Exploring the Effects of Dulbecco’s Modified Eagle’s Medium on Irradiated Layers of Magnesium-Doped Hydroxyapatite in a Chitosan Matrix for Biomedical Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Synthesis of MHA_Ch Powders
2.1.2. Synthesis of Layers by Magnetron Sputtering Technique
2.1.3. Exposure of Mg-HAp/Ch Layers to Electron Beams
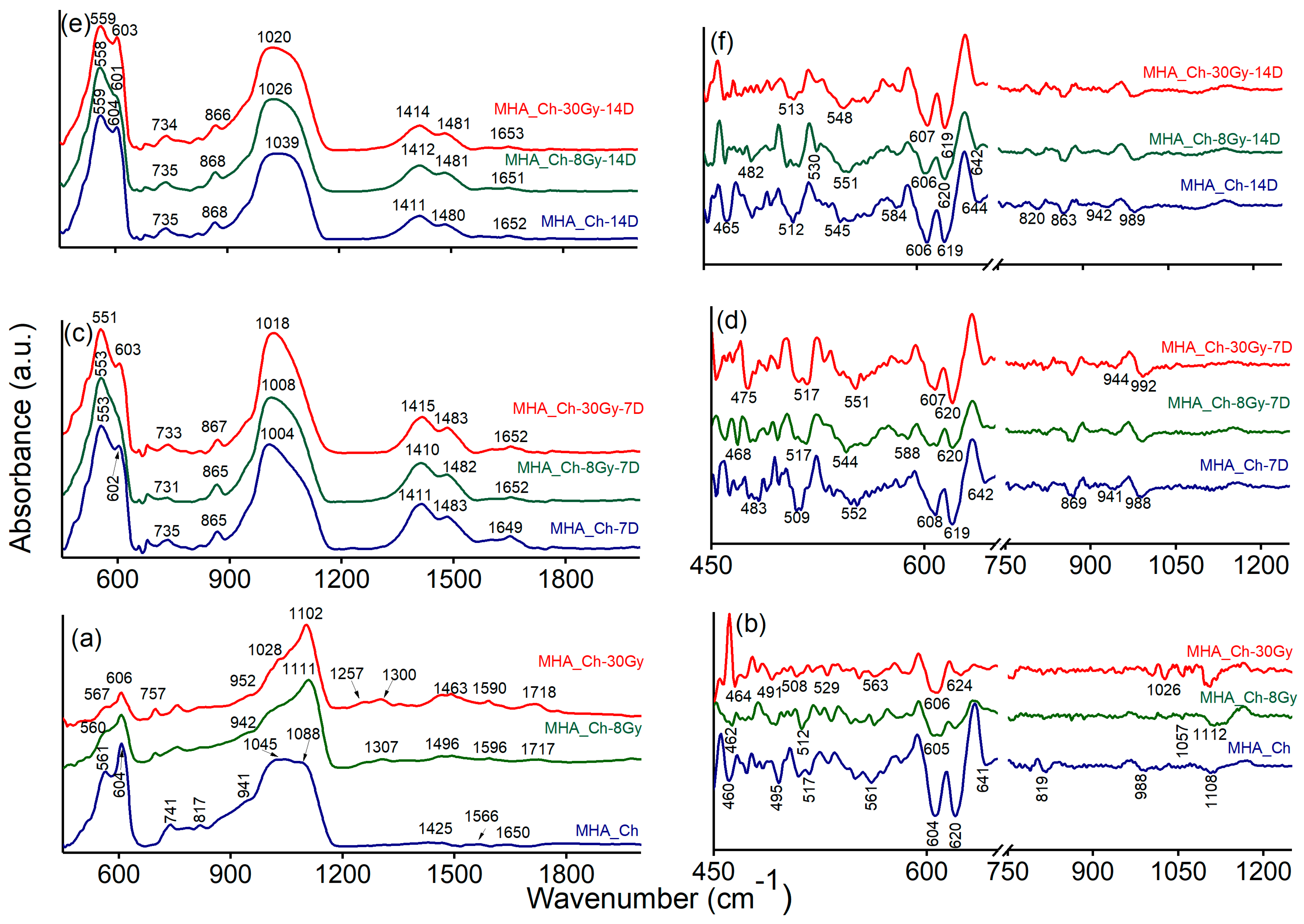
2.2. Physico-Chemical Characterization
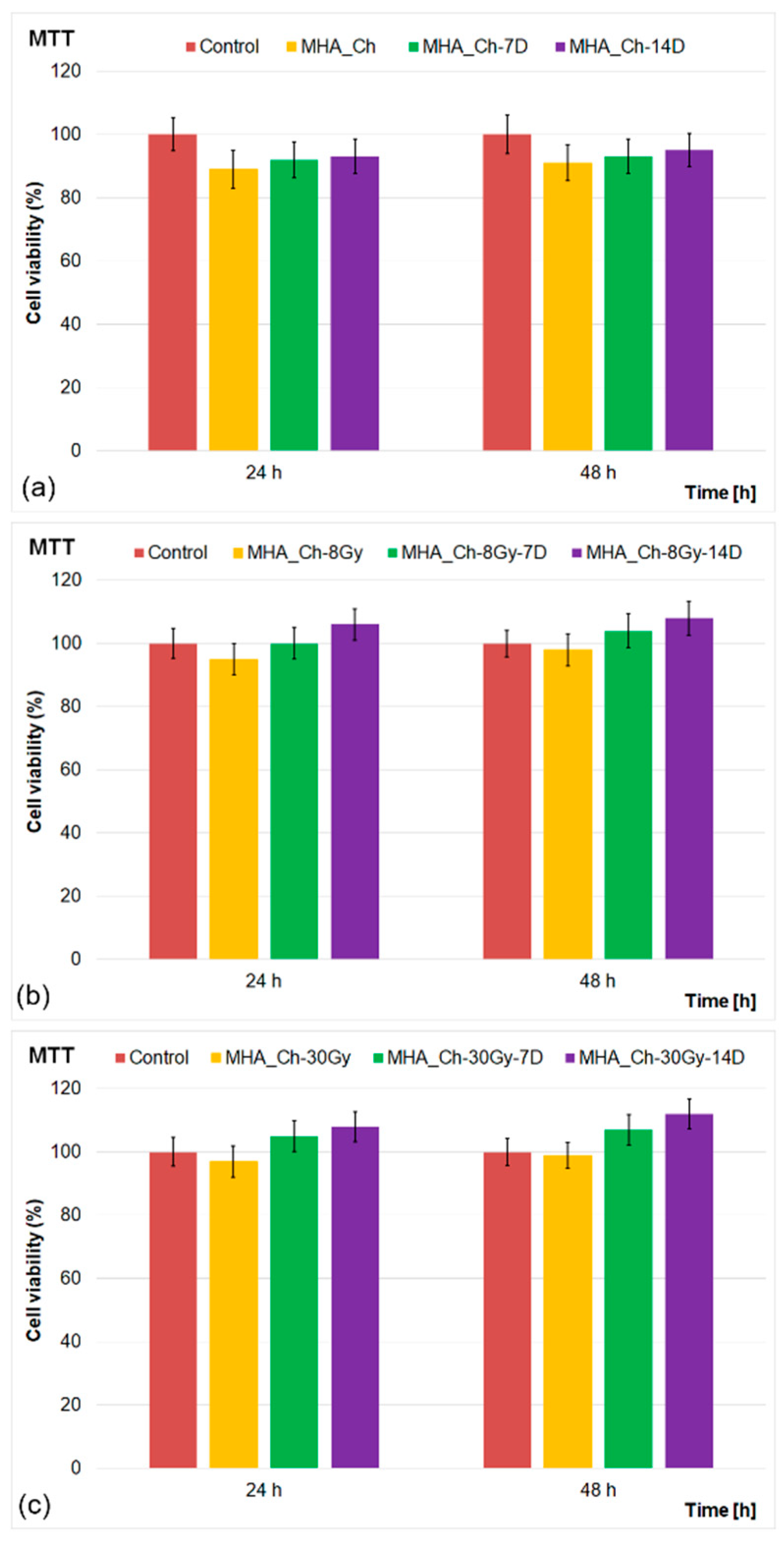
2.3. In Vitro Biological Assays
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jeong, J.; Kim, J.H.; Shim, J.H.; Hwang, N.S.; Heo, C.Y. Bioactive Calcium Phosphate Materials and Applications in Bone Regeneration. Biomater. Res. 2019, 23, 4. [Google Scholar] [CrossRef] [PubMed]
- Filip, D.G.; Surdu, V.-A.; Paduraru, A.V.; Andronescu, E. Current Development in Biomaterials—Hydroxyapatite and Bioglass for Applications in Biomedical Field: A Review. J. Funct. Biomater. 2022, 13, 248. [Google Scholar] [CrossRef] [PubMed]
- Madupalli, H.; Pavan, B.; Tecklenburg, M.M.J. Carbonate substitution in the mineral component of bone: Discriminating the structural changes, simultaneously imposed by carbonate in A and B sites of apatite. J. Solid State Chem. 2017, 255, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Laptoiu, S.A.; Cojocaru, M.O.; Miculescu, M.; Branzei, M. Parameters Tailoring on the Deposition of Hydroxyapatite by Pulsed Electrical Discharge. Materials 2024, 17, 4583. [Google Scholar] [CrossRef]
- Gresita, A.; Raja, I.; Petcu, E.; Hadjiargyrou, M. Collagen-Coated Hyperelastic Bone Promotes Osteoblast Adhesion and Proliferation. Materials 2023, 16, 6996. [Google Scholar] [CrossRef]
- Veronesi, F.; Brogini, S.; De Luca, A.; Bellini, D.; Casagranda, V.; Fini, M.; Giavaresi, G. Cell Adhesion and Initial Bone Matrix Deposition on Titanium-Based Implants with Chitosan–Collagen Coatings: An In Vitro Study. Int. J. Mol. Sci. 2023, 24, 4810. [Google Scholar] [CrossRef] [PubMed]
- Parfenova, L.V.; Galimshina, Z.R.; Parfenov, E.V. Organic-Inorganic Biocompatible Coatings for Temporary and Permanent Metal Implants. Int. J. Mol. Sci. 2024, 25, 11623. [Google Scholar] [CrossRef]
- Krawiec, H.; Kozina, I.; Starowicz, M.; Lekka, M.; Zanella, C.; Fedrizzi, L.; Fedel, M.; Deflorian, F. Corrosion Rate and Mechanism of Degradation of Chitosan/TiO2 Coatings Deposited on MgZnCa Alloy in Hank’s Solution. Int. J. Mol. Sci. 2024, 25, 5313. [Google Scholar] [CrossRef]
- Kołodziejska, B.; Figat, R.; Kolmas, J. Biomimetic Apatite/Natural Polymer Composite Granules as Multifunctional Dental Tissue Regenerative Material. Int. J. Mol. Sci. 2023, 24, 16751. [Google Scholar] [CrossRef]
- McKee, T.J.; Komarova, S.V. Is it time to reinvent basic cell culture medium? Am. J. Physiol. Cell Physiol. 2017, 312, C624–C626. [Google Scholar] [CrossRef]
- Petrakova, N.V.; Teterina, A.Y.; Mikheeva, P.V.; Akhmedova, S.A.; Kuvshinova, E.A.; Sviridova, I.K.; Sergeeva, N.S.; Smirnov, I.V.; Fedotov, A.Y.; Kargin, Y.F.; et al. In Vitro Study of Octacalcium Phosphate Behavior in Different Model Solutions. ACS Omega 2021, 6, 7487–7498. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.T.; Leng, Y.; Chow, K.L.; Ren, F.; Ge, X.; Wang, K.; Lu, X. Cell culture medium as an alternative to conventional simulated body fluid. Acta Biomater. 2011, 7, 2615–2622. [Google Scholar] [CrossRef]
- Um, S.H.; Chung, Y.W.; Seo, Y.; Seo, H.; Ok, M.R.; Kim, Y.C.; Han, H.S.; Chung, J.J.; Edwards, J.R.; Jeon, H. Robust Hydroxyapatite Coating by Laser-Induced Hydrothermal Synthesis. Adv. Funct. Mater. 2020, 30, 2005233. [Google Scholar] [CrossRef]
- Faria, D.; Abreu, C.S.; Buciumeanu, M.; Dourado, N.; Carvalho, O.; Silva, F.S.; Miranda, G. Ti6Al4V laser surface preparation and functionalization using hydroxyapatite for biomedical applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 1534–1545. [Google Scholar] [CrossRef] [PubMed]
- Cojocaru, F.-D.; Gardikiotis, I.; Dodi, G.; Rotaru, A.; Balan, V.; Rezus, E.; Verestiuc, L. Polysaccharides-Calcium Phosphates Composite Beads as Bone Substitutes for Fractures Repair and Regeneration. Polymers 2023, 15, 1509. [Google Scholar] [CrossRef] [PubMed]
- Paul, B.; Lode, A.; Placht, A.M.; Voß, A.; Pilz, S.; Wolff, U.; Oswald, S.; Gebert, A.; Gelinsky, M.; Hufenbach, J. Cell-Material Interactions in Direct Contact Culture of Endothelial Cells on Biodegradable Iron-Based Stents Fabricated by Laser Powder Bed Fusion and Impact of Ion Release. ACS Appl. Mater. Interfaces 2022, 14, 439–451. [Google Scholar] [CrossRef]
- Islam, M.M.; Shahruzzaman, M.; Biswas, S.; Nurus Sakib, M.; Rashid, T.U. Chitosan Based Bioactive Materials in Tissue Engineering Applications—A Review. Bioact. Mater. 2020, 5, 164–183. [Google Scholar] [CrossRef]
- NCCN. Guidelines Version 2. 2020. Available online: https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf (accessed on 30 October 2024).
- Baskar, R.; Dai, J.; Wenlong, N.; Yeo, R.; Yeoh, K.-W. Biological response of cancer cells to radiation treatment. Front. Mol. Biosci. 2014, 1, 24. [Google Scholar] [CrossRef] [PubMed]
- Yarnold, J.R. 8 Gy Single Fraction Radiotherapy for the Treatment of Metastatic Skeletal Pain: Randomised Comparison with a Multifraction Schedule over 12 Months of Patient Follow-up. Bone Pain Trial Working Party. Radiother Oncol. 1999, 52, 111–121. [Google Scholar] [CrossRef]
- Foro Arnalot, P.; Fontanals, A.V.; Galcerán, J.C.; Lynd, F.; Latiesas, X.S.; de Dios, N.R.; Castillejo, A.R.; Bassols, M.L.; Galán, J.L.; Conejo, I.M.; et al. Randomized Clinical Trial with Two Palliative Radiotherapy Regimens in Painful Bone Metastases: 30 Gy in 10 Fractions Compared with 8 Gy in Single Fraction. Radiother. Oncol. 2008, 89, 150–155. [Google Scholar] [CrossRef]
- Debela, D.T.; Muzazu, S.G.Y.; Heraro, K.D.; Ndalama, M.T.; Mesele, B.W.; Haile, D.C.; Kitui, S.K.; Manyazewal, T. New Approaches and Procedures for Cancer Treatment: Current Perspectives. SAGE Open Med. 2021, 9, 20503121211034366. [Google Scholar] [CrossRef]
- Radiation Therapy to Treat Cancer, National Cancer Institute, Updated 9 January, 2019. Available online: https://www.cancer.gov/about-cancer/treatment/types/radiation-therapy/brachytherapy (accessed on 5 December 2024).
- Baskar, R.; Lee, K.A.; Yeo, R.; Yeoh, K.-W. Cancer and Radiation Therapy: Current Advances and Future Directions. Int. J. Med. Sci. 2012, 9, 193–199. [Google Scholar] [CrossRef]
- Bita, B.; Stancu, E.; Stroe, D.; Dumitrache, M.; Ciobanu, S.C.; Iconaru, S.L.; Predoi, D.; Groza, A. The Effects of Electron Beam Irradiation on the Morphological and Physicochemical Properties of Magnesium-Doped Hydroxyapatite/Chitosan Composite Coatings. Polymers 2022, 14, 582. [Google Scholar] [CrossRef] [PubMed]
- Predoi, D.; Ciobanu, C.S.; Iconaru, S.L.; Predoi, S.A.; Chifiriuc, M.C.; Raaen, S.; Badea, M.L.; Rokosz, K. Impact of Gamma Irradiation on the Properties of Magnesium-Doped Hydroxyapatite in Chitosan Matrix. Materials 2022, 15, 5372. [Google Scholar] [CrossRef] [PubMed]
- Gwyddion. Available online: http://gwyddion.net/ (accessed on 30 November 2022).
- ImageJ. Available online: http://imagej.nih.gov/ij (accessed on 20 November 2024).
- Iconaru, S.L.; Motelica-Heino, M.; Predoi, D. Study on europium-doped hydroxyapatite nanoparticles by Fourier transform infrared spectroscopy and their antimicrobial properties. J. Spectrosc. 2013, 2013, 284285. [Google Scholar] [CrossRef]
- Iconaru, S.L.; Predoi, D.; Ciobanu, C.S.; Motelica-Heino, M.; Guegan, R.; Bleotu, C. Development of Silver Doped Hydroxyapatite Thin Films for Biomedical Applications. Coatings 2022, 12, 341. [Google Scholar] [CrossRef]
- Cotrut, C.M.; Blidisel, A.; Vranceanu, D.M.; Vladescu, A.; Ungureanu, E.; Pana, I.; Dinu, M.; Vitelaru, C.; Parau, A.C.; Pruna, V.; et al. Evaluation of the In Vitro Behavior of Electrochemically Deposited Plate-like Crystal Hydroxyapatite Coatings. Biomimetics 2024, 9, 704. [Google Scholar] [CrossRef]
- Motelica-Heino, M.; Predoi, M.V.; Ciobanu, S.C.; Iconaru, S.L.; Predoi, D. Studies of New Layer Formation on the Surface of Zinc Doped Hydroxyapatite/Chitosan Composite Coatings in Biological Medium. Coatings 2023, 13, 472. [Google Scholar] [CrossRef]
- Predoi, D.; Ciobanu, S.C.; Iconaru, S.L.; Predoi, M.V. Influence of the Biological Medium on the Properties of Magnesium Doped Hydroxyapatite Composite Coatings. Coatings 2023, 13, 409. [Google Scholar] [CrossRef]
- Dreghici, D.B.; Butoi, B.; Predoi, D.; Iconaru, S.L.; Stoican, O.; Groza, A. Chitosan–Hydroxyapatite Composite Layers Generated in Radio Frequency Magnetron Sputtering Discharge: From Plasma to Structural and Mor-phological Analysis of Layers. Polymers 2020, 12, 3065. [Google Scholar] [CrossRef] [PubMed]
- Groza, A.; Dreghici, D.B.; Ganciu, M. Calcium Phosphate Layers Deposited on Thermal Sensitive Polymer Substrates in Radio Frequency Magnetron Plasma Discharge. Coatings 2019, 9, 709. [Google Scholar] [CrossRef]
- Berzina-Cimdina, L.; Borodajenko, N. Research of Calcium Phosphates Using Fourier Transform Infrared Spectroscopy. In Infrared Spectroscopy—Materials Science, Engineering and Technology; InTech: Rijeka, Croatia, 2012. [Google Scholar] [CrossRef]
- Zarif, M.E.; Bita, B.; Yehia-Alexe, S.A.; Negut, I.; Gradisteanu Pircalabioru, G.; Andronescu, E.; Groza, A. Biological and Physicochemical Analysis of Sr-Doped Hydroxyapatite/Chitosan Composite Layers. Polymers 2024, 16, 1922. [Google Scholar] [CrossRef] [PubMed]
- Vlădescu, A.; Pârâu, A.; Pană, I.; Cotruț, C.M.; Constantin, L.R.; Braic, V.; Vrânceanu, D.M. In Vitro Activity Assays of Sputtered HAp Coatings with SiC Addition in Various Simulated Biological Fluids. Coatings 2019, 9, 389. [Google Scholar] [CrossRef]
- Singh, G.; Singh, R.P.; Jolly, S.S. Customized hydroxyapatites for bone-tissue engineering and drug delivery applications: A review. J. Sol-Gel Sci. Technol. 2020, 94, 505–530. [Google Scholar] [CrossRef]
- Smith, A.M.; Paxton, J.Z.; Hung, Y.P.; Hadley, M.J.; Bowen, J.; Williams, R.L.; Grover, L.M. Nanoscale crystallinity modulates cell proliferation on plasma sprayed surfaces. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 48, 5–10. [Google Scholar] [CrossRef][Green Version]
- Lim, B.K.; Sun, F.; Ryu, S.C.; Koh, K.; Han, D.W.; Lee, J. Hydroxyapatite coating on damaged tooth surfaces by immersion. Biomed. Mater. 2009, 4, 025017. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Li, K.; Tielens, F.; Wang, J. Effect of sprayed techniques on the surface microstructure and in vitro behavior of nano-HAp coatings. Mater. Sci. Eng. C 2020, 117, 111318. [Google Scholar] [CrossRef] [PubMed]
- Janković, A.; Eraković, S.; Mitrić, M.; Matić, I.Z.; Juranić, Z.D.; Tsui, G.C.; Tang, C.Y.; Mišković-Stanković, V.; Rhee, K.Y.; Park, S.J. Bioactive hydroxyapatite/graphene composite coating and its corrosion stability in simulated body fluid. J. Alloys Compd. 2015, 624, 148–157. [Google Scholar] [CrossRef]
- Safavi, M.S.; Khalil-Allafi, J.; Restivo, E.; Ghalandarzadeh, A.; Hosseini, M.; Dacarro, G.; Malavasi, L.; Milella, A.; Listorti, A.; Visai, L. Enhanced in vitro immersion behavior and antibacterial activity of NiTi orthopedic biomaterial by HAp-Nb2O5 composite deposits. Sci. Rep. 2023, 13, 16045. [Google Scholar] [CrossRef] [PubMed]
- Hankenson, K.D.; Dishowitz, M.; Gray, C.; Schenker, M. Angiogenesis in bone regeneration. Injury 2011, 42, 556–561. [Google Scholar] [CrossRef]
- Cerqueira, A.; García-Arnáez, I.; Romero-Gavilán, F.; Azkargorta, M.; Elortza, F.; Martín de Llanos, J.J.; Carda, C.; Gurruchaga, M.; Goñi, I.; Sua, J. Complex effects of Mg-biomaterials on the osteoblast cell machinery: A proteomic study. Biomater. Adv. 2022, 137, 212826. [Google Scholar] [CrossRef] [PubMed]
- Knapek, A.; Sobola, D.; Tomanek, P.; Pokorna, Z.; Urbanek, M. Field emission from the surface of highly ordered pyrolytic graphite. Appl. Surf. Sci. 2017, 395, 157–161. [Google Scholar] [CrossRef]
- Predoi, D.; Ciobanu, C.S.; Iconaru, S.L.; Raaen, S.; Badea, M.L.; Rokosz, K. Physicochemical and Biological Evaluation of Chitosan-Coated Magnesium-Doped Hydroxyapatite Composite Layers Obtained by Vacuum Deposition. Coatings 2022, 12, 702. [Google Scholar] [CrossRef]
- Iconaru, S.L.; Ciobanu, C.S.; Predoi, G.; Rokosz, K.; Chifiriuc, M.C.; Bleotu, C.; Stanciu, G.; Hristu, R.; Raaen, S.; Raita, S.M.; et al. Biological and Physico-Chemical Properties of Composite Layers Based on Magnesium-Doped Hydroxyapatite in Chitosan Matrix. Micromachines 2022, 13, 1574. [Google Scholar] [CrossRef] [PubMed]
- El Hadad, A.A.G.; Barranco, V.; Jiménez-Morales, A.; Peón, E.; Hickman, G.J.; Perry, C.C.; Galván, J. Enhancing in vitro biocompatibility and corrosion protection of organic–inorganic hybrid sol–gel films with nanocrystalline hydroxyapatite. J. Mater. Chem. B 2014, 2, 3886–3896. [Google Scholar] [CrossRef]
- Shi, Y.; Pei, J.; Zhang, J.; Niu, J.; Zhang, H.; Guo, S.; Li, Z.; Yuan, G. Enhanced corrosion resistance and cytocompatibility of biodegradable Mg alloys by introduction of Mg(OH)2 particles into poly (L-lactic acid) coating. Sci. Rep. 2017, 7, 41796. [Google Scholar] [CrossRef] [PubMed]
- Posada, V.M.; Civantos, A.; Ramírez, J.; Fernández-Morales, P.; Allain, J.P. Tailoring adaptive bioresorbable Mg-based scaffolds with directed plasma nanosynthesis for enhanced osseointegration and tunable resorption. Appl. Surf. Sci. 2021, 550, 149388. [Google Scholar] [CrossRef]
- Zeller-Plumhoff, B.; Laipple, D.; Slominska, H.; Iskhakova, K.; Longo, E.; Hermann, A.; Flenner, S.; Greving, I.; Storm, M.; Willumeit-Römer, R. Evaluating the morphology of the degradation layer of pure magnesium via 3D imaging at resolutions below 40 nm. Bioact. Mater. 2021, 6, 4368–4376. [Google Scholar] [CrossRef] [PubMed]
- Agha, N.A.; Liu, Z.; Feyerabend, F.; Willumeit-Römer, R.; Gasharova, B.; Heidrich, S.; Mihailova, B. The effect of osteoblasts on the surface oxidation processes of biodegradable Mg and Mg-Ag alloys studied by synchrotron IR microspectroscopy. Mater. Sci. Eng. C 2018, 91, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.S.; Huang, H.H.; Tsai, Y.H.; Kuo, Y.L.; Lee, J.W.; Lee, Y.J.; Linn, T.Y.; Chen, P. Creating an extracellular matrix-like three-dimension structure to enhance the corrosion resistance and biological responses of titanium implants. J. Dent. Sci. 2024, 19 (Suppl. 1), S70–S80. [Google Scholar] [CrossRef]
- Zareidoost, A.; Yousefpour, M.; Ghaseme, B.; Amanzadeh, A. The relationship of surface roughness and cell response of chemical surface modification of titanium. J. Mater. Sci. Mater. Med. 2012, 23, 1479–1488. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.H.; Lee, H.H.; Lee, C.H. Substrate properties modulate cell membrane roughness by way of actin filaments. Sci. Rep. 2017, 7, 9068. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Xie, W.; Yu, L.; Camacho, L.C.; Nie, C.; Zhang, M.; Haag, R.; Wei, Q. Surface Roughness Gradients Reveal Topography-Specific Mechanosensitive Responses in Human Mesenchymal Stem Cells. Small 2020, 16, e1905422. [Google Scholar] [CrossRef] [PubMed]
- Gittens, R.A.; McLachlan, T.; Olivares-Navarrete, R.; Cai, Y.; Berner, S.; Tannenbaum, R.; Schwartz, Z.; Sandhage, K.H.; Boyan, B.D. The effects of combined micron-/submicron-scale surface roughness and nanoscale features on cell proliferation and differentiation. Biomaterials 2011, 32, 3395–3403. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, T.; Komatsu, K.; Cheng, J.; Park, G.; Ogawa, T. Beyond microroughness: Novel approaches to navigate osteoblast activity on implant surfaces. Int. J. Implant Dent. 2024, 10, 35. [Google Scholar] [CrossRef]
- Kieswetter, K.; Schwartz, Z.; Dean, D.D.; Boyan, B.D. The role of implant surface characteristics in the healing of bone. Crit. Rev. Oral Biol. Med. 1996, 7, 329–345. [Google Scholar] [CrossRef]
- Martin, J.Y.; Schwartz, Z.; Hummert, T.W.; Schraub, D.M.; Simpson, J.; Lankford, J., Jr.; Dean, D.D.; Cochran, D.L.; Boyan, B.D. Effect of titanium surface-roughness on proliferation, differentiation, and protein-synthesis of human osteoblast-like cells (MG63). J. Biomed. Mater. Res. 1995, 29, 389–401. [Google Scholar] [CrossRef]
- Mendonça, G.; Mendonça, D.B.S.; Simões, L.G.P.; Araújo, A.L.; Leite, E.R.; Duarte, W.R.; Aragão, F.J.L.; Cooper, L.F. The effects of implant surface nanoscale features on osteoblast-specific gene expression. Biomaterials 2009, 30, 4053–4062. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, M.; Zhang, W.; Liu, H.; Jin, F.; Mao, S.; Han, C.; Wang, X. Mechanical strategies to promote vascularization for tissue engineering and regenerative medicine. Burns Trauma 2024, 12, tkae039. [Google Scholar] [CrossRef]
- Degasne, I.; Baslé, M.F.; Demais, V.; Huré, G.; Lesourd, M.; Grolleau, B.; Mercier, L.; Chappard, D. Effects of roughness, fibronectin and vitronectin on attachment, spreading, and proliferation of human osteoblast-like cells (Saos-2) on titanium surfaces. Calcif. Tissue Int. 1999, 64, 499–507. [Google Scholar] [CrossRef]
- Rajtar, A.; Kaluza, G.L.; Yang, Q.; Hakimi, D.; Liu, D.; Tsui, M.; Lien, M.; Smith, D.; Clubb, F.J.; Troczynski, T. Hydroxyapatite-coated cardiovascular stents. EuroIntervention 2006, 2, 113–115. [Google Scholar] [PubMed]
- van der Giessen, W.J.; Sorop, O.; Serruys, P.W.; Peters-Krabbendam, I.; van Beusekom, H.M. Lowering the dose of sirolimus, released from a nonpolymeric hydroxyapatite coated coronary stent, reduces signs of delayed healing. JACC Cardiovasc. Interv. 2009, 2, 284–290. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Balhuc, S.; Campian, R.; Labunet, A.; Negucioiu, M.; Buduru, S.; Kui, A. Dental Applications of Systems Based on Hydroxyapatite Nanoparticles—An Evidence-Based Update. Crystals 2021, 11, 674. [Google Scholar] [CrossRef]
- Ittycheria, P.G.; George, T.; John, M.; Meenu, G.; Thomas, V.; Aswathy, S.; Kuriakose, R.; Thomas, J. Application of Hydroxyapatite in Regenerative Dentistry. In Novel Biomaterials for Tissue Engineering; IntechOpen: London, UK, 2024. [Google Scholar]
- Rau, J.V.; Cacciotti, I.; De Bonis, A.; Fosca, M.; Komlev, V.S.; Latini, A.; Santagata, A.; Teghil, R. Fe-doped hydroxyapatite coatings for orthopedic and dental implant applications. Appl. Surf. Sci. 2014, 307, 301–305. [Google Scholar] [CrossRef]
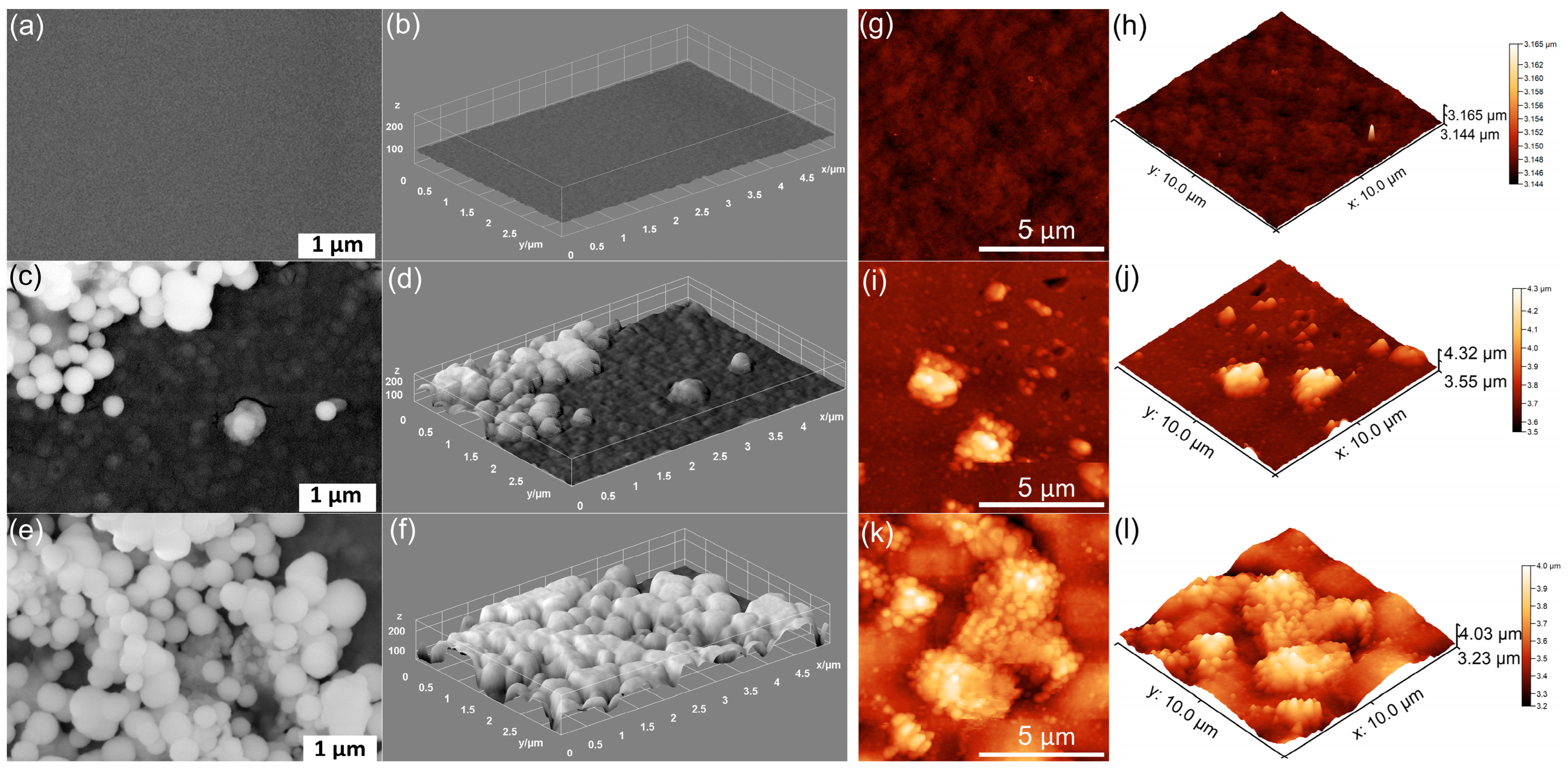
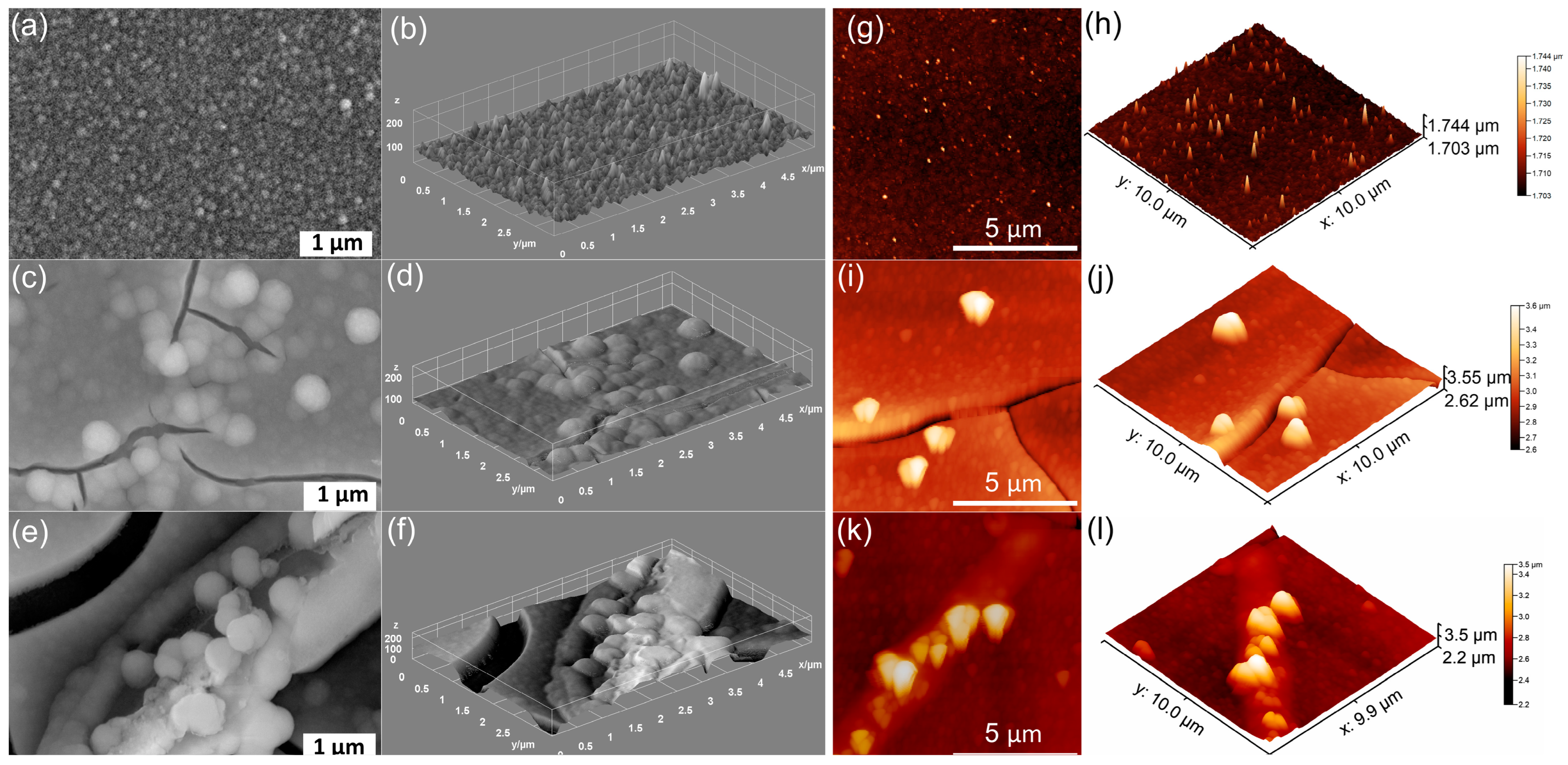
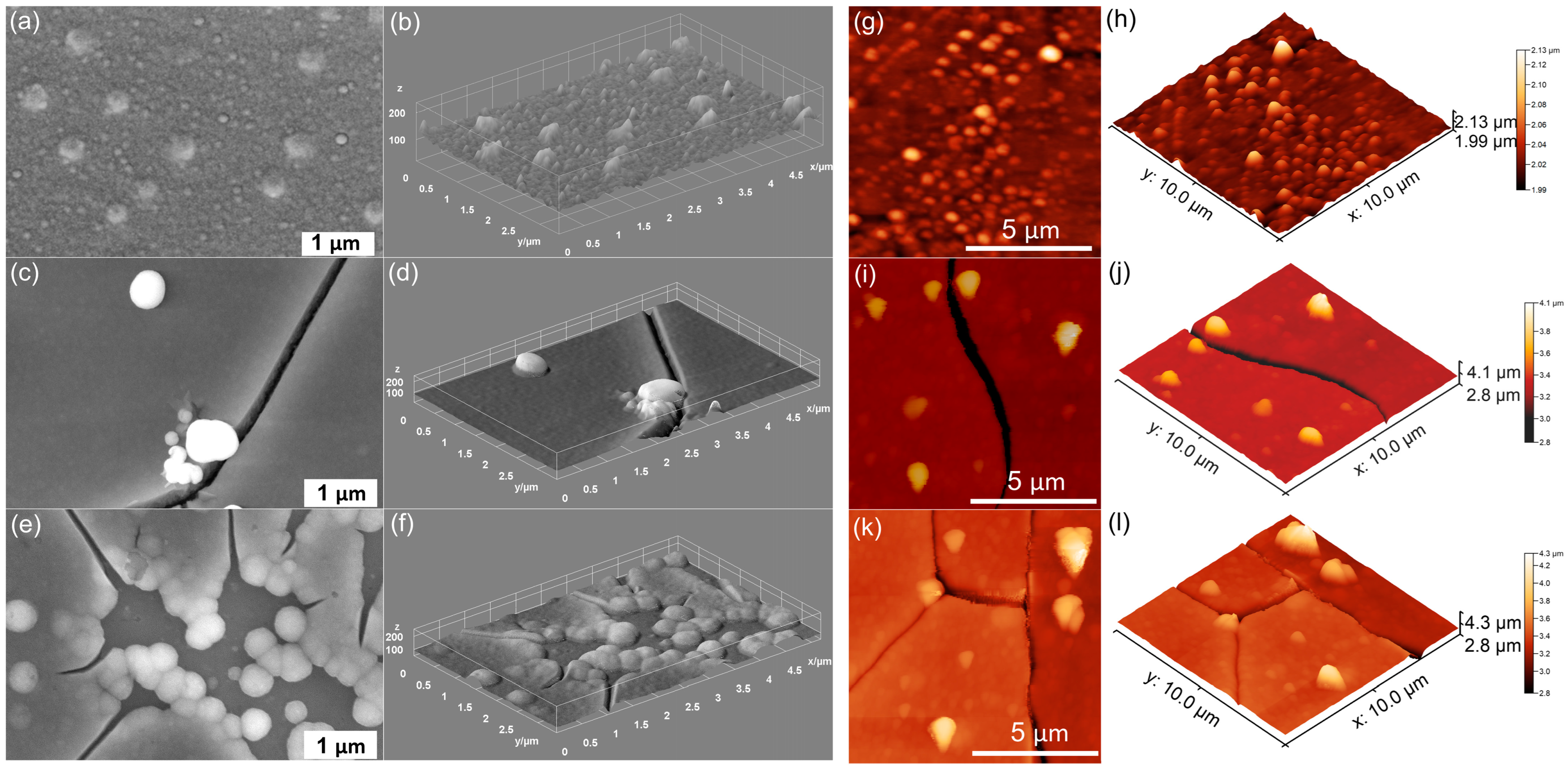
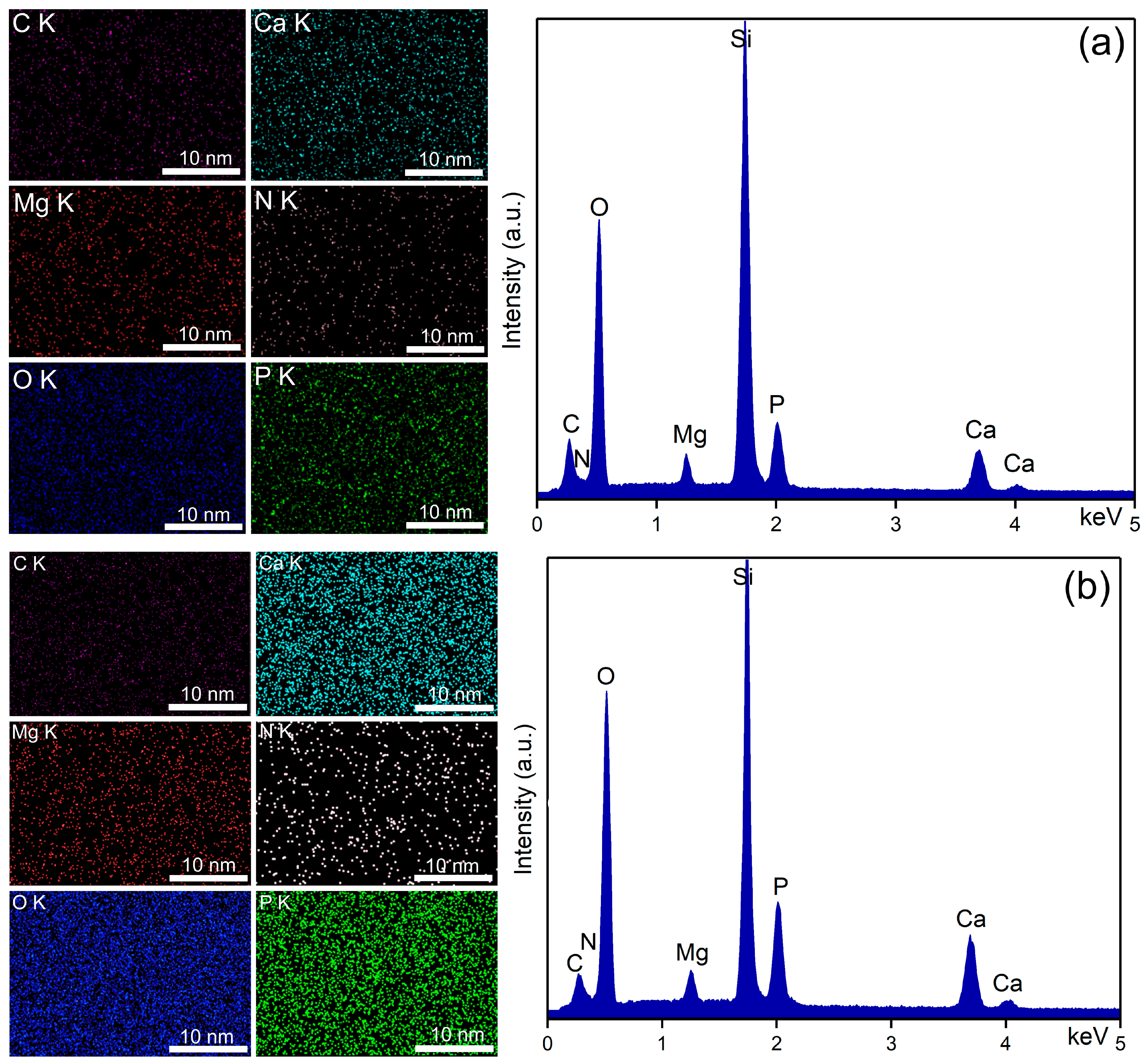
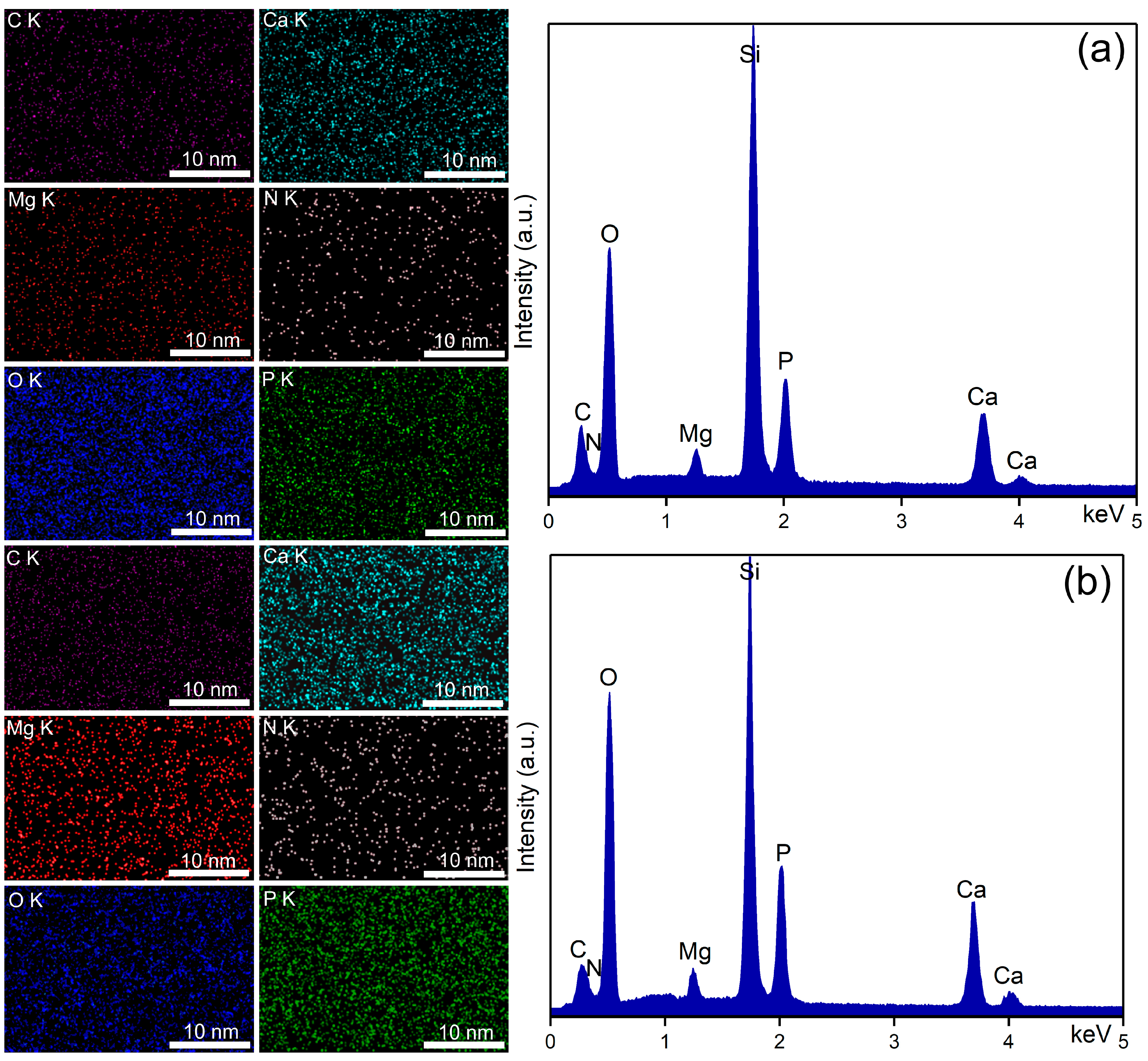
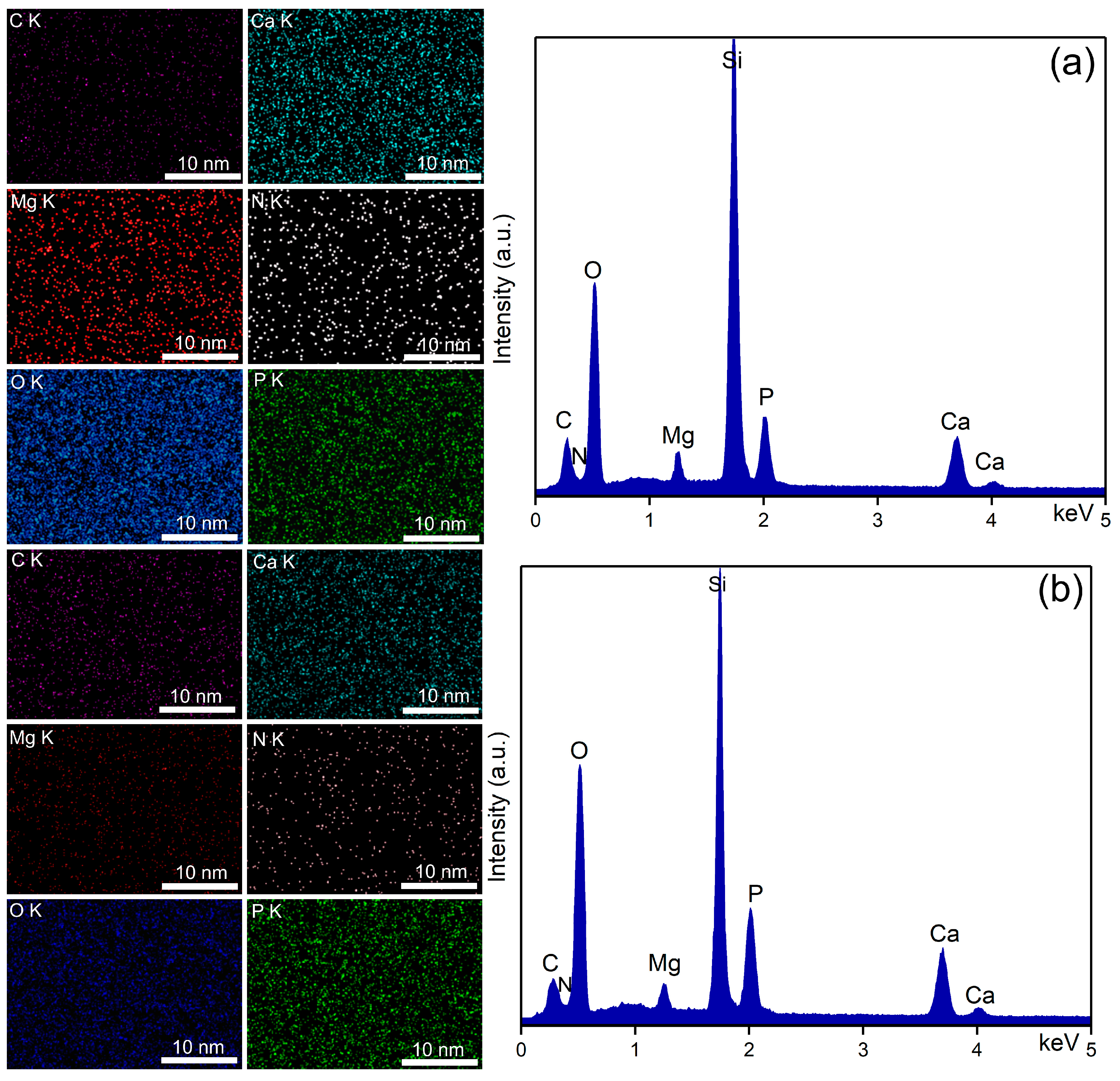
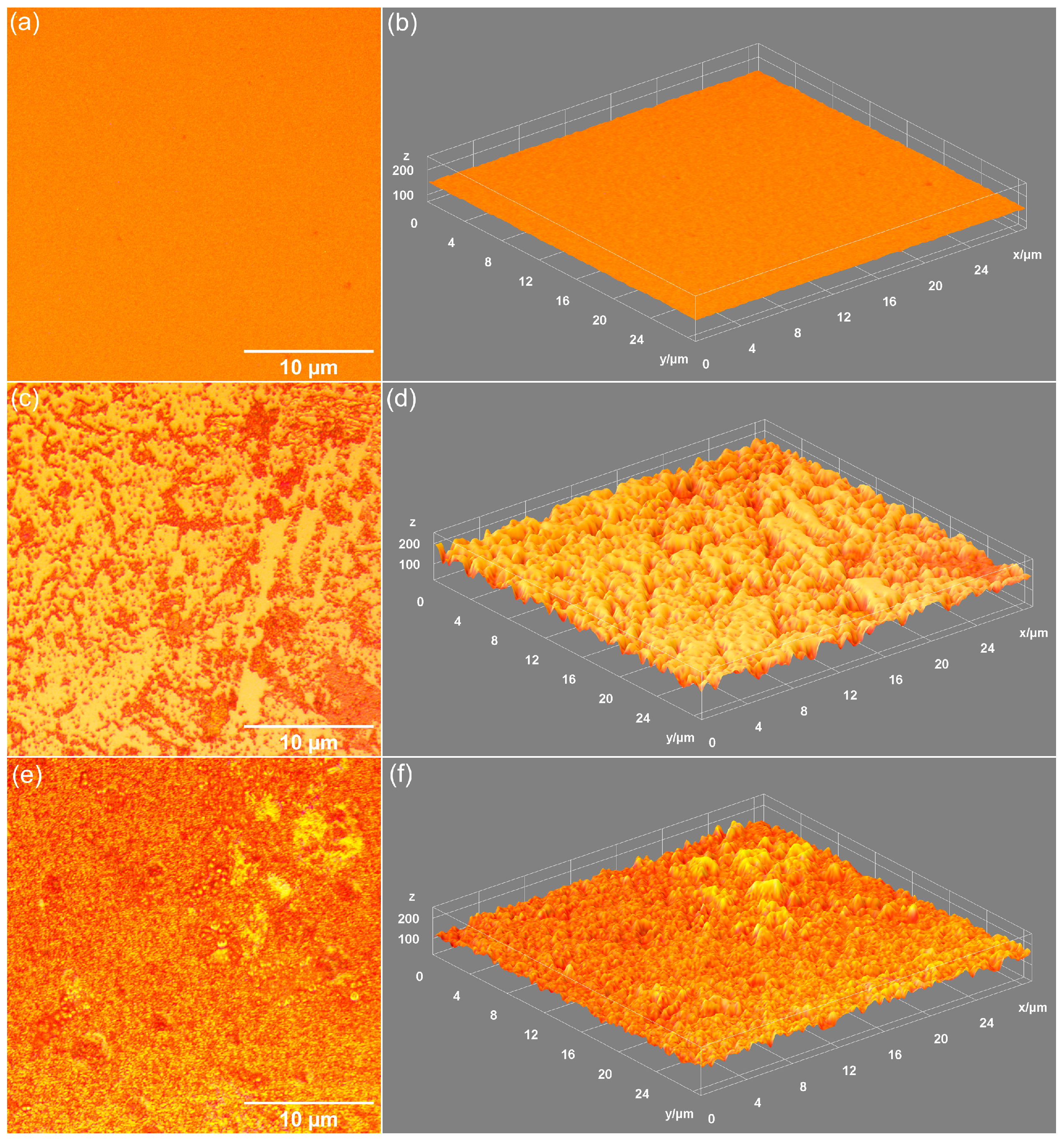
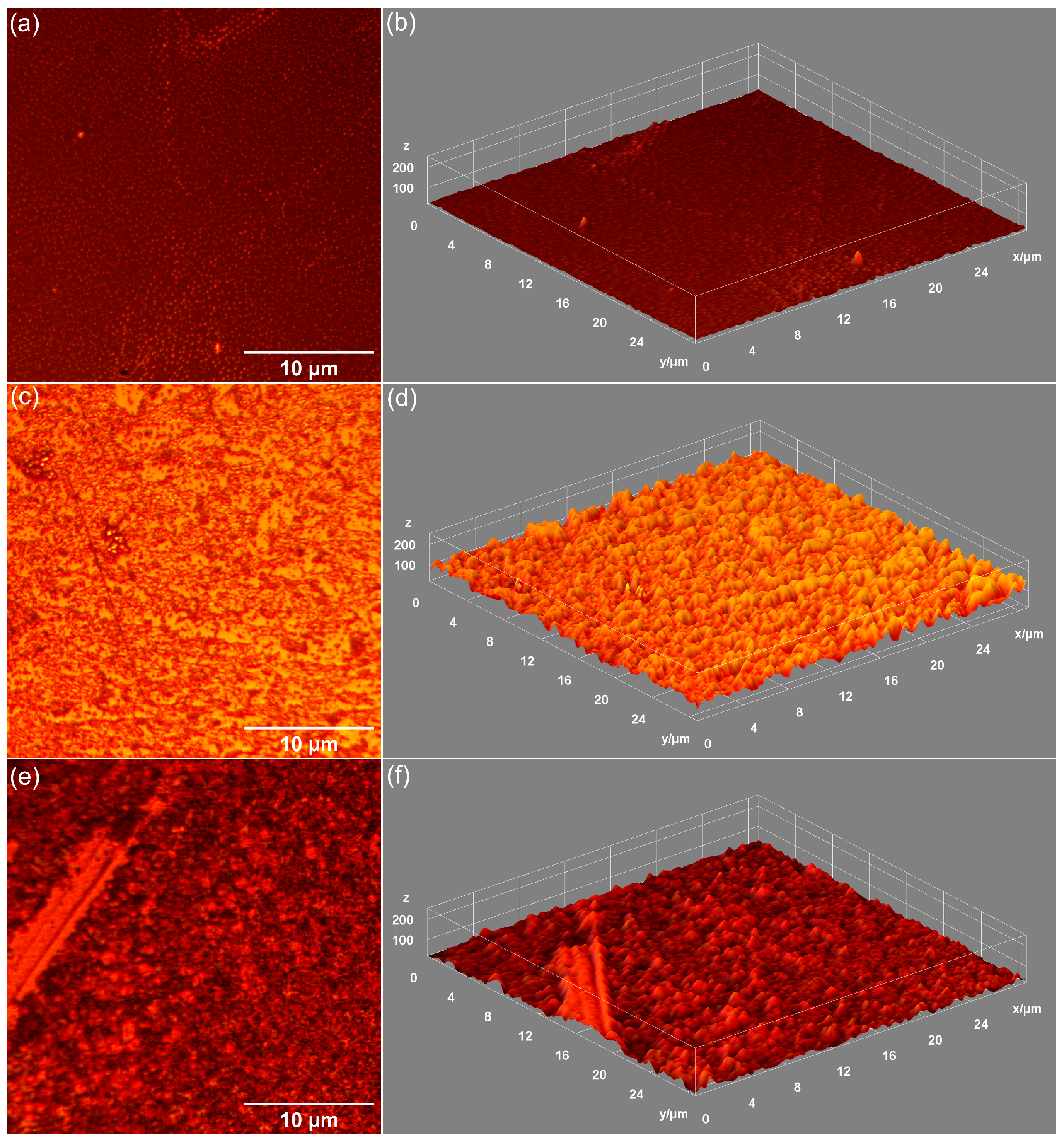
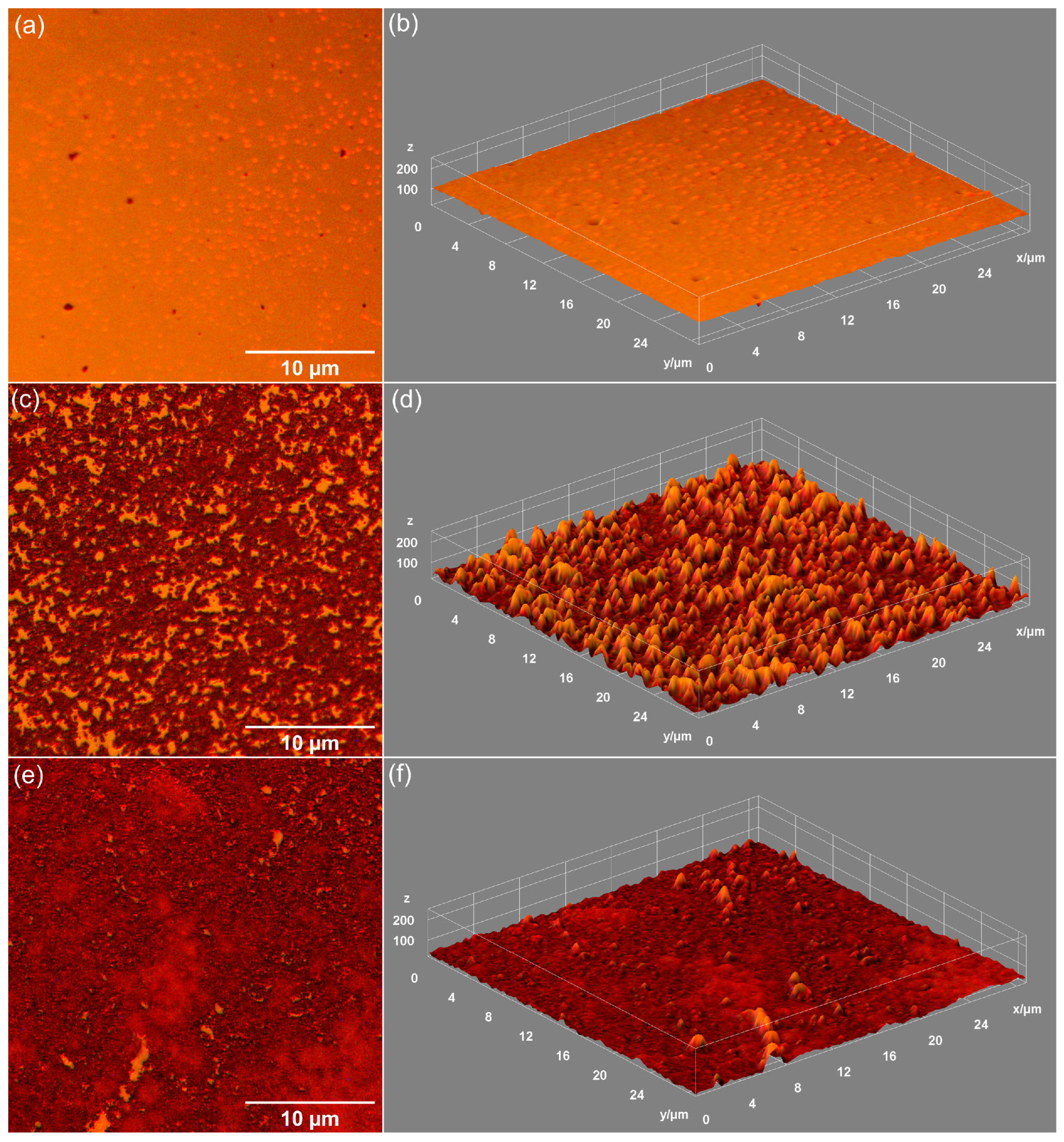

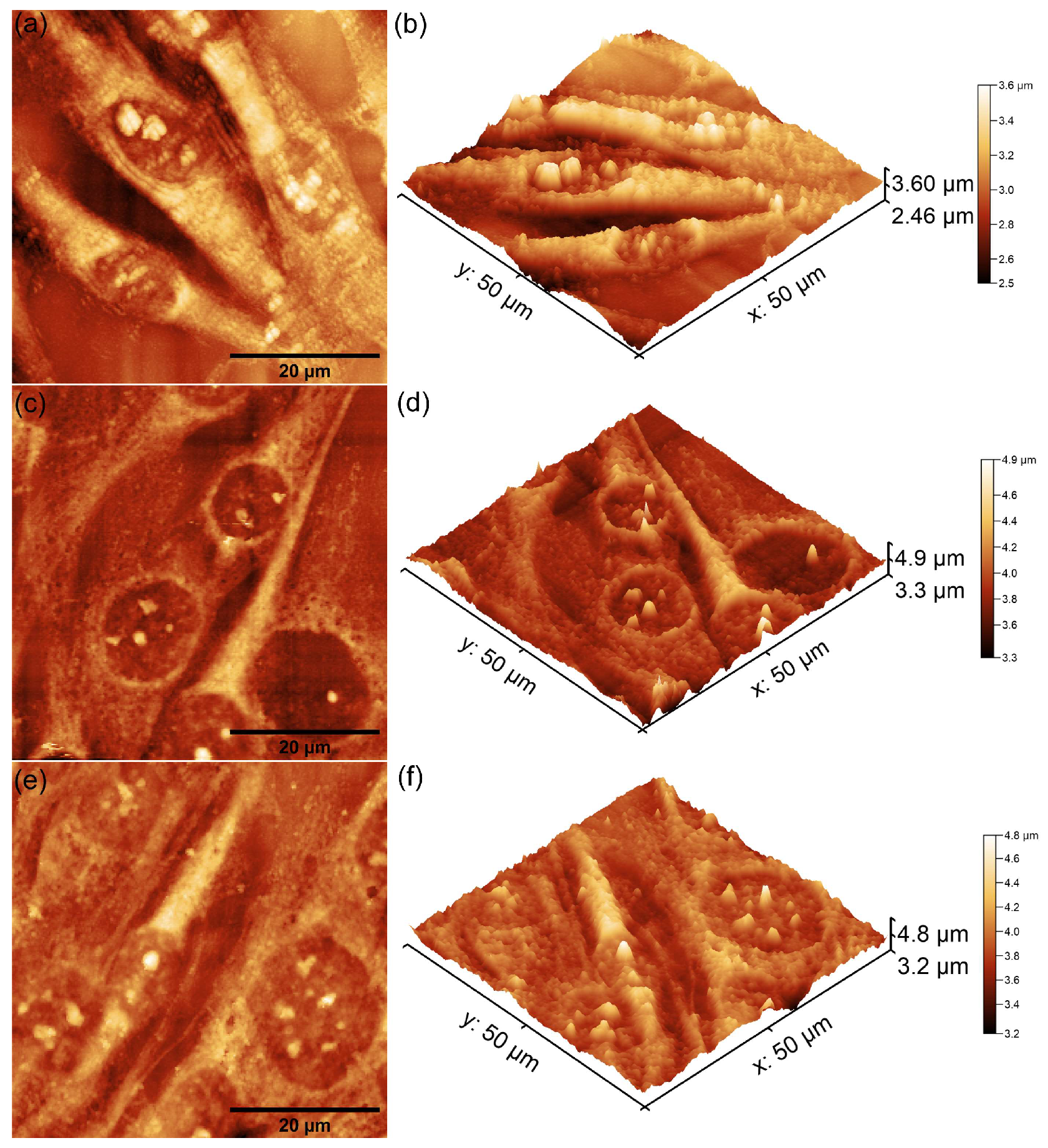


| Sample | RRMS (nm) |
|---|---|
| MHA_Ch | 13.5 |
| MHA_Ch-7D | 87.65 |
| MHA_Ch-14D | 122.7 |
| MHA_Ch-8Gy | 20.01 |
| MHA_Ch-8Gy-7D | 104.7 |
| MHA_Ch-8Gy-14D | 159.5 |
| MHA_Ch-30Gy | 23.57 |
| MHA_Ch-30Gy-7D | 108 |
| MHA_Ch-30Gy-14D | 135.2 |
| Sample | Coatings Thickness (nm) |
|---|---|
| MHA_Ch | 300 ± 3 |
| MHA_Ch-7D | 317 ± 3 |
| MHA_Ch-14D | 332 ± 3 |
| MHA_Ch-8Gy | 273 ± 2 |
| MHA_Ch-8Gy-7D | 281 ± 2 |
| MHA_Ch-8Gy-14D | 297 ± 2 |
| MHA_Ch-30Gy | 258 ± 1 |
| MHA_Ch-30Gy-7D | 263 ± 1 |
| MHA_Ch-30Gy-14D | 274 ± 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bleotu, C.; Iconaru, S.L.; Ciobanu, C.S.; Groza, A.; Predoi, D. Exploring the Effects of Dulbecco’s Modified Eagle’s Medium on Irradiated Layers of Magnesium-Doped Hydroxyapatite in a Chitosan Matrix for Biomedical Applications. Coatings 2025, 15, 209. https://doi.org/10.3390/coatings15020209
Bleotu C, Iconaru SL, Ciobanu CS, Groza A, Predoi D. Exploring the Effects of Dulbecco’s Modified Eagle’s Medium on Irradiated Layers of Magnesium-Doped Hydroxyapatite in a Chitosan Matrix for Biomedical Applications. Coatings. 2025; 15(2):209. https://doi.org/10.3390/coatings15020209
Chicago/Turabian StyleBleotu, Coralia, Simona Liliana Iconaru, Carmen Steluta Ciobanu, Andreea Groza, and Daniela Predoi. 2025. "Exploring the Effects of Dulbecco’s Modified Eagle’s Medium on Irradiated Layers of Magnesium-Doped Hydroxyapatite in a Chitosan Matrix for Biomedical Applications" Coatings 15, no. 2: 209. https://doi.org/10.3390/coatings15020209
APA StyleBleotu, C., Iconaru, S. L., Ciobanu, C. S., Groza, A., & Predoi, D. (2025). Exploring the Effects of Dulbecco’s Modified Eagle’s Medium on Irradiated Layers of Magnesium-Doped Hydroxyapatite in a Chitosan Matrix for Biomedical Applications. Coatings, 15(2), 209. https://doi.org/10.3390/coatings15020209









