Artificial Neural Network-Based Modeling of Atmospheric Zinc Corrosion Rates Using Meteorological and Pollutant Data
Abstract
1. Introduction
2. Experimental Data Collection and Model Development
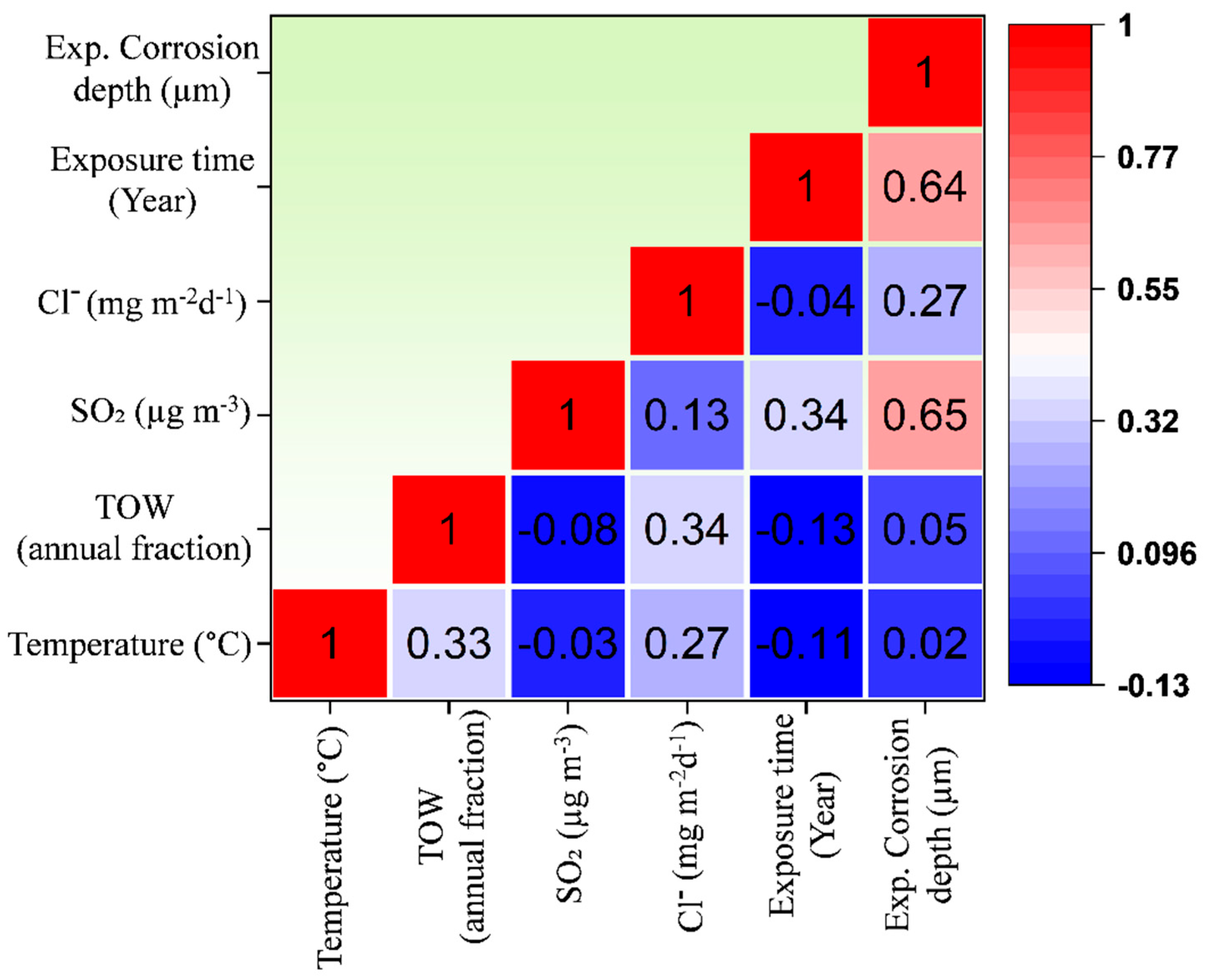
3. Results
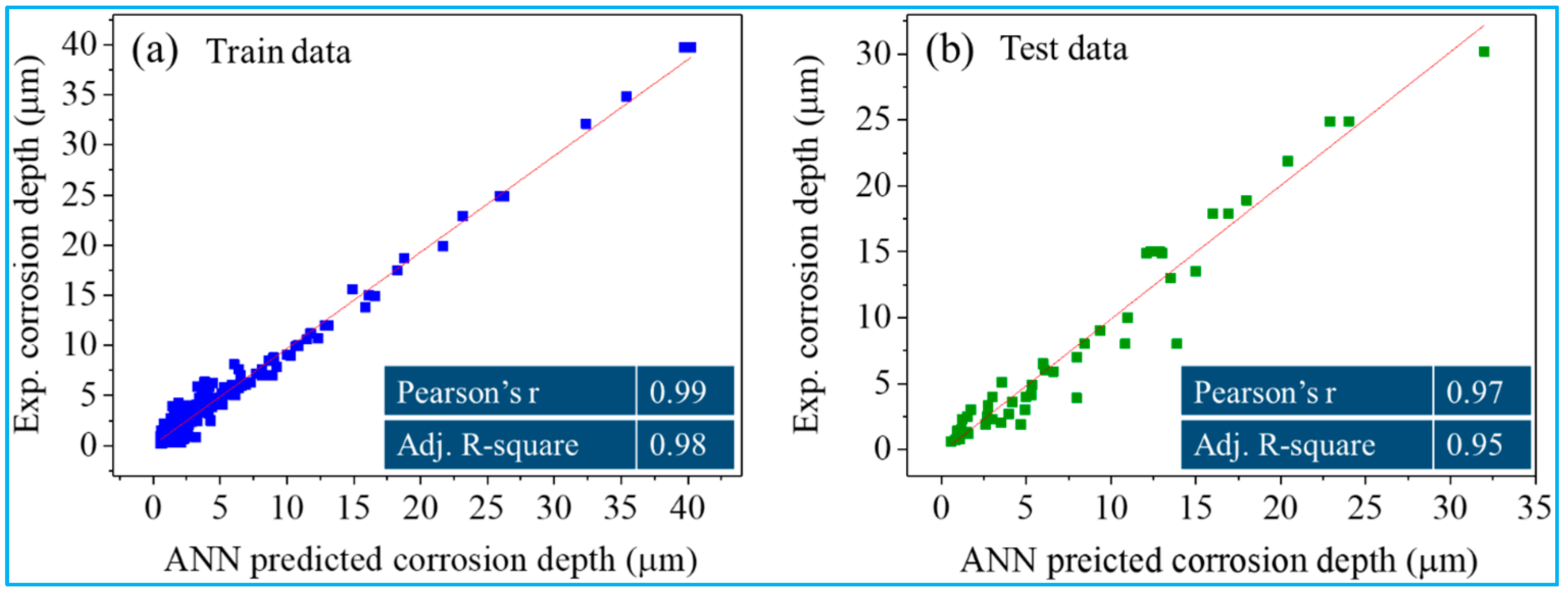
3.1. ANN Model Performance and Comparative Analysis
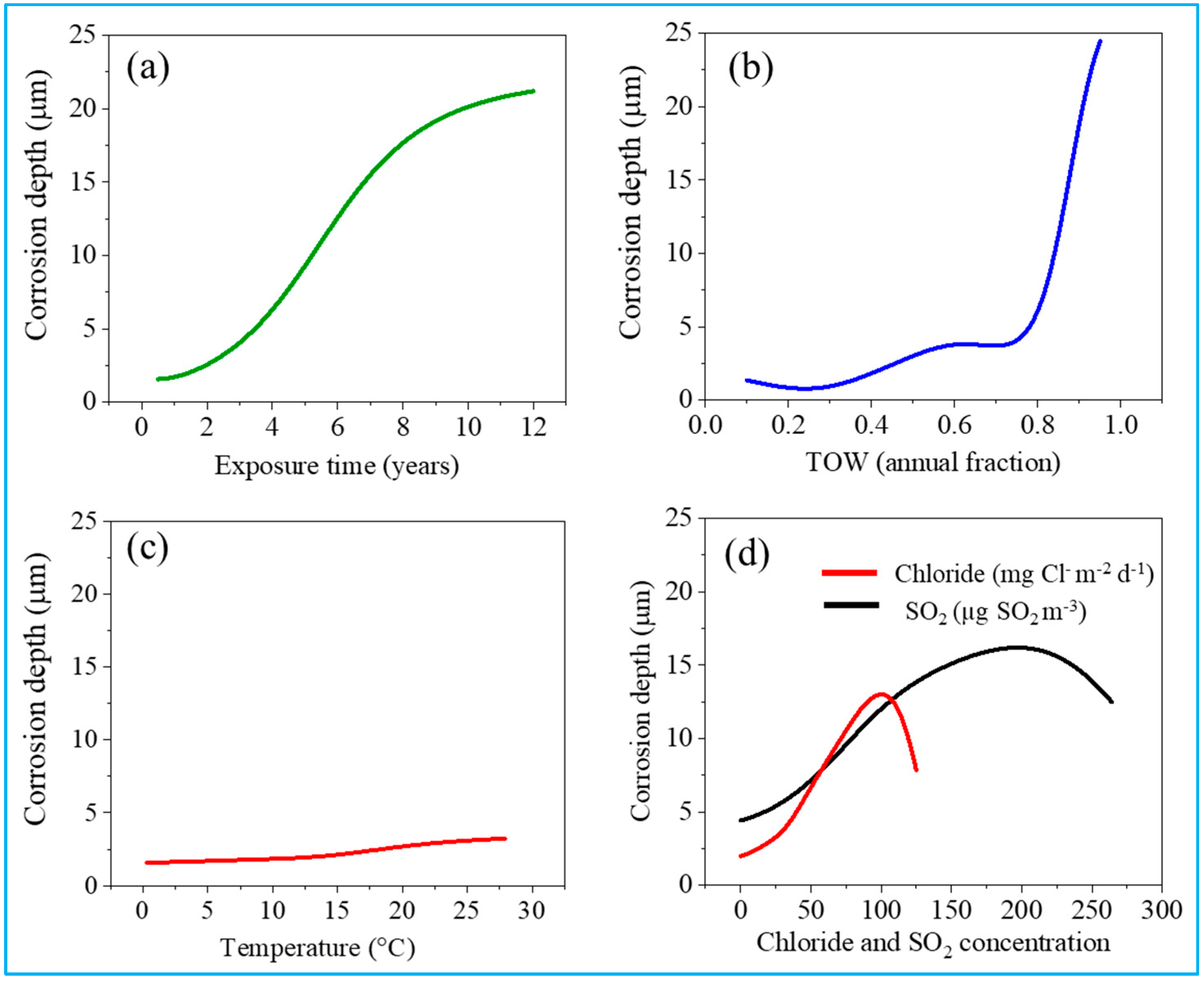
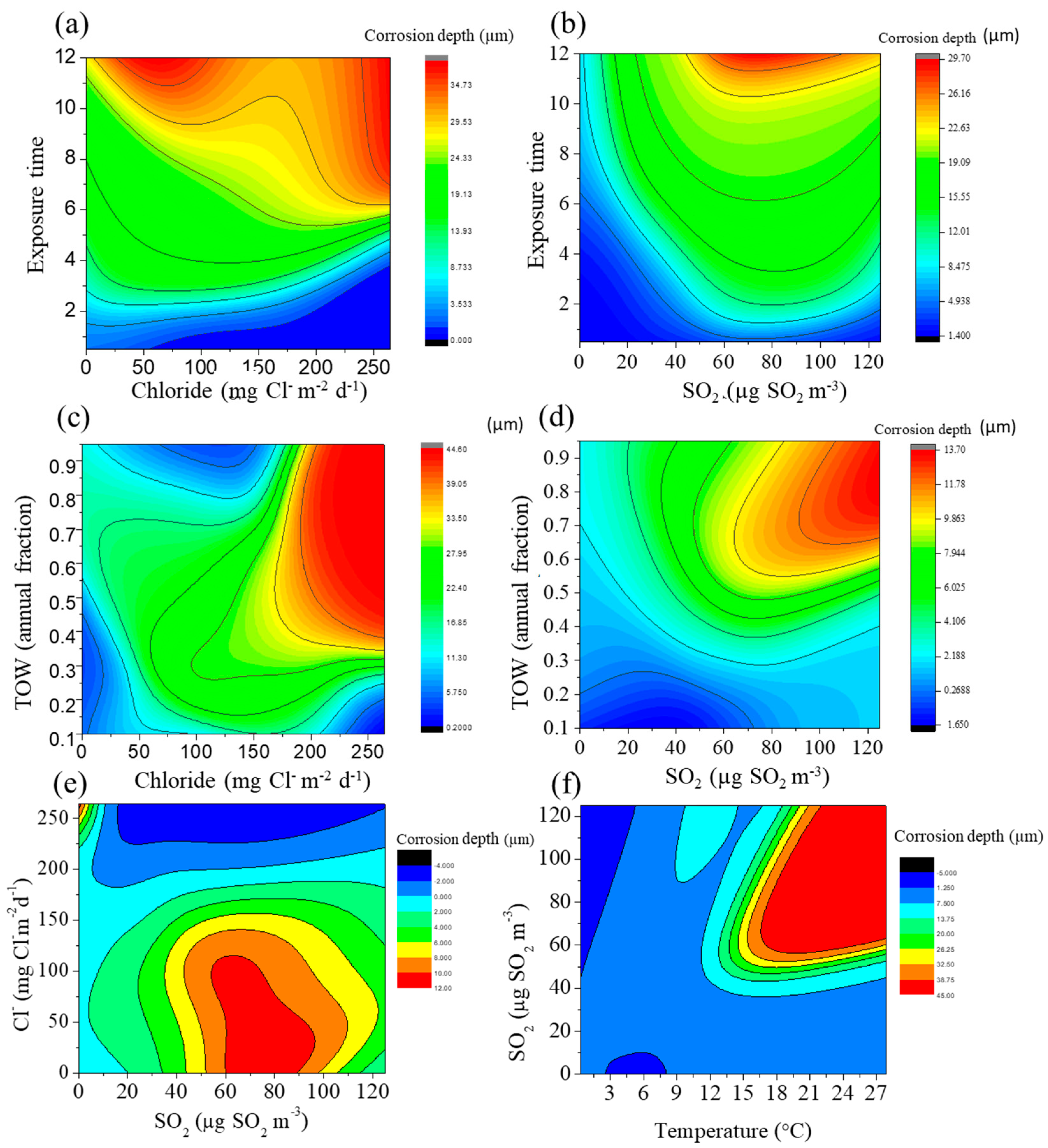
3.2. Prediction of Corrosion Depth
3.3. Index of Relative Importance (IRI)
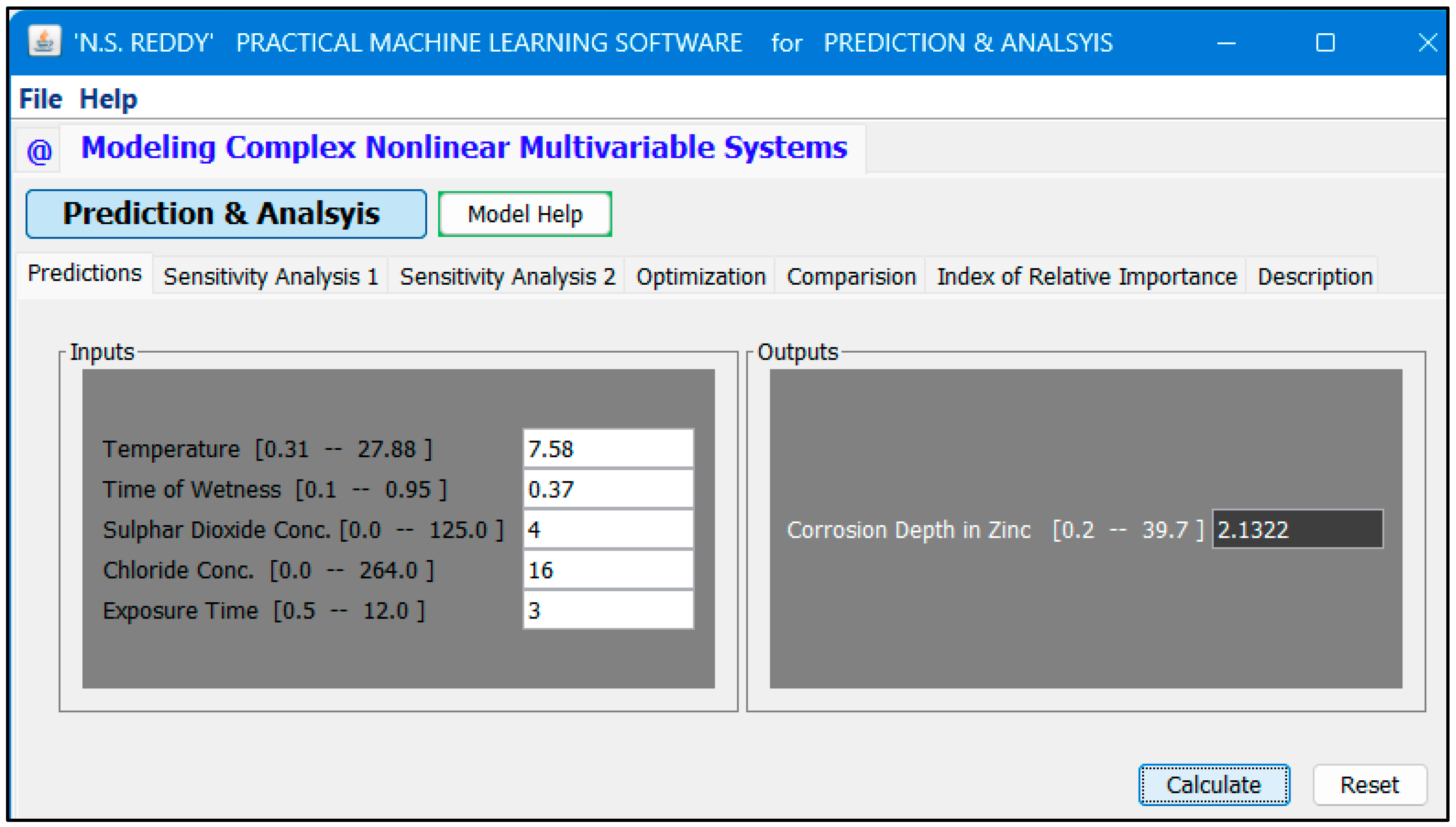
3.4. User-Friendly Neural Network Software for Accurate Prediction of Corrosion Depth
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Z.; Wang, F.; Qiu, D.; Taylor, J.; Zhang, M.-X. The Effect of Solute Elements on the Grain Refinement of Cast Zn. Metal. Mater. Trans. A 2013, 44, 4025–4030. [Google Scholar] [CrossRef]
- Liu, Z.; Qiu, D.; Wang, F.; Taylor, J.; Zhang, M.-X. The Grain Refining Mechanism of Cast Zinc Through Silver Inoculation. Acta Mater. 2014, 79, 315–326. [Google Scholar] [CrossRef]
- Yuan, W.; Xia, D.; Wu, S.; Zheng, Y.; Guan, Z.; Rau, J.V. A Review on Current Research Status of the Surface Modification of Zn-Based Biodegradable Metals. Bioact. Mater. 2021, 7, 192–216. [Google Scholar] [CrossRef]
- Rajaei, M.; Elahi, S.H.; Asefi, A. Modal Properties of Closed-Cell Zinc Foam. Structures 2020, 27, 1380–1383. [Google Scholar] [CrossRef]
- de la Fuente, D.; Castaño, J.G.; Morcillo, M. Long-Term Atmospheric Corrosion of Zinc. Corros. Sci. 2007, 49, 1420–1436. [Google Scholar] [CrossRef]
- Jain, R.; Jain, S.; Dewangan, S.K.; Boriwal, L.K.; Samal, S. Machine Learning-Driven Insights into Phase Prediction for High Entropy Alloys. J. Alloys Metall. Syst. 2024, 8, 100110. [Google Scholar] [CrossRef]
- Marder, A.R. The Metallurgy of Zinc-Coated Steel. Prog. Mater. Sci. 2000, 45, 191–271. [Google Scholar] [CrossRef]
- Kong, L.; Heydari, Z.; Lami, G.H.; Saberi, A.; Baltatu, M.S.; Vizureanu, P. A Comprehensive Review of the Current Research Status of Biodegradable Zinc Alloys and Composites for Biomedical Applications. Materials 2023, 16, 4797. [Google Scholar] [CrossRef]
- Liu, L.; Meng, Y.; Volinsky, A.A.; Zhang, H.; Wang, L.-N. Influences of Albumin on In Vitro Corrosion of Pure Zn in Artificial Plasma. Corros. Sci. 2019, 153, 341–356. [Google Scholar] [CrossRef]
- Azevedo, M.S.; Allely, C.; Ogle, K.; Volovitch, P. Corrosion Mechanisms of Zn (Mg, Al) Coated Steel in Accelerated Tests and Natural Exposure: 1. The Role of Electrolyte Composition in the Nature of Corrosion Products and Relative Corrosion Rate. Corros. Sci. 2015, 90, 472–481. [Google Scholar] [CrossRef]
- Wang, X.; Liu, B.; Li, M.; Liu, Y.; Yu, X.; Sun, X.; Shi, L. Comparative Study of Zinc Base Alloy Coatings: Composition, Microstructure, and Corrosion Resistance. Ferroelectrics 2024, 618, 1655–1665. [Google Scholar] [CrossRef]
- Thierry, D.; Persson, D.; LeBozec, N. Long-Term Atmospheric Corrosion Rates of Zn55Al-Coated Steel. Mater. Corros. 2024, 75, 694–704. [Google Scholar] [CrossRef]
- Maniam, K.K.; Paul, S. Corrosion Performance of Electrodeposited Zinc and Zinc-Alloy Coatings in Marine Environment. Corros. Mater. Degrad. 2021, 2, 163–189. [Google Scholar] [CrossRef]
- Cai, J.; Cottis, R.A.; Lyon, S. Phenomenological Modelling of Atmospheric Corrosion Using an Artificial Neural Network. Corros. Sci. 1999, 41, 2001–2030. [Google Scholar] [CrossRef]
- Feliu, S.; Morcillo, M. The Prediction of Atmospheric Corrosion from Meteorological and Pollution Parameters—II. Long-Term Forecasts. Corros. Sci. 1993, 34, 415–422. [Google Scholar] [CrossRef]
- Weibel, D.; Jovanovic, Z.R.; Gálvez, E.; Steinfeld, A. Mechanism of Zn Particle Oxidation by H2O and CO2 in the Presence of ZnO. Chem. Mater. 2014, 26, 6486–6495. [Google Scholar] [CrossRef]
- Cole, I.S.; Paterson, D.A.; Ganther, W.D. Holistic model for atmospheric corrosion Part 1—Theoretical Framework for Production, Transportation and Deposition of Marine Salts. Corros. Eng. Sci. Technol. 2003, 38, 129–134. [Google Scholar] [CrossRef]
- Spence, J.W.; Haynie, F.H.; Lipfert, F.W.; Cramer, S.D.; McDonald, L.G. Atmospheric Corrosion Model for Galvanized Steel Structures. Corrosion 1992, 48, 1009–1019. [Google Scholar] [CrossRef]
- Mikhailov, A.A.; Tidblad, J.; Kucera, V. The Classification System of ISO 9223 Standard and the Dose–Response Functions Assessing the Corrosivity of Outdoor Atmospheres. Prot. Met. 2004, 40, 541–550. [Google Scholar] [CrossRef]
- Kenny, E.D.; Paredes, R.S.C.; de Lacerda, L.A.; Sica, Y.C.; de Souza, G.P.; Lázaris, J. Artificial Neural Network Corrosion Modeling for Metals in an Equatorial Climate. Corros. Sci. 2009, 51, 2266–2278. [Google Scholar] [CrossRef]
- Zulkifli, F.; Abdullah, S.; Suriani, M.J.; Kamaludin, M.I.A.; Nik, W.W. Multilayer Perceptron Model for the Prediction of Corrosion Rate of Aluminium Alloy 5083 in Seawater via Different Training Algorithms. IOP Conf. Ser. Earth Environ. Sci. 2021, 646, 012058. [Google Scholar] [CrossRef]
- Tidblad, J.; Kucera, V.; Mikhailov, A.A.; Henriksen, J.F.; Kreislova, K.; Yates, T.; Stöckle, B.; Schreiner, M. UN ECE ICP Materials: Dose-Response Functions on Dry and Wet Acid Deposition Effects After 8 Years of Exposure. Water Air Soil Pollut. 2001, 130, 1457–1462. [Google Scholar] [CrossRef]
- Fontana, M.G.; Greene, N.D. Corrosion Engineering; McGraw-Hill: New York, NY, USA, 1984; Volume 1984, pp. 1–99. [Google Scholar]
- Malla, A.D.; Sullivan, J.H.; Penney, D.J.; Dunlop, T.; Barker, P. Mechanistic Study on the Corrosion Behaviour of Zinc and Zinc-Calcium Alloys Designed for Enhanced Metallic Coatings in the Presence of Chloride and Phosphate Ions. Corros. Sci. 2023, 213, 110956. [Google Scholar] [CrossRef]
- Langklotz, U.; Babutzka, M.; Schneider, M.; Burkert, A. The Combination of Minimally Invasive Electrochemical Investigations and FTIR-Spectroscopy to Analyze Atmospheric Corrosion Product Layers on Zinc. Mater. Corros. 2019, 70, 1314–1325. [Google Scholar] [CrossRef]
- Kauffman, G.B. Electrochemical Impedance Spectroscopy. By Mark E. Orazem and Bernard Tribollet. Angew. Chem. 2009, 48, 1532–1533. [Google Scholar] [CrossRef]
- Anderson, E. The Atmospheric Corrosion of Rolled Zinc; ASTM International: West Conshohocken, PA, USA, 1956. [Google Scholar] [CrossRef]
- Ailor, W.; Coburn, S. Metal Corrosion in the Atmosphere; ASTM International: West Conshohocken, PA, USA, 1968. [Google Scholar] [CrossRef]
- Barton, K. Protection Against Atmospheric Corrosion: Theories and Methods; John Wiley & Sons: Hoboken, NJ, USA, 1976. [Google Scholar]
- Shi, Y.Y.; Zhang, Z.; Zhang, J.; Cao, C. Review of Atmospheric Corrosion of Zinc and Zinc Alloy. J. Chin. Soc. Corros. Prot. 2009, 25, 373–379. [Google Scholar]
- Schindelholz, E.J.; Kelly, R.G. Wetting Phenomena and Time of Wetness in Atmospheric Corrosion: A Review. Corros. Rev. 2012, 30, 135–170. [Google Scholar] [CrossRef]
- Marcus, P. Corrosion Mechanisms in Theory and Practice; Marcel Dekker Inc: New York, NY, USA, 1995. [Google Scholar] [CrossRef]
- Liu, Y.; Gu, T.; Liu, M.; Wang, Z.; Cao, G.; Guo, Q.; Wang, C. Corrosion Mechanism of a High Corrosion-Resistance Zn–Al–Mg Coating in Typical Extremely Harsh Marine and Cold Environments. J. Mater. Res. Technol. 2024, 33, 4290–4302. [Google Scholar] [CrossRef]
- Azmat, N.S.; Ralston, K.D.; Muddle, B.C.; Cole, I.S. Corrosion of Zn Under Fine Size Aerosols and Droplets Using Inkjet Printer Deposition and Optical Profilometry Quantification. Corros. Sci. 2011, 53, 3534–3541. [Google Scholar] [CrossRef]
- Azmat, N.S.; Ralston, K.D.; Muddle, B.C.; Cole, I.S. Corrosion of Zn Under Acidified Marine Droplets. Corros. Sci. 2011, 53, 1604–1615. [Google Scholar] [CrossRef]
- Rao, P.; Mulky, L. Microbially Influenced Corrosion and Its Control Measures: A Critical Review. J. Bio- Tribo-Corros. 2023, 9, 57. [Google Scholar] [CrossRef]
- Cai, Y.; Zhao, Y.; Ma, X.; Zhou, K.; Chen, Y. Influence of Environmental Factors on Atmospheric Corrosion in Dynamic Environment. Corros. Sci. 2018, 137, 163–175. [Google Scholar] [CrossRef]
- Guttman, H.; Sereda, P. Metal Corrosion in the Atmosphere. ASTM STP 1968, 435, 223. [Google Scholar]
- Esmaily, M.; Mortazavi, N.; Svensson, J.-E.; Johansson, L.-G. Evidence for an Unusual Temperature Dependence of the Atmospheric Corrosion of Zinc. J. Electrochem. Soc. 2016, 163, 864–872. [Google Scholar] [CrossRef]
- Roberge, P.R.; Klassen, R.D.; Haberecht, P.W. Atmospheric Corrosivity Modeling—A Review. Mater. Des. 2002, 23, 321–330. [Google Scholar] [CrossRef]
- Qu, Q.; Yan, C.; Wan, Y.; Cao, C. Effects of NaCl and SO2 on the Initial Atmospheric Corrosion of Zinc. Corros. Sci. 2002, 44, 2789–2803. [Google Scholar] [CrossRef]
- Oesch, S.; Faller, M. Environmental Effects on Materials: The Effect of the Air Pollutants SO2, NO2, NO and O3 on the Corrosion of Copper, Zinc and Aluminum. A Short Literature Survey and Results of Laboratory Exposures. ChemInform 1997, 28. [Google Scholar] [CrossRef]
- Yin, Q.; Wang, Z.; Liu, M.; Pan, C. Synergistic Effect of NaCl and SO2 on the Initial Atmospheric Corrosion of Zinc Under Wet–Dry Cyclic Conditions. Acta Metall. Sin. Engl. Lett. 2019, 32, 780–796. [Google Scholar] [CrossRef]
- Sziráki, L.; Cziráki, Á.; Geröcs, I.; Vértesy, Z.; Kiss, L. A Kinetic Model of the Spontaneous Passivation and Corrosion of Zinc in Near Neutral Na2SO4 Solutions. Electrochim. Acta 1998, 43, 175–186. [Google Scholar] [CrossRef]
- Yoo, J.D.; Volovitch, P.; Aal, A.A.; Allely, C.; Ogle, K. The Effect of an Artificially Synthesized Simonkolleite Layer on the Corrosion of Electrogalvanized Steel. Corros. Sci. 2013, 70, 1–10. [Google Scholar] [CrossRef]
- Kear, R.W. The Influence of Carbon Smokes on the Corrosion of Metal Surfaces Exposed to Flue Gases Containing Sulphur Trioxide. J. Chem. Technol. Biotechnol. 1951, 1, 393–399. [Google Scholar] [CrossRef]
- Askey, A.; Lyon, S.; Thompson, G.E.; Johnson, J.B.; Wood, G.C.; Sage, P.W.; Cooke, M.J. The Effect of Fly-Ash Particulates on the Atmospheric Corrosion of Zinc and Mild Steel. Corros. Sci. 1993, 34, 1055–1081. [Google Scholar] [CrossRef]
- Falk, T.; Svensson, J.-E.; Johansson, L.-G. The Influence of CO2 and NaCl on the Atmospheric Corrosion of Zinc A Laboratory Study. J. Electrochem. Soc. 1998, 145, 2993–2999. [Google Scholar] [CrossRef]
- Svensson, J.-E.; Johansson, L.-G. A Laboratory Study of the Initial Stages of the Atmospheric Corrosion of Zinc in the Presence of NaCl; Influence of SO2 and NO2. Corros. Sci. 1993, 34, 721–740. [Google Scholar] [CrossRef]
- Wallinder, I.O.; Leygraf, C. A Critical Review on Corrosion and Runoff from Zinc and Zinc-Based Alloys in Atmospheric Environments. Corrosion 2017, 73, 1060–1077. [Google Scholar] [CrossRef]
- Nazarov, A.; Thierry, D. Rate-Determining Reactions of Atmospheric Corrosion. Electrochem. Methods Corros. Res. 2004, 49, 2717–2724. [Google Scholar] [CrossRef]
- Tidblad, J.; Kucera, V.; Ferm, M.; Kreislova, K.; Brüggerhoff, S.; Doytchinov, S.; Screpanti, A.; Grøntoft, T.; Yates, T.; De La Fuente, D.; et al. Effects of Air Pollution on Materials and Cultural Heritage: ICP Materials Celebrates 25 Years of Research. Int. J. Corros. 2012, 2012, 496321. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Persson, D.; Leygraf, C. Initial NaCl-Particle Induced Atmospheric Corrosion of Zinc—Effect of CO2 and SO2. Corros. Sci. 2008, 50, 111–123. [Google Scholar] [CrossRef]
- Parker, M.E.; Kelly, R.G. Improved Atmospheric Corrosion Testing for Aluminum Alloys, Part I: Deconstructing ASTM G85-A2. CORROSION 2019, 76, 39–50. [Google Scholar] [CrossRef]


| Modeling Approach | Pearson’s r | Adjusted R2 | RMSE (mm) | MAE (mm) | Reference |
|---|---|---|---|---|---|
| Our ANN | 0.94 | 0.92 | 0.05 | 0.04 | Current study |
| Traditional Power-Law | 0.82 | 0.76 | 0.15 | 0.12 | Feliu et al. [15] |
| Statistical Regression | 0.85 | 0.81 | 0.12 | 0.10 | Tidblad et al. [22] |
| Previous ANN (3 parameters) | 0.88 | 0.84 | 0.09 | 0.07 | Kenny et al. [20] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maurya, A.K.; Tiwari, S.; Bhavani, A.G.; Park, N.; Reddy, N.G.S. Artificial Neural Network-Based Modeling of Atmospheric Zinc Corrosion Rates Using Meteorological and Pollutant Data. Coatings 2025, 15, 538. https://doi.org/10.3390/coatings15050538
Maurya AK, Tiwari S, Bhavani AG, Park N, Reddy NGS. Artificial Neural Network-Based Modeling of Atmospheric Zinc Corrosion Rates Using Meteorological and Pollutant Data. Coatings. 2025; 15(5):538. https://doi.org/10.3390/coatings15050538
Chicago/Turabian StyleMaurya, Anoop K., Saurabh Tiwari, Annabathini Geetha Bhavani, Nokeun Park, and Nagireddy Gari Subba Reddy. 2025. "Artificial Neural Network-Based Modeling of Atmospheric Zinc Corrosion Rates Using Meteorological and Pollutant Data" Coatings 15, no. 5: 538. https://doi.org/10.3390/coatings15050538
APA StyleMaurya, A. K., Tiwari, S., Bhavani, A. G., Park, N., & Reddy, N. G. S. (2025). Artificial Neural Network-Based Modeling of Atmospheric Zinc Corrosion Rates Using Meteorological and Pollutant Data. Coatings, 15(5), 538. https://doi.org/10.3390/coatings15050538









