Low Molar Mass Carbazole-Based Host Materials for Phosphorescent Organic Light-Emitting Diodes: A Review
Abstract
1. Introduction
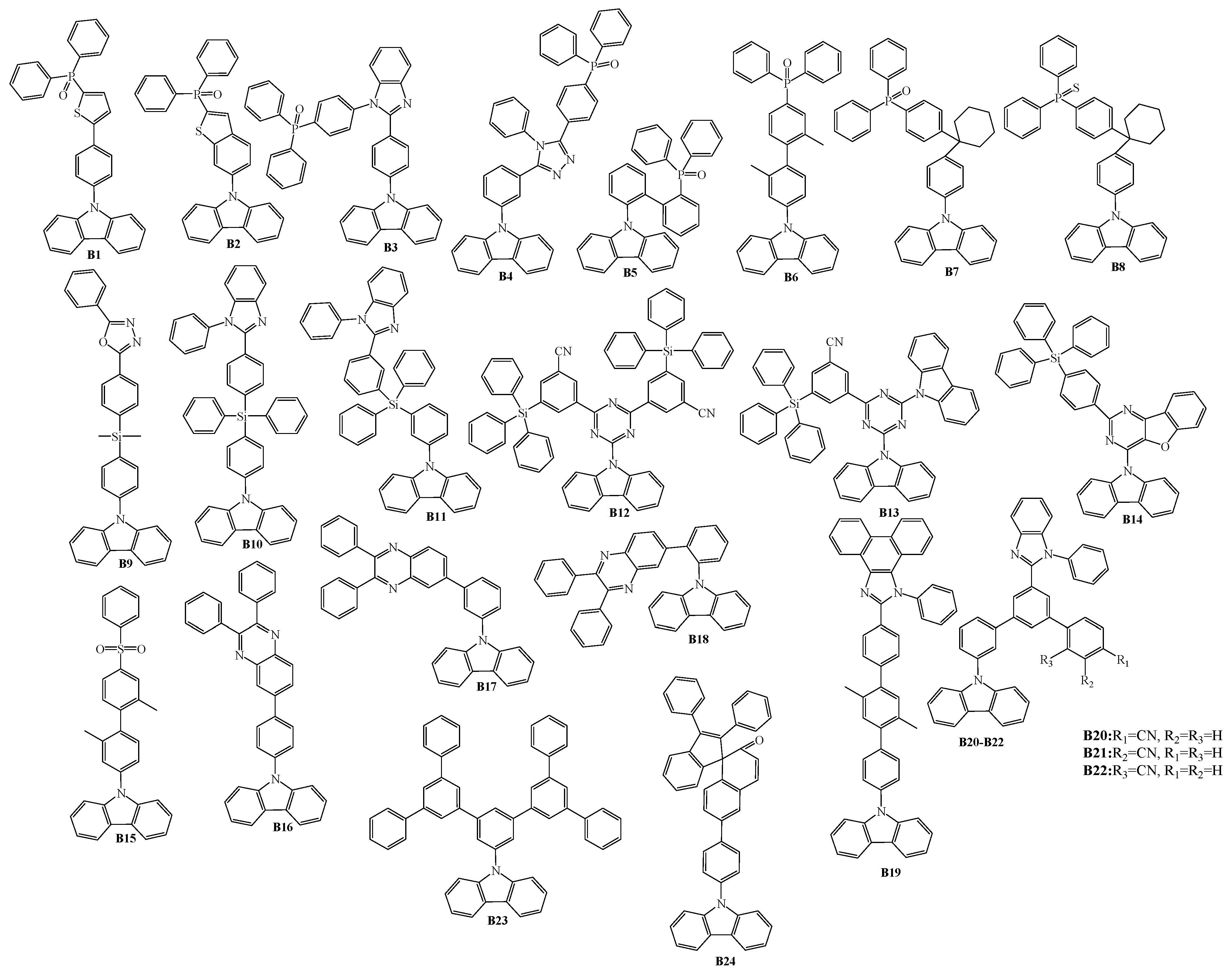
2. 9-Aryl Substituted Carbazoles
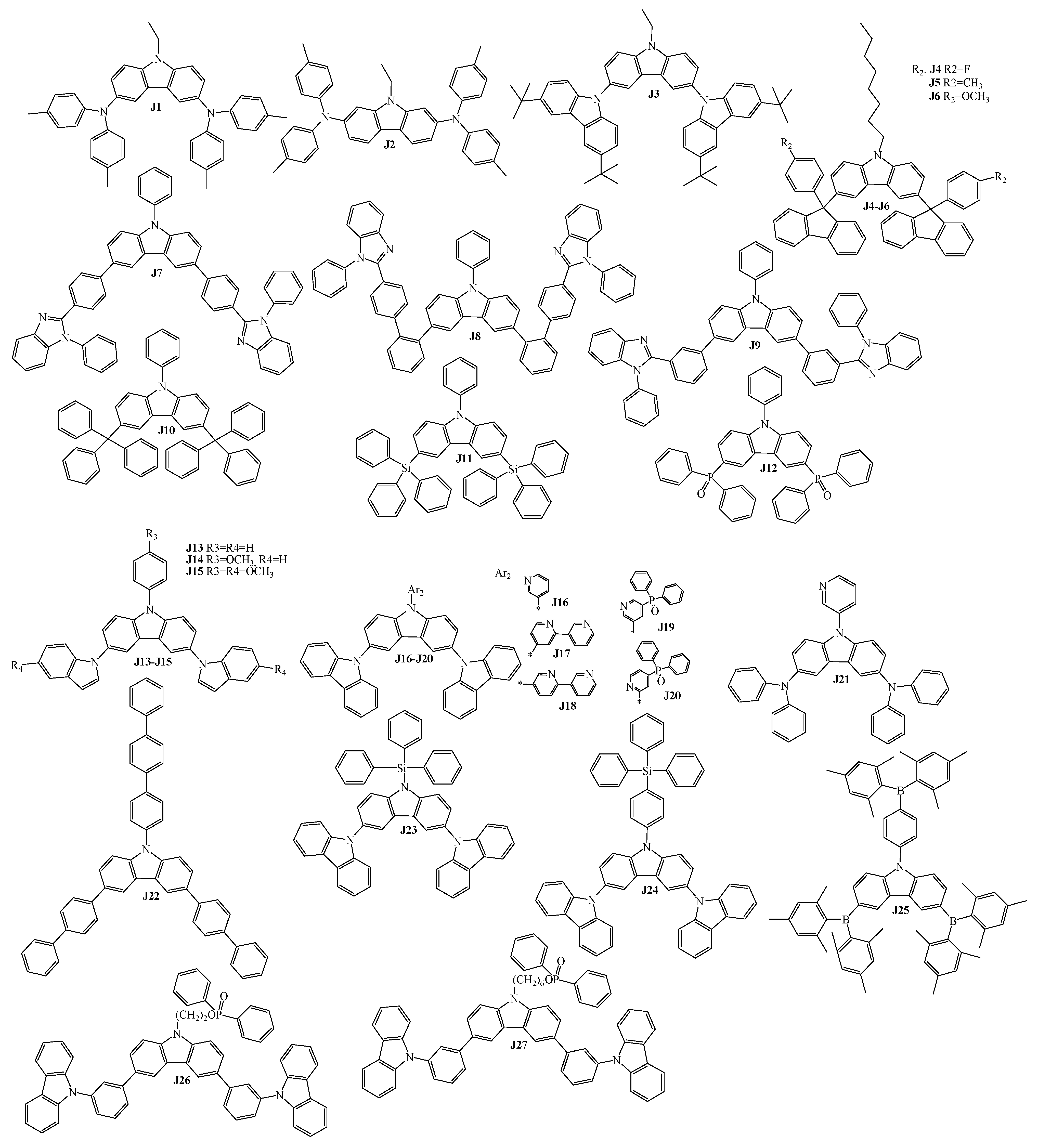
3. Twin Host Derivatives Containing Two Carbazolyl Fragments
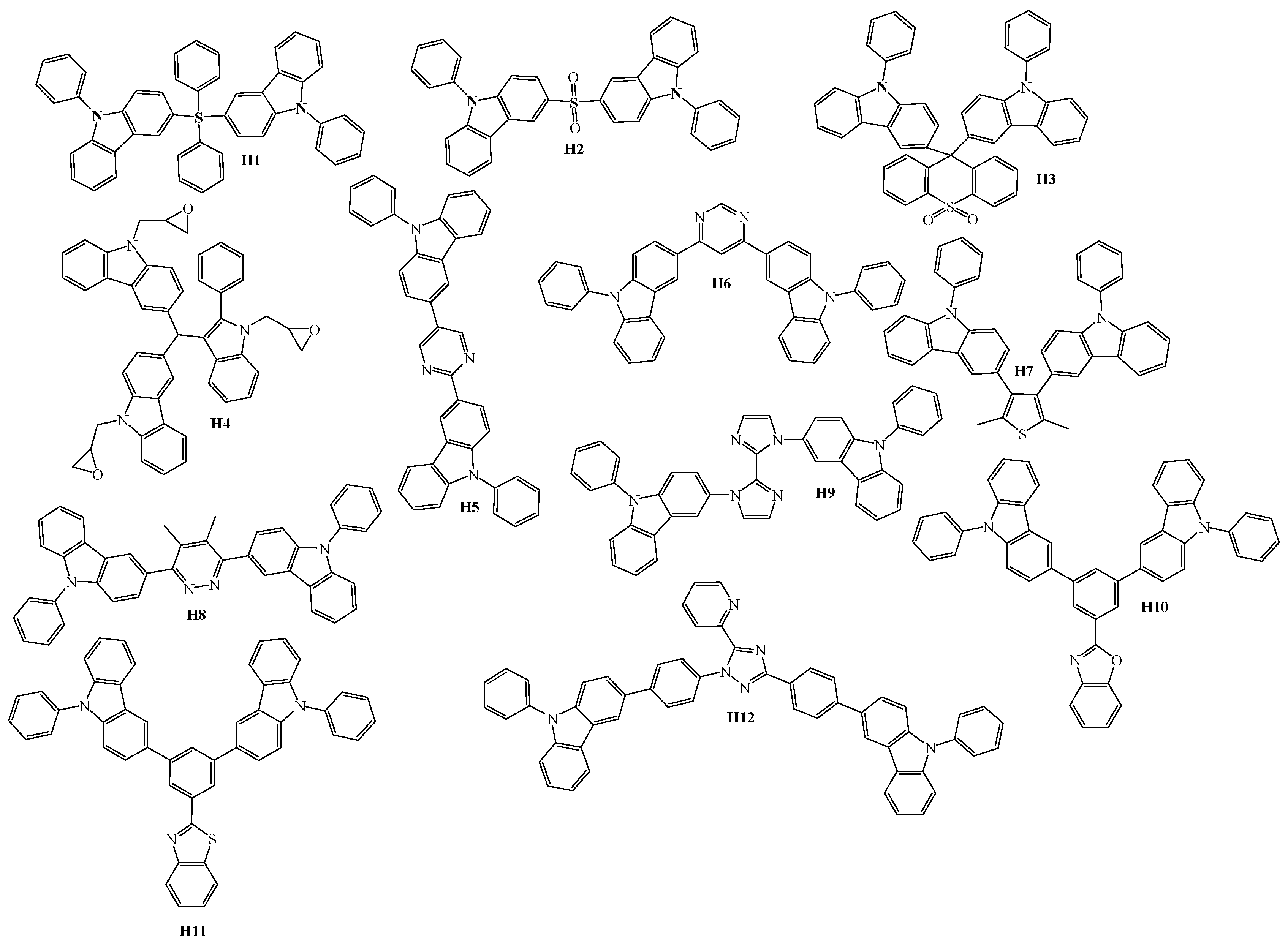
4. Host Materials with 3(2)-Aryl(arylamino)-Substituted Carbazole Fragments
5. Host Materials with 3,6(2,7)-Diaryl(arylamino)-Substituted Carbazole Fragments
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| 2-TNATA | 4,4′,4′′-tris[2-naphthyl(phenyl)amino] triphenylamine |
| 4CzIPN | 1,2,3,5-tetrakis(carbazol-9-yl)-4,6-dicyanobenzene |
| Alq3 | tris(8-hydroxyquinolinato)aluminum |
| AcDbp | 2,7-bis(9,9-dimethylacridin-1′(9H)-yl)dibenzo [a,c]phenazine |
| B3PYMPM | 4,6-bis(3,5-di(pyridin-3-yl)phenyl)-2-methylpyrimidine, 4,6-Bis(3,5-di-3-pyridinylphenyl)-2-methylpyrimidine |
| BCP | 4,7-diphenyl-1,10-phenanthroline |
| BCFA | N-([1,10-biphenyl]-4-yl)-9,9- dimethyl-N-(4-(9-phenyl-9H-carbazol-3-yl)phenyl)-9H-fluoren-2-amine |
| BCFN | N-([1,1′-biphenyl]-4-yl)-9,9-dimethyl-N-(4-(9-phenyl-9H-carbazol-3-yl)phenyl)-9H-fluoren-2-amine |
| Bebq2 | bis(10-hydroxybenzo[h]quinolinato)beryllium |
| Bphen | 4,7-diphenyl-1,10-phenanthroline |
| BPBPA | N,N,N′,N′-Tetra(4-biphenylyl)-4,4′-biphenyldiamine |
| (Bt)2Ir(acac) | bis(2-phenylbenzothiazolato)(acetylacetonate)iridium(III) |
| CE | current efficiencies |
| CuPc | copper phthalocyanine |
| CzSi | 9-(4-tert-Butylphenyl)-3,6-bis(triphenylsilyl)-9H-carbazole |
| DACT-II | 9-[4-(4,6-diphenyl-1,3,5- triazin-2-yl)phenyl]-N,N,N′,N′-tetraphenyl-9H-carbazole-3,6-diamine |
| DBFTrz | 2,8-bis(4,6-diphenyl-1,3,5-triazin-2-yl)dibenzo-[b,d]furan |
| DNTPD | 4,4′-bis[N-[4-{N,N-bis(3-methylphenyl)amino}phenyl]-N-phenylamino]biphenyl |
| DSC | differential scanning calorimetry |
| DPPS | diphenyl-bis [4-(pyridin-3-yl)phenyl]silane |
| DPEPO | bis [2-(diphenylphosphino)phenyl]ether oxide |
| DTAF | 9,9-di [4-(di-p-tolyl)aminophenyl]fluorine |
| Et | triplet energies |
| EQE | external quantum efficiencies |
| FCNIrpic | bis(3,5-difluoro-4-cyano-2-(2-pyridyl)phenyl-(2-carboxypyridyl) iridium(III) |
| FIr6 | bis(2,4-difluorophenylpyridinato)-tetrakis(1-pyrazolyl)borate iridium(III) |
| FIrpic | bis [2-(4,6-difluorophenyl)pyridinato-C2,N](picolinato)iridium |
| HAT-CN | 1,4,5,8,9,11-hexaazatriphenylenehexacarbonitrile |
| HOMO | the highest occupied molecular orbital |
| LUMO | the lowest unoccupied molecular orbital |
| TCTA | tris(4-carbazoyl-9-ylphenyl)amine |
| TAPC | 1,1-bis[(di-4-tolylamino)phenyl]cyclohexane |
| TAZ | 3-(4-biphenyl)-4-phenyl-5-tert -butylphenyl-1,2,4-triazole |
| TmPyPB | 1,3,5-tri(m-pyridin-3-ylphenyl)benzene |
| Tg | glass transition temperatures |
| TCz1 | 3,6-bis(carbazol-9-yl)-9-(2-ethyl-hexyl)-9H -carbazole |
| Tris-PCz | 9-phenyl-3,6-bis(9-phenyl-9Hcarbazol-3-yl)-9H-carbazole |
| Ir(MDQ2(acac) | bis(2-methyldibenzo[f,h]quinoxaline)(acetylacetonate) iridium(III) |
| Ir(mppy)3 | tris [2-(p-tolyl)pyridine]iridium(III) |
| Ir(ppy)3 | tris(2-phenylpyrydine(iridium(III) |
| Ir(dbfmi) | mer-tris(N-dibenzofuranyl-N′-methylimidazole)iridium(III) |
| Ir(pq)2(acac) | bis(1-phenylisoquinoline)(acetylacetonate)iridium(III) |
| Ir (ppz)3 | tris(1-phenylpyrazolato)iridium |
| ITO | indium tin oxide |
| IQE | internal quantum efficiency |
| mCP | N,N′-dicarbazolyl-3,5-benzene |
| (mpq)2Ir(acac) | (2-(3-methylquinolin-2-yl)phenyl) iridium(III) acetylacetonate |
| m-MTDATA | 4,4′,4′′-tris[(3-methylphenyl)phenylamino]triphenylamine |
| MoO3 | Molybdenum trioxide |
| NPB | N,N0-bis-[(1-naphthalenyl)-N,N0-bis-phenyl]-(1,10-biphenyl)-4,40-diamine |
| OLED | organic light-emitting diode |
| Os(bpftz)2 (PPhMe2)2 | bis(3-(trifluoroMethyl)-5-(4-tert-butylpyridyl)-1,2,4-triazolate) dimethylphenylphosphine |
| OXD-7 | 1,3-bis [2-(4-tert-butylphenyl)-1,3,4-oxadiazo-5-yl]benzene |
| PCzAc | 9,10-dihydro-9,9-dimethyl-10- (9-phenyl-9H-carbazol-3-yl)-acridine |
| PE | power efficiencies |
| PEDOT: PSS | poly(3,4-ethylene-dioxythiophene): poly(styrene-sulfonate) |
| PHOLED | phosphorescent organic light-emitting diode |
| PO-01 | bis(4-phenylthieno [3,2-c]pyridinato-N,C2′) (acetylacetonate) iridium(III) |
| poly-TPD | poly(N,N′-bis-4-butylphenyl-N,N′-bisphenyl)benzidine |
| PO-T2TLiq | 2,4,6-tris [3-(diphenylphosphinyl)phenyl]-1,3,5-triazine |
| (PBi)2Ir(acac) | bis(1,2-dipheny1-1H-benzoimidazole) iridium(III) (acetylacetonate) |
| PtN3N-ptb | tetradentate cyclometalated Pt(II) complex |
| TAPC | 1,1-bis[(di-4-tolylamino)phenyl]cyclohexane |
| TCTA | tris(carbazol-9-yl)-triphenylamine |
| TmPyPb | 1,3,5-tris(3-pyridyl-3-phenyl)benzene |
| TPBi | 2,2′,2′′-(1,3,5-benzinetriyl)-tris(1-phenyl-1-H-benzimidazole) |
| (tphpy)2Ir(acac) | bis [2-(2-pyridinyl-N)phenyl-C](acetylacetonato)iridium(III) |
| TTA | triplet-triplet annihilation |
| TGA | thermos-gravimetric analysis |
| Td | thermal decomposition temperatures |
| TSPO1 | Diphenyl [4-(triphenylsilyl)phenyl]phosphine oxide |
| ZADN | 2-[4-(9,10-di-naphthalen-2-yl-anthracen-2-yl)-phenyl]-1-phenyl-1H-benzoimidazole |
References
- Tang, C.W.; VanSlyke, S.A. Organic electroluminescent diodes. Appl. Phys. Lett. 1987, 51, 913. [Google Scholar] [CrossRef]
- Tyan, Y.S.J. Organic light-emitting-diode lighting overview. Photonics Energy 2011, 1, 011009. [Google Scholar] [CrossRef]
- Chen, H.W.; Lee, J.H.; Lin, B.Y.; Chen, S.; Wu, S.T. Liquid crystal display and organic light-emitting diode display: Present status and future perspectives. Sci. Appl. 2018, 7, 17168. [Google Scholar] [CrossRef]
- Xu, R.P.; Li, Y.Q.; Tang, J.X. Recent advances in flexible organic light-emitting diodes. J. Mater. Chem. C 2016, 4, 9116. [Google Scholar] [CrossRef]
- Jou, J.H.; Kumar, S.; Agrawal, A.; Li, T.H.; Sahoo, S. Approaches for fabricating high efficiency organic light emitting diodes. J. Mater. Chem. C 2015, 3, 2974. [Google Scholar] [CrossRef]
- Yokoyama, D.J. Molecular orientation in small-molecule organic light-emitting diodes. Mater. Chem. 2011, 21, 19187. [Google Scholar] [CrossRef]
- Geffroy, B.; Roy, P.L.; Prat, C. Organic light-emitting diode (OLED) technology: Materials, devices and display technologies. Polym. Int. 2006, 55, 572. [Google Scholar] [CrossRef]
- Duan, L.; Hou, L.; Lee, T.W.; Qiao, J.; Zhang, D.; Dong, G.; Wang, L.; Qiu, Y. Solution processable small molecules for organic light-emitting diodes. J. Mater. Chem. 2010, 20, 6392. [Google Scholar] [CrossRef]
- Baldo, M.A.; O’Brien, D.F.; You, Y.; Shoustikov, A.; Sibley, S.; Thompson, M.E.; Forrest, S.R. Highly efficient phosphorescent emission from organic electroluminescent devices. Nature 1998, 395, 151. [Google Scholar]
- Poriel, C.; Rault-Berthelot, J. Pure Hydrocarbons: An Efficient Molecular Design Strategy for the Next Generation of Host Materials for Phosphorescent Organic Light-Emitting Diodes. Acc. Mater. Res. 2022, 3, 379. [Google Scholar] [CrossRef]
- Gong, X.; Ostrowski, J.C.; Moses, D.; Bazan, G.C.; Heeger, A.J. Red electrophosphorescence from polymer doped with iridium complex. Appl. Phys. Lett. 2002, 81, 3711. [Google Scholar] [CrossRef]
- Tsuzuki, T.; Shirasawa, N.; Suzuki, T.; Tokito, S. Color Tunable Organic Light-Emitting Diodes Using Pentafluorophenyl-Substituted Iridium Complexes. Adv. Mater. 2003, 15, 1455. [Google Scholar] [CrossRef]
- Jeon, W.S.; Park, T.J.; Kim, S.Y.; Pode, R.; Jang, J.; Kwona, J.H. Ideal host and guest system in phosphorescent OLEDs. Org. Electron. 2009, 10, 240. [Google Scholar] [CrossRef]
- Strohriegl, P.; Wagner, D.; Schrögel, P.; Hoffmann, S.T.; Köhler, A.; Heinemeyer, U.; Münster, I. Novel host materials for blue phosphorescent OLEDs. Proc. SPIE 2013, 8829, 882906. [Google Scholar]
- Yeh, S.J.; Wu, M.F.; Chen, C.T.; Song, Y.H.; Chi, Y.; Ho, M.H.; Hsu, S.F.; Chen, C.-H. New Dopant and Host Materials for Blue-Light-Emitting Phosphorescent Organic Electroluminescent Devices. Adv. Mater. 2005, 17, 285. [Google Scholar] [CrossRef]
- Yook, K.S.; Lee, J.Y. Bipolar Host Materials for Organic Light-Emitting Diodes. Chem. Rec. 2016, 16, 159. [Google Scholar] [CrossRef]
- Adachi, C.; Kwong, R.C.; Djurovich, P.; Adamovich, V.; Baldo, M.A.; Thompson, M.E.; Forrest, S.R. Endothermic energy transfer: A mechanism for generating very efficient high-energy phosphorescent emission in organic materials. Appl. Phys. Lett. 2001, 79, 2082. [Google Scholar] [CrossRef]
- Holmes, R.J.; Forrest, S.R.; Tung, Y.J.; Kwong, R.C.; Brown, J.J.; Garon, S.; Thompson, M.E. Blue organic electrophosphorescence using exothermic host–guest energy transfer. Appl. Phys. Lett. 2003, 82, 2422. [Google Scholar] [CrossRef]
- Xiao, L.; Su, S.J.; Agata, Y.; Lan, H.; Kido, J. Nearly 100% Internal Quantum Efficiency in an Organic Blue-Light Electrophosphorescent Device Using a Weak Electron Transporting Material with a Wide Energy Gap. Adv. Mater. 2009, 21, 1271. [Google Scholar] [CrossRef]
- Adachi, C.; Baldo, M.A.; Thompson, M.E.; Forrest, S.R. Nearly 100% internal phosphorescence efficiency in an organic light emitting device. J. Appl. Phys. 2001, 10, 5048. [Google Scholar] [CrossRef]
- O’Brien, D.F.; Baldo, M.A.; Thompson, M.E.; Forrest, M.E.S.R. Improved energy transfer in electrophosphorescent devices. Appl. Phys. Lett. 1999, 74, 442. [Google Scholar] [CrossRef]
- Tao, Y.; Yang, C.; Qin, J. Organic host materials for phosphorescent organic light-emitting diodes. Chem. Soc. Rev. 2011, 40, 2943. [Google Scholar] [CrossRef]
- Chaskar, A.; Chen, H.F.; Wong, K.-T. Bipolar Host Materials: A Chemical Approach for Highly Efficient Electrophosphorescent Devices. Adv. Mater. 2011, 23, 3876. [Google Scholar] [CrossRef]
- Jeon, S.O.; Lee, J.Y. Phosphine oxide derivatives for organic light emitting diodes. Mater. Chem. 2012, 22, 4233. [Google Scholar] [CrossRef]
- Jing, L.; Zhensong, Z.; Chunmiao, H.; Dongxue, D.; Zhao, Z.Y.; Huang, H.W.; Hui, X. Tuning peripheral group density in ternary phosphine oxide hosts for low-voltage-driven yellow PhOLEDs. J. Mater. Chem. C 2015, 3, 6709. [Google Scholar] [CrossRef]
- Dongdong, Z.; Juan, Q.; Zhang, Z.D.; Duan, L. Ultrahigh-Efficiency Green PHOLEDs with a Voltage under 3 V and a Power Efficiency of Nearly 110 lm W−1 at Luminance of 10 000 cd m−2. Adv. Mater. 2017, 29, 1702847. [Google Scholar] [CrossRef]
- Wenwen, T.; Qi, Q.; Bo, S.; Chang, Y.; Wei, J.; Xia, C.; Wei, S.; Huanga, H.; Yueming, S. A bipolar homoleptic iridium dendrimer composed of diphenylphosphoryl and diphenylamine dendrons for highly efficient non-doped single-layer green PhOLEDs. J. Mater. Chem. C 2015, 3, 981. [Google Scholar] [CrossRef]
- Uoyama, H.; Goushi, K.; Shizu, K.; Nomura, H.; Adachi, C. Highly efficient organic light-emitting diodes from delayed fluorescence. Nature 2012, 492, 234. [Google Scholar] [CrossRef]
- Ge, Z.; Hayakawa, T.; Ando, S.; Ueda, M.; Akiike, T.; Miyamoto, H.; Kajita, T.; Kakimoto, M.A. Spin-Coated Highly Efficient Phosphorescent Organic Light-Emitting Diodes Based on Bipolar Triphenylamine-Benzimidazole Derivatives. Adv. Funct. Mater. 2008, 18, 584. [Google Scholar] [CrossRef]
- Lee, S.Y.; Yasuda, T.; Yang, Y.S.; Zhang, Q.; Adachi, C. Luminous butterflies: Efficient exciton harvesting by benzophenone derivatives for full-color delayed fluorescence OLEDs. Angew. Chem. Int. Ed. 2014, 126, 6520. [Google Scholar] [CrossRef]
- Lee, C.W.; Lee, J.Y. Above 30% External Quantum Efficiency in Blue Phosphorescent Organic Light-Emitting Diodes Using Pyrido[2,3-b]indole Derivatives as Host Materials. Adv. Mater. 2013, 25, 5450. [Google Scholar] [CrossRef]
- Jiang, H. Hosts for High-Performance Phosphorescent Organic Light-Emitting Diodes Based on Carbazole Derivatives. J. Org. Chem. 2014, 3, 102. [Google Scholar] [CrossRef]
- Romero, D.H.; Schaer, M.; Leclerc, M.; Ades, D.; Siove, A.; Zuppiroli, L. The role of carbazole in organic light-emitting devices. Synthet. Met. 1996, 80, 271. [Google Scholar]
- Shahnawaz; Swayamprabha, S.S.; Nagar, M.R.; Yadav, R.A.K.; Gull, S.; Dubey, D.K.; Jou, J.H. Hole-transporting materials for organic light-emitting diodes: An overview. J. Mater. Chem. C 2019, 7, 7144. [Google Scholar] [CrossRef]
- An, Z.; Zhongfu, A.; Chao, Z.; Ye, T.; Runfeng, C.; Huifang, S.; Ting, C.; Zhixiang, W.; Huanhuan, L.; Renren, D.; et al. Stabilizing triplet excited states for ultralong organic phosphorescence. Nat. Mater. 2015, 14, 685. [Google Scholar] [CrossRef]
- Chengjian, C.; Zhenguo, C.; Kok, C.C.; Andrei, S.; Batsanov, S.; Zhan, Y.; Zhu, M.; Zhiyong, Y.; Bin, L. Carbazole isomer induces ultralong organic phosphorescence. Nat. Mater. 2015, 20, 175. [Google Scholar]
- Joule, J.A. Recent Advances in the Chemistry of 9H-Carbazoles. Adv. Heterocycl. Chem. 1984, 35, 84. [Google Scholar]
- Yang, T.; Xu, H.; Zhao, B.; Tao, P.; Sun, P.; Wang, H.; Xu, B.; Wong, W.Y. Three carbazole-based host materials: Facile synthesis, photophysical properties and performances in PhOLED. Tetrahedron 2016, 72, 8066. [Google Scholar] [CrossRef]
- Lade, J.; Lee, N.Y.; Patil, B.; Deshpande, Y.Y.; Pownthurai, B.; Hsieh, C.A.; Pingale, S.S.; Chen, L.Y.; Chaskar, A. Novel benzothiadiazine 1,1-dioxide based bipolar host materials for efficient red phosphorescent organic light emitting diodes. Org. Electron. 2021, 92, 106104. [Google Scholar] [CrossRef]
- Patil, B.; Pownthurai, B.; Chiou, S.S.; Chen, W.L.; Huang, D.C.; Jadhav, Y.; Chetti, P.; Chang, C.H.; Chaskar, A. Carbazole-pyridine pyrroloquinoxaline/benzothiadiazine 1,1-dioxide based bipolar hosts for efficient red PhOLEDs. Org. Electron. 2021, 96, 106217. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, Y.; Ma, D.; Wang, Q. Phthalonitrile-based bipolar host for efficient green to red phosphorescent and TADF OLEDs. Dye Pigment. 2020, 173, 107895. [Google Scholar] [CrossRef]
- Im, Y.; Lee, J.Y. CN substituted indolocarbazole as a core structure of exciton harvesting and lifetime extending host for green thermally activated delayed fluorescent emitter. Dye Pigment. 2019, 164, 233. [Google Scholar] [CrossRef]
- Liu, X.Y.; Ge, H.T.; Zhao, Y.; Zhao, D.; Fan, J.; Liao, L.S. Novel carbazole derivatives designed by an ortho-linkage strategy for efficient phosphorescent organic light-emitting diodes. J. Mater. Chem. C 2018, 6, 4300. [Google Scholar] [CrossRef]
- Jiang, W.; Zhang, G.; Zhao, G.; Wang, X.; Tian, W.; Sun, Y. Novel benzonitrile-based AIE host with high triplet energy for highly efficient solution-processed blue TADF OLEDs. Dye Pigment. 2023, 210, 111037. [Google Scholar] [CrossRef]
- Gu, Y.; Zhu, L.; Li, Y.; Yu, L.; Wu, K.; Chen, T.; Huang, M.; Wang, F.; Gong, S.; Ma, D.; et al. Adamantane-Based Wide-Bandgap Host Material: Blue Electrophosphorescence with High Efficiency and Very High Brightness. Chem. Eur. J. 2015, 21, 98250. [Google Scholar] [CrossRef]
- Lee, K.H.; Kang, H.J.; Kim, H.M.; Seo, J.H.; Kim, Y.K.; Yoon, S.S. Pyridine/Isoquinoline-Carbazole Containing Bipolar Host Materials for Green Phosphorescent Organic Light-Emitting Diodes. J. Nanosci. Nanotechnol. 2011, 11, 1499. [Google Scholar] [CrossRef]
- Kautny, P.; Wu, Z.; Eichelter, J.; Horkel, E.; Stoger, B.; Chen, J.; Ma, D.; Frohlich, J.; Lumpi, D. Indolo[3,2,1-jk]carbazole based planarized CBP derivatives as host materials for PhOLEDs with low efficiency roll-off. Org. Electron. 2016, 34, 237. [Google Scholar] [CrossRef]
- Pan, B.; Wang, B.; Wang, Y.; Xu, P.; Wang, L.; Chen, J.; Ma, D. A simple carbazole-N-benzimidazole bipolar host material for highly efficient blue and single layer white phosphorescent organic light-emitting diodes. J. Mater. Chem. C 2014, 2, 2466. [Google Scholar] [CrossRef]
- Chang, S.Y.; Lin, G.T.; Cheng, Y.C.; Huang, J.J.; Chang, C.L.; Lin, C.F.; Lee, J.H.; Chiu, T.L.; Leung, M.K. Construction of Highly Efficient Carbazol-9-yl-Substituted Benzimidazole Bipolar Hosts for Blue Phosphorescent Light-Emitting Diodes: Isomer and Device Performance Relationships. ACS Appl. Mater. Interfaces 2018, 10, 42723. [Google Scholar] [CrossRef]
- Park, S.R.; Kim, S.M.; Kong, J.H.; Suh, M.C. Bipolar host materials with carbazole and dipyridylamine groups showing high triplet energy for blue phosphorescent organic light emitting diodes. Dye Pigment. 2017, 141, 217. [Google Scholar] [CrossRef]
- Park, S.R.; Seo, J.S.; Ahna, Y.; Lee, J.H.; Suh, M.C. Thermally stable benzo[f]quinoline based bipolar host materials for green phosphorescent OLEDs. Org. Electron. 2018, 63, 194. [Google Scholar] [CrossRef]
- Hung, W.I.; Tu, G.M.; Chen, S.W.; Chi, Y. Phenylcarbazole-dipyridyl triazole hybrid as bipolar host material for phosphorescent OLEDs. J. Mater. Chem. 2012, 22, 5410. [Google Scholar] [CrossRef]
- An, Z.F.; Chen, R.F.; Yin, J.; Xie, G.H.; Shi, H.F.; Tsuboi, T.; Huang, W. Conjugated Asymmetric Donor-Substituted 1,3,5-Triazines: New Host Materials for Blue Phosphorescent Organic Light-Emitting Diodes. Chem. Eur. J. 2011, 17, 10871. [Google Scholar] [CrossRef]
- Wang, F.; Liu, D.; Li, J.; Ma, M. Molecular Engineering of Host Materials for High-Performance Phosphorescent OLEDs: Zig-Zag Conformation with 3D Gridding Packing Mode Facilitating Charge Balance and Quench Suppression. Adv. Funct. Mater. 2018, 28, 1803193. [Google Scholar] [CrossRef]
- Li, W.; Li, J.; Liu, D.; Jin, Q. Simple Bipolar Host Materials for High-Efficiency Blue, Green, and White Phosphorescence OLEDs. ACS Appl. Mater. Interfaces 2016, 8, 22382. [Google Scholar] [CrossRef]
- Zhang, T.; Zhu, M.; Li, J.; Zhang, Y.; Wang, X. Bipolar host materials comprising carbazole, pyridine and triazole moieties for efficient and stable phosphorescent OLEDs. Dye Pigment. 2021, 192, 109426. [Google Scholar] [CrossRef]
- Jeon, S.K.; Thirupathaiah, B.; Kim, C.; Lim, K.T.; Lee, J.Y.; Seo, S.Y. Novel carbazole derivative as a host material for blue phosphorescent organic light-emitting diodes. Dye Pigment. 2015, 114, 146. [Google Scholar] [CrossRef]
- Seo, C.; Choi, J.M.; Hong, S.S.; Lee, J.Y.; Seo, S.Y. Synthesis of novel benzothiophene derivative as a host material for blue phosphorescent organic light-emitting diodes. Dye Pigment. 2017, 136, 145. [Google Scholar] [CrossRef]
- Mao, H.T.; Song, W.L.; Zang, C.X.; Li, G.F.; Shan, G.G.; Sun, H.Z.; Xie, W.F.; Su, Z.M. Manipulating charge carrier transporting of disubstituted phenylbenzoimidazole-based host materials for efficient full-color PhOLEDs. Org. Electron. 2020, 77, 105513. [Google Scholar] [CrossRef]
- Zhuang, J.; Li, W.; Su, W.; Zhou, M.; Cui, Z. Novel ternary bipolar host material with carbazole, triazole and phosphine oxide moieties for high efficiency sky-blue OLEDs. New J.Chem. 2014, 38, 650. [Google Scholar] [CrossRef]
- Fan, C.; Zhao, F.; Gan, P.; Yang, S.; Liu, T.; Zhong, C.; Ma, D.; Qin, J.; Yang, C. Simple Bipolar Molecules Constructed from Biphenyl Moieties as Host Materials for Deep-Blue Phosphorescent Organic Light-Emitting Diodes. Chem. Eur. J. 2012, 18, 5510. [Google Scholar] [CrossRef] [PubMed]
- Maheshwaran, A.; Sree, V.G.; Park, H.Y.; Kim, H.; Han, S.H.; Lee, J.Y.; Jin, S.H. High Efficiency Deep-Blue Phosphorescent Organic Light-Emitting Diodes with CIE x, y (≤0.15) and Low Efficiency Roll-Off by Employing a High Triplet Energy Bipolar Host Material. Adv. Funct. Mater. 2018, 28, 1802945. [Google Scholar] [CrossRef]
- Park, I.S.; Seo, H.; Tachibana, H.; Kim, J.U.; Zhang, J.; Son, S.M.; Yasuda, T. Cyclohexane-coupled bipolar host materials with high triplet energies for organic light-emitting diodes based on thermally activated delayed fluorescence. ACS Appl. Mater. Interfaces 2017, 9, 2693–2700. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.H.; Lee, J.; Lee, J.; Han, W.S. Silicon-based carbazole and oxadiazole hybrid as a bipolar host material for phosphorescent organic light-emitting diodes. Org. Electron. 2016, 38, 222. [Google Scholar] [CrossRef]
- Gohg, S.; Chen, Y.; Zhang, X.; Cai, P.; Zhong, C.; Ma, D.; Qin, J.; Yang, C.J. High-performance blue and green electrophosphorescence achieved by using carbazole-containing bipolar tetraarylsilanes as host materials. Mater. Chem. 2011, 21, 11197. [Google Scholar] [CrossRef]
- Yun, J.H.; Ha, J.S.; Lee, Y.; Kang, S.W.; Choo, C.; Lee, K.H.; Kim, J.M.; Lee, J.Y.; Jeon, S.O.; Bae, H.J.; et al. More than 25,000 h device lifetime in blue phosphorescent organic light-emitting diodes via fast triplet up-conversion of n-type hosts with sub μs triplet exciton lifetime. Chem. Eng. J. 2022, 450, 137974. [Google Scholar] [CrossRef]
- Ha, J.; Lim, J.; Lee, J.Y. A novel benzo[4,5]furo[3,2-d]pyrimidine-based host as a n-type host for blue phosphorescent organic light-emitting diodes. Sci. China Mater. 2022, 65, 1028. [Google Scholar] [CrossRef]
- Lin, C.C.; Huang, M.J.; Chiu, M.J.; Huang, M.P.; Chang, C.C.; Liao, C.Y.; Chiang, K.M.; Shiau, Y.J.; Chou, T.Y.; Chu, L.K.; et al. Molecular Design of Highly Efficient Thermally Activated Delayed Fluorescence Hosts for Blue Phosphorescent and Fluorescent Organic Light-Emitting Diodes. Chem. Mater. 2017, 29, 1527. [Google Scholar] [CrossRef]
- Hu, M.; Xu, Q.; Jiang, Y.; Mu, H.; Gao, L.; Hu, P.; Huang, J.; Su, J. Bipolar carbazole/quinoxaline-based host materials for efficient red PhOLEDs. Dye Pigment. 2018, 150, 185. [Google Scholar] [CrossRef]
- Peng, L.; Huo, Y.; He, S.; Liu, Y.; Ren, Z.; Ying, S.; Yan, S. A linear deep-blue bipolar fluorescent material with the CIEy < 0.065 serving as the emitter and host for high-performance monochromatic and hybrid white OLEDs. J. Mater. Chem. C 2022, 10, 11642. [Google Scholar] [CrossRef]
- Guo, S.; Zhou, H.; Wang, G.; Jin, X.; Zhang, Y.; Huang, J.; Cui, J.; Cao, J.; Miao, Y.; Wang, H.; et al. Novel benzonitrile- and benzo[d]imidazole-based bipolar hosts for green PhOLEDs with a low turn-on voltage. Dye Pigment. 2022, 200, 110041. [Google Scholar] [CrossRef]
- Jiang, W.; Duan, L.; Qiao, J.; Zhang, D.; Dong, G.; Wang, L.; Qiu, Y. Novel star-shaped host materials for highly efficient solution-processed phosphorescent organic light-emitting diodes. J. Mater. Chem. 2010, 20, 6131. [Google Scholar] [CrossRef]
- Wang, H.; Liub, Y.; Hu, W.; Xu, W.; Wang, P.; Wang, Y.; Luan, X. Novel spironaphthalenone-based host materials for efficient red phosphorescent and thermally activated delayed fluorescent OLEDs. Org. Electron. 2018, 61, 376. [Google Scholar] [CrossRef]
- Moon, B.J.; Yook, K.S.; Lee, J.Y.; Shin, S.; Hwang, S.H. Synthesis, photophysical and electro-optical properties of bis-carbazolyl methane based host material for pure-blue phosphorescent OLED. J. Luminance 2012, 132, 2557. [Google Scholar] [CrossRef]
- Chang, C.H.; Grinienė, R.; Su, Y.D.; Yeh, C.C.; Kao, H.C.; Grazulevicius, J.V.; Volyniuk, D.; Grigalevicius, S. Efficient red phosphorescent OLEDs employing carbazole-based materials as the emitting host. Dye Pigment. 2015, 122, 257. [Google Scholar] [CrossRef]
- Jeon, J.H.; Lee, N.J.; Lee, J.H.; Suh, M.C. Thermally-stable 2,3-diphenylated benzotiophene containing host materials for red phosphorescent organic light-emitting diodes. Dye Pigment. 2014, 111, 116. [Google Scholar] [CrossRef]
- Yoon, J.; Lee, C.; Park, S.H.; Kang, D.W.; Kim, H.; Jeong, J.E.; Woo, H.Y.; Hong, C.S.; Park, S.; Cho, M.J.; et al. Pyrimidine-based bipolar host materials for high efficiency solution processed green thermally activated delayed fluorescence OLEDs. J. Mater. Chem. C 2020, 8, 2196. [Google Scholar] [CrossRef]
- Zhang, D.; Cai, M.; Bin, Z.; Zhang, Y.; Zhang, D.; Duan, L. Highly efficient blue thermally activated delayed fluorescent OLEDs with record-low driving voltages utilizing high triplet energy hosts with small singlet–triplet splittings. Chem. Sci. 2016, 7, 3355. [Google Scholar] [CrossRef]
- Jeong, S.H.; Seo, C.W.; Lee, J.Y.; Cho, N.S.; Kim, J.K.; Yang, J.H. Comparison of Bipolar Hosts and Mixed-Hosts as Host Structures for Deep-Blue Phosphorescent Organic Light Emitting Diodes. Chem. Asian J. 2011, 6, 2895. [Google Scholar] [CrossRef]
- Fei, X.; Zhang, Y.J.; Liu, X.Y.; Fung, M.K.; Fan, J. A series of fluorenone-carbazole based regioisomers as bipolar host materials for efficient organic light emitting diodes. Tertahedron 2019, 75, 2664. [Google Scholar] [CrossRef]
- Jung, J.W.; Lee, K.M.; Kim, O.Y.; Lee, J.Y.; Hwang, S.H. Synthesis of a dibenzothiophene/carboline/carbazole hybrid bipolar host material for green phosphorescent OLEDs. Synth. Met. 2016, 213, 7. [Google Scholar] [CrossRef]
- Song, W.; Su, J. [1,2,4]Triazolo[1,5-a]pyridine based host materials for high-performance red PhOLEDs with external quantum efficiencies over 23%. J. Luminescence 2019, 206, 386. [Google Scholar] [CrossRef]
- Jeon, I.M.; Lee, I.H.; Lee, H.S.; Gong, M.S. Orange phosphorescent organic light-emitting diodes based on spirobenzofluorene type carbazole derivatives as a host material. Dye Pigment. 2011, 89, 29. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, Y.; Shi, N.; Li, X.; Zhao, Y.; Sun, M.; Ding, D.; Xu, H.; Xie, L. Carbazole-endcapped Spiro[fluorene-9,9′-xanthene] with Large Steric Hindrance as Hole-transporting Host for Heavily-doped and High Performance OLEDs. Chin. J. Chem. 2015, 33, 955. [Google Scholar] [CrossRef]
- Quinton, C.; Thiery, S.; Jeannin, O.; Tondelier, D.; Geffroy, B.; Jacques, E.; Rault-Berthelot, J.; Poriel, C. Electron-Rich 4-Substituted Spirobifluorenes: Toward a New Family of High Triplet Energy Host Materials for High-Efficiency Green and Sky Blue Phosphorescent OLEDs. ACS Appl. Mater. Interfaces 2017, 9, 6194. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Zhang, L.; Cui, L.S.; Gao, C.H.; Chen, H.; Li, Q.; Jiang, Z.Q.; Liao, L.S. Control of Conjugation Degree via Position Engineering to Highly Efficient Phosphorescent Host Materials. Org. Lett. 2014, 16, 3748. [Google Scholar] [CrossRef]
- Sun, B.; Tong, K.N.; Liu, S.N.; Fung, M.K.; Fan, J. A series of novel host materials based on the 10,11-dihydro-5H-dibenzo[b,f]azepine unit for highly efficient green and red organic light-emitting diodes. J. Mater. Chem. C 2021, 9, 2969. [Google Scholar] [CrossRef]
- Kim, J.; Kim, S.H.; Lee, H.W.; Lee, S.E.; Kim, Y.K.; Yoon, S.S. Bipolar Host Materials with Carbazole and Isoquinoline/Benzothiazole Moieties for Green Phosphorescent Organic Light-Emitting Diodes. J. Nanosci. Nanotechnol. 2016, 16, 10792. [Google Scholar] [CrossRef]
- Zhang, Z.; Xie, J.; Wang, Z.; Shen, B.; Wang, H.; Li, M.; Zhanga, J.; Cao, J. Manipulation of electron deficiency of δ-carboline derivatives as bipolar hosts for blue phosphorescent organic light-emitting diodes with high efficiency at 1000 cd m−2. J. Mater. Chem. C 2016, 4, 4226. [Google Scholar] [CrossRef]
- Wang, Z.; Zhu, J.; Liu, Z.; Wu, P.; Wang, H.; Zhanga, Z.; Wei, B. Thermally activated delayed fluorescence of co-deposited copper(i) complexes: Cost-effective emitters for highly efficient organic light-emitting diodes. J. Mater. Chem. C 2017, 5, 6982. [Google Scholar] [CrossRef]
- Im, Y.; Lee, J.Y. Effect of the position of nitrogen in pyridoindole on photophysical properties and device performances of α-, β-, γ-carboline based high triplet energy host materials for deep blue devices. Chem. Commun. 2013, 49, 5948. [Google Scholar] [CrossRef]
- Li, J.; Dong, S.C.; Opitz, A.; Liao, L.S.; Koch, N. Design principles of carbazole/dibenzothiophene derivatives as host material in modern efficient organic light-emitting diodes. J. Mater. Chem. C 2017, 5, 6989. [Google Scholar] [CrossRef]
- Godumala, M.; Yoon, J.; Jeong, C.H.; Lee, C.; Jeong, J.E.; Park, S.; Woo, H.Y.; Cho, M.J.; Choi, D.H. An excellent bipolar host material exhibiting EQE of 24.0% with small efficiency roll-off in solution-processable thermally activated delayed fluorescence OLEDs. J. Mater. Chem. C 2019, 7, 13930. [Google Scholar] [CrossRef]
- Wang, S.; Lee, C.W.; Lee, J.Y.; Hwang, S.H. Synthesis and green phosphorescent OLED device performance of cyanofluorene-linked phenylcarbazoles as host material. J. Chem. 2018, 42, 5059. [Google Scholar] [CrossRef]
- Guichen, Y.; Yin, M.; Zhu, J.; Zhou, H.; Miao, Y.; Huang, J.; Wang, H.; Su, J. Novel difluorenyl substituted 1,3,5-triazine and carbazole based bipolar host materials with high thermal stability for efficient green phosphorescent organic light-emitting diodes (PhOLEDs). Tetrahedron 2021, 90, 132175. [Google Scholar] [CrossRef]
- Seo, J.A.; Jeon, S.K.; Lee, J.Y. Acridine derived stable host material for long lifetime blue phosphorescent organic light-emitting diodes. Org. Electron. 2016, 34, 33. [Google Scholar] [CrossRef]
- Grigalevicius, S.; Tavgeniene, D.; Krucaite, G.; Blazevicius, D.; Griniene, R.; Lai, Y.N.; Chiu, H.H.; Chang, C.H. Efficient blue and green phosphorescent OLEDs with host material containing electronically isolated carbazolyl fragments. Opt. Mater. 2018, 79, 446. [Google Scholar] [CrossRef]
- Liang, X.; Liu, Z.; Xia, Y.; Li, D.; Li, J.; Wang, H.; Zhang, Z.; Wang, S.; Zhao, B.; Li, Z.; et al. Lifting Triplet Energy and Bipolar Characteristics by Limiting theRotation of the Peripheral Groups in Host Materials to Achieve High-Efficiency Blue OLED. Chem. Asian J. 2022, 17, 1. [Google Scholar] [CrossRef]
- Xie, G.; Wang, J.; Cao, Y.; Xue, X.; Zhang, X.; Liu, C.; Li, H.; Tao, Y.; Chen, R. Phosphine Sulfide-Based Bipolar Host Materials for Blue Phosphorescent Organic Light-Emitting Diodes. Molecules 2021, 26, 4079. [Google Scholar] [CrossRef]
- Zhao, Z.; Yu, G.; Chang, Q.; Liu, X.; Liu, Y.; Wang, L.; Liu, Z.; Bian, Z.; Liu, W.; Huang, C. Carbazolylphosphines and carbazolylphosphine oxides: Facilely synthesized host materials with tunable mobilities and high triplet energy levels for blue phosphorescent OLEDs. J. Mater. Chem. C 2017, 5, 7344. [Google Scholar] [CrossRef]
- Cho, Y.J.; Lee, J.Y. Modified N,N′-Dicarbazolyl-3,5-benzene as a High Triplet Energy Host Material for Deep-Blue Phosphorescent Organic Light-Emitting Diodes. Chem. Eur. J. 2011, 17, 11415. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Fan, X.; Han, C.; Xu, H. A ternary phosphine oxide host featuring thermally activated delayed fluorescence for blue PHOLEDs with >20% EQE and extremely low roll-offs. J. Mater. Chem. C 2018, 6, 6747. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, G.; Guo, S.; Jin, X.; Luo, X.; Miao, Y.; Huang, J.; Wang, H.; Su, J. A universal bipolar host based on isonicotinonitrile and carbazole for efficient red, green and blue PhOLEDs. New J. Chem. 2022, 46, 15344. [Google Scholar] [CrossRef]
- Cho, Y.J.; Yook, K.S.; Lee, J.Y. A Universal Host Material for High External Quantum Efficiency Close to 25% and Long Lifetime in Green Fluorescent and Phosphorescent OLEDs. Adv. Mater. 2014, 26, 4050. [Google Scholar] [CrossRef]
- Lee, D.R.; Lim, J.; Lee, J.Y. Phthalonitrile based charge transfer type host for yellow phosphorescent organic light-emitting diodes. Org. Electron. 2021, 94, 106166. [Google Scholar] [CrossRef]
- Li, S.W.; Yu, C.H.; Ko, C.L.; Chatterjee, T.; Hung, W.Y.; Wong, K.T. Cyanopyrimidine-Carbazole Hybrid Host Materials for High-Efficiency and Low-Efficiency Roll-Off TADF OLEDs. ACS Appl. Mater. Interfaces 2018, 10, 12930. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Jeon, S.K.; Lee, J.Y. Molecular design approach of increasing the triplet energy of host materials using pyrrole as a core structure. RCS. Adv. 2015, 5, 100378. [Google Scholar] [CrossRef]
- Lu, C.W.; Tsai, C.C.; Li, W.C.; Chiu, T.Y. Triarylboryl-substituted carbazoles as bipolar host materials for efficient green phosphorescent organic light-emitting devices. Dye Pigment. 2019, 163, 145. [Google Scholar] [CrossRef]
- Choi, H.J.; Kim, B.S.; Kim, O.; Chin, B.D.; Lee, C.W. The Correlation between Structure and Physical Properties of Soluble Host for Blue Phosphorescent OLED. Korean Phys. Soc. 2019, 74, 1176. [Google Scholar]
- Lee, C.W.; Lee, J.Y. High quantum efficiency and color stability in white phosphorescent organic light emitting diodes using a pyridine modified carbazole derivative. Dye Pigment. 2014, 103, 34. [Google Scholar] [CrossRef]
- Song, W.; Shi, L.; Gao, L.; Hu, P.; Mu, H.; Xia, Z.; Huang, J.; Su, J. [1,2,4]Triazolo[1,5-a]pyridine as Building Blocks for Universal Host Materials for High-Performance Red, Green, Blue and White Phosphorescent Organic Light-Emitting Devices. ACS Appl. Mater. Interface 2018, 10, 5714. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.J.; Hung, Y.H.; Ting, P.L.; Tsai, Y.N.; Gao, H.J.; Chiu, T.L.; Lee, J.H.; Chen, C.L.; Chou, P.T.; Leung, M.-T. Orthogonally Substituted Benzimidazole-Carbazole Benzene As Universal Hosts for Phosphorescent Organic Light-Emitting Diodes. Org. Lett. 2016, 18, 672. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; He, W.Z.; Liao, H.S.; Hu, Y.X.; Xie, D.D.; Wang, B.Y.; Chi, H.J.; Lv, Y.L.; Zhu, X.; Li, X. Benzimidazole/carbazole-based bipolar host materials for highly efficient green phosphorescent OLEDs with negligible efficiency roll-off. Org. Electron. 2023, 113, 106715. [Google Scholar] [CrossRef]
- Reddy, M.R.; Han, S.H.; Lee, J.Y.; Seo, S.Y. Synthesis and characterization of quinoxaline derivative for high performance phosphorescent organic light-emitting diodes. Dye Pigment. 2018, 153, 132. [Google Scholar] [CrossRef]
- Chen, Z.; Li, H.; Tao, Y.; Chen, L.; Chen, C.; Jiang, H.; Xu, S.; Zhou, X.; Chen, R.; Huang, W. Tuning Intramolecular Conformation and Packing Mode of Host Materials through Noncovalent Interactions for High-Efficiency Blue Electrophosphorescence. ACS Omega. 2019, 4, 9129. [Google Scholar] [CrossRef]
- Hudson, Z.M.; Michael, Z.W.; Helander, H.; Lu, H.Z.; Wang, S. N -Heterocyclic Carbazole-Based Hosts for Simplifi ed Single-Layer Phosphorescent OLEDs with High Effi ciencies. Adv. Mater. 2012, 24, 2922. [Google Scholar] [CrossRef]
- Zhong, Q.; Zeng, S.; Fan, P.; Pang, Y.; Zhu, W.; Wang, Y. Effective bipolar hosts prepared via dipole moment engineering for phosphorescent emitters and white OLEDs. J. Mater. Chem. C 2022, 10, 18415. [Google Scholar] [CrossRef]
- Byeon, S.Y.; Choi, J.M.; Lee, J.Y. Pyridoindole based intramolecular charge transfer type host material for blue phosphorescent organic light-emitting diodes. Dye Pigment. 2016, 134, 285. [Google Scholar] [CrossRef]
- Tao, Y.; Guo, X.; Hao, L.; Chen, R.; Li, H.; Chen, Y.; Zhang, X.; Lai, W.; Huang, W. A Solution-Processed Resonance Host for Highly Efficient Electrophosphorescent Devices with Extremely Low Efficiency Roll-off. Adv. Mater. 2015, 27, 6939. [Google Scholar] [CrossRef]
- Huang, J.J.; Yun, L.K.; Kung, T.J.; Chen, C.L.; Lee, J.H.; Wu, Y.R.; Chiu, T.L.; Chou, P.T.; Leung, M. Networking hole and electron hopping paths by Y-shaped host molecules: Promoting blue phosphorescent organic light emitting diodes. J. Mater. Chem. C 2017, 5, 3600. [Google Scholar] [CrossRef]
- Wen, H.Y.; Ho, S.Y. A bipolar host material for the construction of triplet-energy level for white phosphorescent organic light emitting diodes. RSC Adv. 2022, 12, 28128. [Google Scholar] [CrossRef]
- Yao, C.; Cui, Q.; Peng, J.; Xu, X.; Liu, R.; Wang, J.; Tian, Y.; Li, L. Solution processed blue phosphorescent organic light emitting diodes using a Ge-based small molecular host. J. Mater. Chem. C 2015, 3, 5017. [Google Scholar] [CrossRef]
- Choi, S.; Godumala, M.; Lee, J.H.; Kim, G.H.; Moon, J.S.; Kim, J.Y.; Yoon, D.W.; Yang, J.H.; Kim, J.; Cho, M.J.; et al. Optimized structure of silane-core containing host materials for highly efficient blue TADF OLEDs. J. Mater. Chem. C 2017, 5, 6570. [Google Scholar] [CrossRef]
- Yun, J.H.; Lim, J.; Chung, W.J.; Lee, J.Y. Exciton stabilizing high triplet energy n-type hosts for blue phosphorescent organic light-emitting diodes. Dye Pigment. 2021, 190, 109297. [Google Scholar] [CrossRef]
- Choi, S.; Yoon, J.W.; Godumala, M.; Kim, H.J.; Park, S.H.; Kim, S.K.; Lee, H.; Kwon, J.H.; Cho, M.J.; Choi, D.H. 2D-σ-2A type cruciform host material with silane core for highly efficient solution-processable green thermally activated delayed fluorescence organic light emitting diodes. Dye Pigment. 2019, 167, 120. [Google Scholar] [CrossRef]
- Liu, H.; Bai, Q.; Yao, L.; Hu, D.; Tang, X.; Shen, F.; Zhang, H.; Gao, Y.; Lu, P.; Yang, B.; et al. Solution-Processable Hosts Constructed by Carbazole/PO Substituted Tetraphenylsilanes for Efficient Blue Electrophosphorescent Devices. Adv. Funct. Mater. 2014, 24, 5881. [Google Scholar] [CrossRef]
- Chou, H.H.; Cheng, C.H. A highly efficient universal bipolar host for blue, green, and red phosphorescent OLEDs. Adv. Mater. 2010, 22, 2468. [Google Scholar] [CrossRef]
- Gudeika, D.; Bezvikonnyi, O.; Masimukku, N.; Volyniuk, D.; Chen, C.H.; Ding, W.C.; Lee, J.H.; Chiu, T.L.; Grazulevicius, J.V. Tetraphenyl ornamented carbazolyl disubstituted diphenyl sulfone as bipolar TADF host for highly efficient OLEDs with low efficiency roll-offs. Dye Pigment. 2021, 194, 109573. [Google Scholar] [CrossRef]
- Yan, X.; Cheng, Z.; Yang, T.; Li, C.; Wang, Y. Carbazole-benzonitrile based organic semiconductors: Synthesis, characterization and electroluminescent property. Org. Electron. 2022, 102, 106445. [Google Scholar] [CrossRef]
- Li, W.; Li, J.; Liu, D.; Wang, F.; Zhang, S. Bipolar host materials for high-efficiency blue phosphorescent and delayed-fluorescence OLEDs. J. Mater. Chem. C 2015, 3, 12529. [Google Scholar] [CrossRef]
- Su, S.J.; Sasabe, H.; Takeda, T.; Kido, J. Pyridine-Containing Bipolar Host Materials for Highly Efficient Blue Phosphorescent OLEDs. Chem. Mater. 2008, 20, 1691. [Google Scholar] [CrossRef]
- Yu, J.G.; Han, S.H.; Jeon, H.R.; Chung, H.K.; Lee, J.Y. Pyridazine derived bipolar host materials for phosphorescent organic light-emitting diodes. J. Luminescence 2018, 194, 33. [Google Scholar] [CrossRef]
- Liu, D.; Li, D.; Wang, M.; Li, W. 1,2,4-Triazole-containing bipolar hosts for blue and green phosphorescent organic light-emitting diodes. J. Mater. Chem. C 2016, 4, 7260. [Google Scholar] [CrossRef]
- Wang, Y.; Song, W.; Chen, Y.; Jiang, Y.; Mu, H.; Huang, J.; Su, J. A series of new bipolar CBP derivatives with introduction of a electron-deficient moiety for efficient green organic light-emitting diodes. Org. Electron. 2018, 61, 142. [Google Scholar] [CrossRef]
- Jang, H.G.; Soon, W.; Lee, J.Y.; Heang, S.H. Synthesis of pyrimidine-cored host materials bearing phenylcarbazole for efficient yellow phosphorescent devices: Effect of linkage position. RSC Adv. 2015, 5, 17030. [Google Scholar] [CrossRef]
- Zhuang, J.; Su, W.; Li, W.; Zhou, Y.; Shen, Q.; Zhou, M. Configuration effect of novel bipolar triazole/carbazole-based host materials on the performance of phosphorescent OLED devices. Org. Electron. 2012, 13, 2210. [Google Scholar] [CrossRef]
- Song, W.; Chen, Y.; Xu, Q.; Mu, H.; Cao, J.; Huang, J.; Su, J. [1,2,4]Triazolo[1,5-a]pyridine-Based Host Materials for Green Phosphorescent and Delayed-Fluorescence OLEDs with Low Efficiency Roll-Off. ACS Appl. Mater. Interfaces 2018, 10, 24689. [Google Scholar] [CrossRef]
- Gong, S.; Zhao, Y.; Yang, C.; Zhong, C.; Qin, J.; Ma, D. Tuning the Photophysical Properties and Energy Levels by Linking Spacer and Topology between the Benzimidazole and Carbazole Units: Bipolar Host for Highly Efficient Phosphorescent OLEDs. J. Phys. Chem. C 2010, 114, 5193. [Google Scholar] [CrossRef]
- Li, Q.; Cui, L.S.; Zhong, C.; Yuan, X.D.; Dong, S.H.; Jiang, Z.Q.; Liao, L.S. Synthesis of new bipolar host materials based on 1,2,4-oxadiazole for blue phosphorescent OLEDs. Dye Pigment. 2014, 101, 142. [Google Scholar] [CrossRef]
- Li, Q.; Cui, L.S.; Zhong, C.; Jiang, Z.Q.; Liao, L.S. Asymmetric Design of Bipolar Host Materials with Novel 1,2,4-Oxadiazole Unit in Blue Phosphorescent Device. Org. Lett. 2014, 16, 1622. [Google Scholar] [CrossRef]
- Tao, Y.; Wang, Q.; Yang, C.; Zhong, C.; Zhang, K.; Qin, J.; Ma, D. Tuning the Optoelectronic Properties of Carbazole/Oxadiazole Hybrids through Linkage Modes: Hosts for Highly Efficient Green Electrophosphorescence. Adv. Funct. Mater. 2010, 20, 304. [Google Scholar] [CrossRef]
- Kaunty, P.; Wu, Z.; Stoer, B.; Tissot, A.; Horkel, E.; Chen, J.; Ma, D.; Hagemann, H.; Frohlich, J.; Lumpi, D. Controlling singlet–triplet splitting in carbazole–oxadiazole based bipolar phosphorescent host materials. Org. Electron. 2015, 17, 216. [Google Scholar] [CrossRef]
- Tsik, U.; Volyniuk, D.; Andruleviciene, V.; Leitonas, K.; Sych, G.; Bezvikonnyi, O.; Jasinskas, V.; Gulbinas, V.; Stakhira, P.; Grazulevicius, J.V. Triphenylamino or 9-phenyl carbazolyl-substituted pyrimidine-5-carbonitriles as bipolar emitters and hosts with triplet harvesting abilities. Mater. Today. Chem. 2022, 25, 100955. [Google Scholar] [CrossRef]
- Yao, C.; Peng, C.; Yang, Y.; Li, L.; Bo, M.; Wang, J. Larger VH (Hole Distribution Volume)/VM (Molecular Volume) Induced Higher Charge Mobility of Group IVA Element-Based Host Materials for Potentially Highly Efficient Blue OLEDs. J. Phys. Chem. C 2018, 122, 22273. [Google Scholar] [CrossRef]
- Kim, D.; Coropceanu, V.; Bredas, J.L. Design of Efficient Ambipolar Host Materials for Organic Blue Electrophosphorescence: Theoretical Characterization of Hosts Based on Carbazole Derivatives. J. Am. Chem. Soc. 2011, 133, 17895. [Google Scholar] [CrossRef]
- Seo, J.; Park, S.R.; Kim, M.; Suh, M.C.; Lee, J. The role of electron-transporting Benzo[f]quinoline unit as an electron acceptor of new bipolar hosts for green PHOLEDs. Dye Pigment. 2019, 162, 959. [Google Scholar] [CrossRef]
- Cui, L.S.; Kim, J.U.; Nomura, H.; Nakanotani, H.; Adachi, C. Benzimidazobenzothiazole-Based Bipolar Hosts to Harvest Nearly All of the Excitons from Blue Delayed Fluorescence and Phosphorescent Organic Light-Emitting Diodes. Angew.Chem. Int. Ed. 2016, 55, 6864. [Google Scholar] [CrossRef]
- Cha, J.R.; Lee, C.W.; Gong, M.S. Bipolar Host Material for Phosphorescent OLEDs Based on 2,7-Diazacarbazole as a New Electron-transporting Unit. Bull. Korean Chem. Soc. 2017, 38, 1016. [Google Scholar] [CrossRef]
- Cho, M.J.; Kim, S.J.; Yoon, S.H.; Shin, J.; Hong, T.R.; Kim, H.J.; Son, Y.H.; Kang, J.S.; Um, H.A.; Lee, T.W.; et al. New Bipolar Host Materials for Realizing Blue Phosphorescent Organic Light-Emitting Diodes with High Efficiency at 1000 cd/m2. ACS Appl. Mater. Interfaces 2014, 6, 19808. [Google Scholar] [CrossRef]
- Kang, J.K.; Hong, T.R.; Kim, H.J.; Son, Y.H.; Bin, J.K.; Lee, B.S.; Yang, J.H.; Kim, J.W.; Cho, M.J.; Kwon, J.H.; et al. Molecular design of large-bandgap host materials and their application to blue phosphorescent organic light-emitting diodes. Org. Electron. 2015, 26, 218. [Google Scholar] [CrossRef]
- Moon, J.S.; Ahn, D.H.; Kim, S.W.; Lee, S.Y.; Lee, J.Y.; Kwon, J.H. δ-Carboline-based bipolar host materials for deep blue thermally activated delayed fluorescence OLEDs with high efficiency and low roll-off characteristic. RSC Adv. 2018, 8, 17025. [Google Scholar] [CrossRef]
- Kang, J.S.; Hong, T.R.; Kim, H.J.; Son, Y.H.; Lampande, R.; Kang, B.Y.; Lee, C.; Bin, J.K.; Lee, B.S.; Yang, J.H.; et al. High-performance bipolar host materials for blue TADF devices with excellent external quantum efficiencies. J. Mater. Chem. C 2016, 4, 4512. [Google Scholar] [CrossRef]
- Lin, J.-S.; Lee, M.-T.; Chu, M.-T.; Tseng, M.-R. P-221L: Late-News Poster: New Blue Phosphorescent Host for High-efficiency White OLED. SID Int. Symp. Dig. Tech. Pap. 2011, 42, 1787. [Google Scholar] [CrossRef]
- Liu, K.; Li, X.L.; Liu, M.; Chen, D.; Cai, X.; Wu, Y.C.; Lo, C.C.; Lien, A.; Caoa, Y.; Su, S.J. 9,9-Diphenyl-thioxanthene derivatives as host materials for highly efficient blue phosphorescent organic light-emitting diodes. J. Mater. Chem. C 2015, 3, 9999. [Google Scholar] [CrossRef]
- Choi, K.H.; Kim, J.M.; Chung, W.J.; Lee, J.Y. Effects of Substitution Position of Carbazole-Dibenzofuran Based High Triplet Energy Hosts to Device Stability of Blue Phosphorescent Organic Light-Emitting Diodes. Molecules 2021, 26, 2804. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.J.; Jeon, S.K.; Lee, J.Y. A zig-zag type bidibenzofuran based host material for green phosphorescent organic light-emitting diodes. Dye Pigment. 2015, 114, 278. [Google Scholar] [CrossRef]
- Jiang, H.J.; Sun, J.; Yuan, K.; Zhang, Q.W. Synthesis and characterization of novel topology-varied compounds based on fluorene and carbazole: Potential host materials for phosphorescent organic light-emitting diodes. Synth. Met. 2014, 197, 217. [Google Scholar] [CrossRef]
- Li, G.; Zheng, J.; Klimes, K.; Zhu, Z.Q.; Wu, J.; Zhu, H.; Li, J. Novel Carbazole/Fluorene-Based Host Material for Stable and Efficient Phosphorescent OLEDs. ACS Appl. Mater. Interfaces 2019, 11, 40320. [Google Scholar] [CrossRef]
- Yu, Z.; Zhang, J.; Liu, S.; Zhang, L.; Zhao, Y.; Zhao, H.; Xie, W. High-Efficiency Blue Phosphorescent Organic Light-Emitting Devices with Low Efficiency Roll-Off at Ultrahigh Luminance by the Reduction of Triplet-Polaron Quenching. ACS Appl. Mater. Interfaces 2019, 11, 6292. [Google Scholar] [CrossRef]
- Lin, W.C.; Huang, W.C.; Huang, M.H.; Fan, C.C.; Lin, H.W.; Chen, L.Y.; Liu, Y.W.; Lin, J.S.; Chao, T.C.; Tseng, M.R. A bipolar host containing carbazole/dibenzothiophene for efficient solution-processed blue and white phosphorescent OLEDs. J. Mater. Chem. 2013, C 1, 6835. [Google Scholar]
- Bucinskas, A.; Bezvikonnyi, O.; Gudeika, D.; Volyniuk, D.; Grazulevicius, J.V. Methoxycarbazolyl-disubstituted dibenzofuranes as holes- and electronstransporting hosts for phosphorescent and TADF-based OLEDs. Dye Pigment. 2020, 172, 107781. [Google Scholar] [CrossRef]
- Keruckas, J.; Volyniuk, D.; Simokaitiene, J.; Narbutaitis, E.; Lazauskas, A.; Lee, P.H.; Chiu, T.L.; Lin, C.F.; Arsenyan, P.; Lee, J.H.; et al. Methoxy- and tert-butyl-substituted meta-bis(N-carbazolyl)phenylenes as hosts for organic light-emitting diodes. Org. Electron. 2019, 73, 317. [Google Scholar] [CrossRef]
- Tang, C.; Chen, J.; Li, Y.; Liu, X.; Zhang, L.; Wang, F.; Cao, X.; Jiang, T. Alkyl-Substituted Carbazole/Pyridine Hybrid Host Materials for Efficient Solution-Processable Blue- and Green-Emitting Phosphorescent OLEDs. Electron. Mater. Lett. 2021, 17, 148. [Google Scholar]
- Song, W.; Lee, H.L.; Lee, J.Y. High triplet energy exciplex hosts for deep blue phosphorescent organic light-emitting diodes. J. Mater. Chem. C 2017, 5, 5923. [Google Scholar] [CrossRef]
- Pei, J.; Du, X.; Li, C.; Wang, C.; Fan, C.; Tan, H.; Cao, B.; Huang, F.; Tao, S.; Li, J. Highly twisted organic molecules with ortho linkage as the efficient bipolar hosts for sky-blue thermally activated delayed fluorescence emitter in OLEDs. Org. Electron. 2017, 50, 153. [Google Scholar] [CrossRef]
- Hladka, I.; Lytvyn, R.; Volyniuk, D.; Gudeika, D.; Grazulevicius, J.V. W-shaped bipolar derivatives of carbazole and oxadiazole with high triplet energies for electroluminescent devices. Dye Pigment. 2018, 149, 812. [Google Scholar] [CrossRef]
- Tang, J.; Chen, Y.; Cong, L.; Lin, B.; Sun, Y. Novel tri-carbazole modified fluorene host material for highly efficient solution-processed blue and green electrophosphorescent devices. Tetrahedron 2014, 70, 3847. [Google Scholar] [CrossRef]
- Li, H.; Xu, L.; Tang, Y.; Tao, Y.; Xu, S.; Zheng, C.; Xing, G.; Zhou, X.; Huang, W.; Chen, R. Direct silicon–nitrogen bonded host materials with enhanced σ–π conjugation for blue phosphorescent organic light-emitting diodes. J. Mater. Chem. C 2016, 4, 10047. [Google Scholar] [CrossRef]
- Deng, L.; Wang, X.; Zhang, Z.; Li, J. Durene-decorated CBP derivatives as phosphorescent hosts and exciton-blocking materials for efficient blue OLEDs. J. Mater. Chem. 2012, 22, 19700. [Google Scholar] [CrossRef]
- Oner, S.; Oner, I.; Akdag, H.; Varlikli, C. Tetraphenylsilane group containing carbazoles as high triplet energy host materials for solution-processable PhOLEDs. Turk. J. Chem. 2015, 39, 917. [Google Scholar] [CrossRef]
- Hung, Y.T.; Chen, Z.Y.; Hung, W.Y.; Chen, D.G.; Wong, K.T. Exciplex Cohosts Employing Nonconjugated Linked Dicarbazole Donors for Highly Efficient Thermally Activated Delayed Fluorescence-Based Organic Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2018, 10, 34435. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Jiang, H.; Yang, Q.; Xiang, Y.; Zhang, Y.; Dai, Y.; Li, P.; Zheng, C.; Xie, G.; Chen, R. A solution-processable wholly-aromatic bipolar host material for highly efficient blue electroluminescent devices. J. Mater. Chem. C 2021, 9, 687. [Google Scholar] [CrossRef]
- Cai, M.; Zhang, D.; Xu, J.; Hong, X.; Zhao, C.; Song, X.; Qiu, Y.; Kaji, H.; Duan, L. Unveiling the Role of Langevin and Trap-Assisted Recombination in Long Lifespan OLEDs Employing Thermally Activated Delayed Fluorophores. ACS Appl. Mater. Interfaces 2019, 11, 1096. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.W.; Lee, J.Y.; Hwang, S.H. Synthesis of phenylcarbazole–thiophene-based structural isomers as unipolar host materials for blue PHOLEDs and their device performance. Org. Electron. 2014, 15, 1413. [Google Scholar] [CrossRef]
- Jia, B.; Lian, H.; Sun, T.; Wei, J.; Yang, J.; Zhou, H.; Huang, J.; Dong, Q. New bipolar host materials based on methyl substituted pyridazine for high-performance green and red phosphorescent OLEDs. Dye Pigment. 2019, 168, 212. [Google Scholar] [CrossRef]
- Macdonald, J.; O’Connell, J.; Weber, K.; Hirai, T.; Groarke, M.; Andresen, S.; Bown, M.; Ueno, K. A New Class of Host Materials for Blue Phosphorescent Organic Electroluminescent Devices; SID Symposium Digest of Technical Papers; Wiley: Hoboken, NJ, USA, 2012; DIGEST 447. [Google Scholar]
- Tian, G.; Wei, X.; Xiang, N.; Huang, J.; Cao, J.; Wang, Z.; Zhang, J.; Su, J. Small organic molecules based on oxazole/thiazole with excellent performances in green and red phosphorescent organic light-emitting diodes. RSC Adv. 2016, 6, 51575. [Google Scholar] [CrossRef]
- Wang, F.; Zhao, Y.; Xu, H.; Zhang, J.; Miao, Y.; Guo, K.; Shinar, R.; Shinar, J.; Wang, H.; Xu, B. Two novel bipolar hosts based on 1,2,4-triazole derivatives for highly efficient red phosphorescent OLEDs showing a small efficiency roll-off. Org. Electron. 2019, 70, 272. [Google Scholar] [CrossRef]
- Sun, J.; Jiang, H.J.; Zhang, J.L.; Tao, Y.; Chen, R.F. Synthesis and characterization of heteroatom substituted carbazole derivatives: Potential host materials for phosphorescent organic light-emitting diodes. J. Chem. 2013, 37, 977. [Google Scholar] [CrossRef]
- Vadagaonkar, K.S.; Yang, C.J.; Zeng, W.H.; Chen, J.H.; Patil, B.N.; Chetti, P.; Chen, L.Y.; Chaskar, A.C. Triazolopyridine hybrids as bipolar host materials for green phosphorescent organic light-emitting diodes (OLEDs). Dye Pigment. 2019, 160, 301. [Google Scholar] [CrossRef]
- Jang, H.G.; Kim, B.S.; Lee, J.Y.; Hwang, S.H. Synthesis of dimesitylborane-substituted phenylcarbazoles as bipolar host materials and the variation of the green PHOLED performance with the substituent position of the boron atom. Dalton. Trans. 2014, 43, 7712. [Google Scholar] [CrossRef]
- Li, W.; Li, J.; Wang, F.; Gao, Z.; Zhang, S. Universal Host Materials for High-Efficiency Phosphorescent and Delayed-Fluorescence OLEDs. ACS Appl. Mater. Interface 2015, 7, 26206. [Google Scholar] [CrossRef]
- Sun, M.; Zhu, L.; Kan, W.; Wei, Y.; Ma, D.; Fan, X.; Huang, W.; Xu, H. Ternary donor–acceptor phosphine oxide hosts with peculiar high energy gap for efficient blue electroluminescence. J. Mater. Chem. C 2015, 3, 9469. [Google Scholar] [CrossRef]
- Patil, B.N.; Lade, J.J.; Vadagaonkar, K.S.; Chetti, P.; Chaskar, A.C. Pyrrolo[1, 2-a]quinoxaline-Based Bipolar Host Materials for Efficient Red Phosphorescent OLEDs. Chem. Select. 2018, 3, 10010. [Google Scholar] [CrossRef]
- Yang, T.; Xu, H.; Wang, K.; Tao, P.; Wang, F.; Zhao, B.; Wang, H.; Xu, B. Bipolar host materials based on diphenylphosphine oxide and carbazole derivatives with high triplet energy: Synthesis, characterization and photoelectronic performance in PhOLEDs. Dye Pigment. 2018, 153, 67. [Google Scholar] [CrossRef]
- Huang, H.; Yang, X.; Pan, B.; Wang, L.; Chen, J.; Ma, D.; Yang, C. Benzimidazole–carbazole-based bipolar hosts for high efficiency blue and white electrophosphorescence applications. J. Mater. Chem. 2012, 22, 13223. [Google Scholar] [CrossRef]
- Huixia, X.; Fang, W.; Kexiang, W.; Yanqin, M.; Jie, L.; Jing, Z.; Hua, W.; Yuying, H.; Bingshe, X. Three acceptors based bipolar materials with tunable excited state natures and applications as non-doped blue emitters and hosts in OLEDs. Dye Pigment. 2018, 155, 84. [Google Scholar] [CrossRef]
- Dong, Q.; Tai, F.; Lian, H.; Chen, Z.; Hu, M.; Huang, J.; Wong, W.Y. Thermally stable bipolar host materials for high efficiency phosphorescent green and blue organic light-emitting diodes. Dye Pigment. 2017, 143, 470. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, E.K.; Deeb, I.M.E.; Jung, S.J.; Choi, D.H.; Kim, D.H.; Yoo, K.H.; Kwon, J.H.; Lee, S.H. New Bipolar Green Host Materials Containing Benzimidazole-Carbazole Moiety in Phosphorescent OLEDs. Bull. Korean Chem. Soc. 2011, 32, 3841. [Google Scholar] [CrossRef][Green Version]
- Byeon, S.Y.; Kim, J.H.; Lee, J.Y. CN-Modified Host Materials for Improved Efficiency and Lifetime in Blue Phosphorescent and Thermally Activated Delayed Fluorescent Organic Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2017, 9, 13339. [Google Scholar] [CrossRef]
- Jia, B.; Lian, H.; Sun, T.; Guo, H.; Cheng, X.; Wu, J.; Chen, Y.; Dong, Q.; Huang, J. Efficient green phosphorescent organic light-emitting diodes enabled with new and thermally stable carbazole/pyridine derivatives as hosts. Dye Pigment. 2018, 159, 298. [Google Scholar] [CrossRef]
- Kim, S.M.; Yun, J.H.; Byeon, S.Y.; Jeon, S.K.; Lee, J.Y. Effect of interconnection position of bicarbazole-triazine type bipolar host materials on the photophysical and device performances. J. Ind. Eng. Chem. 2017, 51, 295. [Google Scholar] [CrossRef]
- Chatterjee, T.; Hung, W.Y.; Tang, W.F.; Chen, H.F.; Wong, K.T. Carbazole-bridged triphenylamine-bipyridine bipolar hosts for high-efficiency low roll-off multi-color PhOLEDs. Org. Electron. 2017, 50, 204. [Google Scholar] [CrossRef]
- Braveenth, R.; Ahn, D.H.; Han, J.H.; Moon, J.S.; Kim, S.W.; Lee, H.; Qiong, W.; Kwon, J.H.; Chai, K.Y. Utilizing triazine/pyrimidine acceptor and carbazole-triphenylamine donor based bipolar novel host materials for highly luminescent green phosphorescent OLEDs with lower efficiency roll-off. Dye Pigment. 2018, 157, 377. [Google Scholar] [CrossRef]
- Tomkeviciene, A.; Puckyte, G.; Grazulevicius, J.V.; Kazlauskas, K.; Jursenas, S.; Jankauskas, V. Dimethyldiphenylamino-substituted carbazoles as electronically active molecular materials. Dye Pigment. 2013, 96, 574. [Google Scholar] [CrossRef]
- Jiang, W.; Duan, L.; Qiao, J.; Dong, G.; Zhang, D.; Wang, L.; Qiu, Y. High-triplet-energy tri-carbazole derivatives as host materials for efficient solution-processed blue phosphorescent devices. J. Mater. Chem. 2011, 21, 4918. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, T.; Wei, Q.; Chen, Y.; Guo, X.; Xie, L.; Lai, W.; Fan, Q.; Qian, Y.; Huang, W. Arylfluorene based universal hosts for solution-processed RGB and white phosphorescent organic light-emitting devices. RSC Adv. 2015, 5, 94077. [Google Scholar] [CrossRef]
- Hung, W.Y.; Chi, L.C.; Chen, W.J.; Chen, Y.M.; Chou, S.H.; Wong, K.T. A new benzimidazole/carbazole hybrid bipolar material for highly efficient deep-blue electrofluorescence, yellow–green electrophosphorescence, and two-color-based white OLEDs. J. Mater. Chem. 2010, 20, 10113. [Google Scholar] [CrossRef]
- Chou, S.H.; Hung, W.Y.; Chen, C.M.; Liu, Q.Y.; Liu, Y.H.; Wong, K.T. Manipulation of connecting topology in carbazole/benzimidazole universal bipolar host materials for RGB and White PhOLEDs. RSC Adv. 2013, 3, 13891. [Google Scholar] [CrossRef]
- Seo, J.H.; Kim, H.M.; Choi, E.Y.; Choi, D.H.; Park, J.H.; Lee, K.H.; Yoon, S.S.; Kim, Y.K. Efficient Phosphorescent Green and Red Organic Light-Emitting Diodes Based on the Novel Carbazole-Type Host Material. J. Nanosci. Nanotechnol. 2011, 11, 1373. [Google Scholar] [CrossRef]
- Nagai, Y.; Sasabe, H.; Ohisa, S.; Kido, J. Effect of substituents in a series of carbazole-based host-materials toward high-efficiency carbene-based blue OLEDs. Mater. Chem. C 2016, 4, 9476. [Google Scholar] [CrossRef]
- Keruckas, J.; Grazulevicius, J.V.; Volyniuk, D.; Cherpak, V.; Stakhira, P. 3,6-Bis(indol-1-yl)-9-phenylcarbazoles as electroactive materials for electrophosphorescent diodes. Dye Pigment. 2014, 100, 66. [Google Scholar] [CrossRef]
- Park, M.S.; Lee, J.Y. 9-(Pyridin-3-yl)-9H-carbazole derivatives as host materials for green phosphorescent organic light-emitting diodes. Org. Electron. 2013, 14, 1291. [Google Scholar] [CrossRef]
- Wang, F.; Liu, D.; Li, J.; Ma, M. Modulation of n-Type Units in Bipolar Host Materials toward High-Performance Phosphorescent OLEDs. ACS Appl. Mater. Interfacce 2017, 9, 37888. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.; Lyu, Y.Y.; Lee, H.; Choi, B.; Char, K.; Lee, C. New carbazole-based host material for low-voltage and highly efficient red phosphorescent organic light-emitting diodes. J. Mater. Chem. 2012, 2, 6351. [Google Scholar] [CrossRef]
- Bin, J.K.; Cho, N.S.; Hong, J.I. New host material for high-performance blue phosphorescent organic electroluminescent devices. Adv. Mater. 2012, 24, 2911. [Google Scholar] [CrossRef]
- Li, H.; Bi, R.; Chen, T.; Yuan, K.; Chen, R.; Tao, Y.; Zhang, H.; Zheng, C.; Huang, W. Selectively Modulating Triplet Exciton Formation in Host Materials for Highly Efficient Blue Electrophosphorescence. ACS Appl. Mater. Interface 2016, 8, 7274. [Google Scholar] [CrossRef]
- Shi, H.; Xin, D.; Dong, X.; Dai, J.X.; Wu, X.; Miao, Y.; Fang, L.; Wang, H.; Choi, M.F.F. A star-shaped bipolar host material based on carbazole and dimesitylboron moieties for fabrication of highly efficient red, green and blue electrophosphorescent devices. J. Mater. Chem. C 2014, 2, 2160. [Google Scholar] [CrossRef]
- Ye, H.; Zhou, K.; Wu, H.; Chen, K.; Xie, G.; Hu, J.; Ma, S.; Su, S.J.; Cao, Y. Novel molecular host materials based on carbazole/PO hybrids with wide bandgap via unique linkages for solution-processed blue phosphorescent OLEDs. Opt. Mater. 2016, 60, 244. [Google Scholar] [CrossRef]
- Volyniuk, D.; Cherpak, V.; Stakhira, P.; Minaev, B.; Baryshnikov, G.; Tomkeviciene, A.; Keruckas, J.; Grazulevicius, J.V. Highly Efficient Blue Organic Light-Emitting Diodes Based on Intermolecular Triplet–Singlet Energy Transfer. J. Phys. Chem. C 2013, 117, 22538. [Google Scholar] [CrossRef]
- Xiang, C.; Fu, X.; Wei, W.; Liu, R.; Zhang, Y.; Balema, V.; Nelson, B.; So, F. Efficiency Roll-Off in Blue Emitting Phosphorescent Organic Light Emitting Diodes with Carbazole Host Materials. Adv. Funct. Mater. 2016, 26, 1463. [Google Scholar] [CrossRef]

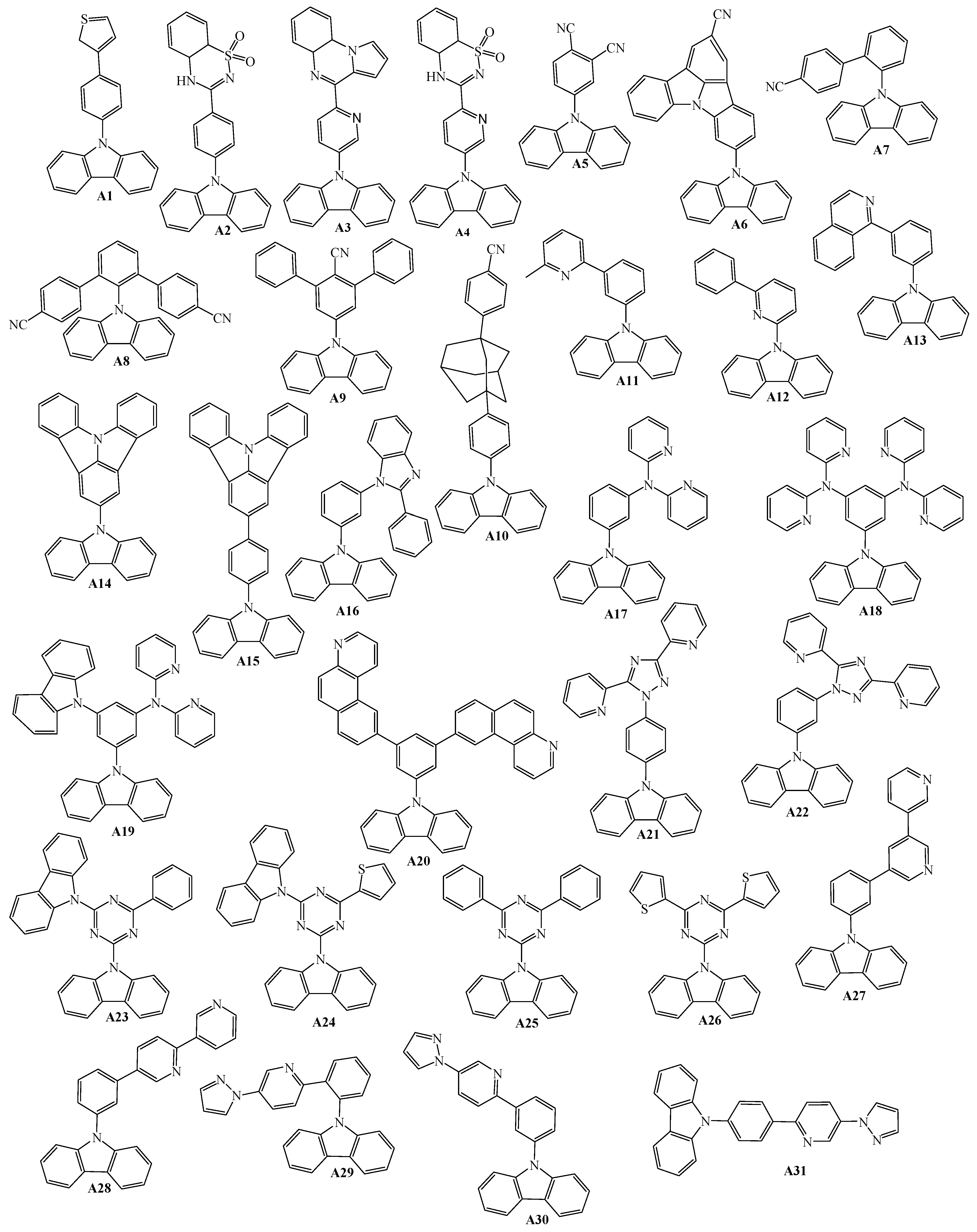
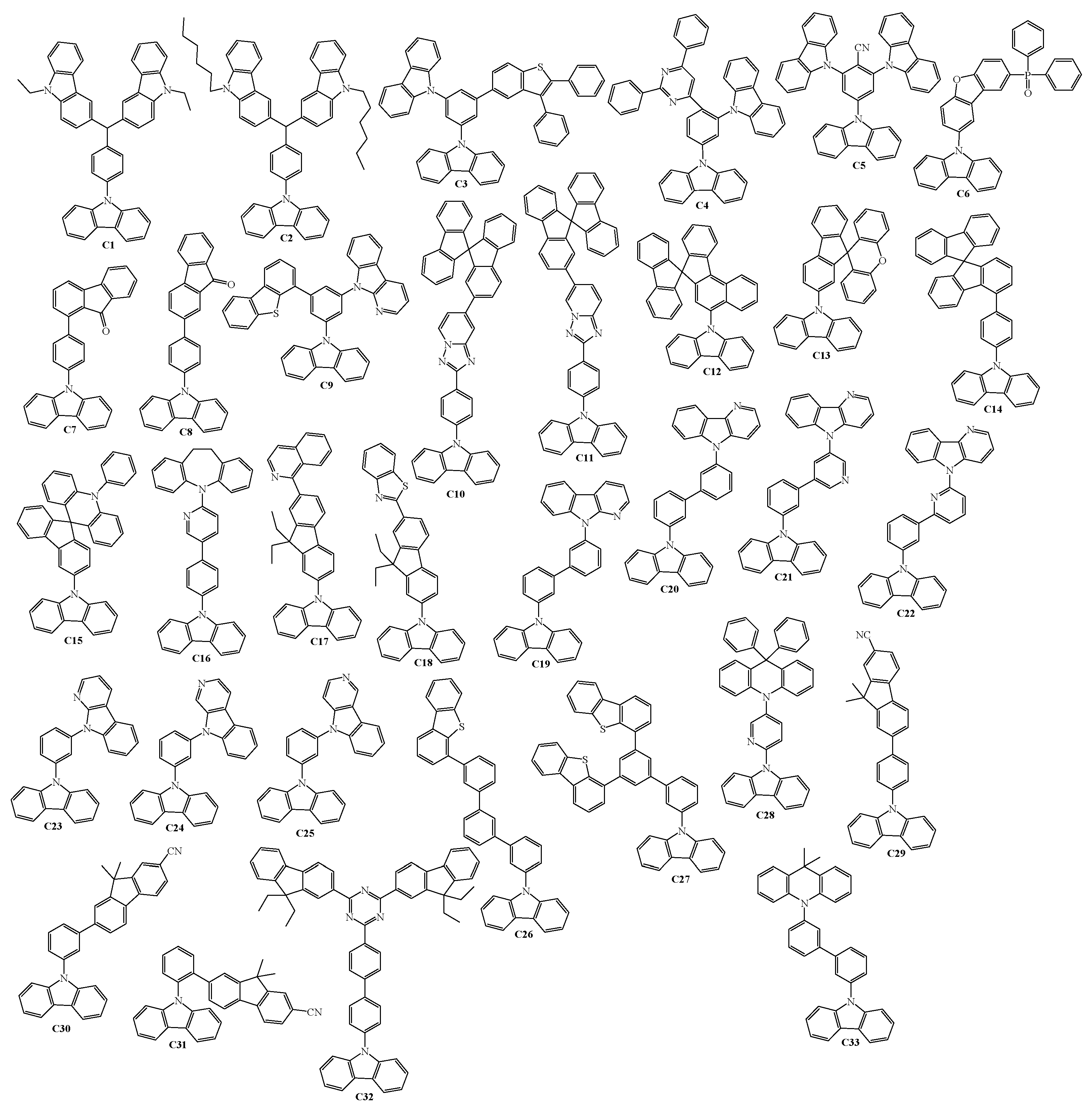
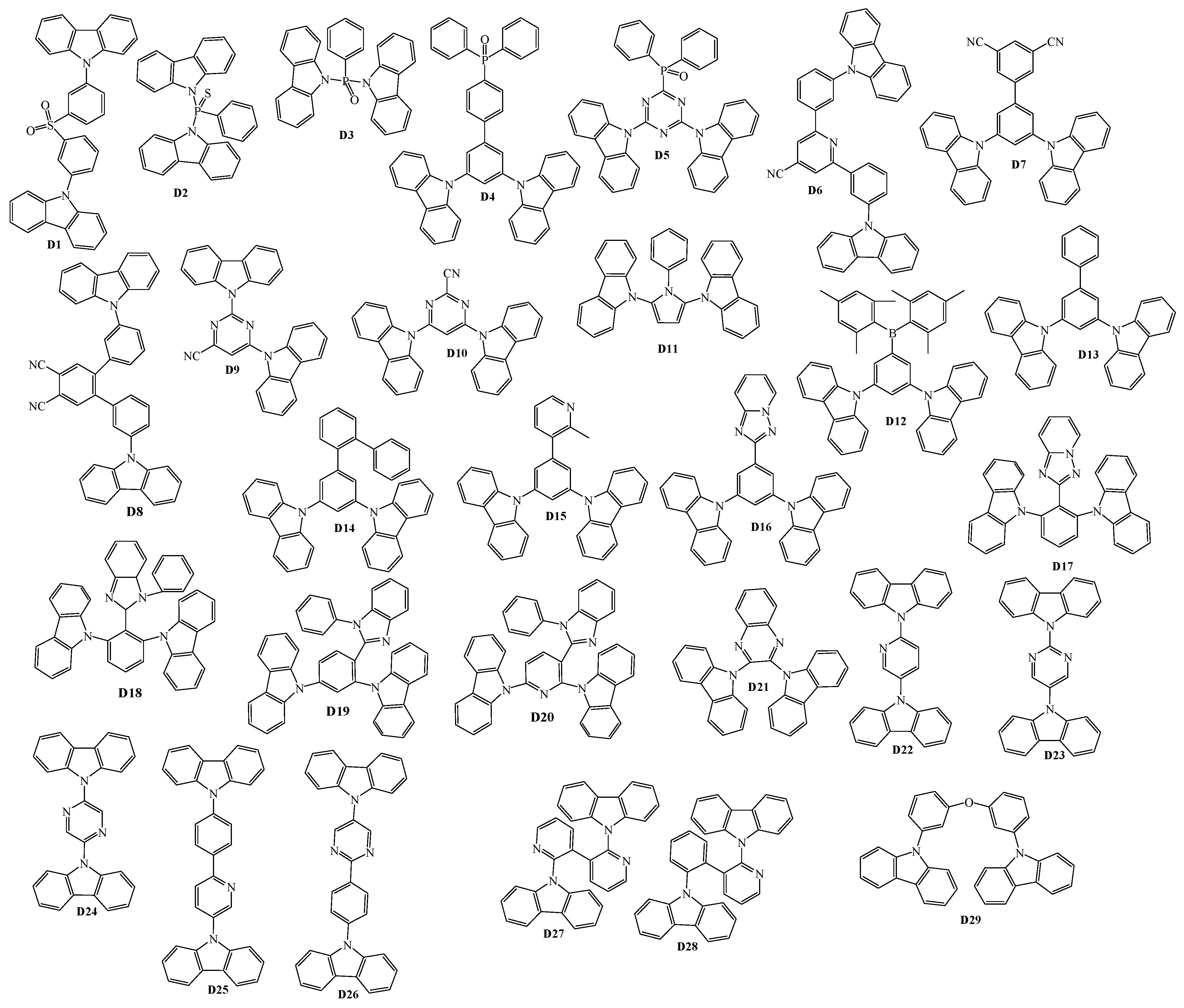
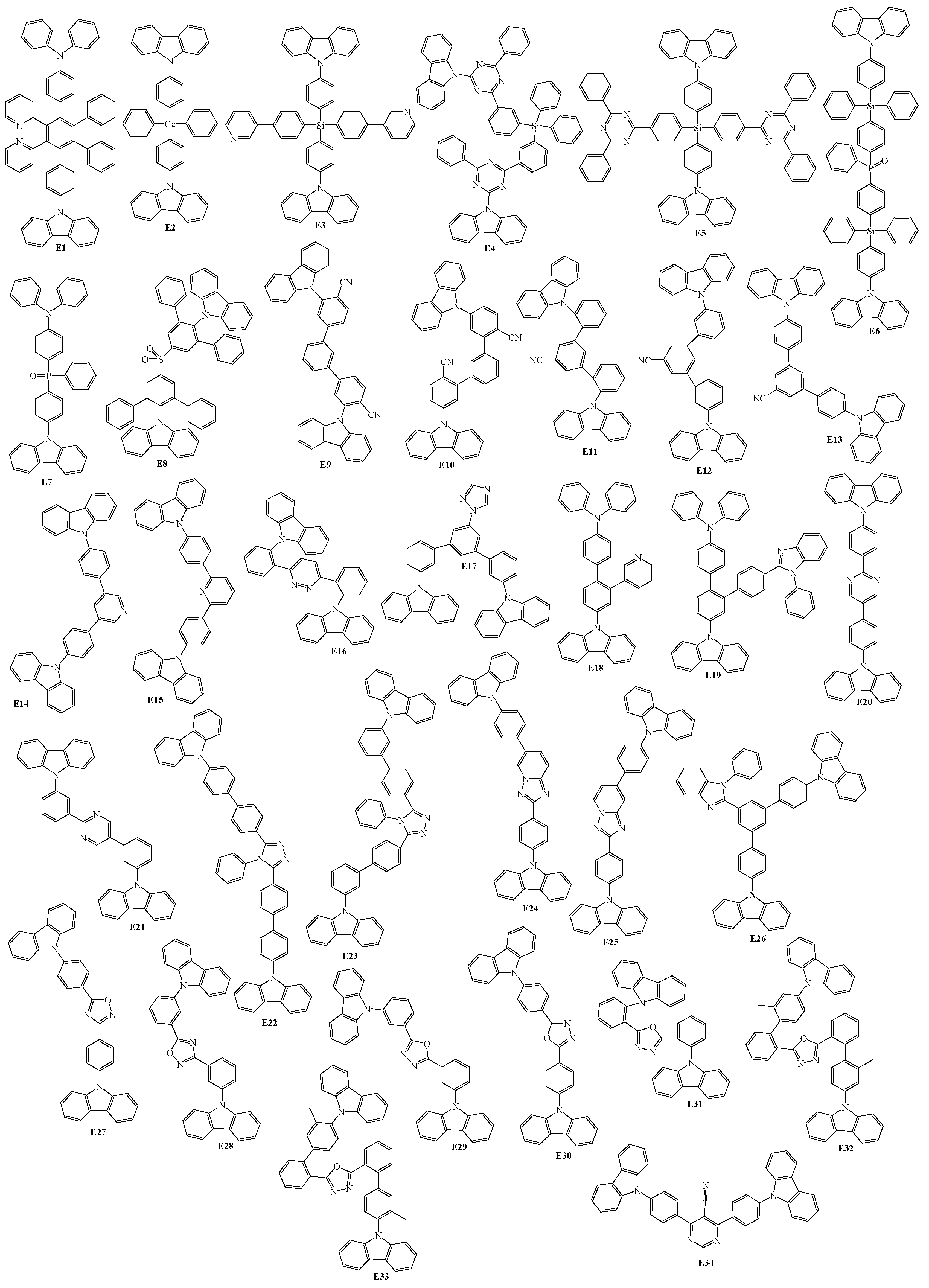
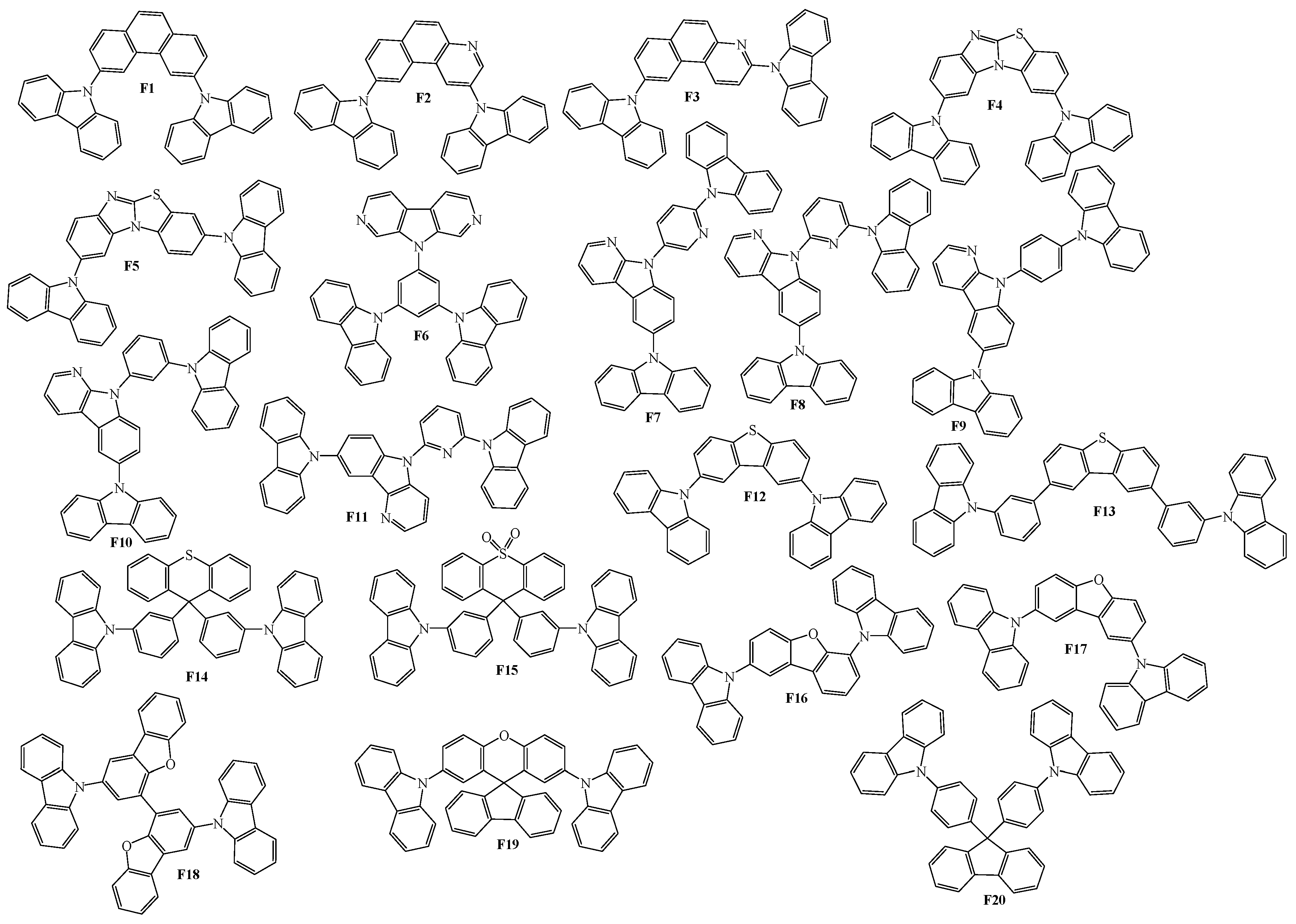
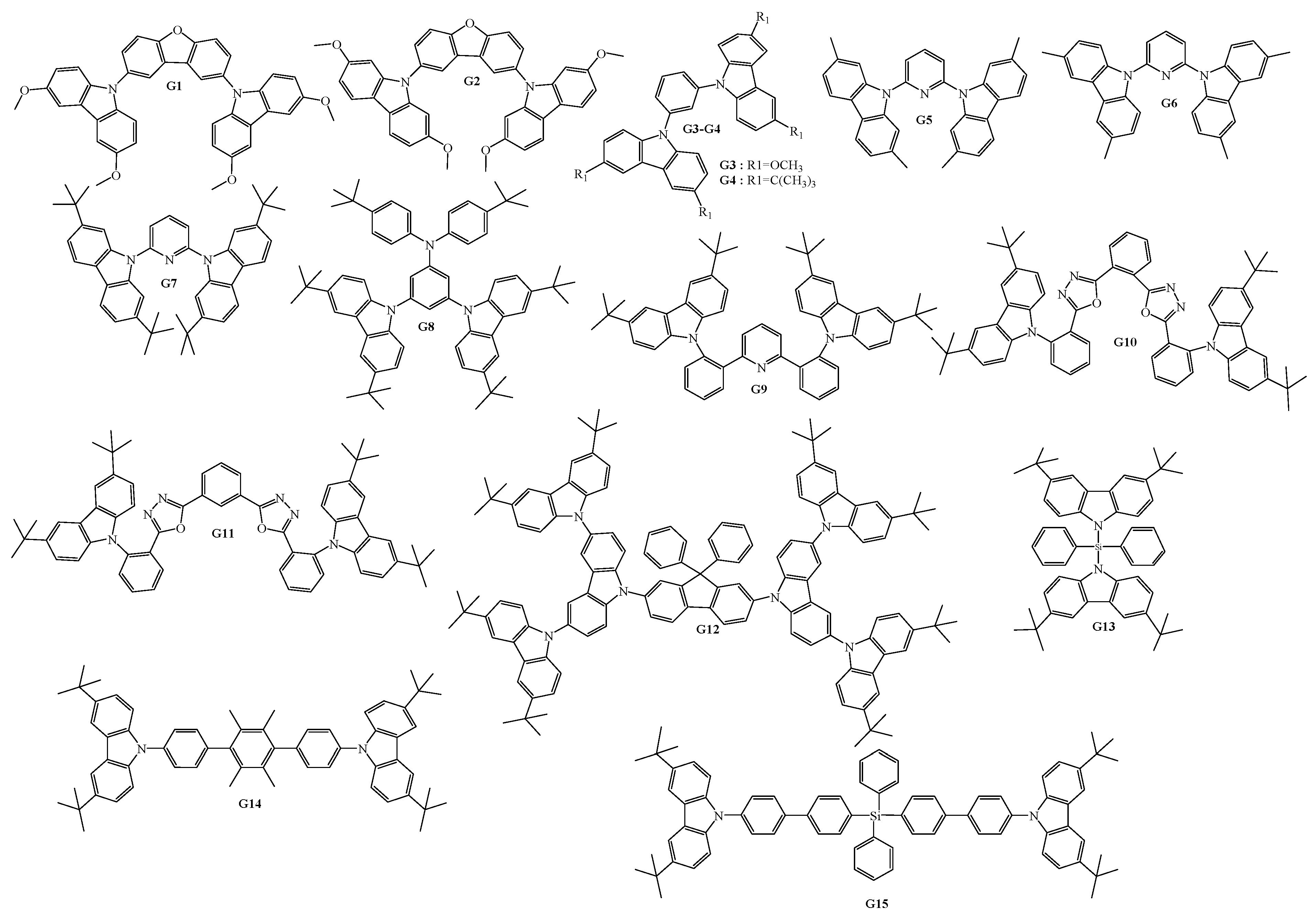
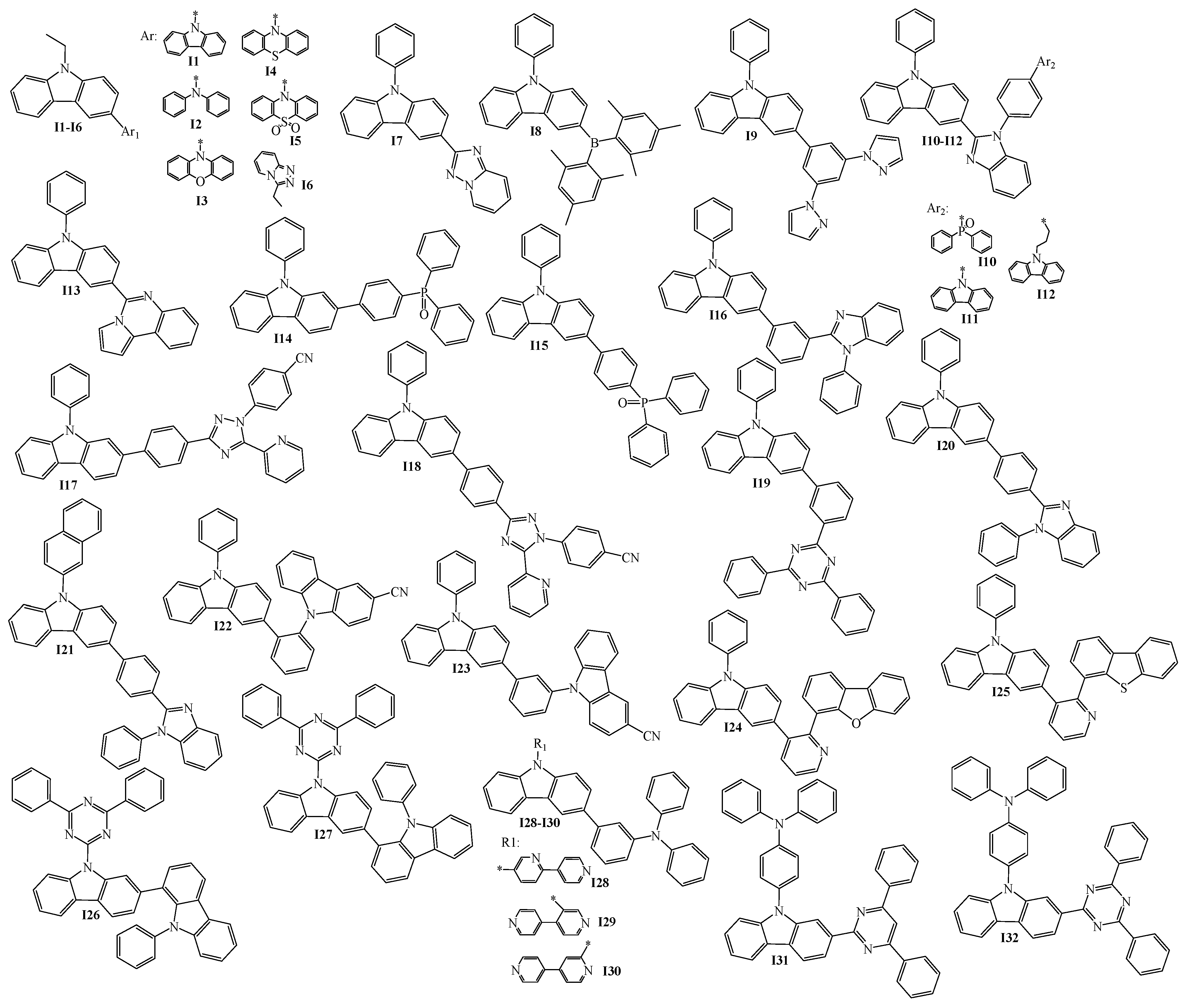
| Host | Td [°C]/Tg [°C] | HOMO/ LUMO/Et [eV] | Device Structure | EQE [%]/CE [cdA−1]/PE [lmW−1] |
|---|---|---|---|---|
| A1 | 299/- | 6.14/2.57/- | ITO/MoO3/TCTA/Ir(ppy)3:A1/TPBi/LiF/Al | 13.08/44.88/37.75 |
| A2 | 417/231 | 5.61/2.56/2.64 | ITO/NPB/TCTA/A2: Ir(piq)2(acac)/TmPyPB/LiF/Al | 12.7/20.6/22.5 |
| A3 | 404/- | 5.66/3.22/2.34 | ITO/TAPC/A3: Ir(piq)2acac/Bebq2/LiF/Al | 13/7.7/7.6 |
| A4 | 401/90 | 5.69/3.20/2.44 | ITO/TAPC/A4: Ir(piq)2acac/Bebq2/LiF/Al | 16.4/9.6/9.4 |
| A5 | 390/- | 5.76/2.72/2.70 | ITO/MoO3/NPB/A5: Ir(ppy)2(acac)/BCP/Alq3/LiF/Al | 21.9/80.1/86.1 |
| A6 | 488/- | 6.10/3.13/2.85 | ITO/DNTPD/BPBPA/PCzAc/A6/DBFTrz/ZADN/LiF/Al. | 17.1/-/- |
| A7 | 287/49 | 5.64/2.08/2.67 | ITO/HAT-CN/TAPC/A7: FIrpic/TmPyPB/Al. | 17.1/34.5/33.1 |
| A8 | 327/- | 5.71/2.13/2.74 | ITO/HAT-CN/TAPC/A8: FIrpic/TmPyPB/Al | 24.4/88.0/86.1 |
| A9 | 316/- | 5.62/2.19/2.87 | ITO/PEDOT:PSS/A9 4TCzBN/TPBi/Cs2CO3/Al | 11.53/21.91/- |
| A10 | 383/87 | 5.62/2.10/3.03 | ITO/MoO3/NPB/TCTA/A10: FIrpic/TmPyPB/LiF/Al. | 24.1/57/45.9 |
| A11 | 271/90 | 5.89/2.19/2.64 | ITO/NPB/TCTA/A11: (tphpy)2Ir(acac)/Bphen//Liq/Al | 4.41/6.44/4.05 |
| A12 | 292/98 | 5.90/2.23/2.62 | ITO/NPB/TCTA/A12: (tphpy)2Ir(acac)/Bphen//Liq/Al | 5.08/17.9/11.6 |
| A13 | 287/84 | 5.85/2.26/2.89 | ITO/NPB/TCTA/A13: (tphpy)2Ir(acac)/Bphen//Liq/Al | 12.3/45/47.1 |
| A14 | 344/111 | 5.56/2.42/2.82 | ITO/MoO3/TCTA/A14: Ir(ppy)2(acac)/BmPyPb/LiF/Al | 15.6/58.5/36.5 |
| A15 | 385/119 | 5.56/2.35/2.84 | ITO/MoO3/TCTA/A15: Ir(ppy)2(acac)/BmPyPb/LiF/Al | 14.8/56.3/35.4 |
| A16 | 349/86 | 5.82/2.25/2.78 | ITO/MoO3/TAPC/TCTA/A16: FIrpic/TmPyPb/LiF/Al. | 26.2/54.5/52.2 |
| A17 | 299/63 | 5.86/2.3/2.94 | ITO/PEDOT:PSS/TAPC/mCP/A17: FIrpic/TmPyPb/LiF/Al | 16.3/31.9/22.3 |
| A18 | 382/97 | 5.78/2.24/2.95 | ITO/PEDOT:PSS/TAPC/mCP/A18: FIrpic/TmPyPb/LiF/Al | 18.9/37.9/26.5 |
| A19 | 383/108 | 5.86/2.30/2.94 | ITO/PEDOT:PSS/TAPC/mCP/A19: FIrpic/TmPyPb/LiF/Al | 21.6/43.3/24.4 |
| A20 | 509/184 | 5.95/2.56/2.55 | ITO/NPB/TCTA/A20: Ir(ppy)2(acac)/BPhen/LiF/Al | 7.07/26.21/26.72 |
| A21 | 358/86 | 5.69/2.12/3.57 | ITO/PEDOT:PSS/TCTA/A21: Os(bpftz)2(PPhMe2)2/TPBi/LiF/Al | 16.5/20.3/18.8 |
| A22 | 331/77 | 5.68/2.15/3.53 | ITO/PEDOT:PSS/TCTA/A22: (Bt)2Ir(acac)/TPBi/LiF/Al | 17.5/43/38 |
| A23 | 445/- | 6.06/2.80/3.14 | ITO/MoO3/m-MTDATA/MoO3/m-MTDATA/Ir (ppz)3/A23: FIrpic/BPhen/LiF/Al | 5.2/10.4/9.3 |
| A24 | 439/- | 6.04/2.82/3.62 | ITO/MoO3/m-MTDATA/MoO3/m-MTDATA/Ir (ppz)3/A24: FIrpic/BPhen/LiF/Al | 9.8/20.9/20.0 |
| A25 | 369/- | 5.99/2.22/3.09 | ITO/MoO3/m-MTDATA/MoO3/m-MTDATA/Ir (ppz)3/A25: FIrpic/BPhen/LiF/Al | 9/17.4/17.1 |
| A26 | 365/- | 6.04/2.88/2.61 | ITO/MoO3/m-MTDATA/MoO3/m-MTDATA/Ir (ppz)3/A26: FIrpic/BPhen/LiF/Al | 9.3/18.3/17.9 |
| A27 | 353./61 | 5.57/2.47/2.84 | ITO/PEDOT:PSS/TAPC/TCTA/A27: Ir(ppy)3/TmPyPB/LiF/Al | 28/97.9/102.5 |
| A28 | 362/63 | 5.58/2.60/2.54 | ITO/PEDOT:PSS/TAPC/TCTA/A28: Ir(ppy)3/TmPyPB/LiF/Al | 23.6/79.7/100.2 |
| A29 | 301/58 | 5.61/2.26/2.95 | ITO/PEDOT:PSS/TAPC/TCTA/A29:Ir(ppy)3/TmPyPB/LiF/Al | 20.9/71.3/56.5 |
| A30 | 328/60 | 5.63/2.34/2.71 | ITO/PEDOT:PSS/TAPC/TCTA/A30:Ir(ppy)3/TmPyPB/LiF/Al | 23.3/80.3/72.0 |
| A31 | 336/- | 5.61/2.35/2.67 | ITO/PEDOT:PSS/TAPC/TCTA/A31:Ir(ppy)3/TmPyPB/LiF/Al | 27.3/91.8/65.0 |
| Host | Td [°C]/Tg [°C] | HOMO/LUMO/Et [eV] | Device Structure | EQE [%]/CE [cdA−1]/PE [lmW−1] |
|---|---|---|---|---|
| B1 | -/109 | 6.15/2.78/2.72 | ITO/PEDOT: PSS/TAPC/mCP/B1: Firpic/TSPO1/LiF/Al | 13.7/35.3/26.1 |
| B2 | -/97 | 6.06/2.88/2.76 | ITO/PEDOT: PSS/TAPC/mCP/B2: Firpic/TSPO1/LiF/Al | 19.1/-/35.5 |
| B3 | 434/127 | 5.31/2.08/2.59 | ITO/MoO3 TAPC/TCTA/B3: Ir(ppy)3/TmPyPB/LiF/Ag:Mg | 13.8/48.4/50.6 |
| B4 | 426/127 | 5.65/2.42/3.06 | ITO/TAPC/B4: Ir(dbi)3)/BCP/LiF/Al | -/41.6/43 |
| B5 | 352/170 | 5.61/2.12/3.03 | ITO/MoO3/NPB/TCTA/B5: Fir6/Tm/TPBi/LiF/Al | 19.5/40/36 |
| B6 | 315/101 | 5.68/2.18/3.02 | ITO/PEDOT:PSS/TAPC/mCP/B5:Ir(cb)3/TSPO1TPBi/LiF/Al | 17.1/18.3/26.3 |
| B7 | 409/104 | 6/2.35/3.03 | ITO/HAT-CN/TAPC/4CzIPN: B7/TPBi/Liq/Al | 20.5/62.1/51.3 |
| B8 | 404/107 | 6/2.35/3.03 | ITO/HAT-CN/TAPC/4CzIPN: B8/TPBi/Liq/Al | 21.7/68.7/59.5 |
| B9 | 441/72 | 5.71/2.17/2.71 | ITO/HAT-CN/TAPC/TCTA/B9: Ir(ppy)3/Liq/Al | 15.83/55.84/54.55 |
| B10 | 425/120 | 5.55/1.88/2.68 | ITO/MoO3/NPB/TCTA/B10: FIrpic/TPBi/LiF/Al | 9.3/22/19.8 |
| B11 | 358/97 | 5.41/1.78/2,72 | ITO/MoO3/NPB/TCTA/B11: Firpic/TPBi/LiF/Al | 11.4/29.3/19.8 |
| B12 | 513/147 | 6.54/3.51/2.79 | ITO/BCFA/mCP/B12: CNIr/DBFTrz//LiF/Al | 13.7/-/- |
| B13 | 484/129 | 6.46/3.44/2.84 | ITO/BCFA/mCP/B13: CNIr/DBFTrz//LiF/Al | 20.9/-/- |
| B14 | 418/116 | 6.34/3.07/3.09 | ITO/BCFN:HATCN/BCFN/mCBP/mCBP:B14 DBFTrz/ZADN/LiF/Al | 21.8/24.8/23.4 |
| B15 | 366/96 | 5.88/2.33/3 | ITO/NPB/mCP/B14: Firpic/TmPyPb/LiF/Al | 31.8/73.5/64.4 |
| B16 | 403/112 | 5.72/2.67/2.37 | ITO/PEDOT:PSS/NPB/TCTA/mCP/B15: Ir(piq)2(acac)/TPBi/LiF/Al | 12.2/21.9/15.4 |
| B17 | 390/107 | 5.81/2.65/2.40 | ITO/PEDOT:PSS/NPB/TCTA/mCP/B16: Ir(piq)2(acac)/TPBi/LiF/Al | 9.2/15.7/11.9 |
| B18 | 357/104 | 5.82/2.46/2.65 | ITO/PEDOT:PSS/NPB/TCTA/mCP/B17: Ir(piq)2(acac)/TPBi/LiF/Al | 13.4/21.2/13.4 |
| B19 | 475/- | 5.20/2.22/2.46 | ITO/HATCN/TAPC/TCTA/B19:PO-01/TPBi/TmPyPB/LiF/Al | 20.1/60.3/57.7 |
| B20 | 436/132 | 5.72/2.35/2.71 | ITO/MoO3/NPB/TCTA/B18: Ir(ppy)3/TPBi/LiF/Al | 17.1/69.2/59.5 |
| B21 | 483/123 | 5.72/2.42/2.7 | ITO/MoO3/NPB/TCTA/B19: Ir(ppy)3/TPBi/LiF/Al | 12.3/46.2/40.7 |
| B22 | 446/117 | 5.72/2.44/2.72 | ITO/MoO3/NPB/TCTA/B20: Ir(ppy)3/TPBi/LiF/Al | 15.3/53.4/43 |
| B23 | 402/128 | 5.67/2.18/2.81 | ITO/PEDOT:PSS/B21: OXD-7:FIrpic/TPBI/Cs2CO3/Al | 9.2/21.7/- |
| B24 | 454/160 | 5.40/2.30/2.6 | ITO/α-NPD/TCTA/mCP/B22: Ir(piq)2acac/TmPyPB/LiF/Al | 16.6/12.4/13.4 l |
| Host | Td [°C]/Tg [°C] | HOMO/LUMO/Et [eV] | Device Structure | EQE [%]/CE [cdA−1]/PE [lmW−1] |
|---|---|---|---|---|
| C1 | 337/140 | 6.16/2.76/2.95 | ITO/DNTPD/NPB/mCP/C1: FCNIrpic/TSPO1/LiF/Al | 13,3/-/- |
| C2 | 417/73 | -/-/2.97 | ITO/TAPC/C2: FIrpic/ETL/LiF/Al | 11.4/39.9/41.8 |
| C3 | 430/150 | 5.66/2.18/2.25 | ITO/NPB/HATCN/NPB/C3: Ir(phq)2(acac)/TpPyPB/LiF/Al | 10.3/19.5/20.4 |
| C4 | 369/223 | 5.69/2.57/2.96 | ITO/PEDOT:PSS/PVK/C4/TPBi/LiF/Al | 18.8/64.1/40.3 |
| C5 | 401/153 | 5.75/2.61/2.87 | ITO/HATCN/NPB/TCTA/mCP/C5/DPEPO/Bphen/LiF/Al | 14.8/-/29.5 |
| C6 | -/- | 5.41/1.39/3 | ITO/DNTPD/NPB/mCP/C6: FCNIrpic/TSPO1/LiF/Al | 20/26.4/22.6 |
| C7 | 367/89 | 5.55/2.89/2.5 | ITO/HATCN/TAPC/C7: Ir(MDQ2(acac) Liq/Al | 15.1/21.4/20 |
| C8 | 388/- | 5.57 3.00/2.36 | ITO/HATCN/TAPC/C8: Ir(MDQ2(acac) Liq/Al | 10.5/12/14.5 |
| C9 | 457/137 | 6.13/2.64/2.67 | ITO/PEDOT:PSS/TAPC/mCP/C9:Ir(ppy)2(acac)/TSPO1/TPBi/LiF/Al | 18.9/-/48.7 |
| C10 | 418/130 | 5.54/1.80/2.43 | ITO/PEDOT: PSS/TAPC/TCTA/Ir(pq)2acac: C10/TmPyPB/LiF/Al. Red PhOLEDs | 23/39.2/26.4 |
| C11 | 451/- | 5.52/1.86/2.47 | ITO/PEDOT: PSS/TAPC/TCTA/Ir(pq)2acac: C11/TmPyPB/LiF/Al. Red PhOLEDs | 22.1/38.6/38.4 |
| C12 | 458/142 | 6.53/3.31/2.29 | ITO/DNTPD/NPB/C12: Ir(pq)2acac/BCP/Alq3/LiF/Al | 9.65/15.4/7.62 |
| C13 | 345/97 | 5.49/2.34/2.99 | ITO/MoO3/m-MTDATA: MoO3/m-MTDATA/Ir(ppy)3/C13: FIrpic/Bphen/LiF/Al. | 8.9/20/17.9 |
| C14 | 311/127 | 5.52/1.97/2.80 | ITO/CuPc/NPB/TCTA/C14: Ir(ppy)3/TPBi/Al | 17.5/67.9/45.4 |
| C15 | 348/150 | 5.74/2.20/2.84 | ITO/HAT-CN/TAPC/C15: FIrpic: PO-01/TmPyPB/Liq/Al. | 21.5/60.2/43.6 |
| C16 | 414/116 | 5.40/1.93/2.80 | TO/HAT-CN/TAPC/C16: Ir(MDQ)2(acac)/TmPyPB/Liq/Al | 23.2/34/33.5 |
| C17 | 332/- | 5.87/2.48/2.54 | ITO/NPB/TcTa/C17: (tphpy)2Ir(acac)/Bphen/Liq/Al | 8.09/29.6/26.6 |
| C18 | 304/- | 5.97/2.80/2.59 | ITO/NPB/TcTa/C18: (tphpy)2Ir(acac)/Bphen/Liq/Al | 5.23/15.1/12.1 |
| C19 | -/- | -/-/- | ITO/MoO3/NPB/mCP/C19: FIrpic/TmPyPB/LiF/Al | 3.24/8.58/6.24 |
| C20 | 386/120 | 5.70/2.46/2.94 | ITO/MoO3/NPB/mCP/C20: FIrpic/TmPyPB/LiF/Al | 19.7/47.7/29.1 |
| C21 | 368/105 | 5.72/2.39/2.96 | ITO/MoO3/NPB/mCP/C21: FIrpic/TmPyPB/LiF/Al | 20.1/42.6/31.7 |
| C22 | 355/101 | 5.73/2.45/2.78 | ITO/MoO3/NPB/mCP/C22: FIrpic/TmPyPB/LiF/Al | 16.5/34.9/23 |
| C23 | -/- | 6.06/2.55/2.89 | deep blue PhOLED | 24.3/-/- |
| C24 | -/- | 6.06/2.62/2.96 | deep blue PhOLED | 21.9/-/- |
| C25 | -/- | 6.08/2.49/2.96 | deep blue PhOLED | 17/-/- |
| C26 | 462/98 | 5.98/2.57/2.79 | ITO/MoO3/TAPC/C26: FIrpic/TmPyPB/Liq/Al | 16.6/36.5/25.6 |
| C27 | 488/140 | 5.99/2.56/2.74 | ITO/MoO3/TAPC/C27: FIrpic/TmPyPB/Liq/Al | 13.9/33/25.9 |
| C28 | 350/300 | 5.7/2.35/2.82 | ITO/PEDOT: PSS/PVK/C28: t4CzIPN/TPBi/LiF/Al | 24/82.2/46.9 |
| C29 | 314/93 | 5.7/2.62/- | ITO/PEDOT: PSS/TSPO1/C29: Ir(ppy)2(acac)/TPBi/LiF/Al | 13.3/39.2/23.4 |
| C30 | 338/103 | 5.78/2.54/- | ITO/PEDOT: PSS/TSPO1/C30: Ir(ppy)2(acac)/TPBi/LiF/Al | 19.6/67/34.5 |
| C31 | 321/110 | 5.74/2.77/- | ITO/PEDOT: PSS/TSPO1/C31: Ir(ppy)2(acac)/TPBi/LiF/Al | 17.3/53.6/25.2 |
| C32 | 512/178 | 5.67/2.39/2.94 | ITO/MoO3/TAPC/TCTA/C32: Ir(ppy)3/TmPyPB/LiF/Al | 7.5/25.7/24.48 |
| C33 | -/- | 5.75/2.09/2.8 | ITO/PEDOT: PSS/TAPC/mCP/C33: Ir(dbi)3/TSPO1/TPBI/LiF/Al | 26.2/ |
| Host | Td [°C]/Tg [°C] | HOMO/LUMO/Et [Ev] | Device Structure | EQE [%]/CE [cdA−1]/PE [lmW−1] |
|---|---|---|---|---|
| D1 | 412/127 | 5.38/2.23/2.78 | ITO/MoO3/mCP/DPEPO/D1: FIrpic/DPEPO/TPBi/LiF/Al | 14.0/30.1/32.2 |
| D2 | 366/209 | 5.74/2.34/3.01 | ITO/PEDOT: PSS/TAPC/D2: FIrpic/TmPyPB/LiF/Al | 17.3/36.7/37.5 |
| D3 | 366/80 | 6.33/2.45/3.03 | ITO/PEDOT: PSS/TAPC/D3: FIrpic/TmPyPB/LiF/Al | 15.8/28.6/15.3 |
| D4 | -/- | 6.13/2.56/2.99 | ITO/DNTPD/NPB/mCP/D4: FCNirpic/TSPO/LiF/Al | 22.4/27.1/- |
| D5 | 412/116 | 5.86/1.74/- | ITO/MoO3/NPB/mCP/D5: FIr6/DPDPOTZ/LiF/Al | 22.9/36.6/41.8 |
| D6 | 460/126 | 5.67/2.55/2.76 | ITO/MoO3/TAPC/TCTA/D6: (Ir(pq)2acac/TmPyPB/LiF/Al, | 26.84/41.14/47.87 |
| D7 | -/- | 5.8/3.4/2.71 | ITO/PEDOT: PSS/TAPC/D7: Ir(ppy)3/TSPO1/LiF/Al | 25.9/-/- |
| D8 | 421/ | 6.14/3.51/- | ITO/PEDOT: PSS/TAPC/mCP/D8/TSPO1/TPBi/LiF/Al | 25.2/81.2/57.2 |
| D9 | 278/150 | 6.04/2.83/3.06 | ITO/ReO3/CzSi/D9: 4CzIPN/PO-T2TLiq/Al | 24/74.4/81.3 |
| D10 | 281/- | 5.95/3.03/2.92 | ITO/ReO3/CzSi/D10: 4CzIPN/PO-T2TLiq/Al | 22.5/65/53.8 |
| D11 | -/- | 6.08/2.38/2.99 | ITO/PEDOT: PSS/TAPC/C11: FIrpic/mCP/TSPO1/TPBi/LiF/Al | 11.1/20.8/7.2 |
| D12 | 342/118 | 5.41/2.39/2.68 | ITO/TAPC/TCTA/D12: Ir(ppy)3/DPEPO/TmPyPB/LiF/Al | 19.3/69.1/88.1 |
| D13 | 335/87 | 5.71/2.17/2.74 | ITO/PEDOT: PSS/poly-TPD/D13 FIrpic/TSPO1/TPBi/LiF/Al | 5.2/9.6/- |
| D14 | 358/102 | 5.67/2.13/2.82 | ITO/PEDOT: PSS/poly-TPD/D14: FIrpic/TSPO1/TPBi/LiF/Al | 3.9/7.5/- |
| D15 | 350/94 | 6.11/2.52/2.99 | ITO/PEDOT: PSS/TAPC/mCP/D15: FIrpic/TPBI/TSPO1/LiF/Al | 19.8/-/- |
| D16 | 417/130 | 5.51/1.57/2.93 | ITO/PEDOT: PSS/TAPC/TCTA/D16: FIrpic/TmPyPB/LiF/Al | 27.1/52.3/40.5 |
| D17 | 435/- | 5.71/2.01/2.92 | ITO/PEDOT: PSS/TAPC/TCTA/D17: FIrpic/TmPyPB/LiF/Al | 23.9/44.8/27.4 |
| D18 | 381/117 | 5.8/2.3/3.1 | ITO/TAPC/mCP/D18: FIrpic/DPPS/LiF/Al | 27/57.5/48.9 |
| D19 | 378/137 | 5.72/2.27/2.49 | ITO/TAPC/D19: Ir(ppy)3/TPBi/Liq/Al | 14.7/52.4/49 |
| D20 | 397/140 | 5.76/2.43/2.46 | ITO/TAPC/D20: Ir(ppy)3/TPBi/Liq/Al | 24.6/88.5/78.5 |
| D21 | 301/115 | 6.3/3.52/2.46 | ITO/PEDOT/TAPC/mCP/D21: PO-01/TSPO1/TPBi/LiF/Al | 24.6/79.3/49.6 |
| D22 | 320/- | 5.71/2.31/3.02 | ITO/PEDOT: PSS/TAPC/mCP/FIrpic:D22/LiF/Al | 16.3/33.0/32.1 |
| D23 | 331/- | 5.82/2.64/2.98 | ITO/PEDOT: PSS/TAPC/mCP/FIrpic:D23/LiF/Al | 11.3/23.7/17.3 |
| D24 | 328/- | 5.73/2.86/2.66 | ITO/PEDOT: PSS/TAPC/mCP/FIrpic:D24/LiF/Al | 6.5/14.6/11.7 |
| D25 | 395/- | 6.05/2.74/2.62 | ITO/MoO3/D25: Ir(ppy)2(acac)/Cs2CO3/Al | 21.5/74.9/56.3 |
| D26 | 403/- | 6.05/2.88/2.61 | ITO/MoO3/D26: Ir(ppy)2(acac)//Cs2CO3/Al | 26.8/92.2/106.1 |
| D27 | 360/- | 5.53/1.63/2.91 | ITO/PEDOT: PSS/TAPC/D27: FIrpic/DPEPO/TmPyPB/LiF/Al | 32.0/66.4/57.9 |
| D28 | 333/- | 5.50/1.36/3.05 | ITO/PEDOT: PSS/TAPC/D28: FIrpic/DPEPO/TmPyPB/LiF/Al | 22.3/48.9/47.9 |
| D29 | -/- | 5.38/1.09/3.02 | ITO/PEDOT: PSS/TAPC/mCP/D29: Ir(dbi)2/TSPO1/TPBi/LiF/Al | 22.81/-/31.37 |
| Host | Td [°C]/Tg [°C] | HOMO/LUMO/Et [eV] | Device Structure | EQE [%]/CE [cdA−1]/PE [lmW−1] |
|---|---|---|---|---|
| E1 | 480/260 | 6.3/3/3 | ITO/NPB/TCTA/E1: Firpic:Ir(piq)2acac/TmPyPB/LiF/Al | 11.3/16.8/15.1 |
| E2 | 377/110 | 5.71/2.30/3.12 | ITO/PEDOT: PSS/PVK/E2: OXD-7/Firpic/Ca/Al | 6.9/15.2/3.8 |
| E3 | -/145 | 5.67/2.15/2.85 | ITO/HATCN/TAPC/DCDPA/TCzTrz: E3/TSPO1/TPBi/LiF/Al | 18.7/32.7/ |
| E4 | 526/153 | 6.40/3.32/2.98 | ITO/BPBPA:HATCN/BPBPA/mCBP/E4:Ir(cb)3/DBFTrz/ZADN/LiF/Al | 20.7/-/- |
| E5 | 575/252 | 5.75/2.32/2.98 | ITO/PEDOT: PSS/PVK/E5: t4CzIPN/TPBi/LiF/Al | 19.1/65.45/41.13 |
| E6 | 485/159 | 5.56/2.21/3.04 | ITO/PEDOT:PSS/E6: Firpic/TmPyPb/TPBi/CsF/Al | 10.4/20.17/7.37 |
| E7 | -/137 | 5.76/2.19/2.56 | ITO/NPB/mCP/E7: Firpic/TAZ/LiF/Al | 23.5/45.1/40.6 |
| E8 | 467/153 | 5.41/1.81/2.93 | ITO/TAPC/mPC/E8: 4CzIPN/DPPS/LiF/Al | 23.38/67.74/60.94 |
| E9 | 492/150 | 5.58/2.23/2.81 | ITO/NPB/TCTA/E9: Ir(ppy)3/TPBi/LiF/Al | 21.95/74.01/67.92 |
| E10 | 486/150 | 5.65/1.82/2.95 | ITO/NPB/TCTA/E10: Ir(ppy)3/TPBi/LiF/Al | 22.28/76.86/89.15 |
| E11 | 350/94 | 5.74/2.16/3.01 | ITO/PEDOT:PSS/TAPC/TCTA/E11: Firpic/TmPyPB/LiF/Al | 19.08/40.93/21.42 |
| E12 | 390/121 | 5.62/2.14/2.81 | ITO/PEDOT:PSS/TAPC/TCTA/E12: Firpic/TmPyPB/LiF/Al | 23.14/46.81/24.50 |
| E13 | 440/140 | 5.59/2.16/2.77 | ITO/PEDOT:PSS/TAPC/TCTA/E13:Firpic/TmPyPB/LiF/Al | 7.03/18.52/13.40 |
| E14 | 455/102 | 6.05/2.65/2.71 | ITO/TPDPES:TBPAH/3DTAPBP/E14: Firpic/TmPyPBP/LiF/Al | 19.1/-/34.6 |
| E15 | 461/107 | 6.15/2.77/2.78 | ITO/TPDPES:TBPAH/3DTAPBP/E15: Firpic/TmPyPBP/LiF/Al | 24.3/-/46.1 |
| E16 | -/167 | 6.14/3.17/2.71 | ITO/PEDOT:PSS/TAPC/PczAc/Mcp/E16:Ir(ppy)3/TSPO1/TPBi/LiF/Al | 15.5/-/- |
| E17 | 415/- | 5.63/2.57/2.86 | ITO/PEDOT:PSS/TAPC/TCTA/E17:Ir(ppy)3/TmPyPB/LiF/Al | 17.5/60.4/44 |
| E18 | 418/122 | 5.88/2.5/2.68 | ITO/NPB/TCTA/E18: Ir(ppy)3/TmPyPB/LiF/Al | 18.9/63.4/56.7 |
| E19 | 375/169 | 5.90 2.52/2.54 | ITO/NPB/TCTA/E19: Ir(ppy)3/TmPyPB/LiF/Al | 17.5/59.8/55.7 |
| E20 | 437/128 | 6.01/2.88/2.4 | ITO/PEDOT:PSS/TAPC/mCP)/E20: PO-01/TSPO1/TPBi/LiF/Al | 22.3/68.3/47.5 |
| E21 | 440/119 | 6.08/2.54/2.5 | ITO/PEDOT:PSS/TAPC/mCP)/E21: PO-01/TSPO1/TPBi/LiF/Al | 19.7/47.5/- |
| E22 | 453/- | 5.61/2.32/2.58 | ITO/NPB/E22: Ip(ppy)3/TPBI/LiF/Al | -/11.7/11.1 |
| E23 | 454/156 | 5.62/2.19/2.56 | ITO/NPB/E23: Ip(ppy)3/TPBI/LiF/Al | -/13/11.2 |
| E24 | 478/144 | 5.28/1.66/2.60 | ITO/PEDOT:PSS/TAPC/TCTA/E24: Ir(ppy)3/TmPyPB/LiF/Al | 22.4/77/43.2 |
| E25 | 502/141 | 5.20/1.38/2.60 | ITO/PEDOT:PSS/TAPC/TCTA/E25: Ir(ppy)3/TmPyPB/LiF/Al | 25.6/90.3/69.6 |
| E26 | 544/63 | 5.59/2.35/2.43 | ITO/MoO3/NPB/E26: Ir(ppy)3/TPBI/LiF/Al | 12.7/39.7/40.8 |
| E27 | -/110 | 5.98/2.72/2.71 | ITO/HAT-CN/TAPC/E27: FIrpic/TmPyPB/Liq/Al | 5.6/13/10,7 |
| E28 | -/100 | 5.99/2.47/2.81 | ITO/HAT-CN/TAPC/E28: FIrpic/TmPyPB/Liq/Al | 6.8/16/16.8 |
| E29 | 464/133 | 5.79/2.28/2.60 | ITO/MoO3/NPB/E29: Ir(ppy)3/BCP/Alq3/LiF/Al | -/21.2/17.0 |
| E30 | 443/112 | 5.80/2.32/2.72 | ITO/MoO3/NPB/E30: Ir(ppy)3/BCP/Alq3/LiF/Al | -/45.9/46.8 |
| E31 | 450/108 | 5.79/2.31/2.64 | ITO/MoO3/NPB/E31: Ir(ppy)3/BCP/Alq3/LiF/Al | -/31.3 20.8 |
| E32 | 401/128 | 5.50/2.02/2.92 | ITO/MoO3/NPB/TCTA/E32: Ir(MDQ)2(acac)/TPBI/LiF/Al | 12.8/17.2/12.8 |
| E33 | 415/- | 5.55/2.02/2.91 | ITO/MoO3/NPB/TCTA/E33: Ir(MDQ)2(acac) TPBI/LiF/Al | 12.7/20.1/13.1 |
| E34 | 423/135 | -/-/2.54 | ITO/HAT-CN/NPB/TCTA/mCBP/E34: AcDbp/nBPhen/Liq/Al | 13.7/14.9/10.2 |
| Host | Td [°C]/Tg [°C] | HOMO/LUMO/Et [Ev] | Device Structure | EQE [%]/CE [cdA−1]/PE [lmW−1] |
|---|---|---|---|---|
| F1 | 402/132 | 5.92/2.68/2.56 | ITO/AQ1200/NPB/TCTA/F1: Ir(ppy)2(acac)/Bphen/LiF/Al | 12.1/44/13.1 |
| F2 | 395/139 | 5.97/2.79/2.60 | ITO/AQ1200/NPB/TCTA/F2: Ir(ppy)2(acac)/Bphen/LiF/Al | 9.8/35.5/12.3 |
| F3 | 427/139 | 5.99/2.84/2.62 | ITO/AQ1200/NPB/TCTA/F3: Ir(ppy)2(acac)/Bphen/LiF/Al | 14.3/48.5/20.6 |
| F4 | -/- | 6.01/2.55/3.02 | ITO/HAT-CN/TAPC/F4: DPAC-TRZ/TSPO1/TPBi/LiF/Al | 20.8/-/ |
| F5 | -/- | 6.07/2.62/3.04 | ITO/HAT-CN/TAPC/F5: DPAC-TRZ/TSPO1/TPBi/LiF/Al | 20.4/-/- |
| F6 | 487/148 | 6.12/2.98/2.88 | ITO/PEDOT: PSS/TAPC/Mcp/F6: Ir(ppy)3/TSPO1/LiF/Al | 21.1/67.7/39.7 |
| F7 | 451/144 | 5.60/2.11/2.92 | ITO/TAPC/F7:Firpic/TmPyPB/LiF/Al | 22.6/40.5/32.6 |
| F8 | 418/128 | 5.73/2.21/2.92 | ITO/TAPC/F8:Firpic/TmPyPB/LiF/Al | 23.7/44.8/31.3 |
| F9 | 578/- | 5.61/2.11/2.92 | ITO/TAPC/F9:Firpic/TmPyPB/LiF/Al | 19.2/39.1/27.2 |
| F10 | 585/- | 5.60/2.09/2.93 | ITO/TAPC/F10:Firpic/TmPyPB/LiF/Al | 25.8/53.1/41.1 |
| F11 | 412/138 | 6.06/2.68/2.97 | ITO/HATCN/TAPC/DCDPA/F11: DMAC-DPS/TSPO1/TPBi/LiF/Al | 18.8/31.2/- |
| F12 | -/- | 5.71/2.19/2.94 | ITO/HATCN/TAPC/2CzIPN: F12/TmPyPB/LiF/Al | 18.5/31.89/- |
| F13 | 380/133 | 6.06/2.68/2.75 | ITO/PEDOT: PSS/F13: Firpic/TmPyPB/CsF/Al | 13.9/29.0/18.6 |
| F14 | 385/125 | 5.24/1.72/3.03 | ITO/HATCN/NPB/TAPC/F14: Firpic/TmPyPB/LiF/Al | 13.4/23.1/21.0 |
| F15 | 420/167 | 5.32/1.81/3.02 | ITO/HATCN/NPB/TAPC/F15: Firpic/TmPyPB/LiF/Al | 29/50.1/47.1 |
| F16 | 457/133 | 6.05/2.66/2.96 | ITO/PEDOT:PSS/F16: mSiTrz/Al | 23/-/27.9 |
| F17 | 467/126 | 6.09/2.55/2.98 | ITO/PEDOT:PSS/F17: mSiTrz/Al | 22.4/-/26.3 |
| F18 | 419/181 | 6.02/2.79/2.75 | ITO/TAPC/mCP/F18: Ir(ppy)3/TSPO1/LiF/Al | 20.6/71.1/43.4 |
| F19 | 370/170 | 5.62/1.81/2.56 | ITO/MoO3/TAPC/F19: Ir(ppy)3/TmPyPb/LiF/Al | -/21/11.9 |
| F20 | 480/- | 5.32/1.82/2.88 | ITO/HATCN/NPD/PtN3N-ptb:F20/BAlq/BPyTP/LiF/Al | 9.5/-/- |
| Host | Td [°C]/Tg [°C] | HOMO/LUMO/Et [Ev] | Device Structure | EQE [%]/CE [cdA−1]/PE [lmW−1] |
|---|---|---|---|---|
| G1 | 424/121 | 5.22/2.91/2.89 | ITO/MoO3/NPB/G1: DACT-II/TSPO1/TmPyPb/LiF/Al | 12.2/41.6/15.1 |
| G2 | 420/123 | 5.29/2.97/3.01 | ITO/HAT-CN/NPB/G2: Ir(ppy)3/TSPO1/TPBi/LiF/Al | 12.6/44.1/36.8 |
| G3 | 379/79 | 5.4/2.4/2.86 | ITO/TAPC/mCP/G3: Firpic/DPPS/LiF/Al | 15.3/33.8/- |
| G4 | 354/145 | 5.8/2.2/2.97 | ITO/TAPC/mCP/G4: Firpic/DPPS/LiF/Al | 22/51.3/- |
| G5 | 342/59 | 5.54/2.01/2.9 | ITO/PEDOT:PSS/G5: FIrpic/TmPyPB/LiF/Al | -/13.8/7 |
| G6 | 344/107 | 5.49/2.07/2.71 | ITO/PEDOT:PSS/G6: FIrpic/TmPyPB/LiF/Al | -/2.9/1 |
| G7 | 366/147 | 5.47/2.03/2.84 | ITO/PEDOT:PSS/G7: FIrpic/TmPyPB/LiF/Al | -/8/2.3 |
| G8 | -/- | 5.8/2.3/3.08 | ITO/PEDOT:PSS/TAPC/Mcp/G8: DBFTrz/TSPO1/TPBI/LiF/Al | 16.4/25.2/- |
| G9 | 300/149 | 5.4/1.98/2.64 | ITO/MoO3/TAPC/TCTA/G9: 2CzPN/DPEPO/TmPyPB/LiF/Al | 11.7/28.9/23.3 |
| G10 | 375/147 | 5.2/1.8//2.97 | ITO/MoO3/TCTA/mCP/G10: FIrpic/TSPO1/TPBi/LiF/Al | 3.6/-/- |
| G11 | 383/143 | 5.1/2/2.97 | ITO/MoO3/TCTA/mCP/G11: FIrpic/TSPO1/TPBi/LiF/Al | 4.2/-/- |
| G12 | 495/304 | 5.25/1.96/2.61 | ITO/PEDOT:PSS/G12:Ir(mppy)3/TPBi/Cs2CO3/Al | 9.8/34.8/- |
| G13 | 350/- | 5.71/2.08/3 | ITO/PEDOT: PSS/TAPC/mCP/G13: FIrpic/TmPyPB/LiF/Al | 7.6/13.7/4.7 |
| G14 | 450/- | 5.48/2.01/3 | ITO/PEDOT:PSS/NPB/TCTA/G14:FIrpic/TPBi/LiF/Al | 7.5/18/8 |
| G15 | -/204 | 5.31/1.40/2.98 | ITO/PEDOT:PSS/G15: Ir(ppy)3/TPBi/Cs2CO3/Al | 2.26/7.6/3 |
| Host | Td [°C]/Tg [°C] | HOMO/LUMO/Et [Ev] | Device Structure | EQE [%]/CE [cdA−1]/PE [lmW−1] |
|---|---|---|---|---|
| H1 | 366/109 | 5.90/2.40/3.03 | ITO/ReO3/H1: PO-T2T: 4CzIPN/PO-T2T/CN-T2T/Liq/Al | 21.1/56.4/59.1 |
| H2 | 331/85 | 5.72/2.27/3.04 | ITO/ReO3/H2: PO-T2T: 4CzIPN/PO-T2T/CN-T2T/Liq/Al | 17.6/56.6/68.5 |
| H3 | 464/138 | 5.53/2.34/3.05 | ITO/PEDOT:PSS/H3: FIrpic/DPEPO/TmPyPBLiq/Al | 9.6/21/8.5 |
| H4 | 242/- | 4.93/0.66/3.04 | ITO/m-MTDATA/H4: FIrpic/DPEPO/Bphen/Ca:Al | 1.2/4.2/12.5 |
| H5 | 420/127 | 5.64/2.33/2.71 | ITO/HAT-CN/NPB/TCTA/mCBP/DACT-II: H5/TPBi/LiF/Al | 19.5/-/41.1 |
| H6 | 403/128 | 5.69/2.44/2.64 | ITO/HAT-CN/NPB/TCTA/mCBP/DACT-II: H6/TPBi/LiF/Al | 22.7/-/53.4 |
| H7 | 390/133 | 6.06/2.53/3.0 | ITO/DNTPD/NPB/TAPC/H7: FIrpic/TSPO1/LiF/Al | 18.9/-/- |
| H8 | 425/102 | 5.58/2.11/2.62 | ITO/PEDOT:PSS/TAPC/TCTA/H8:Ir(ppy)3/TmPyPB/LiF/Al | 22.2/72.5/52.5 |
| H9 | -/- | 5.6/2.2/2.8 | ITO/TcTa/H9: FIrpic/TmPyPB/LiF/Al | -/21.0/12.0 |
| H10 | 371/120 | 5.46/2.16/2.66 | ITO/MoO3/NPB/TCTA/H10: Ir(ppy)2(acac)/TPBi/LiF/Al | -/44.7/42.8 |
| H11 | 442/145 | 5.48/2.22/2.54 | ITO/MoO3/NPB/TCTA/H11: Ir(ppy)2(acac)/TPBi/LiF/Al | -/50.7/50.7 |
| H12 | 432/109 | 5.40/2.12/2.65 | ITO/MoO3/TCTA/H12: Ir(piq)2acac/TPBi/LiF/Al | 14.1/9.7/8.9 |
| Host | Td [°C]/Tg [°C] | HOMO/LUMO/Et [Ev] | Device Structure | EQE [%]/CE [cdA−1]/PE [lmW−1] |
|---|---|---|---|---|
| I1 | 180/- | 5,58/2.34/3.47 | - | - |
| I2 | 334/- | 5.44/1.89/3.39 | - | - |
| I3 | 304/- | 5.45/1.99/3.02 | ITO/MoO3/NPB/TCTA/I3: Ir(ppy)3/TPBI/LiF/Al | 26/34.8/117 |
| I4 | 318/- | 5.46/1.84/3.40 | - | - |
| I5 | 344/- | 5.37/1.86/3.41 | - | - |
| I6 | 145/- | 5.38/2.03/3.43 | - | - |
| I7 | 360/- | 5.4/2.0/2.51 | ITO/TAPC/I7: Ir(ppy)3/B3PYMPM/LiF/Al | 16.7/62.5/69.7 |
| I8 | 290/105 | 6.21/2.86/2.88 | ITO/I7: Ir(ppy)3/LiF/Al | 6.5/-/- |
| I9 | 378/89 | 5.61/2.21/2.78 | ITO/PEDOT:PSS/TAPC/I9: Firpic or Ir(ppy)3/TmPyPB/LiF/Al | 19.5/40.8/28.9 or 29/91.2/57.6 |
| I10 | 423/208 | 5.24/1.16/3.02 | ITO/MoO3/TAPC/I10: FIrpic/TmPyPB/LiF/Al | 17.5/26.4/38.1 |
| I11 | 240/156 | 5.34/0.92/3.01 | ITO/MoO3/TAPC/I11: FIrpic/TmPyPB/LiF/Al | 22.5/46.9/49.1 |
| I12 | 431/- | 5.29/1.02/3.01 | ITO/MoO3/TAPC/I12: FIrpic/TmPyPB/LiF/Al | - |
| I13 | 207.3/- | 5.22/1.35/2.77 | ITO/Tris-PCz: ReO3/Tris-PCz/I13: (bt)2Ir(acac)/3NT2T/LiF/Al | 5/5.5/6.16 |
| I14 | 320/101 | 6.01/2.61/3.40 | ITO/MoO3/TCTA/(Ir(piq)2acac:I14/TPBi/LiF/Al. | 18.7/65.2/74.3 |
| I15 | 444/97 | 6.1/2.82/3.28 | ITO/MoO3/TCTA/(Ir(piq)2acac:I15/TPBi/LiF/Al. | 17.8/62.2/69.3 |
| I16 | 395/103 | 5.6/2.4/2.7/2.63 | ITO/MoO3/NPB/mCP/I16: FIrpic/TmPyPB/LiF/Al | 17/33.5/30.3 |
| I17 | 438/115 | 5.63/2.42/2.59 | ITO/MoO3/TCTA/I17: Ir(piq)2acac/TPBi/LiF/Al | 18.4/64.2/63.3 |
| I18 | 432/116 | 5.73/2.44/2.52 | ITO/MoO3/TCTA/I18: Ir(piq)2acac/TPBi/LiF/Al | 16.9/59.5/59.6 |
| I19 | 384/95 | 6.09/2.87/2.70 | ITO/MoO3/NPB/TCTA/I19: Ir(ppy)3/TPBi/LiF/Al | 15/51.7/37.8 |
| I20 | -/- | 6.10/2.86/2.6 | ITO/2-TNATA/NPB/I20: Ir(ppy)3/Alq3/LiF/Al | -/37.5/46.7 |
| I21 | -/- | 5.93/2.77/2.5 | ITO/2-TNATA/NPB/I21: Ir(ppy)3/Alq3/LiF/Al | -/2.5/3.2 |
| I22 | -/- | 6.1/2.57/2.8 | ITO/PEDOT:PSS/DNTPD/BPBPA/PCZAC/I22:5CzCN/DBFTrz/ZADN | 17.1/-/- |
| I23 | -/- | 6.07/2.57/2.78 | ITO/PEDOT:PSS/DNTPD/BPBPA/PCZAC/I23:5CzCN/DBFTrz/ZADN | 16/-/- |
| I24 | 324/245 | 6.56/3.07/2.66 | ITO/MoO3/TCTA/I24: Ir(ppy)3/TPBi/LiF/Al | 13.5/48.2/50.4 |
| I25 | 326/196 | 5.45/2.01/2.51 | ITO/MoO3/TCTA/I25: Ir(ppy)3/TPBi/LiF/Al | 12.2/42.6/38.2 |
| I26 | -/- | 6.06/3.24/- | ITO/PEDOT:PSS/TAPC/mCP/I26:Ir(ppy)3/TSPO1/TPBi/LiF/Al | 18.8/-/- |
| I27 | -/- | 6.06/3.25/- | ITO/PEDOT:PSS/TAPC/mCP/I27:Ir(ppy)3/TSPO1/TPBi/LiF/Al | 18.4/-/- |
| I28 | 425/110 | 5.47/2.38/2.60 | ITO/Tris-PCz:ReO3/Tris-PCz/I28: (ppy)2Ir(acac)/CN-T2T/Liq/Al | 14.9/53.5/76.5 |
| I29 | 324/- | 5.49/2.41/2.59 | ITO/Tris-PCz:ReO3/Tris-PCz/I29: (bt)2Ir(acac)/CN-T2T/Liq/Al | 22.4/57.3/72.5 |
| I30 | 420/108 | 5.48/2.45/2.57 | ITO/Tris-PCz:ReO3/Tris-PCz/I30: (ppy)2Ir(acac)/CN-T2T/Liq/Al | 22/79.8/102.5 |
| I31 | 425/- | 5.59/2.70/2.44 | ITO/HATCN/TAPC/I31: Ir(ppy)3/TmPyPB/LiF/Al | 15.4/45.6/- |
| I32 | 400/- | 5.63/2.57/2.63 | ITO/HATCN/TAPC/I32: Ir(ppy)3/TmPyPB/LiF/Al | 16.4/48.7/- |
| Host | Td [°C]/Tg [°C] | HOMO/LUMO/Et [Ev] | Device Structure | EQE [%]/CE [cdA−1]/PE [lmW−1] |
|---|---|---|---|---|
| J1 | 373/99 | 4.83/1.94/2.56 | ITO/CuI/BIPC3/J1/TCz1/Ca/Al | 17/13.5/- |
| J2 | 411/100 | 4.81/1.75/2.66 | ITO/CuI/BIPC3/J2/TCz1/Ca/Al | 5/15/- |
| J3 | 417/210 | 5.30/1.78/- | ITO/PEDOT:PSS/TAPC/J3: FIrpic/TmPyPB/LiF/Al | -/124/- |
| J4 | 433/228 | 5.54/1.82/2.86 | ITO/PEDOT:PSS/J4: Ir(mppy)3/TmPyPb/LiF/Al | 7.8/26/- |
| J5 | 366/125 | 5.61/1.87/2.87 | ITO/PEDOT:PSS/J5: Ir(mppy)3/TmPyPb/LiF/Al | 6.3/21.4/- |
| J6 | 459/244 | 5.58/1.77/2.86 | ITO/PEDOT:PSS/J6: Ir(mppy)3/TmPyPb/LiF/Al | 7.2/23.8/- |
| J7 | 480/170 | 5.49/2.37/2.48 | ITO/PEDOT:PSS/DTAF/J7: (pbi)2Ir(acac)/TPBI/LiF/Al | 19.2/62/62 |
| J8 | 359/- | 5.52/2.02/2.63 | ITO/PEDOT:PSS/DTAF/J8: Os(bpftz)2(PPhMe2)2/DPPS/LiF/Al | 19.4/18.4/19.3 |
| J9 | 427/153 | 5.60/2.14/2.63 | ITO/PEDOT:PSS/DTAF/J9: (PBi)2Ir(acac)/DPPS/LiF/Al | 21.4/72.9/64.1 |
| J10 | 428/- | 5.54/1.27/2.99 | ITO/TAPC/Ir(dbfmi):J10/B3PyPB/LiF/Al | 9.42/19.9/10.4 |
| J11 | 428/- | 5.74/1.26/2.99 | ITO/TAPC/Ir(dbfmi):J11/B3PyPB/LiF/Al | 12.21/15.91/9.89 |
| J12 | 440/115 | 6.04/1.50/2.99 | ITO/TAPC/Ir(dbfmi):J12/B3PyPB/LiF/Al | 20.8/29.9/33.4 |
| J13 | 393/89 | -/-/- | ITO/CuI/J13: Ir(Fppy)3/TCz1/Ca/Al | -/12.2/- |
| J14 | 441/77 | -/-/- | ITO/CuI/J14: Ir(Fppy)3/TCz1/Ca/Al | -/-/- |
| J15 | 459/92 | -/-/- | ITO/CuI/J15: Ir(Fppy)3/TCz1/Ca/Al | -/-/- |
| J16 | 446/125 | 5.50/2.19/2.87 | ITO/PEDOT: PSS/TAPC/J16: FIrpic/TmPyPB/LiF/Al | 21.6/41.4/31.2 |
| J17 | 480/133 | 5.51/2.59/2.94 | ITO/PEDOT: PSS/TAPC/J17: FIrpic/TmPyPB/LiF/Al | 25.8/45.6/44.2 |
| J18 | 471/125 | 5.50/2.50/2.68 | ITO/PEDOT: PSS/TAPC/J18: FIrpic/TmPyPB/LiF/Al | 21.6/45/40.4 |
| J19 | 498/156 | 5.61/2.55/2.96 | ITO/PEDOT: PSS/TAPC/J19: FIrpic/TmPyPB/LiF/Al | 23.5/54.4/42.5 |
| J20 | 480/145 | 5.63/2.54/2.94 | ITO/PEDOT: PSS/TAPC/J20: FIrpic/TmPyPB/LiF/Al | 27/51.9/46.5 |
| J21 | -/- | 5.52/2.57/2.92 | ITO/PEDOT: PSS/TAPC/J21: (Ir(ppy)3/BmPyPb/LiF/Al | 7.8/23.8/19.5 |
| J22 | 482/148 | 5.68/3.35/5.21 | ITO/MoO3/NPD/J22:(piq)2Ir(acac)/BCP/Bebq2/LiF/Al | 19.3/16.4/13 |
| J23 | 346/168 | 5.54/2.30/2.62 | ITO/HATCN/NPB/TAPC/J23: Firpic/TmPyPB/LiF/Al | 27.2/46.3/36.5 |
| J24 | 402/168 | 5.11/1.04/2.94 | ITO/MoOx/NPB/TCTA/J24: FIrpic/TmPyPB/Cs2CO3/Al | 20.7/43.7/32.7 |
| J25 | 234/110 | 5.55/1.90/2.83 | ITO/TAPC/J25: Ir(ppy)2(acac)/BPhen/BPhen/Cs2CO3/Ag | -/38.6/- |
| J26 | 384/130 | 6.48/2.83/2.77 | ITO/PEDOT: PSS/PVK/J26: FIrpic/Ba/Al | -/1.14/- |
| J27 | 364/101 | 6.39/2.80/2.76 | ITO/PEDOT: PSS/PVK/J27: FIrpic/Ba/Al | -/4.16/- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krucaite, G.; Grigalevicius, S. Low Molar Mass Carbazole-Based Host Materials for Phosphorescent Organic Light-Emitting Diodes: A Review. Coatings 2025, 15, 398. https://doi.org/10.3390/coatings15040398
Krucaite G, Grigalevicius S. Low Molar Mass Carbazole-Based Host Materials for Phosphorescent Organic Light-Emitting Diodes: A Review. Coatings. 2025; 15(4):398. https://doi.org/10.3390/coatings15040398
Chicago/Turabian StyleKrucaite, Gintare, and Saulius Grigalevicius. 2025. "Low Molar Mass Carbazole-Based Host Materials for Phosphorescent Organic Light-Emitting Diodes: A Review" Coatings 15, no. 4: 398. https://doi.org/10.3390/coatings15040398
APA StyleKrucaite, G., & Grigalevicius, S. (2025). Low Molar Mass Carbazole-Based Host Materials for Phosphorescent Organic Light-Emitting Diodes: A Review. Coatings, 15(4), 398. https://doi.org/10.3390/coatings15040398








