Oxidation Resistance and Mechanical Properties of Mo2N/MoSi2 Multi-Layer Films Prepared by Reactive Magnetron Sputtering
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Films
2.2. Characterization of Films
3. Results and Discussion
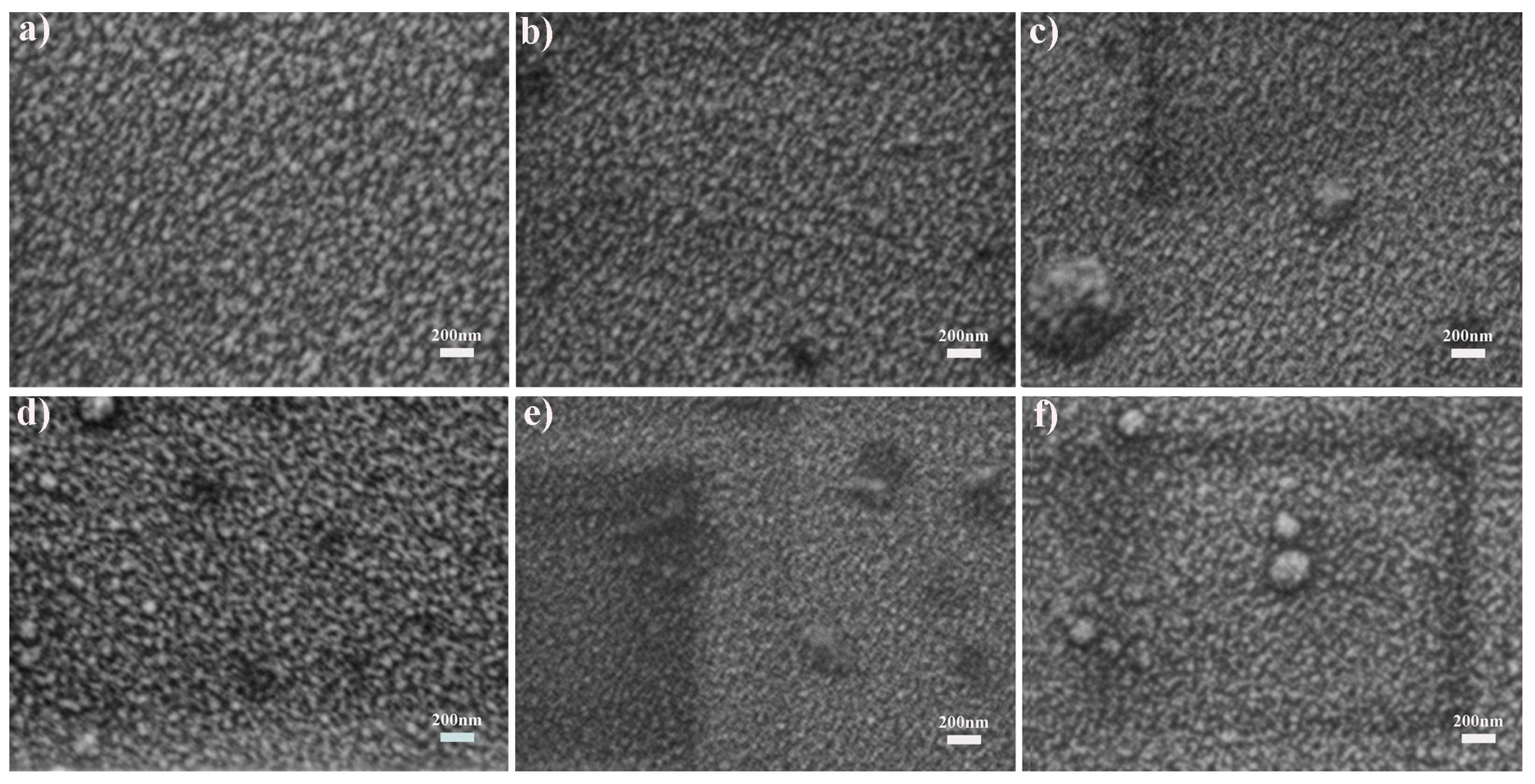
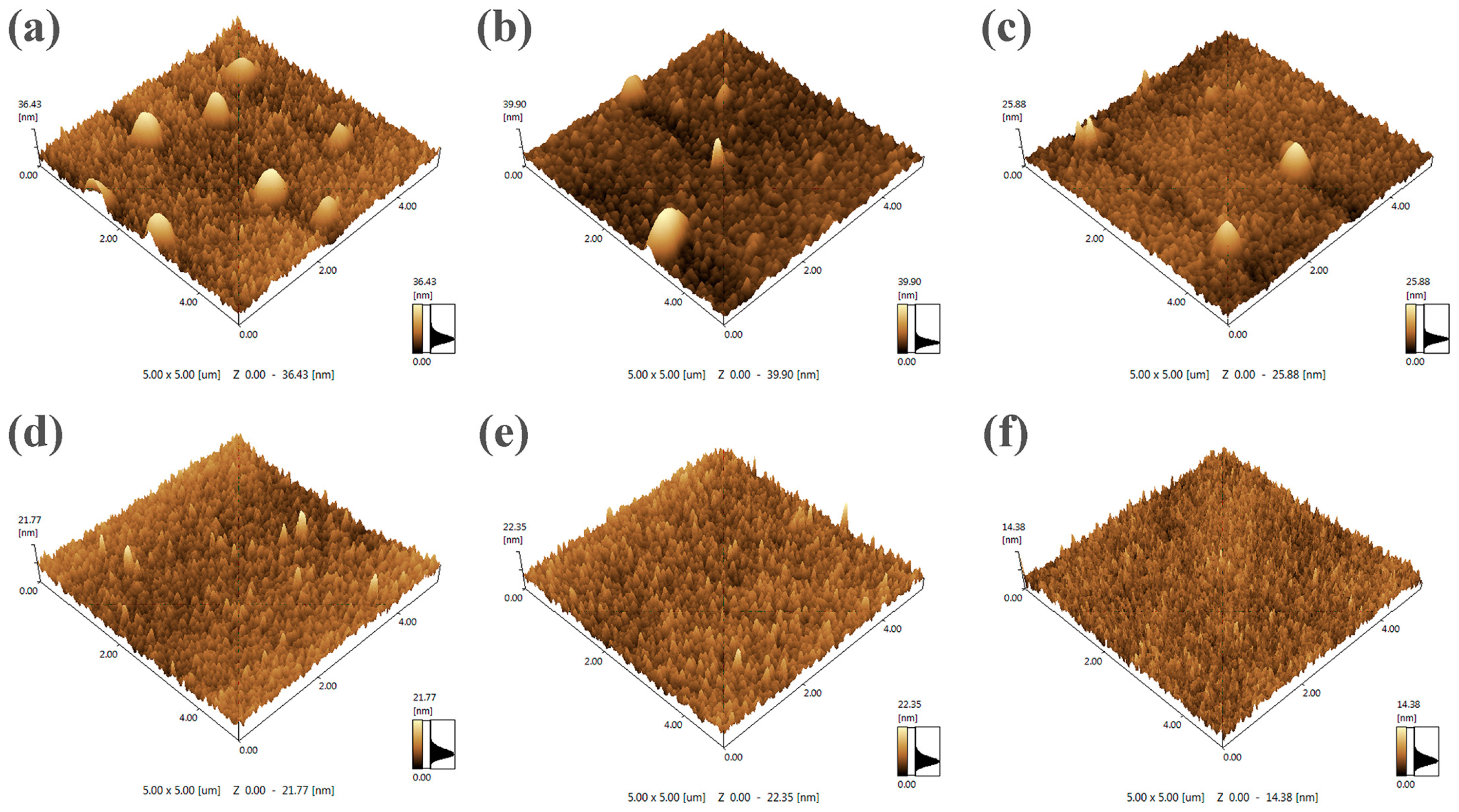
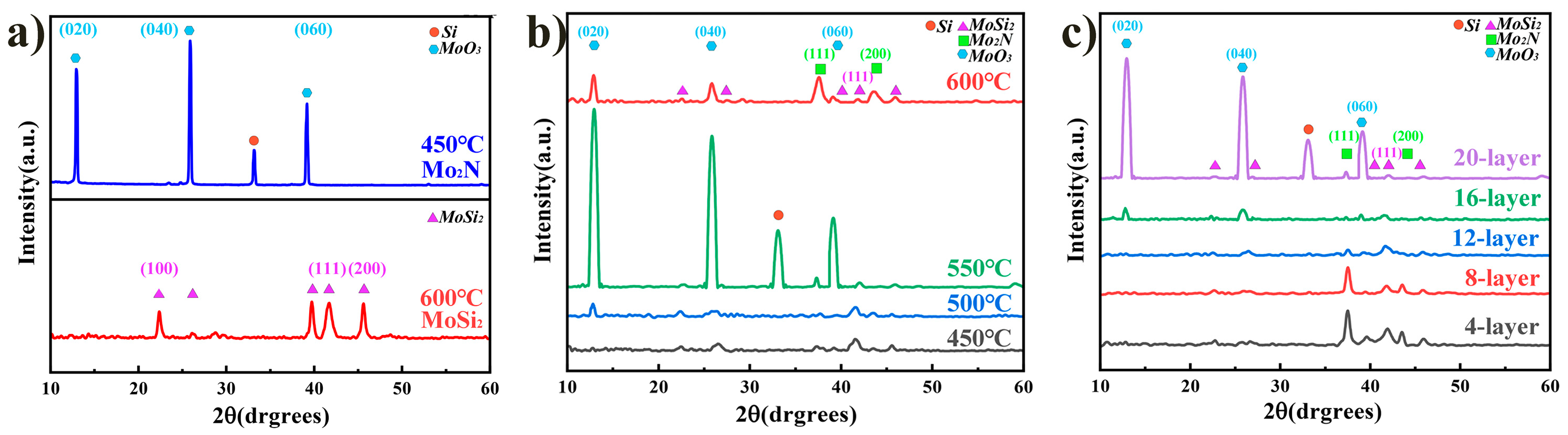
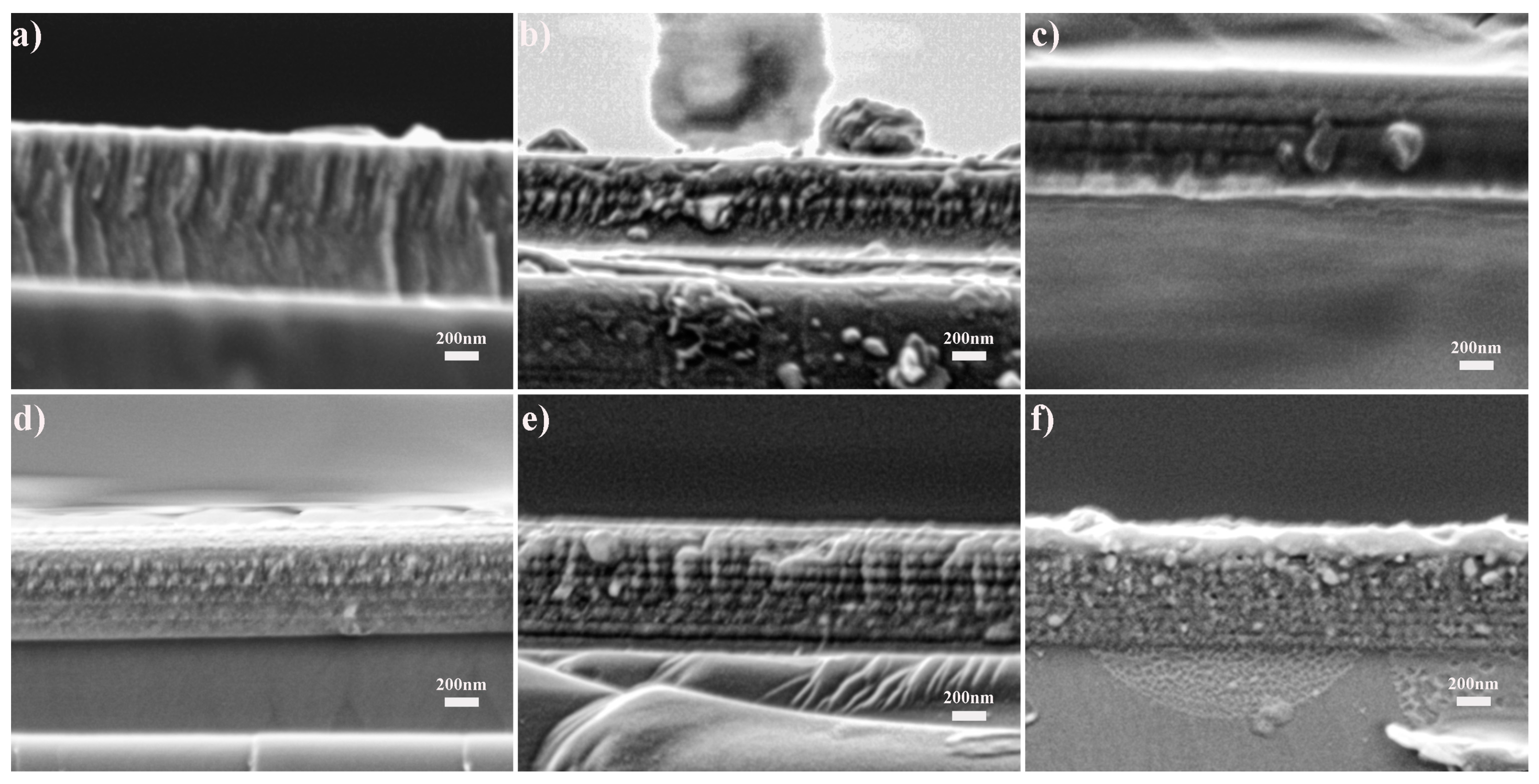
3.1. Structure of As-Received Films
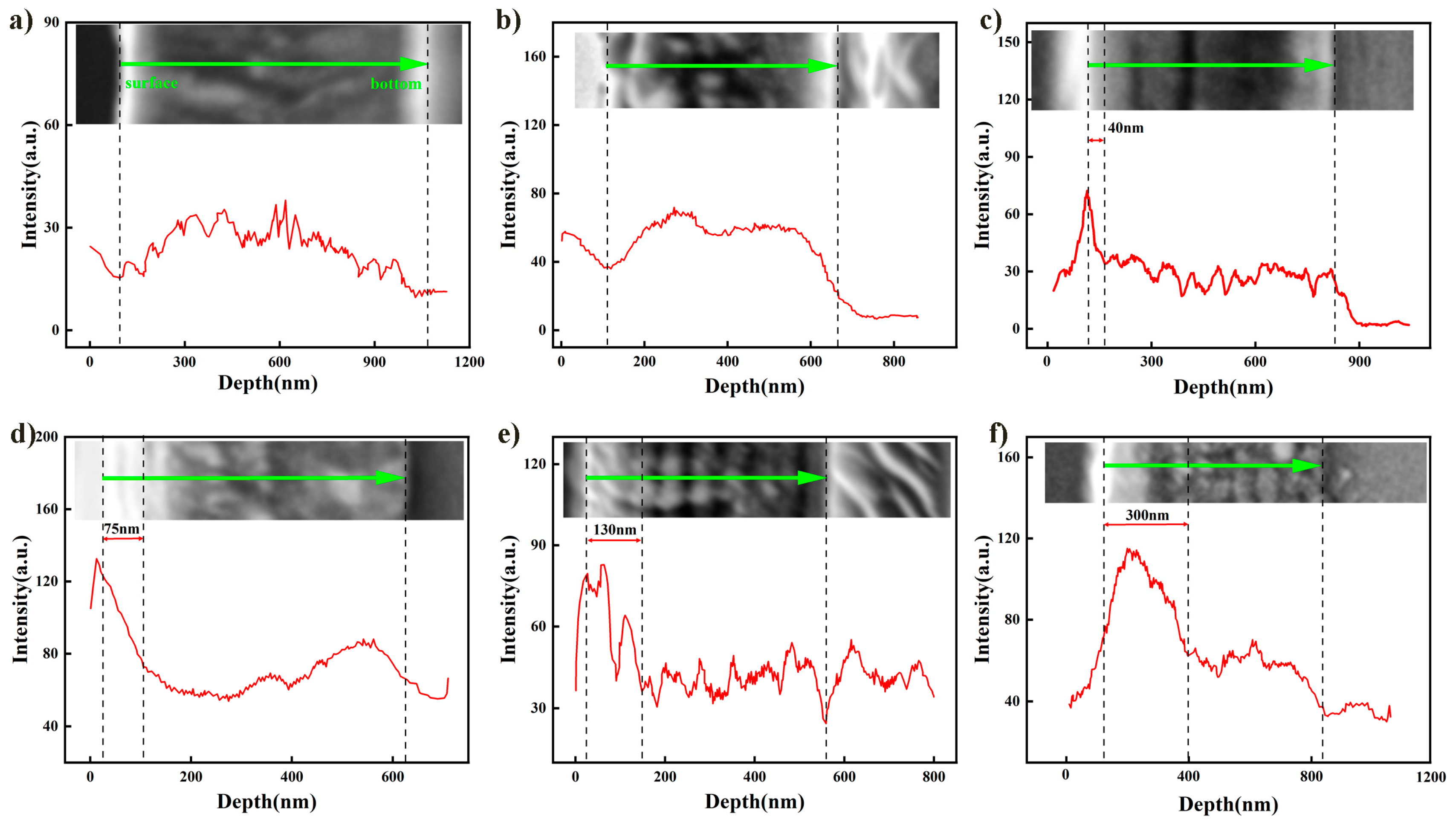
3.2. Oxidation of the Films
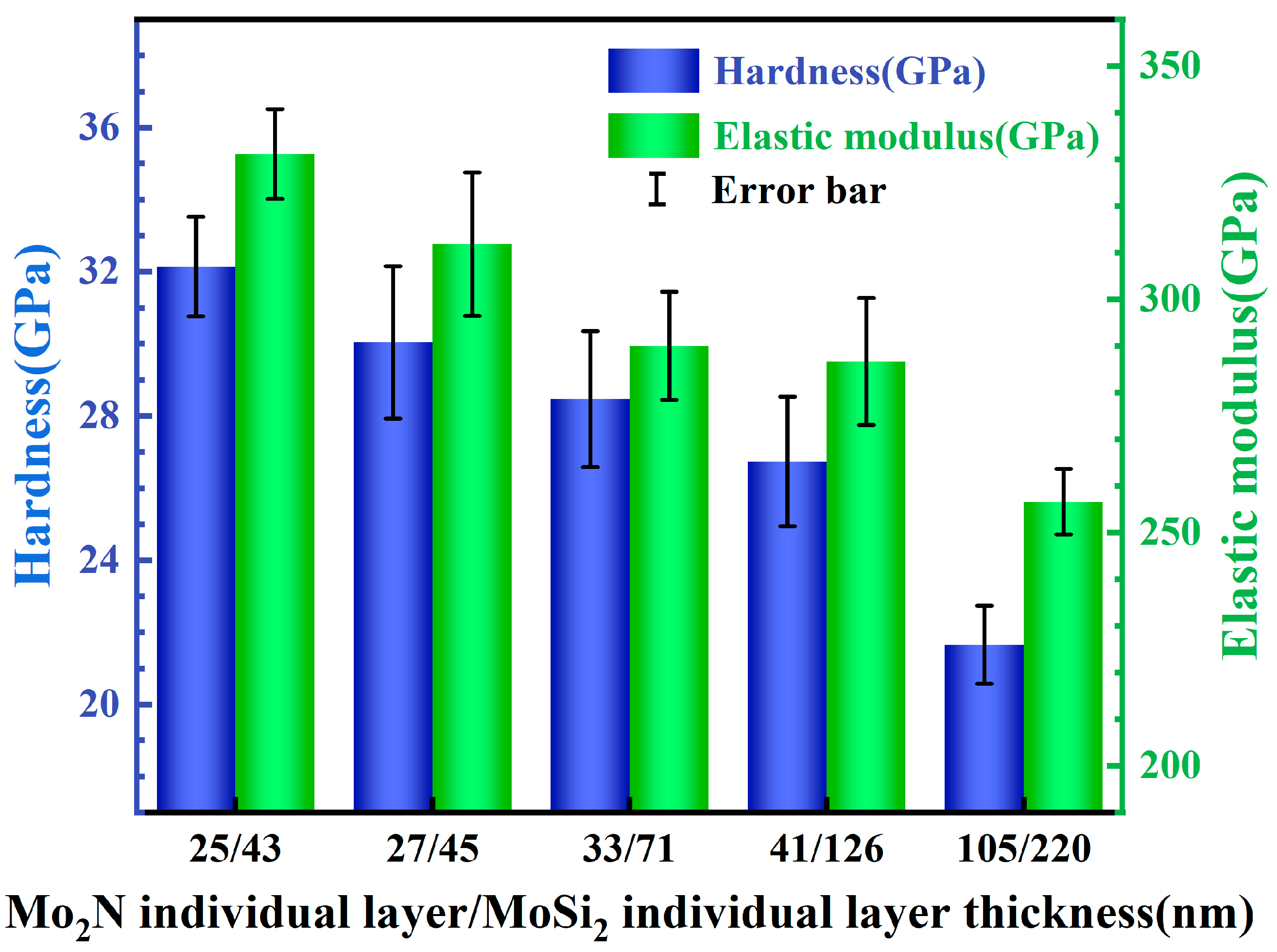
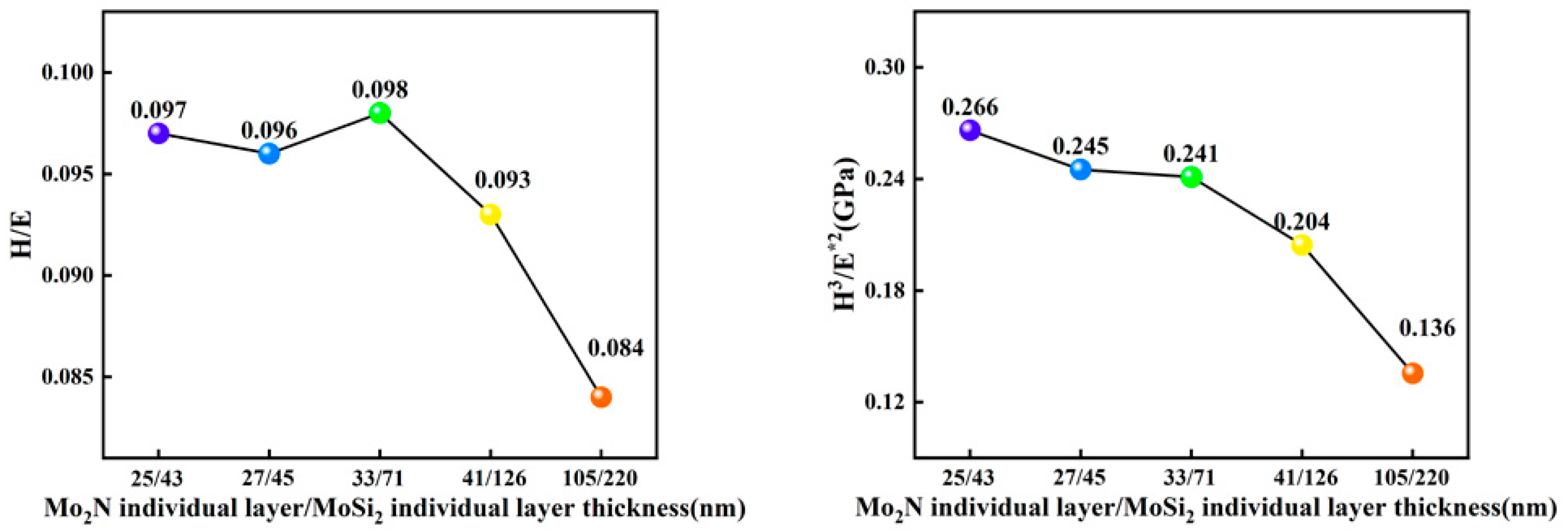
3.3. Nanoindentation of the Films
4. Conclusions
- (1)
- The Mo2N/MoSi2 multi-layer films consist of cubic Mo2N and hexagonal MoSi2 phases, and the average grain size decreases with decreasing thickness of the individual layer. The decrease in grain size is attributed to the confinement effect of the interlayer interfaces, which inhibits grain growth and refines the grain size.
- (2)
- The Mo2N/MoSi2 multi-layer films exhibit better oxidation resistance than the pure Mo2N films. This is primarily attributed to the superior oxidation resistance of the MoSi2 layer and the barrier effect of the multi-layer interfaces. However, as the number of layers increases and the thickness of the individual layers decreases, the pinhole defects on the surface of the films provide oxygen diffusion channels, leading to a gradual decline in the oxidation resistance of the film. Additionally, under high-temperature conditions, the degradation in the oxidation resistance of the multi-layer films may be attributed to the volatilization of the oxidation product MoO3, which creates channels for oxygen ingress.
- (3)
- The Mo2N/MoSi2 multi-layer films exhibit higher elastic modulus and hardness than the MoSi2 and Mo2N films. The elastic modulus and hardness of the Mo2N/MoSi2 multi-layer films increase progressively with an increasing number of layers, which can be ascribed to the combined action of the reduction in the thickness of the individual layer, the increase in the interlayer interface, and grain refinement.
- (4)
- The H/E and H3/E*2 values of the 20-layer Mo2N/MoSi2 multi-layer film are relatively high, indicating that the 20-layer film has excellent tribological properties.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, J.; Ju, H.; Yu, L. Influence of Silicon Content on the Microstructure, Mechanical and Tribological Properties of Magnetron Sputtered Ti-Mo-Si-N Films. Vacuum 2014, 110, 47–53. [Google Scholar] [CrossRef]
- Zhou, R.; Ju, H.; Liu, S.; Zhao, Z.T.; Xu, J.; Yu, L.; Qian, H.; Jia, S.; Song, R.; Shen, J. The Influences of Ag Content on the Friction and Wear Properties of TiCN-Ag Films. Vacuum 2022, 196, 110719. [Google Scholar] [CrossRef]
- Kindlund, H.; Sangiovanni, D.G.; Petrov, I.; Greene, J.E.; Hultman, L. A Review of the Intrinsic Ductility and Toughness of Hard Transition-Metal Nitride Alloy Thin Films. Thin Solid Films 2019, 688, 137479. [Google Scholar] [CrossRef]
- Zhang, X.; Tian, X.B.; Zhao, Z.W.; Gao, J.B.; Zhou, Y.W.; Gao, P.; Guo, Y.Y.; Lv, Z. Evaluation of the Adhesion and Failure Mechanism of the Hard CrN Coatings on Different Substrates. Surf. Coat. Technol. 2019, 364, 135–143. [Google Scholar] [CrossRef]
- Mei, H.; Cai, Z.; Ding, J.; Yan, K.; Li, Q.; Zhao, Z.; Gong, W. The Additions of V and Cu on the Microstructure and Mechanical Properties of Mo-N Coatings. Coatings 2022, 12, 1129. [Google Scholar] [CrossRef]
- Hao, J.; Zhang, K.; Ren, P.; Chen, J.; Wang, M.; Zhang, Y.; Wen, M. Tuning the Wettability, Mechanical and Tribological Properties of NbN Films by Doping Rare Earth Cerium. J. Alloys Compd. 2020, 814, 152339. [Google Scholar] [CrossRef]
- Sun, L.; Mao, J.H.; Li, Y.C.; Zhou, Q.; Yuan, Z.G.; Xiong, X.S.; Fang, Q.F.; Gong, W.B.; Lu, J.J.; Tang, L.B.; et al. Microstructures and Mechanical Properties of MoNx Isomeric Multilayer films with Different Number of Layers Deposited by DC Magnetron Sputtering. Materialia 2023, 28, 101714. [Google Scholar] [CrossRef]
- Krysina, O.V.; Ivanov, Y.F.; Koval, N.N.; Prokopenko, N.A.; Shugurov, V.V.; Petrikova, E.A.; Tolkachev, O.S. Composition, Structure and Properties of Mo-N Coatings Formed by the Method of Vacuum-Arc Plasma-Assisted Deposition. Surf. Coat. Technol. 2021, 416, 127153. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Y.; Wu, X.; Li, X.; Luo, J. The Preparation and Superconducting Properties of Mo2N Nano Films by High Temperature Nitriding Method. Mater. Lett. 2021, 287, 129292. [Google Scholar] [CrossRef]
- Liu, C.; Ju, H.; Yu, L.; Xu, J.; Geng, Y.; He, W.; Jiao, J. Tribological Properties of Mo2N Films at Elevated Temperature. Coatings 2019, 9, 734. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, G.J.; Liu, Z.N.; Jiang, B.L. Oxidation Behavior of Magnetron Sputtered Mo2N /AlN Multilayer Coatings During Heat Treatment. Ceram. Int. 2015, 41, 7028–7035. [Google Scholar] [CrossRef]
- Bouaouina, B.; Besnard, A.; Abaidia, S.E.; Haid, F. Residual Stress, Mechanical and Microstructure Properties of Multilayer Mo2N/CrN Coating Produced by RF Magnetron Discharge. Appl. Surf. Sci. 2017, 395, 117–121. [Google Scholar] [CrossRef]
- Cheng, W.J.; Wang, J.J.; Ma, X.; Liu, P.; Liaw, P.K.; Li, W. A Review on Microstructures and Mechanical Properties of Protective Nano-Multilayered Films or Coatings. J. Mater. Res. Technol. 2023, 27, 2413–2442. [Google Scholar] [CrossRef]
- Yuan, Z.G.; Li, Y.C.; Sun, L.; Yang, J.F.; Mei, S.Q.; Xiong, X.S.; Fang, Q.F.; Chen, Z.; Liu, Y. Microstructures and Tribological Properties of MoS2/ Mo2N Multilayer Gradient Films Deposited by Reactive Magnetron Sputtering. J. Mater. Res. Technol. 2024, 31, 1507–1517. [Google Scholar] [CrossRef]
- Gulbiński, W.; Suszko, T. Thin Films of Mo2N/Ag Nanocomposite—The Structure, Mechanical and Tribological Properties. Surf. Coat. Technol. 2006, 201, 1469–1476. [Google Scholar] [CrossRef]
- Ju, H.B.; Zhou, R.; Luan, J.; Yu, L.H.; Xu, J.H.; Zuo, B.; Yang, J.F.; Geng, Y.X.; Zhao, L.J.; Fernandes, F. Multilayer Mo2N-Ag/SiNx Films for Demanding Applications: Morphology, Structure and Temperature-Cycling Tribological Properties. Mater. Des. 2022, 223, 111128. [Google Scholar] [CrossRef]
- Ju, H.B.; Zhou, R.; Liu, S.J.; Yu, L.H.; Xu, J.H.; Geng, Y.X. Enhancement of the Tribological Behavior of Self-Lubricating Nanocomposite Mo2N/Cu Films by Adding the Amorphous SiNx. Surf. Coat. Technol. 2021, 423, 127565. [Google Scholar] [CrossRef]
- Lin, Z.X.; Liu, Y.C.; Huang, C.R.; Guillon, M.; Wu, F.B. Input Power Effect on Microstructure and Mechanical Properties of MoSiN Multilayer Coatings. Surf. Coat. Technol. 2020, 383, 125222. [Google Scholar] [CrossRef]
- Liang, B.H.; Hsieu, F.S.; Wu, F.B. Modulation Effect on Mechanical Properties of Nanolayered MoN/MoSiN Coatings. Surf. Coat. Technol. 2022, 436, 128278. [Google Scholar] [CrossRef]
- Leonov, A.A.; Denisova, Y.A.; Denisov, V.V.; Syrtanov, M.S.; Shmakov, A.N.; Savostikov, V.M.; Teresov, A.D. Structure and Properties of CrN/TiN Multi-Layer Coatings Obtained by Vacuum-Arc Plasma-Assisted Deposition Method. Coatings 2023, 13, 351. [Google Scholar] [CrossRef]
- Gao, Z.C.; Buchinger, J.; Koutná, N.; Wojcik, T.; Hahn, R.; Mayrhofer, P.H. Ab Initio Supported Development of TiN/MoN Superlattice Thin Films with Improved Hardness and Toughness. Acta Mater. 2022, 231, 117871. [Google Scholar] [CrossRef]
- Hahn, R.; Koutná, N.; Wójcik, T.; Davydok, A.; Kolozsvári, S.; Krywka, C.; Holec, D.; Bartosik, M.; Mayrhofer, P.H. Mechanistic Study of Superlattice-Enabled High Toughness and Hardness in MoN/TaN Coatings. Commun. Mater. 2020, 1, 62. [Google Scholar] [CrossRef]
- Schlögl, M.; Mayer, B.; Paulitsch, J.; Mayrhofer, P.H. Influence of CrN and AlN Layer Thicknesses on Structure and Mechanical Properties of CrN/AlN Superlattices. Thin Solid Films 2013, 545, 375–379. [Google Scholar] [CrossRef]
- Tian, C.X.; Xiang, Y.X.; Zou, C.W.; Yu, Y.J.; Abudouwufu, T.; Yang, B.; Fu, D.J. Mechanical and Tribological Properties of CrWN/MoN Nano-Multilayer Coatings Deposited by Cathodic Arc Ion Plating. Coatings 2024, 14, 367. [Google Scholar] [CrossRef]
- Wu, Z.T.; Ye, R.L.; Bakhit, B.; Petrov, I.; Hultman, L.; Greczynski, G. Improving Oxidation and Wear Resistance of TiB2 Films by Nano-Multilayering with Cr. Surf. Coat. Technol. 2022, 436, 128337. [Google Scholar] [CrossRef]
- Ren, S.M.; Cui, M.J.; Pu, J.B.; Xue, Q.J.; Wang, L.P. Multilayer Regulation of Atomic Boron Nitride Films to Improve Oxidation and Corrosion Resistance of Cu. ACS Appl. Mater. Interfaces 2022, 9, 27152–27165. [Google Scholar] [CrossRef]
- Li, Z.; Ma, G.Z.; Xing, Z.G.; Yong, Q.S.; Zhao, H.C.; Huang, Y.F.; Guo, W.L.; Zhang, Z.N.; Wang, H.D. The Effects of Cr and B Doping on the Mechanical Properties and Tribological Behavior of Multi-Layered Hydrogenated Diamond-Like Carbon Films. Surf. Coat. Technol. 2022, 431, 127977. [Google Scholar] [CrossRef]
- Martinez, E.; Sanjindopinges, R.; Karimi, A.; Esteve, J.; Lévy, F. Mechanical Properties of Nanocomposite and Multilayered Cr-Si-N Sputtered Thin Films. Surf. Coat. Technol. 2004, 180, 570–574. [Google Scholar] [CrossRef]
- Zhu, L.; Chen, P.; Cai, Z.M.; Feng, P.Z.; Kang, X.Q.; Akhtar, F.; Wang, X. Fabrication of MoSi2 Coatings on Molybdenum and Its High-Temperature Anti-Oxidation Properties. Trans. Nonferrous Met. Soc. China 2022, 32, 935–946. [Google Scholar] [CrossRef]
- Ren, X.R.; Lv, J.S.; Li, W.; Hu, Y.W.; Sun, K.; Ma, C.; Chu, H.A.; Wang, W.G.; Xu, L.H.; Li, Z.Y.; et al. Influence of MoSi2 on Oxidation Protective Ability of TaB2-SiC Coating in Oxygen-Containing Environments within a Broad Temperature Range. J. Adv. Ceram. 2020, 9, 703–715. [Google Scholar] [CrossRef]
- Liu, Y.Q.; Shao, G.; Tsakiropoulos, P. On the Oxidation Behaviour of MoSi2. Intermetallics 2001, 9, 125–136. [Google Scholar] [CrossRef]
- Isik, M.; Gasanly, N.M.; Rustamov, F.A. Determination of mechanical properties of Bi12TiO20 crystals by nanoindentation. Mater. Sci. Semicond. Process. 2022, 140, 106389. [Google Scholar] [CrossRef]
- McDonald, I.G.; Moehlenkamp, W.M.; Arola, D.; Wang, J. Residual Stresses in Cu/Ni Multilayer Thin Films Measured Using the Sin2ψ Method. Exp. Mech. 2019, 59, 111–120. [Google Scholar] [CrossRef]
- Cullity, B.D.; Stock, S.R. Elements of X-Ray Diffraction, 3rd ed.; Prentice-Hall: Upper Saddle River, NJ, USA, 2001; ISBN 0-201-61091-4. [Google Scholar]
- Bokuniaeva, A.O.; Vorokh, A.S. Estimation of Particle Size Using the Debye Equation and the Scherrer Formula for Polyphasic TiO2 Powder. J. Phys. Conf. Ser. 2019, 1410, 012057. [Google Scholar] [CrossRef]
- Anu, A.; Khadar, M.A. Charge transport in grain size tuned CuO nanocrystal films. J. Electroceram. 2020, 45, 15–21. [Google Scholar] [CrossRef]
- Mohamed, L.; Noureddine, M.; Mokhtar, B.; Al-Douri, J.; Djillali, B.; Lakhdar, B.; Abd El-Rehim, A.F.; Jadan, M. Dislocations and crystallite size distribution of cadmium oxide thin films synthesized by spray pyrolysis technique. Mater. Sci. Eng. B 2022, 286, 116055. [Google Scholar] [CrossRef]
- Caicedo, J.C.; Amaya, C.; Yate, L.; Gómez, M.E.; Zambrano, G.; Alvarado-Rivera, J.; Muñoz-Saldaña, J.; Prieto, P. TiCN/TiNbCN multilayer coatings with enhanced mechanical properties. Appl. Surf. Sci. 2010, 20, 5898–5904. [Google Scholar] [CrossRef]
- Gao, S.W.; Hu, Y.F. Simultaneously Higher Thermal Stability and Lower Resistance Drifting for Sb/In48.9Sb15.5Te35.6 Nanocomposite Multilayer films. CrystEngComm 2022, 24, 1638–1644. [Google Scholar] [CrossRef]
- Solak, N.; Ustel, F.; Urgen, M.; Aydin, S.; Cakir, A.F. Oxidation Behavior of Molybdenum Nitride Coatings. Surf. Coat. Technol. 2003, 174, 713–719. [Google Scholar] [CrossRef]
- Ellery, A.J.; Baker, R.E.; Simpson, M.J. Communication: Distinguishing between short-time non-Fickian diffusion and long-time Fickian diffusion for a random walk on a crowded lattice. J. Chem. Phys. 2016, 144, 171104. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, H.Q.; Lei, H.; Li, H.Q.; Gong, J.; Sun, C. Influence of Different Coating Structures on the Oxidation Resistance of MoSi2 Coatings. Ceram. Int. 2020, 46, 5993–5997. [Google Scholar] [CrossRef]
- He, Y.X.; Wang, X.B.; Guo, T.; Gao, K.W.; Pang, X.L. Effect of Interface on Oxidation Behavior and Tribological Properties of CrAlN/SiNx multi-layer films. Ceram. Int. 2023, 49, 1369–1380. [Google Scholar] [CrossRef]
- Knittel, S.; Mathieu, S.; Vilasi, M. Oxidation Behaviour of Arc-Melted and Uniaxial Hot Pressed MoSi2 at 500 °C. Intermetallics 2010, 18, 2267–2274. [Google Scholar] [CrossRef]
- Fu, T.; Shen, F.Q.; Zhang, Y.Y.; Yu, L.H.; Cui, K.H.; Wang, J.; Zhang, X. Oxidation Protection of High-Temperature Coatings on the Surface of Mo-Based Alloys—A Review. Coatings 2022, 12, 141. [Google Scholar] [CrossRef]
- Abdollahi, A.; Ehsani, N.; Valefi, Z.; Khalifesoltani, A. SiC Nanoparticles Toughened-SiC/MoSi2-SiC Multilayer Functionally Graded Oxidation Protective Coating for Carbon Materials at High Temperatures. J. Mater. Eng. Perform. 2017, 26, 2878–2889. [Google Scholar] [CrossRef]
- Beltrami, M.; Mavrič, A.; Dal Zilio, S.; Kapun, G. A comparative study of nanolaminate CrN/Mo2N and CrN/W2N as hard and corrosion resistant coatings. Surf. Coat. Technol. 2023, 455, 129209. [Google Scholar] [CrossRef]
- Yuan, Z.G.; Mao, J.H.; Li, Y.C.; Sun, L.; Yang, J.F.; Fang, Q.G.; Gong, W.B.; Xiong, X.S.; Su, G.B. The Oxidation Resistance and Mechanical Properties of MoNx/AlN Multilayer Gradient Films Prepared by Magnetron Sputtering. J. Alloys Compd. 2024, 976, 172989. [Google Scholar] [CrossRef]
- Liu, H.H.; Yan, J.X.; Pei, Z.L.; Gong, J.; Sun, C. Effects of Mo Content on the Grain Size, Hardness and Anti-Wear Performance of Electrodeposited Nanocrystalline and Amorphous Ni-Mo Alloys. Surf. Coat. Technol. 2020, 404, 126476. [Google Scholar] [CrossRef]
- Hansen, N. Hall–Petch Relation and Boundary Strengthening. Scr. Mater. 2004, 51, 801–806. [Google Scholar] [CrossRef]
- Kariper, İ.A. Hardness of Mn2V2O7 Thin Films and Its Influential Factors. Int. J. Miner. Metall. Mater. 2015, 22, 987–991. [Google Scholar] [CrossRef]
- Leyland, A.; Matthews, A. On the Significance of the H/E Ratio in Wear Control: A Nanocomposite Coating Approach to Optimised Tribological Behaviour. Wear 2000, 246, 1–11. [Google Scholar] [CrossRef]
- Beake, B.D. The Influence of the H/E Ratio on Wear Resistance of Coating Systems–Insights from Small-Scale Testing. Surf. Coat. Technol. 2022, 442, 128272. [Google Scholar] [CrossRef]
- Shugurov, A.; Panin, A.; Dmitriev, A.; Nikonov, A. The Effect of Crystallographic Grain Orientation of Polycrystalline Ti on Ploughing Under Scratch Testing. Wear 2018, 408, 214–221. [Google Scholar] [CrossRef]
- Shtanskii, D.V.; Kulinich, S.A.; Levashov, E.A.; Moore, J.J. Structure and Physical-Mechanical Properties of Nanostructured Thin Films. Phys. Solid State 2003, 45, 1177–1184. [Google Scholar] [CrossRef]
- Pshyk, A.V.; Coy, L.E.; Nowaczyk, G.; Kempiński, M.; Peplińska, B.; Pogrebnjak, A.D.; Beresnev, V.M.; Jurga, S. High Temperature Behavior of Functional TiAlBSiN Nanocomposite Coatings. Surf. Coat. Technol. 2016, 305, 49–61. [Google Scholar] [CrossRef]
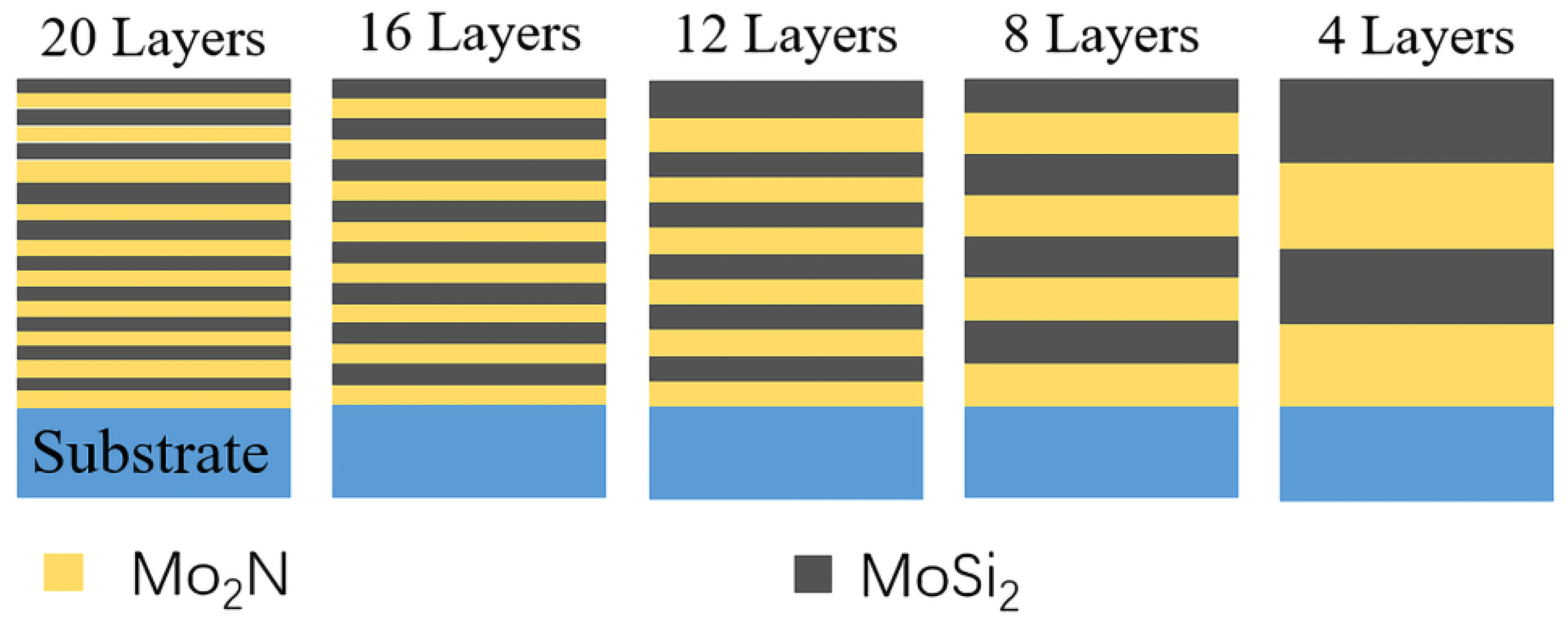




| Deposition Parameters | Mo2N | MoSi2 | |
|---|---|---|---|
| Mo/Si Sputter Power (W) | 80 | 80/100 | |
| N2/Ar Flow Rate (sccm) | 10/20 | 0/25 | |
| Deposition Temperature (°C) | 450 | ||
| Working Pressure (Pa) | 0.78 | ||
| Deposition Time per Layer (s) | pure Mo2N | 7200 | - |
| pure MoSi2 | - | 7200 | |
| 4-layer | 1800 | 1800 | |
| 8-layer | 900 | 900 | |
| 12-layer | 600 | 600 | |
| 16-layer | 450 | 450 | |
| 20-layer | 360 | 360 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, Z.; Yan, G.; Ma, R.; Sun, L.; Yang, J.; Mei, S.; Fang, Q.; Chen, Z.; Liu, Y. Oxidation Resistance and Mechanical Properties of Mo2N/MoSi2 Multi-Layer Films Prepared by Reactive Magnetron Sputtering. Coatings 2025, 15, 457. https://doi.org/10.3390/coatings15040457
Yuan Z, Yan G, Ma R, Sun L, Yang J, Mei S, Fang Q, Chen Z, Liu Y. Oxidation Resistance and Mechanical Properties of Mo2N/MoSi2 Multi-Layer Films Prepared by Reactive Magnetron Sputtering. Coatings. 2025; 15(4):457. https://doi.org/10.3390/coatings15040457
Chicago/Turabian StyleYuan, Zhigang, Guanyu Yan, Ru Ma, Li Sun, Junfeng Yang, Shunqi Mei, Qianfeng Fang, Zhen Chen, and Ying Liu. 2025. "Oxidation Resistance and Mechanical Properties of Mo2N/MoSi2 Multi-Layer Films Prepared by Reactive Magnetron Sputtering" Coatings 15, no. 4: 457. https://doi.org/10.3390/coatings15040457
APA StyleYuan, Z., Yan, G., Ma, R., Sun, L., Yang, J., Mei, S., Fang, Q., Chen, Z., & Liu, Y. (2025). Oxidation Resistance and Mechanical Properties of Mo2N/MoSi2 Multi-Layer Films Prepared by Reactive Magnetron Sputtering. Coatings, 15(4), 457. https://doi.org/10.3390/coatings15040457







