Ni-P Coatings as Hydrogen Permeation Barriers—A Review
Abstract
1. Introduction
2. Hydrogen Permeation Mechanism
2.1. Hydrogen Permeability
2.2. Electrochemical Method for Evaluating the HP: Devanathan–Stachurski’s Cell
- A membrane with a fixed thickness, x = L, is being considered where the in-plane dimensions y and z are significantly larger than L;
- The concentration of hydrogen atoms at the production side of a membrane remains constant over time, and the concentration is zero on the detection side, i.e., C = C0 at x = 0 and C = 0 at x = L for any value of the time t.
- iss: is the steady state permeation current density (A/m2),
- L: is the thickness of the sample (m),
- Deff: is the effective diffusion coefficient (m2/s),
- F: is the Faraday constant (96,485 C/mol),
- MH: is the molar mass of hydrogen (1 g/mol),
- ρFe: is the iron density (7.87 × 106 g/m3).
3. Hydrogen Permeation Barriers
3.1. General
3.2. Ex Situ Surface Analytical Techniques for Evaluating HPBs’ Performance
| Technique | Information | References | |
|---|---|---|---|
| In situ | Gas permeation test | Diffusion coefficient; permeability coefficient; hydrogen solubility coefficient; permeation reduction factor | [44,47,48,49,71] |
| Devanathan–Stachurski’s (D-S) cell Electrochemical test | Diffusion coefficient; trap density; subsurface concentration of hydrogen; permeation reduction factor | [50,51,52,53] | |
| Ex situ | OM | Morphology; particle size | [64,70] |
| SEM | Information about the topography, chemical composition, and film thickness (combined with EDS); 2D detailed images | [64,66,67,68,69,70,71,72] | |
| AFM | Topography; surface roughness | [64,68,71] | |
| XPS/ARXPS/HAXPES | Information about chemical composition, chemical state identification and film thickness; qualitative and quantitative analysis; imaging/mapping. | [65,71,73,74,75,76,77,78,79,80] | |
| ERDA/RBS | H-concentration in films | [80] |
3.3. Production of Electroless Ni-P Coatings
- Source of Ni2+ ions: e.g., nickel sulfate or nickel chloride;
- Reducing agent: usually sodium hypo-phosphite (NaH2PO2);
- Complexing agents: as organic acids or their salts (acetic, malic, succinic, or citric). They prevent the formation of an excessive concentration of free metal ions, and they act as buffers and to delay the precipitation of nickel phosphite. These complexing agents exert a significant influence on the deposits’ quality and porosity levels;
- Stabilizers or accelerators: they are added in small amounts (ppm) in order to raise the deposition rate. The most common stabilizers used are Pb, As, Mo, Cd ions, malic, and thioureas;
- Temperature: it influences the kinetics and speed of deposition and it must be controlled to obtain a high-quality coating. The optimum operating temperature of an acid hypophosphite plating solution ranges from 85 °C to 90 °C. High temperatures beyond 90 °C might lead to solution “plate-out” or bath decomposition [28,88];
- pH regulator: pH is an important parameter since it affects the phosphorus content: the higher the pH value, the lower the phosphorus content obtained. The common pH regulators used are sodium hydroxide and/or sulfuric acid.
3.4. Properties of Electroless Ni-P Coatings
3.5. Nickel and Ni-P Coatings as Hydrogen Permeation Barriers
3.5.1. Pure Nickel
3.5.2. Electrodeposited Nickel (ED)
3.5.3. Amorphous or Electroless Nickel–Phosphorus
- Lcoat: is the thickness of the coating (μm)
- Lsteel: is the thickness of the steel substrate (μm)
- Ltot: is the thickness of the coated sample (μm)
- Dcoat: is the diffusion coefficient of the coating (cm2s−1)
- Dsteel: is the diffusion coefficient of the steel substrate (cm2s−1)
- Deff: is the effective diffusion coefficient of the coated sample (cm2s−1)
3.6. Application for Hydrogen Distribution Pipelines
4. Summary, Perspectives, and Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ARXPS | Angle-resolved X-ray photoelectron spectroscopy |
| AFM | Atomic force microscopy |
| C0 | Subsurface hydrogen concentration |
| CE | Counter electrode |
| Dcoat | Diffusion coefficient of the coating |
| Deff | Effective diffusion coefficient |
| Dsteel | Diffusion coefficient of the steel substrate |
| D-S | Devanathan–Stachurski |
| ED | Energy diffusion |
| ED | Electrodeposited |
| EN | Electroless |
| ERDA | Elastic recoil detection analysis |
| F | Faraday constant |
| HAXPES | Hard X-ray photoelectron spectroscopy |
| HE | Hydrogen embrittlement |
| HP | Hydrogen permeability |
| HPB | Hydrogen permeation barrier |
| iss | Steady-state permeation current density |
| J | Hydrogen diffusion flux |
| Lcoat | Thickness of the coating |
| Lsteel | Thickness of the steel substrate |
| Ltot | Thickness of the coated sample |
| MEM | Maximum entropy method |
| MH | Molar mass of hydrogen |
| Ni-P | Nickel–phosphorous |
| OM | Optical microscopy |
| Pm | Hydrogen permeability |
| PRF | Permeation reduction factor |
| RBS | Rutherford backscattering spectroscopy |
| RE | Reference electrode |
| S | Solubility |
| SEM | Secondary electron microscopy |
| WE | Working electrode |
| XPS | X-ray photoelectron spectroscopy |
| ΔHs | Standard enthalpy of dissolution |
| ρFe | Iron density |
References
- Amir, M.; Deshmukh, R.G.; Khalid, H.M.; Said, Z.; Raza, A.; Muyeen, S.M.; Nizami, A.-S.; Elavarasan, R.M.; Saidur, R.; Sopian, K. Energy Storage Technologies: An Integrated Survey of Developments, Global Economical/Environmental Effects, Optimal Scheduling Model, and Sustainable Adaption Policies. J. Energy Storage 2023, 72, 108694. [Google Scholar] [CrossRef]
- Mazloomi, K.; Gomes, C. Hydrogen as an Energy Carrier: Prospects and Challenges. Renew. Sustain. Energy Rev. 2012, 16, 3024–3033. [Google Scholar] [CrossRef]
- Stolten, D. Hydrogen and Fuel Cells: Fundamentals, Technologies and Applications; John Wiley & Sons: Weinheim, Germany, 2010; ISBN 978-3-527-32711-9. [Google Scholar]
- Stolten, D.; Emonts, B. Hydrogen Science and Engineering, 2 Volume Set: Materials, Processes, Systems, and Technology; John Wiley & Sons: Weinheim, Germany, 2016; ISBN 978-3-527-33238-0. [Google Scholar]
- Ustolin, F.; Campari, A.; Taccani, R. An Extensive Review of Liquid Hydrogen in Transportation with Focus on the Maritime Sector. J. Mar. Sci. Eng. 2022, 10, 1222. [Google Scholar] [CrossRef]
- Meda, U.S.; Bhat, N.; Pandey, A.; Subramanya, K.N.; Lourdu Antony Raj, M.A. Challenges Associated with Hydrogen Storage Systems Due to the Hydrogen Embrittlement of High Strength Steels. Int. J. Hydrogen Energy 2023, 48, 17894–17913. [Google Scholar] [CrossRef]
- Abohamzeh, E.; Salehi, F.; Sheikholeslami, M.; Abbassi, R.; Khan, F. Review of Hydrogen Safety during Storage, Transmission, and Applications Processes. J. Loss Prev. Process Ind. 2021, 72, 104569. [Google Scholar] [CrossRef]
- Wetegrove, M.; Duarte, M.J.; Taube, K.; Rohloff, M.; Gopalan, H.; Scheu, C.; Dehm, G.; Kruth, A. Preventing Hydrogen Embrittlement: The Role of Barrier Coatings for the Hydrogen Economy. Hydrogen 2023, 4, 307–322. [Google Scholar] [CrossRef]
- Li, Y.; Barzagli, F.; Liu, P.; Zhang, X.; Yang, Z.; Xiao, M.; Huang, Y.; Luo, X.; Li, C.; Luo, H.; et al. Mechanism and Evaluation of Hydrogen Permeation Barriers: A Critical Review. Ind. Eng. Chem. Res. 2023, 62, 15752–15773. [Google Scholar] [CrossRef]
- Goyal, A.; Pouya, H.S.; Ganjian, E.; Claisse, P. A Review of Corrosion and Protection of Steel in Concrete. Arab. J. Sci. Eng. 2018, 43, 5035–5055. [Google Scholar] [CrossRef]
- He, K.; Wang, L. A Review of Energy Use and Energy-Efficient Technologies for the Iron and Steel Industry. Renew. Sustain. Energy Rev. 2017, 70, 1022–1039. [Google Scholar] [CrossRef]
- Mousa, E.; Wang, C.; Riesbeck, J.; Larsson, M. Biomass Applications in Iron and Steel Industry: An Overview of Challenges and Opportunities. Renew. Sustain. Energy Rev. 2016, 65, 1247–1266. [Google Scholar] [CrossRef]
- Amezhnov, A.V.; Rodionova, I.G.; Batsalev, A.I.; D’yakonov, D.L.; Shaposhnikov, N.G.; Shatskii, T.E.; Marzoeva, M.E. Effect of Chemical Composition and Microstructure Parameters on Carbon and Low-Alloy Steel Corrosion Resistance Under Oil Industry Pipeline Operation Conditions. Metallurgist 2019, 62, 1030–1038. [Google Scholar] [CrossRef]
- Suzuki, A.; Yukawa, H.; Murata, Y. Consistent Description of Hydrogen Permeation through Metal Membrane Based on Hydrogen Chemical Potential and Its Application to Alloy Design. J. Mater. Res. 2017, 32, 227–238. [Google Scholar] [CrossRef]
- Nanninga, N.E.; Levy, Y.S.; Drexler, E.S.; Condon, R.T.; Stevenson, A.E.; Slifka, A.J. Comparison of Hydrogen Embrittlement in Three Pipeline Steels in High Pressure Gaseous Hydrogen Environments. Corros. Sci. 2012, 59, 1–9. [Google Scholar] [CrossRef]
- Nanninga, N.; Slifka, A.; Levy, Y.; White, C. A Review of Fatigue Crack Growth for Pipeline Steels Exposed to Hydrogen. J. Res. Natl. Inst. Stand. Technol. 2010, 115, 437–452. [Google Scholar] [CrossRef] [PubMed]
- Somerday, B.; Sofronis, P.; Jones, R.H. Effects of Hydrogen on Materials: Proceedings of the 2008 International Hydrogen Conference, September 7–10, 2008, Jackson Lake Lodge, Grand Teton National Park, Wyoming, USA; ASM International: Almere, The Netherlands, 2009; ISBN 978-1-61503-136-8. [Google Scholar]
- Ghosh, G.; Rostron, P.; Garg, R.; Panday, A. Hydrogen Induced Cracking of Pipeline and Pressure Vessel Steels: A Review. Eng. Fract. Mech. 2018, 199, 609–618. [Google Scholar] [CrossRef]
- Symons, D.M. A Comparison of Internal Hydrogen Embrittlement and Hydrogen Environment Embrittlement of X-750. Eng. Fract. Mech. 1999, 68, 751–771. [Google Scholar]
- Flis, J. (Ed.) Corrosion of Metals and Hydrogen-Related Phenomena—Selected Topics; Materials Science Monographs; Elsevier: Amsterdam, The Netherlands, 1991; Volume 59, ISBN 978-0-444-98793-8. [Google Scholar]
- Wach, S.; Miodownik, A.P.; Mackowiak, J. The Diffusion of Hydrogen through Pure Iron Membranes. Corros. Sci. 1966, 6, 271–285. [Google Scholar] [CrossRef]
- Nelson, H.G. Hydrogen Embrittlement Testing; ASTM Special Technical Publication; American Society for Testing and Material: West Conshohocken, PA, USA, 1974; Volume 543. [Google Scholar]
- Capelle, J.; Gilgert, J.; Dmytrakh, I.; Pluvinage, G. Sensitivity of Pipelines with Steel API X52 to Hydrogen Embrittlement. Int. J. Hydrogen Energy 2008, 33, 7630–7641. [Google Scholar] [CrossRef]
- Hardie, D.; Charles, E.A.; Lopez, A.H. Hydrogen Embrittlement of High Strength Pipeline Steels. Corros. Sci. 2006, 48, 4378–4385. [Google Scholar] [CrossRef]
- Zhang, L.; Imade, M.; An, B.; Wen, M.; Iijima, T.; Fukuyama, S.; Yokogawa, K. Internal Reversible Hydrogen Embrittlement of Austenitic Stainless Steels Based on Type 316 at Low Temperatures. ISIJ Int. 2012, 52, 240–246. [Google Scholar] [CrossRef]
- Benassi, G. Critical Analysis of Hydrogen Permeation Techniques. Application to Different Steel Microstructures. Master’s Thesis, Politecnico di Milano, Milano, Italy, 2013. Available online: www.politesi.polimi.it (accessed on 4 February 2025).
- Sudagar, J.; Lian, J.; Sha, W. Electroless Nickel, Alloy, Composite and Nano Coatings—A Critical Review. J. Alloys Compd. 2013, 571, 183–204. [Google Scholar] [CrossRef]
- Loto, C.A. Electroless Nickel Plating—A Review. Silicon 2016, 8, 177–186. [Google Scholar] [CrossRef]
- Mindivan, F.; Mindivan, H.; Bayram, A. The Electroless Monolayer and Duplex Ni–B and Ni–P Coatings for 316L Stainless Steel in Synergistic Combination of Mechanical (Wear) and Chemical (Corrosion) Processes. Adv. Eng. Mater. 2023, 25, 2201501. [Google Scholar] [CrossRef]
- Crobu, M.; Scorciapino, A.; Elsener, B.; Rossi, A. The Corrosion Resistance of Electroless Deposited Nano-Crystalline Ni–P Alloys. Electrochim. Acta 2008, 53, 3364–3370. [Google Scholar] [CrossRef]
- Elsener, B.; Crobu, M.; Scorciapino, M.A.; Rossi, A. Electroless Deposited Ni–P Alloys: Corrosion Resistance Mechanism. J. Appl. Electrochem. 2008, 38, 1053–1060. [Google Scholar] [CrossRef]
- Elsener, B.; Atzei, D.; Krolikowski, A.; Rossi, A. Effect of Phosphorus Concentration on the Electronic Structure of Nanocrystalline Electrodeposited Ni–P Alloys: An XPS and XAES Investigation. Surf. Interface Anal. 2008, 40, 919–926. [Google Scholar] [CrossRef]
- Elsener, B.; Atzei, D.; Krolikowski, A.; Rossi Albertini, V.; Sadun, C.; Caminiti, R.; Rossi, A. From Chemical to Structural Order of Electrodeposited Ni22P Alloy: An XPS and EDXD Study. Chem. Mater. 2004, 16, 4216–4225. [Google Scholar] [CrossRef]
- He, Y.P.; Liu, G.M.; Duan, X.H.; Wu, T.; Dong, M.; Zhu, Y.B. Effects of Nanocrystals on Hydrogen Permeation and Diffusion in Amorphous Electroless Ni-P Coatings. Mater. Today Commun. 2024, 40, 109499. [Google Scholar] [CrossRef]
- Samanta, S.; Mondal, K.; Dutta, M.; Singh, S.B. Electroless NiP Coatings over API X70 Steel: Effect of Composition on the H-Permeation and Corrosion Resistance. Surf. Coat. Technol. 2021, 409, 126928. [Google Scholar] [CrossRef]
- Li, X.; Ma, X.; Zhang, J.; Akiyama, E.; Wang, Y.; Song, X. Review of Hydrogen Embrittlement in Metals: Hydrogen Diffusion, Hydrogen Characterization, Hydrogen Embrittlement Mechanism and Prevention. Acta Metall. Sin. 2020, 33, 759–773. [Google Scholar] [CrossRef]
- Tamura, M. Hydrogen Permeation Characteristics of TiN-Coated Stainless Steels. JMSE-A 2015, 5, 197–201. [Google Scholar] [CrossRef][Green Version]
- Fowler, J.D.; Chandra, D.; Elleman, T.S.; Payne, A.W.; Verghese, K. Tritium Diffusion in A12O3 and BeO. J. Am. Ceram. Soc. 1977, 60, 155–161. [Google Scholar] [CrossRef]
- Wipf, H. Solubility and Diffusion of Hydrogen in Pure Metals and Alloys. Phys. Scr. 2001, 2001, 43. [Google Scholar] [CrossRef]
- Kirchheim, R. Solubility and Diffusivity of Hydrogen in Complex Materials. Phys. Scr. 2001, 2001, 58. [Google Scholar] [CrossRef]
- Rönnebro, E.C.E.; Oelrich, R.L.; Gates, R.O. Recent Advances and Prospects in Design of Hydrogen Permeation Barrier Materials for Energy Applications—A Review. Molecules 2022, 27, 6528. [Google Scholar] [CrossRef]
- Chen, Y.-S.; Huang, C.; Liu, P.-Y.; Yen, H.-W.; Niu, R.; Burr, P.; Moore, K.L.; Martínez-Pañeda, E.; Atrens, A.; Cairney, J.M. Hydrogen Trapping and Embrittlement in Metals—A Review. Int. J. Hydrogen Energy 2024. [Google Scholar] [CrossRef]
- Hatano, Y. Permeation and Permeation Barrier. In Tritium: Fuel of Fusion Reactors; Tanabe, T., Ed.; Springer: Tokyo, Japan, 2017; pp. 207–229. ISBN 978-4-431-56460-7. [Google Scholar]
- Nemanič, V. Hydrogen Permeation Barriers: Basic Requirements, Materials Selection, Deposition Methods, and Quality Evaluation. Nucl. Mater. Energy 2019, 19, 451–457. [Google Scholar] [CrossRef]
- Fick, A. Ueber Diffusion. Ann. Phys. 1855, 170, 59–86. [Google Scholar] [CrossRef]
- Song, R.-H.; Pyun, S. Hydrogen Permeation Through a Bilayer of Fe/Electrodeposited Ni. J. Electrochem. Soc. 1990, 137, 1051. [Google Scholar] [CrossRef]
- Fujiwara, H.; Ono, H.; Onoue, K.; Nishimura, S. High-Pressure Gaseous Hydrogen Permeation Test Method -Property of Polymeric Materials for High-Pressure Hydrogen Devices (1)-. Int. J. Hydrogen Energy 2020, 45, 29082–29094. [Google Scholar] [CrossRef]
- Young, K.T.; Krentz, T.M.; d’Entremont, A.L.; Vogel, E.M.; Hitchcock, D.A. Measurement of Gas-Concentration-Driven Permeation for the Examination of Permeability, Solubility, and Diffusivity in Varying Materials. Rev. Sci. Instrum. 2020, 91, 105105. [Google Scholar] [CrossRef] [PubMed]
- Checchetto, R.; Gratton, L.M.; Miotello, A.; Cestari, C. Hydrogen Permeation Apparatus with Thermal Desorption Spectroscopy Capabilities. Meas. Sci. Technol. 1995, 6, 1605. [Google Scholar] [CrossRef]
- Devanathan, M.a.V.; Stachurski, Z.; Tompkins, F.C. The Adsorption and Diffusion of Electrolytic Hydrogen in Palladium. Proc. R. Soc. Lond. Ser. A Math. Phys. Sci. 1997, 270, 90–102. [Google Scholar] [CrossRef]
- Devanathan, M.a.V.; Stachurski, Z. The Mechanism of Hydrogen Evolution on Iron in Acid Solutions by Determination of Permeation Rates. J. Electrochem. Soc. 1964, 111, 619. [Google Scholar] [CrossRef]
- Standard Practice for Evaluation of Hydrogen Uptake, Permeation, and Transport in Metals by an Electrochemical Technique. Available online: https://www.astm.org/g0148-97r18.html (accessed on 2 November 2023).
- ISO 17081:2004(En); Method of Measurement of Hydrogen Permeation and Determination of Hydrogen Uptake and Transport in Metals by an Electrochemical Technique. International Organization for Standardization: Genève, Switzerland, 2004. Available online: https://www.iso.org/obp/ui#iso:std:iso:17081:ed-1:v1:en (accessed on 8 March 2024).
- Turnbull, A.; Carroll, M.W.; Ferriss, D.H. Analysis of Hydrogen Diffusion and Trapping in a 13% Chromium Martensitic Stainless Steel. Acta Metall. 1989, 37, 2039–2046. [Google Scholar] [CrossRef]
- Turnbull, A.; Saenz de Santa Maria, M.; Thomas, N.D. The Effect of H2S Concentration and pH on Hydrogen Permeation in AISI 410 Stainless Steel in 5% NaCl. Corros. Sci. 1989, 29, 89–104. [Google Scholar] [CrossRef]
- Frappart, S.; Feaugas, X.; Creus, J.; Thebault, F.; Delattre, L.; Marchebois, H. Study of the Hydrogen Diffusion and Segregation into Fe–C–Mo Martensitic HSLA Steel Using Electrochemical Permeation Test. J. Phys. Chem. Solids 2010, 71, 1467–1479. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion; Clarendon Press: Oxford, UK, 1979; ISBN 978-0-19-853411-2. [Google Scholar]
- Laadel, N.-E.; El Mansori, M.; Kang, N.; Marlin, S.; Boussant-Roux, Y. Permeation Barriers for Hydrogen Embrittlement Prevention in Metals—A Review on Mechanisms, Materials Suitability and Efficiency. Int. J. Hydrogen Energy 2022, 47, 32707–32731. [Google Scholar] [CrossRef]
- Levchuk, D.; Koch, F.; Maier, H.; Bolt, H. Deuterium Permeation through Eurofer and α-Alumina Coated Eurofer. J. Nucl. Mater. 2004, 328, 103–106. [Google Scholar] [CrossRef]
- Matějíček, J.; Veverka, J.; Nemanič, V.; Cvrček, L.; Lukáč, F.; Havránek, V.; Illková, K. Characterization of Less Common Nitrides as Potential Permeation Barriers. Fusion Eng. Des. 2019, 139, 74–80. [Google Scholar] [CrossRef]
- Chikada, T.; Suzuki, A.; Koch, F.; Maier, H.; Terai, T.; Muroga, T. Fabrication and Deuterium Permeation Properties of Erbia-Metal Multilayer Coatings. J. Nucl. Mater. 2013, 442, S592–S596. [Google Scholar] [CrossRef]
- Levchuk, D.; Levchuk, S.; Maier, H.; Bolt, H.; Suzuki, A. Erbium Oxide as a New Promising Tritium Permeation Barrier. J. Nucl. Mater. 2007, 367–370, 1033–1037. [Google Scholar] [CrossRef]
- Nemanič, V.; McGuiness, P.J.; Daneu, N.; Zajec, B.; Siketić, Z.; Waldhauser, W. Hydrogen Permeation through Silicon Nitride Films. J. Alloys Compd. 2012, 539, 184–189. [Google Scholar] [CrossRef]
- Panjan, P.; Drnovšek, A.; Gselman, P.; Čekada, M.; Panjan, M. Review of Growth Defects in Thin Films Prepared by PVD Techniques. Coatings 2020, 10, 447. [Google Scholar] [CrossRef]
- Hannachi, R.; Biggio, D.; Elsener, B.; Fantauzzi, M.; Rossi, A. X-Ray Photoelectron Spectroscopy Investigation of X60 Steel. Surf. Sci. Spectra 2024, 31, 024014. [Google Scholar] [CrossRef]
- Harlin, P.; Bexell, U.; Olsson, M. Influence of Surface Topography of Arc-Deposited TiN and Sputter-Deposited WC/C Coatings on the Initial Material Transfer Tendency and Friction Characteristics under Dry Sliding Contact Conditions. Surf. Coat. Technol. 2009, 203, 1748–1755. [Google Scholar] [CrossRef]
- Panjan, P.; Drnovšek, A.; Gselman, P.; Čekada, M.; Panjan, M.; Bončina, T.; Kek Merl, D. Influence of Growth Defects on the Corrosion Resistance of Sputter-Deposited TiAlN Hard Coatings. Coatings 2019, 9, 511. [Google Scholar] [CrossRef]
- Tench, R.J.; Chow, R.; Kozlowski, M.R. Characterization of Defect Geometries in Multilayer Optical Coatings. J. Vac. Sci. Technol. A 1994, 12, 2808–2813. [Google Scholar] [CrossRef]
- Spalvins, T. Characterization of Defect Growth Structrures in Ion-Plated Films by Scanning Electron Microscopy. Thin Solid Film. 1979, 64, 143–148. [Google Scholar] [CrossRef]
- Podgornik, B.; Hogmark, S.; Sandberg, O. Influence of Surface Roughness and Coating Type on the Galling Properties of Coated Forming Tool Steel. Surf. Coat. Technol. 2004, 184, 338–348. [Google Scholar] [CrossRef]
- Shen, X.; Xu, Y.-P.; Li, H.-Z.; Yi, J.; Lyu, Y.-M.; Zhou, H.-S.; Luo, G.-N. Optimization of Alumina Tritium Permeation Barrier with Consideration of the Thickness and the Surface Coverage. Nucl. Mater. Energy 2024, 38, 101574. [Google Scholar] [CrossRef]
- Ortiz, N.; González-Parra, J.R.; Olaya, J.; Agredo, D.; Valdez, R.; Waage, H.; Bolarín, A.M.; Sánchez, F.; Barba-Pingarrón, A. Morphological and Corrosion Characterization of Electroless Ni-P Coatings Deposited on Ductile Iron. Coatings 2024, 14, 1317. [Google Scholar] [CrossRef]
- Krishna, D.N.G.; Philip, J. Review on Surface-Characterization Applications of X-Ray Photoelectron Spectroscopy (XPS): Recent Developments and Challenges. Appl. Surf. Sci. Adv. 2022, 12, 100332. [Google Scholar] [CrossRef]
- Huang, N.K.; Wang, D.Z.; Xiong, Q.; Yang, B. XPS Study of Hydrogen Permeation Effect on SiC–C Films. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2003, 207, 395–401. [Google Scholar] [CrossRef]
- Wang, W.; Yan, G.; Zhang, J.; Ma, Z.; Wang, L.; Guo, Z.; Zhang, S.; Wu, Y. Hydrogen Permeation Behavior of Zirconium Nitride Film on Zirconium Hydride. Materials 2022, 15, 550. [Google Scholar] [CrossRef]
- Cumpson, P.J. Angle-Resolved XPS and AES: Depth-Resolution Limits and a General Comparison of Properties of Depth-Profile Reconstruction Methods. J. Electron. Spectrosc. Relat. Phenom. 1995, 73, 25–52. [Google Scholar] [CrossRef]
- Scorciapino, M.A.; Fantauzzi, M.; Crobu, M.; Navarra, G.; Elsener, B.; Rossi, A. Nanostructure of Surface Films on Ni18P Alloy in Sulfate Solutions by the Maximum Entropy Method. ACS Omega 2017, 2, 7790–7802. [Google Scholar] [CrossRef] [PubMed]
- Artyushkova, K.; Leadley, S.R.; Shard, A.G. Introduction to Reproducible Laboratory Hard X-Ray Photoelectron Spectroscopy. J. Vac. Sci. Technol. A 2024, 42, 052801. [Google Scholar] [CrossRef]
- Cancellieri, C.; Lorenzin, G.; Lyanage, M.; Turlo, V.; Watts, J.F.; Jeurgens, L.P.H. Chemical and Electronic Structure of Buried W/Cu, W/Cr, and W/Mo Interfaces by In Situ XPS/HAXPES Auger Parameter Analysis. Surf. Interface Anal. 2025, 1–11. [Google Scholar] [CrossRef]
- Cancellieri, C.; Gramatte, S.; Politano, O.; Lapeyre, L.; Klimashin, F.F.; Mackosz, K.; Utke, I.; Novotny, Z.; Müller, A.M.; Vockenhuber, C.; et al. Effect of Hydrogen on the Chemical State, Stoichiometry and Density of Amorphous Al2O3 Films Grown by Thermal Atomic Layer Deposition. Surf. Interface Anal. 2024, 56, 293–304. [Google Scholar] [CrossRef]
- Samanta, S.; Singh, C.; Banerjee, A.; Mondal, K.; Dutta, M.; Singh, S.B. Development of Amorphous Ni-P Coating over API X70 Steel for Hydrogen Barrier Application. Surf. Coat. Technol. 2020, 403, 126356. [Google Scholar] [CrossRef]
- Hino, M.; Doi, Y.; Kuwano, R.; Oda, Y.; Horikawa, K. Effect of Phosphorus Content on Hydrogen Embrittlement for High Strength Steel Treated with Electroless Ni–P Plating. Mater. Trans. 2021, 62, 75–81. [Google Scholar] [CrossRef]
- Fayyad, E.M.; Abdullah, A.M.; Hassan, M.K.; Mohamed, A.M.; Jarjoura, G.; Farhat, Z. Recent Advances in Electroless-Plated Ni-P and Its Composites for Erosion and Corrosion Applications: A Review. Emergent Mater. 2018, 1, 3–24. [Google Scholar] [CrossRef]
- Osifuye, C.O.; Popoola, A.P.I.; Loto, C.A.; Oloruntoba, D.T. Effect of Bath Parameters on Electroless Ni-P and Zn-P Deposition on 1045 Steel Substrate. Int. J. Electrochem. Sci. 2014, 9, 6074–6087. [Google Scholar] [CrossRef]
- Electro-Coating Electroless Nickel Plating Services|Electro-Coating. Available online: https://www.electro-coatings.com/electroless-nickel-plating.php (accessed on 4 February 2025).
- Lelevic, A.; Walsh, F.C. Electrodeposition of NiP Alloy Coatings: A Review. Surf. Coat. Technol. 2019, 369, 198–220. [Google Scholar] [CrossRef]
- Daly, B.P.; Barry, F.J. Electrochemical Nickel–Phosphorus Alloy Formation. Int. Mater. Rev. 2003, 48, 326–338. [Google Scholar] [CrossRef]
- Guo, R.H.; Jiang, S.X.; Yuen, C.W.M.; Ng, M.C.F.; Lan, J.W.; Zheng, G.H. Influence of Deposition Parameters and Kinetics of Electroless Ni-P Plating on Polyester Fiber. Fibers Polym. 2012, 13, 1037–1043. [Google Scholar] [CrossRef]
- Mousavi, M.; Rahimi, E.; Mol, J.M.C.; Gonzalez-Garcia, Y. The Effect of Phosphorous Content on the Microstructure and Localised Corrosion of Electroless Nickel-Coated Copper. Surf. Coat. Technol. 2024, 492, 131174. [Google Scholar] [CrossRef]
- Standard Specification for Autocatalytic (Electroless) Nickel-Phosphorus Coatings on Metal. Available online: https://www.astm.org/b0733-22.html (accessed on 6 February 2025).
- Mai, Q.X.; Daniels, R.D.; Harpalani, H.B. Structural Changes Induced by Heating in Electroless Nickel-Phosphorus Alloys. Thin Solid Film. 1988, 166, 235–247. [Google Scholar] [CrossRef]
- Martyak, N.M. Characterization of Thin Electroless Nickel Coatings. Chem. Mater. 1994, 6, 1667–1674. [Google Scholar] [CrossRef]
- Lambert, M.R.; Duquette, D.J. A Study of Electroless Nickel Coatings Containing Low Phosphorus. Thin Solid Film. 1989, 177, 207–223. [Google Scholar] [CrossRef]
- Martyak, N.M.; Drake, K. Peak-Profile Analysis of Electroless Nickel Coatings. J. Alloys Compd. 2000, 312, 30–40. [Google Scholar] [CrossRef]
- Pillai, A.M.; Rajendra, A.; Sharma, A.K. Electrodeposited Nickel–Phosphorous (Ni–P) Alloy Coating: An in-Depth Study of Its Preparation, Properties, and Structural Transitions. J. Coat. Technol. Res. 2012, 9, 785–797. [Google Scholar] [CrossRef]
- Bredael, E.; Blanpain, B.; Celis, J.P.; Roos, J.R. On the Amorphous and Crystalline State of Electrodeposited Nickel-Phosphorus Coatings. J. Electrochem. Soc. 1994, 141, 294. [Google Scholar] [CrossRef]
- Keong, K.G.; Sha, W. Crystallisation and Phase Transformation Behaviour of Electroless Nickel-Phosphorus Deposits and Their Engineering Properties. Surf. Eng. 2002, 18, 329–343. [Google Scholar] [CrossRef]
- Hu, C.-C.; Bai, A. Influences of the Phosphorus Content on Physicochemical Properties of Nickel–Phosphorus Deposits. Mater. Chem. Phys. 2003, 77, 215–225. [Google Scholar] [CrossRef]
- Brenner, A.; Riddell, G.E. Nickel Plating on Steel by Chemical Reduction. J. Res. Natl. Bur. Stand. 1946, 37, 31–34. [Google Scholar] [CrossRef]
- Hari Krishnan, K.; John, S.; Srinivan, K.N.; Praveen, J.; Ganesan, M.; Kavimani, P.M. An Overall Aspect of Electroless Ni-P Depositions—A Review Article. Metall. Mater. Trans. A 2006, 37, 1917–1926. [Google Scholar]
- Manna, M.; Bandyopadhyay, N.; Bhattacharjee, D. Effect of Plating Time for Electroless Nickel Coating on Rebar Surface: An Option for Application in Concrete Structure. Surf. Coat. Technol. 2008, 202, 3227–3232. [Google Scholar] [CrossRef]
- Vitry, V.; Francq, E.; Bonin, L. Mechanical Properties of Heat-Treated Duplex Electroless Nickel Coatings. Surf. Eng. 2019, 35, 158–166. [Google Scholar] [CrossRef]
- Mainier, F.B.; Fonseca, M.P.C.; Tavares, S.S.M.; Pardal, J.M. Quality of Electroless Ni-P (Nickel-Phosphorus) Coatings Applied in Oil Production Equipment with Salinity. J. Mater. Sci. Chem. Eng. 2013, 1, 1–8. [Google Scholar] [CrossRef]
- Sahoo, P.; Das, S.K. Tribology of Electroless Nickel Coatings—A Review. Mater. Des. 2011, 32, 1760–1775. [Google Scholar] [CrossRef]
- ISO 4527:2003; Metallic Coatings—Autocatalytic (Electroless) Nickel-Phosphorus Alloy Coatings—Specification and Test Methods. International Organization for Standardization: Genève, Switzerland, 2003. Available online: https://www.iso.org/standard/36258.html (accessed on 6 February 2025).
- Yaktiti, A.; Dreano, A.; Carton, J.F.; Christien, F. Hydrogen Diffusion and Trapping in a Steel Containing Porosities. Corros. Sci. 2022, 199, 110208. [Google Scholar] [CrossRef]
- Luu, W.C.; Kuo, H.S.; Wu, J.K. Hydrogen Permeation through Nickel-Plated Steels. Corros. Sci. 1997, 39, 1051–1059. [Google Scholar] [CrossRef]
- Samanta, S.; Vishwanath, K.; Mondal, K.; Dutta, M.; Singh, S.B. Electroless Amorphous NiP Coatings Over API X70 Steel: Resistance to Wear and Hydrogen Embrittlement. Met. Mater. Int. 2022, 28, 397–411. [Google Scholar] [CrossRef]
- Tanabe, T.; Miyata, Y.; Imoko, S. Hydrogen Permeation Through Nickel. Technol. Rep. Osaka Univ. 1977, 27, 389–396. [Google Scholar]
- Robertson, W.M. Hydrogen Permeation, Diffusion and Solution in Nickel. Int. J. Mater. Res. 1973, 64, 436–443. [Google Scholar] [CrossRef]
- Chatterjee, S.S.; Ateya, B.G.; Pickering, H.W. Effect of Electrodeposited Metals on the Permeation of Hydrogen through Iron Membranes. Met. Trans. A 1978, 9, 389–395. [Google Scholar] [CrossRef]
- Paatsch, W. Morphology and Permeability of Nickel Electro-Deposits. Plat. Surf. Finish. 1988, 75, 52–55. [Google Scholar]
- Kim, K.B.; Park, K.; Lee, J.S. Hydrogen Permeation Behavior of Nickel Electroplated AISI 4340 Steel. Met. Mater. 1998, 4, 1013–1016. [Google Scholar] [CrossRef]
- Nishimura, R.; Okitsu, K.; Inoue, H.; Latanision, R.; Hubler, G. Hydrogen Permeation Behavior in Polycrystalline Nickel Implanted with Various Elements; Wit Press Limited: Cambridge, MA, USA, 2007; Volume 55, p. 51. ISBN 978-1-84564-073-6. [Google Scholar]
- Sakamoto, Y.; Takao, K.; Baba, K. Diffusivity of Hydrogen in Amorphous Ni81P19 and Ni70Cr6.7Fe2.5Si8.0B12.8 Alloys. Mater. Sci. Eng. 1988, 97, 437–440. [Google Scholar] [CrossRef]
- Santos, D.S.D.; Miranda, P.E.V.D. Hydrogen Solubility in Amorphous and Crystalline Materials. Int. J. Hydrogen Energy 1998, 23, 1011–1017. [Google Scholar] [CrossRef]
- Nishimura, R.; Latanision, R.M.; Hubler, G.K. Hydrogen Permeation Behavior in Polycrystalline Nickel Implanted with Helium, Argon, Nickel, Yttrium and Platinum. Mater. Sci. Eng. 1987, 90, 243–251. [Google Scholar] [CrossRef]
- Li, Y.; Huard, M.; Wong, K.; Wang, X.; Adane, K.F. Coatings and Liners for Hydrogen Service Pipelines; Canadian Standards Association: Toronto, ON, Canada, 2024; Available online: https://www.csagroup.org/article/research/coatings-and-liners-for-hydrogen-service-pipelines/?srsltid=AfmBOopIuX2eH5ywjflCYZGOvd7Dfce4HVyVg-4OFkTPjd_zYekX--Ey (accessed on 4 February 2025).
- Lipiäinen, S.; Lipiäinen, K.; Ahola, A.; Vakkilainen, E. Use of Existing Gas Infrastructure in European Hydrogen Economy. Int. J. Hydrogen Energy 2023, 48, 31317–31329. [Google Scholar] [CrossRef]
- Adam, P.; von dem Bussche, C.; Engelshove, S.; Thiemann, T. Hydrogen Infrastructure—The Pillar of Energy Transition. In the Practical Conversion of Long-Distance Gas Networks to Hydrogen Operation; Siemens Energy, Gascade Gastransport GmbH, Nowega GmbH: Munich, Germany, 2020. [Google Scholar]
- AMPP TR21473-2024; In-Situ Coating of Steel Pipelines via Pigging. Association for Materials Protection and Performance: Houston, TX, USA, 2024. Available online: https://store.ampp.org/ampp-tr21473-2024-in-situ-coating-of-steel-pipelines-via-pigging (accessed on 4 February 2025).




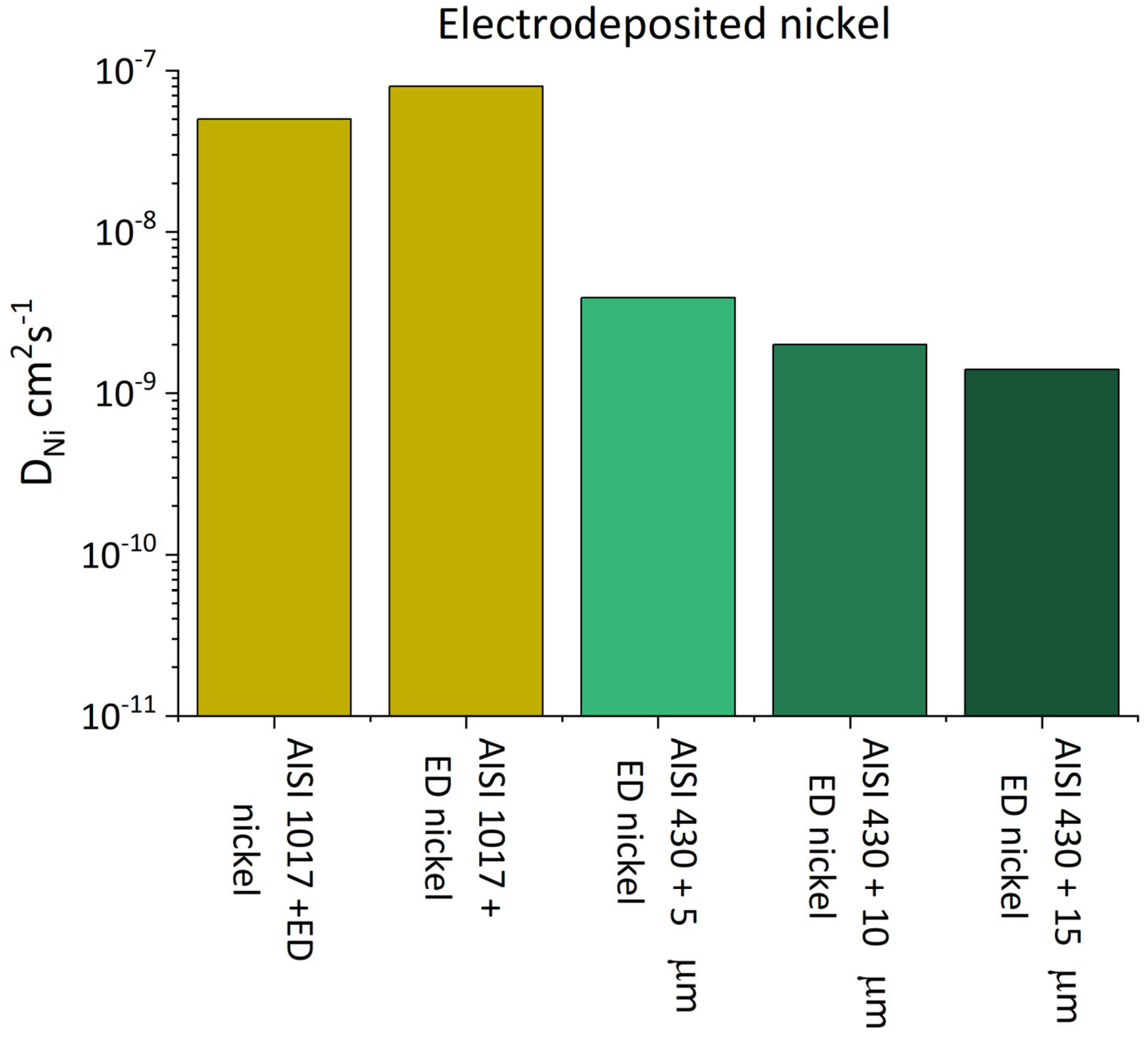
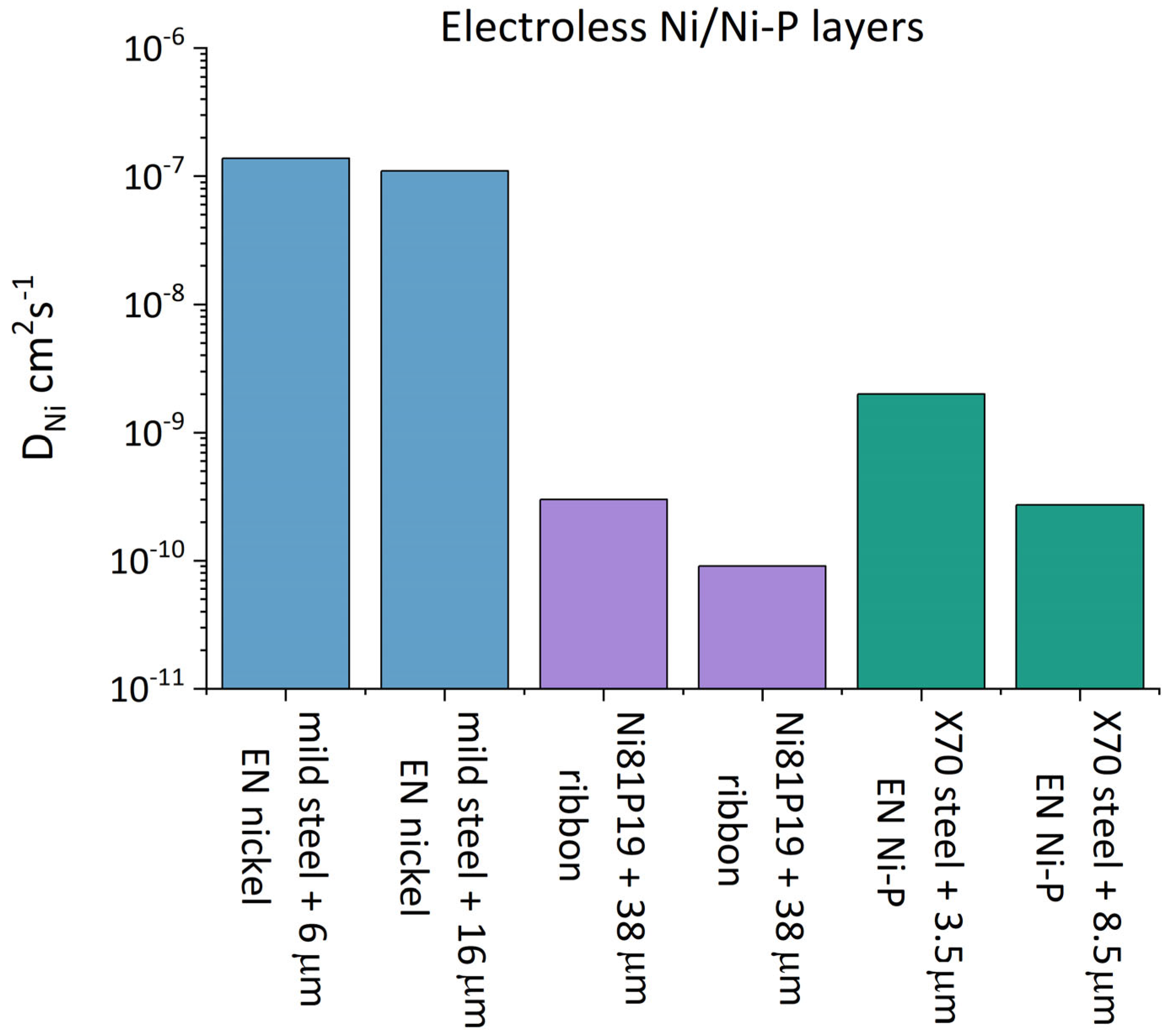
| Material | Coating | Thickness µm | Deff cm2s−1 | DNi cm2s−1 | C0 | Reference |
|---|---|---|---|---|---|---|
| Nickel | - | - | 9.5 × 10−5 | - | - | [109] |
| - | - | 1.33 × 10−4 | [110] | |||
| AISI 1017 | ED nickel | 50 | 5.0 × 10−8 | [112] | ||
| ED nickel | 50 | 8.0 × 10−8 | ||||
| AISI 430 | - | - | 1.2 × 10−6 | - | 6.9 × 10−6 | [113] |
| ED nickel | 5 | 5.0 × 10−7 | 3.9 × 10−9 | 4.8 × 10−6 | ||
| ED nickel | 10 | 2.2 × 10−7 | 2.0 × 10−9 | 4.8 × 10−6 | ||
| ED nickel | 15 | 8.4 × 10−8 | 1.4 × 10−9 | 4.8 × 10−6 | ||
| Mild steel | - | - | 1.04 × 10−6 | - | [107] | |
| ED nickel | 8 | 6.78 × 10−7 | 1.9 × 10−8 | |||
| EN nickel EN nickel | 6 16 | 1.38 × 10−7 1.10 × 10−7 | 1.2–2.4 × 10−9 | |||
| Ni81P19 | Ribbon | 38 | - | 3 × 10−10 | [115] | |
| Ni81P19 | Ribbon | 9 × 10−11 | 1823 × 10−6 | [116] | ||
| X70 steel | - | - | 0.75 × 10−6 | - | [108] | |
| ED nickel | 3.8 | 0.54 × 10−6 | * 4.7 × 10−9 | |||
| EN nickel | 3.5 | 0.39 × 10−6 | * 2 × 10−9 | |||
| EN nickel | 8.5 | 0.042 × 10−6 | * 2.7 × 10−10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biggio, D.; Elsener, B.; Rossi, A. Ni-P Coatings as Hydrogen Permeation Barriers—A Review. Coatings 2025, 15, 365. https://doi.org/10.3390/coatings15040365
Biggio D, Elsener B, Rossi A. Ni-P Coatings as Hydrogen Permeation Barriers—A Review. Coatings. 2025; 15(4):365. https://doi.org/10.3390/coatings15040365
Chicago/Turabian StyleBiggio, Deborah, Bernhard Elsener, and Antonella Rossi. 2025. "Ni-P Coatings as Hydrogen Permeation Barriers—A Review" Coatings 15, no. 4: 365. https://doi.org/10.3390/coatings15040365
APA StyleBiggio, D., Elsener, B., & Rossi, A. (2025). Ni-P Coatings as Hydrogen Permeation Barriers—A Review. Coatings, 15(4), 365. https://doi.org/10.3390/coatings15040365







