Impact of Surface Tension and Surface Energy on Spray Coating Paper with Polysaccharide-Based Biopolymers
Abstract
1. Introduction
2. Materials and Methods
2.1. Paper Properties
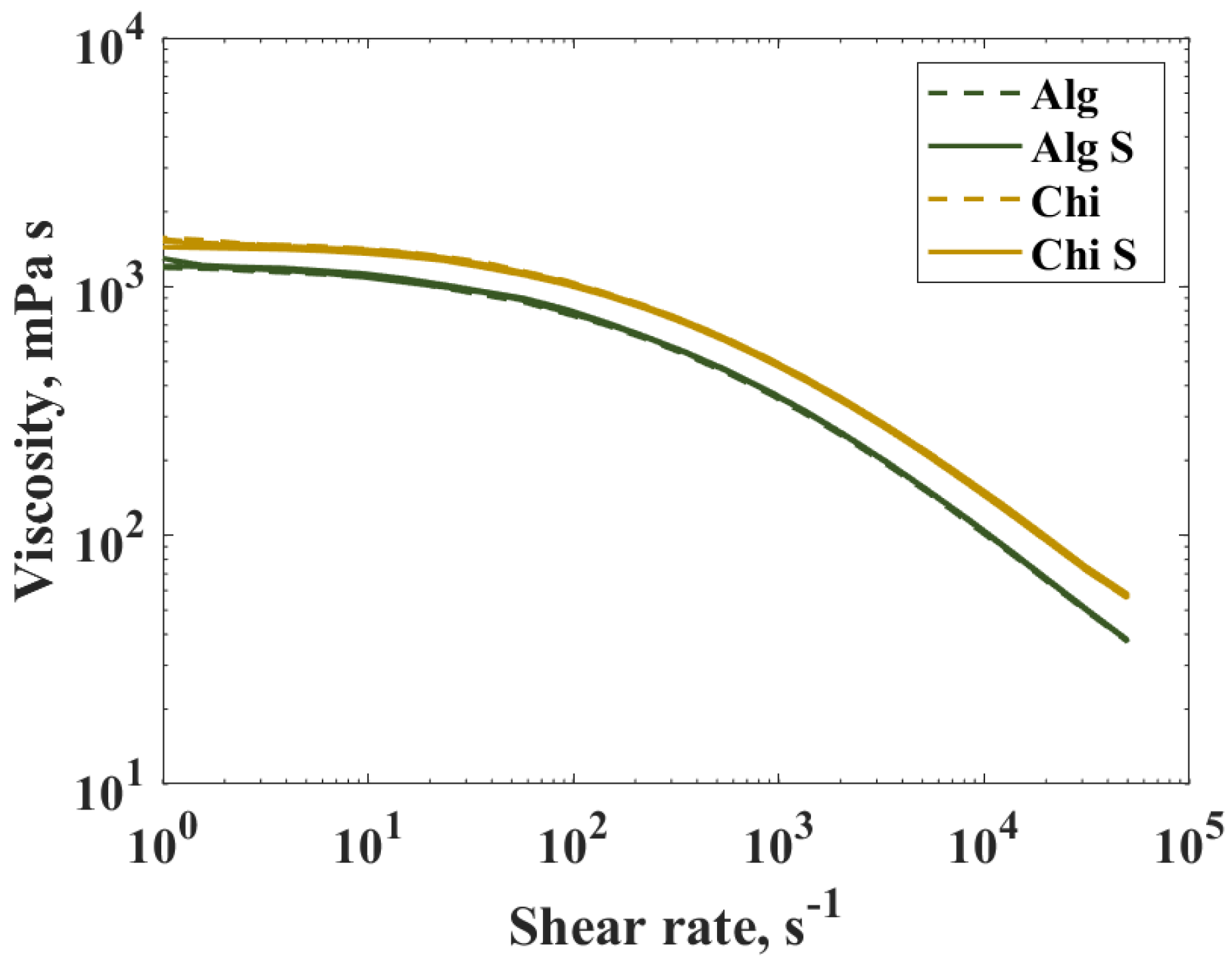
2.2. Biopolymer Solutions
2.3. Biopolymer Contact Angles
2.4. Coating
2.5. Coated Paper Characterization
2.6. Use of AI in This Article
3. Results and Discussion
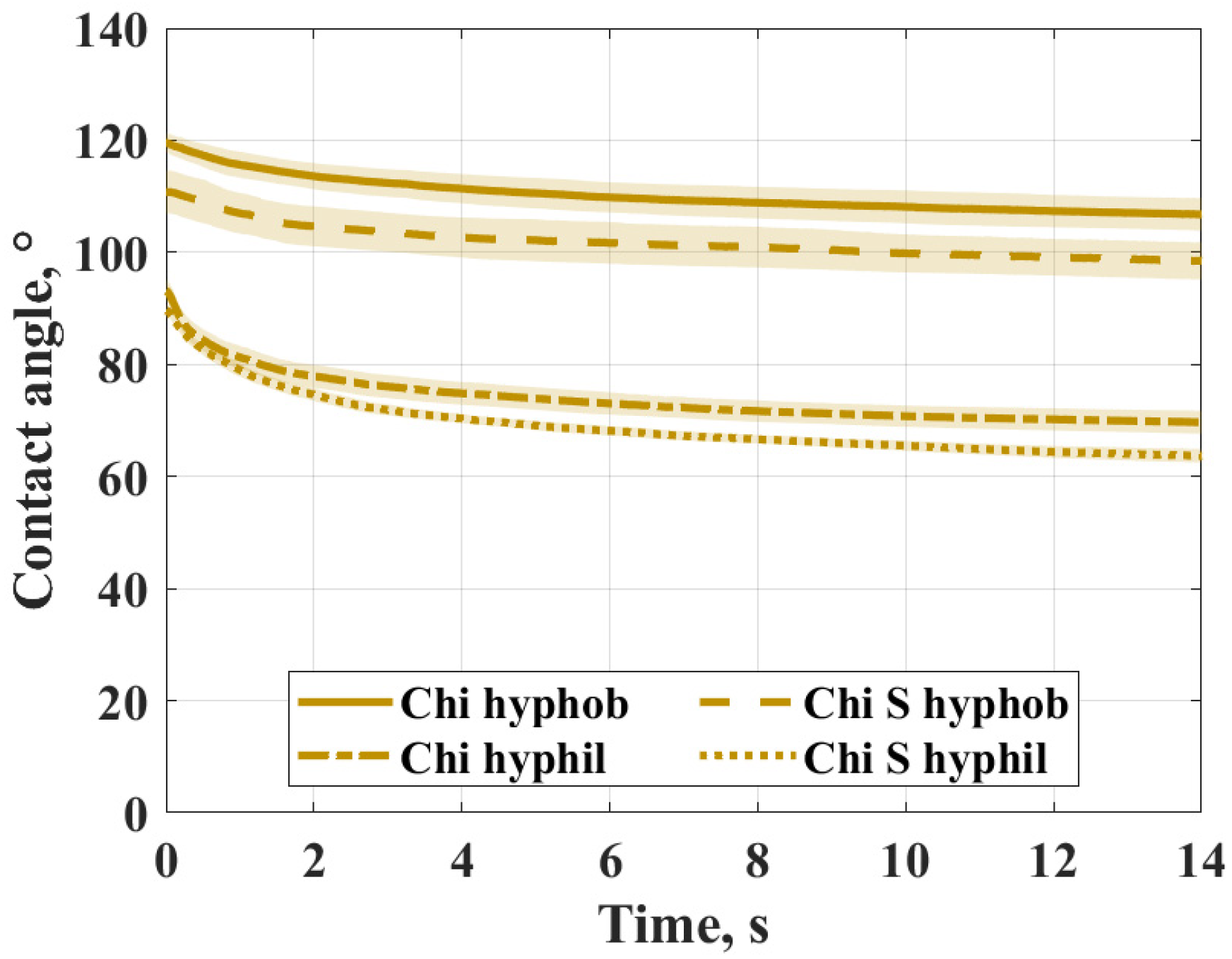
3.1. Contact Angle
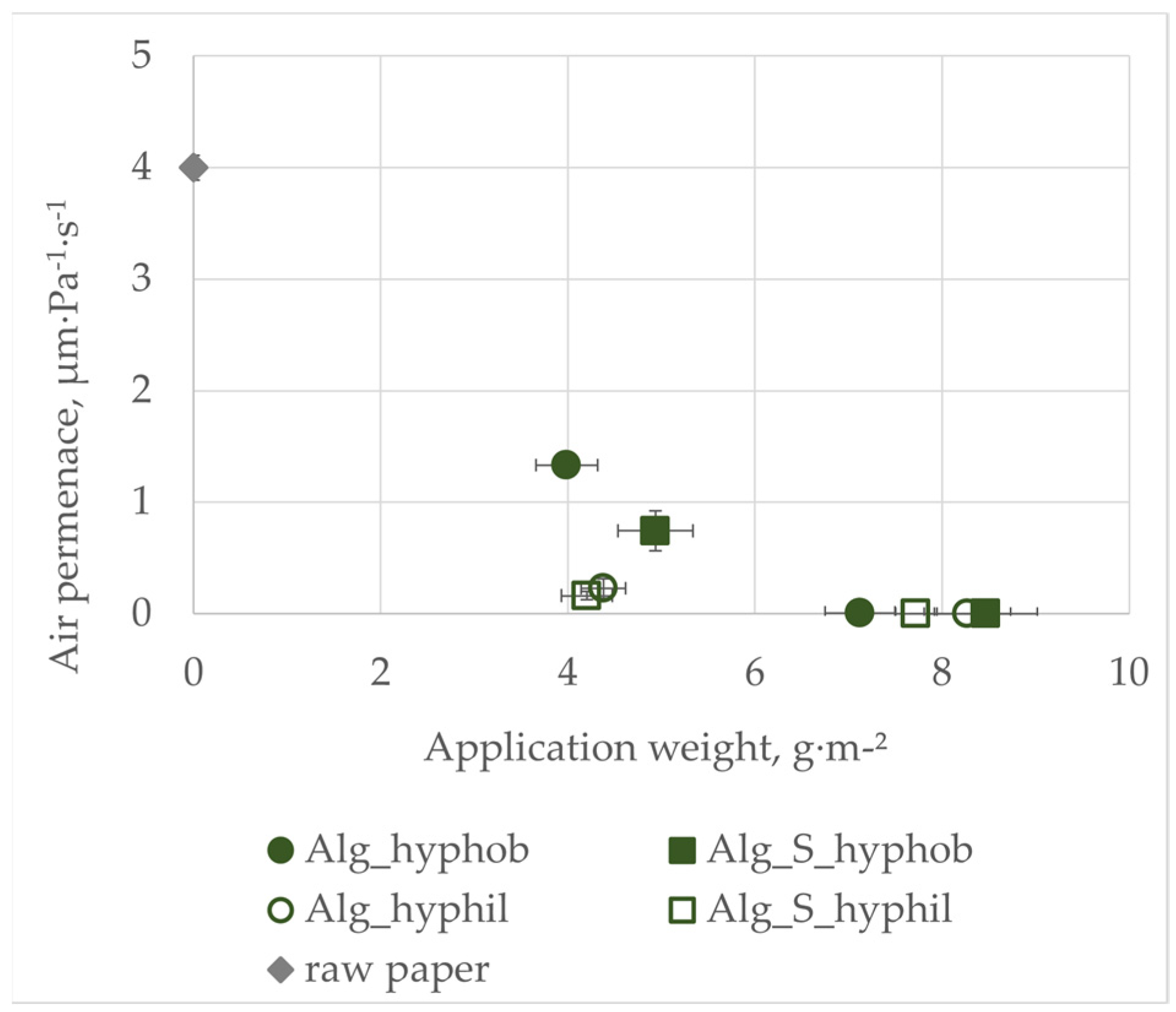
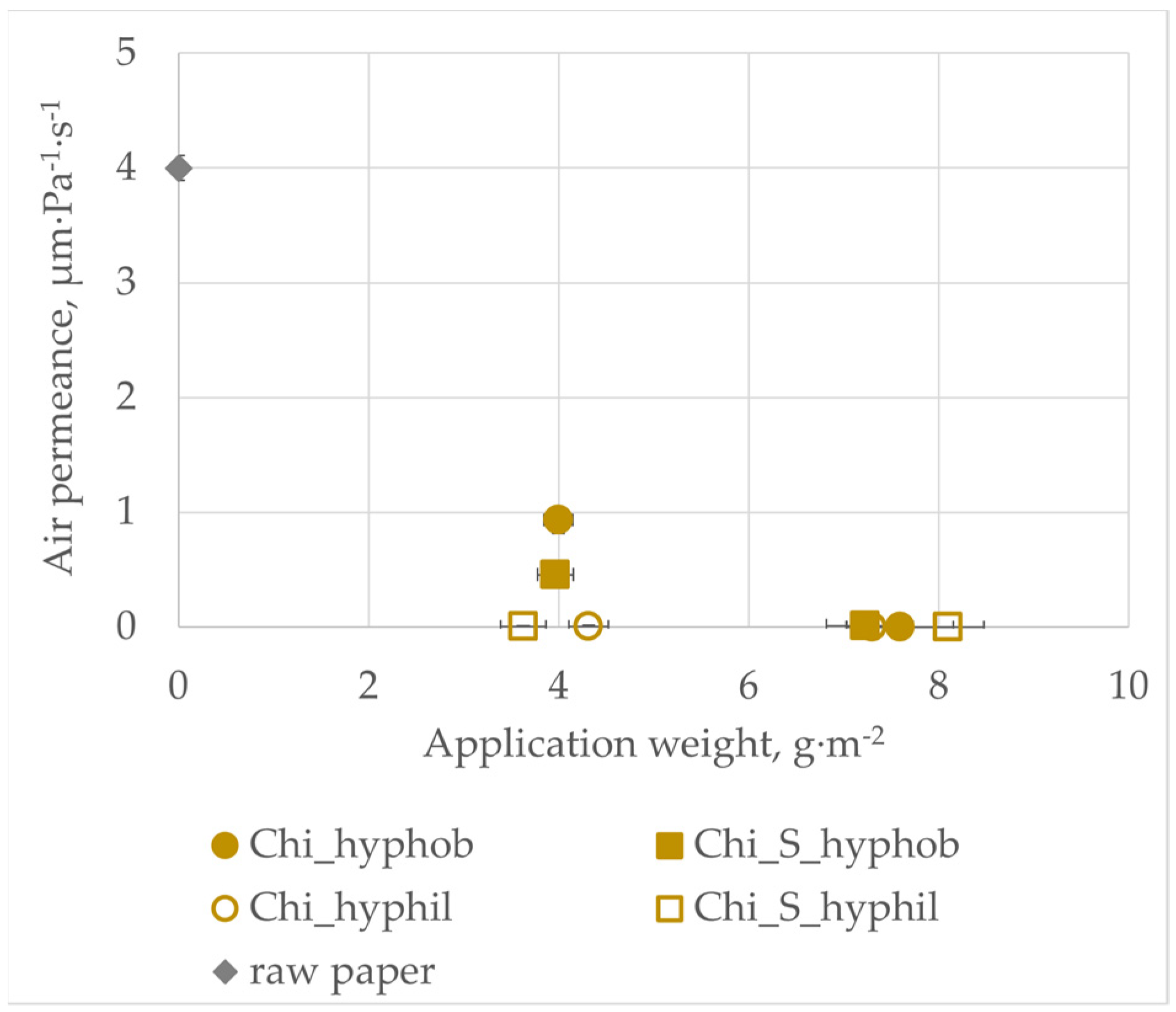
3.2. Air Permeance
3.3. Grease Resistance (KIT)
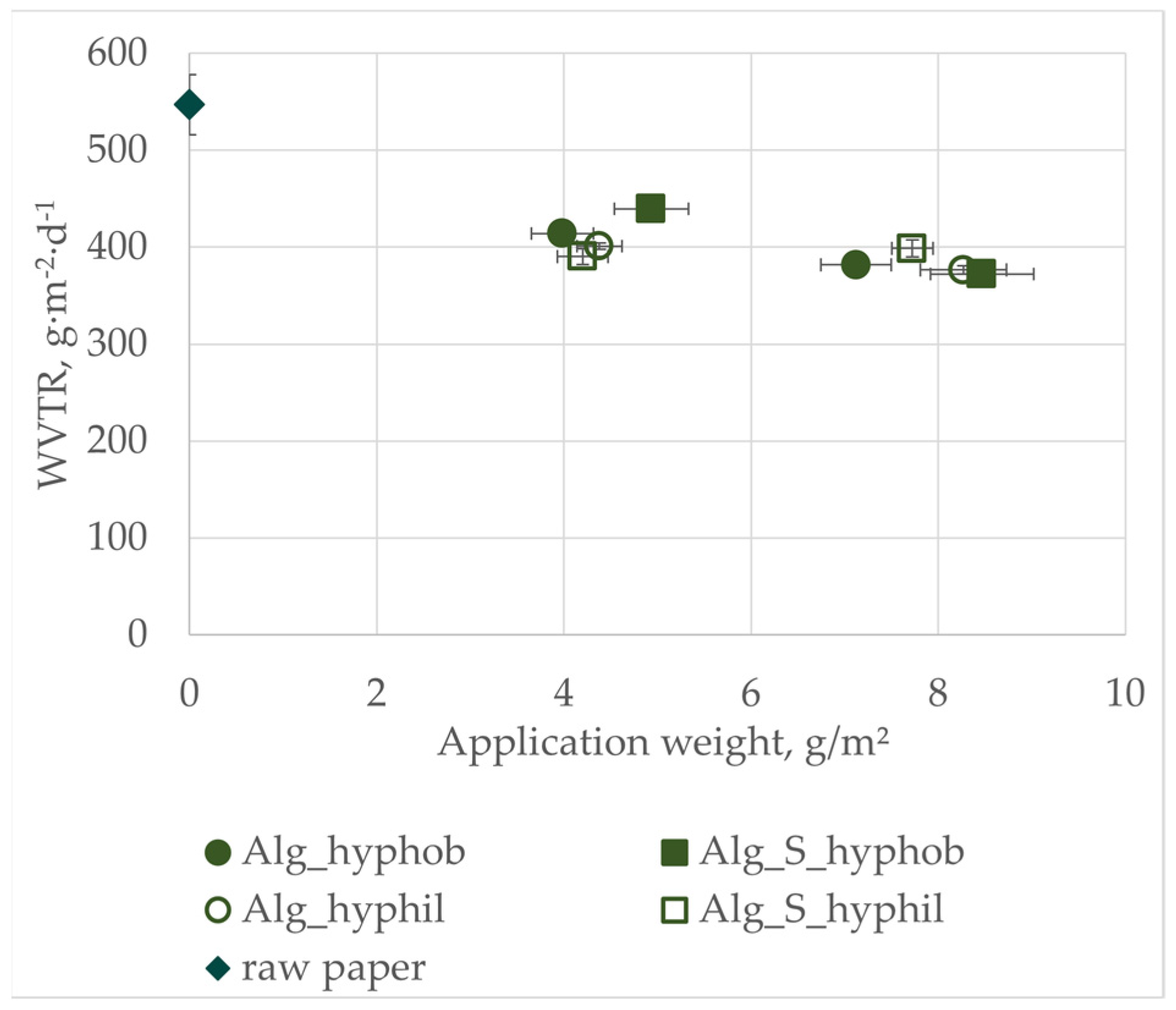
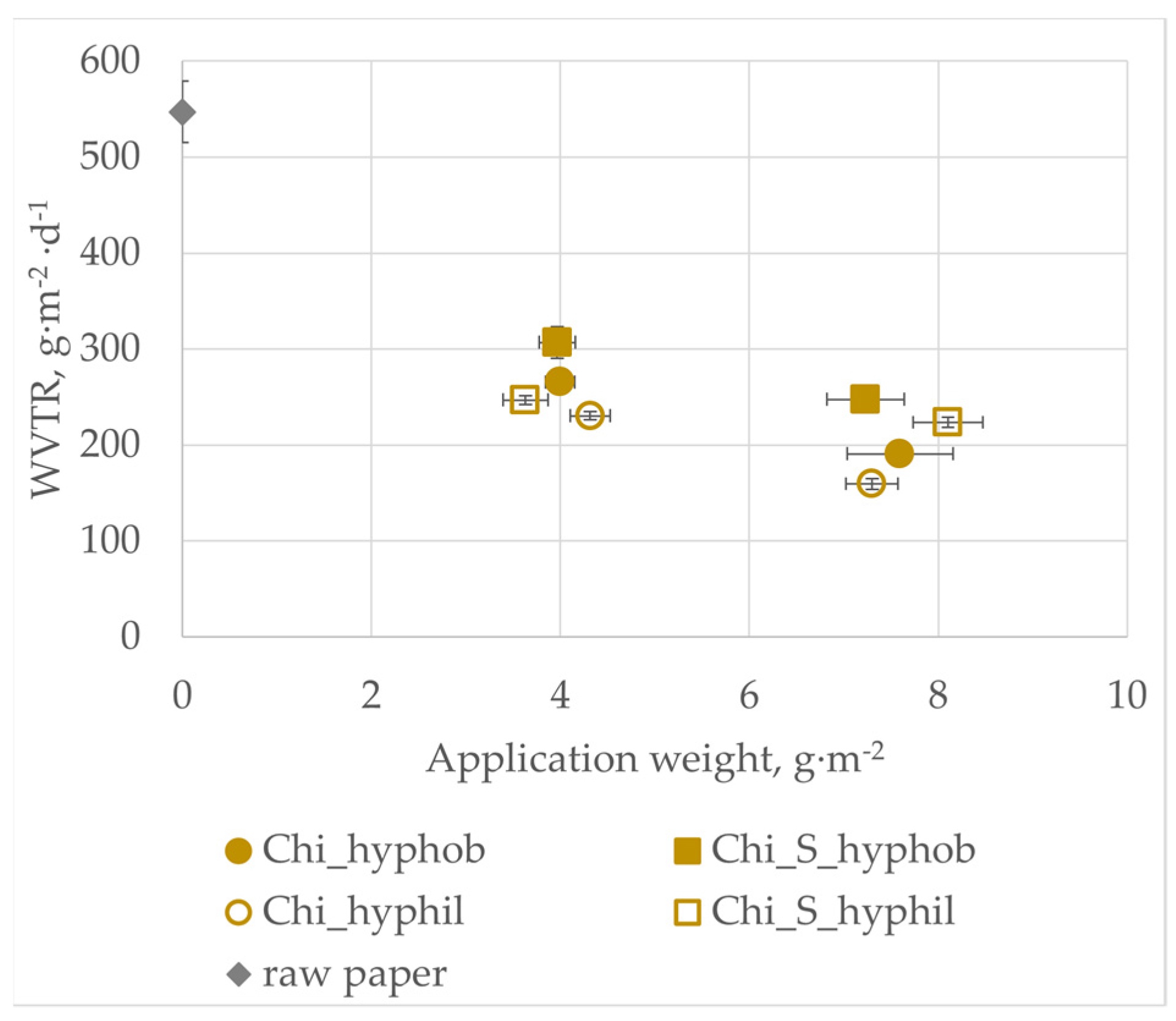
3.4. Water Vapor Transmission Rate
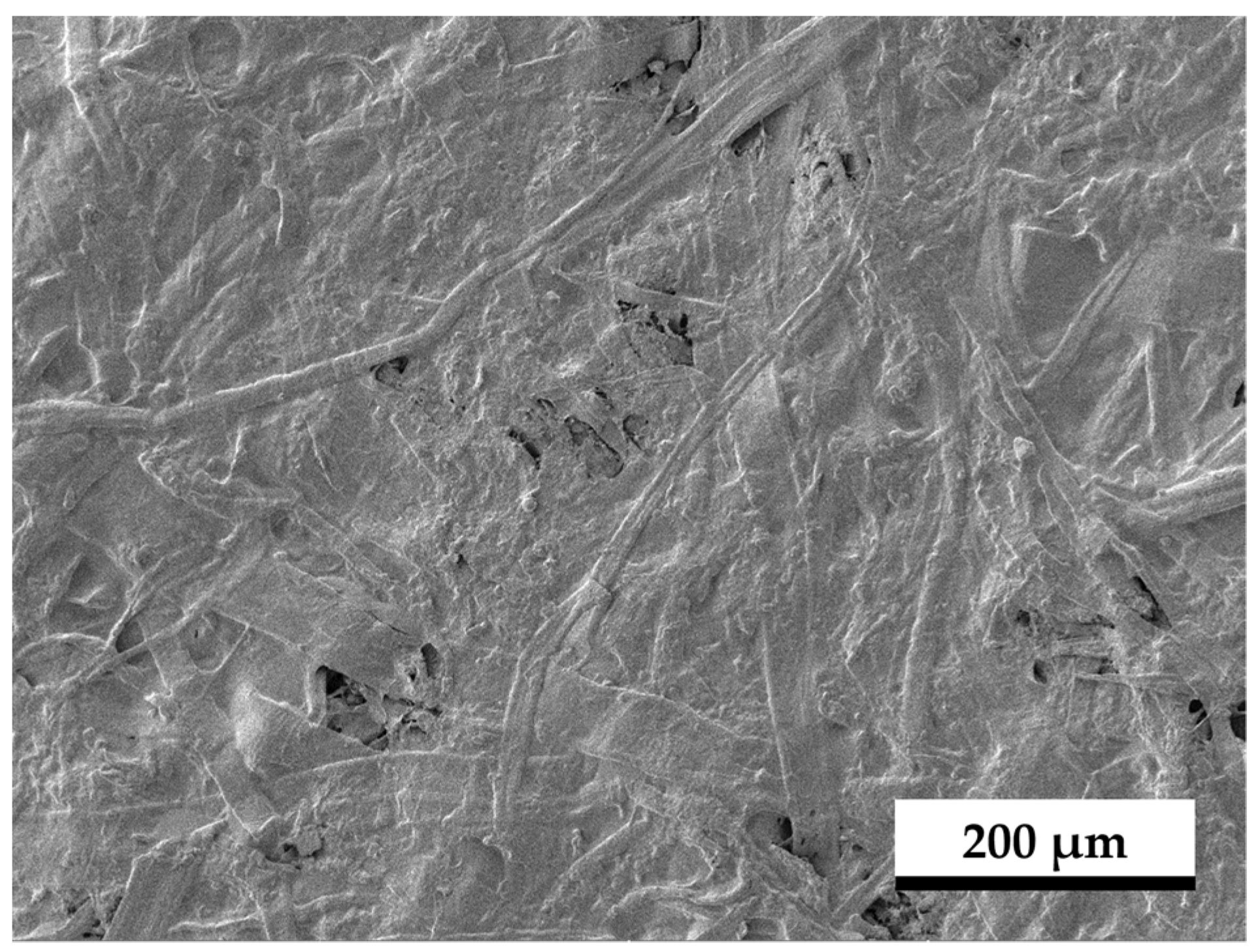
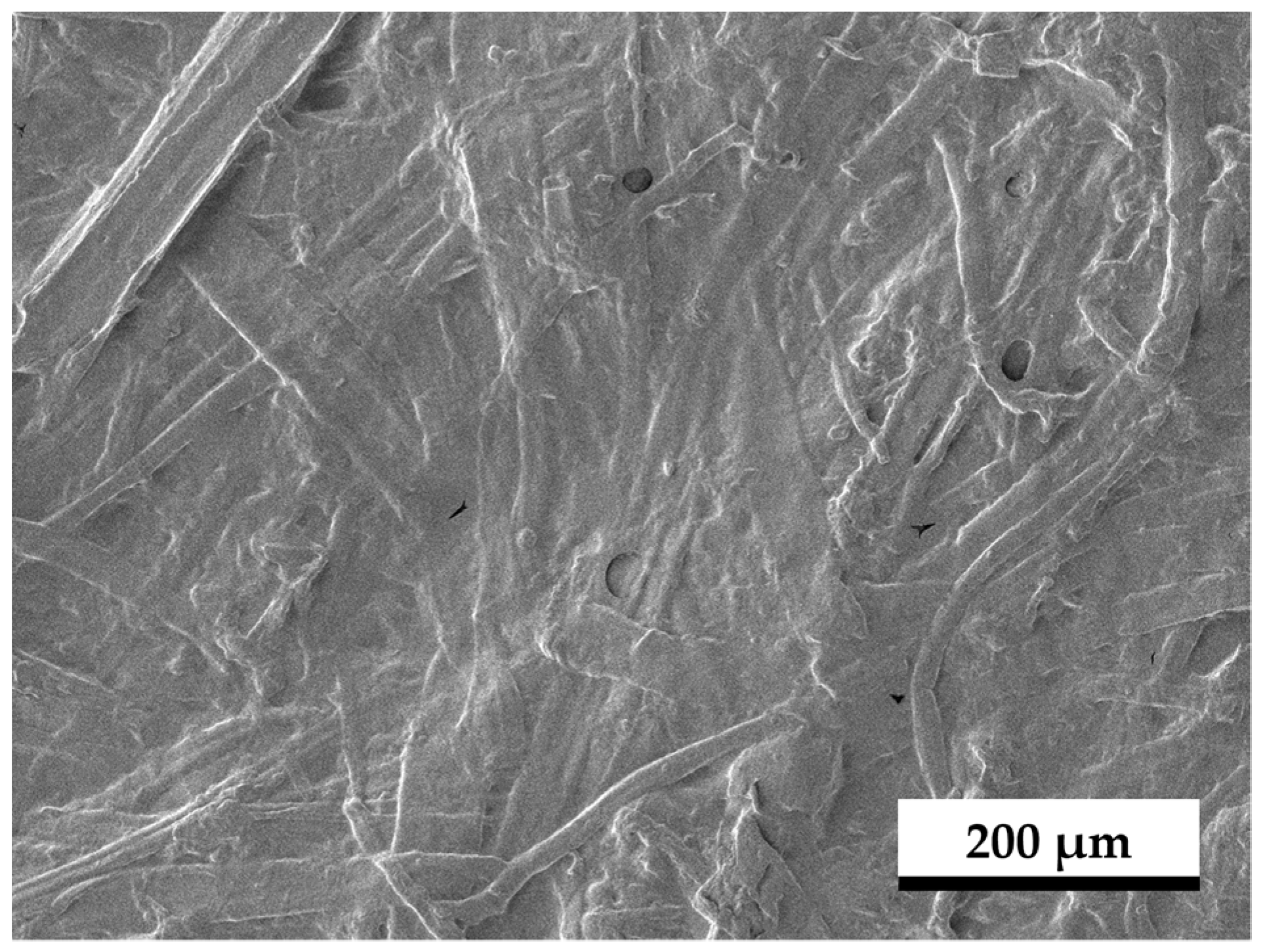
3.5. SEM Images
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Alg | Alginate |
| Chi | Chitosan |
| hyphob | Hydrophobic |
| hyphil | Hydrophilic |
| S | Surfactant |
References
- Udayakumar, G.P.; Muthusamy, S.; Selvaganesh, B.; Sivarajasekar, N.; Rambabu, K.; Banat, F.; Sivamani, S.; Sivakumar, N.; Hosseini-Bandegharaei, A.; Show, P.L. Biopolymers and Composites: Properties, Characterization and Their Applications in Food, Medical and Pharmaceutical Industries. J. Environ. Chem. Eng. 2021, 9, 105322. [Google Scholar] [CrossRef]
- Detzel, A.; Bodrogi, F.; Kauertz, B.; Bick, C.; Welle, F.; Schmid, M.; Schmitz, K.; Müller, K.; Käb, H. Biobasierte Kunststoffe Als Verpackung von Lebensmitteln; Freising: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Marsh, K.; Bugusu, B. Food Packaging—Roles, Materials, and Environmental Issues: Scientific Status Summary. J. Food Sci. 2007, 72, 39–55. [Google Scholar] [CrossRef] [PubMed]
- Khalid, M.Y.; Arif, Z.U. Novel Biopolymer-Based Sustainable Composites for Food Packaging Applications: A Narrative Review. Food Packag. Shelf Life 2022, 33, 100892. [Google Scholar] [CrossRef]
- Kontominas, M.G. Use of Alginates as Food Packaging Materials. Foods 2020, 9, 1440. [Google Scholar] [CrossRef]
- Reichert, C.L.; Bugnicourt, E.; Coltelli, M.B.; Cinelli, P.; Lazzeri, A.; Canesi, I.; Braca, F.; Martínez, B.M.; Alonso, R.; Agostinis, L.; et al. Bio-Based Packaging: Materials, Modifications, Industrial Applications and Sustainability. Polymers 2020, 12, 1558. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Han, F.; Zhang, S.; Li, P.; Shang, N. Novel Bio-Based Materials and Applications in Antimicrobial Food Packaging: Recent Advances and Future Trends. Int. J. Mol. Sci. 2021, 22, 9663. [Google Scholar] [CrossRef] [PubMed]
- Mujtaba, M.; Lipponen, J.; Ojanen, M.; Puttonen, S.; Vaittinen, H. Trends and Challenges in the Development of Bio-Based Barrier Coating Materials for Paper/Cardboard Food Packaging; a Review. Sci. Total Environ. 2022, 851, 158328. [Google Scholar] [CrossRef]
- Khwaldia, K.; Arab-Tehrany, E.; Desobry, S. Biopolymer Coatings on Paper Packaging Materials. Compr. Rev. Food Sci. Food Saf. 2010, 9, 82–91. [Google Scholar] [CrossRef]
- European Paper Recycling Council. Monitoring Report 2022 European Declaration on Paper Recycling 2021–2023; European Paper Recycling Council c/o Cepi aisbl: Brussels, Belgium, 2023. [Google Scholar]
- Kunam, P.K.; Ramakanth, D.; Akhila, K.; Gaikwad, K.K. Bio-Based Materials for Barrier Coatings on Paper Packaging. Biomass Convers. Biorefin. 2022, 14, 12637–12652. [Google Scholar] [CrossRef]
- Brodnjak, U.V.; Tihole, K. Chitosan Solution Containing Zein and Essential Oil as Bio Based Coating on Packaging Paper. Coatings 2020, 10, 497. [Google Scholar] [CrossRef]
- Songok, J.; Toivakka, M. Modelling of Capillary-Driven Flow for Closed Paper-Based Microfluidic Channels. J. Micromech. Microeng. 2017, 27, 65001. [Google Scholar] [CrossRef]
- Parreidt, T.S.; Müller, K.; Schmid, M. Alginate-Based Edible Films and Coatings for Food Packaging Applications. Foods 2018, 7, 170. [Google Scholar] [CrossRef]
- Lan, W.; He, L.; Liu, Y. Preparation and Properties of Sodium Carboxymethyl Cellulose/Sodium Alginate/Chitosan Composite Film. Coatings 2018, 8, 291. [Google Scholar] [CrossRef]
- Wu, W.; Liu, T.; He, H.; Wu, X.; Cao, X.; Jin, J.; Sun, Q.; Roy, V.A.L.; Li, R.K.Y. Rhelogical and Antibacterial Performance of Sodium Alginate/Zinc Oxide Composite Coating for Cellulosic Paper. Colloids Surf. B Biointerfaces 2018, 167, 538–543. [Google Scholar] [CrossRef] [PubMed]
- Kopacic, S.; Walzl, A.; Zankel, A.; Leitner, E.; Bauer, W. Alginate and Chitosan as a Functional Barrier for Paper-Based Packaging Materials. Coatings 2018, 8, 235. [Google Scholar] [CrossRef]
- Gatto, M.; Ochi, D.; Yoshida, C.M.P.; da Silva, C.F. Study of Chitosan with Different Degrees of Acetylation as Cardboard Paper Coating. Carbohydr. Polym. 2019, 210, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Kopacic, S.; Walzl, A.; Hirn, U.; Zankel, A.; Kniely, R.; Leitner, E.; Bauer, W. Application of Industrially Produced Chitosan in the Surface Treatment of Fibre-Based Material: Effect of Drying Method and Number of Coating Layers on Mechanical and Barrier Properties. Polymers 2018, 10, 1232. [Google Scholar] [CrossRef]
- Tanpichai, S.; Srimarut, Y.; Woraprayote, W.; Malila, Y. Chitosan Coating for the Preparation of Multilayer Coated Paper for Food-Contact Packaging: Wettability, Mechanical Properties, and Overall Migration. Int. J. Biol. Macromol. 2022, 213, 534–545. [Google Scholar] [CrossRef]
- Mayrhofer, A.; Kopacic, S.; Bauer, W. Extensive Characterization of Alginate, Chitosan and Microfibrillated Cellulose Cast Films to Assess Their Suitability as Barrier Coating for Paper and Board. Polymers 2023, 15, 3336. [Google Scholar] [CrossRef]
- Vartiainen, J.; Harlin, A. Crosslinking as an Efficient Tool for Decreasing Moisture Sensitivity of Biobased Nanocomposite Films. Mater. Sci. Appl. 2011, 02, 346–354. [Google Scholar] [CrossRef]
- Zhu, Q.; Tan, J.; Li, D.; Zhang, T.; Liu, Z.; Cao, Y. Cross-Linked Chitosan/Tannin Extract as a Biodegradable and Repulpable Coating for Paper with Excellent Oil-Resistance, Gas Barrier and UV-Shielding. Prog. Org. Coat. 2023, 176, 107399. [Google Scholar] [CrossRef]
- Oguzlu, H.; Tihminlioglu, F. Preparation and Barrier Properties of Chitosan-Layered Silicate Nanocomposite Films. Macromol. Symp. 2010, 298, 91–98. [Google Scholar] [CrossRef]
- Brodnjak, U.V. Experimental Investigation of Novel Curdlan/Chitosan Coatings on Packaging Paper. Prog. Org. Coat. 2017, 112, 86–92. [Google Scholar] [CrossRef]
- Rhim, J.W.; Lee, J.H.; Hong, S.I. Water Resistance and Mechanical Properties of Biopolymer (Alginate and Soy Protein) Coated Paperboards. Lwt 2006, 39, 806–813. [Google Scholar] [CrossRef]
- Zhang, W.; Xiao, H.; Qian, L. Enhanced Water Vapour Barrier and Grease Resistance of Paper Bilayer-Coated with Chitosan and Beeswax. Carbohydr. Polym. 2014, 101, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Nehchiri, N.; Amiri, S.; Radi, M. Improving the Water Barrier Properties of Alginate Packaging Films by Submicron Coating with Drying Linseed Oil. Packag. Technol. Sci. 2021, 34, 283–295. [Google Scholar] [CrossRef]
- Wang, S.; Sha, J.; Wang, W.; Qin, C.; Li, W.; Qin, C. Superhydrophobic Surfaces Generated by One-Pot Spray-Coating of Chitosan-Based Nanoparticles. Carbohydr. Polym. 2018, 195, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Liu, M.; Hou, Y.; Zhang, L.; Wang, D.; Shen, H.; Ding, C.; Li, C.; Fu, S. Nonfluorinated Multifunctional Superhydrophobic Cellulose Sheet with Polysaccharide B Biopolymer-Based Hierarchical Rough Composite Structure. ACS Sustain. Chem. Eng. 2020, 8, 8505–8518. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, H.; Cheng, Y.; Zhang, L.; Shen, W. Superhydrophobic Surfaces with Dual-Scale Roughness and Water Vapor-Barrier Property for Sustainable Liquid Packaging Applications. Cellulose 2022, 29, 9777–9790. [Google Scholar] [CrossRef]
- Milbreta, U.; Andze, L.; Filipova, I.; Dortins, E. Effect of Nanofibrillated Cellulose on Alginate and Chitosan Film Properties as Potential Barrier Coatings for Paper Food Packaging. Bioresources 2024, 19, 3375–3389. [Google Scholar] [CrossRef]
- Afra, E.; Mohammadnejad, S.; Saraeyan, A. Cellulose Nanofibils as Coating Material and Its Effects on Paper Properties. Prog. Org. Coat. 2016, 101, 455–460. [Google Scholar] [CrossRef]
- Herrera, M.A.; Mathew, A.P.; Oksman, K. Barrier and Mechanical Properties of Plasticized and Cross-Linked Nanocellulose Coatings for Paper Packaging Applications. Cellulose 2017, 24, 3969–3980. [Google Scholar] [CrossRef]
- Ruberto, Y.; Vivod, V.; Grkman, J.J.; Lavrič, G.; Graiff, C.; Kokol, V. Slot-Die Coating of Cellulose Nanocrystals and Chitosan for Improved Barrier Properties of Paper. Cellulose 2024, 31, 3589–3606. [Google Scholar] [CrossRef]
- Kumar, V.; Bousfield, D.W.; Toivakka, M. Slot Die Coating of Nanocellulose on Paperboard. Tappi J. 2018, 17, 11–19. [Google Scholar] [CrossRef]
- Strand, A.; Kouko, J.; Oksanen, A.; Salminen, K.; Ketola, A.; Retulainen, E.; Sundberg, A. Enhanced Strength, Stiffness and Elongation Potential of Paper by Spray Addition of Polysaccharides. Cellulose 2019, 26, 3473–3487. [Google Scholar] [CrossRef]
- Mayrhofer, A.; Mandlez, D.; Bauer, W. Comparison of the Application of Polysaccharide-Based Barrier Coatings on Paper Using Film Press and Spray Coating. Tappi J. 2025, 24, 25–35. [Google Scholar] [CrossRef]
- Desbrieres, J. Viscosity of Semiflexible Chitosan Solutions: Influence of Concentration, Temperature, and Role of Intermolecular Interactions. Biomacromolecules 2002, 3, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Mazur, K.; Buchner, R.; Bonn, M.; Hunger, J. Hydration of Sodium Alginate in Aqueous Solution. Macromolecules 2014, 47, 771–776. [Google Scholar] [CrossRef]
- Beneventi, D.; Chaussy, D.; Curtil, D.; Zolin, L.; Gerbaldi, C.; Penazzi, N. Highly Porous Paper Loading with Microfibrillated Cellulose by Spray Coating on Wet Substrates. Ind. Eng. Chem. Res. 2014, 53, 10982–10989. [Google Scholar] [CrossRef]
- Shanmugam, K.; Varanasi, S.; Garnier, G.; Batchelor, W. Rapid Preparation of Smooth Nanocellulose Films Using Spray Coating. Cellulose 2017, 24, 2669–2676. [Google Scholar] [CrossRef]
- Kirubanandan, S. Spray Coated Cellulose Nanofiber Laminates on the Paper to Enhance Its Barrier and Mechanical Properties. J. Sustain. Environ. Manag. 2022, 1, 10–17. [Google Scholar]
- Vishtal, A.; Khakalo, A.; Rojas, O.J.; Retulainen, E. Improving the Extensibility of Paper: Sequential Spray Addition of Gelatine and Agar. Nord. Pulp Paper Res. J. 2015, 30, 452–460. [Google Scholar] [CrossRef]
- Nasr, G.G.; Yule, A.J.; Bendig, L. Industrial Sprays and Atomization, 1st ed.; Springer: London, UK, 2002. [Google Scholar] [CrossRef]
- Lefebvre, A.H.; McDonell, V.G. Atomization and Sprays, 2nd ed.; Combustion: An international series; Taylor & Francis, CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Felton, L.A. Mechanisms of Polymeric Film Formation. Int. J. Pharm. 2013, 457, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Young, T., III. An Essay on the Cohesion of Fluids. Philos. Trans. R. Soc. Lond. 1805, 95, 65–87. [Google Scholar] [CrossRef]
- Karbowiak, T.; Debeaufort, F.; Voilley, A. Importance of Surface Tension Characterization for Food, Pharmaceutical and Packaging Products: A Review. Crit. Rev. Food Sci. Nutr. 2006, 46, 391–407. [Google Scholar] [CrossRef] [PubMed]
- Cooper, W.F.; Nuttall, W.H. The Theory of Wetting, and the Determination of the Wetting Power of Dipping and Spraying Fluids Containing a Soap Basis. J. Agric. Sci. 1915, 7, 219–239. [Google Scholar] [CrossRef]
- Chen, L.; Bonaccurso, E. Effects of Surface Wettability and Liquid Viscosity on the Dynamic Wetting of Individual Drops. Phys. Rev. E 2014, 90, 022401. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C. Estimation of the Surface Free Energy of Polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Kaelble, D.H. Dispersion-Polar Surface Tension Properties of Organic Solids. J. Adhes. 1970, 2, 66–81. [Google Scholar] [CrossRef]
- Rabel, W. Einige Aspekte Der Benetzungstheorie Und Ihre Anwendung Auf Die Untersuchung Und Veränderung Der Oberflächeneigenschaften von Polymeren. Farbe Lack. 1971, 77, 997–1005. [Google Scholar]
- Waldner, C.; Hirn, U. Modeling Liquid Penetration into Porous Materials Based on Substrate and Liquid Surface Energies. J. Colloid Interface Sci. 2023, 640, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Lee, T.R. Contact Angle and Wetting Properties. In Surface Science Techniques; Bracco, G., Holst, B., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 3–34. [Google Scholar] [CrossRef]
- Lavric, G.; Oberlintner, A.; Filipova, I.; Novak, U.; Blaz, L.; Vrabic-Brodnjak, U. Nanocomposites Designed as Active Film Packaging Materials. Polymers 2021, 13, 2523. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.X.; Kim, K.M.; Hanna, M.A.; Nag, D. Chitosan-Starch Composite Film: Preparation and Characterization. Ind. Crops Prod. 2005, 21, 185–192. [Google Scholar] [CrossRef]
- Bourbon, A.I.; Pinheiro, A.C.; Cerqueira, M.A.; Rocha, C.M.R.; Avides, M.C.; Quintas, M.A.C.; Vicente, A.A. Physico-Chemical Characterization of Chitosan-Based Edible Films Incorporating Bioactive Compounds of Different Molecular Weight. J. Food Eng. 2011, 106, 111–118. [Google Scholar] [CrossRef]
- Alvarado, S.; Sandoval, G.; Palos, I.; Tellez, S.; Aguirre-Loredo, Y.; Velazquez, G. The Effect of Relative Humidity on Tensile Strength and Water Vapor Permeability in Chitosan, Fish Gelatin and Transglutaminase Edible Films. Food Sci. Technol. 2015, 35, 690–695. [Google Scholar] [CrossRef]
- Chinma, C.E.; Ariahu, C.C.; Alakali, J.S. Effect of Temperature and Relative Humidity on the Water Vapour Permeability and Mechanical Properties of Cassava Starch and Soy Protein Concentrate Based Edible Films. J. Food Sci. Technol. 2015, 52, 2380–2386. [Google Scholar] [CrossRef]






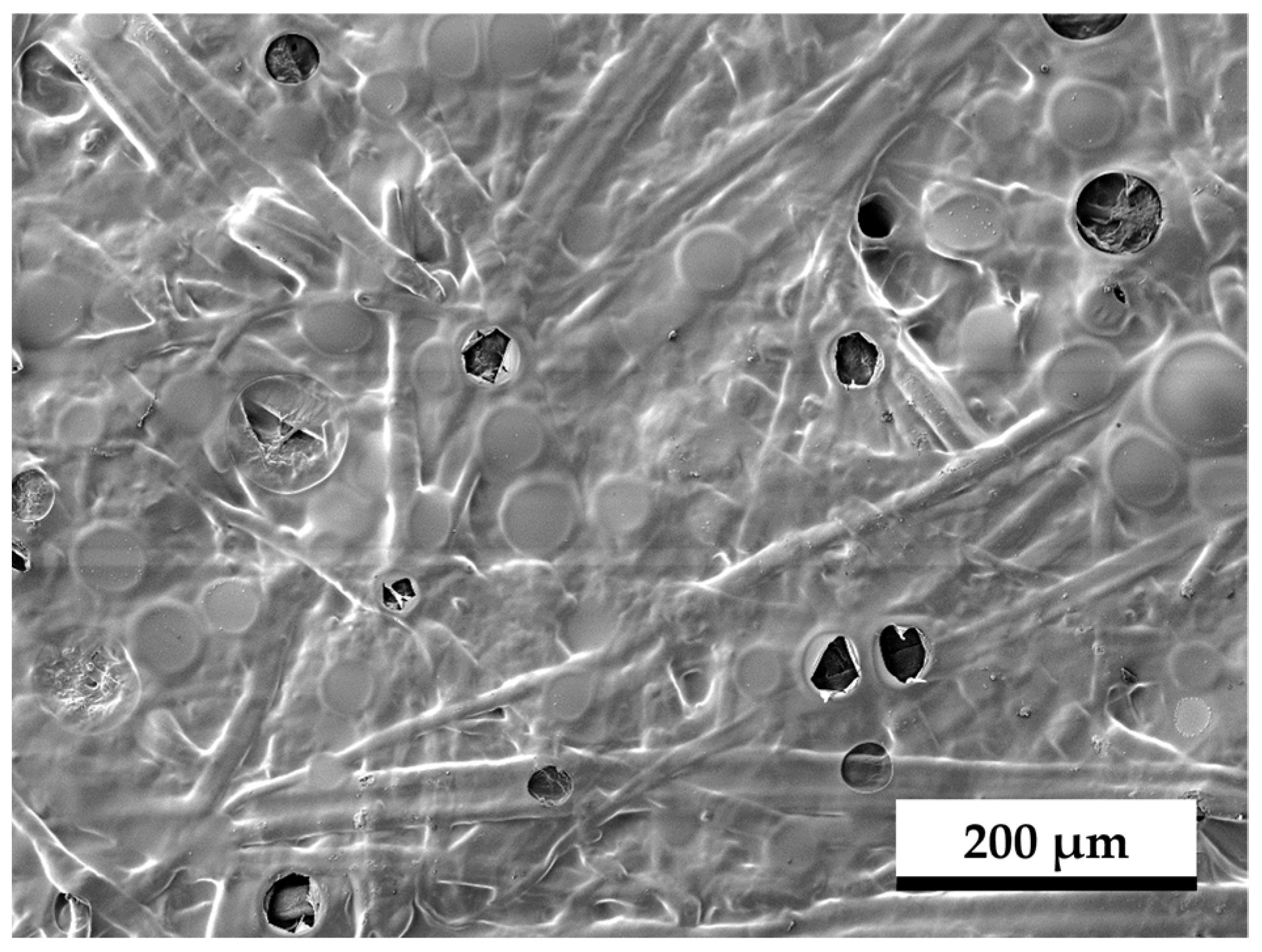
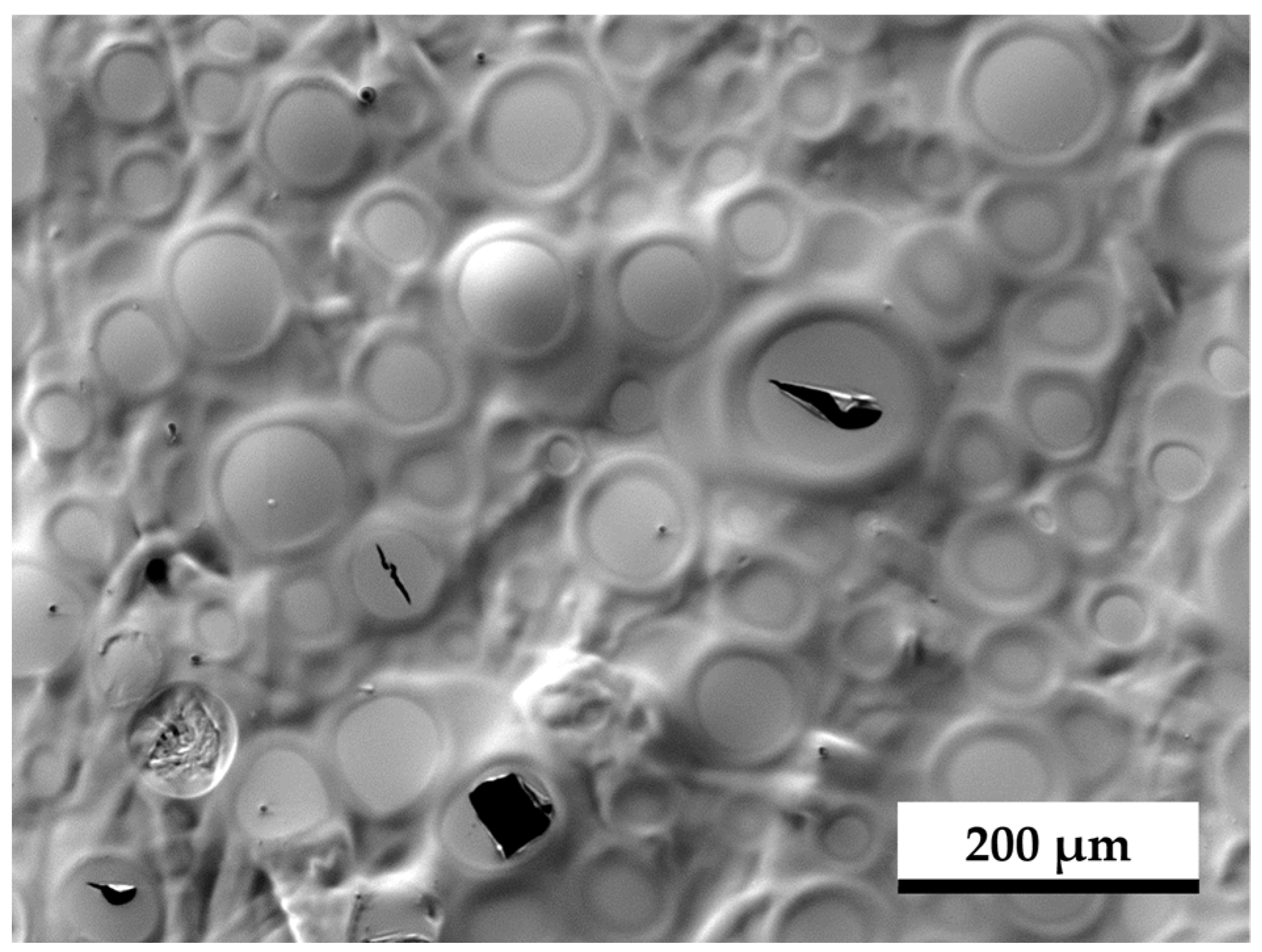


| Paper Property | Standard/Test Procedure | No. of Samples | Hydrophobic Side | Hydrophilic Side |
|---|---|---|---|---|
| Grammage | ISO 536:2019 “Paper and board—Determination of grammage” | 10 | 121.9 g·m−3 ± 0.7 | |
| Roughness | ISO 8791-2:2013 “Paper and board—Determination of roughness/smoothness (air leak methods) Part 2: Bendtsen method” | 10 | 970 mL·min−1 ± 77 | 1691 mL·min−1 ± 72 |
| Air permeance | ISO 5636-3:2013 “Paper and board—Determination of air permeance (medium range)—Part 3: Bendtsen method” | 10 | 4.0 µm·Pa−1·s−1 ± 0.1 | |
| Water vapor transmission rate | ISO 2528:2017 “Sheet materials—Determination of water vapour transmission rate (WVTR)—Gravimetric (dish) method” | 3 | 547.1 g·m−2·d−1 ± 31.2 | |
| Grease resistance (KIT) | T 559 cm-12 “Grease resistance test for paper and paperboard” | 3 | 1.0 ± 0 | 1.8 ± 0.3 |
| Cobb60 | ISO 535:2023 “Paper and board—Determination of water absorptiveness—Cobb method” | 10 | 42.1 g·m−2 ± 6.1 | 117.5 g·m−2 ± 2.7 |
| Total surface energy | OWRK theory | 12 | 15.9 mN·m−1 | 24.8 mN·m−1 |
| Surface energy polar | OWRK theory | 12 | 0.1 mN·m−1 | 11.0 mN·m−1 |
| Surface energy dispersive | OWRK theory | 12 | 15.8 mN·m−1 | 13.7 mN·m−1 |
| Surfactant Added | Alginate | Chitosan |
|---|---|---|
| No | 58.9 mN m−1 ± 0.2 | 62.7 mN m−1 ± 0.1 |
| Yes | 43.1 mN m−1 ± 0.2 | 42.5 mN m−1 ± 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mayrhofer, A.; Bauer, W. Impact of Surface Tension and Surface Energy on Spray Coating Paper with Polysaccharide-Based Biopolymers. Coatings 2025, 15, 335. https://doi.org/10.3390/coatings15030335
Mayrhofer A, Bauer W. Impact of Surface Tension and Surface Energy on Spray Coating Paper with Polysaccharide-Based Biopolymers. Coatings. 2025; 15(3):335. https://doi.org/10.3390/coatings15030335
Chicago/Turabian StyleMayrhofer, Anna, and Wolfgang Bauer. 2025. "Impact of Surface Tension and Surface Energy on Spray Coating Paper with Polysaccharide-Based Biopolymers" Coatings 15, no. 3: 335. https://doi.org/10.3390/coatings15030335
APA StyleMayrhofer, A., & Bauer, W. (2025). Impact of Surface Tension and Surface Energy on Spray Coating Paper with Polysaccharide-Based Biopolymers. Coatings, 15(3), 335. https://doi.org/10.3390/coatings15030335






