Anticorrosive Effect of New Polymer Composite Coatings on Carbon Steel in Aggressive Environments by Electrochemical Procedures
Abstract
1. Introduction
2. Experimental
2.1. Materials and Methods
2.2. Instruments
3. Results and Discussion
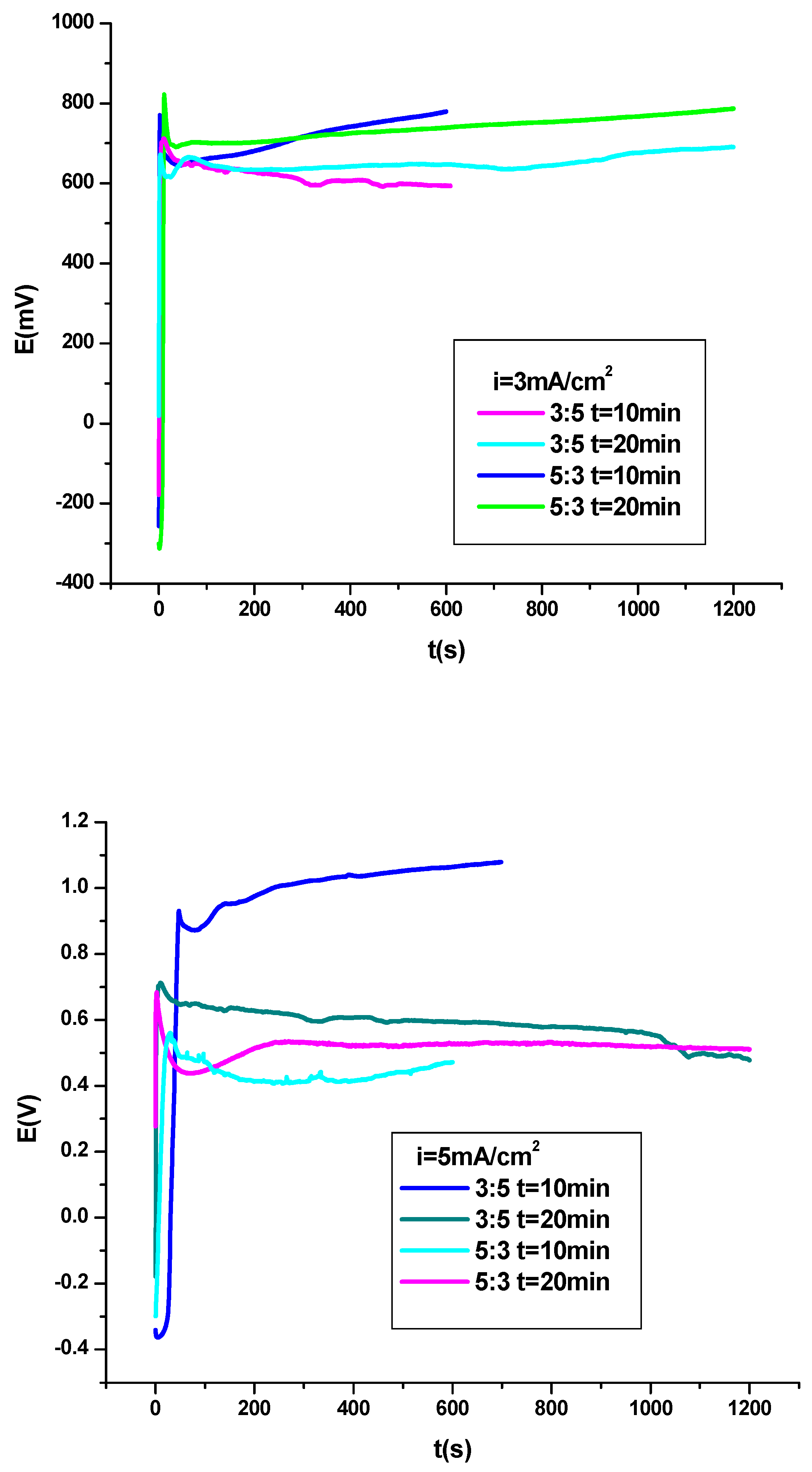
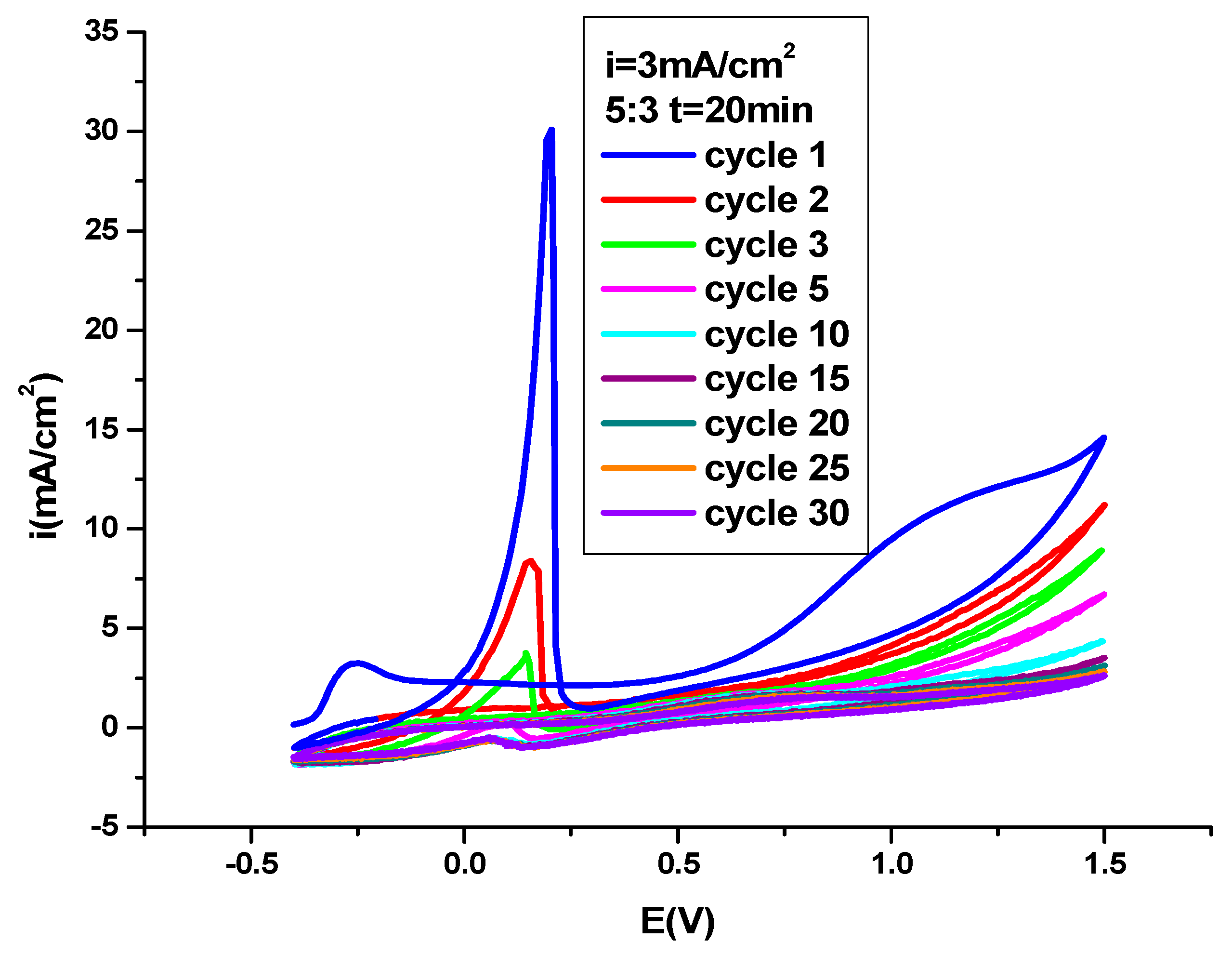
3.1. Electrochemical Polymerization of PNMPY-SDS/PEDOT Coating on OL 37 Substrate
3.2. Electroanalysis of P3MPY-SDS/PEDOT Composite Coating
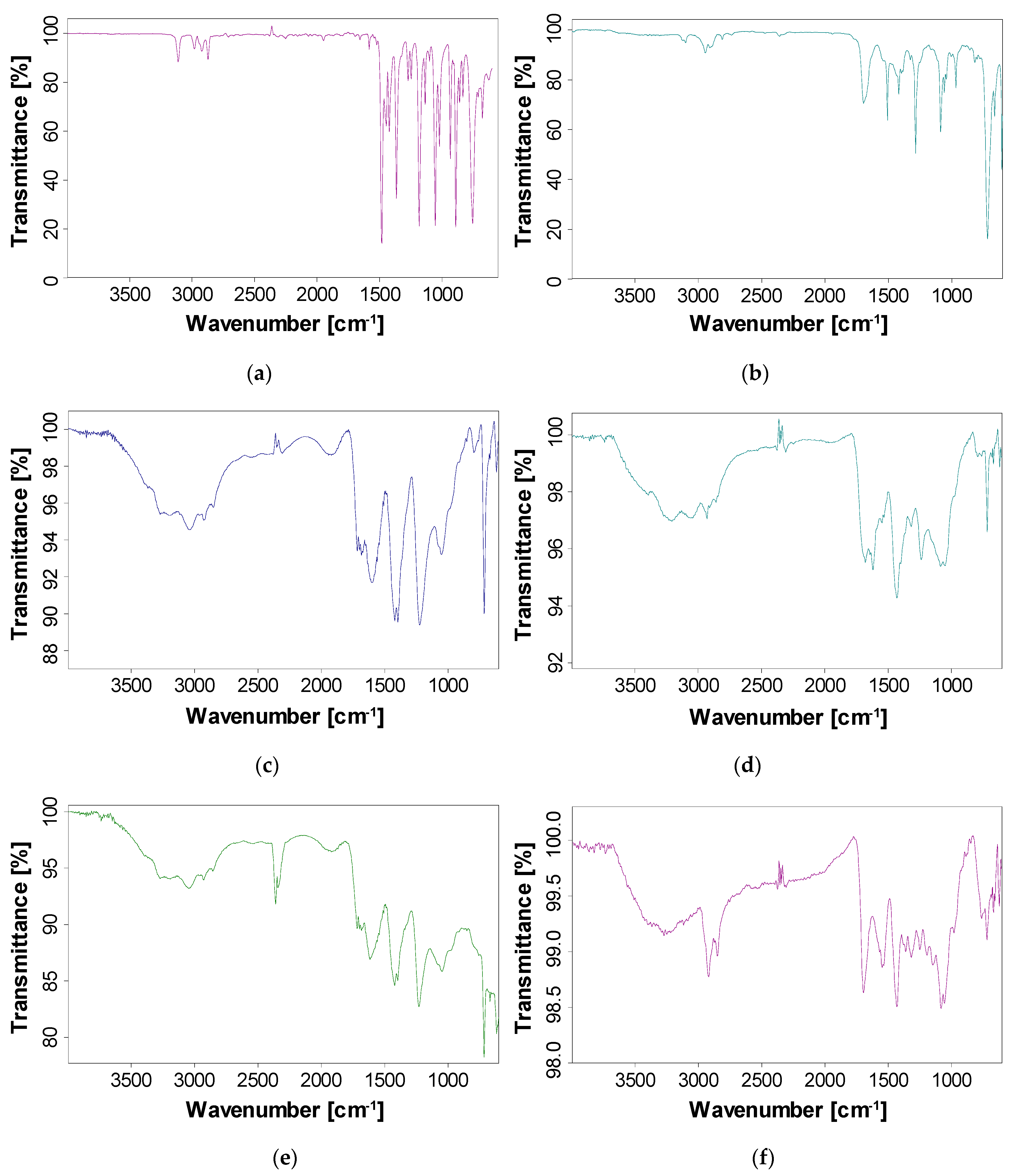
3.3. FT-IR Explorations
3.4. Electrochemical Examination
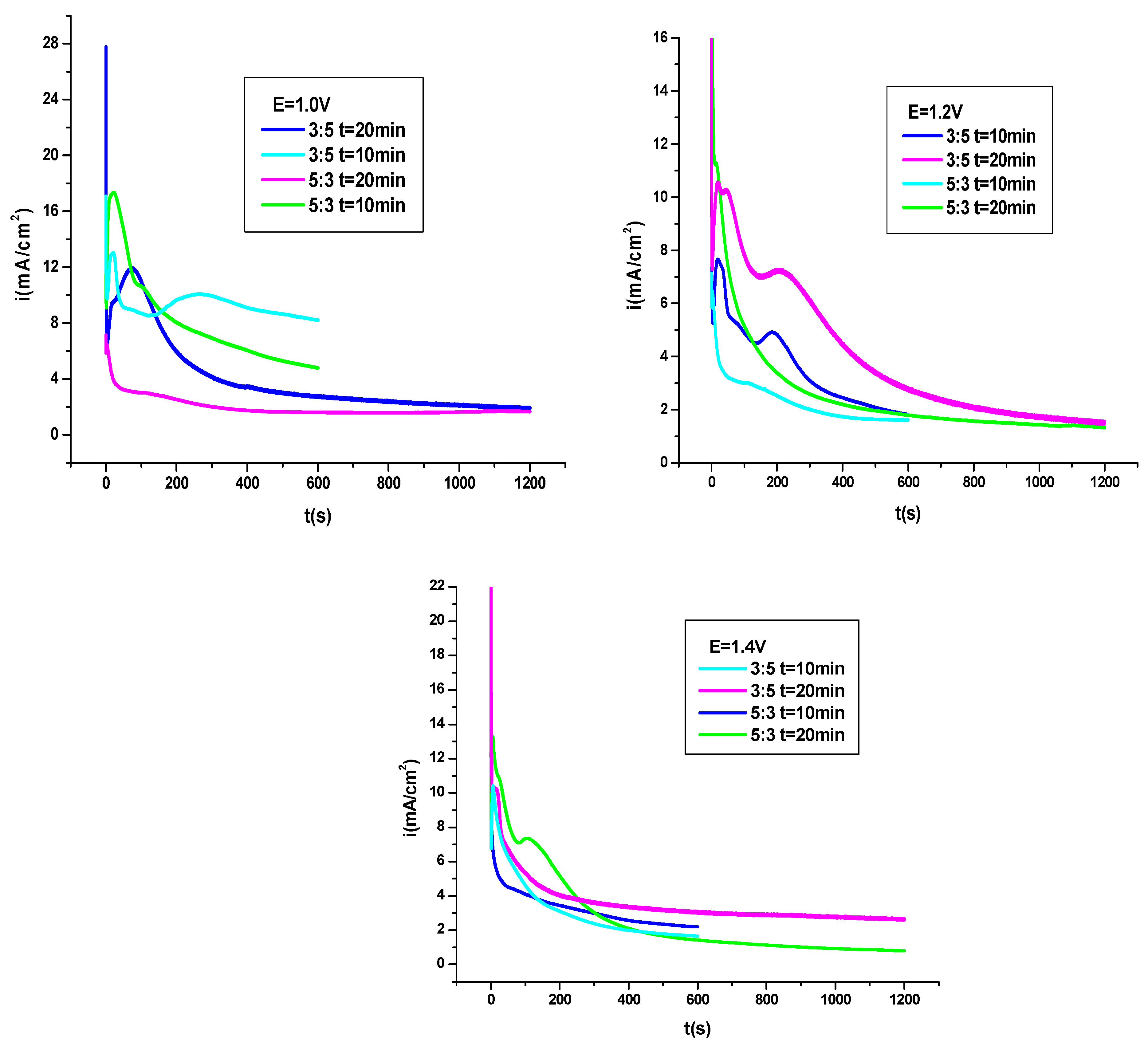
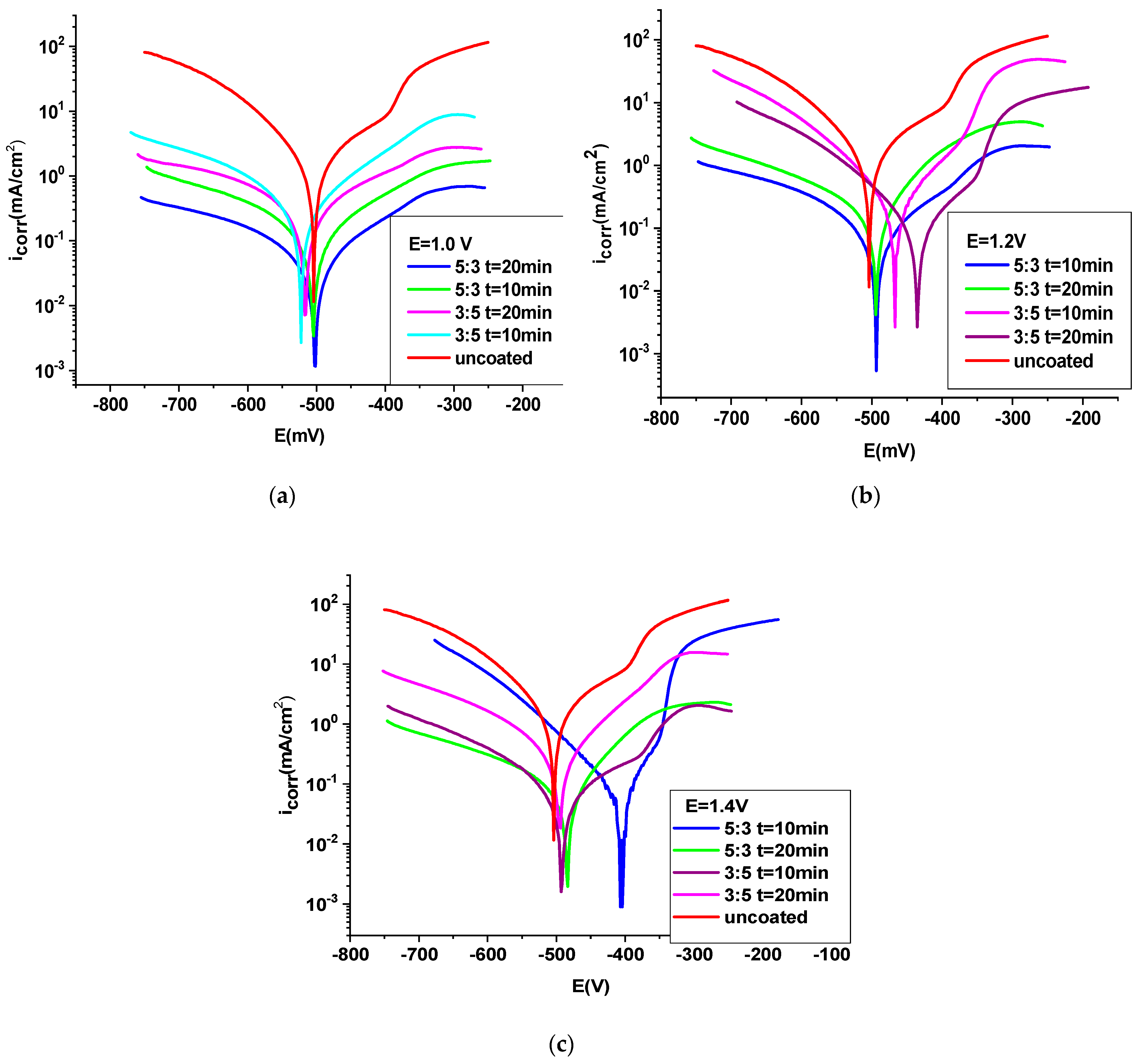
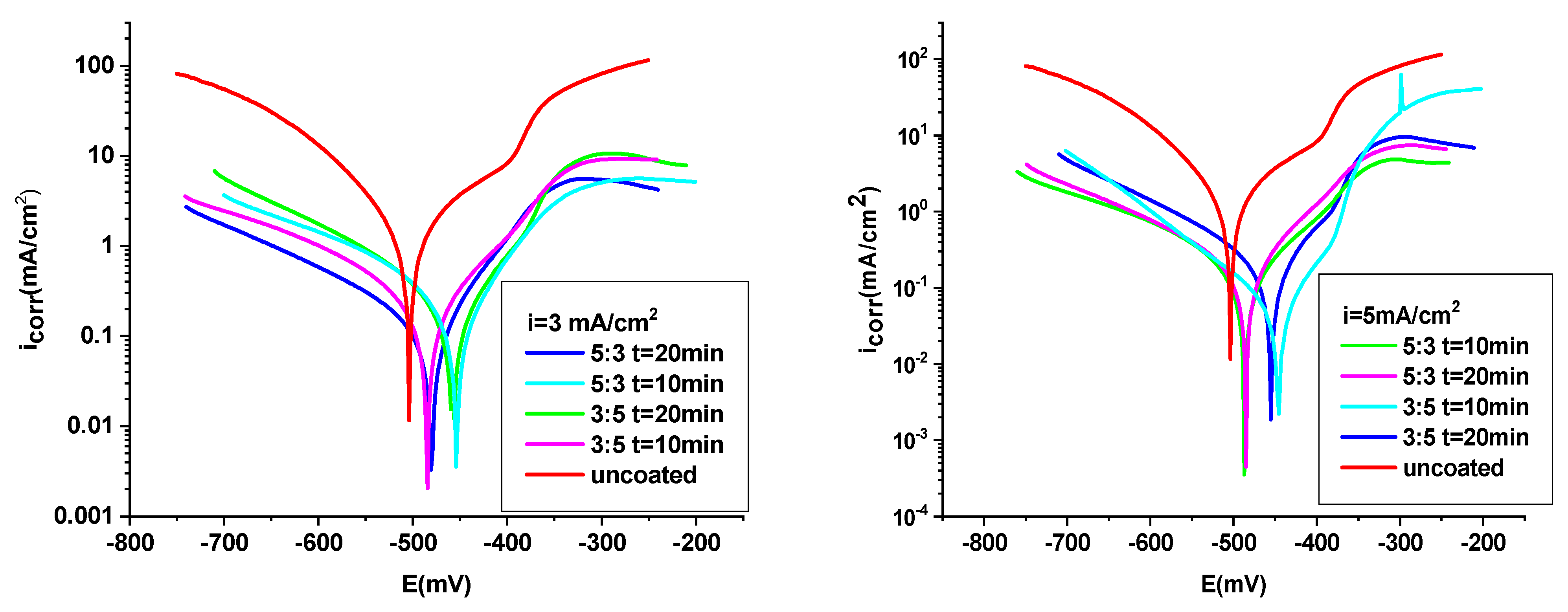
3.4.1. Potentiodynamic Polarization Procedure
- P = film porosity (dimensionless),
- Rp = polarization resistance (Ω cm2),
- ΔEcorr = the difference between corrosion potential for covered and uncovered sample (mV),
- βa = anodic Tafel slope for OL 37 (mV).
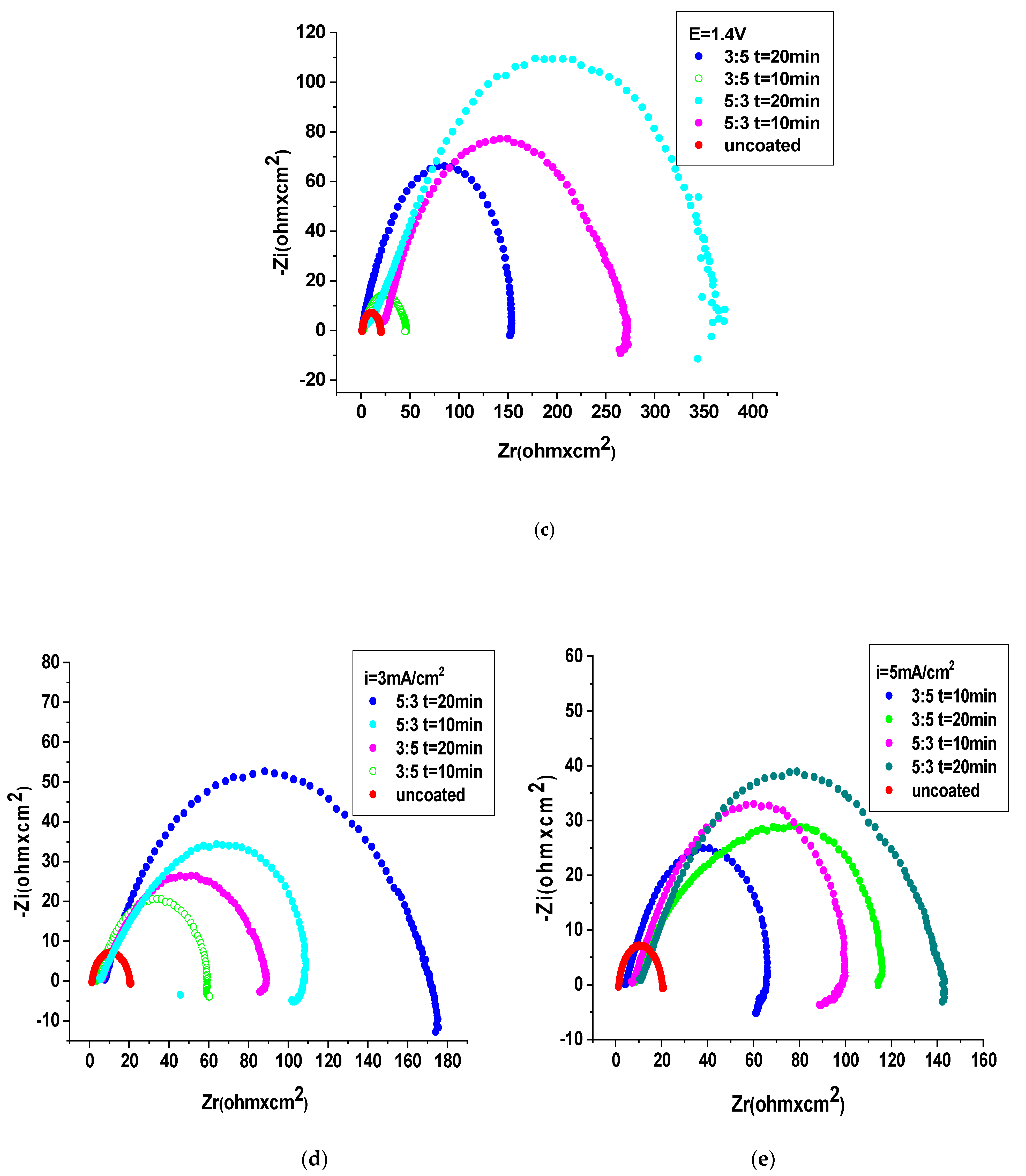
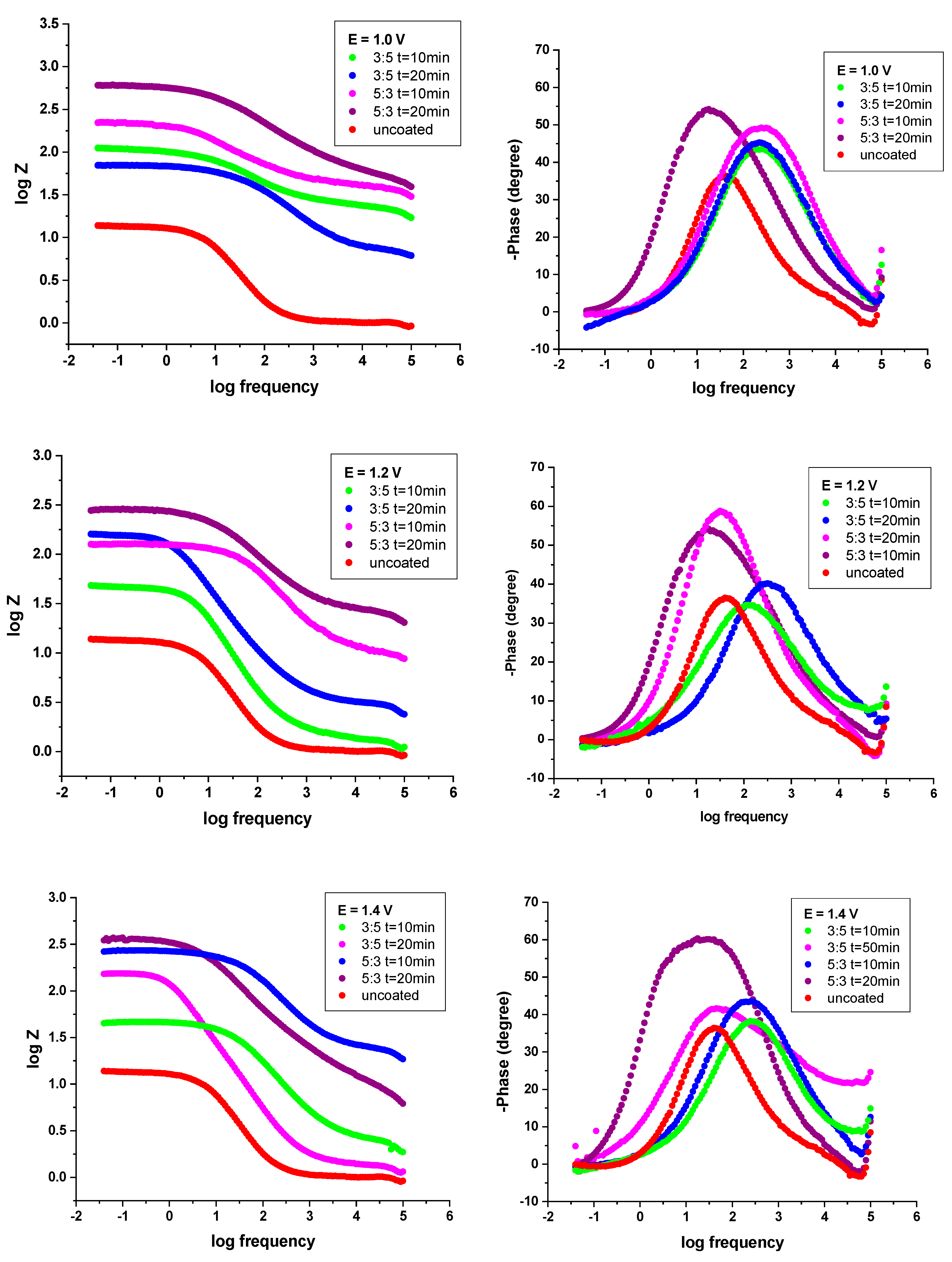
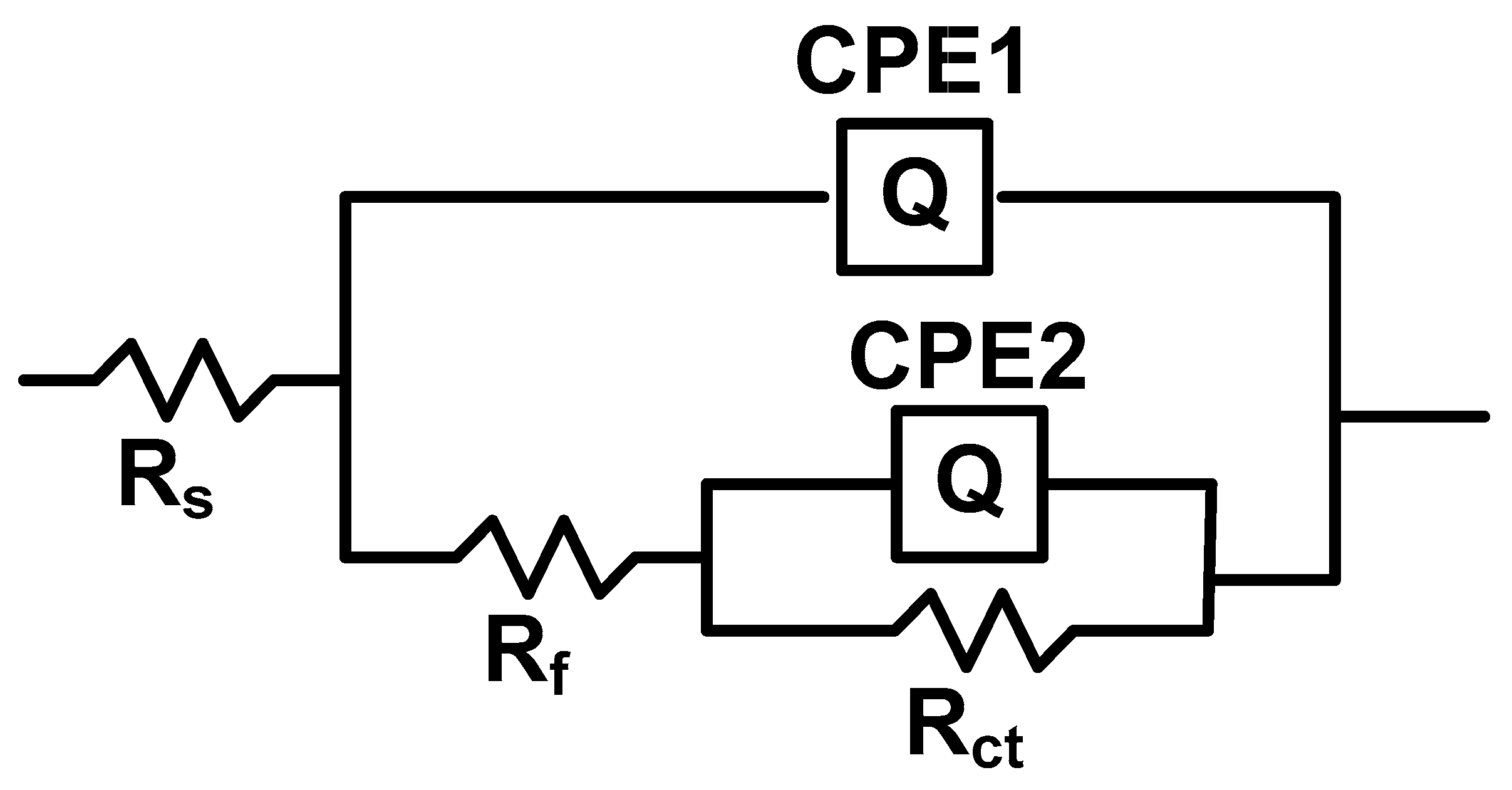
3.4.2. Electrochemical Impedance Spectroscopy (EIS) Studies
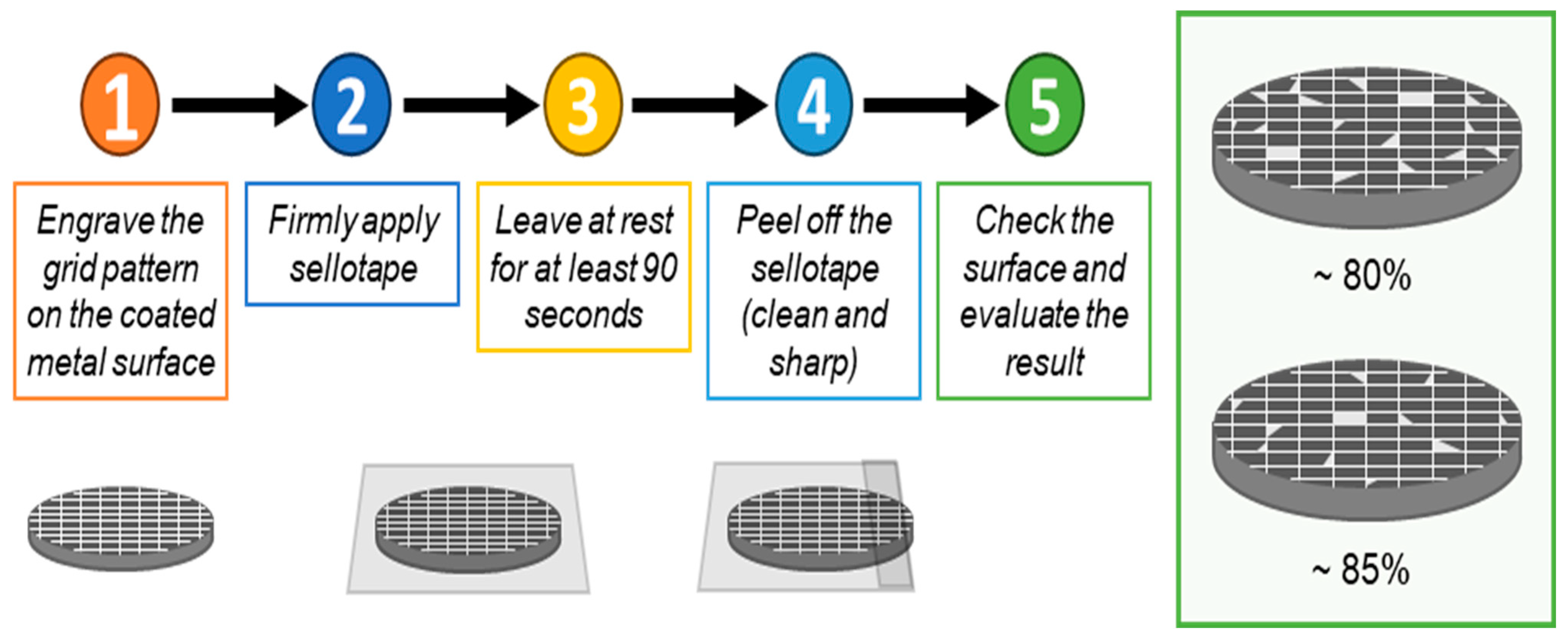
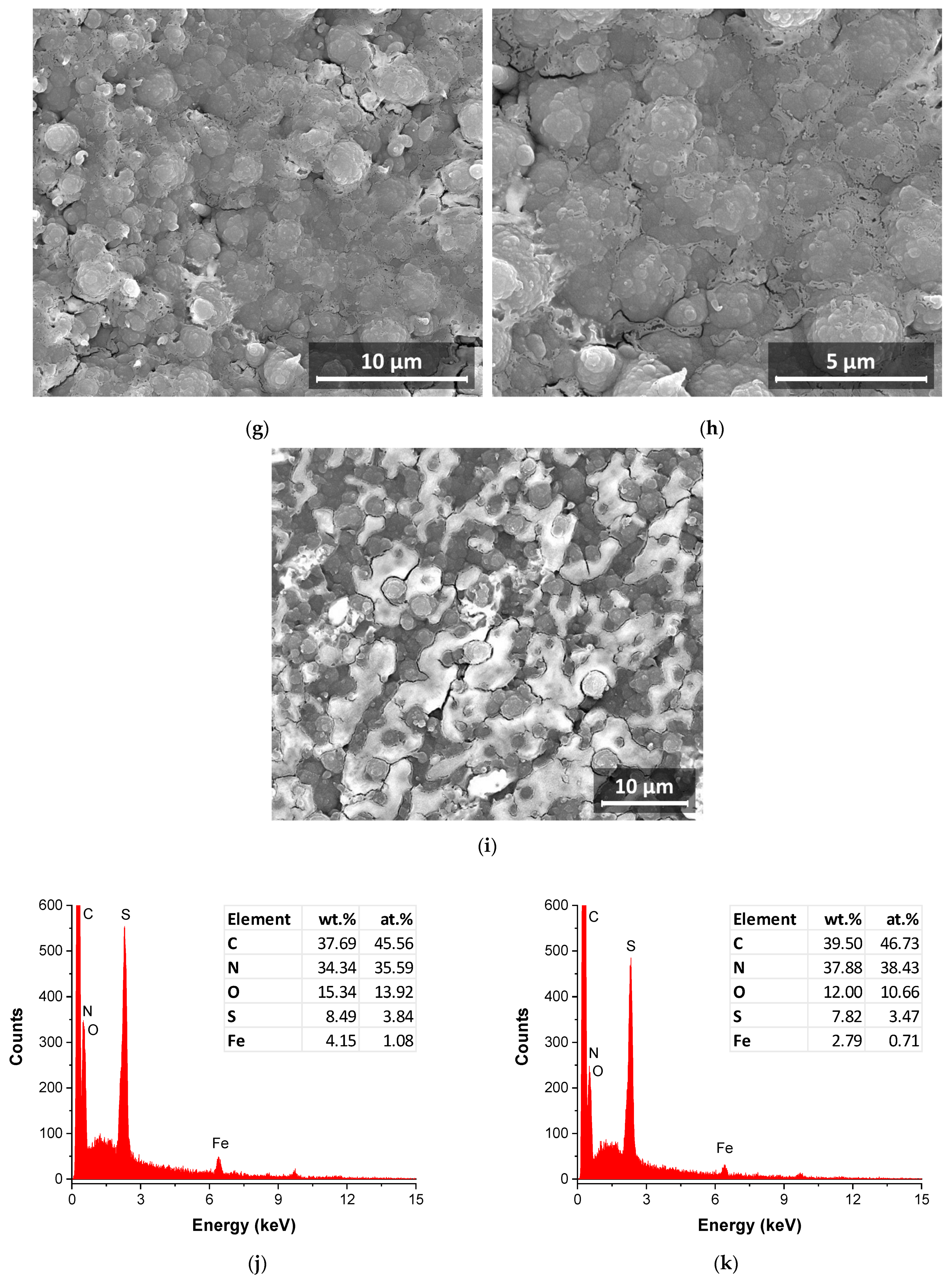
3.5. SEM Exploration
4. Conclusions
- As revealed by SEM, electrochemical measurements, and adhesion tests, uniform, homogeneous, dense, and adherent P3MPY-SDS/PEDOT composite coatings were successfully deposited on the OL 37 substrate under various conditions in oxalic acid solution via electrodeposition—an efficient and cost-effective method widely used for corrosion protection. Electrochemical tests demonstrate that the P3MPY-SDS/PEDOT coating serves as a protective layer for OL 37 in a sulfuric acid medium. Furthermore, by optimizing the film composition and deposition conditions, the protective performance was enhanced and evaluated using potentiodynamic and EIS measurements in 0.5 M H2SO4.
- The OL 37 substrate coated with the P3MPY-SDS/PEDOT composite exhibits a corrosion rate approximately nine times lower than the uncoated sample, with a protective capacity exceeding 90%. The parameters of the anticorrosion protection of P3MPY-SDS/PEDOT are ranked as follows: 1.4 V > 1.2 V > 1.0 V > 5 mA/cm2 > 3 mA/cm2, indicating that these composite coatings significantly reduce corrosion activity.
- Evaluation of FT-IR spectra shows that the P3MPY-SDS/PEDOT layer is created on the OL 37 substrate. Owing to the perfection of the physical barrier action, the coating is uniform, compact through low porosity, and has greater inhibitive properties and, in conclusion, P3MPY-SDS/PEDOT composite films obtained potentiostatically at 1.4 V 5:3, t = 20 min are much better and more protective than those obtained at 1.0 V under the same conditions, and coatings obtained galvanostatically at 5 mA/cm2 have higher protection efficiency than those at 3 mA/cm2. The higher substrate coverage and superior adsorption activity also suggested a higher corrosion protection action of the coatings according to the experimental data presented.
- Composite coatings deposited at potentials between 1.4 V and 1.2 V offered superior corrosion protection compared to those deposited at current densities ranging from 3 mA/cm2 to 5 mA/cm2. In addition to deposition conditions, a 5:3 molar ratio and a longer deposition time, i.e., 20 min, produce more protective films compared to a 3:5 molar ratio and shorter deposition time (10 min).
- We can state that the composite coatings obtained under the presented conditions prevent corrosive attack on the carbon steel substrate and the new composite P3MPY-SDS/PEDOT obtained by electrochemical polymerization methods can be successfully used in technological practices for the protection of various metallic materials.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y. Strengthening, Corrosion and Protection of High-Temperature Structural Materials. Coatings 2022, 12, 1136. [Google Scholar] [CrossRef]
- Xie, J.; Lu, Z.; Zhou, K.; Li, C.; Ma, J.; Wang, B.; Xu, K.; Cui, H.; Liu, J. Researches on corrosion behaviors of carbon steel/copper alloy couple under organic coating in static and flowing seawater. Prog. Org. Coat. 2022, 166, 106793. [Google Scholar] [CrossRef]
- Wang, T.; Xu, Y.; Liu, Z.; Li, G.; Guo, Y.; Lian, J.; Zhang, Z.; Ren, L. A chitosan/polylactic acid composite coating enhancing the corrosion resistance of the bio-degradable magnesium alloy. Prog. Org. Coat. 2023, 178, 107469. [Google Scholar] [CrossRef]
- Yu, H.; Guo, Q.; Wang, C.; Cao, G.; Liu, Y. Preparation and performance of PANI/CNTs composite coating on 316 stainless steel bipolar plates by pulsed electrodeposition. Prog. Org. Coat. 2023, 182, 107611. [Google Scholar] [CrossRef]
- Liao, F.-Q.; Chen, Y.-C. Siloxane-based epoxy coatings through cationic photopolymerization for corrosion protection. Prog. Org. Coat. 2022, 174, 107235. [Google Scholar] [CrossRef]
- Li, J.; Liu, J.-C.; Gao, C.-J. On the mechanism of conductivity enhancement in PEDOT/PSS film doped with multi-walled carbon nanotubes. J. Polym. Res. 2009, 17, 713–718. [Google Scholar] [CrossRef]
- Branzoi, F.; Mihai, A.M.; Zaki, M.Y. Anticorrosion Protection of New Composite Coating for Cobalt-Based Alloy in Hydrochloric Acid Solution Obtained by Electrodeposition Methods. Coatings 2024, 14, 106. [Google Scholar] [CrossRef]
- Sun, Y.; Hu, C.; Cui, J.; Shen, S.; Qiu, H.; Li, J. Electrodeposition of polypyrrole coatings doped by benzenesulfonic acid-modified graphene oxide on metallic bipolar plates. Prog. Org. Coat. 2022, 170, 106995. [Google Scholar] [CrossRef]
- Chou, T.; Chandrasekaran, C.; Limmer, S.; Seraji, S.; Wu, Y.; Forbess, M.; Nguyen, C.; Cao, G. Organic–inorganic hybrid coatings for corrosion protection. J. Non-Cryst. Solids 2001, 290, 153–162. [Google Scholar] [CrossRef]
- Özyılmaz, A.T.; Çolak, N.; Ozyilmaz, G.; Sangün, M.K. Protective properties of polyaniline and poly(aniline-co-o-anisidine) films electrosynthesized on brass. Prog. Org. Coat. 2007, 60, 24–32. [Google Scholar] [CrossRef]
- Ramírez, A.; Mieres, F.; Pineda, F.; Grez, P.; Heyser, C. Electrosynthesis of polyindole-carboxylic acids on stainless steel and their corrosion protection at different temperatures in acidic solution. Prog. Org. Coat. 2022, 177, 107075. [Google Scholar] [CrossRef]
- Zhai, Y.; Pan, K.; Zhang, E. Anti-Corrosive Coating of Carbon-Steel Assisted by Polymer-Camphorsulfonic Acid Embedded within Graphene. Coatings 2020, 10, 879. [Google Scholar] [CrossRef]
- Song, L.; Gao, Z.; Sun, Q.; Chu, G.; Shi, H.; Xu, N.; Li, Z.; Hao, N.; Zhang, X.; Ma, F.; et al. Corrosion protection performance of a coating with 2-aminino-5-mercato-1,3,4-thiadizole-loaded hollow mesoporous silica on copper. Prog. Org. Coat. 2022, 175, 107331. [Google Scholar] [CrossRef]
- Hernández-Martínez, D.; León-Silva, U.; Nicho, M.E. Corrosion protection of steel by poly(3-hexylthiophene) polymer blends. Anti-Corrosion Methods Mater. 2015, 62, 229–240. [Google Scholar] [CrossRef]
- Güven, N.C.; Ozkazanc, H. Corrosion protection behavior of poly(N-methylpyrrole)/boron nitride composite film on alumi-num-1050. Prog. Org. Coat. 2022, 164, 106696. [Google Scholar] [CrossRef]
- Patil, D.; Patil, P.P. Electrodeposition of poly(o-toluidine) on brass from aqueous salicylate solution and its corrosion protection performance. J. Appl. Polym. Sci. 2010, 118, 2084–2091. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, A. Study on the synthesis of PANI/CNT nanocomposite and its anticorrosion mechanism in waterborne coatings. Prog. Org. Coat. 2021, 159, 106447. [Google Scholar] [CrossRef]
- Dararatana, N.; Seidi, F.; Crespy, D. Polymer conjugates for dual functions of reporting and hindering corrosion. Polymer 2020, 194, 122346. [Google Scholar] [CrossRef]
- Luo, Y.; Li, X.; Luo, Z.; Chen, L.; Yang, Y.; Li, J.; Han, G. Enhanced adhesive and anti-corrosive performances of polymer composite coating for rusted metallic substrates by capillary filling. Prog. Org. Coat. 2023, 178, 107467. [Google Scholar] [CrossRef]
- Liu, A.; Tian, H.; Li, S.; Ju, X.; Yang, H.; Sun, Y.; Wang, L.; Li, W. Bioinspired layered hybrid coatings with greatly enhanced barrier effect and active corrosion protection performance. Prog. Org. Coat. 2021, 152, 106131. [Google Scholar] [CrossRef]
- Shinde, V.; Gaikwad, A.; Patil, P. Synthesis and characterization of corrosion protective poly(2,5-dimethylaniline) coatings on copper. Appl. Surf. Sci. 2006, 253, 1037–1045. [Google Scholar] [CrossRef]
- Ren, S.; Barkey, D. Electrochemically Prepared Poly(3-methylthiophene) Films for Passivation of 430 Stainless Steel. J. Electrochem. Soc. 1992, 139, 1021–1026. [Google Scholar] [CrossRef]
- Martí, M.; Armelin, E.; Iribarren, J.I.; Alemán, C. Soluble polythiophenes as anticorrosive additives for marine epoxy paints. Mater. Corros. 2013, 66, 23–30. [Google Scholar] [CrossRef]
- Gupta, D.K.; Neupane, S.; Singh, S.; Karki, N.; Yadav, A.P. The effect of electrolytes on the coating of polyaniline on mild steel by electrochemical methods and its corrosion behavior. Prog. Org. Coat. 2021, 152, 106127. [Google Scholar] [CrossRef]
- Yağan, A.; Pekmez, N.Ö.; Yıldız, A. Poly(N-methylaniline) coatings on stainless steel by electropolymerization. Corros. Sci. 2007, 49, 2905–2919. [Google Scholar] [CrossRef]
- Rui, M.; Zhu, A. The synthesis and corrosion protection mechanisms of PANI/CNT nanocomposite doped with organic phosphoric acid. Prog. Org. Coat. 2021, 153, 106134. [Google Scholar] [CrossRef]
- Fuseini, M.; Zaghloul, M.M.Y. Investigation of Electrophoretic Deposition of PANI Nano fibers as a Manufacturing Technology for corrosion protection. Prog. Org. Coat. 2022, 171, 107015. [Google Scholar] [CrossRef]
- Yağan, A.; Pekmez, N.Ö.; Yıldız, A. Poly(N-ethylaniline) coatings on 304 stainless steels for corrosion protection in aqueous HCl and NaCl solutions. Electrochimica Acta 2008, 53, 2474–2482. [Google Scholar] [CrossRef]
- Davoodi, A.; Honarbakhsh, S.; Farzi, G.A. Evaluation of corrosion resistance of polypyrrole/functionalized multi-walled carbon nanotubes composite coatings on 60Cu–40Zn brass alloy. Prog. Org. Coat. 2015, 88, 106–115. [Google Scholar] [CrossRef]
- Yan, Q.; Pan, W.; Zhong, S.; Zhu, R.; Li, G. Effect of solvents on the preparation and corrosion protection of polypyrrole. Prog. Org. Coat. 2019, 132, 298–304. [Google Scholar] [CrossRef]
- Yılmaz, S.M.; Atun, G. Corrosion protection efficiency of the electrochemically synthesized polypyrrole-azo dye composite coating on stainless steel. Prog. Org. Coat. 2022, 169, 106942. [Google Scholar] [CrossRef]
- Branzoi, F.; Branzoi, V.; Musina, A. Fabrication and characterisation of conducting composite films based on conducting polymers and functionalised carbon nanotubes. Surf. Interface Anal. 2012, 44, 1076–1080. [Google Scholar] [CrossRef]
- Asan, G.; Asan, A.; Çelikkan, H. The effect of 2D-MoS2 doped polypyrrole coatings on brass corrosion. J. Mol. Struct. 2019, 1203, 127318. [Google Scholar] [CrossRef]
- Su, W.; Iroh, J.O. Electrodeposition mechanism, adhesion and corrosion performance of polypyrrole and poly (N-methylpyrrole) coatings on steel substrates. Synth. Met. 2000, 114, 225–234. [Google Scholar] [CrossRef]
- González-Tejera, M.; García, M.; de la Blanca, E.S.; Redondo, M.; Raso, M.; Carrillo, I. Electrochemical synthesis of N-methyl and 3-methyl pyrrole perchlorate doped copolymer films. Thin Solid Films 2007, 515, 6805–6811. [Google Scholar] [CrossRef]
- Duran, B.; Bereket, G. Cyclic Voltammetric Synthesis of Poly(N-methyl pyrrole) on Copper and Effects of Polymerization Parameters on Corrosion Performance. Ind. Eng. Chem. Res. 2012, 51, 5246–5255. [Google Scholar] [CrossRef]
- Zeybek, B.; Aksun, E. Electrodeposition of poly(N-methylpyrrole) on stainless steel in the presence of sodium dodecylsulfate and its corrosion performance. Prog. Org. Coat. 2015, 81, 1–10. [Google Scholar] [CrossRef]
- Çakmakcı, I.; Duran, B.; Duran, M.; Bereket, G. Experimental and theoretical studies on protective properties of poly(pyrrole-co-N-methyl pyrrole) coatings on copper in chloride media. Corros. Sci. 2013, 69, 252–261. [Google Scholar] [CrossRef]
- Bazzaoui, M.; Martins, J.; Bazzaoui, E.; Martins, L.; Machnikova, E. Sweet aqueous solution for electrochemical synthesis of polypyrrole part 1B: On copper and its alloys. Electrochim. Acta 2007, 52, 3568–3581. [Google Scholar] [CrossRef]
- Redondo, M.; de la Blanca, E.S.; García, M.; González-Tejera, M. Poly(N-methylpyrrole) electrodeposited on copper: Corrosion protection properties. Prog. Org. Coat. 2009, 65, 386–391. [Google Scholar] [CrossRef]
- Aradilla, D.; Azambuja, D.; Estrany, F.; Iribarren, J.I.; Ferreira, C.A.; Alemán, C. Poly(3,4-ethylenedioxythiophene) on self-assembled alkanethiol monolayers for corrosion protection. Polym. Chem. 2011, 2, 2548–2556. [Google Scholar] [CrossRef]
- Kumar, A.M.; Hussein, M.A.; Adesina, A.Y.; Ramakrishna, S.; Al-Aqeeli, N. Influence of surface treatment on PEDOT coatings: Surface and electrochemical corrosion aspects of newly developed Ti alloy. RSC Adv. 2018, 8, 19181–19195. [Google Scholar] [CrossRef]
- Lallemand, F.; Plumier, F.; Delhalle, J.; Mekhalif, Z. Electrochemical elaboration of adherent poly(3,4-ethylene-dioxythiophene) films and hybride nanowires on nickel. Appl. Surf. Sci. 2008, 254, 3318–3323. [Google Scholar] [CrossRef]
- Zhang, S.; Li, M.; Zhai, L. Preparation and Corrosion Inhibition of Single and Biphase Composite Coating Based on PEDOT in 0.1M NaOH. Int. J. Electrochem. Sci. 2019, 14, 4828–4837. [Google Scholar] [CrossRef]
- Kumara, R.; Karadea, S.S.; Shindec, S.K.; Warkhade, S.K. Enhanced corrosion protection of Cu&Al in Saline media using a new PEDOT based waterborn polyurethane coating. Results Surf. Interfaces 2023, 12, 100139. [Google Scholar]
- Kumar, A.M.; Adesina, A.Y.; Hussein, M.A.; Ramakrishna, S.; Al-Aqeeli, N.; Akhtar, S.; Saravanan, S. PEDOT/FHA nanocomposite coatings on newly developed Ti-Nb-Zr implants: Biocompatibility and surface protection against corrosion and bacterial infections. Mater. Sci. Eng. C 2019, 98, 482–495. [Google Scholar] [CrossRef] [PubMed]
- Branzoi, F.; Petrescu, S. The Electrodeposition of Derivatives of Pyrrole and Thiophene on Brass Alloy in the Presence of Dodecane-1-Sulfonic Acid Sodium Salt in Acidic Medium and Its Anti-Corrosive Properties. Coatings 2023, 13, 953. [Google Scholar] [CrossRef]
- Zhou, W.; Wu, K.; Zhang, K.; Wang, Z.; Liu, Z.; Hu, S.; Fang, Y.; He, C. Studies on Corrosion Behaviors of Q235 Steel Coated by the Polypyrrole Films Doped with different dopants. Int. J. Electrochem. Sci. 2020, 15, 2594–2603. [Google Scholar] [CrossRef]
- Gopi, D.; Saraswathy, R.; Kavitha, L.; Kim, D.-K. Electrochemical synthesis of poly(indole-co-thiophene) on low-nickel stainless steel and its anticorrosive performance in 0.5 mol L−1 H2SO4. Polym. Int. 2014, 63, 280–289. [Google Scholar] [CrossRef]
- Menkuer, M.; Ozkazanc, H. Anticorrosive polypyrrole/zirconium-oxide composite film prepared in oxalic acid and dodecylbenzene sulfonic acid mix electrolyte. Prog. Org. Coat. 2020, 147, 105815. [Google Scholar] [CrossRef]
- Branzoi, F.; Băran, A.; Petrescu, S. Evaluation of Corrosion Protection Performance of New Polymer Composite Coatings on Carbon Steel in Acid Medium by Electrodeposition Methods. Coatings 2021, 11, 903. [Google Scholar] [CrossRef]
- Gallegos-Melgar, A.; Serna, S.A.; Lázaro, I.; Gutiérrez-Castañeda, E.J.; Mercado-Lemus, V.H.; Arcos-Gutierrez, H.; Hernández-Hernández, M.; Porcayo-Calderón, J.; Mayen, J.; Monroy, M.D. Potentiodynamic Polarization Performance of a Novel Composite Coating System of Al2O3/Chitosan-Sodium Alginate, Applied on an Aluminum AA6063 Alloy for Protection in a Chloride Ions Environment. Coatings 2020, 10, 45. [Google Scholar] [CrossRef]






| The System P3MPY-SDS/PEDOT OL 37 | Ecorr (mV) | icorr (mA/cm2) | Rp Ω cm2 | Rmpy | Pmm/year | Kg (g/m2h) | ba (mV/ decade) | −bc (mV/ decade) | E (%) | %P |
|---|---|---|---|---|---|---|---|---|---|---|
| OL 37 + 0.5 M H2SO4 | −507 | 0.902 | 14 | 419 | 10.63 | 9.45 | 98 | 95 | - | |
| P3MPY-SDS/PEDOT 3 mA/cm2 3:5 molar ratio, t = 10 min | −487 | 0.118 | 114 | 55.06 | 1.39 | 1.24 | 77 | 98 | 87 | 0.06 |
| P3MPY-SDS/PEDOT 3 mA/cm2 5:3 molar ratio, t = 10 min | −454 | 0.096 | 119 | 44.8 | 1.13 | 1.01 | 63 | 82 | 90 | 0.08 |
| P3MPY-SDS/PEDOT 3 mA/cm2 3:5 molar ratio, t = 20 min | −458 | 0.114 | 116 | 53.2 | 1.35 | 1.20 | 68 | 88 | 88 | 0.02 |
| P3MPY-SDS/PEDOT 3 mA/cm2 5:3 molar ratio, t = 20 min | −481 | 0.076 | 180 | 35.46 | 0.90 | 0.80 | 64 | 103 | 92 | 0.009 |
| P3MPY-SDS/PEDOT 5 mA/cm2 3:5 molar ratio, t = 10 min | −457 | 0.121 | 127 | 54.46 | 1.43 | 1.22 | 75 | 109 | 87 | 0.02 |
| P3MPY-SDS/PEDOT 5 mA/cm2 5:3 molar ratio, t = 10 min | −487 | 0.087 | 162 | 40.6 | 1.03 | 0.91 | 83 | 97 | 91 | 0.04 |
| P3MPY-SDS/PEDOT 5 mA/cm2 3:5 molar ratio, t = 20 min | −488 | 0.085 | 168 | 39.66 | 1.01 | 0.89 | 80 | 101 | 91 | 0.04 |
| P3MPY-SDS/PEDOT 5 mA/cm2 5:3 molar ratio, t = 20 min | −449 | 0.041 | 304 | 19.13 | 1.48 | 0.43 | 65 | 96 | 95 | 0.008 |
| The System P3MPY-SDS/PEDOT OL 37 | Ecorr (mV) | icorr (mA/cm2) | Rp Ω cm2 | Rmpy | Pmm/year | Kg (g/m2h) | ba (mV/ decade) | −bc (mV/ decade) | E (%) | %P |
|---|---|---|---|---|---|---|---|---|---|---|
| OL 37 + 0.5 M H2SO4 | −507 | 0.902 | 14 | 419 | 10.63 | 9.45 | 98 | 95 | - | - |
| P3MPY-SDS/PEDOT 1.0 V 3:5 molar ratio, t = 10 min | −500 | 0.16 | 90 | 74.6 | 1.89 | 1.68 | 88 | 93 | 82 | 0.095 |
| P3MPY-SDS/PEDOT 1.0 V 5:3 molar ratio, t = 10 min | −499 | 0.063 | 240 | 29.4 | 0.74 | 0.66 | 96 | 102 | 92 | 0.024 |
| P3MPY-SDS/PEDOT 1.0 V 3:5 molar ratio, t = 20 min | −497 | 0.144 | 106 | 67.2 | 1.70 | 1.51 | 101 | 103 | 85 | 0.07 |
| P3MPY-SDS/PEDOT 1.0 V 5:3 molar ratio, t = 20 min | −503 | 0.027 | 586 | 13.1 | 0.33 | 0.29 | 94 | 108 | 97 | 0.001 |
| P3MPY-SDS/PEDOT 1.2 V 3:5 molar ratio, t = 10 min | −467 | 0.18 | 89 | 84 | 2.13 | 1.89 | 77 | 78 | 80 | 0.052 |
| P3MPY-SDS/PEDOT 1.2 V 5:3 molar ratio, t = 10 min | −502 | 0.074 | 230 | 34.5 | 0.87 | 0.77 | 99 | 105 | 92 | 0.005 |
| P3MPY-SDS/PEDOT 1.2 V 3:5 molar ratio, t = 20 min | −448 | 0.081 | 190 | 37.8 | 0.95 | 0.85 | 89 | 85 | 91 | 0.014 |
| P3MPY-SDS/PEDOT 1.2 V 5:3 molar ratio, t = 20 min | −494 | 0.052 | 327 | 24.2 | 0.61 | 0.54 | 92 | 104 | 94 | 0.07 |
| P3MPY-SDS/PEDOT 1.4 V 3:5 molar ratio, t = 10 min | −408 | 0.059 | 212 | 27.5 | 0.69 | 0.62 | 62 | 84 | 93 | 0.006 |
| P3MPY-SDS/PEDOT 1.4 V 5:3 molar ratio, t = 10 min | −487 | 0.046 | 300 | 21.4 | 0.54 | 0.48 | 69 | 107 | 95 | 0.002 |
| P3MPY-SDS/PEDOT 1.4 V 3:5 molar ratio, t = 20 min | −496 | 0.17 | 89 | 79.3 | 2.01 | 1.79 | 76 | 87 | 81 | 0.08 |
| P3MPY-SDS/PEDOT 1.4 V 5:3 molar ratio, t = 20 min | −481 | 0.039 | 379 | 18.2 | 0.46 | 0.41 | 101 | 87 | 96 | 0.001 |
| The P3MPY-SDS/PEDOT/OL 37 | Ecorr (mV) | icorr (µA/cm2) | Rp Ω cm2 | Rmpy | Pmm/year | Kg (g/m2h) | ba (mV/ decade) | −bc (mV/ decade) | E (%) |
|---|---|---|---|---|---|---|---|---|---|
| P3MPY-SDS/PEDOT 1.0 V 5:3 molar ratio, t = 20 min, 0 h | −503 | 0.028 | 587 | 13.1 | 0.33 | 0.29 | 94 | 108 | 97 |
| P3MPY-SDS/PEDOT 1.0 V 5:3 molar ratio, t = 20 min 24 h | −551 | 0.020 | 684 | 9.38 | 0.24 | 0.22 | 101 | 102 | 98 |
| P3MPY-SDS/PEDOT 1.0 V 5:3 molar ratio, t = 20 min 48 h | −574 | 0.029 | 617 | 13.53 | 0.34 | 0.30 | 105 | 107 | 97 |
| P3MPY-SDS/PEDOT 1.0 V 5:3 molar ratio, t = 20 min 72 h | −573 | 0.036 | 544 | 16.81 | 0.43 | 0.38 | 106 | 93 | 96 |
| P3MPY-SDS/PEDOT 1.0 V 5:3 molar ratio, t = 20 min 96 h | −572 | 0.045 | 370 | 21 | 0.53 | 0.47 | 107 | 103 | 95 |
| P3MPY-SDS/PEDOT 1.0 V 5:3 molar ratio, t = 20 min 120 h | −581 | 0.055 | 301 | 25.66 | 0.65 | 0.58 | 113 | 114 | 94 |
| P3MPY-SDS/PEDOT 1.0 V 5:3 molar ratio, t = 20 min 144 h | −580 | 0.061 | 291 | 28.46 | 0.72 | 0.64 | 114 | 116 | 93 |
| The System P3MPY-SDS/PEDOT/OL 37 | Rs ohm·cm2 | Q-Yo S·s−n·cm−2 | Q-n | Rf ohm·cm2 | Q-Yo S·s−n·cm−2 | Q-n | Rct ohm·cm2 | χ |
|---|---|---|---|---|---|---|---|---|
| OL 37 + 0.5 M H2SO4 | 1.03 | 0.0014 | 0.99 | 1.3 | 0.0026 | 0.75 | 11.6 | 5.7 × 10−3 |
| P3MPY-SDS/PEDOT 1.0 V 3:5 molar ratio, t = 10 min | 6.68 | 0.000036 | 0.98 | 21 | 0.00040 | 0.64 | 78 | 2.31 × 10−4 |
| P3MPY-SDS/PEDOT 1.0 V 5:3 molar ratio, t = 10 min | 18.6 | 0.000055 | 0.63 | 46 | 0.000416 | 0.64 | 184 | 4.24 × 10−4 |
| P3MPY-SDS/PEDOT 1.0 V 3:5 molar ratio, t = 20 min | 14.5 | 0.000531 | 0.60 | 28 | 0.000274 | 0.71 | 112 | 7.97 × 10−5 |
| P3MPY-SDS/PEDOT 1.0 V 5:3 molar ratio, t = 20 min | 22.5 | 0.000025 | 0.61 | 64 | 0.000074 | 0.62 | 522 | 1.40 × 10−4 |
| P3MPY-SDS/PEDOT 1.2 V 3:5 molar ratio, t = 10 min | 1.3 | 0.000320 | 0.91 | 22 | 0.000631 | 0.88 | 56 | 7.58 × 10−4 |
| P3MPY-SDS/PEDOT 1.2 V 5:3 molar ratio, t = 10 min | 10.36 | 0.000015 | 0.71 | 42 | 0.000091 | 0.68 | 260 | 2.27 x10−4 |
| P3MPY-SDS/PEDOT 1.2 V 3:5 molar ratio, t = 20 min | 14.5 | 0.000541 | 0.61 | 32 | 0.000264 | 0.72 | 110 | 8.18 × 10−5 |
| P3MPY-SDS/PEDOT 1.2 V 5:3 molar ratio, t = 20 min | 6.5 | 0.000009 | 0.91 | 38 | 0.000094 | 0.74 | 120 | 1.61 × 10−4 |
| P3MPY-SDS/PEDOT 1.4 V 3:5 molar ratio, t = 10 min | 2.46 | 0.000462 | 0.72 | 24 | 0.00008 | 0.92 | 58 | 5.17 × 10−4 |
| P3MPY-SDS/PEDOT 1.4 V 5:3 molar ratio, t = 10 min | 2.6 | 0.000039 | 0.64 | 52 | 0.000031 | 0.77 | 240 | 1.89 × 10−4 |
| P3MPY-SDS/PEDOT 1.4 V 3:5 molar ratio, t = 20 min | 1.38 | 0.000895 | 0.83 | 60 | 0.000283 | 0.98 | 108 | 1.33 × 10−3 |
| P3MPY-SDS/PEDOT 1.4 5:3 molar ratio, t = 20 min | 6.4 | 0.000093 | 0.64 | 34 | 0.000130 | 0.70 | 347 | 8.79 × 10−4 |
| The System P3MPY-SDS/PEDOT/OL 37 | Rs ohm·cm2 | Q-Yo S·s−n·cm−2 | Q-n | Rf ohm·cm2 | Q-Yo S·s−n·cm−2 | Q-n | Rct ohm·cm2 | χ |
|---|---|---|---|---|---|---|---|---|
| OL 37 + 0.5 M H2SO4 | 1.03 | 0.0014 | 0.99 | 1.3 | 0.0026 | 0.75 | 11.6 | 5.71 × 10−3 |
| P3MPY-SDS/PEDOT 3 mA/cm2 3:5 molar ratio, t = 10 min | 6.09 | 0.00016 | 0.74 | 26 | 0.00010 | 0.78 | 72 | 3.81 × 10−4 |
| P3MPY-SDS/PEDOT 3 mA/cm2 5:3 molar ratio, t = 10 min | 5.6 | 0.000224 | 0.83 | 40 | 0.00017 | 0.72 | 94 | 1.57 × 10−3 |
| P3MPY-SDS/PEDOT 3 mA/cm2 3:5 molar ratio, t = 20 min | 3.82 | 0.000203 | 8.22 | 28 | 0.000194 | 8.85 | 66 | 1.27 × 10−3 |
| P3MPY-SDS/PEDOT 3 mA/cm2 5:3 molar ratio, t = 20 min | 6.2 | 0.000072 | 0.77 | 32 | 0.000065 | 0.70 | 150 | 7.53 × 10−4 |
| P3MPY-SDS/PEDOT 5 mA/cm2 3:5 molar ratio, t = 10 min | 2.72 | 0.00014 | 0.89 | 26 | 0.00012 | 0.83 | 88 | 2.05 × 10−3 |
| P3MPY-SDS/PEDOT 5 mA/cm2 5:3 molar ratio, t = 10 min | 7.24 | 0.000484 | 0.61 | 42 | 0.000496 | 0.98 | 104 | 2.48 × 10−4 |
| P3MPY-SDS/PEDOT 5 mA/cm2 3:5 molar ratio, t = 20 min | 4.49 | 0.000151 | 0.85 | 28 | 0.0001342 | 0.92 | 60 | 2.38 × 10−3 |
| P3MPY-SDS/PEDOT 5 mA/cm2 5:3 molar ratio, t = 20 min | 8.52 | 0.000071 | 0.72 | 44 | 0.000097 | 0.68 | 120 | 1.08 × 10−4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Branzoi, F.; Băran, A.; Mihai, M.A.; Praschiv, A. Anticorrosive Effect of New Polymer Composite Coatings on Carbon Steel in Aggressive Environments by Electrochemical Procedures. Coatings 2025, 15, 359. https://doi.org/10.3390/coatings15030359
Branzoi F, Băran A, Mihai MA, Praschiv A. Anticorrosive Effect of New Polymer Composite Coatings on Carbon Steel in Aggressive Environments by Electrochemical Procedures. Coatings. 2025; 15(3):359. https://doi.org/10.3390/coatings15030359
Chicago/Turabian StyleBranzoi, Florina, Adriana Băran, Marius Alexandru Mihai, and Alexandru Praschiv. 2025. "Anticorrosive Effect of New Polymer Composite Coatings on Carbon Steel in Aggressive Environments by Electrochemical Procedures" Coatings 15, no. 3: 359. https://doi.org/10.3390/coatings15030359
APA StyleBranzoi, F., Băran, A., Mihai, M. A., & Praschiv, A. (2025). Anticorrosive Effect of New Polymer Composite Coatings on Carbon Steel in Aggressive Environments by Electrochemical Procedures. Coatings, 15(3), 359. https://doi.org/10.3390/coatings15030359








