Efficacy of Air-Polishing with Sodium Bicarbonate vs. Erythritol in the Decrease of the Bacterial Concentration on the Surface of Dental Implants: In Vitro Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
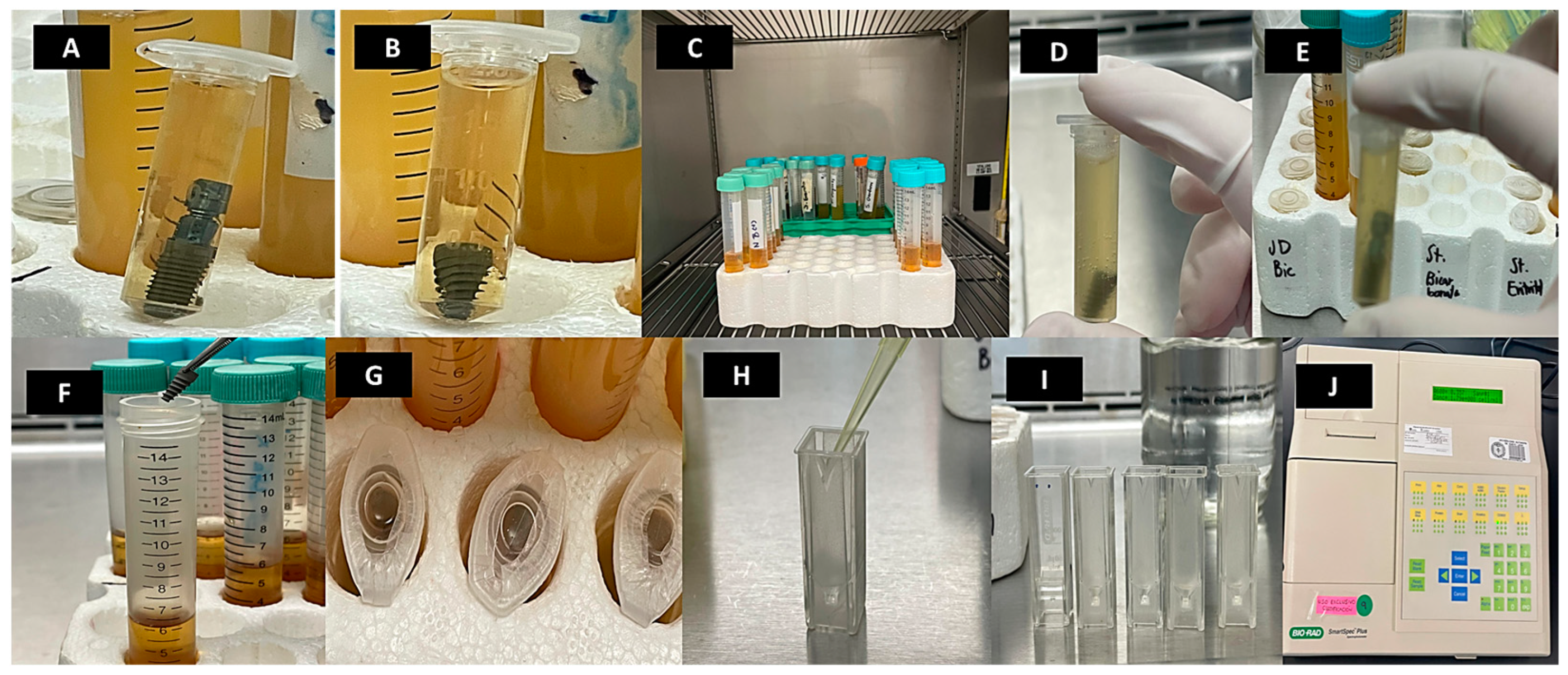
2.2. Sterilization and Decontamination Protocol for Dental Implants
2.3. Preparation of Bacterial Culture
2.4. Inoculation of Bacterial Culture on Dental Implants
2.5. Optical Density Evaluation for Bacterial Growth Assessment
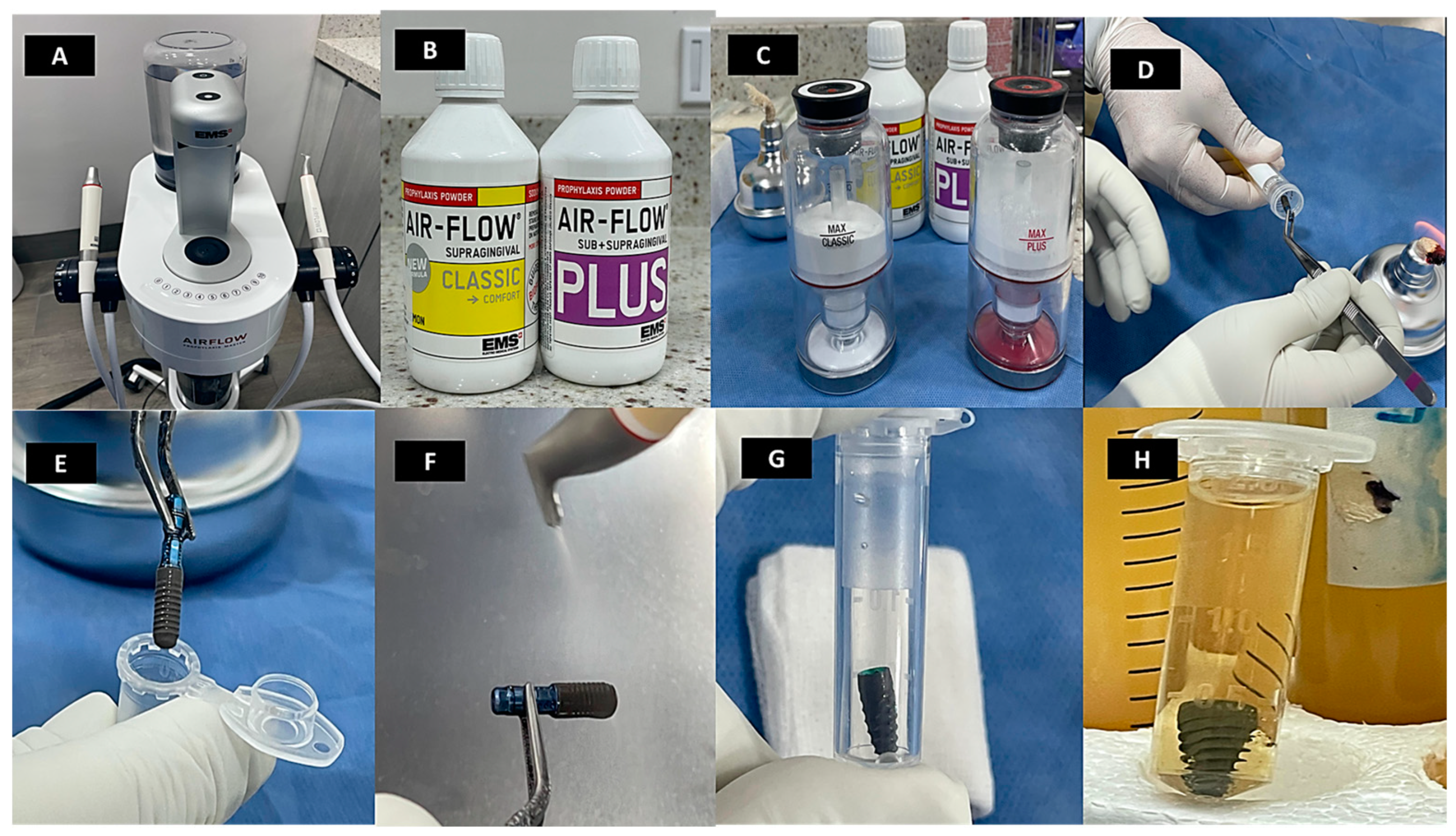
2.6. Implant Surface Treatment
2.7. Treatment Efficacy Evaluation
2.8. Implant Surface Characterization
2.9. Statistical Analysis
3. Results
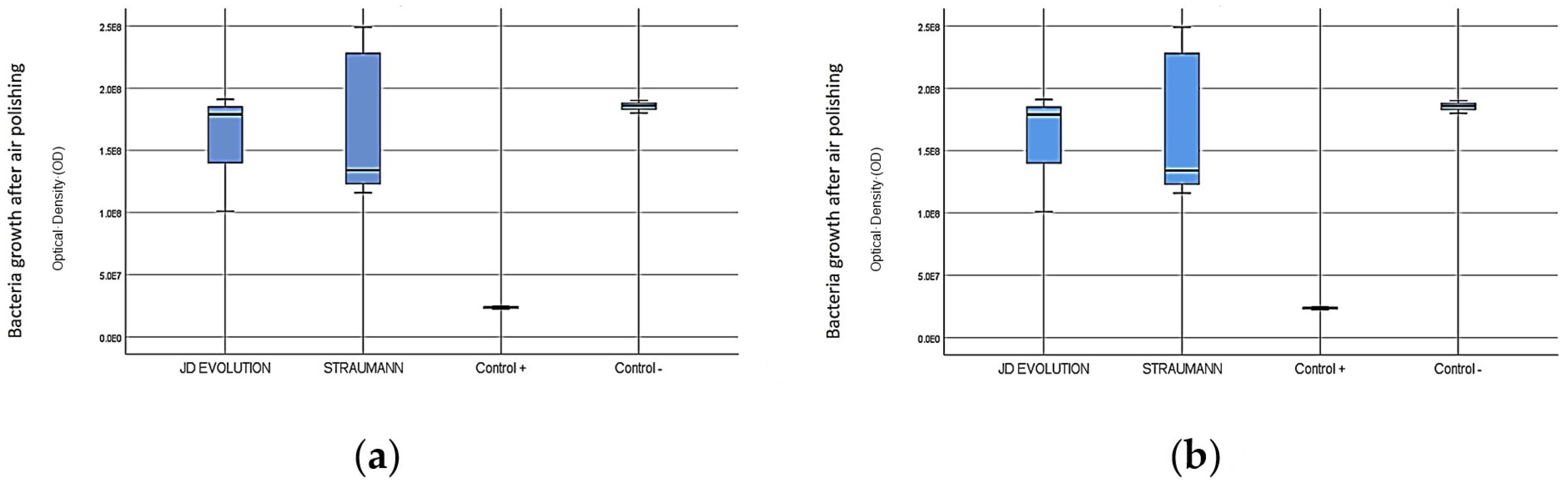
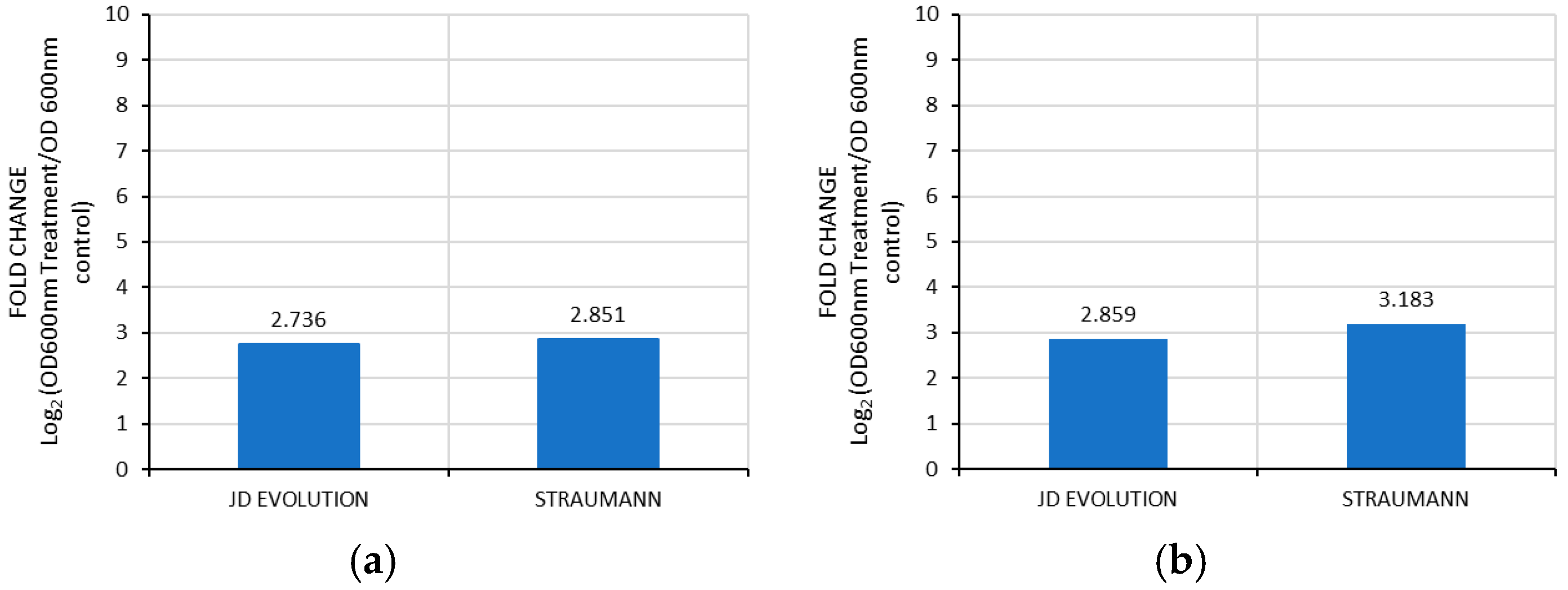
3.1. Bacterial Growth on Implant Surface Treated with Sodium Bicarbonate and Erythritol on Implant Surfaces
3.2. Comparative Analysis of Control Groups with Sodium Bicarbonate and Erythritol on Implant Surfaces
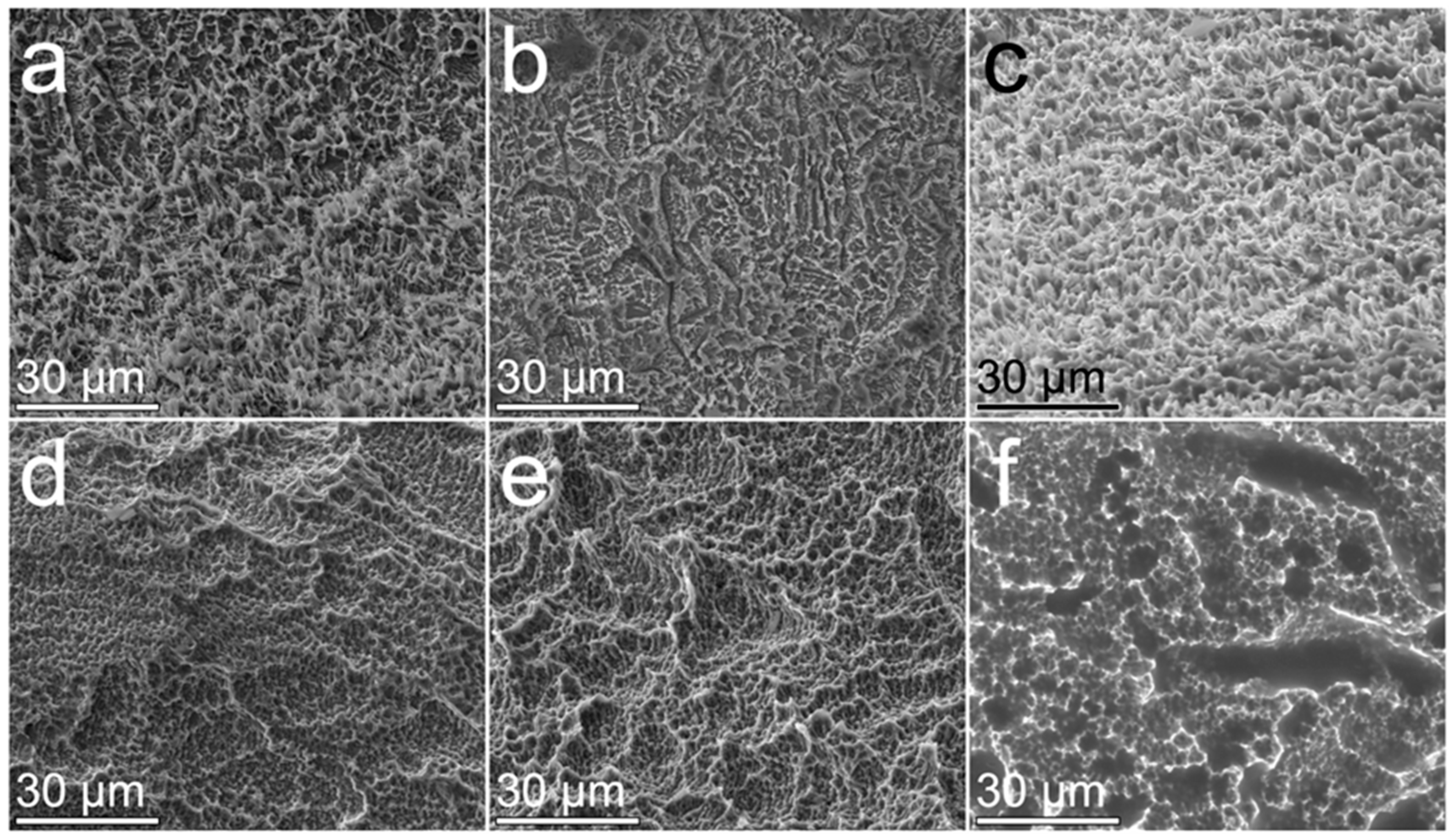
3.3. The Surface Morphology of Implants Treated with Powders by Air Polishing
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Emfietzoglou, R.; Dereka, X. Survival Rates of Short Dental Implants (≤6 mm) Used as an Alternative to Longer (>6 mm) Implants for the Rehabilitation of Posterior Partial Edentulism: A Systematic Review of RCTs. Dent. J. 2024, 12, 185. [Google Scholar] [CrossRef] [PubMed]
- Axe, A.; Patel, N.; Qaqish, J.; Ling, M.R.; Araga, M.; Parkinson, C.; Goyal, C.R. Efficacy of an experimental toothpaste containing sodium bicarbonate, sodium hyaluronate and sodium fluoride on gingivitis. BMC Oral Health 2024, 24, 209. [Google Scholar] [CrossRef] [PubMed]
- De Bruyn, H.; Christiaens, V.; Doornewaard, R.; Jacobsson, M.; Cosyn, J.; Jacquet, W.; Vervaeke, S. Implant surface roughness and patient factors on long-term peri-implant bone loss. Periodontology 2000 2016, 73, 218–227. [Google Scholar] [CrossRef]
- Buser, D.; Sennerby, L.; De Bruyn, H. Modern implant dentistry based on osseointegration: 50 years of progress, current trends and open questions. Periodontology 2000 2016, 73, 7–21. [Google Scholar] [CrossRef]
- Caton, J.G.; Armitage, G.; Berglundh, T.; Chapple, I.L.C.; Jepsen, S.; Kornman, K.S.; Mealey, B.L.; Papapanou, P.N.; Sanz, M.; Tonetti, M.S. A new classification scheme for periodontal and peri-implant diseases and conditions—Introduction and key changes from the 1999 classification. J. Clin. Periodontol. 2018, 45, S1–S8. [Google Scholar] [CrossRef]
- Rokaya, D.; Srimaneepong, V.; Wisitrasameewon, W.; Humagain, M.; Thunyakitpisal, P. Peri-implantitis Update: Risk Indicators, Diagnosis, and Treatment. Eur. J. Dent. 2020, 14, 672–682. [Google Scholar] [CrossRef] [PubMed]
- Abushahba, F.; Gürsoy, M.; Hupa, L.; Närhi, T.O. Effect of bioactive glass air-abrasion on Fusobacterium nucleatum and Porphyromonas gingivalis biofilm formed on moderately rough titanium surface. Eur. J. Oral Sci. 2021, 129, e12783. [Google Scholar] [CrossRef]
- Fürst, M.M.; Salvi, G.E.; Lang, N.P.; Persson, G.R. Bacterial colonization immediately after installation on oral titanium implants. Clin. Oral Implant. Res. 2007, 18, 501–508. [Google Scholar] [CrossRef]
- Pokrowiecki, R.; Mielczarek, A.; Zaręba, T.; Tyski, S. Oral microbiome and peri-implant diseases: Where are we now? Ther. Clin. Risk Manag. 2017, 13, 1529–1542. [Google Scholar] [CrossRef]
- Polymeri, A.; van der Horst, J.; Buijs, M.J.; Zaura, E.; Wismeijer, D.; Crielaard, W.; Loos, B.G.; Laine, M.L.; Brandt, B.W. Submucosal microbiome of peri-implant sites: A cross-sectional study. J. Clin. Periodontol. 2021, 48, 1228–1239. [Google Scholar] [CrossRef]
- Mason, S.; Patil, P.; Karad, V. A Randomised Clinical Study to Evaluate Efficacy on Gingival Health of 62% and 67% Sodium Bicarbonate Toothpastes. Oral Health Prev. Dent. 2021, 19, 609–618. [Google Scholar] [CrossRef]
- Pratten, J.; Wiecek, J.; Mordan, N.; Lomax, A.; Patel, N.; Spratt, D.; Middleton, A.M. Physical disruption of oral biofilms by sodium bicarbonate: An in vitro study. Int. J. Dent. Hyg. 2015, 14, 209–214. [Google Scholar] [CrossRef]
- Putt, M.S.; Milleman, K.R.; Ghassemi, A.; Vorwerk, L.M.; Hooper, W.J.; Soparkar, P.M.; Winston, A.E.; Proskin, H.M. Enhancement of plaque removal efficacy by tooth brushing with baking soda dentifrices: Results of five clinical studies. J. Clin. Dent. 2008, 19, 111–119. [Google Scholar] [PubMed]
- Drago, L.; Del Fabbro, M.; Bortolin, M.; Vassena, C.; De Vecchi, E.; Taschieri, S. Biofilm Removal and Antimicrobial Activity of Two Different Air-Polishing Powders: An In Vitro Study. J. Periodontol. 2014, 85, e363–e369. [Google Scholar] [CrossRef]
- Zhu, M.; Zhao, M.; Hu, B.; Wang, Y.; Li, Y.; Song, J. Efficacy of glycine powder air-polishing in supportive periodontal therapy: A systematic review and meta-analysis. J. Periodontal Implant. Sci. 2021, 51, 147–162. [Google Scholar] [CrossRef] [PubMed]
- Giacomelli, L.; Salerno, M.; Derchi, G.; Genovesi, A.; Paganin, P.P.; Covani, U. Effect of air polishing with glycine and bicarbonate powders on a nanocomposite used in dental restorations: An in vitro study. Int. J. Periodontics Restor. Dent. 2011, 31, e51–e56. [Google Scholar]
- Biazussi, B.R.; Perrotti, V.; D’Arcangelo, C.; Elias, C.N.; Bianchini, M.A.; Tumedei, M.; de Vasconcellos, D.K. Evaluation of the Effect of Air Polishing With Different Abrasive Powders on the Roughness of Implant Abutment Surface: An In Vitro Study. J. Oral Implantol. 2019, 45, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Mensi, M.; Cochis, A.; Sordillo, A.; Uberti, F.; Rimondini, L. Biofilm Removal and Bacterial Re-Colonization Inhibition of a Novel Erythritol/Chlorhexidine Air-Polishing Powder on Titanium Disks. Materials 2018, 11, 1510. [Google Scholar] [CrossRef]
- Elmeligy, S.M.A.; Saleh, W.; Elewa, G.M.; Abu El-Ezz, H.Z.; Mahmoud, N.M.; Elmeadawy, S. The efficacy of diode laser and subgingival air polishing with erythritol in treatment of periodontitis (clinical and microbiological study). BMC Oral Health 2024, 24, 763. [Google Scholar] [CrossRef]
- Abdulbaqi, H.R.; Shaikh, M.S.; Abdulkareem, A.A.; Zafar, M.S.; Gul, S.S.; Sha, A.M. Efficacy of erythritol powder air-polishing in active and supportive periodontal therapy: A systematic review and meta-analysis. Int. J. Dent. Hyg. 2021, 20, 62–74. [Google Scholar] [CrossRef]
- Liu, C.C.; Dixit, N.; Hatz, C.R.; Janson, T.M.; Bastendorf, K.D.; Belibasakis, G.N.; Cosgarea, R.; Karoussis, I.K.; Mensi, M.; O’Neill, J.; et al. Air powder waterjet technology using erythritol or glycine powders in periodontal or peri-implant prophylaxis and therapy: A consensus report of an expert meeting. Clin. Exp. Dent. Res. 2024, 10, e855. [Google Scholar] [CrossRef] [PubMed]
- Gheorghe, D.N.; Bennardo, F.; Silaghi, M.; Popescu, D.-M.; Maftei, G.-A.; Bătăiosu, M.; Surlin, P. Subgingival Use of Air-Polishing Powders: Status of Knowledge: A Systematic Review. J. Clin. Med. 2023, 12, 6936. [Google Scholar] [CrossRef]
- Oh, S.-L.; Shiau, H.J.; Reynolds, M.A. Survival of dental implants at sites after implant failure: A systematic review. J. Prosthet. Dent. 2020, 123, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Zhao, A.; Sun, J.; Liu, Y. Understanding bacterial biofilms: From definition to treatment strategies. Front. Cell. Infect. Microbiol. 2023, 13, 1137947. [Google Scholar] [CrossRef] [PubMed]
- Batalha, V.C.; Bueno, R.A.; Fronchetti Junior, E.; Mariano, J.R.; Santin, G.C.; Freitas, K.M.S.; Ortiz, M.A.L.; Salmeron, S. Dental Implants Surface in vitro Decontamination Protocols. Eur. J. Dent. 2020, 15, 407–411. [Google Scholar] [CrossRef]
- Periasamy, S.; Kolenbrander, P.E. Mutualistic Biofilm Communities Develop with Porphyromonas gingivalis and Initial, Early, and Late Colonizers of Enamel. J. Bacteriol. 2009, 191, 6804–6811. [Google Scholar] [CrossRef]
- Biesta-Peters, E.G.; Reij, M.W.; Joosten, H.; Gorris, L.G.M.; Zwietering, M.H. Comparison of Two Optical-Density-Based Methods and a Plate Count Method for Estimation of Growth Parameters of Bacillus cereus. Appl. Environ. Microbiol. 2010, 76, 1399–1405. [Google Scholar] [CrossRef]
- Delucchi, F.; Ingegnieros, L.; Pesce, P.; Baldi, D.; Canullo, L.; Bagnasco, F.; Zunino, P.; Menini, M. Efficacy and safety of erythritol air-polishing in implant dentistry: A systematic review. Int. J. Dent. Hyg. 2024, 23, 44–62. [Google Scholar] [CrossRef]
- Beal, J.; Farny, N.G.; Haddock-Angelli, T.; Selvarajah, V.; Baldwin, G.S.; Buckley-Taylor, R.; Gershater, M.; Kiga, D.; Marken, J.; Sanchania, V.; et al. Robust estimation of bacterial cell count from optical density. Commun. Biol. 2020, 3, 512. [Google Scholar] [CrossRef]
- Pujarern, P.; Klaophimai, A.; Amornsettachai, P.; Panyayong, W.; Chuenjitkuntaworn, B.; Rokaya, D.; Suphangul, S. Efficacy of Biofilm Removal on the Dental Implant Surface by Sodium Bicarbonate and Erythritol Powder Airflow System. Eur. J. Dent. 2024, 18, 1022–1029. [Google Scholar] [CrossRef]
- Ronay, V.; Merlini, A.; Attin, T.; Schmidlin, P.R.; Sahrmann, P. In vitro cleaning potential of three implant debridement methods. Simulation of the non-surgical approach. Clin. Oral Implant. Res. 2016, 28, 151–155. [Google Scholar] [CrossRef]
- Hakki, S.S.; Tatar, G.; Dundar, N.; Demiralp, B. The effect of different cleaning methods on the surface and temperature of failed titanium implants: An in vitro study. Lasers Med. Sci. 2017, 32, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-H.; Kim, O.-J.; Chung, H.-J.; Kim, O.-S. Effect of a Er, Cr:YSGG laser and a Er:YAG laser treatment on oral biofilm-contaminated titanium. J. Appl. Oral Sci. 2020, 28, e20200528. [Google Scholar] [CrossRef] [PubMed]
- Tzanakakis, E.-G.C.; Skoulas, E.; Pepelassi, E.; Koidis, P.; Tzoutzas, I.G. The Use of Lasers in Dental Materials: A Review. Materials 2021, 14, 3370. [Google Scholar] [CrossRef] [PubMed]
- Ţălu, Ş.; Stach, S.; Alb, S.F.; Salerno, M. Multifractal characterization of a dental restorative composite after air-polishing. Chaos Solitons Fractals 2015, 71, 7–13. [Google Scholar] [CrossRef]
- Schwarz, F.; Becker, K.; Renvert, S. Efficacy of air polishing for the non-surgical treatment of peri-implant diseases: A systematic review. J. Clin. Periodontol. 2015, 42, 951–959. [Google Scholar] [CrossRef]
- Matsubara, V.H.; Leong, B.W.; Leong, M.J.L.; Lawrence, Z.; Becker, T.; Quaranta, A. Cleaning potential of different air abrasive powders and their impact on implant surface roughness. Clin. Implant. Dent. Relat. Res. 2019, 22, 96–104. [Google Scholar] [CrossRef]
- Amate-Fernández, P.; Figueiredo, R.; Blanc, V.; Àlvarez, G.; León, R.; Valmaseda-Castellón, E. Erythritol-enriched powder and oral biofilm regrowth on dental implants: An in vitro study. Med. Oral Patol. Oral Cir. Bucal 2021, e602–e610. [Google Scholar] [CrossRef]
- Luengo, F.; Sanz-Esporrín, J.; Noguerol, F.; Sanz-Martín, I.; Sanz-Sánchez, I.; Sanz, M. In vitro effect of different implant decontamination methods in three intraosseous defect configurations. Clin. Oral Implant. Res. 2022, 33, 1087–1097. [Google Scholar] [CrossRef]
- Junior, E.; Zubek, M.; Queiroz, P.; Freitas, K.; Ortiz, M.; Salmeron, S. Sodium Bicarbonate Jet Reduces Contamination of Dental Implants In Vitro Without Causing Visible Surface Changes. Int. J. Oral Maxillofac. Implant. 2022, 37, 587–592. [Google Scholar] [CrossRef]
- Haude, S.; Matthes, R.; Pitchika, V.; Holtfreter, B.; Schlüter, R.; Gerling, T.; Kocher, T.; Jablonowski, L. In-vitro biofilm removal from TiUnite® implant surface with an air polishing and two different plasma devices. BMC Oral Health 2024, 24, 558. [Google Scholar] [CrossRef] [PubMed]
- Sinjari, B.; D’Addazio, G.; Bozzi, M.; Santilli, M.; Traini, T.; Murmura, G.; Caputi, S. SEM Analysis of Enamel Abrasion after Air Polishing Treatment with Erythritol, Glycine and Sodium Bicarbonate. Coatings 2019, 9, 549. [Google Scholar] [CrossRef]
- Onisor, F.; Mester, A.; Mancini, L.; Voina-Tonea, A. Effectiveness and Clinical Performance of Erythritol Air-Polishing in Non-Surgical Periodontal Therapy: A Systematic Review of Randomized Clinical Trials. Medicina 2022, 58, 866. [Google Scholar] [CrossRef]
- Blum, I.R.; Jagger, D.C.; Wilson, N.H.F. Defective dental restorations: To repair or not to repair? part 1: Direct composite restorations. Dent. Update 2011, 38, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Junior, S.A.R.; Ferracane, J.L.; Bona, Á.D. Influence of surface treatments on the bond strength of repaired resin composite restorative materials. Dent. Mater. 2009, 25, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Rathke, A.; Tymina, Y.; Haller, B. Effect of different surface treatments on the composite–composite repair bond strength. Clin. Oral Investig. 2008, 13, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Reis, A.; Loguercio, A.D.; Klein-Júnior, C.A.; Ferreira, S.Q.; Costa, T.R.F. Durability of Surface Treatments and Intermediate Agents Used for Repair of a Polished Composite. Oper. Dent. 2010, 35, 231–237. [Google Scholar] [CrossRef]
- Németh, K.D.; Told, R.; Szabó, P.; Maróti, P.; Szénai, R.; Pintér, Z.B.; Lovász, B.V.; Szalma, J.; Lempel, E. Comparative Evaluation of the Repair Bond Strength of Dental Resin Composite after Sodium Bicarbonate or Aluminum Oxide Air-Abrasion. Int. J. Mol. Sci. 2023, 24, 11568. [Google Scholar] [CrossRef]
- Graumann, S.J.; Sensat, M.L.; Stoltenberg, J.L. Air polishing: A review of current literature. J. Dent. Hyg. 2013, 87, 173–180. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Rueda, A.Y.; Garza-Ramos, M.A.D.L.; Rodríguez-Franco, N.I.; Rodríguez-Pulido, J.I.; Elizalde-Molina, C.L.; Elizondo-Cantú, O. Efficacy of Air-Polishing with Sodium Bicarbonate vs. Erythritol in the Decrease of the Bacterial Concentration on the Surface of Dental Implants: In Vitro Study. Coatings 2025, 15, 327. https://doi.org/10.3390/coatings15030327
Gómez-Rueda AY, Garza-Ramos MADL, Rodríguez-Franco NI, Rodríguez-Pulido JI, Elizalde-Molina CL, Elizondo-Cantú O. Efficacy of Air-Polishing with Sodium Bicarbonate vs. Erythritol in the Decrease of the Bacterial Concentration on the Surface of Dental Implants: In Vitro Study. Coatings. 2025; 15(3):327. https://doi.org/10.3390/coatings15030327
Chicago/Turabian StyleGómez-Rueda, Ashley Yaressi, Myriam Angélica De La Garza-Ramos, Norma Idalia Rodríguez-Franco, Jesús Israel Rodríguez-Pulido, Claudia Lucía Elizalde-Molina, and Omar Elizondo-Cantú. 2025. "Efficacy of Air-Polishing with Sodium Bicarbonate vs. Erythritol in the Decrease of the Bacterial Concentration on the Surface of Dental Implants: In Vitro Study" Coatings 15, no. 3: 327. https://doi.org/10.3390/coatings15030327
APA StyleGómez-Rueda, A. Y., Garza-Ramos, M. A. D. L., Rodríguez-Franco, N. I., Rodríguez-Pulido, J. I., Elizalde-Molina, C. L., & Elizondo-Cantú, O. (2025). Efficacy of Air-Polishing with Sodium Bicarbonate vs. Erythritol in the Decrease of the Bacterial Concentration on the Surface of Dental Implants: In Vitro Study. Coatings, 15(3), 327. https://doi.org/10.3390/coatings15030327






