Evaluation of Pectin-Based Coatings, Olive Leaf Extract, and Chitosan Nanoparticles for Acrylamide and Hydroxymethylfurfural Mitigation in French Fries: A Comparative Study of the Deep Frying and Air Frying Methods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Film Forming Solution Preparation
2.2.1. Determination of Zeta Potential and Z-Average and Polydispersity Index (PDI) for Coating Solutions
2.2.2. Determination of Antioxidant Activity for Coating Solutions
2.3. Dipping Method
2.4. Frying Method
2.5. Acrylamide and HMF Standard Preparation
2.6. Extraction of ACR and HMF from the French Fries
2.7. Determination of ACR by HPLC Method
2.8. Determination of HMF by HPLC Method
2.9. Determination of Water Content
2.10. Determination of Oil Content
2.11. Determination of Color
2.12. Sensory Evaluation
2.13. Statistical Analysis
3. Results and Discussion
3.1. Effect of OLE and CH-NPs on Zeta Potential, Z-Average and PDI of the PEC-Based Coating Solution
3.2. Effect of OLE and CH-NPs on the Antioxidant Activity of the PEC-Based Coating Solution
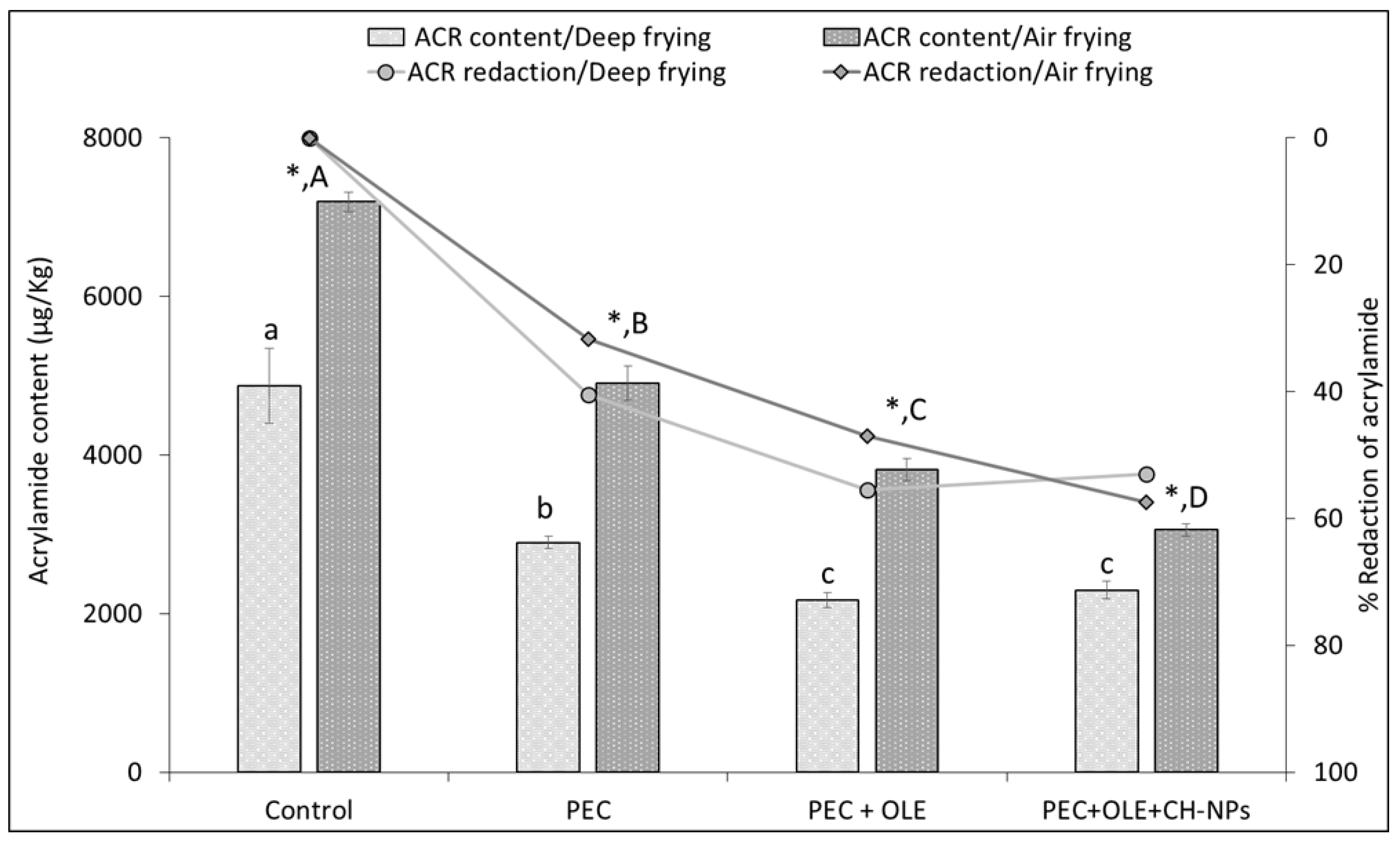
3.3. Effect of Coating Solutions on ACR and HMF Formation for Both Frying Methods
3.4. Effect of Coating Solutions on Oil Uptake for French Fries in the Deep Fat Frying Method
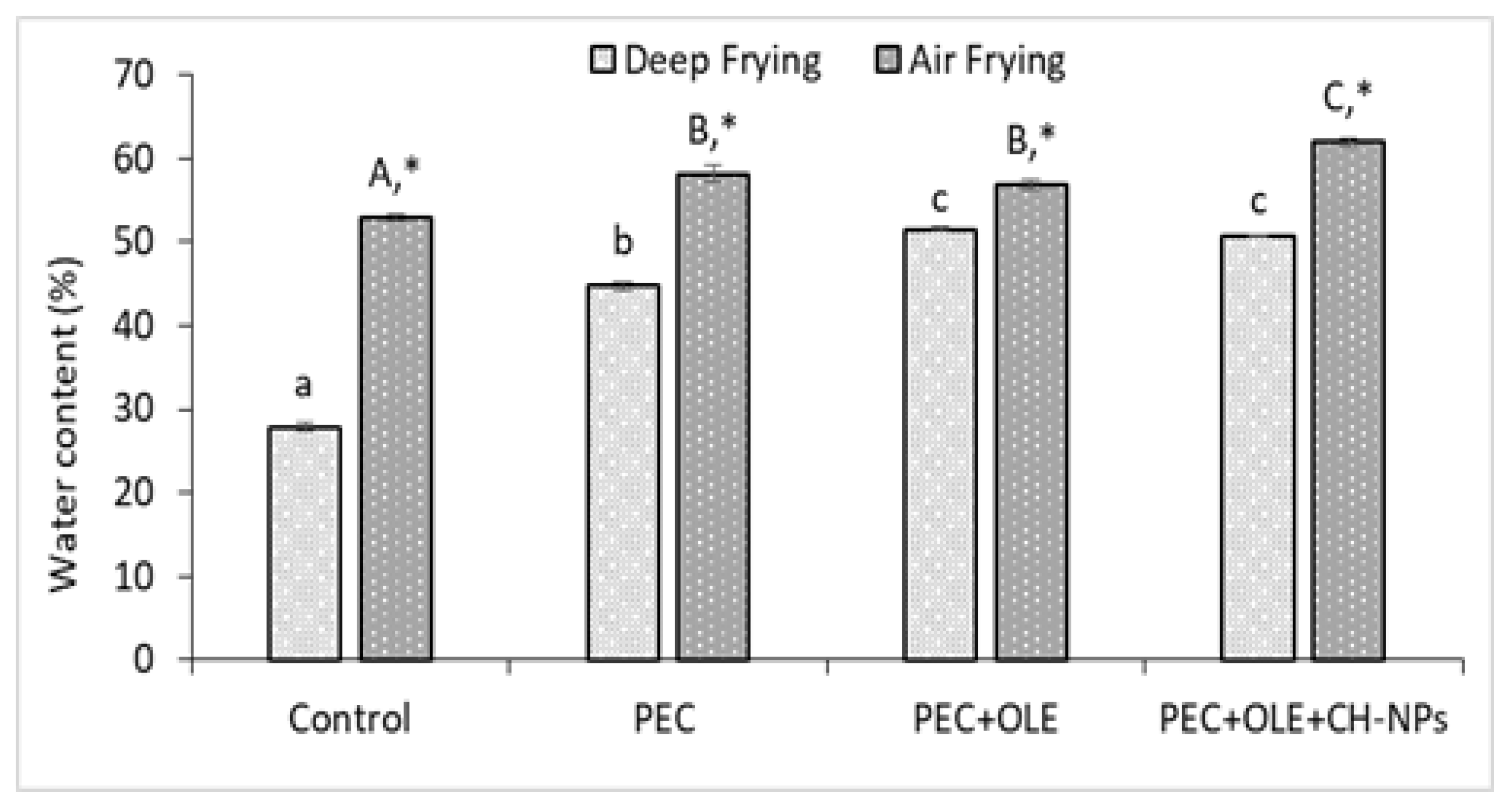
3.5. Effect of Different Coating Solutions on the Water Content of French Fries Fried at Different Frying Methods
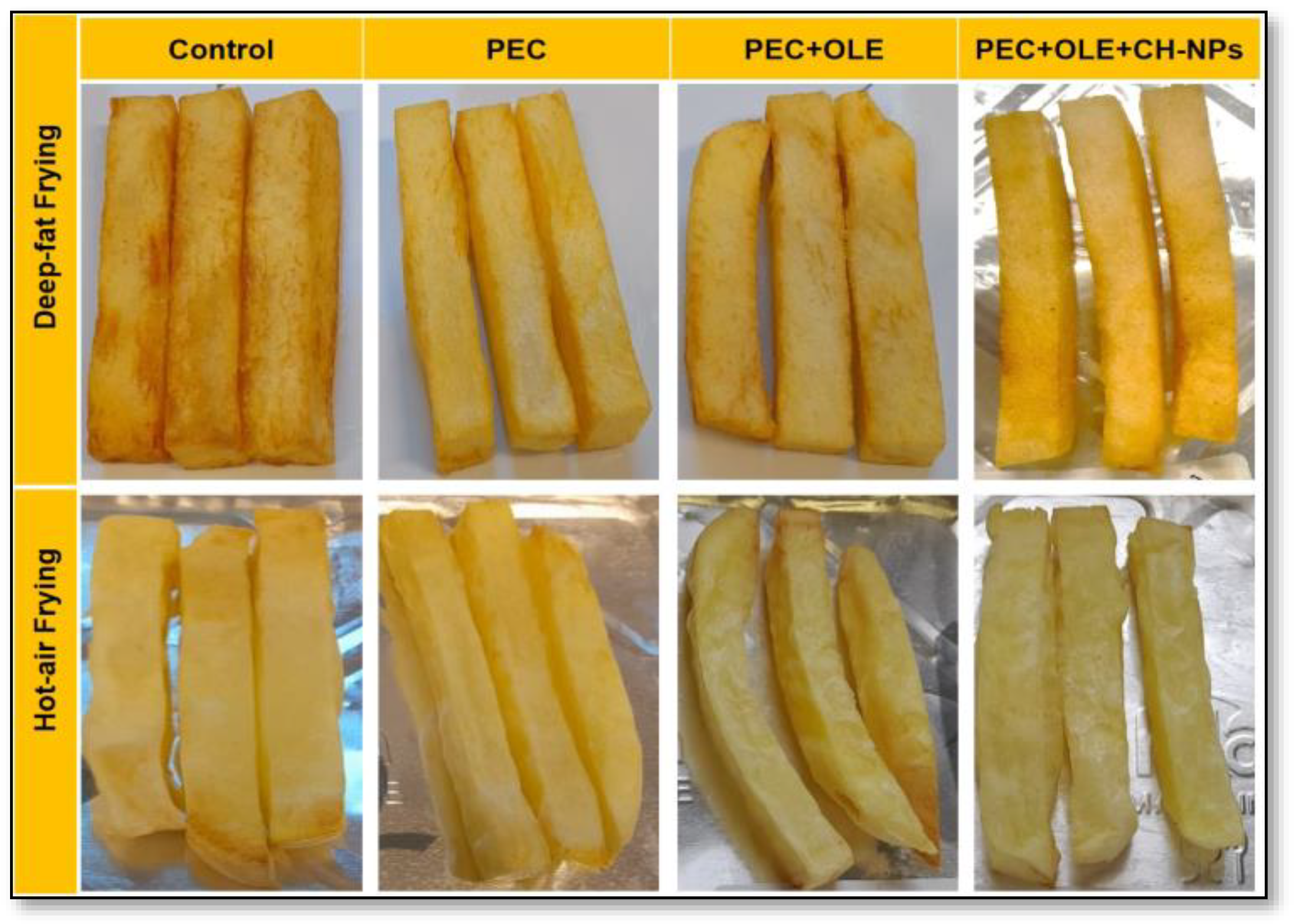
3.6. Effect of Coating Solutions on Color Properties for Both Frying Methods
3.7. Effect of Coating Solutions on Sensory Properties for Both Frying Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pinto, T.; Pinto, A.; Vilela, A. Edible Coatings and Films for Preparation of Grapevine By-Product Infusions and in Freshly Processed Products. Coatings 2023, 13, 1350. [Google Scholar] [CrossRef]
- Galus, S.; Aytunga, E.; Kibar, A.; Gniewosz, M.; Kra’sniewska, K.K. Coatings Novel Materials in the Preparation of Edible Films and Coatings-A Review. Coatings 2020, 10, 674. [Google Scholar] [CrossRef]
- Syarifuddin, A.; Muflih, M.H.; Izzah, N.; Fadillah, U.; Ainani, A.F.; Dirpan, A. Pectin-Based Edible Films and Coatings: From Extraction to Application on Food Packaging towards Circular Economy—A Review. Carbohydr. Polym. Technol. Appl. 2025, 9, 100680. [Google Scholar] [CrossRef]
- Nascimento, C.P.F.; Lopes, M.V.A.; Bessa, J.M.R.; Andrade, A.P.C.; Oliveira, L.S.; Eça, K.S. Effect of Pectin Coating Incorporated with Guava Leaf Extract (Psidium guajava L.) on the Stability of Fresh Beef Steak. Int. J. Food Sci. Technol. 2024, 59, 2068–2078. [Google Scholar] [CrossRef]
- Ngo, T.M.P.; Nguyen, T.H.; Dang, T.M.Q.; Do, T.V.T.; Reungsang, A.; Chaiwong, N.; Rachtanapun, P. Effect of Pectin/Nanochitosan-based Coatings and Storage Temperature on Shelf-life Extension of “Elephant” Mango (Mangifera indica L.) Fruit. Polymers 2021, 13, 3430. [Google Scholar] [CrossRef]
- Al-Asmar, A.; Giosafatto, C.V.L.; Sabbah, M.; Sanchez, A.; Santana, R.V.; Mariniello, L. Effect of Mesoporous Silica Nanoparticles on the Physicochemical Properties of Pectin Packaging Material for Strawberry Wrapping. Nanomaterials 2020, 10, 52. [Google Scholar] [CrossRef]
- Giosafatto, C.V.L.; Sabbah, M.; Al-Asmar, A.; Esposito, M.; Sanchez, A.; Santana, R.V.; Cammarota, M.; Mariniello, L.; Di Pierro, P.; Porta, R. Effect of Mesoporous Silica Nanoparticles on Glycerol-Plasticized Anionic and Cationic Polysaccharide Edible Films. Coatings 2019, 9, 172. [Google Scholar] [CrossRef]
- Lee, C.-H.; Chen, K.-T.; Lin, J.-A.; Chen, Y.-T.; Chen, Y.-A.; Wu, J.-T.; Hsieh, C.-W. Recent Advances in Processing Technology to Reduce 5-Hydroxymethylfurfural in Foods. Trends Food Sci. Technol. 2019, 93, 271–280. [Google Scholar] [CrossRef]
- Jin, C.; Wu, X.; Zhang, Y. Relationship between Antioxidants and Acrylamide Formation: A Review. Food Res. Int. 2013, 51, 611–620. [Google Scholar] [CrossRef]
- Capuano, E.; Fogliano, V. Acrylamide and 5-Hydroxymethylfurfural (HMF): A Review on Metabolism, Toxicity, Occurrence in Food and Mitigation Strategies. LWT Food Sci. Technol. 2011, 44, 793–810. [Google Scholar] [CrossRef]
- Pedreschi, F.; Mariotti, M.S.; Granby, K. Current Issues in Dietary Acrylamide: Formation, Mitigation and Risk Assessment. J. Sci. Food Agric. 2014, 94, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Erkekoglu, P.; Baydar, T. Acrylamide Neurotoxicity. Nutr. Neurosci. 2014, 17, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Göncüoğlu, N.; Gökmen, V. Accumulation of 5-Hydroxymethylfurfural in Oil During Frying of Model Dough. J. Am. Oil Chem. Soc. 2013, 90, 413–417. [Google Scholar] [CrossRef]
- Gökmen, V.; Kocadağlı, T.; Göncüoğlu, N.; Mogol, B.A. Model Studies on the Role of 5-Hydroxymethyl-2-Furfural in Acrylamide Formation from Asparagine. Food Chem. 2012, 132, 168–174. [Google Scholar] [CrossRef]
- Al-Asmar, A.; Giosafatto, C.V.L.; Sabbah, M.; Mariniello, L. Hydrocolloid-Based Coatings with Nanoparticles and Transglutaminase Crosslinker as Innovative Strategy to Produce Healthier Fried Kobbah. Foods 2020, 9, 698. [Google Scholar] [CrossRef]
- Champrasert, O.; Orifila, C.; Suwannaporn, P. Acrylamide Mitigation Using Zein–Polysaccharide Complex Particles. Food Hydrocoll. 2022, 124, 107317. [Google Scholar] [CrossRef]
- Rifai, L.; Saleh, F.A. A Review on Acrylamide in Food: Occurrence, Toxicity, and Mitigation Strategies. Int. J. Toxicol. 2020, 39, 93–102. [Google Scholar] [CrossRef]
- Ledbetter, M.; Blidi, S.; Ackon, S.; Bruno, F.; Sturrock, K.; Pellegrini, N.; Fiore, A. Effect of Novel Sequential Soaking Treatments on Maillard Reaction Products in Potato and Alternative Vegetable Crisps. Heliyon 2021, 7, e07441. [Google Scholar] [CrossRef]
- Al-Asmar, A.; Naviglio, D.; Giosafatto, C.V.L.; Mariniello, L. Hydrocolloid-Based Coatings Are Effective at Reducing Acrylamide and Oil Content of French Fries. Coatings 2018, 8, 147. [Google Scholar] [CrossRef]
- Zhu, R.-G.; Sun, S.-C.; Li, Y.-F.; Zang, H.; Sun, X.-Y.; Wei, J.; Song, L.-F.; Li, T.-J.; Wang, Y.-X.; Ning, C.; et al. Comparative Effects of Pectin and Hydrolyzed Pectin Coating as Pre-Frying Treatments on Acrylamide Formation in Potato Chips. Int. J. Biol. Macromol. 2024, 269, 132015. [Google Scholar] [CrossRef]
- Champrasert, O.; Chu, J.; Meng, Q.; Viney, S.; Holmes, M.; Suwannaporn, P.; Orfila, C. Inhibitory Effect of Polysaccharides on Acrylamide Formation in Chemical and Food Model Systems. Food Chem. 2021, 363, 130213. [Google Scholar] [CrossRef] [PubMed]
- Odetayo, T.; Tesfay, S.; Ngobese, N.Z. Nanotechnology-Enhanced Edible Coating Application on Climacteric Fruits. Food Sci. Nutr. 2022, 10, 2149–2167. [Google Scholar] [CrossRef]
- Lin, H.-T.V.; Ting, Y.-S.; Ndraha, N.; Hsiao, H.-I.; Sung, W.-C. Effect of Chitosan Incorporation on the Development of Acrylamide during Maillard Reaction in Fructose–Asparagine Model Solution and the Functional Characteristics of the Resultants. Polymers 2022, 14, 1565. [Google Scholar] [CrossRef] [PubMed]
- Sabbah, M.; Al-Asmar, A.; Younis, D.; Al-Rimawi, F.; Famiglietti, M.; Mariniello, L. Production and Characterization of Active Pectin Films with Olive or Guava Leaf Extract Used as Soluble Sachets for Chicken Stock Powder. Coatings 2023, 13, 1253. [Google Scholar] [CrossRef]
- Zam, W. Effect of Alginate and Chitosan Edible Coating Enriched with Olive Leaves Extract on the Shelf Life of Sweet Cherries (Prunus avium L.). J. Food Qual. 2019, 2019, 8192964. [Google Scholar] [CrossRef]
- Famiglietti, M.; Savastano, A.; Gaglione, R.; Arciello, A.; Naviglio, D.; Mariniello, L. Edible Films Made of Dried Olive Leaf Extract and Chitosan: Characterization and Applications. Foods 2022, 11, 2078. [Google Scholar] [CrossRef]
- Hu, H.; Wang, Y.; Huang, Y.; Yu, Y.; Shen, M.; Li, C.; Nie, S.; Xie, M. Natural Antioxidants and Hydrocolloids as a Mitigation Strategy to Inhibit Advanced Glycation End Products (AGEs) and 5-Hydroxymethylfurfural (HMF) in Butter Cookies. Foods 2022, 11, 657. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, X.; McClements, D.J.; Chen, L.; Miao, M.; Jin, Z. Effect of New Frying Technology on Starchy Food Quality. Foods 2021, 10, 1852. [Google Scholar] [CrossRef]
- Göncüoğlu Taş, N.; Kocadağlı, T.; Balagiannis, D.P.; Gökmen, V.; Parker, J.K. Effect of Salts on the Formation of Acrylamide, 5-Hydroxymethylfurfural and Flavour Compounds in a Crust-like Glucose/Wheat Flour Dough System during Heating. Food Chem. 2023, 410, 135358. [Google Scholar] [CrossRef]
- Téllez-Morales, J.A.; Rodríguez-Miranda, J.; Aguilar-Garay, R. Review of the Influence of Hot Air Frying on Food Quality. Meas. Food 2024, 14, 100153. [Google Scholar] [CrossRef]
- Teruel, M.d.R.; Gordon, M.; Linares, M.B.; Garrido, M.D.; Ahromrit, A.; Niranjan, K. A Comparative Study of the Characteristics of French Fries Produced by Deep Fat Frying and Air Frying. J. Food Sci. 2015, 80, E349–E358. [Google Scholar] [CrossRef] [PubMed]
- The European Food Safety Authority [EFSA]. Acrylamide. Available online: https://www.efsa.europa.eu/en/topics/topic/acrylamide (accessed on 24 March 2025).
- Geirola, N.; Greco, S.; Mare, R.; Ricupero, D.; Settino, M.; Tirinato, L.; Maurotti, S.; Montalcini, T.; Pujia, A. Assessment of 5-Hydroxymethylfurfural in Food Matrix by an Innovative Spectrophotometric Assay. Int. J. Mol. Sci. 2024, 25, 8501. [Google Scholar] [CrossRef] [PubMed]
- Jozinović, A.; Šarkanj, B.; Ačkar, D.; Balentić, J.P.; Šubarić, D.; Cvetković, T.; Ranilović, J.; Guberac, S.; Babić, J. Simultaneous Determination of Acrylamide and Hydroxymethylfurfural in Extruded Products by LC-MS/MS Method. Molecules 2019, 24, 1971. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Lu, J.; Li, M.; Li, C.; Wang, Y.; Shen, M.; Chen, Y.; Nie, S.; Zeng, M.; Chen, J.; et al. Effect of Acidity Regulators on Acrylamide and 5-Hydroxymethylfurfural Formation in French Fries: The Dual Role of PH and Acid Radical Ion. Food Chem. 2022, 371, 131154. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists. Official Methods of Analysis, 15th ed.; Helrich, K., Ed.; AOAC International: Arlington, VA, USA, 1990. [Google Scholar]
- Leclercq, C.; Arcella, D.; Piccinelli, R.; Sette, S.; Le Donne, C. The Italian National Food Consumption Survey INRAN-SCAI 2005–06: Main Results in Terms of Food Consumption. Public Health Nutr. 2009, 12, 2504–2532. [Google Scholar] [CrossRef]
- Tavera-Quiroz, M.J.; Urriza, M.; Pinotti, A.; Bertola, N. Plasticized Methylcellulose Coating for Reducing Oil Uptake in Potato Chips. J. Sci. Food Agric. 2012, 92, 1346–1353. [Google Scholar] [CrossRef]
- Mohammed, M.A.; Syeda, J.T.M.; Wasan, K.M.; Wasan, E.K. An Overview of Chitosan Nanoparticles and Its Application in Non-Parenteral Drug Delivery. Pharmaceutics 2017, 9, 53. [Google Scholar] [CrossRef]
- Lorevice, M.V.; Otoni, C.G.; de Moura, M.R.; Mattoso, L.H.C. Chitosan Nanoparticles on the Improvement of Thermal, Barrier, and Mechanical Properties of High- and Low-Methyl Pectin Films. Food Hydrocoll. 2016, 52, 732–740. [Google Scholar] [CrossRef]
- Albertos, I.; Avena-Bustillos, R.J.; Martín-Diana, A.B.; Du, W.-X.; Rico, D.; McHugh, T.H. Antimicrobial Olive Leaf Gelatin Films for Enhancing the Quality of Cold-Smoked Salmon. Food Packag. Shelf Life 2017, 13, 49–55. [Google Scholar] [CrossRef]
- Mi, Y.; Zhang, J.; Tan, W.; Miao, Q.; Li, Q.; Guo, Z. Preparation of Doxorubicin-Loaded Carboxymethyl-β-Cyclodextrin/Chitosan Nanoparticles with Antioxidant, Antitumor Activities and PH-Sensitive Release. Mar. Drugs 2022, 20, 278. [Google Scholar] [CrossRef]
- Kongkaoroptham, P.; Piroonpan, T.; Pasanphan, W. Chitosan Nanoparticles Based on Their Derivatives as Antioxidant and Antibacterial Additives for Active Bioplastic Packaging. Carbohydr. Polym. 2021, 257, 117610. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhao, N.; Huang, X.; Wang, X.; Bao, T.; Zhang, H.; Xia, S.; Hayat, K. Distribution Characteristics and Formation Mechanism of Acrylamide in Air-Fried Potato Strips. Food Chem. 2025, 478, 143664. [Google Scholar] [CrossRef]
- Dong, L.; Qiu, C.; Wang, R.; Zhang, Y.; Wang, J.; Liu, J.; Yu, H.; Wang, S. Effects of Air Frying on French Fries: The Indication Role of Physicochemical Properties on the Formation of Maillard Hazards, and the Changes of Starch Digestibility. Front. Nutr. 2022, 9, 889901. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; McClements, D.J.; Xu, Z.; Meng, M.; Qiu, C.; Long, J.; Jin, Z.; Chen, L. Recent Advances in the Optimization of the Sensory Attributes of Fried Foods: Appearance, Flavor, and Texture. Trends Food Sci. Technol. 2023, 138, 297–309. [Google Scholar] [CrossRef]
- Haddarah, A.; Naim, E.; Dankar, I.; Sepulcre, F.; Pujolà, M.; Chkeir, M. The Effect of Borage, Ginger and Fennel Extracts on Acrylamide Formation in French Fries in Deep and Electric Air Frying. Food Chem. 2021, 350, 129060. [Google Scholar] [CrossRef]
- Morales, G.; Jimenez, M.; Garcia, O.; Mendoza, M.R.; Beristain, C.I. Effect of Natural Extracts on the Formation of Acrylamide in Fried Potatoes. LWT Food Sci. Technol. 2014, 58, 587–593. [Google Scholar] [CrossRef]
- Verma, V.; Yadav, N. Effect of Plant Extracts on the Reduction of Acrylamide and Hydroxymethylfurfural Formation in French Fries. Food Chem. Adv. 2024, 4, 100708. [Google Scholar] [CrossRef]
- Li, Y.; Bai, X.; Zhao, M.; Wang, H.; Feng, J.; Xia, X.; Liu, Q. Sodium Alginate Edible Coating to Reduce Oil Absorption of French Fries with Maintaining Overall Acceptability: Based on a Water Replacement Mechanism. Int. J. Biol. Macromol. 2023, 236, 124042. [Google Scholar] [CrossRef]
- Yang, D.; Wu, G.; Li, P.; Zhang, H.; Qi, X. Comparative Analysis of the Oil Absorption Behavior and Microstructural Changes of Fresh and Pre-Frozen Potato Strips during Frying via MRl, SEM, and XRD. Food Res. Int. 2019, 122, 295–302. [Google Scholar] [CrossRef]
- Dehghannya, J.; Ngadi, M. Recent Advances in Microstructure Characterization of Fried Foods: Different Frying Techniques and Process Modeling. Trends Food Sci. Technol. 2021, 116, 786–801. [Google Scholar] [CrossRef]
- Kizito, K.F.; Abdel-Aal, M.H.; Ragab, M.H.; Youssef, M.M. Quality Attributes of French Fries as Affected by Different Coatings, Frozen Storage and Frying Conditions. J. Agric. Sci. Bot. 2017, 1, 23–29. [Google Scholar] [CrossRef]
- Kurek, M.; Ščetar, M.; Galić, K. Edible Coatings Minimize Fat Uptake in Deep Fat Fried Products: A Review. Food Hydrocoll. 2017, 71, 225–235. [Google Scholar] [CrossRef]
- Al-Asmar, A.; Giosafatto, C.V.L.; Panzella, L.; Mariniello, L. The Effect of Transglutaminase to Improve the Quality of Either Traditional or Pectin-Coated Falafel (Fried Middle Eastern Food). Coatings 2019, 9, 331. [Google Scholar] [CrossRef]
- Liu, Y.; Tian, J.; Hu, B.; Yu, P.; Fan, L. Relationship between Crust Characteristics and Oil Uptake of Potato Strips with Hot-Air Pre-Drying during Frying Process. Food Chem. 2021, 360, 130045. [Google Scholar] [CrossRef] [PubMed]
- Mellema, M. Mechanism and Reduction of Fat Uptake in Deep-Fat Fried Foods. Trends Food Sci. Technol. 2003, 14, 364–373. [Google Scholar] [CrossRef]
- Gouyo, T.; Mestres, C.; Maraval, I.; Fontez, B.; Hofleitner, C.; Bohuon, P. Assessment of Acoustic-Mechanical Measurements for Texture of French Fries: Comparison of Deep-Fat Frying and Air Frying. Food Res. Int. 2020, 131, 108947. [Google Scholar] [CrossRef]
- Heredia, A.; Castelló, M.L.; Argüelles, A.; Andrés, A. Evolution of Mechanical and Optical Properties of French Fries Obtained by Hot Air-Frying. LWT Food Sci. Technol. 2014, 57, 755–760. [Google Scholar] [CrossRef]
- Starowicz, M.; Zieliński, H. How Maillard Reaction Influences Sensorial Properties (Color, Flavor and Texture) of Food Products? Food Rev. Int. 2019, 35, 707–725. [Google Scholar] [CrossRef]
- Iglesias-Carres, L.; Racine, K.C.; Chadwick, S.; Nunn, C.; Kalambur, S.B.; Neilson, A.P.; Ferruzzi, M.G. Mechanism of Off-Color Formation in Potato Chips Fried in Oil Systems Containing Ascorbic Acid as a Stabilizer. LWT 2023, 179, 114682. [Google Scholar] [CrossRef]
- Coria-Hernández, J.; Arjona-Román, J.L.; Meléndez-Pérez, R. Comparative Study of Conventional Frying and Air Frying on the Quality of Potatoes (Solanum tuberosum L.). Food Sci. Nutr. 2023, 11, 6676–6685. [Google Scholar] [CrossRef]
- Chen, L.; Ma, R.; Zhang, Z.; McClements, D.J.; Qiu, L.; Jin, Z.; Tian, Y. Impact of Frying Conditions on Hierarchical Structures and Oil Absorption of Normal Maize Starch. Food Hydrocoll. 2019, 97, 105231. [Google Scholar] [CrossRef]
- Zhang, J.; Xie, T.; Fan, L. Improving the Quality and Reducing Oil Absorption of Fried Potato Chips by Ultrasound Pretreatment. LWT 2021, 148, 111763. [Google Scholar] [CrossRef]


| Zeta Potential (mV) | Z-Average (d.nm) | Polydispersity Index (PDI) | Antioxidant Activity (%) | |
|---|---|---|---|---|
| Control OLE 0.2% (w/v) | −10.31 ± 1.5 | 1210.1 ± 204 | 0.289 ± 0.01 | 33.68 ± 0.5 |
| CH-NPs 1% | 4.93 ± 1.1 | 407.9 ± 29.5 | 0.121 ± 0.06 | 4.11 ± 0.2 |
| Coating solutions PEC 1% | −36.08 ± 2.4 | 3833 ± 316 | 0.213 ± 0.06 | 6.92 ± 1.1 |
| PEC 1% + OLE 0.2% | −36.53 ± 2.8 c,d | 3369± 224 c,d | 0.252 ± 0.05 a,c,d | 62.06 ± 1.7 a,c,d |
| PEC 1% + OLE 0.2% + CH-NPs 1% | −35.23 ± 1.4 c,d | 3378± 173 c,d | 0.290 ± 0.13 a,d | 78.99 ± 1.2 a,b,c,d |
| Coating Solutions | L* | a* | b* | ∆E | BI |
|---|---|---|---|---|---|
| Deep Fat Frying | |||||
| Control | 66.6 ± 3.2 a | 7.3 ± 0.4 a | 40.7 ± 1.9 a | n.a | 97.2 ± 1.0 a |
| PEC | 79.0 ± 1.8 b | 1.0 ± 0.6 b | 37.3 ± 1.2 b | 14.3 ± 1.3 a | 62.5 ± 2.1 b |
| PEC + OLE | 75.0 ± 1.3 c | 2.9 ± 0.2 c | 39.5 ± 0.4 a | 9.6 ± 0.3 b | 74.4 ± 1.2 c |
| PEC + OLE + CH-NPs | 77.0 ± 1.4 c | 1.0 ± 0.5 b | 35.3 ± 0.5 c | 13.3 ± 1.5 a | 60.2 ± 3.1 b |
| Hot Air Frying | |||||
| Control | 86.7 ± 0.8 a* | −5.7 ± 0.6 a* | 28.2 ± 0.6 a* | n.a | 33.3 ± 1.1 a* |
| PEC | 81.0 ± 0.7 b* | −3.7 ± 0.8 b* | 26.3 ± 0.8 b* | 6.3 ± 0.8 a* | 34.6 ± 2.3 a* |
| PEC + OLE | 82.2 ± 1.3 b* | −3.5 ± 1.0 b* | 33.5 ± 1.3 c* | 7.4 ± 1.6 a* | 47.4 ± 3.1 b* |
| PEC + OLE + CH-NPs | 81.0 ± 1.3 b* | −3.3 ± 0.5 b* | 28.1 ± 1.5 a* | 6.3 ± 1.0 a* | 38.2 ± 2.9 a* |
| Coating Material | Appearance | Odor | Texture | Flavor | Overall Acceptability |
|---|---|---|---|---|---|
| Deep Fat Frying | |||||
| Control | 6.0 ± 1.7 a | 6.2 ± 1.5 a | 5.7 ± 2.0 a | 6.3 ± 1.7 a | 6.1 ± 1.7 a |
| PEC | 6.1 ± 1.5 a | 6.5 ± 1.4 a | 5.8 ± 1.8 a | 6.1 ± 1.8 a | 6.1 ± 1.6 a |
| PEC + OLE | 6.4 ± 1.9 a | 6.3 ± 1.4 a | 5.6 ± 2.0 a | 5.5 ± 2.1 a | 5.7 ± 1.9 a |
| PEC + OLE + CH-NPs | 6.7 ± 1.4 a | 6.4 ± 1.3 a | 5.7 ± 1.9 a | 6.1 ± 1.9 a | 6.2 ± 1.4 a |
| Hot Air Frying | |||||
| Control | 3.0 ± 1.5 b | 4.8 ± 1.8 b | 3.2 ± 1.9 b | 3.3 ± 2.0 b | 3.1 ± 2.0 b |
| PEC | 3.6 ± 1.9 b | 4.6 ± 2.3 b | 3.9 ± 2.5 b | 3.3 ± 2.2 b | 3.4 ± 2.0 b |
| PEC + OLE | 3.9 ± 2.2 b | 5.2 ± 2.0 b | 3.8 ± 2.0 b | 3.7 ± 2.3 b | 3.8 ± 2.2 b |
| PEC + OLE + CH-NPs | 4.7 ± 1.8 b | 5.5 ± 2.0 b | 4.3 ± 2.3 b | 4.4 ± 2.6 b | 4.4 ± 2.3 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abu-Thabet, A.; Al-Asmar, A.; Sabbah, M.; Mayyala, A.; Mariniello, L. Evaluation of Pectin-Based Coatings, Olive Leaf Extract, and Chitosan Nanoparticles for Acrylamide and Hydroxymethylfurfural Mitigation in French Fries: A Comparative Study of the Deep Frying and Air Frying Methods. Coatings 2025, 15, 562. https://doi.org/10.3390/coatings15050562
Abu-Thabet A, Al-Asmar A, Sabbah M, Mayyala A, Mariniello L. Evaluation of Pectin-Based Coatings, Olive Leaf Extract, and Chitosan Nanoparticles for Acrylamide and Hydroxymethylfurfural Mitigation in French Fries: A Comparative Study of the Deep Frying and Air Frying Methods. Coatings. 2025; 15(5):562. https://doi.org/10.3390/coatings15050562
Chicago/Turabian StyleAbu-Thabet, Asmaa, Asmaa Al-Asmar, Mohammed Sabbah, Abdallatif Mayyala, and Loredana Mariniello. 2025. "Evaluation of Pectin-Based Coatings, Olive Leaf Extract, and Chitosan Nanoparticles for Acrylamide and Hydroxymethylfurfural Mitigation in French Fries: A Comparative Study of the Deep Frying and Air Frying Methods" Coatings 15, no. 5: 562. https://doi.org/10.3390/coatings15050562
APA StyleAbu-Thabet, A., Al-Asmar, A., Sabbah, M., Mayyala, A., & Mariniello, L. (2025). Evaluation of Pectin-Based Coatings, Olive Leaf Extract, and Chitosan Nanoparticles for Acrylamide and Hydroxymethylfurfural Mitigation in French Fries: A Comparative Study of the Deep Frying and Air Frying Methods. Coatings, 15(5), 562. https://doi.org/10.3390/coatings15050562










