Mechanical and Electrochemical Properties of Titanium Aluminum Nitride Coatings with Different Nitrogen Flow Rates on CrMnSi Steel by Filter Cathode Vacuum Arc Technology
Abstract
:1. Introduction
2. Materials and Methods
3. Results
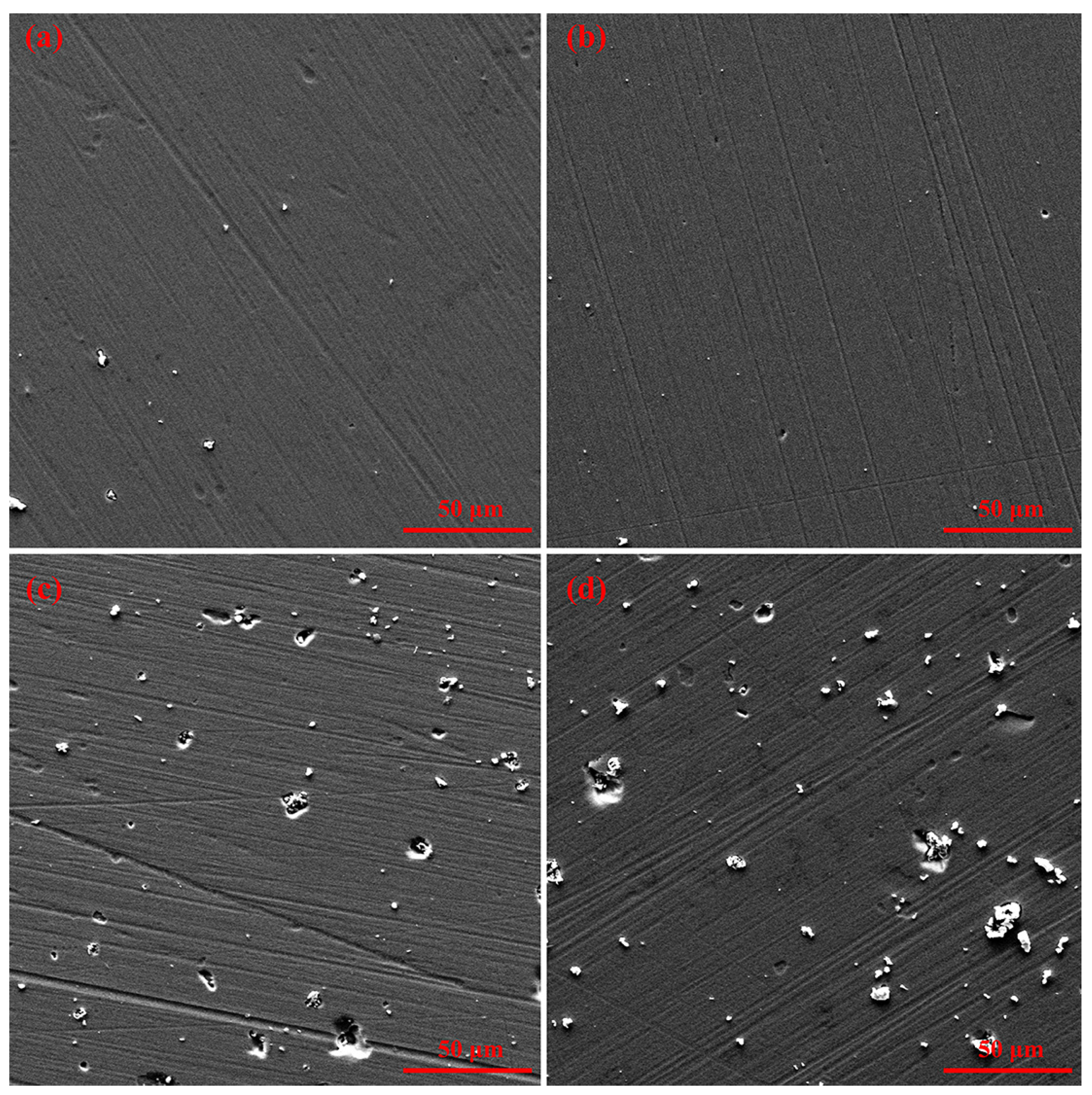
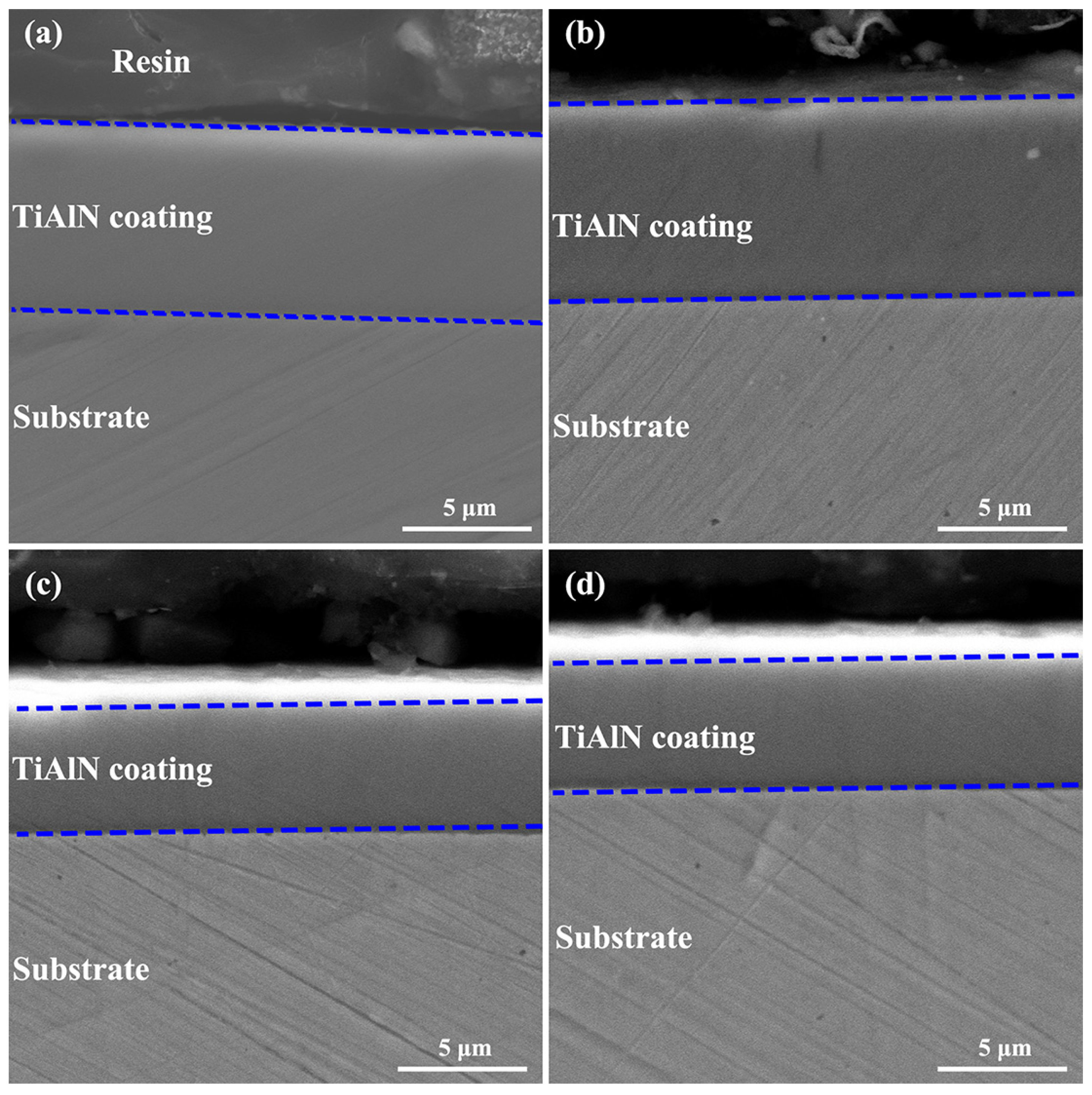
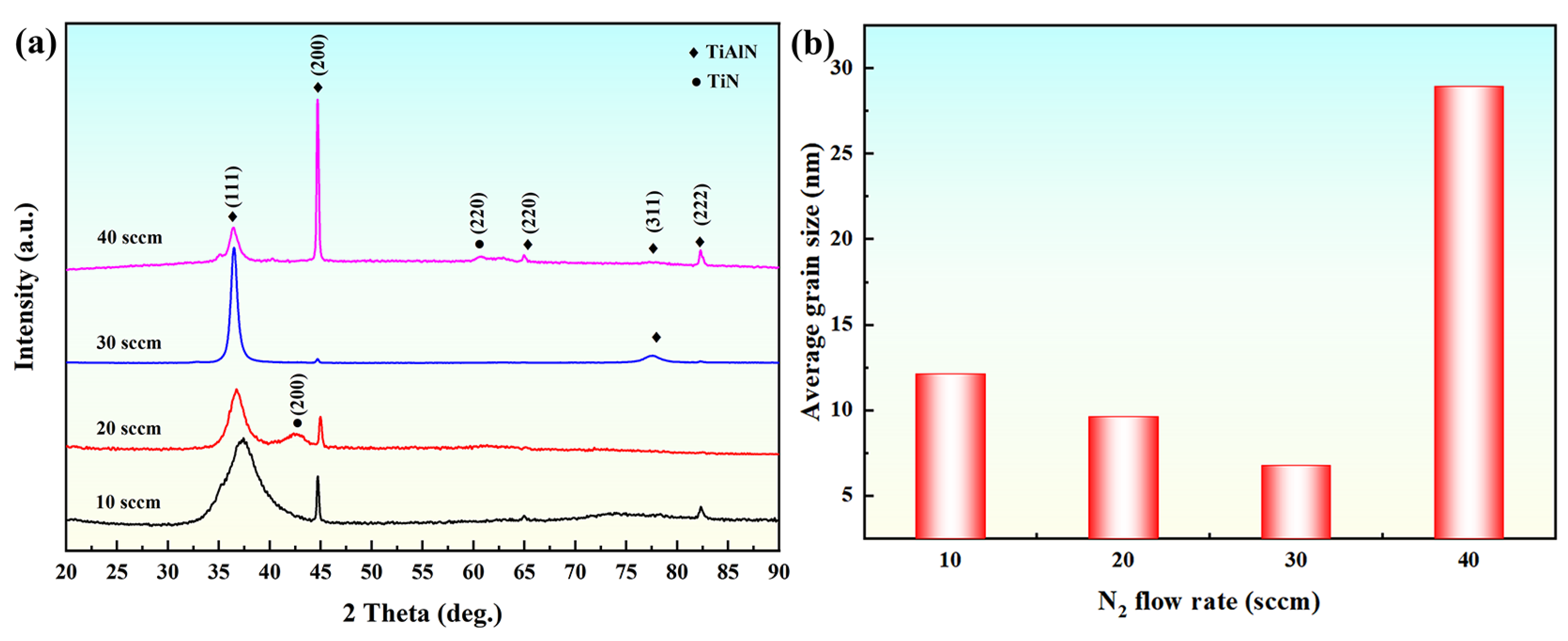
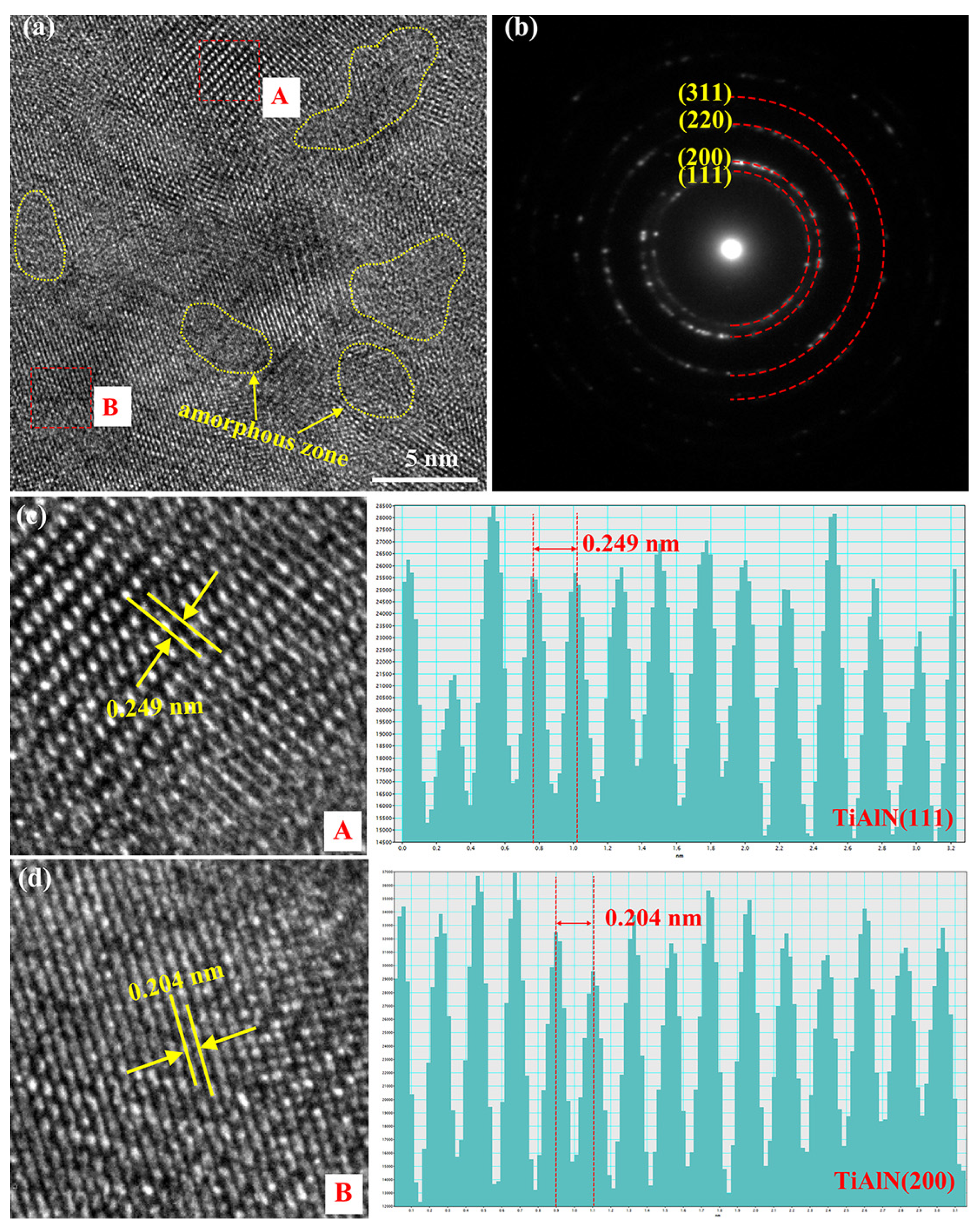
3.1. Structural Regulation of Coatings by Nitrogen Flow Rate
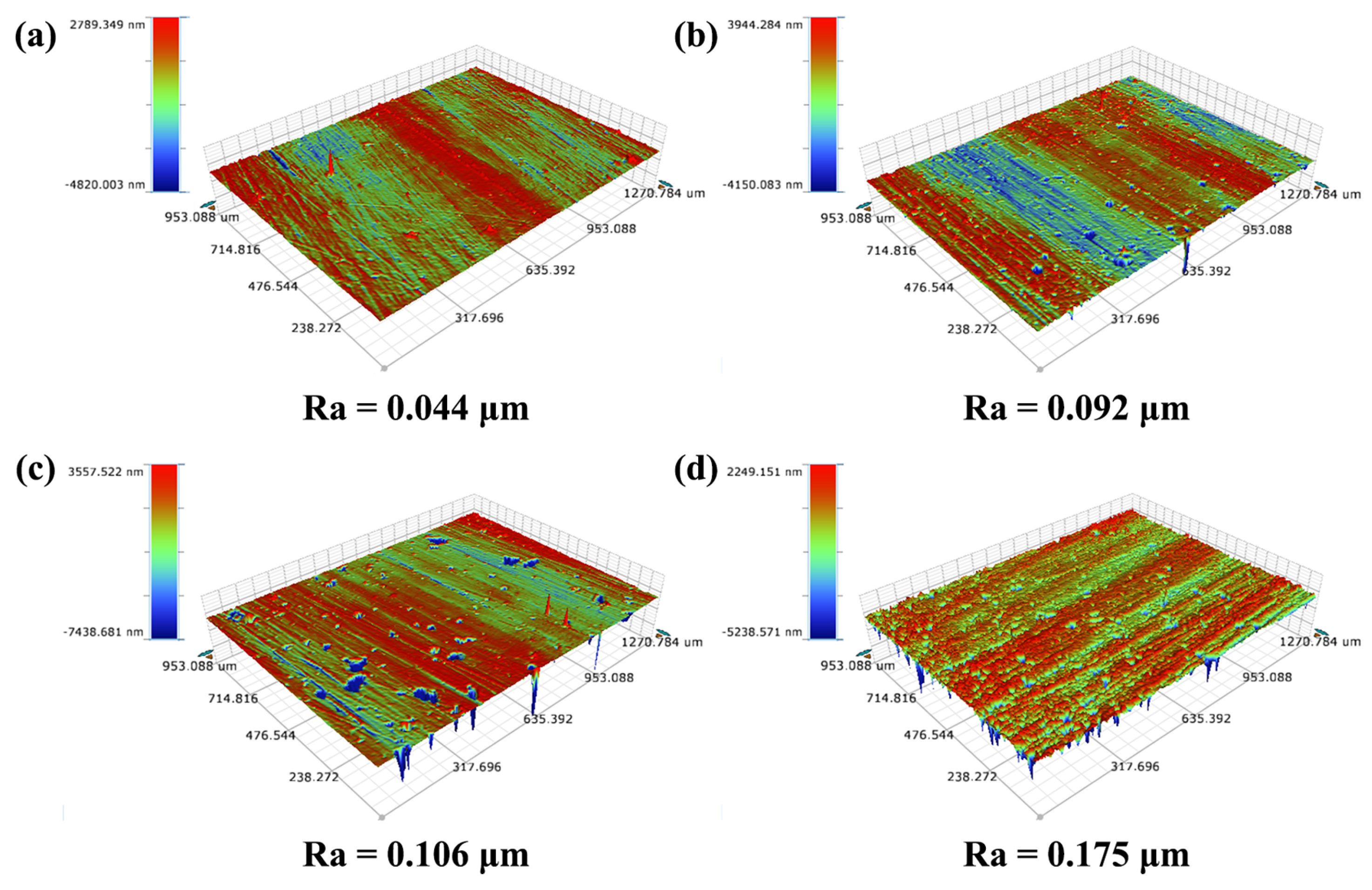
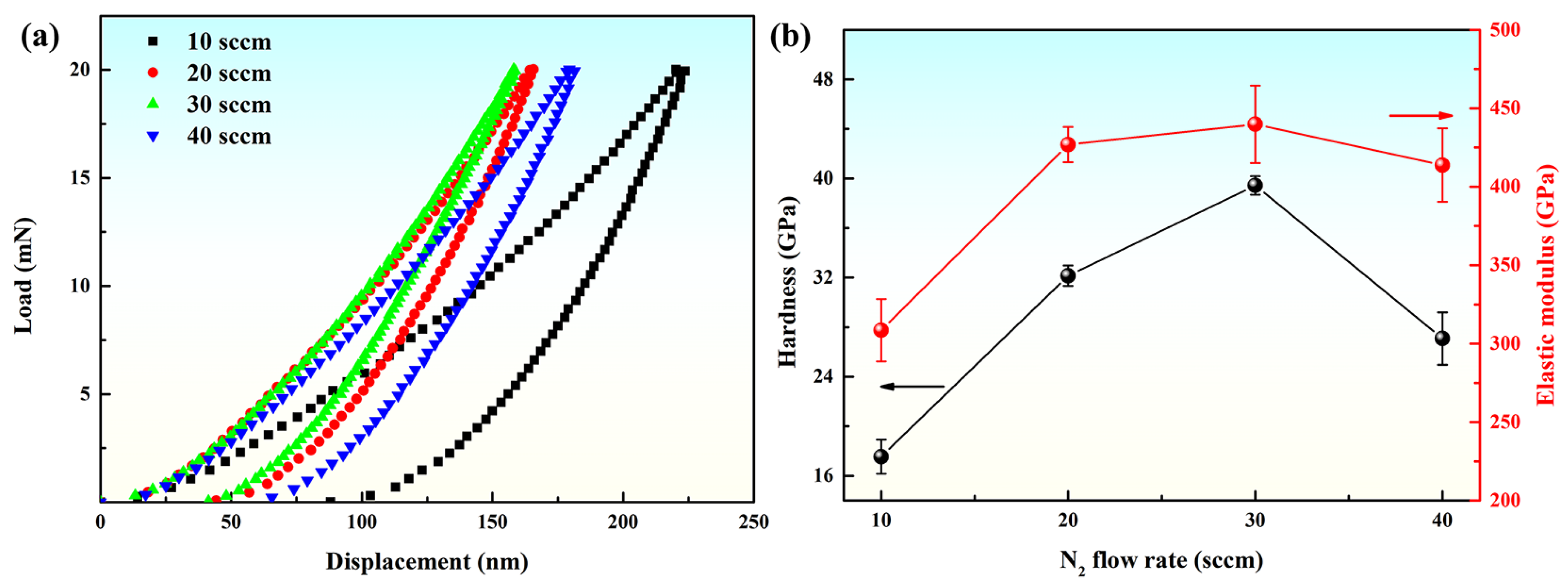
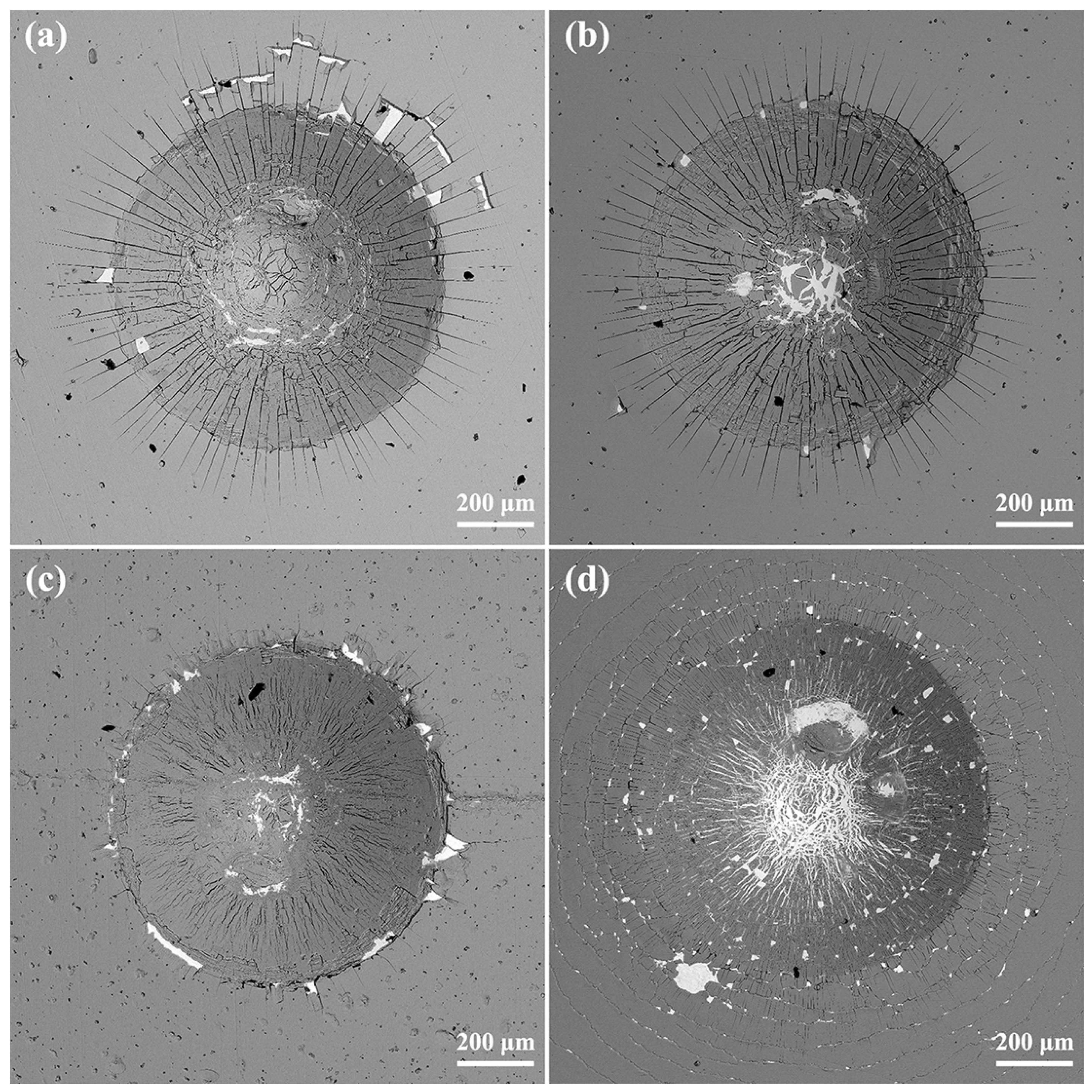
3.2. Mechanical Behaviors Regulation of Coatings by Nitrogen Flow Rate
3.3. Electrochemical Performance Regulation of Coatings by Nitrogen Flow Rate
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fan, W.; Wang, J.; Peng, Y.; Tan, H.; Qi, Y.; Zhang, Y.F.; He, B.; Wang, X.; Lin, X. Anisotropic dynamic compression response of an ultra-high strength steel fabricated by laser hybrid additive manufacturing. Addit. Manuf. 2024, 86, 104186. [Google Scholar] [CrossRef]
- Li, J.; Zhan, D.; Jiang, Z.; Zhang, H.; Yang, Y.; Zhang, Y. Progress on improving strength-toughness of ultra-high strength martensitic steels for aerospace applications: A review. J. Mater. Res. Technol. 2023, 23, 172–190. [Google Scholar]
- Fan, W.; Wang, J.; Peng, Y.; Tan, H.; Wang, Y.; Qi, Y.; Feng, Z.; Zhang, F.; He, B.; Lin, X. Microstructure and mechanical properties of an ultra-high strength steel fabricated by laser hybrid additive manufacturing. Mater. Sci. Eng. A 2023, 885, 145594. [Google Scholar] [CrossRef]
- Wang, F.; Wang, W.; Du, J.; Zhang, L.; Sohn, I.L.; Zeng, J. Oxidation behavior of Cr–Mn–Si alloyed steel by mold flux melt in high temperatures. J. Mater. Res. Technol. 2024, 29, 3898–3909. [Google Scholar]
- Jiang, C.C.; Xiao, G.Y.; Zhang, X.; Zhu, R.F.; Lu, Y.P. Formation and corrosion resistance of a phosphate chemical conversion coating on medium carbon low alloy steel. New J. Chem. 2016, 40, 1347–1353. [Google Scholar]
- Meng, J.; Shi, X.; Zhang, S.; Wang, M.; Xue, F.; Liu, B.; Cui, W.; Bian, L. Friction and wear properties of TiN-TiB2-Ni based composite coatings by argon arc cladding technology. Surf. Coat. Technol. 2019, 374, 437–447. [Google Scholar]
- Sun, S.; Wu, Z.; Pang, M.; Chang, J.; Xuan, Y.; Qi, H.; Yang, R.; Wu, Y. Microstructure and corrosion behavior of chromium-rich stainless steel coatings deposited by different laser cladding processes. J. Mater. Res. Technol. 2024, 29, 3879–3890. [Google Scholar]
- Wang, B.; Chen, Q.X.; Liu, H.X.; Yasuo, N.; Okido, S. Method to improve the wear and corrosion resistance of piston rods by thermal spray. Hydraul. Pneum. Seals 2013, 10, 59–61. [Google Scholar]
- Büker, L.; Böttcher, R.; Leimbach, M.; Hahne, T.; Dickbreder, R.; Bund, A. Influence of carboxylic acids on the performance of trivalent chromium electrolytes for the deposition of functional coatings. Electrochim. Acta 2022, 411, 140054. [Google Scholar]
- Zhao, Y.; Wang, K.; Wang, Z.; Shi, M.; Li, F.; Ye, C. Effect of different surface treatments of 42CrMo steel piston rod on its corrosion resistance in sodium chloride solution. Int. J. Electrochem. Sci. 2022, 17, 220850. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, X.; Gao, J.; Shi, Y.; Geng, L.; Zhang, Y. Surface crack propagation of electroplated chromium coating and cotton fabric with dry friction. Eng. Failure Anal. 2024, 163, 108485. [Google Scholar]
- Huang, C.-Y.; Chen, Y.; Lin, C.-S. High-temperature oxidation resistance of hot stamping steel with chromium coating electroplated in trivalent chromium bath. Mater. Today Commun. 2022, 33, 104663. [Google Scholar]
- Cui, Y.; Zheng, G.; Zhang, J.; Jing, C.; Cheng, X.; Yang, X. Life cycle comprehensive performance assessment of the TiAlN coated tool based on surface treatment. J. Clean. Prod. 2024, 434, 139811. [Google Scholar]
- Liang, J.; Gao, H.; Li, D.; Lei, Y.; Li, S.; Guo, L.; Chen, L.; Leng, Z.; Sun, Y.; Li, C. Study on milling tool wear morphology and mechanism during machining superalloy GH4169 with PVD-TiAlN coated carbide tool. Tribol. Int. 2023, 182, 108298. [Google Scholar]
- Guan, Q.; Han, J.; Zhou, S.; Guan, J.; Zhang, C.; Cao, F.; Chen, X. Improved mechanical and tribological properties of TiAlN coatings by high current pulsed electron beam irradiation. Int. J. Refract. Met. Hard Mater. 2024, 118, 106435. [Google Scholar]
- Zhang, M.; Li, Y.; Feng, Y.; Xin, L.; Niu, Y.; Su, J.; Zhu, S.; Wang, F. Studies on different oxidation behaviors of TiAlN on titanium alloy and stainless steel under thermal cycling. Corros. Sci. 2021, 192, 109865. [Google Scholar] [CrossRef]
- Sivagnanam Chandra, N.P.; Otsuka, Y.; Mutoh, Y.; Yamamoto, K. Effect of coating thickness on fatigue behavior of TiAlN coated Ti-alloys. Int. J. Fatigue 2020, 140, 105767. [Google Scholar]
- Moreno, M.; Andersson, J.M.; M’Saoubi, R.; Kryzhanivskyy, V.; Johansson-Jöesaar, M.P.; Johnson, L.J.S.; Odén, M.; Rogström, L. Adhesive wear of TiAlN coatings during low speed turning of stainless steel 316 L. Wear 2023, 524–525, 204838. [Google Scholar]
- Yin, Z.; Wu, S.; Zhang, Y.; Yan, W.; Dai, S.; Peng, X.; Liao, B.; Zhang, X.; Wang, J.; Ouyang, X.; et al. A comparative study on the structure and properties of TiAlSiN coatings deposited by FCVA and HiPIMS. J. Alloys Compd. 2024, 1005, 175844. [Google Scholar]
- Zhang, R.; Lee, W.-Y.; Umehara, N.; Tokoroyama, T.; Murashima, M.; Takimoto, Y. The development of B/Cr co-doped DLC coating by FCVA deposition system and its tribological properties at 300 °C. Surf. Coat. Technol. 2024, 487, 130968. [Google Scholar]
- Guo, L.; Wang, X.; Lu, L.; Cao, H.; Dai, Y.; Tang, K.; Zhao, N.; Qi, F.; Ouyang, X. Different corrosion behaviors of Sn-based modification coatings on magnesium alloy surface via plasma-involved processes: FCVA deposition vs MEVVA ion implantation. Appl. Surf. Sci. 2025, 684, 161842. [Google Scholar]
- Zhang, L.; Shen, Y.-Q.; Zhao, Y.-m.; Chen, S.-N.; Ouyang, X.; Zhang, X.; Liang, H.; Liao, B.; Chen, L. Structure control of high-quality TiAlN Monolithic and TiAlN/TiAl multilayer coatings based on filtered cathodic vacuum arc technique. Surf. Interfaces 2023, 38, 204838. [Google Scholar] [CrossRef]
- Ahlgren, M.; Blomqvist, H. Influence of bias variation on residual stress and texture in TiAIN PVD coatings. Surf. Coat. Technol. 2005, 200, 157–160. [Google Scholar]
- Skordaris, G.; Bouzakis, K.D.; Charalampous, P.; Kotsanis, T.; Bouzakis, E.; Bejjani, R. Bias voltage effect on the mechanical properties, adhesion and milling performance of PVD films on cemented carbide inserts. Wear 2018, 404–405, 50–61. [Google Scholar]
- Zhao, B.; Zhao, X.; Lin, L.; Zou, L. Effect of bias voltage on mechanical properties, milling performance and thermal crack propagation of cathodic arc ion-plated TiAlN coatings. Thin Solid Film. 2020, 708, 138116. [Google Scholar]
- Li, D.; Chen, J.; Zou, C.; Ma, J.; Li, P.; Li, Y. Effects of Al concentrations on the microstructure and mechanical properties of Ti–Al–N films deposited by RF-ICPIS enhanced magnetron sputtering. J. Alloys Compd. 2014, 609, 239–243. [Google Scholar]
- Schramm, I.C.; Johansson Jõesaar, M.P.; Jensen, J.; Mücklich, F.; Odén, M. Impact of nitrogen vacancies on the high temperature behavior of (Ti1−x Alx)Ny alloys. Acta Mater. 2016, 119, 218–228. [Google Scholar] [CrossRef]
- Liu, L.; Tang, W.; Zhou, L.; Wu, Z.; Ruan, Q.; Li, X.; Qasim, A.M.; Cui, S.; Li, T.; Fu, R.K.Y.; et al. Comparative study of TiAlN coatings deposited by different high-ionization physical vapor deposition techniques. Ceram. Int. 2020, 46, 10814–10819. [Google Scholar]
- Schramm, I.C.; Pauly, C.; Johansson Jõesaar, M.P.; Slawik, S.; Suarez, S.; Mücklich, F.; Odén, M. Effects of nitrogen vacancies on phase stability and mechanical properties of arc deposited (Ti0.52Al0.48)Ny (y < 1) coatings. Surf. Coat. Technol. 2017, 330, 77–86. [Google Scholar]
- Zhang, G.P.; Gao, G.J.; Wang, X.Q.; Lv, G.H.; Zhou, L.; Chen, H.; Pang, H.; Yang, S.Z. Influence of pulsed substrate bias on the structure and properties of Ti–Al–N films deposited by cathodic vacuum arc. Appl. Surf. Sci. 2012, 258, 7274–7279. [Google Scholar]
- Cai, F.; Chen, M.; Li, M.; Zhang, S. Influence of negative bias voltage on microstructure and property of Al-Ti-N films deposited by multi-arc ion plating. Ceram. Int. 2017, 43, 3774–3783. [Google Scholar] [CrossRef]
- Cao, H.; Yang, J.; Luo, W.; Li, Y.; Qi, F.; Zhao, N.; Lu, L.; Ouyang, X. Influence of film structure on the microstructure and properties of TiAlN coatings on Al-Si alloys. Mater. Charact. 2022, 189, 111996. [Google Scholar]
- Cao, H.-S.; Liu, F.-J.; Li, H.; Luo, W.-Z.; Qi, F.-G.; Lu, L.-W.; Zhao, N.; Ouyang, X.-P. Effect of bias voltage on microstructure, mechanical and tribological properties of TiAlN coatings. Trans. Nonferrous Met. Soc. China 2022, 32, 3596–3609. [Google Scholar] [CrossRef]
- Li, Y.; Cao, H.; Li, H.; Yang, J.; Qi, F.; Lu, L.; Zhao, N.; Zhou, Y.; Ouyang, X. Effect of buffer layer on oxidation and corrosion resistance of CrN coatings on Zr alloy prepared by FCVAD technology. Surf. Coat. Technol. 2022, 448, 128942. [Google Scholar] [CrossRef]
- Liu, F.; Cao, H.; Li, H.; Yang, J.; Zhao, N.; Qi, F.; Ouyang, X. Effect of annealing on the microstructure, mechanical and electrochemical properties of CrAlC coatings. Surf. Coat. Technol. 2022, 447, 128800. [Google Scholar] [CrossRef]
- Hussein, M.; Kumar, M.; Ankah, N.; Abdelaal, A. Surface, mechanical, and in vitro corrosion properties of arc-deposited TiAlN ceramic coating on biomedical Ti6Al4V alloy. Trans. Nonferrous Met. Soc. China 2023, 33, 494–506. [Google Scholar] [CrossRef]
- Zhao, S.-S.; Du, H.; Zheng, J.-D.; Yang, Y.; Wang, W.; Gong, J.; Sun, C. Deposition of thick TiAlN coatings on 2024 Al/SiCp substrate by Arc ion plating. Surf. Coat. Technol. 2008, 202, 5170–5174. [Google Scholar]
- Poláková, H.; Musil, J.; Vlček, J.; Allaart, J.; Mitterer, C. Structure-hardness relations in sputtered Ti–Al–V–N films. Thin Solid Film. 2003, 444, 189–198. [Google Scholar]
- Khamseh, S.; Nose, M.; Kawabata, T.; Matsuda, K.; Ikeno, S. Influence of total gas pressure on the microstructure and properties of CrAlN films deposited by a pulsed DC balanced magnetron sputtering system. J. Alloys Compd. 2010, 503, 389–391. [Google Scholar]
- Chen, J.T.; Wang, J.; Zhang, F.; Zhang, G.A.; Fan, X.Y.; Wu, Z.G.; Yan, P.X. Characterization and temperature controlling property of TiAlN coatings deposited by reactive magnetron co-sputtering. J. Alloys Compd. 2009, 472, 91–96. [Google Scholar]
- Wang, Y.Q.; Zong, X.Y.; Jiang, Z.Q.; Tian, X.B. Characterization and mechanical properties of TiN/TiAlN multilayer coatings with different modulation periods. Int. J. Adv. Manuf. Technol. 2018, 96, 1677–1683. [Google Scholar]
- Anders, A. Ion charge state distributions of vacuum arc plasmas: The origin of species. Phys. Rev. A 1997, 55, 969–981. [Google Scholar] [CrossRef]
- Pinot, Y.; Tuilier, M.H.; Pac, M.J.C.; Rousselot, D.; Thiaudière, C. Ulhaq-Bouillet, Influence of film thickness on the structural transition cubic/hexagonal within Ti0.38Al0.62N films. Thin Solid Film. 2018, 649, 160–166. [Google Scholar]
- Yang, F.-C.; Lin, C.-Y.; Tang, J.-F.; Chang, C.-L. Effect of mid-frequency pulse insertion on the microstructural and mechanical properties of AlTiN coatings prepared using superimposed HiPIMS process. Surf. Coat. Technol. 2020, 388, 125597. [Google Scholar]
- Niu, X.; Dong, G.; Wei, S.; Wang, Y.; Wang, B.; Tian, H. Effects of nitrogen flow rate on the microstructure and mechanical and tribological properties of TiAlN films prepared via reactive magnetron sputtering. Ceram. Int. 2023, 49, 19885–19894. [Google Scholar]
- Chakrabarti, K.; Jeong, J.J.; Hwang, S.K.; Yoo, Y.C.; Lee, C.M. Effects of nitrogen flow rates on the growth morphology of TiAlN films prepared by an rf-reactive sputtering technique. Thin Solid Films 2002, 406, 159–163. [Google Scholar]
- Patsalas, P.; Gravalidis, C.; Logothetidis, S. Surface kinetics and subplantation phenomena affecting the texture, morphology, stress, and growth evolution of titanium nitride films. J. Appl. Phys. 2004, 96, 6234–6246. [Google Scholar] [CrossRef]
- Cao, X.; He, W.; He, G.; Liao, B.; Zhang, H.; Chen, J.; Lv, C. Sand erosion resistance improvement and damage mechanism of TiAlN coating via the bias-graded voltage in FCVA deposition. Surf. Coat. Technol. 2019, 378, 125009. [Google Scholar]
- Kumar, D.D.; Rani, R.; Kumar, N.; Panda, K.; Kirubaharan, A.M.K.; Kuppusami, P.; Baskaran, R. Tribochemistry of TaN, TiAlN and TaAlN coatings under ambient atmosphere and high-vacuum sliding conditions. Appl. Surf. Sci. 2020, 499, 143989. [Google Scholar]
- Alishahi, M.; Mahboubi, F.; Mousavi Khoie, S.M.; Aparicio, M.; Lopez-Elvira, E.; Méndez, J.; Gago, R. Structural properties and corrosion resistance of tantalum nitride coatings produced by reactive DC magnetron sputtering. RSC Adv. 2016, 6, 89061–89072. [Google Scholar] [CrossRef]
- Rizzo, A.; Mirenghi, L.; Massaro, M.; Galietti, U.; Capodieci, L.; Terzi, R.; Tapfer, L.; Valerini, D. Improved properties of TiAlN coatings through the multilayer structure. Surf. Coat. Technol. 2013, 235, 475–483. [Google Scholar]
- Zhou, W.; Liang, J.; Zhang, F.; Mu, J.; Zhao, H. A comparative research on TiAlN coatings reactively sputtered from powder and from smelting TiAl targets at various nitrogen flow rates. Appl. Surf. Sci. 2014, 313, 10–18. [Google Scholar]
- Li, G.; Zhang, L.; Cai, F.; Yang, Y.; Wang, Q.; Zhang, S. Characterization and corrosion behaviors of TiN/TiAlN multilayer coatings by ion source enhanced hybrid arc ion plating. Surf. Coat. Technol. 2019, 366, 355–365. [Google Scholar] [CrossRef]
- Cedeño-Vente, M.L.; Manríquez, J.; Mondragón-Rodríguez, G.C.; Camacho, N.; Gómez-Ovalle, A.E.; Gonzalez-Carmona, J.M.; Alvarado-Orozco, J.M.; Espinosa-Arbelaez, D.G. Application of a transmission line model to evaluate the influence of structural defects on the corrosion behavior of arc-PVD CrN coatings. Ceram. Int. 2021, 47, 20885–20899. [Google Scholar]
- Wang, H.W.; Stack, M.M.; Lyon, S.B.; Hovsepian, P.; Münz, W.D. The corrosion behaviour of macroparticle defects in arc bond-sputtered CrN/NbN superlattice coatings. Surf. Coat. Technol. 2000, 126, 279–287. [Google Scholar]
- Sugumaran, A.A.; Purandare, Y.; Ehiasarian, A.P.; Hovsepian, P.E.J.C. Corrosion Behavior of post-deposition polished droplet-embedded arc evaporated and droplet-free high power impulse magnetron sputtering/direct current magnetron sputtering. Coatings 2017, 73, 685–693. [Google Scholar]
- Chen, W.; Hu, T.; Wang, C.; Xiao, H.; Meng, X. The effect of microstructure on corrosion behavior of a novel AlCrTiSiN ceramic coating. Ceram. Int. 2020, 46, 12584–12592. [Google Scholar]
- Ananthakumar, R.; Subramanian, B.; Kobayashi, A.; Jayachandran, M. Electrochemical corrosion and materials properties of reactively sputtered TiN/TiAlN multilayer coatings. Ceram. Int. 2012, 38, 477–485. [Google Scholar]




| Deposition Parameters | Values |
|---|---|
| Arc source | TiAl alloy |
| Arc current | 90 A |
| Deposition temperature | 25 °C |
| Negative bias | −75 V |
| Filter duct current | 2.5 A |
| Based vacuum | 4.0 × 10−3 Pa |
| Duty cycle | 50% |
| N2 flow rate | 10 sccm; 20 sccm; 30 sccm, 40 sccm |
| Deposition time | 60 min |
| N2/sccm | Ti/at.% | Al/at.% | N/at.% | Al/(Ti + Al) Ratio | (Ti + Al)/N Ratio |
|---|---|---|---|---|---|
| 10 | 67.51 | 6.69 | 25.80 | 0.090 | 2.88 |
| 20 | 50.66 | 7.89 | 41.45 | 0.135 | 1.41 |
| 30 | 39.64 | 8.34 | 52.02 | 0.174 | 0.92 |
| 40 | 31.77 | 9.19 | 59.04 | 0.224 | 0.69 |
| N2/sccm | Hardness/GPa | Elastic Modulus/GPa | H/E | H3/E2/GPa | We/% |
|---|---|---|---|---|---|
| 10 | 17.5 ± 1.4 | 308.5 ± 19.8 | 0.057 | 0.057 | 58.7 |
| 20 | 32.2 ± 0.8 | 426.9 ± 11.2 | 0.075 | 0.182 | 72.7 |
| 30 | 39.5 ± 0.7 | 439.8 ± 24.7 | 0.090 | 0.317 | 74.3 |
| 40 | 27.1 ± 2.1 | 413.8 ± 23.5 | 0.065 | 0.116 | 66.7 |
| N2/sccm | Ecorr/V | icorr/μA∙cm−2 | βa/V∙dec−1 | βc/V∙dec−1 | Rp/kΩ∙cm2 |
|---|---|---|---|---|---|
| 10 | −0.298 | 0.671 | 0.238 | −0.139 | 56.83 |
| 20 | −0.472 | 0.820 | 0.109 | −0.144 | 32.87 |
| 30 | −0.682 | 1.17 | 0.131 | −0.050 | 13.43 |
| 40 | −0.605 | 1.795 | 0.100 | −0.133 | 13.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, H.; Ouyang, X.; Wu, X.; Chen, L.; Wu, J.; Wu, J.; Wang, J.; Liao, B. Mechanical and Electrochemical Properties of Titanium Aluminum Nitride Coatings with Different Nitrogen Flow Rates on CrMnSi Steel by Filter Cathode Vacuum Arc Technology. Coatings 2025, 15, 379. https://doi.org/10.3390/coatings15040379
Cao H, Ouyang X, Wu X, Chen L, Wu J, Wu J, Wang J, Liao B. Mechanical and Electrochemical Properties of Titanium Aluminum Nitride Coatings with Different Nitrogen Flow Rates on CrMnSi Steel by Filter Cathode Vacuum Arc Technology. Coatings. 2025; 15(4):379. https://doi.org/10.3390/coatings15040379
Chicago/Turabian StyleCao, Hongshuai, Xiao Ouyang, Xianying Wu, Lin Chen, Jiakun Wu, Jie Wu, Junfeng Wang, and Bin Liao. 2025. "Mechanical and Electrochemical Properties of Titanium Aluminum Nitride Coatings with Different Nitrogen Flow Rates on CrMnSi Steel by Filter Cathode Vacuum Arc Technology" Coatings 15, no. 4: 379. https://doi.org/10.3390/coatings15040379
APA StyleCao, H., Ouyang, X., Wu, X., Chen, L., Wu, J., Wu, J., Wang, J., & Liao, B. (2025). Mechanical and Electrochemical Properties of Titanium Aluminum Nitride Coatings with Different Nitrogen Flow Rates on CrMnSi Steel by Filter Cathode Vacuum Arc Technology. Coatings, 15(4), 379. https://doi.org/10.3390/coatings15040379




