Sharma, M.; Patel, J.; Conway, W.; Ferquson, S.; Sulakvelidze, A. Effectiveness of bacteriophages in reducing Escherichia coli O157:H7 on fresh-cut cantaloupes and lettuce. J. Food Prot. 2009, 72, 1481–1485. [Google Scholar] [CrossRef]
- Viazis, S.; Akhtar, M.; Feirtag, J.; Diez-Gonzalez, F. Reduction of Escherichia coli O157:H7 viability on leafy green vegetables by treatment with a bacteriophage mixture and trans-cinnamaldehyde. Food Microbiol. 2011, 28, 149–157. [Google Scholar] [CrossRef]
- Nie, X.; Zhang, R.; Cheng, L.; Li, S.; Zhao, X.; Chen, X. Combining the biocontrol yeast Pichia kluyveri with UV-C treatment to control postharvest decay of king oyster mushrooms (Pleurotus eryngii) caused by Lactococcus lactis subsp. Lactis. Biol. Control 2020, 149, 104327. [Google Scholar] [CrossRef]
- Jin, T.; Gurtler, J.B. Inactivation of Salmonella on tomato stem scars by edible chitosan and organic acid coatings. J. Food Prot. 2012, 75, 1368–1372. [Google Scholar] [CrossRef]
- Jovanovic, G.; Klaus, A.; Niksic, M. Antimicrobial activity of chitosan coatings and films against Listeria monocytogenes on black radish. Rev. Argent. Microbiol. 2016, 48, 128–136. [Google Scholar] [CrossRef]
- Kang, J.-H.; Song, K.B. Antimicrobial activity of honeybush (Cyclopia intermedia) ethanol extract against foodborne pathogens and its application in washing fresh-cut Swiss chard. Food Control 2021, 121, 107674. [Google Scholar] [CrossRef]
- Lee, C.-L.; Kim, G.-H.; Yoon, K.-S. Effects of combined aerosolization with ultraviolet C light-emitting diode on enterohemorrhagic Escherichia coli and Staphylococcus aureus attached to soft fresh produce. Foods 2021, 10, 1834. [Google Scholar] [CrossRef]
- Priya, K.; Thirunavookarasu, N.; Chidanand, D.V. Recent advances in edible coating of food products and its legislations: A review. J. Agric. Food Res. 2023, 12, 100623. [Google Scholar] [CrossRef]
- Liyanapathiranage, A.; Dassanayake, R.S.; Gamage, A.; Karri, R.R.; Manamperi, A.; Evon, P.; Jayakodi, Y.; Madhujith, T.; Merah, O. Recent developments in edible films and coatings for fruits and vegetables. Coatings 2023, 13, 1177. [Google Scholar] [CrossRef]
- Vanaraj, R.; Suresh Kumar, S.M.; Mayakrishnan, G.; Rathinam, B.; Kim, S.C. A Current trend in efficient biopolymer coatings for edible fruits to enhance shelf life. Polymers 2024, 16, 2639. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Lall, A.; Kumar, S.; Patil, T.D.; Gaikwad, K.K. Plant-based edible films and coatings for food packaging applications: Recent advances, applications, and trends. Sustain. Food Technol. 2024, 2, 1428. [Google Scholar] [CrossRef]
- Matloob, A.; Ayub, H.; Mohsin, M.; Ambreen, S.; Khan, F.A.; Oranab, S.; Rahim, M.A.; Khalid, W.; Nayik, G.A.; Ramniwas, S.; et al. A review on edible coatings and films: Advances, composition, production methods, and safety concerns. ACS Omega 2023, 8, 28932–28944. [Google Scholar] [CrossRef]
- Guaña-Escobar, F.; Vaca-Tenorio, M.; Aguilar-Morales, J. Biopelículas y envases activos, nuevas tecnologías en la industria alimentaria. FACSALUD-UNEMI 2022, 6, 18–32. [Google Scholar] [CrossRef]
- Peerzada Gh, J.; Sinclair, B.J.; Perinbarajan, G.K. An overview on smart and active edible coatings: Safety and regulations. Eur. Food Res. Technol. 2023, 249, 1935–1952. [Google Scholar] [CrossRef]
- Hamed, I.; Nordeng, A.; Lerfall, J.J. Sustainable edible packaging systems based on active compounds fromfood processing byproducts: A review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 198–226. [Google Scholar] [CrossRef] [PubMed]
- Ungureanu, C.; Tihan, G.; Zgârian, R.; Pandelea, G. Bio-coatings for preservation of fresh fruits and vegetables. Coatings 2023, 13, 1420. [Google Scholar] [CrossRef]
- Solano-Doblado, L.G.; Alamilla-Beltrán, L.; Jiménez-Martínez, C. Películas y recubrimientos comestibles funcionalizados. Tip. Rev. Esp. Cienc. 2018, 21, 30–42. [Google Scholar] [CrossRef]
- Maringgal, B.; Hashim, N.; Tawakkal, I.; Mohamedc, M. Recent advance in edible coating and its effect on fresh/fresh-cut fruits quality. Trends Food Sci. Technol. 2020, 96, 253–267. [Google Scholar] [CrossRef]
- Yousuf, B.; Qadri, O.; Srivastava, A. Recent developments in shelf-life extension of fresh-cut fruits and vegetables by application of different edible coatings: A review. LWT-Food Sci. Technol. 2018, 75, 124–130. [Google Scholar] [CrossRef]
- Du, H.; Yang, F.; Yu, H.; Yao, W.; Xie, Y. Controllable fabrication of edible coatings to improve the match between barrier and fruits respiration through layer-by-layer assembly. Food Bioprocess Technol. 2022, 15, 1778–1793. [Google Scholar] [CrossRef]
- Chaudhary, S.; Kumar, S.; Kumar, V.; Sharma, R. Chitosan nanoemulsions as advanced edible coatings for fruits and vegetables: Composition, fabrication and developments in last decade. Int. J. Biol. Macromol. 2020, 152, 154–170. [Google Scholar] [CrossRef]
- Weng, S.; Marcet, I.; Rendueles, M.; Díaz, M. Edible films from the laboratory to industry: A review of the different production methods. Food Bioprocess Technol. 2024, 18, 3245–3271. [Google Scholar] [CrossRef]
- De Ancos, B.; González-Peña, D.; Colina-Coca, C.; Sánchez-Moreno, C. Uso de películas/recubrimientos comestibles en los productos de iv y v gama. Rev. Iberoam. Tecnol. Postcosecha 2015, 16, 8–17. [Google Scholar]
- Ahmed, M.; Saini, P.; Iqbal, U.; Sahu, K. Edible microbial cellulose-based antimicrobial coatings and films containing clove extract. Food Prod. Proc. Nutr. 2024, 6, 65. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, H.; Luo, W.; Chen, G.; Xiao, N.; Xiao, G.; Liu, C. Development of functional hydroxyethyl cellulose based composite films for food packaging applications. Front. Bioeng. Biotechnol. 2022, 10, 989893. [Google Scholar] [CrossRef]
- Popescu, P.-A.; Palade, L.M.; Nicolae, I.-C.; Popa, E.E.; Mitelut, A.C.; Drăghici, M.C.; Matei, F.; Popa, M.E. Chitosan-based edible coatings containing essential oils to preserve the shelf life and postharvest quality parameters of organic strawberries and apples during cold storage. Foods 2022, 11, 3317. [Google Scholar] [CrossRef] [PubMed]
- Dinh, T.A.; Le, Y.N.; Pham, N.Q.; Ton-That, P.; Van-Xuan, T.; Ho, T.G.H.; Nguyen, T.; Phuong, H.H.K. Fabrication of antimicrobial edible films from chitosan incorporated with guava leaf extract. Prog. Org. Coat. 2023, 183, 107772. [Google Scholar] [CrossRef]
- Das, S.K.; Vishakha, K.; Das, S.; Chakraborty, D.; Ganguli, A. Carboxymethyl cellulose and cardamom oil in a nanoemulsion edible coating inhibit the growth of foodborne pathogens and extend the shelf life of tomatoes. Biocatal. Agric. Biotechnol. 2022, 42, 102369. [Google Scholar] [CrossRef]
- Filgueiras, C.T.; Fakhouri, F.M.; Garcia, V.A.d.S.; Velasco, J.I.; Nogueira, G.F.; Ramos da Silva, L.; Oliveira, R.A.d. Effect of adding red propolis to edible biodegradable protein films for coating grapes: Shelf life and sensory analysis. Polymers 2024, 16, 888. [Google Scholar] [CrossRef] [PubMed]
- Garcia, F.; Lin, W.J.; Mellano, V.; Davidov-Pardo, G. Effect of biopolymer coatings made of zein nanoparticles and ε-polylysine as postharvest treatments on the shelf-life of avocados (Persea americana Mill. Cv. Hass). J. Agric. Food Res. 2022, 7, 100260. [Google Scholar] [CrossRef]
- Ngubane, S.; Tesfay, S.Z.; Magwaza, L.S.; Mditshwa, A. The effect of composite edible coatings on the postharvest quality of “Hass” avocado fruit treated at different harvest maturities. Front. Sustain. Food Syst. 2024, 8, 1473731. [Google Scholar] [CrossRef]
- Ghasemi, S.; Jaldani, S.; Sanaei, F.; Ghiafehshirzadi, A.; Alidoost, A.; Hashemi, M.; Marashi, S.M.H.; Khodaiyan, F.; Noori, S.M.A. Application of alginate polymer films and coatings incorporated with essential oils in foods: A review of recent literature with emphasis on nanotechnology. Int. J. Food Eng. 2023, 19, 73–86. [Google Scholar] [CrossRef]
- Ozuna-Valencia, K.H.; Moreno-Vásquez, M.J.; Graciano-Verdugo, A.Z.; Rodríguez-Félix, F.; Robles-García, M.Á.; Barreras-Urbina, C.G.; Quintero-Reyes, I.E.; Cornejo-Ramírez, Y.I.; Tapia-Hernández, J.A. The application of organic and inorganic nanoparticles incorporated in edible coatings and their effect on the physicochemical and microbiological properties of seafood. Processes 2024, 12, 1889. [Google Scholar] [CrossRef]
- Pandey, V.K.; Islam, R.U.; Shams, R.; Dar, A.H. A comprehensive review on the application of essential oils as bioactive compounds in nano-emulsion based edible coatings of fruits and vegetables. Appl. Food Res. 2022, 2, 100042. [Google Scholar] [CrossRef]
- Fadiji, T.; Rashvand, M.; Daramola, M.O.; Iwarere, S.A. A Review on antimicrobial packaging for extending the shelf life of food. Processes 2023, 11, 590. [Google Scholar] [CrossRef]
- Yousuf, B.; Wu, S.; Siddiqui, M.W. Incorporating essential oils or compounds derived thereof into edible coatings: Effect on quality and shelf life of fresh/fresh-cut produce. Trends Food Sci. Tech. 2021, 108, 245–257. [Google Scholar] [CrossRef]
- Angane, M.; Swift, S.; Huang, K.; Butts, C.A.; Quek, S.Y. Essential oils and their major components: An updated review on antimicrobial activities, mechanism of action and their potential application in the food industry. Foods 2022, 11, 464. [Google Scholar] [CrossRef]
- Beniwal, D.; Gupta, S. Bio-efficacies of essential oils against food-borne bacteria. Plant Sci. Today 2023, 11, 358–364. [Google Scholar] [CrossRef]
- Gaowa, S.; Feng, K.; Li, Y.; Long, Y.; Hu, W. Effect of alginate-based edible coating containing thyme essential oil on quality and microbial safety of fresh-cut potatoes. Horticulturae 2023, 9, 543. [Google Scholar] [CrossRef]
- Zhang, W.; Goksen, G.; Zhou, Y.; Yang, J.; Khan, M.R.; Ahmad, N.; Fei, T. Application of a chitosan–cinnamon essential oil composite coating in inhibiting postharvest apple diseases. Foods 2023, 12, 3518. [Google Scholar] [CrossRef]
- Vakili-Ghartavol, M.; Arouiee, H.; Golmohammadzadeh, S.; Naseri, M.; Bandian, L. Edible coatings based on solid lipid nanoparticles containing essential oil to improve antimicrobial activity, shelf-life, and quality of strawberries. J. Stored Prod. Res. 2024, 106, 102262. [Google Scholar] [CrossRef]
- Atarés, L.; Chiralt, A. Essential oils as additives in biodegradable films and coatings for active food packaging. Trends Food Sci. Technol. 2016, 48, 51–62. [Google Scholar] [CrossRef]
- Gammage, S.; Marangoni, A.G. Safety of edible coatings on fruits and vegetables. Compr. Rev. Food Sci. Food Saf. 2025, 24, e70108. [Google Scholar] [CrossRef]
- Gupta, R.K.; Guha, P.; Srivastav, P.P. Investigating the toxicological effect of nanomaterials in food packaging associated with human health and the environment. J. Hazard. Mater. Lett. 2024, 5, 100125. [Google Scholar] [CrossRef]
- Ansari, M.A. Nanotechnology in Food and Plant Science: Challenges and Future Prospects. Plants 2023, 12, 2565. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Huang, Y.; Mou, Z.; Li, R.; Hossen, M.A.; Dai, J.; Qin, W.; Lee, K. Characterization and preliminary safety evaluation of nano-SiO2 isolated from instant coffee. Ecotox. Environ. Saf. 2021, 224, 112694. [Google Scholar] [CrossRef]
- Huang, J.; Zou, L.; Bao, M.; Feng, Q.; Xia, W.; Zhu, C. Toxicity of polystyrene nanoparticles for mouse ovary and cultured human granulosa cells. Ecotox. Environ. Saf. 2023, 249, 114371. [Google Scholar] [CrossRef]
- El-Atrash, A.; Zaki, S.; Tousson, E.; Negm, M. Copper oxide nanoparticles induced liver and kidney toxicity in rat. Asian J. Biochem. Genet. Mol. Biol. 2022, 12, 154–160. [Google Scholar] [CrossRef]
- Firouzamandi, M.; Hejazy, M.; Mohammadi, A.; Shahbazfar, A.A.; Norouzi, R. In vivo toxicity of oral administrated nano-SiO2: Can food additives increase apoptosis? Biol. Trace Elem. Res. 2022, 201, 4769–4778. [Google Scholar] [CrossRef]
- Hernández, G.; Aguilar, L.; Barrera-Necha, L. Toxicological and genotoxicological evaluation of chitosan nanoparticles loaded with pinene in a murine model. Int. J. Green Herb. Chem. 2020, 9, 437–448. [Google Scholar]
- Gibbons, E.; Winder, C.; Barron, E.; Fernandez, D.; Krysmann, M.; Kelarakis, A.; Parry, A.; Yeates, S. Layer by layer antimicrobial coatings based on Nafion, lysozyme and chitosan. Nanomaterials 2019, 9, 1563. [Google Scholar] [CrossRef]
- Otoni, C.; Avena-Bustillos, R.; Azeredo, H.; Lorevice, M.; Moura, M.; Mattoso, L.; McHugh, T. Recent advances on edible films based on fruits and vegetables—A review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1151–1169. [Google Scholar] [CrossRef] [PubMed]
- Torres-León, C.; Vicente, A.; Flores-López, A.; Rojas, R.; Serna-Cock, L.; Alvarez-Perez, O.; Aguilar, C. Edible films and coatings based on mango (var. Ataulfo) by-products to improve gas transfer rate of peach. LWT-Food Sci. Technol. 2018, 97, 624–631. [Google Scholar] [CrossRef]
- Duhan, J.; Kumar, R.; Kumar, N.; Kaur, P.; Nehra, S.; Duhan, S. Nanotechnology: The new perspective in precision agriculture. Biol. Rep. 2017, 15, 11–23. [Google Scholar] [CrossRef]
- Vasile, C. Polymeric nanocomposites and nanocoatings for food packaging: A review. Materials 2018, 11, 1834. [Google Scholar] [CrossRef] [PubMed]
- Correa-Pacheco, Z.N.; Bautista-Baños, S.; Ramos-García, M.; Martínez-González, M.; Hernández-Romano, J. Physicochemical characterization and antimicrobial activity of edible propolis-chitosan nanoparticle films. Prog. Org. Coat. 2019, 137, 105326. [Google Scholar] [CrossRef]
- Denis-Rohr, A.; Bastarrachea, L.; Goddard, J. Antimicrobial efficacy of N-halamine coatings prepared via dip and spray layer-by-layer deposition. Food Bioprod. Process 2015, 96, 12–19. [Google Scholar] [CrossRef]
- Calvacante, A.; Alves de Souza, M.; Toscano de Barros, S.; Vinhosa, N.; Simões, M.; de Andrade, C.E.E. Development and evaluation of biodegradable films and coatings obtained from fruit and vegetable residues applied to fresh-cut carrot (Daucus carota L.). Postharvest Biol. Technol. 2016, 112, 194–204. [Google Scholar]
- Cortez-Vega, W.; Pizato, S.; Andreghetto de Souza, J.; Prentice, C. Using edible coatings from Whitemouth croaker (Micropogonias furneri) protein isolate and organo-clay composite for improve the conservation properties of fresh-cut ‘Formosa’ papaya. Innov. Food Sci. Emerg. 2014, 22, 197–202. [Google Scholar] [CrossRef]
- Aquinas, N.; Chithra, C.H.; Bhat, M.R. Progress in bioproduction, characterization and applications of pullulan: A review. Polym. Bull. 2024, 81, 12347–12382. [Google Scholar] [CrossRef]
- Rashid, A.; Qayum, A.; Liang, Q.; Kang, L.; Ekumah, J.; Han, X.; Ren, X.; Ma, H. Exploring the potential of pullulan-based films and coatings for effective food preservation: A comprehensive analysis of properties, activation strategies and applications. Int. J. Biol. Macromol. 2024, 260, 129479. [Google Scholar] [CrossRef]
- Kang, L.; Liang, Q.; Chen, H.; Zhou, Q.; Chi, Z.; Rashid, A.; Ma, H.; Ren, X. Insights into ultrasonic treatment on the properties of pullulan/oat protein/ nisin composite film: Mechanical, structural and physicochemical properties. Food Chem. 2023, 402, 134237. [Google Scholar] [CrossRef]
- Priyadarshi, R.; Riahi, Z.; Rhim, J. Antioxidant pectin/pullulan edible coating incorporated with Vitis vinifera grape seed extract for extending the shelf life of peanuts. Postharvest Biol. Technol. 2022, 183, 111740. [Google Scholar] [CrossRef]
- Yener, F.Y.; Korel, F.; Yemenicioğlu, A. Antimicrobial activity of lactoperoxidase system incorporated into cross-linked alginate films. J. Food Sci. 2009, 74, M73–M79. [Google Scholar] [CrossRef]
- Anacarso, I.; De Niederhaeusern, S.; Iseppi, R.; Sabia, C.; Bondi, M.; Messi, P. Anti-listerial activity of chitosan and Enterocin 416K1 in artificially contaminated RTE products. Food Control 2011, 22, 2076–2080. [Google Scholar] [CrossRef]
- Meira, S.M.M.; Zehetmeyer, G.; Werner, J.O.; Brandelli, A. A novel active packaging material based on starch-halloysite nanocomposites incorporating antimicrobial peptides. Food Hydrocoll. 2017, 63, 561–570. [Google Scholar] [CrossRef]
- Amarillas, L.; Lightbourn-Rojas, L.; Angulo-Gaxiola, A.K.; Basilio Heredia, J.; González-Robles, A.; León-Félix, J. The antibacterial effect of chitosan-based edible coating incorporated with a lytic bacteriophage against Escherichia coli O157:H7 on the surface of tomatoes. J. Food Saf. 2018, 38, e12571:1-10. [Google Scholar] [CrossRef]
- Lai, T.T.; Pham, T.T.H.; van Lingen, M.; Desaulniers, G.; Njamen, G.; Tolnai, B.; Jabrane, T.; Moineau, S.; Barnabé, S. Development of antimicrobial paper coatings containing bacteriophages and silver nanoparticles for control of foodborne pathogens. Viruses 2022, 14, 2478. [Google Scholar] [CrossRef] [PubMed]
- Alves, D.; Cerqueira, M.A.; Pastrana, L.M.; Sillankorva, S. Entrapment of a phage cocktail and cinnamaldehyde on sodium alginate emulsion-based films to fight food contamination by Escherichia coli and Salmonella enteritidis. Food Res. Int. 2020, 128, 108791. [Google Scholar] [CrossRef] [PubMed]
- Tomat, D.; Soazo, M.; Verdini, R.; Casabonne, C.; Aquili, V.; Balagué, C.; Quiberoni, A. Evaluation of an WPC edible film added with a cocktail of six lytic phages against foodborne pathogens such as enteropathogenic and Shigatoxigenic Escherichia coli. LWT-Food Sci. Technol. 2019, 113, 108316. [Google Scholar] [CrossRef]
- Eswaranandam, S.; Hettiarachchy, N.S.; Johnson, M.G. Antimicrobial activity of citric, lactic, malic, or tartaric acids and nisin-incorporated soy protein film against Listeria monocytogenes, Escherichia coli O157:H7, and Salmonella gaminara. J. Food Sci. 2004, 6, FMS79–FMS84. [Google Scholar] [CrossRef]
- Abarca, R.L.; Medina, J.; Alvarado, N.; Ortiz, P.A.; Carrillo López, B. Biodegradable gelatin-based films with nisin and EDTA that inhibit Escherichia coli. PLoS ONE 2022, 17, e0264851. [Google Scholar] [CrossRef]
- Pintado, C.M.; Ferreira, M.A.; Sousa, I. Properties of whey protein–based films containing organic acids and nisin to control Listeria monocytogenes. J. Food Prot. 2009, 72, 1891–1896. [Google Scholar] [CrossRef]
- Yuan, G.; Lv, H.; Yang, B.; Chen, X.; Sun, H. Physical properties, antioxidant and antimicrobial activity of chitosan films containing carvacrol and pomegranate peel extract. Molecules 2015, 20, 11034–11045. [Google Scholar] [CrossRef]
- Abdollahi, M.; Rezaei, M.; Farzi, G. Improvement of active chitosan film properties with rosemary essential oil for food packaging. Int. J. Food Sci. Technol. 2012, 47, 847–853. [Google Scholar] [CrossRef]
- He, S.; Yang, Q.; Ren, X.; Zi, J.; Lu, S.; Wang, S.; Zhang, Y.; Wang, Y. Antimicrobial efficiency of chitosan solutions and coatings incorporated with clove oil and/or ethylenediaminetetraacetate. J. Food Saf. 2014, 34, 345–352. [Google Scholar] [CrossRef]
- Jin, T.Z.; Fan, X.; Mukhopadhyay, S. Antimicrobial coating with organic acids and essential oil for the enhancement of safety and shelf life of grape tomatoes. Int. J. Food Microbiol. 2022, 378, 109827. [Google Scholar] [CrossRef]
- Ramos-García, M.; Bosquez-Molina, E.; Hernández-Romano, J.; Zavala-Padilla, G.; Terrés-Rojas, E.; Alia-Tejacal, I.; Barrera-Necha, L.; Hernández-López, M.; Bautista-Baños, S. Use of chitosan-based edible coatings in combination with other natural compounds, to control Rhizopus stolonifer and Escherichia coli DH5α in fresh tomatoes. Crop Prot. 2012, 38, 1–6. [Google Scholar] [CrossRef]
- Hernández-Ochoa, L.; Gonzales-Gonzales, A.; Gutiérrez-Mendez, N.; Muñoz-Castellanos, L.N.; Quintero-Ramos, A. Study of the antibacterial activity of chitosan-based films prepared with different molecular weights including spices essential oils and functional extracts as microbial agents. Rev. Mex. Ing. Quím. 2011, 10, 455–463. [Google Scholar]
- Wang, H.; Yang, C.; Wang, J.; Chen, M.; Luan, D.; Li, L. EVOH films containing antimicrobials geraniol and α-terpilenol extend the shelf life of snakehead slices. Packag. Technol. Sci. 2017, 30, 587–600. [Google Scholar] [CrossRef]
- Aziz, S.G.G.; Almasi, H. Physical characteristics, release properties, and antioxidant and antimicrobial activities of whey protein isolate films incorporated with thyme (Thymus vulgaris L.) extract-loaded nanoliposomes. Food Bioprocess Technol. 2018, 11, 1552–1565. [Google Scholar] [CrossRef]
- Amjadi, S.; Almasi, H.; Ghorbani, M.; Ramazani, S. Reinforced ZnONPs/rosemary essential oil-incorporated zein electrospun nanofibers by κ-carrageenan. Carbohyd. Polym. 2020, 232, 115800. [Google Scholar] [CrossRef]
- El Fawal, G.F.; Omer, A.M.; Tamer, T.M. Evaluation of antimicrobial and antioxidant activities for cellulose acetate films incorporated with Rosemary and Aloe vera essential oils. J. Food Sci. Technol. 2019, 56, 1510–1518. [Google Scholar] [CrossRef]
- Sivarooban, T.; Hettiarachchy, N.S.; Johnson, M.G. Physical and antimicrobial properties of grape seed extract, nisin, and EDTA incorporated soy protein edible films. Food Res. Int. 2008, 41, 781–785. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Q.; Tong, J.; Zhou, J. Physicochemical properties of chitosan films incorporated with honeysuckle flower extract for active food packaging. J. Food Process Eng. 2015, 40, e12305. [Google Scholar] [CrossRef]
- Shankar, S.; Rhim, J.W. Preparation of antibacterial poly (lactide)/poly (butylene adipate-co-terephthalate) composite films incorporated with grapefruit seed extract. Int. J. Biol. Macromol. 2018, 120, 846–852. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Sokorai, K.; Ukuku, D.O.; Jin, T.; Fan, X.; Olanya, M.; Juneja, V. Inactivation of Salmonella in grape tomato stem scars by organic acid wash and chitosan-allyl isothiocyanate coating. Int. J. Food Microbiol. 2018, 266, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Maruthupandy, M.; Seo, J. Allyl isothiocyanate encapsulated halloysite covered with polyacrylate as a potential antibacterial agent against food spoilage bacteria. Mater. Sci. Eng. C 2019, 105, 110016. [Google Scholar] [CrossRef]
- Chen, W.; Jin, T.Z.; Gurtler, J.B.; Geveke, D.J.; Fan, X. Inactivation of Salmonella on whole cantaloupe by application of an antimicrobial coating containing chitosan and allyl isothiocyanate. Int. J. Food Microbiol. 2012, 155, 165–170. [Google Scholar] [CrossRef]
- Li, W.; Liu, L.; Jin, T.Z. Antimicrobial activity of allyl isothiocyanate used to coat biodegradable composite films as affected by storage and handling conditions. J. Food Prot. 2012, 75, 2234–2237. [Google Scholar] [CrossRef]
- Durango, A.M.; Soares, N.F.F.; Benevides, S.; Teixeira, J.; Carvalho, M.; Wobeto, C.; Andrade, N.J. Development and evaluation of an edible antimicrobial film based on yam starch and chitosan. Packag. Technol. Sci. 2006, 19, 55–59. [Google Scholar] [CrossRef]
- Rodríguez-Núñez, J.R.; López-Cervantes, J.; Sánchez-Machado, D.I.; Ramírez-Wong, B.; Torres-Chavez, P.; Cortez-Rocha, M.O. Antimicrobial activity of chitosan-based films against Salmonella Typhimurium and Staphylococcus aureus. Int. J. Food Sci. Technol. 2012, 47, 2127–2133. [Google Scholar] [CrossRef]
- Tripathi, S.; Mehrotra, G.K.; Dutta, P.K. Preparation and physicochemical evaluation of chitosan/poly (vinyl alcohol)/pectin ternary film for food-packaging applications. Carbohyd. Polym. 2010, 79, 711–716. [Google Scholar] [CrossRef]
- Romainor, A.N.B.; Chin, S.F.; Pang, S.C.; Bilung, L.M. Preparation and characterization of chitosan nanoparticles-doped cellulose films with antimicrobial property. J. Nanomater. 2014, 2014, 710459. [Google Scholar] [CrossRef]
- Otoni, C.G.; de Moura, M.R.; Aouada, F.A.; Camilloto, G.P.; Cruz, R.S.; Lorevice, M.V.; de FF Soares, N.; Mattoso, L.H. Antimicrobial and physical-mechanical properties of pectin/papaya puree/cinnamaldehyde nanoemulsion edible composite films. Food Hydrocoll. 2014, 41, 188–194. [Google Scholar] [CrossRef]
- Pagno, C.H.; Costa, T.M.; de Menezes, E.W.; Benvenutti, E.V.; Hertz, P.F.; Matte, C.R.; Tosati, J.V.; Monteiro, A.R.; Rios, A.O.; Flôres, S.H. Development of active biofilms of quinoa (Chenopodium quinoa W.) starch containing gold nanoparticles and evaluation of antimicrobial activity. Food Chem. 2015, 173, 755–762. [Google Scholar] [CrossRef]
- Yu, N.; Peng, H.; Qiu, L.; Wang, R.; Jiang, C.; Cai, T.; Xiong, H. New pectin-induced green fabrication of Ag@AgCl/ZnO nanocomposites for visible-light triggered antibacterial activity. Int. J. Biol. Macromol. 2019, 141, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Jeong, D.; Kanmani, P. Study on physical and mechanical properties of the biopolymer/silver based active nanocomposite films with antimicrobial activity. Carbohyd. Polym. 2019, 224, 115159. [Google Scholar] [CrossRef]
- Bruna, J.E.; Galotto, M.J.; Guarda, A.; Rodríguez, F. A novel polymer based on MtCu2+/cellulose acetate with antimicrobial activity. Carbohyd. Polym. 2014, 102, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Nouri, A.; Yaraki, M.T.; Ghorbanpour, M.; Agarwal, S.; Gupta, V.K. Enhanced Antibacterial effect of chitosan film using Montmorillonite/CuO nanocomposite. Int. J. Biol. Macromol. 2018, 109, 1219–1231. [Google Scholar] [CrossRef]
- Adibelli, M.; Ozcelik, E.; Batibay, G.S.; Arasoglu, T.O.; Arsu, N. A facile and versatile route for preparation AgNp nanocomposite thin films via thiol-acrylate photopolymerization: Determination of antibacterial activity. Prog. Org. Coat. 2020, 143, 105620. [Google Scholar] [CrossRef]
- Vishnuvarthanan, M.; Rajeswari, N. Food packaging: Pectin–laponite–Ag nanoparticle bionanocomposite coated on polypropylene shows low O2 transmission, low Ag migration and high antimicrobial activity. Environ. Chem. Lett. 2019, 17, 439–445. [Google Scholar] [CrossRef]
- Kim, S.; Song, K.B. Antimicrobial activity of buckwheat starch films containing zinc oxide nanoparticles against Listeria monocytogenes on mushrooms. Int. J. Food Sci. Technol. 2018, 53, 1549–1557. [Google Scholar] [CrossRef]
- Du, W.L.; Niu, S.S.; Xu, Y.L.; Xu, Z.R.; Fan, C.L. Antibacterial activity of chitosan tripolyphosphate nanoparticles loaded with various metal ions. Carbohyd. Polym. 2009, 75, 385–389. [Google Scholar] [CrossRef]
- Huang, C.; Zhang, B.; Wang, S.; Zhang, L.; Wang, J.; Huang, X.; Huang, L. Moisture-triggered release of self-produced ClO2 gas from microcapsule antibacterial film system. J. Mater. Sci. 2018, 53, 12704–12717. [Google Scholar] [CrossRef]
- Zhang, B.; Huang, C.; Zhang, L.; Wang, J.; Huang, X.; Zhao, Y.; Li, C. Application of chlorine dioxide microcapsule sustained-release antibacterial films for preservation of mangos. J. Food Sci. Technol. 2019, 56, 1095–1103. [Google Scholar] [CrossRef] [PubMed]
- Lund, P.A.; De Biase, D.; Liran, O.; Scheler, O.; Mira, N.P.; Cetecioglu, Z.; Noriega- Fernández, E.; Bover-Cid, S.; Hall, R.; Sauer, M.; et al. Understanding how microorganisms respond to acid ph is central to their control and successful exploitation. Front. Microbiol. 2020, 11, 556140. [Google Scholar] [CrossRef]
- Yap, P.S.X.; Yusoff, K.; Lim, S.H.E.; Chong, C.M.; Lai, K.S. Membrane disruption properties of essential oils—A double-edged sword? Processes 2021, 9, 595. [Google Scholar] [CrossRef]
- Hochma, E.; Yarmolinsky, L.; Khalfin, B.; Nisnevitch, M.; Ben-Shabat, S.; Nakonechny, F. Antimicrobial Effect of Phytochemicals from Edible Plants. Processes 2021, 9, 2089. [Google Scholar] [CrossRef]
- Pikhtirova, A.; Pecka-Kiełb, E.; Zigo, F. Antimicrobial activity of saponin-containing plants: Review. J. Dairy Vet. Anim. Res. 2023, 2, 121–127. [Google Scholar]
- Rodríguez, B.; Pacheco, L.; Bernal, I.; Piña, M. Mechanisms of action of flavonoids: Antioxidant, antibacterial and antifungal properties. Cienc. Ambiente Clima 2023, 6, 33–66. [Google Scholar] [CrossRef]
- Rao, J.; Chen, B.; McClements, D.J. Improving the efficacy of essential oils as antimicrobials in foods: Mechanisms of action. Annu. Rev. Food Sci. Technol. 2019, 10, 365–387. [Google Scholar] [CrossRef]
- Gálvez-Iriqui, A.C.; Plascencia-Jatomea, M.; Bautista-Baños, S. Lysozymes: Characteristics, mechanism of action and technological applications on the control of pathogenic microorganisms. Mex. J. Phytopathol. 2020, 38, 360–383. [Google Scholar] [CrossRef]
- Kadirvel, V.; Palanisamy, Y.; Ganesan, N.D. Active Packaging System—An Overview of Recent Advances for Enhanced Food Quality and Safety. Packag. Technol. Sci. 2024, 38, 145–162. [Google Scholar] [CrossRef]
- Sablon, E.; Contreras, B.; Vandamme, E. Antimicrobial peptides of lactic acid bacteria: Mode of action, genetics and biosynthesis. In Advances in Biochemical Engineering/Biotechnology, 68. New Products and New Areas of Bioprocess Engineering; Sheper, T., Ed.; Springer: Berlin/Heidelberg, Germany, 2000; pp. 21–60. [Google Scholar]
- Yousefi, M.; Nematollahi, A.; Shadnoush, M.; Mortazavian, A.M.; Khorshidian, N. Antimicrobial Activity of Films and Coatings Containing Lactoperoxidase System: A Review. Front. Nutr. 2022, 9, 828065. [Google Scholar] [CrossRef] [PubMed]
- Khorshidian, N.; Khanniri, E.; Koushki, M.R.; Sohrabvandi, S.; Yousefi, M. An Overview of Antimicrobial Activity of Lysozyme and Its Functionality in Cheese. Front. Nutr. 2022, 9, 833618. [Google Scholar] [CrossRef]
- Sanca, F.M.M.; Blanco, I.R.; Dias, M.; Moreno, A.M.; Martins, S.M.M.K.; Stephano, M.A.; Mendes, M.A.; Mendonça, C.M.N.; Pereira, W.A.; Azevedo, P.O.S.; et al. Antimicrobial activity of peptides produced by Lactococcus lactis subsp. lactis on Swine pathogens. Animals 2023, 13, 2442. [Google Scholar]
- Charest, A.M.; Reed, E.; Bozorgzadeh, S.; Hernandez, L.; Getsey, N.V.; Smith, L.; Galperina, A.; Beauregard, H.E.; Charest, H.A.; Mitchell, M.; et al. Nisin Inhibition of Gram-Negative Bacteria. Microorganisms 2024, 12, 123. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Chen, L.; Wu, J.; He, Y.; Yang, H. Elucidating antimicrobial mechanism of nisin and grape seed extract against Listeria monocytogenes in broth and on shrimp through NMR-based metabolomics approach. Int. J. Food Microbiol. 2020, 319, 108494. [Google Scholar] [CrossRef] [PubMed]
- Lárez-Velazquez, C. Chitosan an overview of its multiple advantages for creating sustainable development poles. Polímeros 2023, 33, e20230005. [Google Scholar] [CrossRef]
- Nasaj, M.; Chehelgerdi, M.; Asghari, B.; Ahmadieh, A.; Asgari, M.; Kabiri-Samani, S.; Sharifi, E.; Arabestani, M. Factors influencing the antimicrobial mechanism of chitosan action and its derivatives: A review. Int. J. Biol. Macromol. 2024, 277, 134321. [Google Scholar] [CrossRef]
- Bautista-Baños, S.; Laura, L.; Barrera-Necha, L.L.; Hernández-López, M.; Rodríguez-González, F. Morphological and ultrastructural modifications of chitosan-treated fungal phytopathogens. In Chitosan in the Preservation of Agricultural Commodities; Bautista-Baños, S., Romanazzi, G., Jiménez-Aparicio, A., Eds.; Academic Press: Cambridge, MA, USA; Elsevier: Cambridge, MA, USA, 2016; pp. 251–275. [Google Scholar]
- Ke, C.L.; Deng, F.S.; Chuang, C.Y.; Lin, C.H. Antimicrobial Actions and Applications of Chitosan. Polymers 2021, 13, 904. [Google Scholar] [CrossRef]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Heras Caballero, A.; Acosta, N. Chitosan: An overview of its properties and applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef]

| Year | Country | Pathogenic Bacteria | Agricultural Product | References |
|---|---|---|---|---|
| 2013 | Sweden | E. coli O157:H7 | Mixed salad | [18] |
| 2013 | Canada | E. coli O157:H7 | Lettuce | [19] |
| 2013 | United States | S. Saintpaul | Cucumber (Cucumis sativus) | [20] |
| 2013 | Unites States | E. coli O157:H7 | Lettuce | [21] |
| 2013–2014 | Norway | S. Coeln | Mixed salad | [22] |
| 2013–2014 | Republic of Korea | E. coli O6 | Kimchi (Brassica pekinensis) | [23] |
| 2014 | Finland | Y. pseudotuberculosis | Grated carrot | [24] |
| 2014 | Noway | Y. enterocolítica O9 | Mixed salad | [25] |
| 2014 | United States | S. Newport | Cucumber | [26] |
| 2014 | United States | E. coli O157:H7 | Celery (Apium graveolens) | [27] |
| 2014 | Luxemburg | Staphylococcus aureus | Mixed salad | [28] |
| 2015 | Canada | E. coli O157:H7 | Leafy vegetables | [29] |
| 2015–2016 | United States | Salmonella Poona | Cucumber | [30] |
| 2016 | Australia | S. Hvittingtoss | Melon | [31] |
| 2016 | Australia | S. Anatum | Lettuce | [32] |
| 2017 | Canada | E. coli O157:H7 | Romain lettuce | [33] |
| 2017 | United States | E. coli O157:H7 | Romain lettuce | [34] |
| 2018 | Canada, United States | E. coli O157:H7 | Romain lettuce | [35] |
| 2018 | Austria, Denmark, Finland, Sweden, England | Listeria monocytogenes | Frozen corn | [36] |
| 2019 | Norway | S. Agbeni | Snack mix | [37] |
| 2019 | New Zeland | S. Typhimurium | Alfalfa sprouts (Medicago sativa) | [38] |
| 2019 | Sweden | S. Typhimurium | Tomato (Solanum lycopersicum) | [39] |
| 2019 | United States | S. Uganda | Papaya | [40] |
| 2019 | United States | S. Carrau | Melon | [41] |
| 2019 | Italy, Sweden, Denmark | Y. enterocolítica | Spinach | [42] |
| 2020 | United States | S. Javiana | Cut fruit | [43] |
| 2021 | United States | S. Typhimurium | Mixed salad | [44] |
| 2021 | United States | S. Oranienburg | Onion (Allium cepa) | [45] |
| 2022 | United States | S. Typhimurium | Alfalfa sprouts | [46] |
| 2023 | United States | S. Thompson | Diced onion | [47] |
| 2023 | United States | S. Newport | Cantaloup | [48] |
| 2024 | United States | Salmonella | Organic basil (Ocimum basilicum) | [49] |
| 2024 | United States | E. coli O157:H7 | Onion | [50] |
| 2024 | United States | E. coli O157:H7 | Baby carrots | [51] |
| Control Method | Fruit/Vegetable | Treatment Strategy | Pathogen | Final Microbial Load | Refs. |
|---|---|---|---|---|---|
| Chemical | Spinach | Sodium hypochlorite (100 ppm, 5 min) | E. coli O157:H7 | 1.1 UFC g−1 | [84] |
| Chemical | Lettuce | Sodium hypochlorite (200 ppm, 2 min) | E. coli O157:H7 | 1.0 UFC g−1 | [85] |
| Chemical | Red chard (Beta vulgaris subs. vulgaris) | Sodium hypochlorite (6%, 6 min) | E. coli O157:H7 | 0.85 UFC g−1 | [86] |
| Chemical | Cabbage, lettuce, mint | Sodium hypochlorite (100 mg Kg−1, 5 min) | E. coli E. coli O157:H7 | 6 log CFU g−1 | [87] |
| Chemical | Peach | Sodium hypochlorite (21 ppm, 5 s) | Listeria innocua | 1.10–2.02 log CFU mL−1 | [88] |
| Chemical | Lettuce | Sodium hypochlorite (1.5 mL L−1, 15 min) | E. coli | 0.7 log10 UFC mL−1 | [89] |
| Chemical | Blueberry (Vaccinium corymbosum) | Sodium hypochlorite (100 mg L−1, 5 min) | E. coli O157:H7 | 4.4 UFC g−1 | [59] |
| Chemical | Papaya | Calcium hypochlorite (10 ppm, 5min) | Salmonella Typhimurium Listeria monocytogenes | 1.8 log UFC 1.24 log UFC | [90] |
| Chemical | Green pepper (Capsicum annum) | Ozone (1.8 mg L−1, 1 min) | Total coliforms | 1.0 UFC g−1 | [91] |
| Chemical | Chilli (C. frutescens) | Ozone (800 mg h−1 15 min) | S. Typhimurium E. coli | complete inhibition | [92] |
| Chemical | Starfruit (Averrhoa carambola) and guava (Psidium guava) | Hydrogen peroxide (5.0%, 1 min) | E. coli ATCC25922 S. aureus (ATCC 25955) | 2. 0 -4.0 UFC mL (starfruit), 1.5–2.2 UFC mL (guava) | [93] |
| Chemical | Strawberries | Levulinic acid 0.5%; or 5 %+ sodium dodecyl sulfate 0.5%; or 2 %(2 min) | Salmonella, Enterococcus faecium, E. coli O157:H7, E. coli P1, and L. monocytogenes | 2.0, 2.6, 1.9, 1.8, 2.2-log reductions | [94] |
| Physical | Cantaloupe | Heat (84 °C, 240 s) | L. innocua | 4.0 log10 UFC g−1 | [95] |
| Physical | Chinese cabbage | Heat (55 °C, 3 s) | Enterobacterias | 1.0 UFC 102 g−1 | [78] |
| Physical | Grape (Vitis vinifera) and tomato | UV (0.60–6.0 kJ/m2, 1 min) | S. enterica and E. coli O157:H7 | 2.15–3.1 and 2.3–3.5 log10 CFU g−1, respectively | [96] |
| Physical | Broccoli (Brasica oleracea) | UV (15 kJ/m2, 37 s) | E. coli, S. enteritidis, and L. monocytogenes | 4.1, 2.9, and 4.2 log10 UFC g−1, respectively | [97] |
| Physical | Peach | UV (442 mJ/cm2, 60 s) | E. coli O157:H7 | 0.9 log UFC | [98] |
| Physical | Apple slices | Cold plasma (200 mbar, 3 min) | S. enterica serovar Typhimurium (ATCC 13311) | 6 log UFC g−1 | [99] |
| Physical + chemical | Blueberries | Pulsed electric fields (2 kV/cm field strength, 1 ms pulse width, and 100 pulses per s), 2, 4 and 6 min | E. coli K12 and L. innocua | 1.0 and 0.5 log CFU/g E. coli and 2.3 and 2.6 log CFU/g L. innocua | [100] |
| Fruit/Vegetable | Treatment Strategy | Pathogen | Final Microbial Load | Refs. |
|---|---|---|---|---|
| Cantaloupe | Antagonist E. coli O157:H7-specific bacteriophages (ECP-100) 6.69 log PFU ml−1 | E. coli O157:H7 | 0.7 UFC mL−1 | [108] |
| Romaine lettuce (Lactuca sativa var. longifolia) | Antagonist E. coli O157:H7-specific phage strains: 38, 39, 41, CEV2, AR1, 42, ECA1, and ECB (107 PFU mL−1) | E. coli O157:H7 and ATCC 43895 | 1.5 UFC/leaf | [109] |
| Mushrooms (Agaricus bisporus) | Antagonist Pichia kluyveri 1 × 108 cells/mL + UV-C 6 Kj/m2 | Lactococcus lactis subsp. lactis | Disease incidence 40% and natural infection 15% | [110] |
| Tomato | Chitosan (200 mg) + lactic, acetic, and levulinic acid (2%, 10 min) | S. Montevideo ATCC 8387, S. Newport, S. Saintpaul 02-517-1, and S. Typhimurium ATCC 14028 | 2.5 log10 UFC/stem | [111] |
| Black radish (Raphanus sativus var. sativus) | Chitosan (1%) + essential oil (0.2%) | L. monocytogenes ATCC 19115, and 19112 | 2.4 and 2.1 log10 UFC g−1, respectively | [112] |
| Swiss chard | Honeybush ethanol extract 6 m L−1 | L. monocytogenes and E. coli O157:H7 | 2.31–2.67 log reductions | [113] |
| Romain lettuce | Peanut skin extract + benzethonium chloride emulsion 5 mg L-1 | L. monocytogenes and E. coli O157:H7 | 3.0 and 2.8 log reductions | [114] |
| Antimicrobials | Polymer/Carrier | Target Microorganisms | Effect | Refs. |
|---|---|---|---|---|
| Enzymes | ||||
| Lactoperoxidase system (0.1, 0.5, 1%), H2O2 (0.2, 0.4, 0.8 mM), KSCN (1, 2, 4 mM). | Alginate | E. coli, L. innocua, and P. fluorescens | Growth of all tested bacteria prevented for a 6 h period. | [171] |
| Enterocin 416K1 (1280 IU/mL). | Chitosan | L. monocytogenes | Anti-listerial activity for most combinations. | [172] |
| Nisin or pediocin | Corn starch | L. monocytogenes and Clostridium perfringens | Active films against these two bacteria. The halloysite retained antimicrobial activity when comparing to films without nanofiller addition. | [173] |
| Bacteriophages | ||||
| vB_EcoMH2W | Chitosan | E. coli O157:H7 | Chitosan-based edible coating can stabilize phage vB_EcoMH2W without significant loss in lytic activity of phage over a period of one week. | [174] |
| Listeria bacteriophage P100 and silver nanoparticles | Alginate | L. monocytogenes | Antimicrobial activity of the alginate–silver coating was achieved with an alginate concentration of 1%. Adding phage P100 (109 PFU mL−1) into the alginate–silver coating led to a synergic effect that resulted in a 5-log reduction in L. monocytogenes. | [175] |
| Bacteriophages (EC4 and φ135) and cinnamaldehyde (CNMA) | Sodium alginate | S. enteritidis and E. coli | A combination of both phages with the higher concentration of CNMA resulted in a synergic antimicrobial effect against E. coli and a facilitative effect against Salmonella. | [176] |
| Myoviridae bacteriophages (T-even type), DT1 to DT6 | Whey protein concentrate (WPC) matrix | E. coli O157:H7 and STEC strains | The six-phage cocktail added into WPC films was highly stable, effectively released from films, and proved highly effective as a biocontrol. | [177] |
| Nisin and organic acids | ||||
| Citric, lactic, malic, and tartaric acids (0.9, 1.8, 2.6) in combination with nisin (205 IU g −1of protein). | Soy protein | L. monocytogenes, E. coli O157:H7, and S. Gaminara | Malic acid (2.6%) was the most effective acid into soy protein films for controlling these bacteria. | [178] |
| Nisin-EDTA | Gelatin | E. coli | 1 and 3 logarithmic cycles of the films with 5%, 10%, and 20% of the compound (nisin/Na-EDTA) distributed in the polymer matrix, respectively. | [179] |
| Malic, citric, lactic, formic, ascorbic, fumaric, acetic acids (1.5–3%), and Nisin 50 IU/mL, pH 6.2. | Whey protein | L. monocytogenes | Nisin (50 IUmL−1) improved the antilisterial effects of lactic, citric, and malic acids. | [180] |
| Acetic acid (2%), lactic acid (2%), levulinic acid (2%), and 10 ppm of allyl isothiocyanate. | Chitosan | Cocktail of four Salmonella strains | The three-acid solution without chitosan reduced the populations of Salmonella by 6.0, 3.6, and 5.3 log CFU mL−1. | [107] |
| Essential oils | ||||
| Carvacrol (10 g L−1) and pomegranate peel extract (PPE, 10 g L−1). | Chitosan | E. coli and S. aureus | Except for PPE-incorporated film, all exhibited antibacterial activity | [181] |
| Rosemary essential oil (REO, 0.5, 1.0, and 1.5%). | Chitosan | L. monocytogenes, P. putida, S. agalactiae, L. lactis, and E. coli | Higher antibacterial activity | [182] |
| Clove oil (CO, 0.05% v/v) and/or ethylenediaminetetraacetate (E, 10 mM). | Chitosan | E. coli and S. aureus | All coating solutions exhibited moderate to strong antimicrobial activity | [183] |
| Essential oils and functional extracts (FE) (50, 100, 250, 500, 750, and 1000 mg L−1). | Chitosan | S. Typhimurium, E. coli O157:H7, S. aureus, B. cereus, and L. monocytogenes | Antimicrobial activity against most strains tested | [184] |
| Lime and thyme essential oil | Chitosan—beeswax | Escherichia coli DH5α | In vitro experiments showed the best coatings were those of chitosan (1%), beeswax (0.1%), and lime essential oil (0.1%), since no growth of E. coli DH5α took place. | [185] |
| Oregano oil (0, 15.7, 25.9, 36.1 mg mL−1) | Pectin | E. coli O157:H7, S. choleraesuis, S. aureus, and L. monocytogenes. | Pectin-OEO film was effective against E. coli O157:H7, S. aureus, and L. monocytogenes. All concentrations with pectin showed inhibition of violacein production and total coliforms, yeast, and molds. | [186] |
| Geraniol (Ger) and α-terpilenol (Ter) | Ethylene–vinyl alcohol copolymer (EVOH) | E. coli, S. enterica, and L. monocytogenes | The incorporation of Ger and Ter inhibited the growth of the three bacteria. (EVOH/Ter). | [187] |
| Thyme extract-loaded nanoliposomes | Whey protein isolate | S. aureus and E. coli | The possible antimicrobial activity of the films containing TE-loaded nanoliposomes against S. aureus and E. coli decreased in comparison to the free TE-incorporated films. | [188] |
| ZnONPs and rosemary essential oil | Zein nanofibers with κ-carrageenan (Z/KC/ZnONPs/RE) | S. aureus and E. coli | The Z/KC/ZnONPs/RE sample showed inhibition activity against S. aureus (18.5 mm) and E. coli (14.7 mm). | [189] |
| Rosemary and Aloe vera oil | Cellulose acetate | E. coli and B. subtilis | The antimicrobial activity against E. coli and B. subtilis increased as rosemary and A. vera oil percentage increased in cellulose acetate membranes. | [190] |
| Plant extracts | ||||
| Grape seed extract (1%), nisin (10,000 IU/g), and EDTA (0.16%). | Soy protein | L. monocytogenes, E. coli O157:H7, and S. Typhimurium | Film incorporated with the combined GSE, nisin, and EDTA demonstrated the greatest inhibitory activity against L. monocytogenes, E. coli O157:H7, and S. Typhimurium. | [191] |
| Honeysuckle flower extract (5, 10, 20, and 30% | Chitosan | E. coli | The film-forming solution of chitosan with 30% HFE exhibited the best antimicrobial effect. | [192] |
| Grapefruit seed extract | Poly(lactide) (PLA)/poly(butylene adipate-co-terephthalate) | L. monocytogenes and E. coli | Antibacterial activity against L. monocytogenes, but only bacteriostatic activity against E. coli. | [193] |
| Allyl isothiocyanate | Halloysite nanotubes (HNTs) coated with sodium polyacrylate (PA) | S. aureus and E. coli | The activity was pronounced against E. coli at 100 μg mL−1 with concentrations of 25 μg mL−1 and 200 μg mL−1, reducing the viable cell population by 41% and 96%, respectively. | [194] |
| Allyl isothiocyanate (AIT) and nisin | Chitosan | Salmonella | The chitosan + 60 AIT coating reduced populations of native bacteria on cantaloupes to ca. 2 log10 CFU cm−2 during the first 6 days, and populations remained unchanged through day 14 at 10 °C. | [195] |
| Allyl isothiocyanate | Polylactic acid and sugar beet pulp | Salmonella Stanley | The films (8.16 μL of AIT per cm2 of surface area) inhibited the growth of Salmonella during 24 h of incubation at 22 °C, while the populations of Salmonella in controls increased from ca. 4 to over 8 log CFU mL−1, indicating a minimum inactivation of 4 log CFU mL−1 on films in comparison to the growth on controls. | [196] |
| Allyl isothiocyanate (AIT), nisin, 2%, acetic acid, 2% lactic acid, and 2% levulinic acid | Chitosan | Cocktail of three Salmonella strains | The addition of nisin to the chitosan-AIT coating synergistically increased the antibacterial effect. | [197] |
| Chitosan | ||||
| Chitosan (1, 3, and 5%). | Yam starch 4% and chitosan | S. enteritidis | The chitosan-treated film caused a reduction of 1–2 log cycles, the pure chitosan presented a reduction of 4–6 log cycles. | [198] |
| Polyamide 6/66 chitosan (1, 2, 2.5, and 3% w/v). | Plastic films chitosan films chitosan solution | S. typhimurium and S. aureus | Antimicrobial activity was lessened when chitosan was combined with the plastic matrix. | [199] |
| Chitosan (1%)/poly(vinyl alcohol)/pectin (1, 1.5, and 2 g) ternary film. | - | E. coli, S. aureus, B. subtilis, Pseudomonas, and C. albicans | Antimicrobial activity of the film against pathogenic bacteria. | [200] |
| Chitosan nanoparticles (0.1, 0.5, 1, 5, 10, and 30% (v/v)). | Cellulose films | E. coli | A maximum E. coli inhibition of 85% was achieved at 5% (v/v) by doping chitosan nanoparticles into the cellulose films. | [201] |
| Nanoemulsions | ||||
| Cinnamaldehyde 1.5%. | Pectin/papaya puree films | E. coli, S. enterica, L. monocytogenes, and S. aureus | Cinnamaldehyde provided antimicrobial properties against all bacteria tested. L. monocytogenes and S. aureus were more susceptible. | [202] |
| Gold nanoparticles (2.5 and 5% v/v). | Quinoa starch (4%) | E. coli and S. aureus | The active biofilms (2.5% gold nanoparticles) exhibited strong antibacterial activity against foodborne pathogens with inhibition percentages of 99% against E. coli and 98% against S. aureus. | [203] |
| Ag@AgCl/ZnO nanocomposites | Pectin | S. aureus | Nanocomposites with excellent photocatalytic antibacterial activity. | [204] |
| Silver nanoparticles (AgNPs) | Biopolymer pullulan | E. coli O157:H7, L. monocytogenes, S. Typhimurium, S. aureus, and B. cereus | Nanocomposite films, especially pullulan/AgNPs and pullulan/pectin/AgNPs films exhibited good antimicrobial activity against them. | [205] |
| Copper montmorillonite modified (MtCu2+) antimicrobial nanocomposites | Cellulose acetate (CA) | E. coli | Antimicrobial effect was observed for nanocomposites films, obtaining a 98% reduction against E. coli. | [206] |
| Montmorillonite −copper oxide (MMT-CuO) nanocomposites | Chitosan | E. coli, P. aeruginosa, B. aureus, and B. cereus | CSG3MMT-CuO90 films showed more intense antibacterial activity against S. aureus and B. cereus than E. coli and P. aeruginosa. | [207] |
| Silver nanoparticles | Pectin–laponite | E. coli and S. aureus | Coated bionanocomposite exhibited a strong antimicrobial activity against the E. coli and S. aureus. | [208] |
| Silver nanoparticles (AgNps) | Polymer matrix (thiol-acrylato) | E. coli, P. aeruginosa, S. aureus, and B. cereus | Antimicrobial results show that photochemically prepared nanocomposites considerably increase the antimicrobial activity on Gram-negative bacteria compared to Gram-positive bacteria. | [209] |
| Zinc oxide nanoparticles (ZnO-N; 0%, 1.5%, 3%, and 4.5%) | Buckwheat starch (BS) | L. monocytogenes | The BS/ZnO-N films reduced this bacterium in a range of 2.96–3.74 log CFU mL−1. | [210] |
| Others | ||||
| Ag+, Cu2+, Zn2+, Mn2+, or Fe2+ (120 µg mL−1). | Chitosan | E. coli, S. Sholeraesuis, and S. aureus | Antibacterial activity was enhanced by the metal ions loaded, except for Fe2+. Especially for chitosan nanoparticles loaded Cu2+, the MIC and MBC against E. coli, S. Sholeraesuis, and S. aureus were 21–42 times lower than that of Cu2+, respectively. | [211] |
| Chlorine dioxide (ClO2) microcapsule | Polylactic acid (PLA) | E. coli and S. aureus | Addition of ClO2 microcapsules at a concentration of 20% imparted PLA films with excellent antimicrobial properties, with a growth inhibition of E. coli and S. aureus by 4.95 log CFU mL−1, and the antibacterial rate was 99%. | [212] |
| Chlorine dioxide microcapsule | Polylactic acid film | Foodborne pathogens | Cross-sections of the antimicrobial film showed that the film was covered with voids due to deliberate release of chlorine dioxide gas during the packaging process. | [213] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bautista-Baños, S.; Correa-Pacheco, Z.N.; Ventura-Aguilar, R.I.; Landa-Salgado, P.; Cortés-Higareda, M.; Ramos-García, M.d.L. Traditional and Recent Alternatives for Controlling Bacterial Foodborne Pathogens in Fresh Horticultural Commodities—A Review. Coatings 2025, 15, 597. https://doi.org/10.3390/coatings15050597
Bautista-Baños S, Correa-Pacheco ZN, Ventura-Aguilar RI, Landa-Salgado P, Cortés-Higareda M, Ramos-García MdL. Traditional and Recent Alternatives for Controlling Bacterial Foodborne Pathogens in Fresh Horticultural Commodities—A Review. Coatings. 2025; 15(5):597. https://doi.org/10.3390/coatings15050597
Chicago/Turabian StyleBautista-Baños, Silvia, Zormy Nacary Correa-Pacheco, Rosa Isela Ventura-Aguilar, Patricia Landa-Salgado, Mónica Cortés-Higareda, and Margarita de Lorena Ramos-García. 2025. "Traditional and Recent Alternatives for Controlling Bacterial Foodborne Pathogens in Fresh Horticultural Commodities—A Review" Coatings 15, no. 5: 597. https://doi.org/10.3390/coatings15050597
APA StyleBautista-Baños, S., Correa-Pacheco, Z. N., Ventura-Aguilar, R. I., Landa-Salgado, P., Cortés-Higareda, M., & Ramos-García, M. d. L. (2025). Traditional and Recent Alternatives for Controlling Bacterial Foodborne Pathogens in Fresh Horticultural Commodities—A Review. Coatings, 15(5), 597. https://doi.org/10.3390/coatings15050597








