Abstract
This study concerns the effects of the addition of crystalline nanocellulose (CNC) and ionizing radiation on the properties of cornstarch–poly(vinyl alcohol) (PVA) films. Moreover, ESR spectroscopy and gas chromatography were used for a comparison of the reactivity of CNC and two micro-sized celluloses (microfibrinal (MFC) and microcrystalline (MCC)) under the influence of irradiation. This showed that the highest reactivity of CNC was related to the lowest sizes of the particles (observed by SEM). A series of starch/PVA/CNC films characterized by a starch/PVA ratio equal to 40:60 and a CNC addition in a range from 0.5 wt% to 10.0 wt% with 30 wt% of glycerol were prepared by solution casting. The films were irradiated in a gamma chamber (in a vacuum) or in an e-beam (in the air) using a dose of 25 kGy. The mechanical properties, contact angle to water, swelling and solubility in water, moisture absorption in a humid atmosphere, and the gel content of the films were determined. The functional properties of the films strongly depended on the addition of CNC. The films formed with 1.0 wt% of CNC had the best mechanical properties and the lowest surface and bulk hydrophilicity, which could be improved further after irradiation. The results can be related to the increased homogeneity and modified distribution of the nanoparticles in the films after irradiation (as shown by SEM). Degradation is a predominant process that occurs due to irradiation; however, the crosslinking processes also have some role. The protective effect of CNC against degradation was discovered by diffuse reflectance spectroscopy.
1. Introduction
The interest in the substitution of traditional packaging for biodegradable plastics makes it necessary to search for new materials based on natural and biodegradable synthetic polymers [1,2,3,4]. The preparation of films in mixed systems composed of a variety of natural polymers (polysaccharides or proteins), as well as those containing polysaccharides and biodegradable synthetic polymers, seems to be one of the methods with which to obtain better and more environmentally friendly packaging materials. Additionally, most of these materials can be used for the packaging of food as well as for edible coatings for food and pharmaceuticals. Recently, Amini et al. [5] presented the application of a nanocomposite material based on carboxymethyl-cellulose–PVA and nanoclay for packing wheat flour, achieving very good results for its antifungal activity.
Starch is an abundant and cheap biopolymer with a good film-forming ability and is an appropriate material for the preparation of biodegradable and edible packaging. However, a serious disadvantage of this material is its high affinity to water and, consequently, the dependence of the mechanical properties of a material on the content of this plasticizer (related to the moisture level in the environment). Moreover, although starch films have shown good mechanical strength, films prepared with the use of native starch alone are brittle. The use of modified starches and the blending of starch with other polymers are considered interesting routes for improving the functional properties of starch-based plastics. PVA can be used for packaging purposes and forms films with excellent mechanical properties. It is also known to be an appropriate polymer for blending with starch [4,6,7,8,9,10,11,12,13,14]. However, our previous results showed that films prepared with the use of PVA alone are even more hydrophilic compared to starch films and those prepared in a mixed starch–PVA system [6].
A variety of commercial biodegradable plastics are already available on the market, especially as packaging materials for food (like Mater-bi by Novamont and BASF and PLA Nativia by Taghleef Industry). Films and foams based on starch or PVA are the prospective materials [1,2,3,4], especially because the appropriate blending of starch with PVA makes it possible to obtain products with improved functional properties compared to materials based on each component alone [4,6]. The data on global migration [15,16] have shown that packaging based on a starch–PVA system can be used for food with a high-fat content or covered with a fatty layer, except for dry food.
Another solution to improve the properties of starch- or PVA-based plastics is to introduce a reinforcing agent, like inorganic particles [17,18] or natural fibers. It is known that the presence of cellulose fibers in synthetic and natural polymers also appears useful in the case of both starch and PVA [19,20,21].
Nanocelluloses (NCs) [22,23,24] are described as crystals (CNC) or fibers (CNF) characterized by at least one dimension in a nanoscale. Besides bacterial secretions, NCs are obtained from cellulose raw materials through the destruction of the less resistant amorphous blocks by mechanical (CNF) or chemical (CNC) treatment. Due to their unique supramolecular structure, nanocelluloses can be used to synthesize advanced composite materials with well-controlled structures from the molecular to the micrometric level. Thus, materials made with the addition of nano-sized celluloses might have better properties compared to those prepared with the use of micro-sized celluloses. Crystalline nanocellulose (CNC) forms crystallites in a three-dimensional nanoscale. Because of its crystalline structure, excellent mechanical properties can be expected, while highly developed specific surface areas and linkages exposed to chemical or physical attack might determine its unique reactivity.
Currently, intensive research is being conducted on the preparation of nanocomposites based on natural and biodegradable polymers, including those based on starch and/or PVA. There have been reports of the obtainment of some improved materials thanks to the addition of NCs [16,19,20,21,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39], but new studies are still needed.
Ionizing radiation (gamma or electron) induces chemical and physical changes in polymers. The advantage of using this radiation modification is that no initializing or lethal agents are necessary to conduct the process. Therefore, there is no need for an intensive purification of the resulting product, and the structure of the obtained product may differ from a product obtained chemically because chemical reagents do not participate in the reactions at any time. Moreover, it is easy to control processes like degradation, crosslinking, or grafting by changing the conditions of irradiation. Accordingly, radiation modification appears to be an alternative prospective method that might substitute chemical and enzymatic procedures, applied until now on an industrial scale for the modification of polymers.
The development of methods for radiation decontamination and sterilization has been observed during the last decades. This also concerns the decontamination of food. There have also been some attempts to modify the functional properties of food using radiation techniques. Accordingly, the need has emerged for the search for appropriate packaging materials that are resistant to irradiation and, thus, can be used for products that require radiation treatment [40,41]. An additional benefit may be obtained after irradiation of a packaging material with better properties as compared to the initial one. Among the other types of action, irradiation of the “ready” films seems to have, at present, high practical impact, both in relation to polymer modification and because the sterile packaging material is obtained. In particular, the irradiation of “ready” food in packages might enable the preparation of more stable, safe, and durable products.
Therefore, research on the influence of radiation on potential packaging materials appears to be an interesting line of inquiry. Only a few studies have been presented so far on the impact of ionizing radiation on starch/PVA films [6,7,12,13,14,40,41], and it is difficult to find information dealing with the influence of ionizing radiation on the starch–PVA–CNC system, although data concerning partial systems have been reported [21,28] and some studies dealing with the effect of irradiation on films made from other polysaccharides reinforced with NC have also been performed [42].
Our previous results have shown that the use of irradiated starch enables films with better functional properties compared to those prepared with native starch to be obtained [43]. We have also studied the effects of irradiation on the starch/PVA films and found that such films can be used for food packaging [7,40,41]. Additionally, we obtained promising results showing an improvement in starch/PVA films due to the addition of NCs supported by radiation treatment [27].
Accordingly, our present studies focused on the elaboration of a method for the preparation of new biodegradable films based on starch and PVA with the addition of crystalline nanocellulose (CNC) and the examination of the effect of ionizing radiation on the properties of the obtained materials. Our efforts concerned the determination of the effect of the modified content of CNC being introduced to the starch/PVA composition. Additionally, in the first stage of the research, the reactivity of CNC to irradiation was compared to the reactivity of two micro-sized celluloses (microfibrillated and microcrystalline).
2. Experimental
2.1. Materials
Cornstarch (Sigma product S412), poly(vinyl alcohol) PVA (Merck, Ettlingen, Germany) with a mean molecular mass of 145,000 kDa (fully hydrolyzed) were employed. The starch was degraded before use by the way of irradiation in a gamma chamber with an absorbed dose of 10 kGy and a dose rate of 5.0 kGy/h (Cieśla et al. [43]) (see below). Hydrophilic crystalline nanocellulose CNC (powder) was sourced from the Process Development Center, University of Maine. It was obtained by hydrolysis in sulfuric acid and contained some sulfate groups (SO42−). According to the producer’s information, the dimensions of the CNC nanowhiskers were as follows: diameter 5–20 nm and length 150–200 nm. Moreover, two micro-sized celluloses (both cotton linters from Sigma-Aldrich, Saint Louis, MO, USA products No. 43526 and No. C6288) were examined and were described by the producer as microcrystalline cellulose (MCC) and microfibrillated cellulose (MFC), respectively. Accordingly, we also maintained the MCC and MFC designations for these preparations, respectively. Analytical grade glycerol (Chempur, Piekary Śląskie, Poland) and deionized water were used for the film preparation.
2.2. Films Preparation
A series of the starch/PVA/CNC films were prepared by solution casting according to the modified procedure of Abramowska et al. [6]. The starch/PVA ratio in all the films was equal to 40:60 (in terms of the weight ratio of starch/PVA). CNC was introduced to the film’s composition at the levels of 0.5 wt%, 1.0 wt%, 2.5 wt%, 5.0 wt%, 7.5 wt%, and 10.0 wt% (in terms of the total polymer mass comprising starch, PVA, and CNC). Glycerol was introduced as a plasticizer at the level of 30 wt% in terms of the total polymer mass.
A homogeneous solution of PVA (1.64 wt%) was prepared by the way of heating in water at a temperature of 90 °C with vigorous stirring over 4 h. Starch was suspended in glycerol/water solutions (1.71% of starch in a glycerol/water mixture), and then homogenized at a temperature of 90 °C over 40 min. Starch, pre-irradiated with a dose of 10 kGy, was found to be an appropriate substrate, enabling a gel solution with the required concentration that possesses sufficiently low viscosity and good homogeneity for obtaining. Consequently, it was possible to obtain homogeneous films; however, using the native starch led to viscous gels under the same conditions and resulted in the preparation of non-homogeneous films. The gelatinized starch–glycerol dispersion was then added stepwise into the PVA solution with continuous mixing. The resulting mixtures were then continuously heated over 1 h at 90 °C. Simultaneously, a CNC gel solution was prepared by the way of homogenization in water at a temperature of 60 °C for 30 min (15 min with a magnetic stirrer and 15 min in an ultrasound bath). The CNC solution was then added stepwise to the starch/PVA gel solution, and the whole solution was homogenized for another 40 min. The solution was then poured into polystyrene Petri dishes, dried over 20 h in a heating chamber at a temperature of 50 °C, and then allowed to dry at room temperature.
Finally, the films were peeled from the substrate and conditioned prior to radiation treatment and measurements at ambient temperature (25 °C) with a relative humidity of 43%.
2.3. Irradiation
Irradiation of the films was conducted at ambient temperature with an electron beam in air or gamma rays (60Co) in a vacuum. A 25 kGy dose was applied in both cases. Irradiation with electrons was carried out in an Elektronika 10/10 accelerator, generating a 10 MeV electron beam at an average dose rate of approximately 3 kGy/min to the films packed in polyethylene bags. Gamma irradiation was performed in a GC-5000 gamma chamber, applying a dose rate of 5.1 kGy/h to the films closed in a glass vessel.
2.4. Examination of Radiation Effects on Cellulose Preparations
2.4.1. Scanning Electron Microscopy (SEM)
A DSM 942 Scanning Electron Microscope (Zeiss-Leo, Jena, Germany) was used for SEM studies of the cellulose preparations at a voltage of 5 kV and magnifications of 100×, 500×, and 1000×. Observations were conducted at room temperature for the samples being covered with a thin layer of gold.
2.4.2. Electron Spin Resonance (ESR)
ESR measurements in the X-band (frequency of 9.5 GHz) were performed on a Bruker ESP 300 spectrometer at ambient temperature. The microwave power was 1 mW, and the modulation amplitude was 1 G. Samples placed in an ESR tube under vacuum were irradiated in the gamma chamber with a dose of 5 kGy using a dose rate of 5.1 kGy/h prior to the ESR measurements. The Apollo program was used to analyze the spectra. The intensities of the ESR signals were recalculated to provide values per 1 mg of substance (I/mg).
2.4.3. Gas Chromatography (GC)
The process of cellulose radiolysis starts from the formation of a primary free radical by the removal of an electron from one of the carbon atoms and is followed by the abstraction of a hydrogen atom, leading to the disruption of the glycosidic bonds, as well as the subsequent abstraction of hydrogen from the glucopyranose rings. This causes fragmentation and oxidation, consisting of the replacement of some OH groups by C=O and COOH groups—this process being associated with the evolution of gaseous products (mainly H2, with a negligible participation of CO2, CO, CH4, and others). When the process is conducted in the presence of atmospheric oxygen, the latter is consumed by the oxidation reaction. Accordingly, the effectiveness of the ongoing radiation processes can be described in terms of the effectiveness of releasing or absorbing gaseous products to or from the surrounding atmosphere.
The yield of hydrogen evolution to the surrounding atmosphere and of oxygen absorption was determined for the samples subjected to irradiation in tightly closed vials (as described by Głuszewski et al. [44,45]. Irradiation was performed in the gamma chamber with a dose of 5 kGy. A Shimadzu GC-2014 gas chromatograph (Shimadzu, Kyoto, Japan, assembled and purchased by Shim-Pol A.Borzymowski, Izabelin, Poland), with a thermal conductivity detector and columns packed with 5 Å molecular sieves, was employed. The chromatographic system was working at 100 °C, the columns were kept at 40 °C, and the detector at 120 °C. The rate of flow of the carrier gas was 10 mL/min. The efficiencies of hydrogen evolution and oxygen consumption (GH2 and GO2) were expressed as µmol/J.
2.5. Characterization of the Films
2.5.1. Mechanical Properties
Mechanical tests were performed using a Type 5565 Instron testing machine applying a ramp velocity of 20 mm/min. Tensile strength (TS), elongation at break (Δl, %), and Young’s Modulus (YM) values were determined on the basis of eight measurements performed on pieces of material with the dimensions ca. 60 × 10 mm cut from separate films.
In the next sections, the terms “higher extensibility” or “lower extensibility” are applied in order to designate higher or lower Δl values. A low value of the YM showed high film elasticity.
2.5.2. Contact Angle to Water
Measurements of the water contact angle (wetting angle, υ), enabling the hydrophilic/hydrophobic properties of the film surface to be evaluated, were performed using an instrument constructed in the Laboratory of Materials Research, INCT. The procedure that was described in Abramowska et al. [6] was applied. A water drop (volume of 5 μL) was placed on the film’s surface, and the shape of the drop image was analyzed. The contact angle was determined for each individual object according to the following equation:
where h and r are the height and radius of the drop image.
The average values for each composition were calculated on the basis of 15 measurements performed on at least three pieces cut from separate films.
2.5.3. Swelling in Water
The procedure described by Abramowska et al. [6] was applied with modifications.
Square pieces of the films (with dimensions of 10 × 10 mm) were weighed and immersed in distilled water for 24 h at a temperature of 25 °C. The films were then drained with filter paper, re-weighed, and dried at a temperature of 110 °C for 24 h (until the sample reached a constant (“dry”) mass. The increase in mass after swelling was related to the initial mass or to the “dry” mass of the sample. Swelling parameters (A and B) were then determined as a weight percentage (wt%) by applying the following formulas:
A: Swelling (initial)
B: Swelling (dry)
where W0, Ws, and Wd are the weight of the initial sample, the weight of the swollen sample (a solid film with captured water), and the weight of the dried sample (determined after the swelling experiment), respectively.
The swelling parameters were determined based on three independent measurements.
2.5.4. Solubility in Water
The procedure described by Cieśla and Abramowska [41] was applied. The solubility was determined as the mass of the fraction that was dissolved in water during the immersion of the film for 24 h at 25 °C (weight percentage). Accordingly, the total mass loss after the above swelling experiment (Section 2.5.3) was evaluated for each individual sample using the formula:
The average values were calculated for each particular composition based on three repetitions.
Simultaneously, the moisture content in the samples was determined. The samples (150 mg each) were dried at a temperature of 110 °C until a constant mass was achieved. The moisture content (weight percentage) was calculated according to the following formula:
where W0s and Wds are the weight of the initial sample and the weight of the sample after drying. The moisture content determinations were carried out with an accuracy of 0.35%. The following average values were calculated for the non-irradiated films and the films irradiated in the gamma chamber and in the e-beam, respectively, with a CNC level of: 0.0 wt% CNC—7.77 wt%, 8.35 wt%, and 9.18 wt%; 0.05 wt% CNC—8.47 wt%, 9.04 wt% and 8.39 wt%; 1.0 wt% CNC—7.97 wt%, 7.85 wt% and 8.93 wt%; CNC 2.5 wt%—8.28 wt%, 8.20 wt% and 8.59 wt%; 5 wt% of CNC: 7.89 wt%, 8.81 wt% and 8.76 wt%; 7.5 wt% CNC—8.60 wt%, 9.73 wt% and 8.12 wt%; and 10 wt% CNC—9.22 wt%, 9.57 wt%, 10.11 wt%).
The soluble fraction (weight percentage) was calculated as the difference between the average values of the total mass loss resulting after the swelling experiment and the moisture content in the sample:
Solubility = Total mass loss − Moisture content
Three repetitions were performed for each sample.
2.5.5. Moisture Uptake
The tests were carried out in conditions of 100% RH (relative humidity) at a temperature of 4 °C. Pieces of films were weighted (0.100 g each) and placed in a glass weighing vessel, which was placed into a desiccator over a container with distilled water, and the whole apparatus was placed in the refrigerator. The mass of the samples was measured after the required time from 1 to 28 days. The mass change related to the moisture uptake was determined according to the formula:
where Wi is the initial weight of the sample, and Wm is the weight of the moist sample.
2.5.6. Gel Fraction Analysis
To evaluate the polymer degradation/crosslinking, the gel content was determined after heating in water (gel fraction). The procedure described in [6] was applied. Portions of the samples (ca. 150 mg) were placed in water in pre-weighed tubes and heated at a temperature of 105 °C over 2 h with intermittent stirring. The liquid phase was then separated from the gel and transferred into the pre-weighed tubes. Both the gel fraction and the liquid phase were dried at a temperature of 110 °C until a constant mass was achieved and re-weighed. The mass of the dried soluble part (Ws) and the mass of the dried gel (Wg) were then calculated. The content of the gel fraction (weight percentage) was determined on the basis of three separate measurements according to the following formula:
In addition, RGF values representing the ratio of the gel fraction content found for the irradiated samples to that found for the appropriate non-irradiated reference sample were determined for better evaluation of the changes caused by irradiation.
2.5.7. Diffuse Reflectance Spectroscopy (DRS)
Diffuse reflectance spectroscopy measurements were conducted using a Jasco V-670 spectrophotometer (producer JASCO Corporation, Tokyo, Japan) equipped with a reflection device (Model ISN-723 with a 60 mm integrating sphere, the same producer JASCO Corporation, Tokyo, Japan). Prior to the measurements, the film was irradiated with gamma rays in air at ambient temperature with an absorbed dose of 25 kGy. Measurements were taken for each individual irradiated film against the corresponding reference non-irradiated film.
2.5.8. Scanning Electron Microscopy (SEM)
SEM observations were conducted for the films with the use of an Ultra Plus SEM microscope (Zeiss, Jena, Germany) at a 1 kV voltage. Magnifications of 5000×, 10,000×, 100,000× and 250,000× were applied.
3. Results and Discussion
3.1. Evaluation of the Properties of the Selected Celluloses
3.1.1. SEM Studies of Celluloses
Granules and fibrils were observed for both micro-sized celluloses at magnifications of 100× and 1000× (Figure 1). The fibrils constituted the predominant fraction of the particles in the MFC sample, but some granules were also visible. In contrast, in the case of the MCC sample, although the majority of the particles were in the form of granules, a small fraction of fibrils was also observed. This basically corresponded to the description given by the manufacturer. The granules in the MCC preparation had irregular shapes and were larger than the fibrils visible in the MFC preparation. Accordingly, a larger specific surface area could be expected for the MFC sample compared to the MCC preparation.

Figure 1.
SEM images of the cellulose preparations. Photos taken at 100× magnification (a–c): (a)—MFC, (b)—MCC, and (c)—CNC. Photos taken at 1000× magnification (d–f): (d)—MFC, (e)—MCC, and (f)—CNC. Distances of 200 µm (a–c) and 20 µm (d–f) are shown, respectively.
The CNC preparation was in the form of a sponge that readily crumbled. No granules or fibrils were observed at the magnifications used. However, the surface of the preparation was not smooth: at a magnification of 100×, irregularities in its structure became visible, indicating the presence of “holes”, and observations made at a higher magnification of 1000× indicated a much greater specific surface area compared to the relatively smooth particle surfaces seen in the MFC and MCC specimens.
3.1.2. Electron Spin Resonance (ESR)
Similarly to other polysaccharides (including those built from glucose units), different types of ESR signals can be detected for celluloses [46]. Such signals are known to depend on the position of the free radical (unpaired electron) in the molecule, considering that the unpaired electron can be located at different carbon atoms in the ring or on a side chain (Scheme 1). Multiple ESR signals result due to interactions of the unpaired electron with nearby carbon atoms, with it being able to interact simultaneously with several carbon (or hydrogen) atoms [46,47,48,49,50,51,52]. Accordingly, each radical can give rise to a spectrum that can be composed of one or more signal lines and can be described as a singlet, doublet, or triplet (isotropic or anisotropic) [47]. Taking into account that several free radicals can be present in the same sample, a very complex ESR pattern can be recorded. In fact, the computer simulations indicated that the experimental complex ESR spectra could be deconvoluted into singlets, doublets, and triplets [47,48], which can be attributed to specific free radicals formed on particular carbon atoms and, additionally, to splitting from several radicals [47]. Wencka et al. [49] found more than 20 lines in the ESR spectra in irradiated cellobiose and identified six different types of stable free radicals.
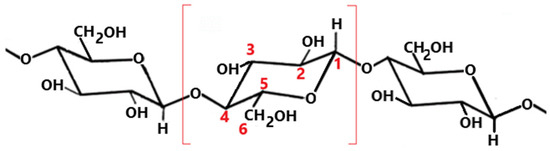
Scheme 1.
Molecular structure of cellulose The part in brackets highlighted in red represents the glycosidic unit. The numbering marked in red shows the positions of the individual carbon atoms in the glycosidic unit.
Most researchers have stated that free radical processes are initiated by the creation of C1 or C4 radicals (Scheme 1), starting from the disruption of the glycosidic linkage [51]. Sasai et al. [47] pointed out that the end chain radicals formed in glucose-based polymers in the process of cleavage of the 1,4-glycosidic bond are highly reactive and thus undergo rearrangement. This starts with the abstraction of a hydrogen atom from the glucose unit, giving a C1 radical, as well as C2 and/or C3 radicals [48]. Rintarak et al. [48] presented a simulation of free radical formation in the glucose units, postulating the formation of free radicals at the C1 position (resulting in a doublet spectrum) or at a C6 position (doublet or triplet). However, these authors also identified a singlet as an additional component in their spectra. Simultaneously, Kameya et al. [51] reported that the triplet signal originates from interactions of the two hydrogen atoms at C6 with an unpaired electron formed by the removal of the hydrogen atom at C5. This results in a radical located in the cellulose ring and might be followed by the breakage of the bond at C5.
In the present study, spectra composed of several lines were observed in the cases of the cellulose samples (Figure 2). The MFC and CNC spectra had a similar character and could be described as a triplet with a g factor equal to 2.003 (similar to the spectra shown in [48,51]). Therefore, the intensity of the spectral lines could be compared. The height of the signal detected for CNC (2.25/mg) indicated its high reactivity compared to MFC (1.16/mg).
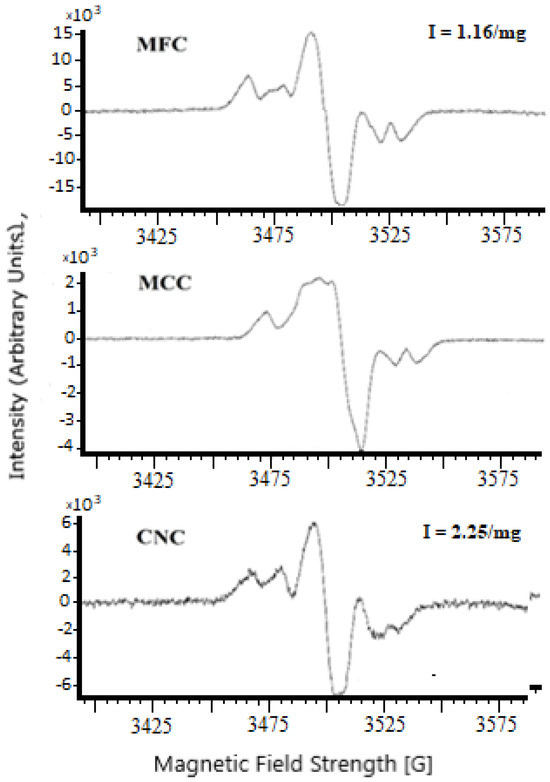
Figure 2.
ESR patterns of the cellulose samples.
The ESR pattern of the MCC sample differed from those of MFC and CNC, showing the formation of at least some of the free radicals at different positions compared to the latter two samples. Therefore, the intensity of the major signal could not be compared to the intensity of the signals found in the cases of MFC and CNC. However, the intensity at the maximum of the major signal could be evaluated as ca. 0.2/mg.
3.1.3. Gas Chromatography
Table 1 presents data on the hydrogen evolution and oxygen consumption during irradiation of the cellulose samples in air. Both the parameters GH2 and GO2 had higher values in the case of the radiolysis of MFC compared to the MCC sample. Simultaneously, both parameters were meaningfully higher in the case of CNC. The results show that MFC is more susceptible to radiation processes compared to MCC and that the reactivity of CNC is considerably higher compared to both micro-sized celluloses. This effect can be related to the differences in the specific surface area between the particular cellulose samples. In particular, the high reactivity of CNC is related to its large specific surface area (Section 3.1.1).

Table 1.
The efficiency of hydrogen evolution and oxygen consumption is determined by gas chromatography.
3.2. Evaluation of the Properties of the Starch:PVA/CNC Films
It was found that the addition of CNC to the films resulted in the modification of their functional properties and depended strongly on the nanocellulose content.
3.2.1. Overall Discussion of the Processes Taking Place in the Starch–PVA–CNC System
Scheme 1 and Scheme 2 show the molecular structure of cellulose, starch, and PVA. The most likely process that occurred due to the irradiation of both polysaccharides in the solid state was the cleavage of glycosidic bonds. In the case of PVA, chain degradation may also occur under irradiation in the process of disruption of the C-C linkages (Scheme 2b). However, at the same time, crosslinking processes may occur due to the formation of bridges between adjacent PVA chains via OH groups. Simultaneously, possibly partial crosslinking of the polysaccharide with PVA can take place. This process occurs via OH groups present in the molecules of both reagents.
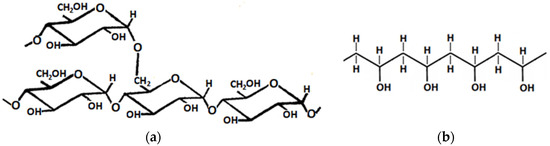
Scheme 2.
Molecular structures of (a) starch amylopectin (branched polymer) and (b) poly(vinyl alcohol) (PVA).
The schemes of the possible processes that can occur in starch–PVA systems [53] under irradiation (the degradation of starch and PVA, formation of crosslinks between two PVA molecules and between starch molecules and PVA molecules) were indicated in a previous paper [53].
In the present paper, the possibility of creating crosslinks between PVA macromolecules and cellulose macromolecules is indicated (Scheme 3). It also seems possible that due to the small dimensions of the CNC molecules, steric factors played a smaller role and were less likely to disturb the formation of crosslinks between starch macromolecules and CNC. It was also possible that the small CNC chains participated in the formation of crosslinks between starch and PVA.
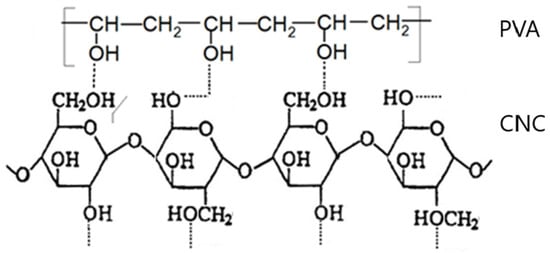
Scheme 3.
Possible formation of crosslinks between CNC and PVA.
3.2.2. Mechanical Properties of the Starch/PVA/CNC Films
The results (Figure 3) show that all the films exhibited a relatively high TS, varying in the case of the non-irradiated samples from 23.68 ± 0.82 MPa to 28.95 ± 0.95 MPa.
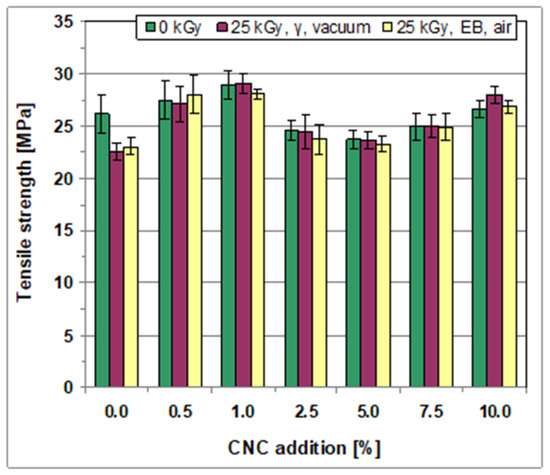
Figure 3.
Tensile strength of the films, non-irradiated and irradiated.
The films prepared based on the starch–PVA system (no addition of CNC) revealed a relatively high TS (Figure 3) (the TS was equal to 26.15 ± 1.79 MPa), and acceptable Δl (the Δl was equal to 112.8% ± 15.9%) (Table 2). The relatively high value of YM showed the rather low elasticity of these films (Table 2).

Table 2.
Elongation at break and Young’s Modulus of the of the starch/PVA/CNC films, non-irradiated and irradiated.
Adding CNC to the composition of the films caused some changes in tensile strength but especially strongly affected their elongation at break (Figure 3, Table 2). Moreover, the direction and degree of the modification of mechanical properties in relation to the films produced based on the pure starch–PVA system depends on the CNC level. Therefore, the TS of the films produced with a small amount of CNC added (0.5 wt% and 1.0 wt%) was higher than that of the films prepared without adding CNC. Simultaneously, an increased elongation at break was observed in these films, with the highest Δl value at 1.0 wt% (Table 2). Conversely, lower values of the TS were obtained at a higher level of CNC (2.5 and 5.0 wt%), but this parameter began increasing at 7.5 wt%, and at 10 wt% of CNC, it again exceeded the value obtained for pure starch/PVA films. Meanwhile, the extensibility of the films produced with added CNC equal to 5 wt%, 7.5 wt%, and 10 wt% decreased as compared to the films prepared based on the pure starch–PVA system or those obtained with less CNC added (Δl decrease from 112.8 ± 15.9 wt% to 68.2 ± 8.5 wt% at 10.0 wt% of CNC.
These results can be interpreted in relation to the crosslinked polymer networks, whose structures differ depending on the amount of the additive (compare Section 3.2.7 and Section 3.2.10). At the same time, inserting a larger amount of CNC may cause CNC aggregates to appear, the number and size of which increases as the amount of the CNC additive increases.
Irradiation, both with γ-rays and fast electrons, induced a decrease in TS in the case of the starch/PVA films (Figure 3). This was accompanied by a considerable decrease in extensibility (shown by lower Δl values; Table 2). This is due to the prevalence during irradiation of degradation processes over crosslinking processes.
Conversely, only small or negligible changes in TS were observed after irradiation of all the films with added CNC (Figure 3). Simultaneously, although irradiation reduced the elongation at break of most of these films, the relative changes in the Δl values were considerably lower as compared to the changes induced in the case of pure starch/PVA films. It is worth mentioning that in the case of the films prepared with additional CNC equal to 0.5, 1.0, and 2.5 wt%, the irradiated films were still characterized by a high tensile strength and acceptable extensibility.
Young’s Modulus (YM) values of the non-irradiated films prepared based on the starch/PVA and starch/PVA/CNC compositions with CNC of up to 5.0 wt% were similar, showing comparable elasticity (Table 2).
A small decrease in YM can be deduced after irradiation in the case of the films based on the pure starch–PVA system, suggesting their somewhat increased elasticity, while no particular changes in this parameter could be concluded after irradiation in the case of the films obtained with a small amount of CNC added.
Contrasting, higher values of Young’s Modulus were detected at the level of CNC of 7.5 and 10.0 wt% (the higher the CNC content, the higher the values of Young’s Modulus). Moreover, the irradiation of these films led to an additional increase in YM. This result showed greater stiffness of these films as compared to the previous cases, and additionally, the stiffness increased after irradiation (Table 2). This corresponds to the decreased Δl values detected for these films (Table 2).
It can be concluded that the films produced with added CNC at the levels of 0.5, 1.0, and 2.5 wt% revealed acceptable mechanical properties (a high tensile strength, appropriate extensibility, and elasticity) with sufficient resistance to irradiation. Also, the films with the best mechanical properties were manufactured with an added CNC of 1.0 wt%.
3.2.3. Contact Angle of the Films to Water
The films prepared based on the pure starch–PVA system were hydrophilic, as shown by a low contact angle (64.16° ± 5.59°); Figure 4). The contact angle values of most of the samples produced with added CNC were considerably higher as compared to those determined for the starch/PVA films (i.e., reaching 77.50° ± 4.84° after adding 5 wt% of CNC), showing an essentially decreased surface hydrophilicity. Irradiation led to a further decrease in surface hydrophilicity. In the cases of starch/PVA and the majority of starch/PVA/CNC compositions, the contact angle exceeded 85° in some cases, for example, in the case of the films produced with added CNC of 0.5 wt% or 2.5 wt%.
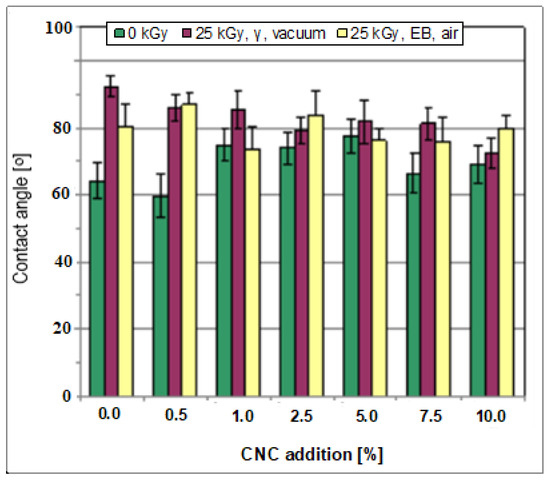
Figure 4.
The contact angle of the films, non-irradiated and irradiated.
The decrease in surface hydrophilicity connected with the addition of CNC can be related to the formation of a more crosslinked polymer network with the participation of CNC molecules. In turn, the effect of irradiation can be attributed to the formation of a more crosslinked polymer structure but, above all, to the formation of oxidation products (as described in Section 3.2.9). However, when it comes to the formation of oxidation products, it is noteworthy that an increase in the contact angle was observed both after irradiation performed in the air (in an e-beam) but also in a vacuum (in a gamma chamber). Therefore, it can be stated that the formation of the products with lower hydrophilicity as compared to the initial polymers was possibly only partially due to reactions with atmospheric oxygen. Thus, suppose that the essential transformations of the OH groups of all components (both polysaccharides and PVA) were due to free radical processes occurring directly in the polymer skeleton.
3.2.4. Swelling of the Films in Water
The addition of CNC to the initial non-irradiated films usually induced an increase in swelling capability (shown by both parameters A and B) (Figure 5 and Figure 6). The only exceptions were the films prepared with the addition of 1.0 wt% of CNC, where a considerable decrease in the swelling parameters was detected. The increase in swelling capacity strongly depended on the amount of CNC; the introduction of CNC at a level of 0.5 wt% already led to an increase in swelling capacity. It was followed by a decrease at 1.0 wt% and by a further increase at higher CNC levels. Thus, the highest swelling was noted in the case of the sample (non-irradiated) prepared with the addition of 2.5 wt% of CNC, while swelling parameter A was practically unaffected by a further increase in CNC levels from 2.5 wt% up to 10 wt%. On the contrary, swelling parameter B was lowered due to an increase in the amount of CNC to over 2.5 wt% but then remained at a similar value at CNC levels of 5.0, 7.5, and 10.0 wt%.
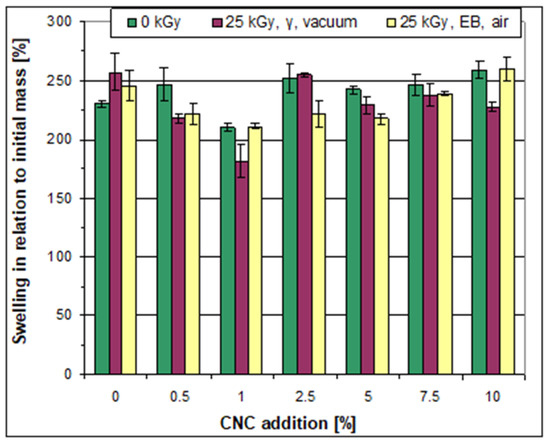
Figure 5.
Swelling of the films in water (the A parameter), non-irradiated and irradiated.
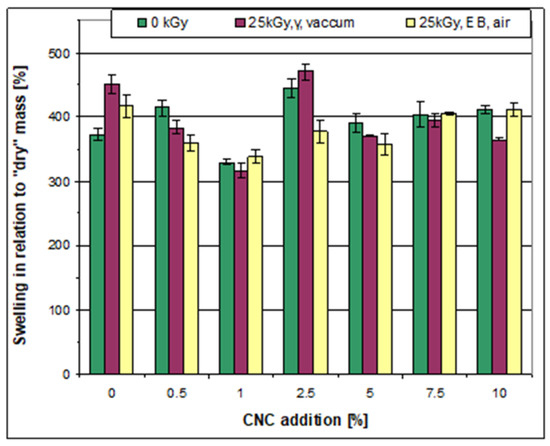
Figure 6.
Swelling of the films in water (the B parameter), non-irradiated and irradiated.
Irradiation of the films based on the pure starch–PVA system contributed to an increasing swelling capacity. On the contrary, for the majority of the compositions obtained with the addition of CNC, the swelling capacity decreased after irradiation. This was the case, for example, with the films obtained after adding 0.5% CNC or 5.0% CNC. The majority of the films irradiated in the accelerator were characterized by a lower swelling capacity compared to the films with the same composition irradiated in the gamma chamber.
Differences were also noted between the effect of irradiation on the A and B swelling parameters. For example, swelling parameter A (related to the initial mass of the sample) was much lower after irradiation with γ-rays in the case of the sample that contained 1.0 wt% of CNC compared to the non-irradiated sample and that irradiated in the e-beam. However, no particular difference was noted between the swelling parameter B (related to “dry” mass) determined for these films, although in some cases, the B parameter showed more evident differences between the samples irradiated under various conditions (Figure 5 and Figure 6).
It is worth mentioning that the B parameter of most of the irradiated samples prepared with the addition of CNC was lower compared to the non-irradiated pure starch/PVA films (thus, it was also lower than the irradiated pure starch/PVA films). This observation might be related to the creation of a denser polymer network with the contribution of CNC. It can thus be supposed that CNC forms crosslinks with PVA and with starch (via OH groups) and is additionally able to participate in the creation of crosslinks between starch and PVA macromolecules. It can also be deduced that irradiation supports such processes.
Accordingly, it can be concluded that the composition characterized by the lowest swelling capability was obtained with the addition of 1.0 wt% of CNC and that this capability additionally decreased after irradiation with γ-rays. Simultaneously, it can be concluded that the majority of the irradiated films based on the starch/PVA/CNC system revealed a lower ability for bonding water molecules compared to the non-irradiated (as well as the irradiated) films based on the pure starch–PVA system.
3.2.5. Solubility of the Films in Water
Some differences between the conclusions that can be derived on the basis of the analysis of each swelling parameter (A and B) suggest that possibly some other phenomenon contributes to the experimental results besides swelling. Therefore, dissolution of the samples during their immersion in water should also be considered.
Immersion of the starch/PVA films (no CNC) in water for 24 h resulted in the dissolution of 22.37 ± 1.22 wt% of the material. The effect of the addition of CNC on the solubility depended on the amount that was introduced to the composition. Additions at a level of 0.5 wt%, 5.0 wt%, or 7.5 wt% had a relatively small impact on this property (Figure 5). However, an addition of 1.0 wt% of CNC led to a decrease in solubility (to 19.87 ± 0.70%). Subsequently, an increase in solubility was observed at a CNC level of 2.5 wt%. This was decreased on further addition of the additive (5.0 and 7.5 wt%) and finally again reached a value lower than in the case of pure starch/PVA films at 10 wt% of CNC.
The increase in solubility due to the CNC addition was probably connected to the presence of “free” CNC (neither bound to starch nor PVA chains), which possibly resulted in some deterioration in the polymer microstructure. On the other hand, the creation of a crosslinked and ordered polymer network is expected to result in a decrease in solubility. It seems that there is no strict correlation between the CNC addition and the ability to create an ordered structure. Thus, 0.5 wt% of CNC seemed to be insufficient, while 1.0 wt% of CNC was appropriate, and 2.5 wt% appeared too high. Surprisingly, a decreased solubility was observed for the films obtained with a CNC addition of 10.0 wt%. It can thus be supposed that the above results occur because, at a high level of CNC, crosslinks between two CNC particles (CNC-CNC) became more probable. Such species are expected to be more resistant to an interaction with water compared to a single CNC chain. Moreover, they might subsequently participate in the creation of crosslinks with starch or PVA macromolecules (with the formation of products that are also less soluble compared to those obtained with single CNC chains). Additionally, considering the possibility of the formation of crosslinks between the adjacent chains of starch or PVA and the probable participation of CNC in the resulting network, it can be supposed that the increasing level of CNC (over 5.0 wt%) will lead to a more crosslinked structure. Accordingly, the dense structure created at the CNC level of 10.0 wt% (although accompanied by a degraded product) was characterized by a lower solubility in water.
Generally, irradiation of the films in the gamma chamber facilitated dissolution in water (Figure 7). It was difficult to draw a clear conclusion concerning the effect of irradiation in the e-beam on solubility. Depending on the films’ composition, irradiation in the e-beam caused an increase or decrease in solubility or did not meaningfully affect the property. Therefore, it can be supposed that the contribution of the crosslinking processes became considerable in such cases (see explanation in Section 3.2.7). The preference given to degradation under irradiation in a gamma chamber is connected to the fact that a prolonged time of exposure led to chain scission, regardless of whether the specimens contained separate starch, PVA, or CNC, or a crosslinked macromolecule composed from two or all of the polymers.
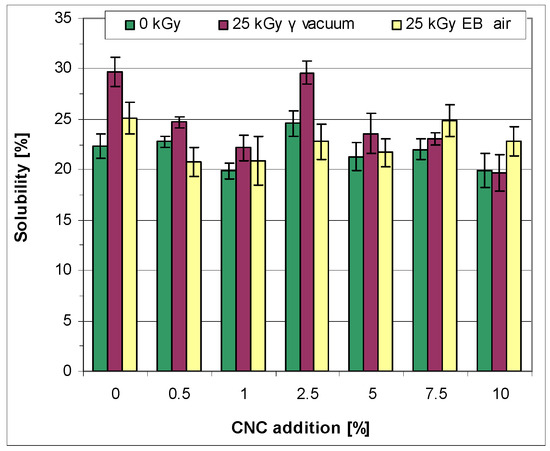
Figure 7.
Solubility of the non-irradiated and irradiated films in water.
3.2.6. Moisture Uptake
The example curves representing moisture uptake are shown in Figure 8. The films already reached a considerable level of moisture uptake after one day of exposure to water vapor, and the increase in moisture uptake then continued for the next few days. However, prolonged exposure caused a decrease in the sample mass. As a result, specific maxima were observed, while masses of the samples finally stabilized at the level that was reached after one or two days of exposure. For example, in the case of pure starch/PVA films, the maximum mass increase was reached after 4 days (at ca. 28 wt%), and the mass increase stabilized at a level of ca. 15 wt.
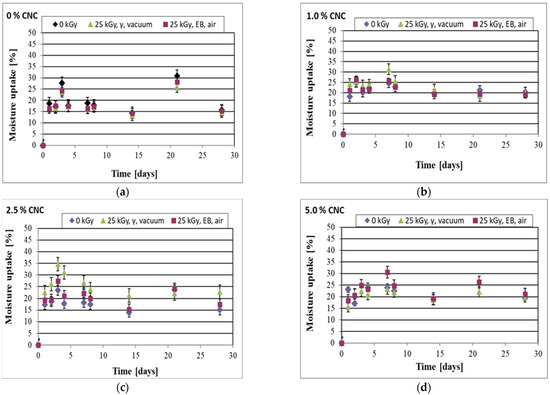
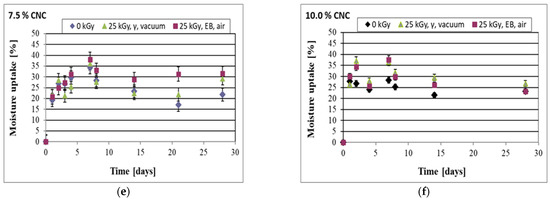
Figure 8.
Moisture uptake in the films was obtained with various additions of CNC, non-irradiated and irradiated. Foils prepared without CNC addition (a) and with CNC addition at the levels of 1 wt% (b), 2.5 wt% (c), 5.0 wt% (d), 7.5 wt% (e), and 10.0 wt% (f).
It is difficult to provide a clear explanation of such anomalous behavior. It can be supposed that after the absorption of a meaningful amount of water molecules, the structure loosened and was easily reorganized. However, the newly formed structure was able to include less water compared to the primary structure, so excess moisture was removed. On the other hand, it can also be assumed that in the samples with essential moisture content, chemical reactions occur, resulting in partial decomposition of the film components with the formation of gaseous products that can be easily lost from the material. Both these phenomena might have reduced the mass of the polymer in the later stages of the processes.
The samples prepared with the addition of CNC absorbed more water compared to the samples obtained based on the pure starch–PVA system. In particular, for the non-irradiated samples the following approximate values of the final mass increase were determined at particular CNC levels: at 1.0% CNC ca. 20 wt% increase; at 2.5 wt% CNC ca. 17 wt% increase; at 5 wt% CNC ca. 20 wt% increase; at 7.5 wt% CNC ca. 22 wt% increase; and at 10 wt% CNC ca. 23 wt% increase.
Irradiation led to an increase in moisture uptake (Figure 8), and it was noted that irradiation in the gamma chamber induced a higher increase in this property compared to irradiation in the e-beam. This result corresponded well to the changes in the swelling parameters, which were also found to be higher after irradiation was performed in the gamma chamber compared to those irradiated in the e-beam (Section 3.2.4). On the other hand, the increase in moisture uptake after irradiation remained somewhat in contradiction with the results of swelling, the latter showing that there was a decrease in swelling after irradiation (Section 3.2.4). This might be connected to the mode of calculation of the particular parameters and to the fact that the majority of irradiated samples showed somewhat higher moisture content compared to the non-irradiated samples stored under the same conditions (Section 2.5.4); thus the initial state of the sample before exposure to water vapor differed in the case of the non-irradiated and irradiated samples. Simultaneously, the stable moisture uptake was higher in the case of the films prepared with the addition of 10 wt% of CNC compared to the other samples, in line with the higher level of absorbed moisture content under the storage conditions (Section 2.5.4).
3.2.7. Gel Fraction
The results showed that the sample prepared with pure starch/PVA (no addition of CNC) contained a considerable gel fraction of 34.70 ± 1.10 wt% (Figure 8). The gel content strongly increased on the addition of CNC up to a level of 2.5 wt%, but it then stabilized, and the future addition of CNC over 2.5 wt% affected the amount present relatively slightly. Considering that gel formation occurred due to the presence of a crosslinked fraction of polymer, it could be concluded that the addition of CNC resulted in the formation of a more crosslinked polymer network compared to that created in the pure starch–PVA system.
Irradiation induced an essential decrease in gel content (Figure 9). The RGF parameters showing the remaining gel fraction were in a range from 79 wt% to 90 wt%. Also, the reduction in gel content was larger after irradiation was performed in the gamma chamber compared to irradiation in the e-beam. Additionally, the RGF parameter increased with the increase in the content of CNC. Therefore, the remaining content of gel after irradiation in the gamma chamber was as follows: 0.79 in the case of the samples obtained with or without the addition of 0.5, 1.0, and 2.5 wt% of CNC, but 0.80, 0.82, and 0.85 in the case of the samples prepared at CNC levels of 5.0, 7.5, and 10.0 wt%, respectively. Simultaneously, the following RGF values were obtained for particular samples after irradiation in the e-beam: 0.83 (for pure starch/PVA films), 0.82 (CNC level of 0.5 wt%), 0.84 (1.0 wt% of CNC), 0.83 (2.5 wt% CNC), and 0.92, 0.87, and 0.88 at CNC levels of 5.0, 7.5, and 10.0 wt%, respectively.
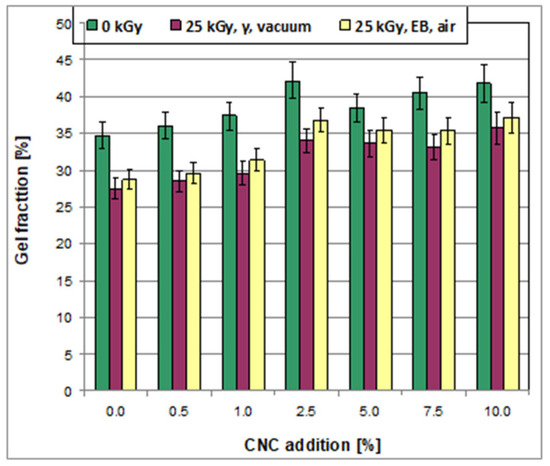
Figure 9.
Gel fraction in the non-irradiated and irradiated films.
The reduction in gel content proved that degradation was the prevailing process caused by irradiation. Moreover, the predominance of degradation processes over crosslinking processes was greater after irradiation in the gamma chamber in vacuum compared to irradiation in the e-beam in air, probably because irradiation in a gamma chamber with a dose of 25 kGy at the applied conditions needed a prolonged time of ca 5 h, while the same dose in the e-beam was obtained in less than 1 min. Accordingly, there was more time for free radical reactions during irradiation in the gamma chamber compared to the e-beam (independently of the post-radiation processes that took place in both cases after irradiation). It can be supposed that these effects occurred because prolonged irradiation in the gamma chamber led to chain scission, independently of whether the samples contained separate starch, PVA, or CNC or whether they were crosslinked macromolecules composed from two or all the polymers.
On the contrary, regarding the high dose rate in the e-beam, a large number of free radicals were formed during a short time, and these radicals were present in the sample simultaneously. Due to the high density of free radicals in the material, their reaction to give crosslinks was more probable compared to irradiation in a gamma chamber, where the density of the free radicals, which were formed within a few hours (and could be terminated), was low.
3.2.8. Scanning Electron Microscopy
SEM images were taken for the sample prepared with the addition of 2.5 wt% of nanocellulose, either non-irradiated or irradiated with the use of γ-rays (Figure 10).
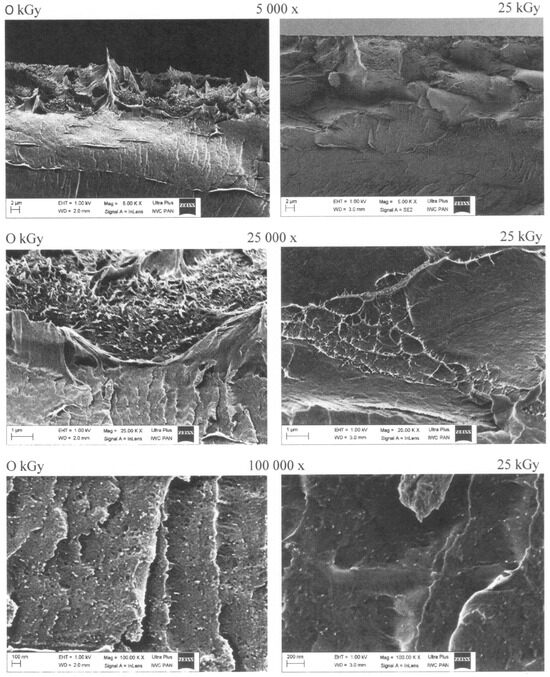
Figure 10.
SEM images of the films prepared with the addition of 2.5 wt% of CNC, non-irradiated and irradiated with γ-rays using a dose of 25 kGy.
SEM photos (Figure 10) of the non-irradiated sample showed numerous structural irregularities with clearly visible cracks on the fractures and significant areas with protruding precipitations (fibers) on the surface. Moreover, porosity was detected at higher magnifications (of 25,000× and 100,000×), where a large number of nanoparticles clearly separated from the polymer matrix were also observed. Similarly, separated nanoparticles were previously shown for PVA nanocomposite films prepared with a 5% addition of CNF/graphene oxide (16:1) nanocomposites [34].
Both the surfaces and fractures of the films became smoother after irradiation (Figure 10), as shown by the lack of porosity, less evident cracks, and diminished areas with irregularities on the surfaces. The nanoparticles were less visible after irradiation, showing a decrease in the size of agglomerates resulting from specific “melting” in the polymer matrix. Possibly, these changes were caused by better bonding of the CNC with chains of starch and PVA, which possibly thickened and sealed the polymer structure and consequently led to stronger films. Simultaneously, some “disappearance” of the nanoparticles was accompanied by the formation of “ribbons,” which possibly accumulated nanoparticles and created paths that additionally strengthened the polymer matrix.
3.2.9. UV–VIS Diffuse Reflectance Spectroscopy
Figure 11 presents the UV–VIS DRS patterns of the radiolysis products formed in the pure starch–PVA system and the sample prepared with the addition of 2.5 wt% of CNC.
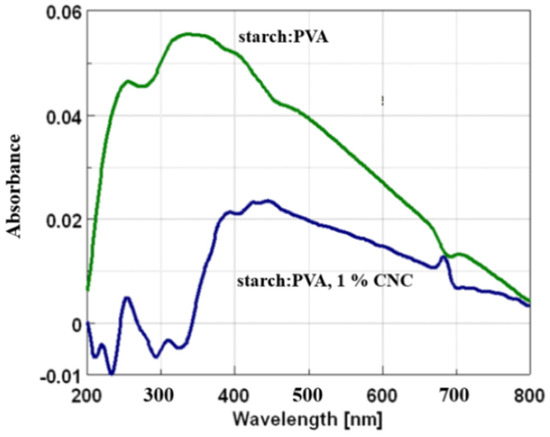
Figure 11.
UV–VIS DRS spectra were recorded for the starch/PVA (40:60) sample and the sample containing 1.0 wt% of CNC.
In the case of the pure starch/PVA sample, absorption was observed over a wide wavelength starting from 200 nm, with the highest intensity at ca. 340 nm (Figure 11). Several maxima showed the superposition of the bands originating from different chemical groups. However, the most interesting bands appeared in the region with maxima at ca. 340 nm (the highest) and ca. 255 nm, with the shoulder at ca. 400 nm. The result corresponds to that shown in Cieśla et al. [41] for a similar composition.
According to Wang et al. and Głuszewski et al. [44,54], the maximum at ca. 255 nm might be assigned to carbonyl and carboxyl groups. Głuszewski et al. [44] stated that this band could be due to vibrations of C=O groups placed in the middle of a polymer chain (ketone groups). Moreover, these authors [44] have suggested that the maximum at ca. 340 nm originates from the vibrations of the C=O (and COOH) groups located at the ends of the chain or near chain ends. However, the bands in the high wavelength region can also be attributed to the presence of C=C linkages [55] and C-O-C (ether) linkages (others not excluded). The energy of vibrations of individual groups depends additionally on their position in the polymer chain. Accordingly, their summarized contributions resulted in the appearance of a broad tailored band.
It seems worth mentioning that the radiolysis of the polysaccharides and PVA led to the substitution of some OH groups by C=O groups and, consequently, to the formation of carbonyl and carboxyl derivatives. According to von Sontag and Relieve et al. [56,57], the formation of C=O groups in polysaccharides occurs via the disruption of glycosidic linkages and ring-opening reactions with subsequent reorganization of the macromolecules. However, besides the carbonyl derivatives, carboxylic products might occur from possible reactions with atmospheric oxygen [58]. Simultaneously, apart from the formation of carbonyl and carboxyl derivatives, C=C double bonds may result from PVA dehydroxylation taking place during crosslinking of the polymers in the solid phase [59,60]. Zainuddin et al. [61] detected C=C linkages accompanied by C=O, COOH, and C-O-C (ether) groups in solid PVA crosslinked by irradiation.
The pattern recorded for the irradiated starch/PVA/CNC sample differed considerably from the pattern obtained for the sample prepared without the CNC addition. A maximum detected in the case of the starch/PVA film at ca. 340 nm almost disappeared, and thus, the band attributed to C=O bonds placed inside the chains was more visible and possibly higher (ketone; max. at ca. 270 nm). A strong, wide, and tailored band was, however, still observed in the range above ca 320 nm, with multiple maxima (at ca. 389, 423, 449, and 681 nm).
Simultaneously, additional bands appeared in the low wavelength region (not observed in the case of the pure starch–PVA system). The band with a low intensity at ca. 220 nm indicated the presence of peroxides and hydroperoxides [62,63], which was evidence of chain scission. It is difficult to give a clear interpretation; however, one possibility is that the radicals created at the CNC molecules might result in their cutting, especially in breaking linkages in “free” CNC or in CNC loosely bonded with the starch–PVA network.
A comparison of the patterns recorded for both samples showed that the addition of CNC, even at a level as low as 1.0 wt%, strongly influenced the radiation processes and led to the formation of different products compared to the pure starch–PVA system.
Differences between the profiles of the patterns recorded for the films prepared with and without CNC indicated strong interactions between CNC and the other film components (starch, PVA, and glycerol), possibly leading to the formation of a specific network with the participation of CNC.
Regarding the hypothesis that the vibrations of C=O groups located at or near the ends of the chains had an essential contribution to the band with a maximum at ca. 340 nm [44], it can be assumed that the formation of such groups takes place with considerable efficiency in the case of the irradiated starch/PVA film, while the presence of CNC blocks their creation. While the presence of such groups can be related to a possible increase in the number of chain endings after irradiation, the weakness of these bands in the case of the sample prepared with the addition of CNC possibly shows that the scission of chains is restricted. However, such a conclusion seems to be in contradiction with data that indicate an overall lower gel content (thus lower crosslinking) in irradiated samples compared to the corresponding non-irradiated references. Therefore, it can be assumed that the presence of nanocellulose only partially prevents the degradation processes. However, in the presence of CNC, the cutting of chains of the building polymers led to the creation of other products in which other functional groups may have played an important role, i.e., groups with C=C or C-O-C linkages (mentioned above). A decrease in total gel fraction might have been connected to the appearance of products with shorter chains, which, however, did not reveal the absorption of UV rays around 340 nm. On the other hand, the products that were formed in the irradiated starch–PVA–CNC system (1.0 wt% CNC) may have been less capable of forming a gel, independently of whether they were crosslinked or not.
The results described in the previous sections suggest an additional partial crosslinking taking place under irradiation in the compositions created with the addition of CNC. This concerns, for example, the lower swelling in water of films obtained at a low CNC level. On the other hand, the data showed a decreased gel fraction and increased solubility, indicating degradation processes. Simultaneously, an increased ability for moisture absorption might be connected to both crosslinking and degradation. Importantly, degradation and crosslinking are concurrent processes that take place simultaneously in the same sample subjected to radiation treatment.
In turn, a decreased hydrophilicity of the radiolysis products indicated the creation of new functional groups with lower hydrophilicity, which were probably different from the functional groups formed in the case of a film without CNC (considering the possible lack or decrease of the C=O and COOH groups located at or near chain endings.
3.2.10. Discussion of the Effect of the Addition of CNC on the Films’ Properties in Relation to the Literature Data
The results of this study indicated that the addition of a small amount of CNC improved the films’ properties, as shown by a meaningful amelioration in Δl accompanied by a slight increase in TS, a considerably increased contact angle, and a marked decrease in swelling parameters and solubility. However, further increasing the addition of CNC contributed to a deterioration in the functional properties of the films, but after even greater incorporation of CNC, an improvement occurred again in relation to the films obtained with an intermediate additive. It is significant that the “limit values” of additives at which deterioration or re-improvement was observed were specific for individual parameters. In particular, deterioration of the TS, swelling, and solubility occurred at 2.5 wt% or 5 wt% and a re-improvement at 5 or 7.5 wt%. Exceptionally, the contact angle deteriorated with the addition of CNC at 7.5 wt% and improved again with the addition of 10 wt%. Another exception was Δl, which, after reaching a maximum (optimum) with the addition of 1.0 wt% nanocellulose, decreased successively without re-improvement. These results might be related to the formation of crosslinked polymer networks. As discussed in Section 3.2.5, it can be presumed that, initially, crosslinks were formed between CNC particles and the polymer matrix (starch and PVA). A further increase in CNC may have caused the appearance of “free” CNC (which may have resulted in a deterioration in the film’s properties). The probability of forming crosslinks between two CNC molecules also increased, which could have been followed by further crosslinking of the matrix via CNC-CNC groups. This process could be associated with further improvement in the film’s properties. However, based on the data shown in Section 3.2.2, it is possible that the first type of crosslinking contributed to an increase, while the second type of crosslinking caused a decrease in extensibility. It can also be considered that the introduction of a greater amount of nanocellulose led to the formation of NC aggregates [16,33,34] and that the greater the NC content, the larger the aggregates were [16]. The presence of large aggregates caused a deterioration in structure, influencing the functional (particularly mechanical) properties of the films.
The above supposition is consistent with the interpretation presented in the literature. Most authors have attributed an improvement in the mechanical properties (in particular, an increase in TS) and a decrease in the affinity to water to the formation of hydrogen bonds between the NC molecules and the polymer matrix [16,33,34,35,36,37]. These authors indicated that blending with nanocellulose removes some of the free OH groups from the original blend of polymers. These groups become inaccessible, which causes a decrease in the hydrophilicity of the samples. Meanwhile, the creation of a new polymer network, more strongly linked by hydrogen bonds, helps to increase the mechanical resistance of the film. This explanation is similar to the interpretation presented in the current article.
Strong interactions between NC and the polymer blend (attributed to the formation of hydrogen bonds) were confirmed by FTIR spectroscopy [16,33,36]. Moreover, FTIR spectroscopy enabled such interactions to be observed in the cases of the starch–NC [38] and the PVA–NC systems [34,37]. It can thus be supposed that hydrogen bonds in the starch/PVA/NC are formed between NC and starch as well as between NC and PVA. The above results support the interpretation presented in this study (Section 3.2.1).
Independently of the above explanation regarding the formation of a crosslinked polymer network with the participation of NC and its physicochemical properties, the literature data do not always fit the presented concept. Although in most of the described cases, there was a simultaneous increase in TS and Δl [20,33,34,35,36,37,38,39], in other cases, an increase in TS was accompanied by a decrease in Δl [16,38]. Also, only in some of the reports was a decrease in water affinity associated with an increase in TS [34,36,39], while in other papers, on the contrary, a simultaneous increase in TS and affinity for water was noted [16,33].
The effects of the addition of NCs on the properties of starch/PVA films differed in their dependence on the starch type, starch/PVA ratio, NC type, the conditions of its preparation, the level of NC in the films, and the method and conditions of the film syntheses. Thus, it is difficult to relate the results of the current study to individual articles. This applies in particular to the “gap” in the nanocellulose addition in which an improvement in individual parameters occurred (except for the direction of changes associated with their incorporation.
It seems most reasonable to compare the data shown in the current article with the data obtained for systems that are characterized by similar starch/PVA weight ratios (thus containing simultaneously relatively large amounts of starch and PVA) and with a CNC addition in the range up to 10 wt%. Since the composition of the films has often been presented in different ways, the weight ratios were recalculated for the purpose of discussion so that their presentation would match their presentation in the present study.
Therefore, Cano et al. [16] reported that the incorporation of CNCs to a pea starch–PVA (33:67) system at a level of 1 wt% led to an increase in TS, while it decreased at levels of 3 and 5 wt% CNC. This result is similar to that presented in this paper (maximum TS at 1.0 wt% of CNC). However, contrary to our results, Δl was already lowered after the addition of 1 wt% CNC, while our data showed an increase in this parameter in relation to the films formed without CNC at a low level of CNC (with a maximum at 1 wt%) and a reduction starting from 5 wt% CNC, becoming gradually lower with an increasing CNC percentage. Simultaneously, Cano et al. noted an increase in moisture content and water vapor permeability (WVP) at a 1 wt% addition of CNC (which they attributed to an increase in water affinity), followed by a decrease in both parameters at 3 wt% and 5 wt%. Our data did not show any changes in moisture content and rather suggested a decrease in water affinity (as shown by the swelling and solubility data).
At the same time, similarly to our results, Jia et al. [33] detected an increase in the TS of cassava starch/PVA (67:33) after the addition of 1 wt% or 2 wt% CNC, accompanied by an increase in Δl. As in the present research, the highest Δl value was also reached at a level of 1 wt% CNC. However, the solubility in water (and WVP) was the highest at 1 wt% of CNC, and the contact angle was practically uninfluenced. This differs from our data showing the lowest solubility at 1 wt% of CNC and (in the same range of CNC addition) a much higher contact angle compared to the sample prepared without CNC.
Simultaneously, Jia et al. [33] also found that using ultrasonication during the preparation of the polymer solution enabled more homogeneous films to be obtained; these films revealed better mechanical properties but simultaneously higher hydrophilicity. In the current experiments, ultrasound treatment was also used for preparing CNC solutions, which were then used to synthesize the films.
Das et al. [20] examined the effect of the addition of jute nanofibrils (up to 15 wt%) into a soluble starch/PVA (50:50) composition. In contrast to the current results, the TS increased gradually with the increase in CNF addition, obtaining a maximum value at a CNF level equal to 15 wt%, while the Δl reached a maximum at 10 wt% of CNF, being at a level at which an essential reduction in the sample Δl was found in the present study. The authors pointed out, however, that the values of the selected parameters strongly depended on the size and homogeneity of the nanoparticles (which they synthesized themselves) as well as on the duration of film storage.
The results reported by Das et al. [20] can be compared additionally with the results obtained by Priya et al. [19] for films characterized by the same starch/PVA ratio (50:50) but prepared with cellulosic fibers (not nanoscale) and cornstarch. When the films were doped with the fibers at a level of 5–30 wt%, the best mechanical properties were found at 20 wt% (the highest TS and Δl), and were, therefore, higher than those obtained for their nanocomposite by Das et al. [20]. A comparison of these results seems to indicate that the use of nanofibers instead of microfibers allows for the optimization of composites while reducing the cellulose component.
Cases of films characterized by a higher content of PVA (lower starch/PVA weight ratios) were reported by Guimarăes et al. [39] and Zhou et al. [35]. Guimarăes et al. [39] presented the effect of the addition of bamboo CNF (0.5, 1.5, 4.5, and 6.5 wt%) on the properties of cassava starch/PVA (19:81) composites. In contrast to the present data, reductions in both TS and Δl were discovered at low levels of CNF, while a further increase in CNF resulted in an increase in both parameters. The best mechanical properties were determined for the films containing 4.5 wt% of CNF (in our case, at 1 wt% of CNC). Simultaneously, a gradual decrease in solubility (and in WVP) was observed, while in the case of the current data, solubility and swelling were the lowest at a CNC level of 1 wt%. Zhou et al. [35] examined cornstarch/PVA (9:91) prepared by a melt process with the addition of 10 or 20 wt% CNF and discovered an increase in TS and Δl at both these levels of CNF. This result differs from the results obtained now, which indicated similar TS levels for the films prepared with 0 and 10 wt% CNC, and a considerably lower Δl at a CNC level of 10 wt%, compared to the reference sample (prepared without CNC.
The case of high starch content was described by Noshirvani et al. [36]. These authors examined films of potato starch/PVA (90:10) prepared with the addition of 3, 5, 7, 10, 15, and 20 wt% of CNC. The authors found that the introduction of CNC at the lowest level of 3 wt% caused a decrease in TS (this differs from the results obtained in the current study) while increasing extensibility. With the increase in CNC addition, an increase in TS and Δl was observed initially, while at a higher level of CNC, a deterioration in both parameters occurred. However, the strength of the films obtained after adding 5, 7, and 10 wt% of CNC did not differ from the strength of the reference films, although these films were characterized by a higher Δl. This differs from our data, which showed a reduction in Δl with an increase in CNC in the sequence of 5.0, 7.5, and 10.0 wt%). As a result, the films with the best mechanical properties were obtained after adding 7 wt% CNC (in our case, 1 wt%). With a further increase in CNC addition, a deterioration in mechanical properties was observed (similar to the present study), indicated by a reduction in both TS and Δl. At the same time, with the increase in CNC addition, a gradual decrease in the affinity for water was also observed, indicated by a decrease in solubility and WVP and an increase in the contact (wetting) angle. This is different from the results of the present study, which indicated the lowest affinity to water at 1 wt% of the CNC (shown by minimum values of solubility and swelling parameters and the increased contact angle value).
Additionally, the results presented by Noshirvani et al. [36] can be compared with those of Jia et al. [34], obtained for a nanocomposite formed with pure PVA. An increase in TS and a decrease in capacity for moisture uptake were observed after the addition of 5 wt% of CNF. It is also worth mentioning that the results of Handoko et al. [37] indicated that the addition of CNC to PVA films contributed to an increase in TS and Δl, while a small addition of CNC caused a deterioration in mechanical properties, the best properties being obtained with a moderate addition of CNF.
Similar effects of the addition of CNC were observed with taro starch films prepared with the addition of 5, 10, or 15 wt%. CNC [38]. However, the TS increased, while the Δl decreased when the CNC addition increased. The change in Δl under such CNC addition resembled the results of the present study. No change in moisture content and water solubility was observed, and the swelling did not change at 5 wt%, while it increased at 10 and 15 wt% CNC. This differs from the current data showing a higher solubility and swelling in water already present at a CNC level of 5 wt%. At the same time, the contact angle values were lower at a moderate CNC content (5 wt%) compared to the reference films (showing a deterioration in the film’s structure), whereas it increased with an increasing CNC addition (10 and 15 wt%). In the case of the current study, higher contact angle values were observed at both 5 and 10 wt% CNC.
At the same time, in the reported cases, a decrease in equilibrium moisture system absorption in a humid atmosphere [20,34,36] was observed with an increase in the addition of NC, regardless of the composition of the polymer matrix. This differs from our results, which indicated an increase in the equilibrium level of moisture content with an increase in CNC addition. The increase in the mass of the samples after 1–2 days of exposure, with a subsequent reduction to the equilibrium level, was reported only by Das et al. [20] for the soluble starch/PVA composition (without any NC).
4. Conclusions
Differences were observed between the free radicals created by ionizing radiation in nanocrystalline and microfibrinal celluloses (CNC and MFC) and those formed in microcrystalline cellulose (MCC.) The reactivity of the samples under irradiation was higher for MCC > MFC > CNC, which can be related to the decreasing size of micro-nanoparticles.
The addition of CNC to the film composition resulted in the modification of its functional properties, while the direction and degree of the modifications depended on the CNC level. Besides surface hydrophilicity (which always decreased), the addition of CNC at a level of 0.5, 1.0, or 2.5% led to an improvement in mechanical properties (especially to greater extensibility). However, a further increase in the CNC level caused their deterioration.
In most cases, the swelling and solubility increased after the addition of CNC. Exceptionally, lower swelling and solubility were observed at a CNC level of 1.0 wt%. Moisture uptake in a humid atmosphere revealed anomalous behavior, with the equilibrium value increasing gradually when the CNC level increased.
Irradiation of the reference films (no CNC) resulted in a decrease in TS and extensibility (Δl), while it either did not influence or even increase the TS of the films formed with the addition of CNC. Moreover, irradiation led to a decrease in the surface hydrophilicity of all the films and in the bulk hydrophilicity of the films formed with the addition of CNC. The solubility increased in all cases, and the moisture uptake increased in the case of the films prepared at a CNC level of 2.5 wt% and higher. Thus, with certain reservations, it can be considered that irradiation contributes to an improvement in film properties.
It can be deduced that the addition of CNC to the film’s composition resulted in the creation of a more crosslinked polymer network. Modification of the film properties after irradiation can be related to a change in morphology, including a decreased size of the agglomerates of nanoparticles and their modified distribution. Simultaneously, a decrease in surface hydrophilicity could be explained in terms of the substitution of some OH groups by less hydrophilic C=O and COOH groups.
Degradation is the predominant process that took place under irradiation, while the prevalence of degradation over concurring crosslinking was greater after irradiation in a gamma chamber compared to an e-beam. Simultaneously, the addition of CNC to the films somewhat shifted the direction of the reaction toward crosslinking.
Superior films were obtained at low levels of CNC. The films formed with 1.0 wt% of CNC showed the best properties, which additionally could be improved by irradiation.
Author Contributions
K.C.: Conceptualization, methodology, data curation, supervision, writing—original draft preparation, review and editing, and visualization. A.A.: investigations and raw data curation. All authors have read and agreed to the published version of the manuscript.
Funding
This work was sponsored in the frame of statutory activity of the Institute of Nuclear Chemistry and Technology. Additionally, a research grant from the International Atomic Energy Agency (IAEA Research Contract 26851), received as part of the Co-ordinated Research Project CRP F22081, is kindly acknowledged.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article. Any additional raw data can be obtained upon request.
Acknowledgments
The authors wish to thank Grażyna Strzelczak for performing the ESR experiments and Wojciech Głuszewski for the measurements by means of gas chromatography.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Jimenez, A.; Fabra, M.J.; Talens, P.; Chiralt, A. Edible and biodegradable starch films: A review. Food Bioprocess. Tech. 2012, 5, 2058–2076. [Google Scholar] [CrossRef]
- Cabusas Alvarado, M. Recent progress in polyvinyl alcohol (PVA)/nanocellulose composite films for packaging applications: A comprehensive review of the impact on physico-mechanical properties. Food Bioeng. 2024, 3, 189–209. [Google Scholar] [CrossRef]
- Bangar, S.P.; Purewal, S.S.; Trif, M.; Maqsood, S.; Kumar, M.; Manjunatha, V.; Alexandru Rusu, V. Functionality and applicability of starch-based films: An eco-friendly approach. Foods 2021, 10, 2181. [Google Scholar] [CrossRef]
- Abedi-Firoozjah, R.; Chabook, N.; Rostami, O.; Heydari, M.; Kolahdouz-Nasiri, A.; Javanmardi, F.; Khadije, K.; Khaneghah, A.M. PVA/starch films: An updated review of their preparation, characterization, and diverse applications in the food industry. Polym. Test. 2023, 118, 107903. [Google Scholar] [CrossRef]
- Amini, M.; Rasouli, M.; Shoja, S.; Mozaffar, M.; Bekeschus, S. Preserving wheat flour with cellulose nanocomposite packaging and cold plasma treatment: Eliminating fungal contamination and improving functionality. Innov. Food Sci. Emerg. Technol. 2024, 93, 103632. [Google Scholar] [CrossRef]
- Abramowska, A.; Cieśla, K.A.; Buczkowski, M.J.; Nowicki, A.; Głuszewski, W.J. The influence of ionizing radiation on the properties of starch:PVA films. Nukleonika 2015, 60, 669–677. [Google Scholar] [CrossRef]
- Cieśla, K.; Abramowska, A.; Boguski, J.; Drewnik, J. The effect of PVA type and radiation treatment on the properties of starch:PVA films. Radiat. Phys. Chem. 2017, 141, 142–148. [Google Scholar] [CrossRef]
- Cano, A.I.; Cháfer, M.; Chiralt, A.; Gonzalez-Martinez, C. Physical and microstructural properties of biodegradable films based on pea starch and PVA. J. Food Eng. 2015, 167, 59–64. [Google Scholar] [CrossRef]
- Aydin, A.A.; Ilberg, V. Effect of different polyol-based plasticizers on thermal properties of polyvinyl alcohol: Starch blends. Carbohyd. Polym. 2016, 136, 441–448. [Google Scholar] [CrossRef]
- Mathew, S.; Jayakumar, A.; Kumar, V.P.; Mathew, J.; Radhakrishnan, E.K. One-step synthesis of eco-friendly boiled rice starch blended polyvinylalcohol bionanocomposite films decorated with in situ generated silver nanoparticles for food packaging purpose. Int. J. Biol. Macromol. 2019, 139, 475–485. [Google Scholar] [CrossRef]
- Tak, H.-Y.; Yun, Y.-H.; Lee, C.-M.; Yoon, S.-D. Sulindac imprinted mungbean starch/PVA biomaterial films as a transdermal drug delivery patch. Carbohyd Polym. 2019, 208, 261–268. [Google Scholar] [CrossRef]
- Parvin, F.; Khan, M.; Saadat, A.H.M.; Khan, M.A.H.; Islam, J.M.M.; Ahmed, M.; Gafur, M.A. Preparation and characterization of gamma irradiated sugar containing starch/poly(vinyl alcohol)-based blend films. J. Polym. Environ. 2011, 19, 1013–1022. [Google Scholar] [CrossRef]
- Senna, M.M.; El-Shahat, H.A.; El Naggar, A.W.M. Characterization of gamma irradiated plasticized starch/poly(vinyl alcohol) (PLST/PVA) blends and their application as protected edible materials. J. Polym. Res. 2011, 18, 763–771. [Google Scholar] [CrossRef]
- Naznin, M.; Abedin, M.-Z.; Khan, M.-A.; Gafur, M.D. Influence of Acacia Catechu extracts and urea and gamma irradiation on the mechanical properties of starch/PVA-based material. Int. Sch. Res. Netw. ISRN Polym. Sci. 2012, 12, 348685. [Google Scholar] [CrossRef][Green Version]
- Drewnik, J.; Cieśla, K. The effect of composition and ionising radiaton on the migration phenomena in starch-PVA films placed in isooctane. In Book of Abstracts, Proceedings of 9th International Conference on Modification, Degradation and Stabilization of Polymers, Cracow, Poland, 4–8 September 2016; Industrial Chemistry Research Institute: Warsaw, Poland, 2016. [Google Scholar]
- Cano, A.; Fortunati, E.; Cháfer, M.; González-Martıíínez, C.; Chiralt, A.; Kenny, J.M. Effect of cellulose nanocrystals on the properties of pea starch-poly(vinyl alcohol) blend films. J. Mater. Sci. 2015, 50, 6979–6992. [Google Scholar] [CrossRef]
- Kim, M.H.; Kyoo, L.H.; Lee, H.S. Transparent and high gas barrier films based on poly(vinyl alcohol)/grapheme oxide composites. Thin Solid. Films 2011, 519, 7766–7771. [Google Scholar] [CrossRef]
- Yao, K.; Cai, J.; Liu, M.; Yu, Y.; Xiong, H.; Tang, S.; Ding, S. Structure and properties of starch/PVA/nano-SiO2 hybrid films. Carbohyd. Polym. 2011, 86, 1784–1789. [Google Scholar] [CrossRef]
- Priya, B.; Gupta, V.K.; Pathania, D.; Singha, A.S. Synthesis, characterization and antibacterial activity of biodegradable starch/PVA composite films reinforced with cellulosic fibre. Carbohyd. Polym. 2014, 109, 171–179. [Google Scholar] [CrossRef]
- Das, K.; Ray, D.; Bandyopadhyay, N.R.; Sahoo, S.; Mohanty, A.K.; Misra, M. Physico-mechanical properties of the jute micro/nanofibril reinforce starch/polyvinyl alcohol biocomposite films. Compos. Part. B-Eng. 2011, 42, 376–381. [Google Scholar] [CrossRef]
- Stoica-Guzun, A.; Stroescu, M.; Jipa, I.; Dobre, L.; Zaharescu, T. Effect of γ irradiation on poly(vinylalcohol) and bacterial cellulose composites used as packaging. Radiat. Phys. Chem. 2013, 84, 200–204. [Google Scholar] [CrossRef]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose nanocrystals: Chemistry, self-assembly, and applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef] [PubMed]
- Jonoobi, R.; Oladi, R.; Davoudpour, Y.; Oksman, K.; Dufresne, A.; Hamzeh, Y.; Davoodi, R. Different preparation methods and properties of nanostructured cellulose from various natural resources and residues: A review. Cellulose 2015, 22, 935–969. [Google Scholar] [CrossRef]
- Kargarzadeh, H.; Mariano, M.; Gopakumar, D.; Ahmad, I.; Thomas, S.; Dufresnee, A.; Huang, J.; Ling, N. Advances in cellulose nanomaterials. Cellulose 2018, 25, 2151–2189. [Google Scholar] [CrossRef]
- Kagarzadeh, H.; Huang, J.; Lin, N.; Ahmad, I.; Mariano, M.A.; Gałęski, A. Recent developments in nanocellulose-based biodegradablepolymers, thermoplastic polymers, and porous nanocomposites. Prog. Polym. Sci. 2018, 87, 197–227. [Google Scholar] [CrossRef]
- Monteiro Cordeiro de Azeredo, H.; Camparelli Mattoso, L.H.; Habig McHugh, T. Nanocomposites in Food Packaging—A review. In Advances in Deverse Industrial Applications of Nanocomposites; Boreddy, R., Ed.; Intech Open FRSC: London, UK, 2011; Chapter 4; pp. 157–177. ISBN 978-953-307/978-953-51-4511-0. [Google Scholar]
- Cieśla, K. Biodegradowalna Folia na Bazie Układu Skrobia: PVA Oraz Sposób jej Wytwarzania (Biodegradable Film Based on the Starch: PVA System and the Method for Manufacturing the Same). Polish Patent No P.429513, 24 March 2023. [Google Scholar]
- Jipa, J.M.; Stroescu, M.; Stoica-Guzun, A.; Dobre, T.; Jinga, S.; Zaharescu, T. Effect of gamma irradiation on biopolymer composite films of poly(vinylalcohol) and bacterial cellulose. Nucl. Instrum. Meth B 2012, 278, 82–87. [Google Scholar] [CrossRef]
- Popescu, M.-C.; Dogaru, B.-I.; Goanta, M.; Timpu, D. Structural and morphological evaluation of CNC reinforced PVA/starch biodegradable films. Int. J. Biol. Macromol. 2018, 116, 385–393. [Google Scholar] [CrossRef]
- Goncalves de Oliveira Filho, J.; Albiero, B.R.; Cipriano, L.; de Oliveira Nobre Bezerra, C.; Campos Alencar Oldoni, F.; Buranelo Egea, M.; Monteiro Cordeiro de Azeredo, H.; Ferreira, M.D. Arrowroot starch-based films incorporated with a carnauba wax nanoemulsion, cellulose nanocrystals, and essential oils: A new functional material for food packaging applications. Cellulose 2021, 28, 6499–6511. [Google Scholar] [CrossRef]
- Qiao, J.; Dong, Y.; Chen, C.; Xie, J. Development and characterization of starch/PVA antimicrobial active films with controlled release property by utilizing electrostatic interactions between nanocellulose and lauroyl arginate ethyl ester. Int. J. Biol. Macromol. 2024, 261, 129415. [Google Scholar] [CrossRef]
- Almeida, T.; Karamysheva, A.; Valente, B.F.A.; Silva, J.M.; Braz, M.; Almeida, A.; Silvestre, A.J.D.; Vilela, C.; Freire, C.S.R. Biobased ternary films of thermoplastic starch, bacterial nanocellulose and gallic acid for active food packaging. Food Hydrocolloid. 2023, 144, 108934. [Google Scholar] [CrossRef]
- Jia, R.-J.; Teng, K.-Y.; Huang, J.-Y.; Wei, X.; Qin, Z.-Y. Hydrogen bonding crosslinking of starch-polyvinyl alcohol films reinforced by ultrasound-assisted and cellulose nanofibers dispersed cellulose nanocrystals. Starch–Stärke 2022, 74, 2100227. [Google Scholar] [CrossRef]
- Jia, Y.; Hu, C.; Shi, P.; Xu, Q.; Zhu, W.; Liu, R. Effects of cellulose nanofibrils/graphene oxide hybrid nanofiller in PVA nanocomposites. Int. J. Biol. Macromol. 2020, 161, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Luo, Y.; Lv, Z.; Xunwen Sun, X.; Tian, Y.; Zhang, X. Melt-processed poly (vinyl alcohol)/corn starch/nanocellulose composites with improved mechanical properties. .Int. J. Biol. Macromol. 2021, 183, 1903–1910. [Google Scholar] [CrossRef] [PubMed]
- Noshirvani, N.; Hong, W.; Ghanbarzadeh, B.; Fasihi, H.; Montazami, R. Study of cellulose nanocrystal doped starch-polyvinyl alcohol bionanocomposite films. Int. J. Biol. Macromol. 2018, 107, 2065–2074. [Google Scholar] [CrossRef] [PubMed]
- Handoko, F.; Yusuf, Y. Synthesis and physicochemical properties of poly(vinyl)alcohol nanocomposites reinforced with nanocrystalline cellulose from tea (Camellia sinensis) waste. Materials 2021, 14, 7154. [Google Scholar] [CrossRef]
- Daza-Orsini, S.M.; Medina-Jaramillo, C.; Caicedo-Chacon, W.D.; Ayala-Valencia, G.; López-Córdoba, A. Isolation of taro peel cellulose nanofibers and its application in improving functional properties of taro starch nanocomposites films. Int. J. Biol. Macromol. 2024, 273, 132951. [Google Scholar] [CrossRef]
- Guimarăes, M., Jr.; Botaro, V.R.; Monteiro Novack, K.; Gomes Teixeira, F.; Denzin Tonoli, G.H. Starch/PVA-based nanocomposites reinforced with bamboo nanofibrils. Ind. Crop Prod. 2015, 70, 72–83. [Google Scholar] [CrossRef]
- Cieśla, K.; Abramowska, A. Effect of absorbed dose on starch:PVA films irradiated with gamma rays. Radiat. Phys. Chem. 2021, 180, 109290. [Google Scholar] [CrossRef]
- Cieśla, K.; Abramowska, A. The influence of electron and gamma irradiation on the properties of starch: PVA films. The effect of irradiation dose. Nukleonika 2021, 66, 3–9. [Google Scholar] [CrossRef]
- Khan, R.A.; Salmieri, S.; Dussault, D.; Uribe-Calderon, J.; Kamal, M.R.; Safrany, A.; Lacroix, M. Production and properties of nanocellulose-reinforced methylcellulose-based biodegradable films. J. Agric. Food Chem. 2010, 58, 7878–7885. [Google Scholar] [CrossRef]
- Cieśla, K.A.; Nowicki, A.; Buczkowski, M.J. Radiation modification of the functional properties of the edible films prepared using starch and starch—Lipid system. Nukleonika 2010, 55, 233–242. [Google Scholar]
- Głuszewski, W.; Boruc, B.; Kubera, H.; Abbasowa, D. The use of DRS and GC to studies the effects of ionizing radiation on paper artifacts. Nukleonika 2015, 60, 665–668. [Google Scholar] [CrossRef]
- Głuszewski, W.; Cieśla, K.; Łyczko, K. Studies of the radical processes in selected polysaccharides by gas chromatography. Nukleonika, 2025; to be submitted. [Google Scholar]
- Leonor, S.J.; Gómez, J.A.; Kinoshita, A.; Calandreli, I.; Tfouni, E.; Baffa, O. Spectroscopic properties of irradiated gum arabic. Food Chem. 2013, 141, 1860–1864. [Google Scholar] [CrossRef] [PubMed]
- Sasai, Y.; Kondo, S.-I.; Kuzuya, M. Nature of Mechanoradical Formation of Substituted Celluloses as Studied by Electron Spin Resonance. Chem. Pharm. Bull. 2004, 52, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Rintarak, P.; Pattanasiri, B.; Anupong, S.; Ninlaphruk, S.; Dangtip, S. Electron Spin Resonance Analysis of γ-induced free radicals in riceberry rice grain. Proceedings of the Siam Physics Congress 2018 (SPC2018): A Creative Path to Sustainable Innovation. J. Phys. Conf. Ser. 2018, 1144, 12173. [Google Scholar] [CrossRef]
- Wencka, M.; Wichlacz, K.; Kasprzyk, H.; Lijewski, S.; Hoffmann, S.K. Free radicals and their electron spin relaxation in cellobiose. X-band and W-band ESR and electron spin echo studies. Cellulose 2007, 14, 183–194. [Google Scholar] [CrossRef]
- Yamaoki, R.; Kimura, S.; Ohta, M. Electron spin resonance characterization of radical components in irradiated black pepper skin and core. Radiat. Phys. Chem. 2011, 80, 282–1288. [Google Scholar] [CrossRef]
- Kameya, H.; Ukai, M.; Shimoyama, Y. An ESR study of radiation induced radicals in glucose polymers. Radiat. Phys. Chem. 2013, 84, 232–234. [Google Scholar] [CrossRef]
- Wach, R.A.; Mitomo, H.; Yoshii, F. ESR investigation on gamma-irradiated methylcellulose and hydroxyethylcellulose in dry state and in aqueous solution. J. Radioanal. Nucl. Chem. 2004, 261, 113–118. [Google Scholar] [CrossRef]
- Cieśla, K.; Grabowska, M. The effect of treatment with HEMA and gamma irradiation on the starch:PVA films studied by differential scanning calorimetry and thermogravimetry. Radiat. Phys. Chem. 2022, 197, 110168. [Google Scholar] [CrossRef]
- Wang, S.-M.; Huang, Q.-Z.; Wang, Q.-S. Study on the synergetic degradation of chitosan with ultraviolet light and hydrogen peroxide. Carbohyd Res. 2005, 340, 1143–1147. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Zhang, H.; Song, Y.; Zhang, J.; Yang, H.; Jiang, L.; Dan, Y. Investigation of polypyrrole/polyvinyl alcohol–titanium dioxide composite films for photo-catalytic applications. Appl. Surf. Sci. 2015, 342, 55–63. [Google Scholar] [CrossRef]
- von Sontag, C. Carbohydrates. In Radiation Chemistry. Present Status and Future Trends; Jonah, C.D., Rao, B.S.M., Eds.; Elsevier Sciences BV: Amsterdam, The Netherlands; London, UK; New York, NY, USA; Oxford, UK; Paris, France; Shannon, Ireland; Tokyo, Japan, 2001; pp. 481–511. [Google Scholar]
- Relleve, L.; Nagasawa, N.; Luan, L.Q.; Yagi, T.; Aranilla, C.; Abad, L.; Kume, T.; Yoshii, F.; dela Rosa, A. Degradation of carrageenan by radiation. Polym. Degrad. Stabil. 2005, 87, 403–410. [Google Scholar] [CrossRef]
- Sharpatyi, V.A. Radiation chemistry of polysaccharides. 1. Mechanism of carbon monoxide and formic acid formation. High. Energ. Chem. 2003, 37, 369–372. [Google Scholar] [CrossRef]
- .Akhter, S.; Allan, K.; Buchanan, D.; Cook, J.A.; Campion, A.; White, J.M. XPS and IR study of X-ray induced degradation of PVA polymer film. Appl. Surf. Sci. 1988, 35, 241–258. [Google Scholar] [CrossRef]
- El-Sawy, N.M.; El-Arnaouty, M.B.; Abdel, G. γ-irradiation effect on the non-cross-linked and cross-linked polyvinyl alcohol films. Polymer Plast. Technol. 2010, 49, 169–177. [Google Scholar] [CrossRef]
- Zainuddin; Hill, D.J.T.; Le, T.T. An ESR study on γ-irradiated poly(vinyl alcohol). Radiat. Phys. Chem. 2001, 62, 283–291. [Google Scholar] [CrossRef]
- Zagórski, Z.P.; Rafalski, A. Free radicals in irradiated unstabilized polypropylene, as seen by DRS absorption-spectrophotometry. Radiat. Phys. Chem. 1998, 52, 257–260. [Google Scholar] [CrossRef]
- Milosavljevic, B.H.; Thomas, J.K. Effects of the degree of hydrolysis on radiation induced reactions in the poly(vinyl alcohol)–poly(vinyl acetate) system. Radiat. Phys. Chem. 2001, 62, 3–10. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).