Antibacterial and Film Characteristics of Copper-Doped Diamond-like Carbon Films via Sputtering Using a Mixed Target of Copper and Graphite
Abstract
1. Introduction
2. Experimental
2.1. Preparation of Cu-DLC Films
2.2. Structure of Cu-DLC Film
2.3. Surface Properties of Cu-DLC Films
2.4. Antibacterial Properties of Cu-DLC Films
2.5. Release of Copper from Cu-DLC Films in Wet Environments
3. Results and Discussion
3.1. Structural Change in Cu-DLC Film with Different C/Cu Target Ratios
3.2. Change in Surface Properties of Cu-DLC Film for C/Cu Mixed Target Ratio
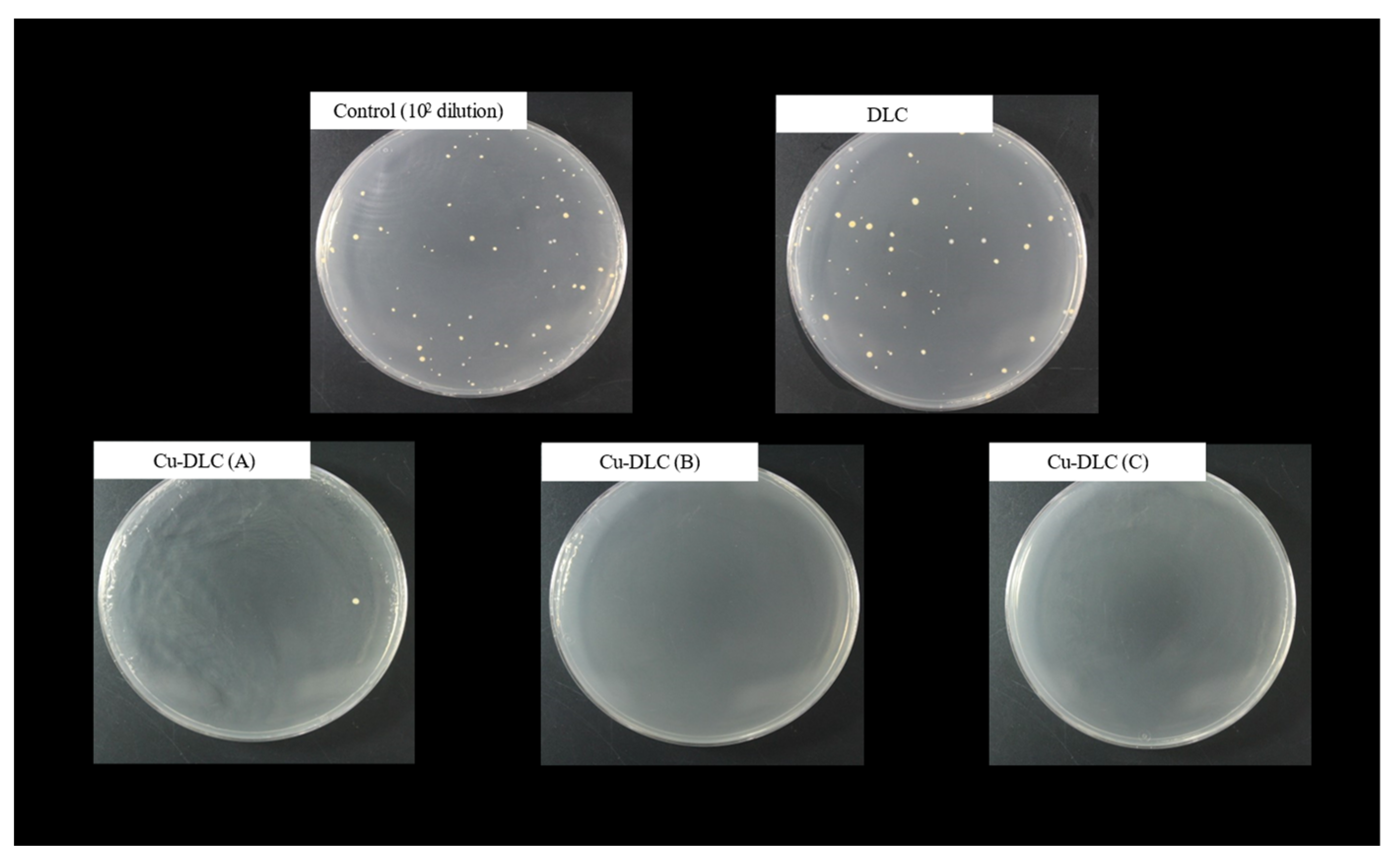
3.3. Antimicrobial Properties of Cu-DLC Films with Different C/Cu Mixed Target Ratios
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Robertson, J. Diamond-like amorphous carbon. Mater. Sci. Eng. R 2002, 37, 129–281. [Google Scholar] [CrossRef]
- Bewilogua, K.; Hofmann, D. History of diamond-like carbon films—From first experiments to worldwide applications. Surf. Coat. Technol. 2014, 242, 214–225. [Google Scholar] [CrossRef]
- Roy, R.K.; Lee, K.-R. Biomedical applications of diamond-like carbon coatings: A review. J. Biomed. Mater. Res. 2007, 83, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Radoń-Kobus, K.; Madej, M.; Kowalczyk, J.; Piotrowska, K. Properties of Diamond-like Tungsten-Doped Carbon Coatings Lubricated with Cutting Fluid. Coatings 2024, 14, 342. [Google Scholar] [CrossRef]
- Hou, M.-H.; Jia, L.; Kang, R.-Z.; Lu, Z.-L.; Ma, G.-Z.; Cui, J. The performance prediction and mechanism study of doped DLC films by the combination of molecular dynamics and first-principles calculations. Diam. Relat. Mater. 2024, 149, 111644. [Google Scholar] [CrossRef]
- Antimicrobial coatings market worth $7.36 bn by 2025, growing at a CAGR of 8.2%. Focus Powder Coat. 2020, 2020, 6, ISSN 1364-5439. [CrossRef]
- Takayuki, O.; Yuto, K. Antimicrobial coating using copper-doped diamond-like carbon film deposited by dual magnetron sputtering. Jpn. J. Appl. Phys. 2023, 62, 078002. [Google Scholar]
- Dwivedi, N.; Kumar, S.; Malik, H.K.; Sreekumar, C.; Dayal, S.; Rauthan, C.M.S.; Panwar, O.S. Investigation of properties of Cu containing DLC films produced by PECVD process. J. Phys. Chem. Solids. 2012, 73, 308–316. [Google Scholar] [CrossRef]
- Zhou, B.; Liu, Z.; Piliptsou, D.G.; Wang, S.; Yu, Z.; Rogachev, A.V.; Rudenkov, A.S.; Balmakou, A. Structure and optical properties of Cu-DLC composite films deposited by cathode arc with double-excitation source. Diam. Relat. Mater. 2016, 69, 191–197. [Google Scholar] [CrossRef]
- Chan, Y.H.; Huang, C.F.; Ou, K.L.; Peng, P.W. Mechanical properties and antibacterial activity of copper doped diamond-like carbon films. Surf. Coat. Technol. 2011, 206, 1037–1040. [Google Scholar] [CrossRef]
- Khan, I.; Aadil, F.; Farook, A.; Muhammed, S. u-H. Structural, morphological, electrical and optical properties of Cu doped DLC thin films. Mater. Res. Express. 2019, 6, 126420. [Google Scholar] [CrossRef]
- Yang, S.; Sun, B.; Liu, Y.; Zhu, J.; Song, J.; Hao, Z.; Zeng, X.; Zhao, X.; Shu, Y.; Chen, J.; et al. Effect of ITO target crystallinity on the properties of sputtering deposited ITO films. Ceram. Int. 2020, 46, 6342–6350. [Google Scholar] [CrossRef]
- Bakhet, S.; Tamulevičienė, A.; Vasiliauskas, A.; Andrulevičius, M.; Meškinis, Š.; Tamulevičius, S.; Kašėtienė, N.; Malakauskas, M.; Lelešius, R.; Zienius, D.; et al. Antiviral and antibacterial efficacy of nanocomposite amorphous carbon films with copper nanoparticles. Appl. Surf. Sci. 2024, 670, 160642. [Google Scholar] [CrossRef]
- Kanasugi, K.; Ichijo, E.; Alanazi, A.; Ohgoe, Y.; Manome, Y.; Hiratsuka, M.; Hirakuri, K. Effects of ultraviolet sterilization exposure on the structural and surface properties of graphite-/polymer-like carbon films. Thin Solid Film. 2024, 807, 140549. [Google Scholar] [CrossRef]
- ISO 22196:2007; Plastics—Measurement of Antibacterial Activity on Plastics Surfaces. ISO: Geneva, Switzerland.
- Onodera, S.; Fujii, S.; Moriguchi, H.; Tsujioka, M.; Hirakuri, K. Antibacterial property of F doped DLC film with plasma treatment. Diam. Relat. Mater. 2020, 107, 107835. [Google Scholar] [CrossRef]
- Kanasugi, K.; Arimura, K.; Alanazi, A.; Ohgoe, Y.; Manome, Y.; Hiratsuka, M.; Hirakuri, K. UV sterilization effects and osteoblast proliferation on amorphous carbon films classified based on optical constants. Bioengineering 2022, 9, 505. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.C.; Robertson, J. Raman spectroscopy of amorphous, nanostructured, diamond–like carbon, and nanodiamond. Philos. Trans. R. Soc. Lond. 2004, 362, 2477–2512. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Wang, A.; Wang, Q. Microstructure and mechanical property of diamond-like carbon films with ductile copper incorporation. Surf. Coat. Technol. 2015, 272, 33–38. [Google Scholar] [CrossRef]
- Kang, F.; Pei, L.; Li, H.; Ji, L.; Liu, X.; Zhou, H.; Chen, J. Cu doping and friction-induced self-migration in a-C:H films to improve anti-wear life in vacuum. Wear 2024, 538–539, 205204. [Google Scholar] [CrossRef]
- Goto, M. Preparations and tribological properties of soft-metal/DLC composite coatings by RF magnetron sputter using composite targets. Int. J. Mech. Mater. Des. 2018, 14, 313–327. [Google Scholar] [CrossRef]

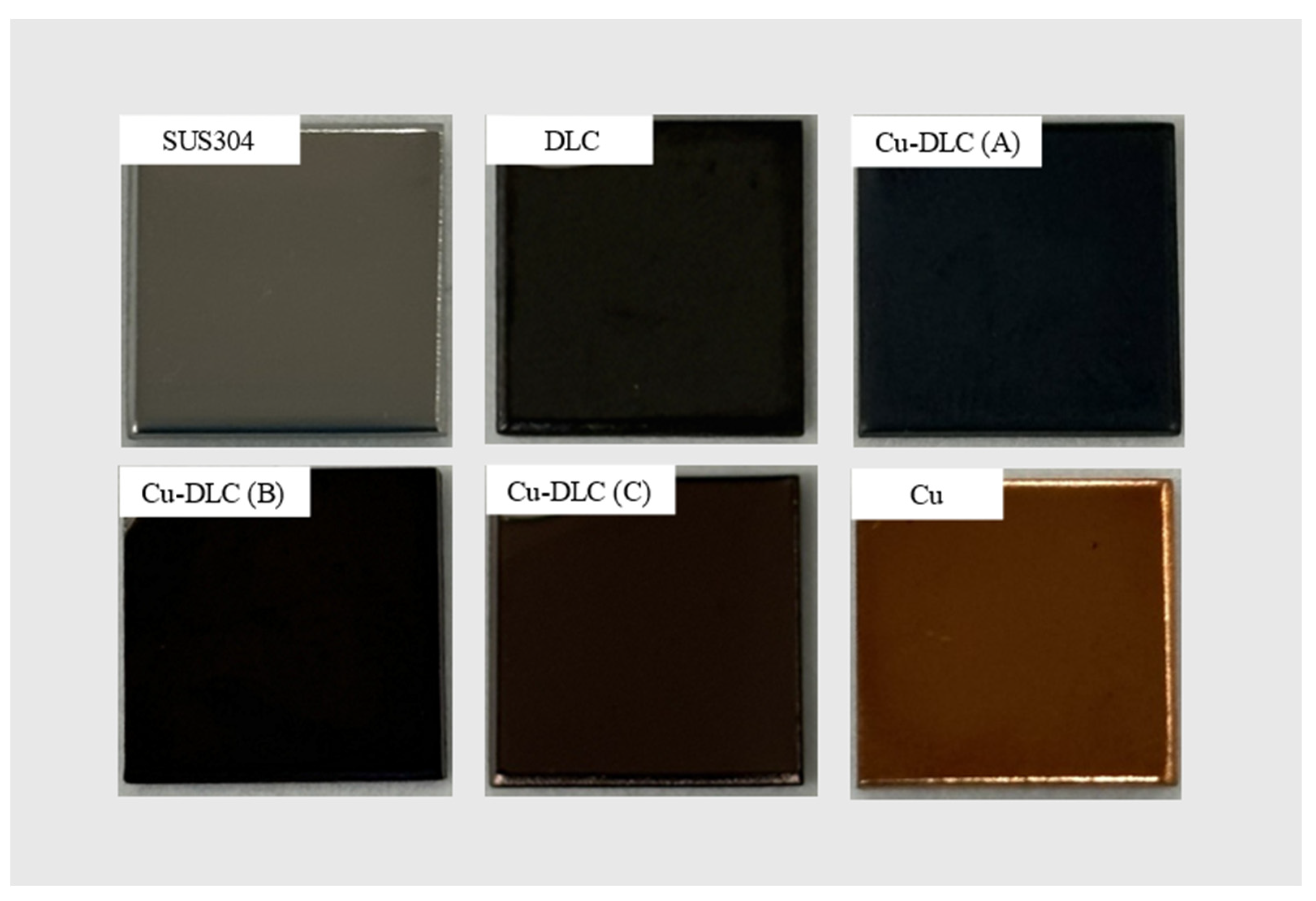

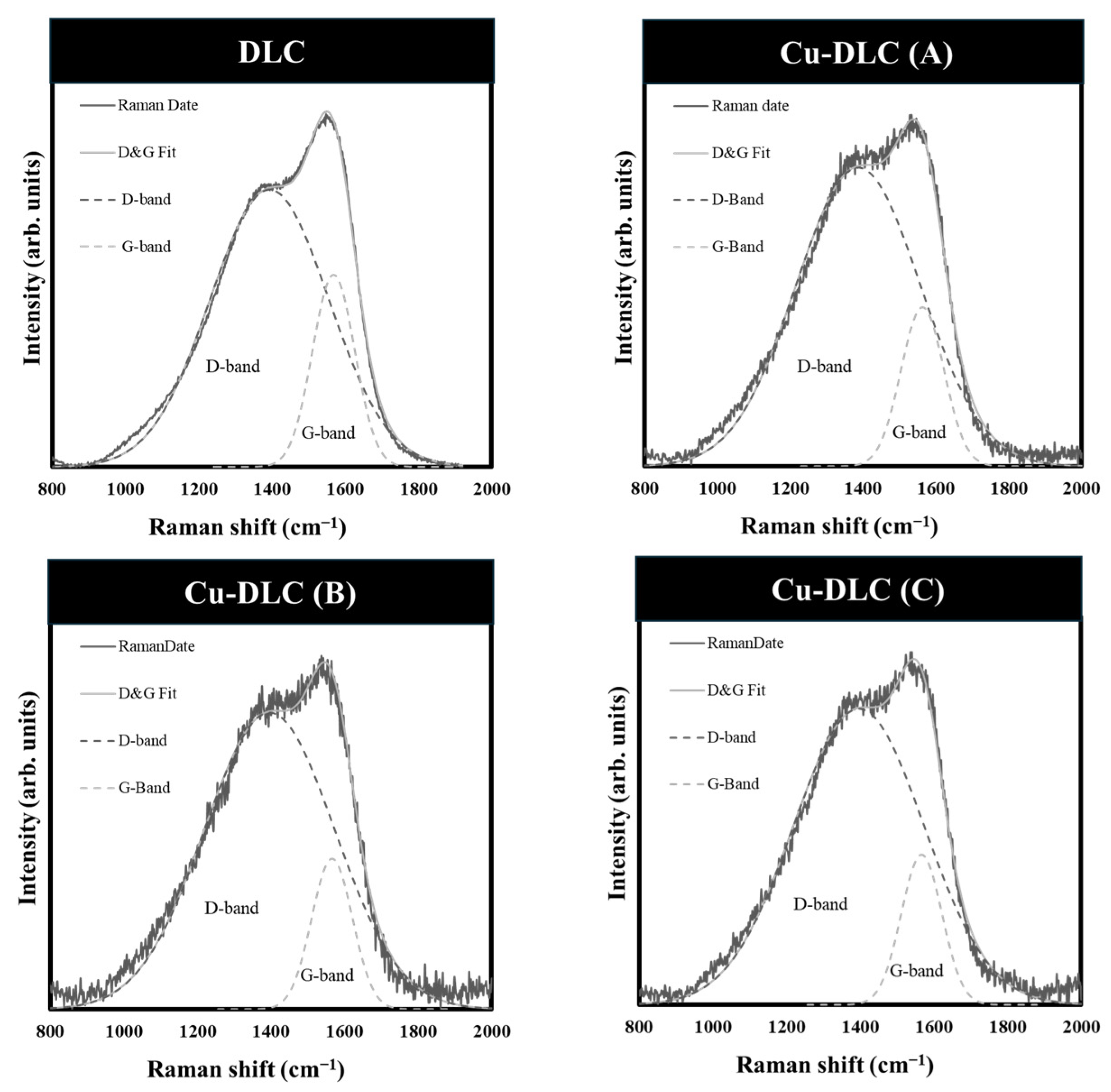
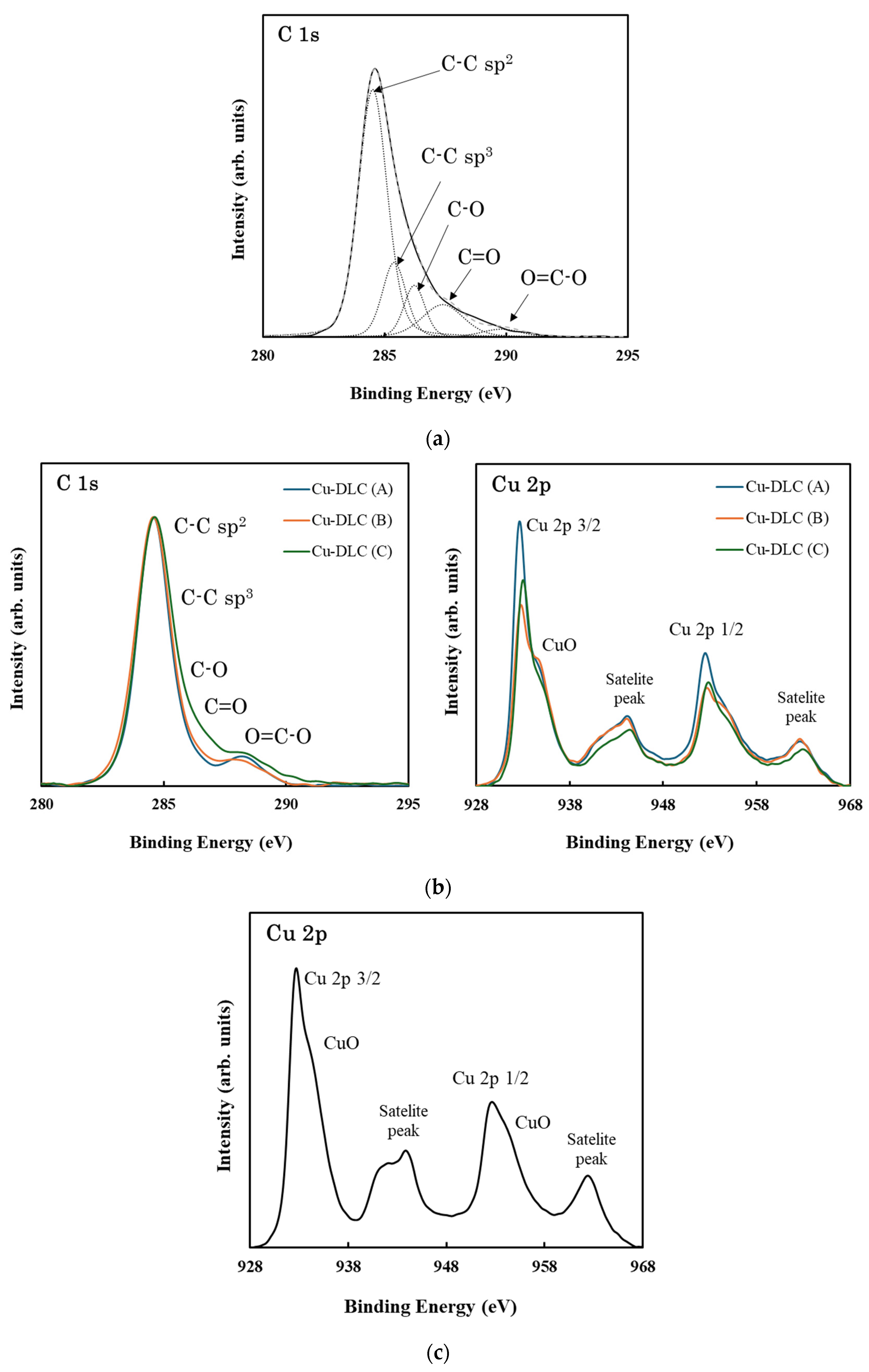

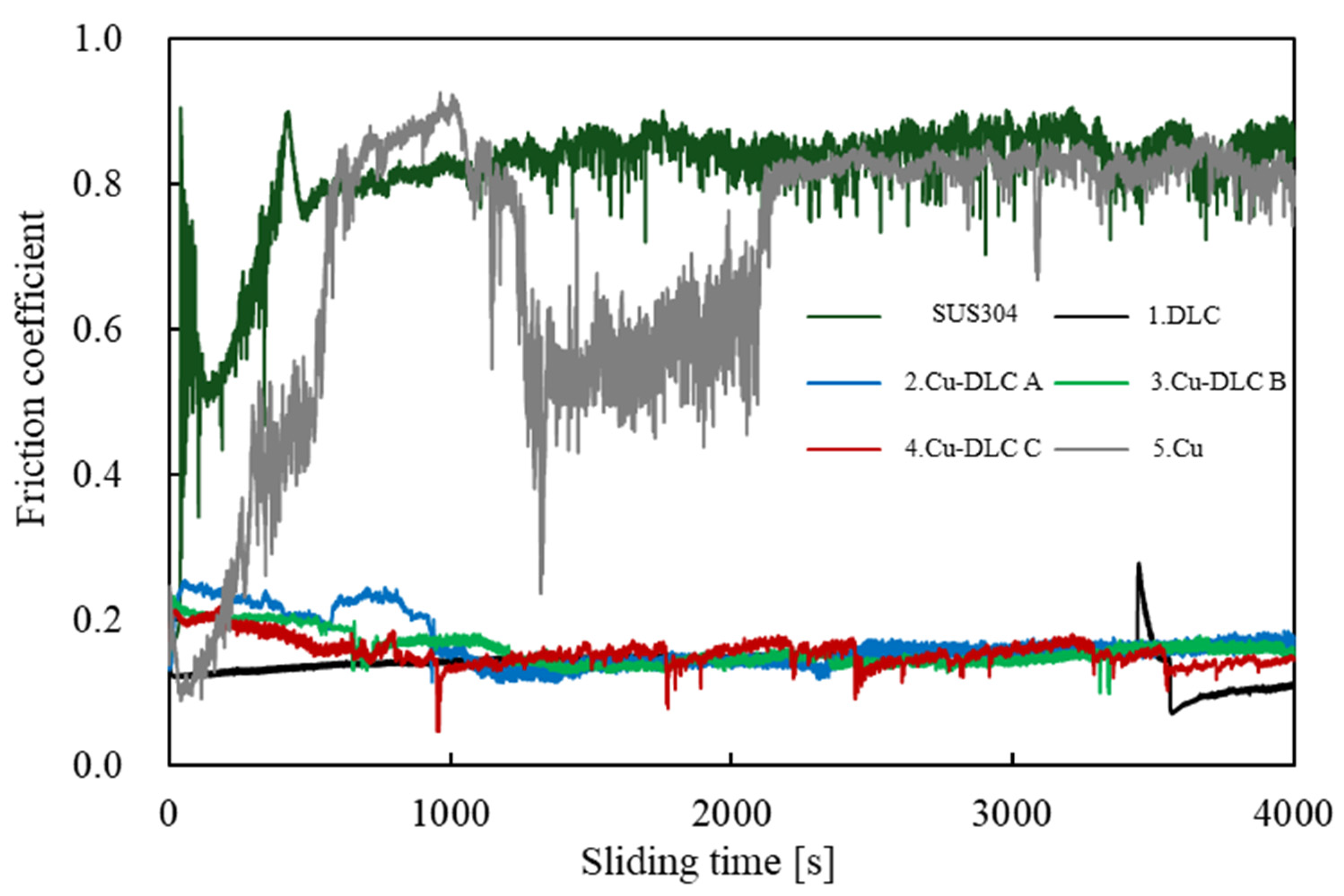

| Sample | C:Cu Target Ratio | Ar Gas Flow Rate [sccm] | Gas Pressure [Pa] | Bias Voltage [V] | Bias Current [A] | Deposition Time [min] |
|---|---|---|---|---|---|---|
| DLC | 100:0 | 20 | 3.7 × 10−1 | 530 | 1.44 | 180 |
| Cu-DLC (A) | 70:30 | 20 | 3.5 × 10−1 | 540 | 1.61 | 80 |
| Cu-DLC (B) | 60:40 | 20 | 3.6 × 10−1 | 530 | 1.92 | 60 |
| Cu-DLC (C) | 50:50 | 20 | 3.6 × 10−1 | 530 | 1.84 | 40 |
| Cu | 0:100 | 20 | 3.7 × 10−1 | 390 | 2.40 | 13 |
| Sample | Film Thickness (nm) | Deposition Rate (nm/min) | EPMA | Raman | ||||
|---|---|---|---|---|---|---|---|---|
| C (at%) | Cu (at%) | O (at%) | D-Peak Position (cm−1) | G-Peak Position (cm−1) | ID/IG Intensity Ratio | |||
| DLC | 1132 | 6.3 | 86.2 | - | 13.8 | 1393.0 ± 1.1 | 1568.5 ± 0.4 | 1.44 |
| Cu-DLC (A) | 944 | 11.8 | 53.0 | 40.0 | 7.0 | 1386.7 ± 1.7 | 1563.2 ± 0.9 | 1.88 |
| Cu-DLC (B) | 1287 | 21.5 | 44.2 | 47.3 | 8.5 | 1391.8 ± 2.1 | 1562.7 ± 1.3 | 2.04 |
| Cu-DLC (C) | 1058 | 26.5 | 34.9 | 54.1 | 11.0 | 1395.7 ± 1.6 | 1566.9 ± 0.9 | 1.97 |
| Cu | 930 | 71.5 | - | 96.9 | 3.1 | - | - | - |
| Sample | Surface Composition | C 1s Curve Fitting Area | Surface Roughness [nm] | Friction Coefficient | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C (at%) | Cu (at%) | O (at%) | C-C sp2 | C-C sp3 | C-O | C=O | O=C-O | |||
| DLC | 86.8 | - | 13.3 | 0.58 | 0.14 | 0.09 | 0.11 | 0.03 | 3.36 ± 0.96 | 0.14 ± 0.02 |
| Cu-DLC (A) | 46.7 | 27.9 | 25.4 | 0.59 | 0.17 | 0.05 | 0.10 | 0.02 | 4.29 ± 0.12 | 0.16 ± 0.02 |
| Cu-DLC (B) | 52.0 | 26.4 | 21.6 | 0.48 | 0.25 | 0.05 | 0.09 | 0.01 | 4.02 ± 0.15 | 0.17 ± 0.03 |
| Cu-DLC (C) | 53.2 | 22.4 | 24.5 | 0.49 | 0.20 | 0.08 | 0.11 | 0.03 | 5.97 ± 0.11 | 0.16 ± 0.02 |
| Cu | - | 62.9 | 37.1 | - | - | - | - | - | 2.36 ± 0.05 | 0.70 ± 0.20 |
| Sample | EPMA | ICP-OES |
|---|---|---|
| ΔCu Content (at%) | Cu Release Amount (ppm) | |
| Cu-DLC (A) | 1.3 | 0.37 |
| Cu-DLC (C) | 1.7 | 0.56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanasugi, K.; Nakajima, T.; Hirakuri, K. Antibacterial and Film Characteristics of Copper-Doped Diamond-like Carbon Films via Sputtering Using a Mixed Target of Copper and Graphite. Coatings 2025, 15, 559. https://doi.org/10.3390/coatings15050559
Kanasugi K, Nakajima T, Hirakuri K. Antibacterial and Film Characteristics of Copper-Doped Diamond-like Carbon Films via Sputtering Using a Mixed Target of Copper and Graphite. Coatings. 2025; 15(5):559. https://doi.org/10.3390/coatings15050559
Chicago/Turabian StyleKanasugi, Kazuya, Takayoshi Nakajima, and Kenji Hirakuri. 2025. "Antibacterial and Film Characteristics of Copper-Doped Diamond-like Carbon Films via Sputtering Using a Mixed Target of Copper and Graphite" Coatings 15, no. 5: 559. https://doi.org/10.3390/coatings15050559
APA StyleKanasugi, K., Nakajima, T., & Hirakuri, K. (2025). Antibacterial and Film Characteristics of Copper-Doped Diamond-like Carbon Films via Sputtering Using a Mixed Target of Copper and Graphite. Coatings, 15(5), 559. https://doi.org/10.3390/coatings15050559






