DFT-Based Investigation of Pd-Modified WO3/Porous Silicon Composites for NO2 Gas Sensors: Enhanced Synergistic Effect and High-Performance Sensing
Abstract
1. Introduction
2. Materials and Methods
2.1. First-Principles Calculations
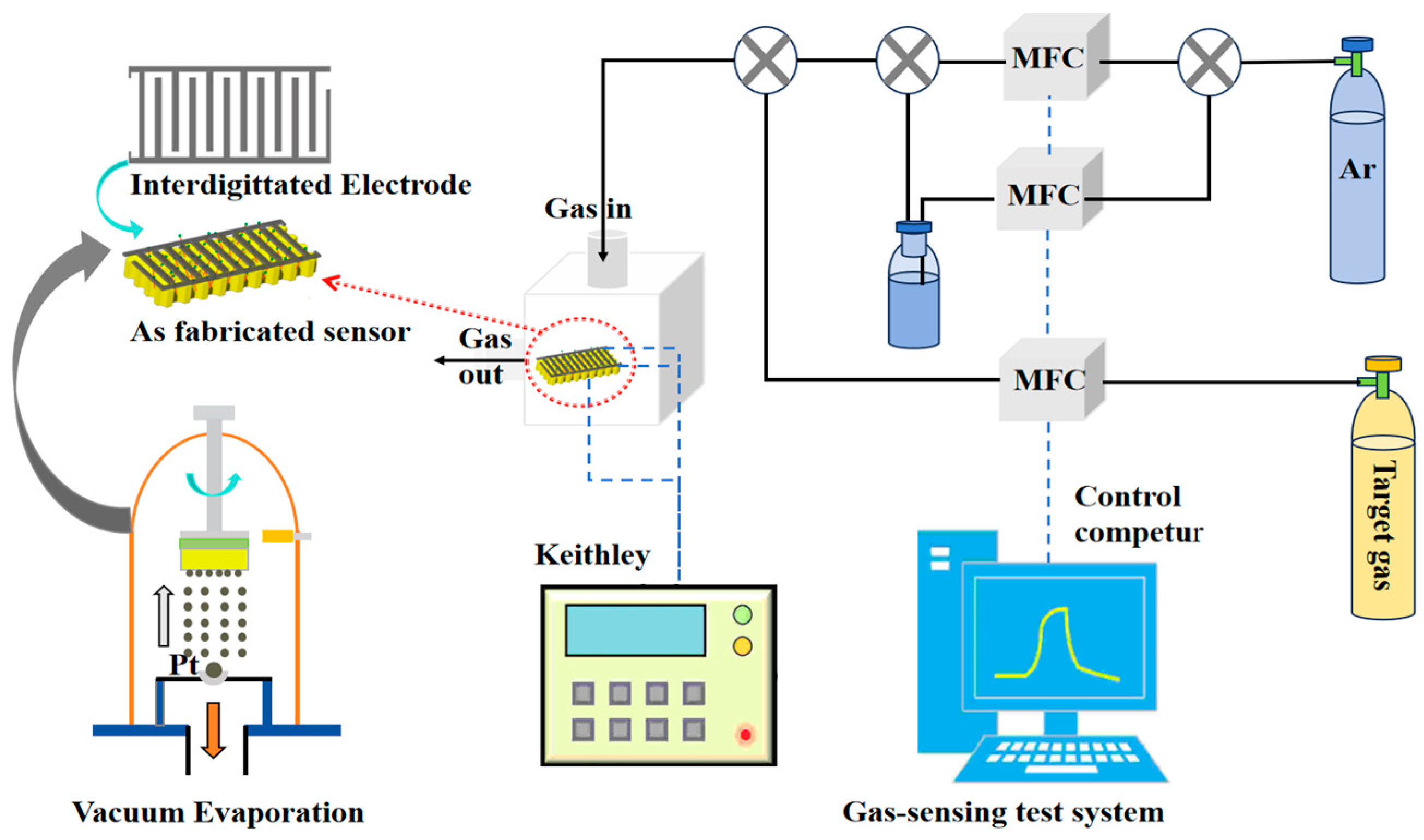
2.2. Synthesis of Pd-WO3/PSi Composite and Its Gas Sensing Measurements
3. Results and Discussion
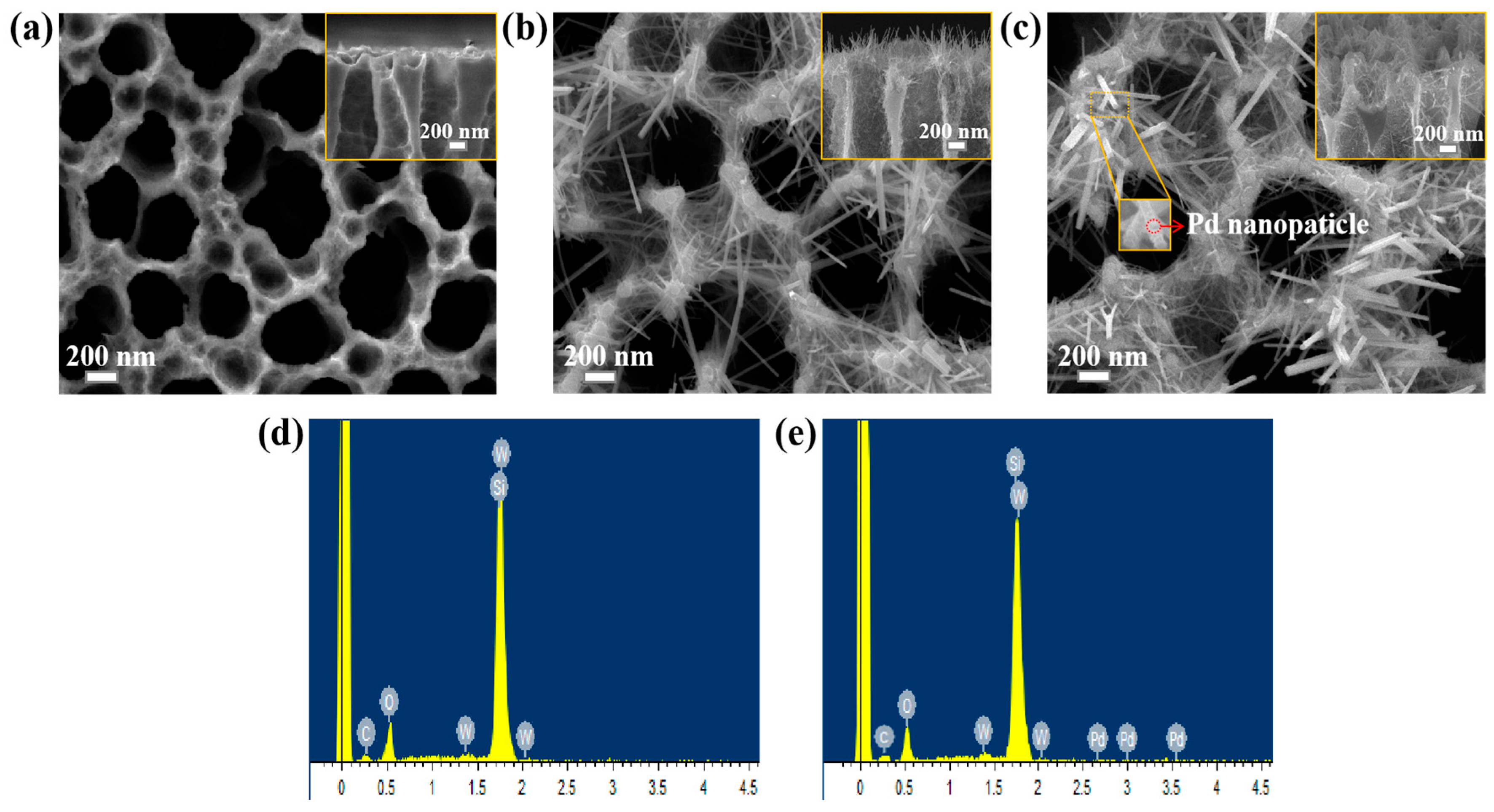
3.1. Morphology Analysis
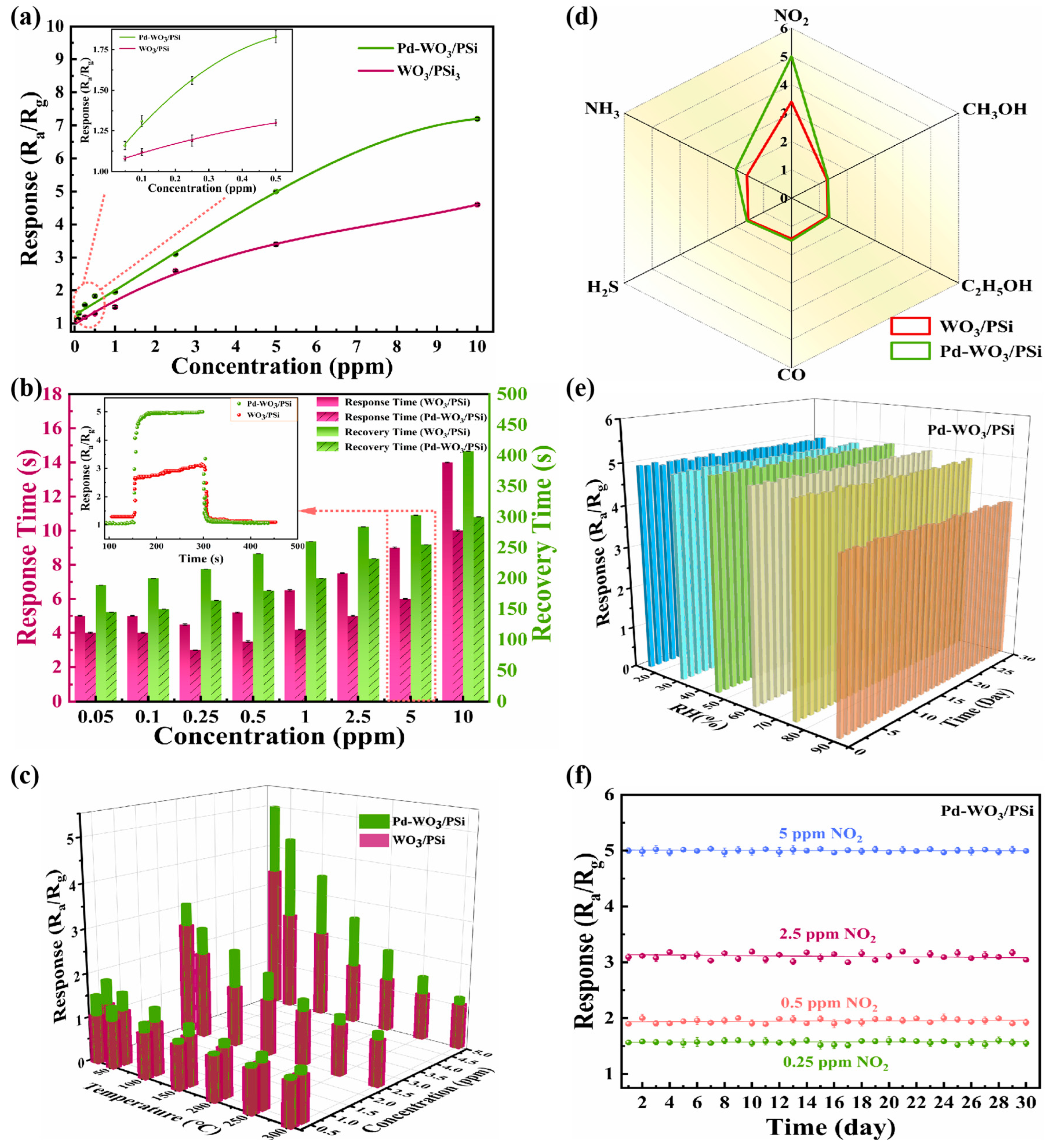
3.2. Gas Sensing Characterization
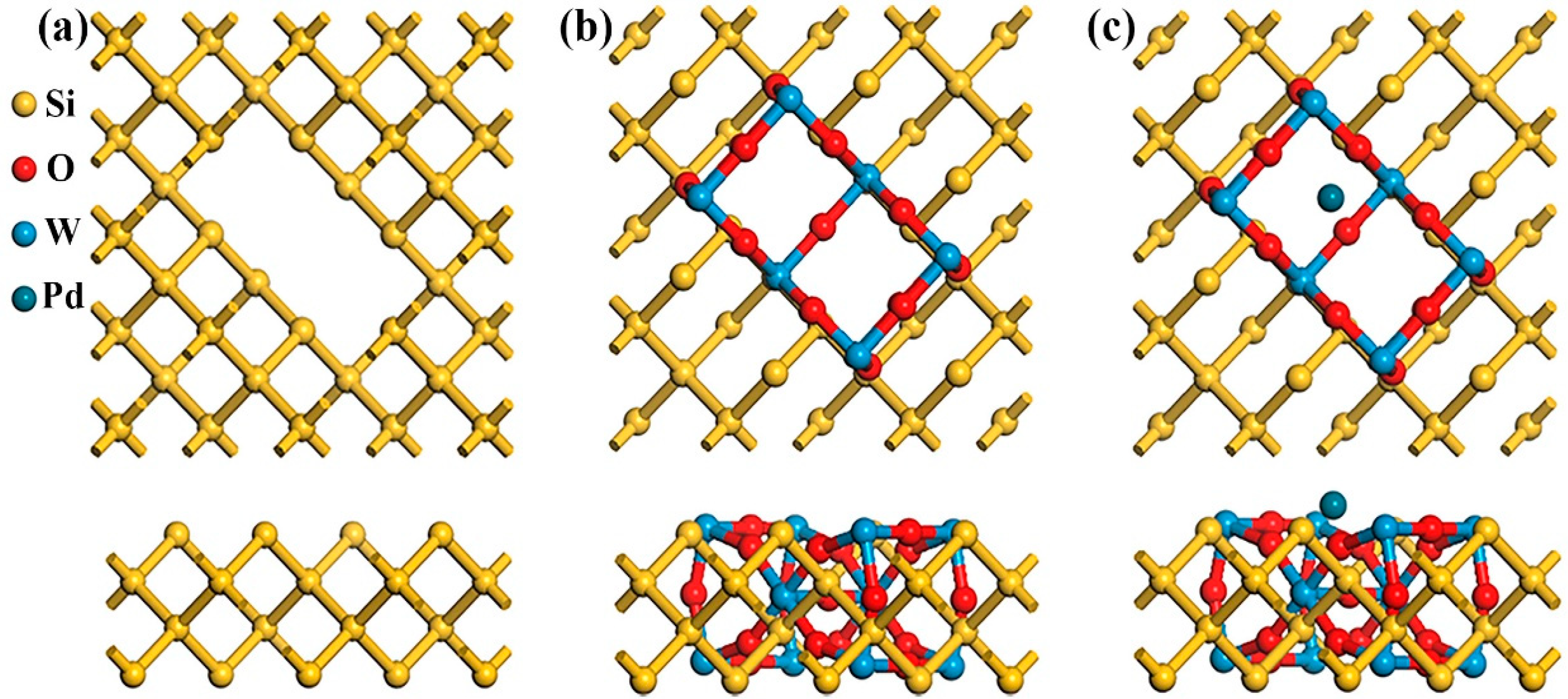
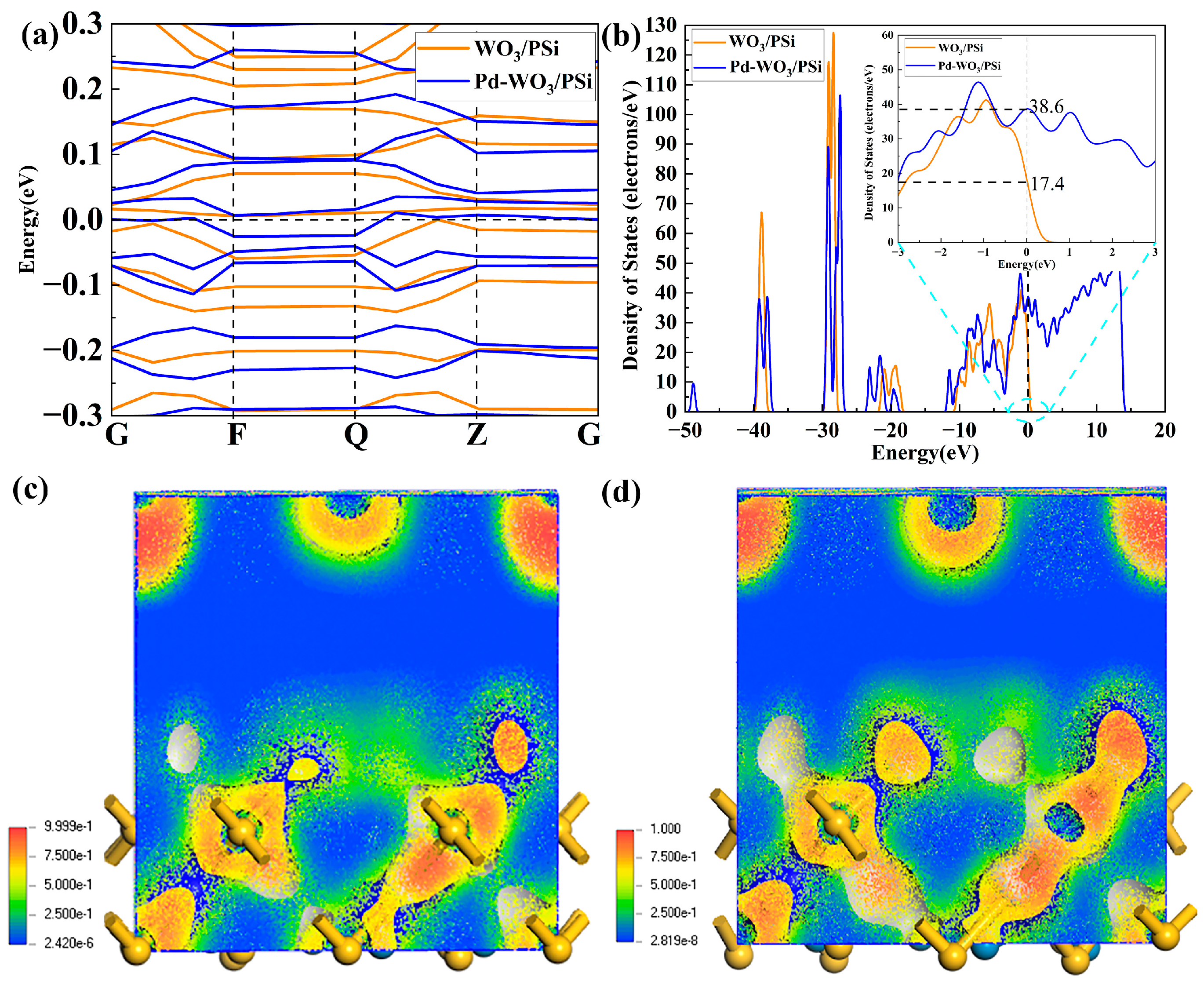
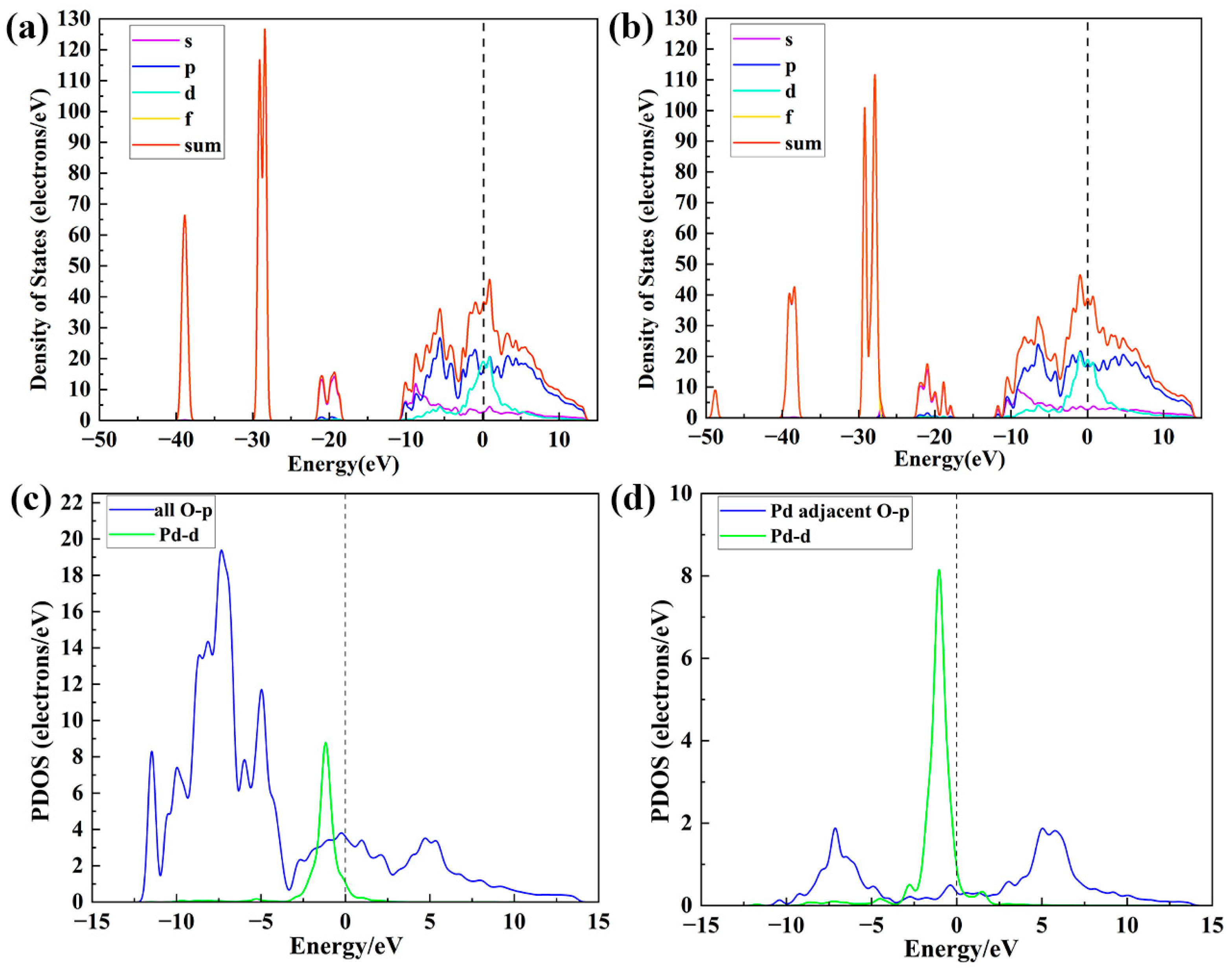
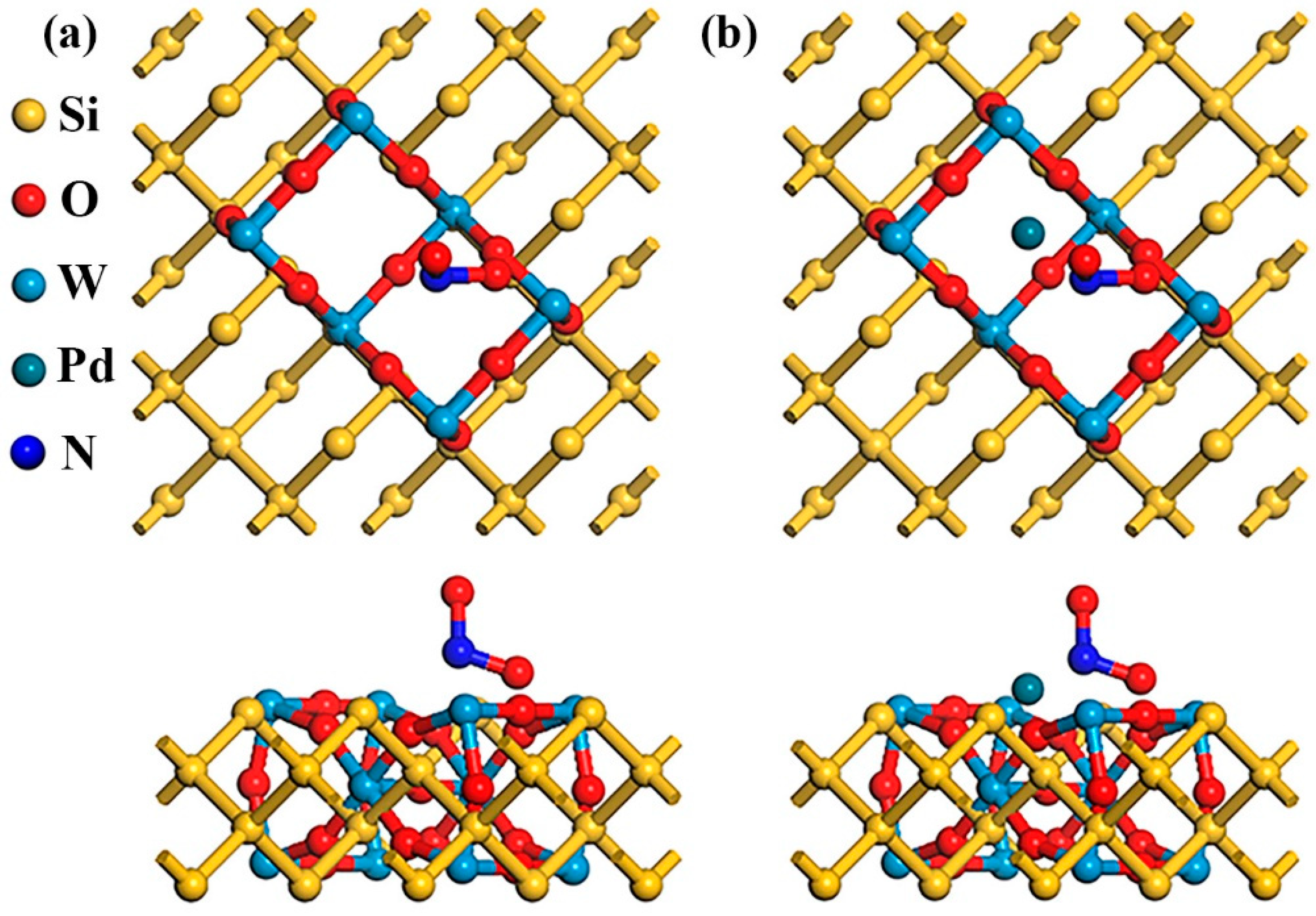
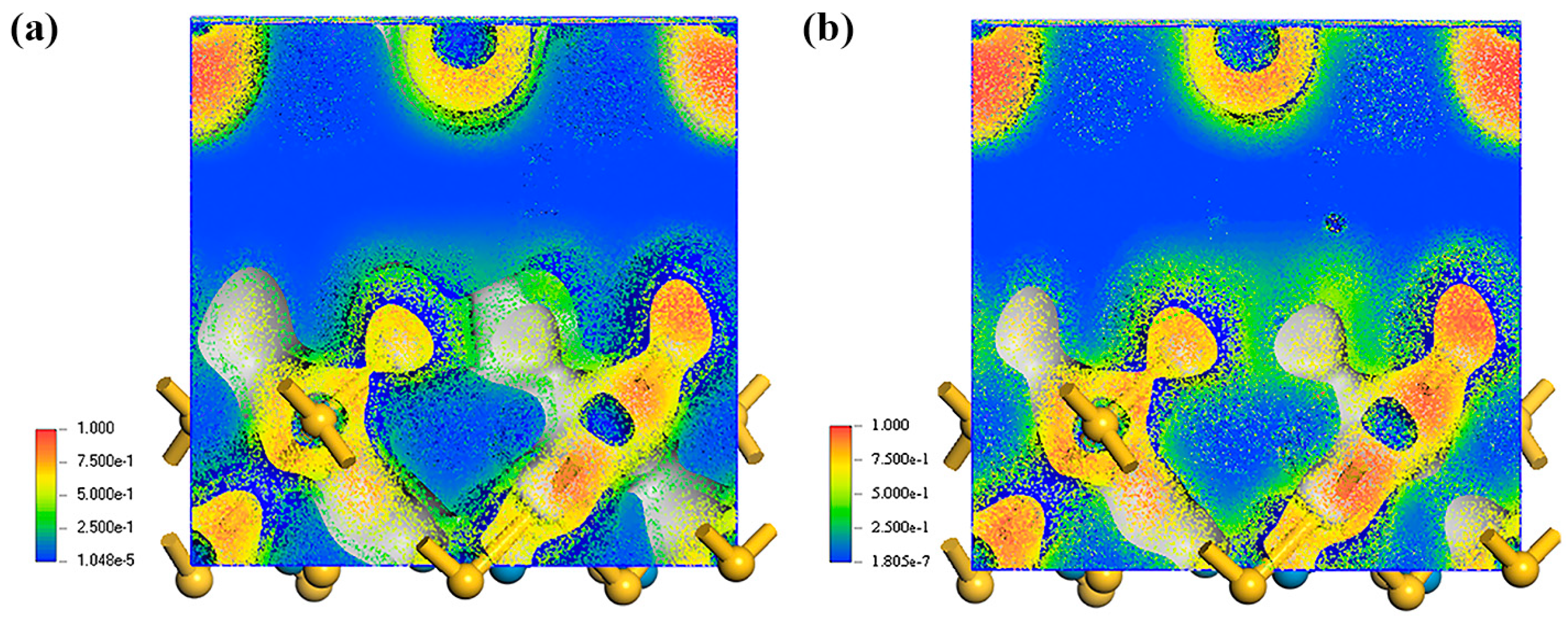
3.3. DFT Analysis Without NO2
3.4. DFT Analysis with NO2
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qiu, P.; Qin, Y.; Zhu, L. Memristive Gas Sensor (Gasistor) Based on Ag/Ordered TiO2 Nanorods/FTO Sandwich Structure for Evaluation of Ethanol Concentration in Mixed Ambient. Sens. Actuators B Chem. 2024, 421, 136548. [Google Scholar] [CrossRef]
- Tokimori, S.; Funato, K.; Wada, K.; Matsuyama, T.; Okamoto, K. Emission Enhancement of ZnO Thin Films in Ultraviolet Wavelength Region Using Au Nano-Hemisphere on Al Mirror Structures. Nanomaterials 2025, 15, 400. [Google Scholar] [CrossRef]
- Ren, L.; Li, Y.; Li, Z.; Lin, X.; Lu, C.; Ding, W.; Zou, J. Boosting Hydrogen Storage Performance of MgH2 by Oxygen Vacancy-Rich H-V2O5 Nanosheet as an Excited H-Pump. Nanomicro Lett. 2024, 16, 160. [Google Scholar] [CrossRef]
- Park, K.R.; Cho, H.B.; Lee, J.; Song, Y.; Kim, W.B.; Choa, Y.H. Design of Highly Porous SnO2-CuO Nanotubes for Enhancing H2S Gas Sensor Performance. Sens. Actuators B Chem. 2020, 302, 127179. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, G.J.; Zhang, J.X.; Bai, F.Q.; Zhang, H.X. First-Principles Investigation on the Interfacial Interaction and Electronic Structure of BiVO4/WO3 Heterostructure Semiconductor Material. Appl. Surf. Sci. 2021, 549, 149309. [Google Scholar] [CrossRef]
- Szindler, M.M.; Szindler, M.; Bicz, J.; Matus, K. Al2O3/ZnO Multilayer Coatings for Improvement in Functional Properties of Surgical Scalpel Blades. Coatings 2025, 15, 436. [Google Scholar] [CrossRef]
- Leonard, R.L.; Bull, A.B.; Xue, F.; Haycook, C.P.; Gray, S.K.; Bond, C.W.; Bond, P.E.; Brown, L.R.; Giorgio, T.D.; Johnson, J.A. Biocompatibility of Al2O3-Doped Diamond-like Carbon Laparoscope Coatings. Coatings 2025, 15, 437. [Google Scholar] [CrossRef]
- Hyun, S.K.; Nam, B.; Ko, T.K.; Lee, C.; Choi, S.B.; Lee, W.I. Optimal Composition of ZnO/WO3 Composite Nanoparticle Gas Sensors. Phys. Status Solidi (A) Appl. Mater. Sci. 2020, 217, 1900874. [Google Scholar] [CrossRef]
- Boudiba, A.; Roussel, P.; Zhang, C.; Olivier, M.G.; Snyders, R.; Debliquy, M. Sensing Mechanism of Hydrogen Sensors Based on Palladium-Loaded Tungsten Oxide (Pd-WO3). Sens. Actuators B Chem. 2013, 187, 84–93. [Google Scholar] [CrossRef]
- Wang, S.; Ren, C.; Tian, H.; Yu, J.; Sun, M. MoS2/ZnO van Der Waals Heterostructure as a High-Efficiency Water Splitting Photocatalyst: A First-Principles Study. Phys. Chem. Chem. Phys. 2018, 20, 13394–13399. [Google Scholar] [CrossRef]
- Liu, H.; Zhou, J.; Yu, L.; Wang, Q.; Liu, B.; Li, P.; Zhang, Y. High-Sensitivity SO2 Gas Sensor Based on Noble Metal Doped WO3 Nanomaterials. Int. J. Electrochem. Sci. 2021, 16, 12. [Google Scholar] [CrossRef]
- Lee, J.; Jung, Y.; Sung, S.H.; Lee, G.; Kim, J.; Seong, J.; Shim, Y.S.; Jun, S.C.; Jeon, S. High-Performance Gas Sensor Array for Indoor Air Quality Monitoring: The Role of Au Nanoparticles on WO3, SnO2, and NiO-Based Gas Sensors. J. Mater. Chem. A Mater. 2021, 9, 1159–1167. [Google Scholar] [CrossRef]
- Yang, W.; Wen, Y.; Zeng, D.; Wang, Q.; Chen, R.; Wang, W.; Shan, B. Interfacial Charge Transfer and Enhanced Photocatalytic Performance for the Heterojunction WO3/BiOCl: First-Principles Study. J. Mater. Chem. A Mater. 2014, 2, 20770–20775. [Google Scholar] [CrossRef]
- Takács, M.; Zámbó, D.; Deák, A.; Pap, A.E.; Bársony, I. Gas Sensitivity Enhancement of WO3 Nano-Rods by Gold Nanoparticles. Procedia Eng. 2015, 120, 1128–1131. [Google Scholar] [CrossRef]
- Ma, J.; Ren, Y.; Zhou, X.; Liu, L.; Zhu, Y.; Cheng, X.; Xu, P.; Li, X.; Deng, Y.; Zhao, D. Pt Nanoparticles Sensitized Ordered Mesoporous WO3 Semiconductor: Gas Sensing Performance and Mechanism Study. Adv. Funct. Mater. 2018, 28, 1705268. [Google Scholar] [CrossRef]
- Han, S.I.; Lee, S.Y.; Duy, L.T.; Seo, H. Reversible Gasochromic Hydrogen Sensing of Mixed-Phase MoO3 with Multi-Layered Pt/Ni/Pt Catalyst. Int. J. Hydrogen Energy 2021, 46, 33339–33348. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, M.; Liang, K.; Turak, A.; Zhang, B.; Meng, D.; Wang, C.; Qu, F.; Cheng, W.; Yang, M. An Acetone Gas Sensor Based on Nanosized Pt-Loaded Fe2O3 Nanocubes. Sens. Actuators B Chem. 2019, 290, 59–67. [Google Scholar] [CrossRef]
- Kattel, S.; Yan, B.; Chen, J.G.; Liu, P. CO2 Hydrogenation on Pt, Pt/SiO2 and Pt/TiO2: Importance of Synergy between Pt and Oxide Support. J. Catal. 2016, 343, 115–126. [Google Scholar] [CrossRef]
- Han, X.; Wu, X.; Deng, Y.; Liu, J.; Lu, J.; Zhong, C.; Hu, W. Ultrafine Pt Nanoparticle-Decorated Pyrite-Type CoS2 Nanosheet Arrays Coated on Carbon Cloth as a Bifunctional Electrode for Overall Water Splitting. Adv. Energy Mater. 2018, 8, 1800935. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, B.; Li, J.; Duan, Z.; Yang, Y.; Yuan, Z.; Jiang, Y.; Tai, H. Pd-Decorated ZnO Hexagonal Microdiscs for NH3 Sensor. Chemosensors 2024, 12, 43. [Google Scholar] [CrossRef]
- Cui, H.; Jia, P.; Peng, X. Adsorption of SO2 and NO2 Molecule on Intrinsic and Pd-Doped HfSe2 Monolayer: A First-Principles Study. Appl. Surf. Sci. 2020, 513, 145863. [Google Scholar] [CrossRef]
- Nong, S.; Dong, W.; Yin, J.; Dong, B.; Lu, Y.; Yuan, X.; Wang, X.; Bu, K.; Chen, M.; Jiang, S.; et al. Well-Dispersed Ruthenium in Mesoporous Crystal TiO2 as an Advanced Electrocatalyst for Hydrogen Evolution Reaction. Am. Chem. Soc. 2018, 140, 5719–5727. [Google Scholar] [CrossRef]
- Li, G.; Cheng, Z.; Xiang, Q.; Yan, L.; Wang, X.; Xu, J. Bimetal Pd Au Decorated SnO2 Nanosheets Based Gas Sensor with Temperature-Dependent Dual Selectivity for Detecting Formaldehyde and Acetone. Sens. Actuators B Chem. 2019, 283, 590–601. [Google Scholar] [CrossRef]
- Chen, S.; Li, S.; You, R.; Guo, Z.; Wang, F.; Li, G.; Yuan, W.; Zhu, B.; Gao, Y.; Zhang, Z.; et al. Elucidation of Active Sites for CH4 Catalytic Oxidation over Pd/CeO2 via Tailoring Metal−support Interactions. ACS Catal. 2021, 11, 5666–5677. [Google Scholar] [CrossRef]
- Ramizy, A.; Salih, E.Y.; Dahham, N.A.; Jasem, M. Fabrication and detection characteristics evaluation of SnO2@WO3 film as an effective NO2 gas sensor. Mater. Lett. 2024, 377, 137392. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, W.; Li, Y. NO2 and H2 Sensing Properties for Urchin-like Hexagonal WO3 Based on Experimental and First-Principle Investigations. Ceram. Int. 2019, 45, 6043–6050. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, A.; Chandra, R. Fabrication of Porous Silicon Filled Pd/SiC Nanocauliflower Thin Films for High Performance H2 Gas Sensor. Sens. Actuators B Chem. 2018, 264, 10–19. [Google Scholar] [CrossRef]
- Mhamdi, H.; Azaiez, K.; Fiorido, T.; Zaghouani, R.B.; Lazzari, J.L.; Bendahan, M.; Dimassi, W.; Benabderrahmane Zaghouani, R.; Bendahan, M. Room Temperature NO2 Gas Sensor Based on Stain-Etched Porous Silicon: Towards a Low-Cost Gas Sensor Integrated on Silicon. Inorg. Chem. Commun. 2022, 139, 109325. [Google Scholar] [CrossRef]
- Li, X.; Fu, L.; Karimi-Maleh, H.; Chen, F.; Zhao, S. Innovations in WO3 Gas Sensors: Nanostructure Engineering, Functionalization, and Future Perspectives. Heliyon 2024, 10, 6, 27740. [Google Scholar] [CrossRef]
- Van Toan, N.; Hung, C.M.; Duy, N.V.; Hoa, N.D.; Le, D.T.T.; Van Hieu, N. Bilayer SnO2–WO3 Nanofilms for Enhanced NH3 Gas Sensing Performance. Mater. Sci. Eng. B 2017, 224, 163–170. [Google Scholar] [CrossRef]
- Nimbalkar, T.M.; Kadam, S.A.; Ma, Y.R.; Selvaraj, M.; Assiri, M.A.; Patil, V.B. Tailoring Zn Mixed WO3 Nanoflowers for Highly Efficient NO2 Gas Detection. Ceram. Int. 2024, 50, 38514–38521. [Google Scholar] [CrossRef]
- Nilima Kandhare, V.L. Mathe, Sunita Bhagwat, Room temperature NO2 gas sensor using h-WO3 nanorod based thin films. Mater. Sci. Eng. B 2024, 305, 117422. [Google Scholar] [CrossRef]
- Bellucci, A.; De Bonis, A.; Curcio, M.; Santagata, A.; Pace, M.L.; Bolli, E.; Mastellone, M.; Polini, R.; Salerno, R.; Valentini, V.; et al. WO3-Based Thin Films Grown by Pulsed Laser Deposition as Gas Sensors for NO2 Detection. Sensors 2024, 24, 7366. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhang, P.; Ning, T.; Sun, Y.; Ren, Q.; Xu, M.; Zhao, X.; Luo, X.; Zhai, C.; Yan, J.; et al. Gas Sensor Based on Flower-like NiO Modified with WO3 Nanoparticles for NO2 Detection. ACS Appl. Nano Mater. 2024, 7, 7856–7864. [Google Scholar] [CrossRef]



| Materials | Working Temp. (°C) | Response | NO2 Conc. (ppm) | Ref. |
|---|---|---|---|---|
| SnO2@WO3 | 150 | 39.5 | 20 | [25] |
| WO3 | 250 | 45 | 50 | [26] |
| Zn mixed WO3 | 150 | 321.81 | 100 | [31] |
| h-WO3 nanorod | 150 | 1.55 | 5 | [32] |
| WO3-Based film | 75 | 5 | 5 | [33] |
| NiO/WO3 NPs | 200 | 16.6 | 10 | [34] |
| Pd-WO3/PSi | 25 | 3.1 | 2.5 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiang, X.; Wang, Z.; Guo, Y.; Zhou, W. DFT-Based Investigation of Pd-Modified WO3/Porous Silicon Composites for NO2 Gas Sensors: Enhanced Synergistic Effect and High-Performance Sensing. Coatings 2025, 15, 570. https://doi.org/10.3390/coatings15050570
Qiang X, Wang Z, Guo Y, Zhou W. DFT-Based Investigation of Pd-Modified WO3/Porous Silicon Composites for NO2 Gas Sensors: Enhanced Synergistic Effect and High-Performance Sensing. Coatings. 2025; 15(5):570. https://doi.org/10.3390/coatings15050570
Chicago/Turabian StyleQiang, Xiaoyong, Zhipeng Wang, Yongliang Guo, and Weibin Zhou. 2025. "DFT-Based Investigation of Pd-Modified WO3/Porous Silicon Composites for NO2 Gas Sensors: Enhanced Synergistic Effect and High-Performance Sensing" Coatings 15, no. 5: 570. https://doi.org/10.3390/coatings15050570
APA StyleQiang, X., Wang, Z., Guo, Y., & Zhou, W. (2025). DFT-Based Investigation of Pd-Modified WO3/Porous Silicon Composites for NO2 Gas Sensors: Enhanced Synergistic Effect and High-Performance Sensing. Coatings, 15(5), 570. https://doi.org/10.3390/coatings15050570






