Hydrogen Embrittlement Resistance of Ferritic–Pearlitic Pipeline Steel with Non-Electrochemically Deposited Copper- or Nickel–Phosphorus-Based Coating
Abstract
1. Introduction
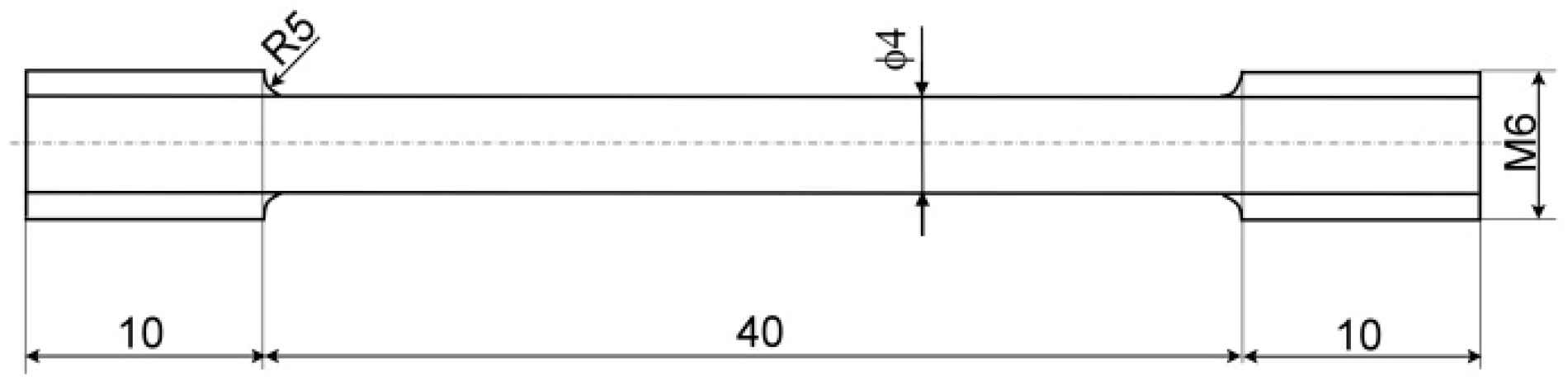
2. Materials and Methods
3. Results and Discussion
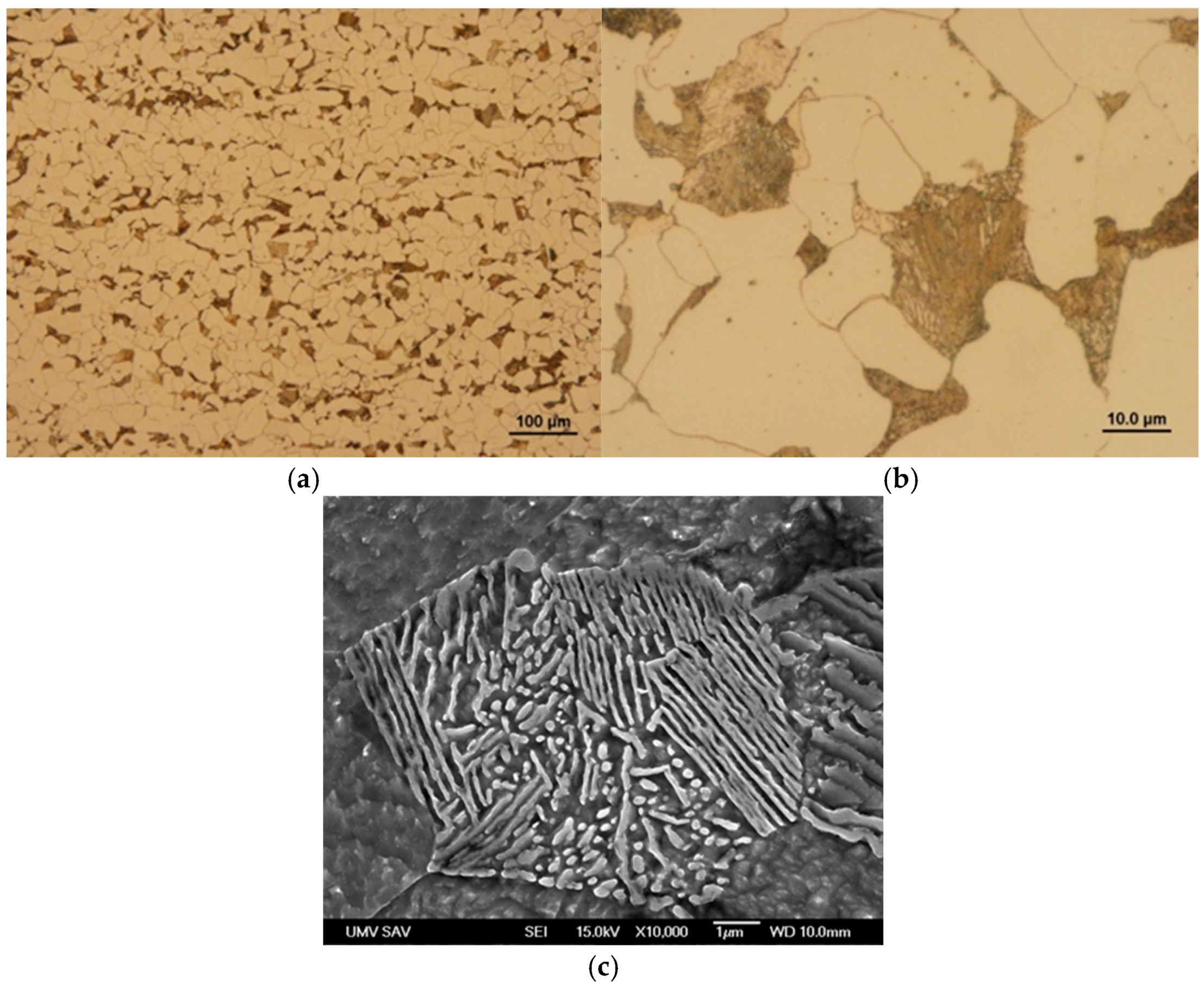
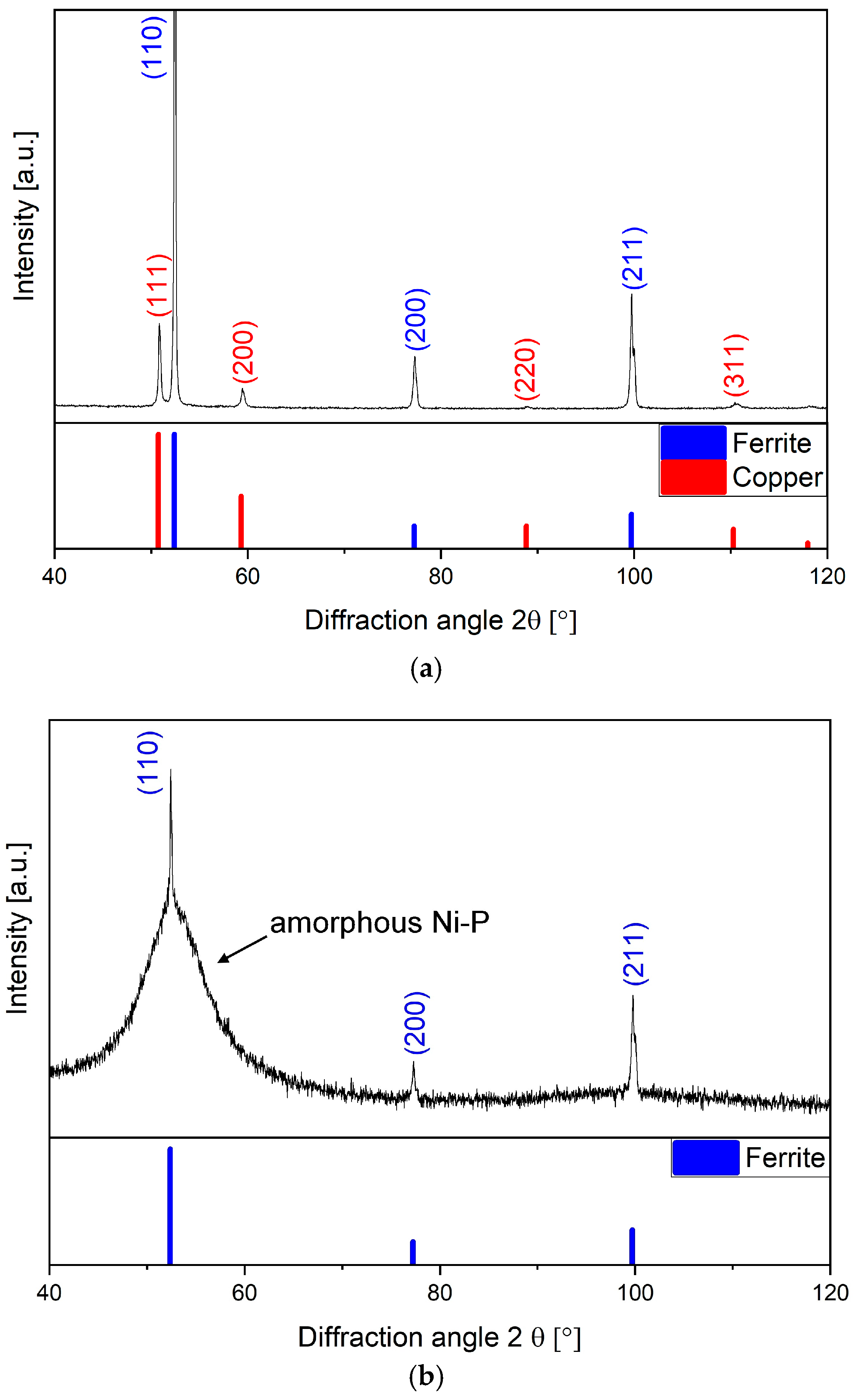
3.1. Microstructure and Phase Analyses
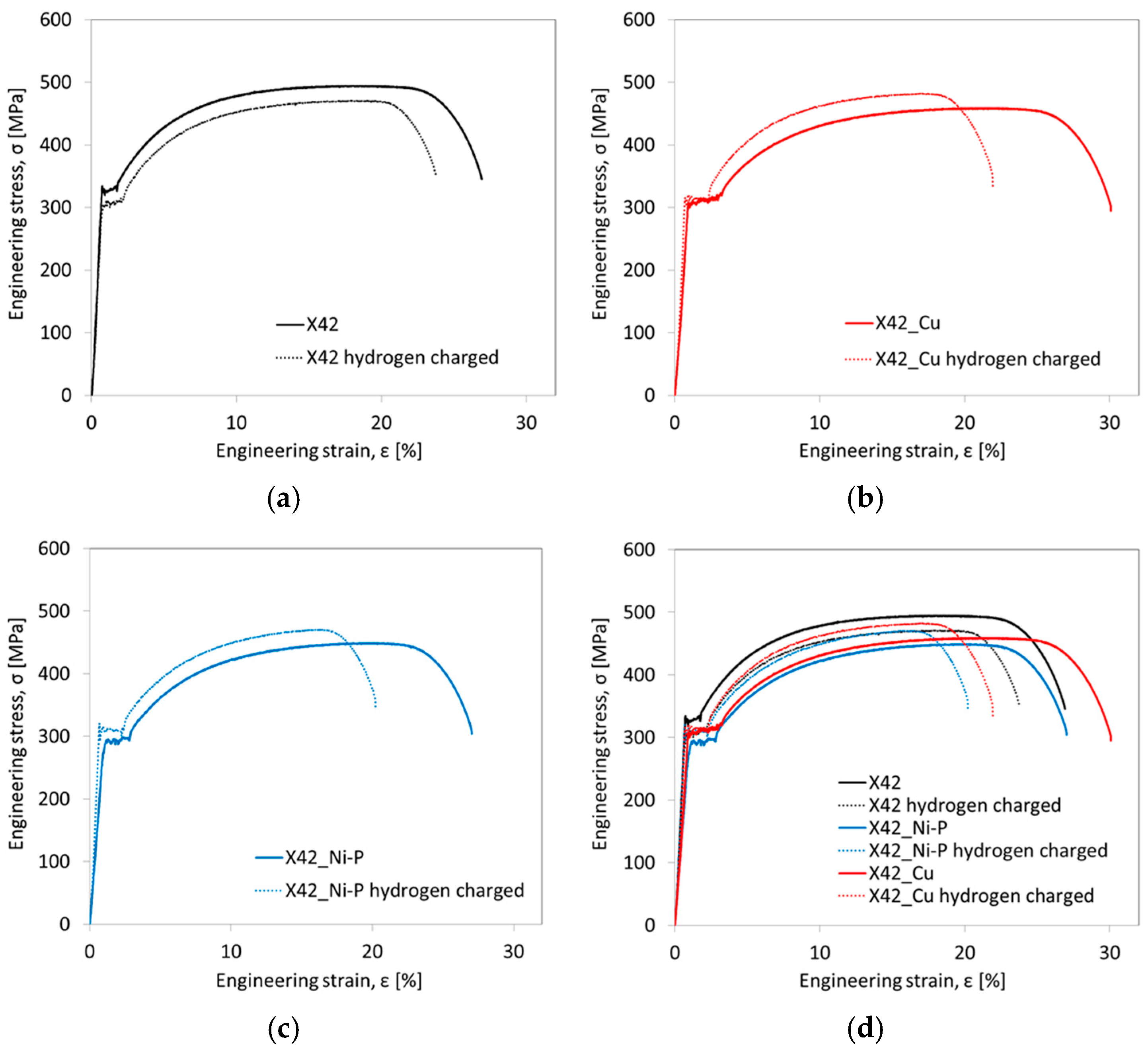
3.2. Tensile Properties and HE Resistance
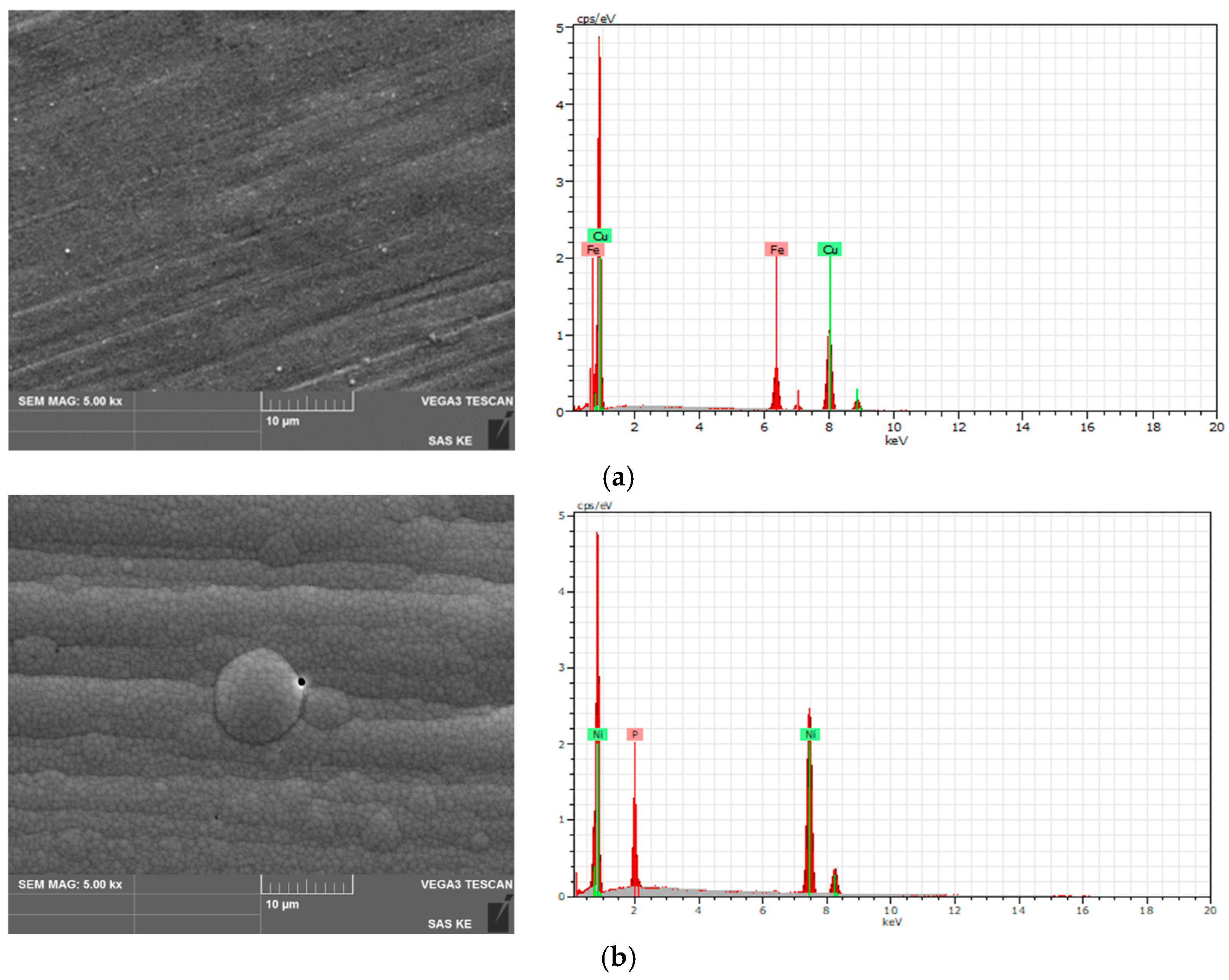
3.3. Fractographic Characterization
4. Conclusions
- The electroless plating procedures used resulted in chemically stable copper- and nickel–phosphorus-based coatings on the surface of the X42 steel base material. The Cu-based coating had a fully crystalline lattice structure, whereas the Ni–P-based coating was of an amorphous nature.
- The room-temperature tensile properties of the X42 material in the non-hydrogenated material condition were unaffected by the coatings used. The thickness of the studied coatings did not play a role in the view of the HE resistance of the material coating systems studied under the used electrolytic hydrogenation conditions.
- Both applied coatings considerably improved the HEI values related to the changes in the yield stress, ultimate tensile strength, and reduction of area. On the contrary, the HEI value related to total elongation deteriorated, which was ascribed to the earlier onset of necking during tensile straining due to observed surface imperfections within the coatings.
- Based on the obtained results of tensile tests and related fractographic observations, it is concluded that the studied Cu- and Ni–P-based coatings served as hydrogen permeation barriers, reducing the hydrogen migration into the steel matrix beneath the coatings. Nevertheless, further research efforts are needed in terms of the surface quality improvement of the coatings under consideration.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gajdzik, B.; Nagaj, R.; Wolniak, R.; Bałaga, D.; Žuromskaitė, B.; Grebski, W.W. Renewable Energy Share in European Industry: Analysis and Extrapolation of Trends in EU Countries. Energies 2024, 17, 2476. [Google Scholar] [CrossRef]
- Khurshid, H.; Mohammed, B.S.; Al-Yacouby, A.M.; Liew, M.S.; Zawawi, N.A.W.A. Analysis of hybrid offshore renewable energy sources for power generation: A literature review of hybrid solar, wind, and waves energy systems. Dev. Built Environ. 2024, 19, 100497. [Google Scholar] [CrossRef]
- Zhang, X.; Feng, D.; Wang, J.; Sui, A. Integrating renewable energy systems: Assessing financial innovation, renewable energy generation intensity, energy transition and environmental regulation with renewable energy sources. Energy Strat. Rev. 2024, 56, 101567. [Google Scholar] [CrossRef]
- Urhan, B.B.; Erdoğmuş, A.; Dokuz, A.S.; Gökçek, M. Predicting green hydrogen production using electrolyzers driven by photovoltaic panels and wind turbines based on machine learning techniques: A pathway to on-site hydrogen refuelling stations. Int. J. Hydrogen Energy 2025, 101, 1421–1438. [Google Scholar] [CrossRef]
- Ďurovič, M.; Hnát, J.; Strečková, M.; Bouzek, K. Efficient cathode for the hydrogen evolution reaction in alkaline membrane water electrolysis based on NiCoP embedded in carbon fibres. J. Power Sources 2023, 556, 232506. [Google Scholar] [CrossRef]
- Nigutová, K.; Oroszová, L.; Molčanová, Z.; Csík, D.; Gáborová, K.; Möllmer, J.; Lange, M.; Saksl, K. Experimental Validation of Hydrogen Affinity as a Design Criterion for Alloys. Materials 2024, 17, 6106. [Google Scholar] [CrossRef]
- Zhang, R.; Ai, S.; Long, M.; Wan, L.; Li, Y.; Jia, D.; Duan, H.; Chen, D. Quantitative Study on Hydrogen Concentration–Hydrogen Embrittlement Sensitivity of X80 Pipeline Steel Based on Hydrogen Permeation Kinetics. Metals 2024, 14, 763. [Google Scholar] [CrossRef]
- Gubóová, A.; Oriňaková, R.; Strečková, M.; Paračková, M.; Petruš, O.; Plešingerová, B.; Mičušík, M. Iron-nickel metal foams modified by phosphides as robust catalysts for a hydrogen evolution reaction. Mater. Today Chem. 2023, 34, 101778. [Google Scholar] [CrossRef]
- Varcholová, D.; Kušnírová, K.; Oroszová, L.; Möllmer, J.; Lange, M.; Gáborová, K.; Buľko, B.; Demeter, P.; Saksl, K. New-Generation Materials for Hydrogen Storage in Medium-Entropy Alloys. Materials 2024, 17, 2897. [Google Scholar] [CrossRef]
- Bera, C.; Streckova, M.; Orinakova, R.; Guboova, A.; Bystron, T.; Girman, V.; Kromka, F.; Podobova, M.; Bouzek, K. NiCoP fibers as novel catalysts for hydrogen evolution in alkali and acidic environment. Int. J. Hydrogen Energy 2024, 60, 118–132. [Google Scholar] [CrossRef]
- Tashie-Lewis, B.C.; Nnabuife, S.G. Hydrogen Production, Distribution, Storage and Power Conversion in a Hydrogen Economy—A Technology Review. Chem. Eng. J. Adv. 2021, 8, 100172. [Google Scholar] [CrossRef]
- Akhtar, M.S.; Khan, H.; Liu, J.J.; Na, J. Green hydrogen and sustainable development—A social LCA perspective highlighting social hotspots and geopolitical implications of the future hydrogen economy. J. Clean. Prod. 2023, 395, 136438. [Google Scholar] [CrossRef]
- Ghadiani, H.; Farhat, Z.; Alam, T.; Islam, M.A. Fracture Toughness Assessment of Pipeline Steels Under Hydrogen Exposure for Blended Gas Applications. Metals 2025, 15, 29. [Google Scholar] [CrossRef]
- Falat, L.; Čiripová, L.; Petruš, O.; Puchý, V.; Petryshynets, I.; Kovaľ, K.; Džunda, R. The Effects of Electrochemical Hydrogen Charging on Charpy Impact Toughness and Dry Sliding Tribological Behavior of AISI 316H Stainless Steel. Crystals 2023, 13, 1249. [Google Scholar] [CrossRef]
- Li, Q.; Ghadiani, H.; Jalilvand, V.; Alam, T.; Farhat, Z.; Islam, M.A. Hydrogen Impact: A Review on Diffusibility, Embrittlement Mechanisms, and Characterization. Materials 2024, 17, 965. [Google Scholar] [CrossRef]
- Ghadiani, H.; Farhat, Z.; Alam, T.; Islam, M.A. Assessing Hydrogen Embrittlement in Pipeline Steels for Natural Gas-Hydrogen Blends: Implications for Existing Infrastructure. Solids 2024, 5, 375–393. [Google Scholar] [CrossRef]
- Johnson, W.H. On some remarkable changes produced in iron and steel by the action of hydrogen and acids. Proc. R. Soc. Lond. 1875, 23, 168–179. [Google Scholar] [CrossRef]
- Padhy, G.K.; Komizo, Y.I. Diffusible Hydrogen in Steel Weldments-A Status Review. Trans. JWRI 2013, 42, 39–62. [Google Scholar] [CrossRef]
- Depover, T.; Laureys, A.; Pérez Escobar, D.; Van den Eeckhout, E.; Wallaert, E.; Verbeken, K. Understanding the Interaction between a Steel Microstructure and Hydrogen. Materials 2018, 11, 698. [Google Scholar] [CrossRef]
- Dwivedi, S.K.; Vishwakarma, M. Hydrogen embrittlement in different materials: A review. Int. J. Hydrogen Energy 2018, 43, 21603–21616. [Google Scholar] [CrossRef]
- Islam, A.; Alam, T.; Sheibley, N.; Edmonson, K.; Burns, D.; Hernandez, M. Hydrogen blending in natural gas pipelines: A comprehensive review of material compatibility and safety considerations. Int. J. Hydrogen Energy 2024, 93, 1429–1461. [Google Scholar] [CrossRef]
- Rosa, N.; Fereidani, N.A.; Cardoso, B.J.; Martinho, N.; Gaspar, A.; Silva, M.G. Advances in hydrogen blending and injection in natural gas networks: A review. Int. J. Hydrogen Energy 2025, 105, 367–381. [Google Scholar] [CrossRef]
- Cui, D.; Bai, Y.; Xiong, L.; Yu, B.; Wei, B.; Sun, C. Effects of hydrogen blending ratios and CO2 on hydrogen embrittlement of X65 steel in high-pressure offshore hydrogen-blended natural gas pipelines. J. Mater. Res. Technol. 2024, 33, 4763–4771. [Google Scholar] [CrossRef]
- Kim, W.J.; Park, Y.; Park, D.J. Quantitative risk assessments of hydrogen blending into transmission pipeline of natural gas. J. Loss Prev. Process Ind. 2024, 91, 105412. [Google Scholar] [CrossRef]
- Mahajan, D.; Tan, K.; Venkatesh, T.; Kileti, P.; Clayton, C.R. Hydrogen Blending in Gas Pipeline Networks—A Review. Energies 2022, 15, 3582. [Google Scholar] [CrossRef]
- Toribio, J. HELP versus HEDE in progressively cold-drawn pearlitic steels: Between Donatello and Michelangelo. Eng. Fail. Anal. 2018, 94, 157–164. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, H.; Yang, M.; Jia, W.; Qiu, Y.; Lan, L. From the perspective of new technology of blending hydrogen into natural gas pipelines transmission: Mechanism, experimental study, and suggestions for further work of hydrogen embrittlement in high-strength pipeline steels. Int. J. Hydrogen Energy 2022, 47, 8071–8090. [Google Scholar] [CrossRef]
- Turnbull, A. Perspectives on hydrogen uptake, diffusion and trapping. Int. J. Hydrogen Energy 2015, 40, 16961–16970. [Google Scholar] [CrossRef]
- Dadfarnia, M.; Sofronis, P.; Neeraj, T. Hydrogen interaction with multiple traps: Can it be used to mitigate embrittlement? Int. J. Hydrogen Energy 2011, 36, 10141–10148. [Google Scholar] [CrossRef]
- Stopher, M.A.; Lang, P.; Kozeschnik, E.; Pedro, J.; del-Castillo, R.D. Modelling hydrogen migration and trapping in steels. Mater. Des. 2016, 106, 205–215. [Google Scholar] [CrossRef]
- Ohaeri, E.; Eduok, U.; Szpunar, J. Hydrogen related degradation in pipeline steel: A review. Int. J. Hydrogen Energy 2018, 43, 14584–14617. [Google Scholar] [CrossRef]
- Atrens, A.; Liu, Q.; Tapia-Bastidas, C.; Gray, E.; Irwanto, B.; Venezuela, J.; Liu, Q. Influence of Hydrogen on Steel Components for Clean Energy. Corros. Mater. Degrad. 2018, 1, 3–26. [Google Scholar] [CrossRef]
- Yi, K.; Ma, R.; Xiang, S.; Liu, X.; Liu, C.; Zhang, X.; Fu, Y. Eliminating reversible hydrogen embrittlement in high-strength martensitic steel by an electric current pulse. Int. J. Hydrogen Energy 2022, 47, 17045–17055. [Google Scholar] [CrossRef]
- Sobola, D.; Dallaev, R. Exploring Hydrogen Embrittlement: Mechanisms, Consequences, and Advances in Metal Science. Energies 2024, 17, 2972. [Google Scholar] [CrossRef]
- Kolachev, B.A. Hydrogen Brittleness of Metals; Metallurgy: Moscow, Russia, 1985; p. 216. [Google Scholar]
- Myagkikh, P.N. Hydrogenation and Hydrogen Embrittlement. Master’s Thesis, Toglyatti State University, Institute of Mechanical Engineering, Togliatti, Russia, 2016. [Google Scholar]
- Li, H.; Niu, R.; Li, W.; Lu, H.; Cairney, J.; Chen, Y.S. Hydrogen in pipeline steels: Recent advances in characterization and embrittlement mitigation. J. Nat. Gas Sci. Eng. 2022, 105, 104709. [Google Scholar] [CrossRef]
- Lee, J.Y.; Lee, S.M. Hydrogen trapping phenomena in metals with bcc and fcc crystal structures by the desorption thermal-analysis technique. Surf. Coat. Technol. 1986, 28, 301–314. [Google Scholar] [CrossRef]
- Hirata, K.; Iikubo, S.; Koyama, M.; Tsuzaki, K.; Ohtani, H. First principles study on hydrogen diffusivity in BCC, FCC, and HCP iron. Metall. Mater. Trans. A 2018, 49, 5015–5022. [Google Scholar] [CrossRef]
- Balaji, V.; Jeyapandiarajan, P.; Joel, J.; Arivazhagan Anbalagan, P.; Ashwath, S.; Anouncia, M.; Batako, A.; Xavior, M.A. Mitigating hydrogen embrittlement in high-entropy alloys for next-generation hydrogen storage systems. J. Mater. Res. Technol. 2024, 33, 7681–7697. [Google Scholar] [CrossRef]
- Nnoka, M.; Jack, T.A.; Szpunar, J. Effects of different microstructural parameters on the corrosion and cracking resistance of pipeline steels: A review. Eng. Fail. Anal. 2024, 159, 108065. [Google Scholar] [CrossRef]
- Shao, Y.; Liu, X.; Song, Y.; Zhang, Z.; Wang, P.; Xing, X.; Peng, W.; Xing, S.; Bian, J.; Cao, X. Experimental study on slow tensile, fatigue, and impact on X42 steel and #20 carburizing steel. Int. J. Press. Vessel. Pip. 2024, 208, 105139. [Google Scholar] [CrossRef]
- Villavicencio, J.; Ulloa, N.; Lozada, L.; Moreno, M.; Castro, L. The role of non-metallic Al2O3 inclusions, heat treatments and microstructure on the corrosion resistance of an API 5L X42 steel. J. Mater. Res. Technol. 2020, 9, 5894–5911. [Google Scholar] [CrossRef]
- Rahimi, S.; Verbeken, K.; Depover, T.; Proverbio, E. Hydrogen embrittlement of pipeline steels under gaseous and electrochemical charging: A comparative review on tensile properties. Eng. Fail. Anal. 2025, 167, 108956. [Google Scholar] [CrossRef]
- Lima dos Santos, M.; Filgueira de Almeida, A.; de Sousa Figueiredo, G.G.; da Silva, M.M.; Maciel, T.M.; Santos, T.F.A.; de Santana, R.A.C. Influence of Centerline Segregation Region on the Hydrogen Embrittlement Susceptibility of API 5L X80 Pipeline Steels. Metals 2024, 14, 1154. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Park, J.S.; Nahm, S.H.; Baek, U.B. Evaluation of hydrogen related degradation of API X42 pipeline under hydrogen/natural gas mixture conditions using small punch test. Theor. Appl. Fract. Mech. 2021, 113, 102961. [Google Scholar] [CrossRef]
- Walallawita, R.; Hinchliff, M.C.; Sediako, D.; Quinn, J.; Chou, V.; Walker, K.; Hill, M. Evaluating the Effect of Blended and Pure Hydrogen in X60 Pipeline Steel for Low-Pressure Transmission Using Hollow-Specimen Slow-Strain-Rate Tensile Testing. Metals 2024, 14, 1132. [Google Scholar] [CrossRef]
- Wang, H.; Ming, H.; Wang, J.; Ke, W.; Han, E.H. Hydrogen permeation behavior at different positions in the normal direction of X42 and X52 pipeline steels. Int. J. Hydrogen Energy 2024, 72, 1105–1115. [Google Scholar] [CrossRef]
- Bhadeshia, H.K.D.H. Prevention of Hydrogen Embrittlement in Steels. ISIJ Int. 2016, 56, 24–36. [Google Scholar] [CrossRef]
- Nemanič, V. Hydrogen permeation barriers: Basic requirements, materials selection, deposition methods, and quality evaluation. Nucl. Mater. Energy 2019, 19, 451–457. [Google Scholar] [CrossRef]
- Chatterjee, S.S.; Ateya, B.G.; Pickering, H.W. Effect of electrodeposited metals on the permeation of hydrogen through iron membranes. Metall. Trans. A 1978, 9, 389–395. [Google Scholar] [CrossRef]
- Samanta, S.; Singh, C.; Banerjee, A.; Mondal, K.; Dutta, M.; Singh, S.B. Development of amorphous Ni-P coating over API X70 steel for hydrogen barrier application. Surf. Coat. Technol. 2020, 403, 126356. [Google Scholar] [CrossRef]
- Biggio, D.; Elsener, B.; Rossi, A. Ni-P Coatings as Hydrogen Permeation Barriers—A Review. Coatings 2025, 15, 365. [Google Scholar] [CrossRef]
- Chen, J.-M.; Wu, J.-K. Hydrogen diffusion through copper-plated AISI 4140 steels. Corros. Sci. 1992, 33, 657–666. [Google Scholar] [CrossRef]
- Michler, T.; Naumann, J. Coatings to reduce hydrogen environment embrittlement of 304 austenitic stainless steel. Surf. Coat. Technol. 2009, 203, 1819–1828. [Google Scholar] [CrossRef]
- ISO 6892-1:2019; Metallic Materials—Tensile Testing Part 1: Method of Test at Room Temperature. ISO: London, UK, 2019. Available online: https://www.iso.org/standard/78322.html (accessed on 5 March 2025).
- Walton, W. Feret‘s Statistical Diameter as a Measure of Particle Size. Nature 1948, 162, 329–330. [Google Scholar] [CrossRef]
- He, Y.P.; Liu, G.M.; Duan, X.H.; Wu, T.; Dong, M.; Zhu, Y.B. Effects of nanocrystals on hydrogen permeation and diffusion in amorphous electroless Ni-P coatings. Mater. Today Commun. 2024, 40, 109499. [Google Scholar] [CrossRef]
- Sun, C.; Li, J.; Shuang, S.; Zeng, H.; Luo, J.L. Effect of defect on corrosion behavior of electroless Ni-P coating in CO2-saturated NaCl solution. Corros. Sci. 2018, 134, 23–37. [Google Scholar] [CrossRef]
- Xu, X.; Miao, J.; Bai, Z.; Feng, Y.; Ma, Q.; Zhao, W. The corrosion behavior of electroless Ni–P coating in Cl−/H2S environment. Appl. Surf. Sci. 2012, 258, 8802–8806. [Google Scholar] [CrossRef]
- Cottrell, A.H.; Bilby, B.A. Dislocation theory of yielding and strain ageing of iron. Proc. Phys. Soc. 1949, 62, 49–62. [Google Scholar] [CrossRef]
- Zhou, Q.J.; Qiao, L.J.; Qi, H.B.; Li, J.X.; He, J.Y.; Chu, W.Y. Hydrogen blistering and hydrogen-induced cracking in amorphous nickel phosphorus coating. J. Non-Crystal. Solids 2007, 353, 4011–4014. [Google Scholar] [CrossRef]
- Popov, B.N. Chapter 8—Hydrogen Permeation and Hydrogen-Induced Cracking. In Corrosion Engineering Principles and Solved Problems, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2025; pp. 333–382. ISBN 978-0-443-22011-1. [Google Scholar] [CrossRef]
- Gathimba, N.; Kitane, Y. Effect of surface roughness on tensile ductility of artificially corroded steel plates. J. Constr. Steel Res. 2021, 176, 106392. [Google Scholar] [CrossRef]





| C | N | Mn | Si | P | S | Cu | Cr | Mo | V | Ni | Al | Sn | Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.16 | 0.009 | 0.51 | 0.24 | 0.014 | 0.01 | 0.19 | 0.09 | 0.02 | 0.007 | 0.08 | 0.027 | 0.012 | Balance |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falat, L.; Čiripová, L.; Kromka, F.; Homolová, V.; Džunda, R.; Motýľová, M. Hydrogen Embrittlement Resistance of Ferritic–Pearlitic Pipeline Steel with Non-Electrochemically Deposited Copper- or Nickel–Phosphorus-Based Coating. Coatings 2025, 15, 585. https://doi.org/10.3390/coatings15050585
Falat L, Čiripová L, Kromka F, Homolová V, Džunda R, Motýľová M. Hydrogen Embrittlement Resistance of Ferritic–Pearlitic Pipeline Steel with Non-Electrochemically Deposited Copper- or Nickel–Phosphorus-Based Coating. Coatings. 2025; 15(5):585. https://doi.org/10.3390/coatings15050585
Chicago/Turabian StyleFalat, Ladislav, Lucia Čiripová, František Kromka, Viera Homolová, Róbert Džunda, and Marcela Motýľová. 2025. "Hydrogen Embrittlement Resistance of Ferritic–Pearlitic Pipeline Steel with Non-Electrochemically Deposited Copper- or Nickel–Phosphorus-Based Coating" Coatings 15, no. 5: 585. https://doi.org/10.3390/coatings15050585
APA StyleFalat, L., Čiripová, L., Kromka, F., Homolová, V., Džunda, R., & Motýľová, M. (2025). Hydrogen Embrittlement Resistance of Ferritic–Pearlitic Pipeline Steel with Non-Electrochemically Deposited Copper- or Nickel–Phosphorus-Based Coating. Coatings, 15(5), 585. https://doi.org/10.3390/coatings15050585






