Abstract
In this paper, we present the development of magnesium-doped hydroxyapatite in chitosan matrix (MHA_Ch) powder by an adapted coprecipitation method. The MHA_Ch powder was then deposited as thin layers by radio frequency magnetron sputtering. The MHA_Ch layers were exposed to various irradiation doses and immersed in Dulbecco’s Modified Eagle’s Medium (DMEM) for various time intervals. We report, for the first time, the effects of DMEM on irradiated layers of magnesium-doped hydroxyapatite in a chitosan matrix. The surface morphology of the layers before and after irradiation and immersion in DMEM was evaluated by SEM, AFM, and MM studies. Additionally, data about the functional groups present in the layers and the changes induced by exposure of the layers to irradiation and DMEM were obtained by FTIR studies. In vitro biological assays were conducted using an MG63 cell line (ATCC CRL1427). Our results suggest that the magnesium-doped hydroxyapatite in chitosan matrix layers may be suitable candidates for applications in the biomedical domain.
Keywords:
DMEM; magnesium; hydroxyapatite; chitosan; layers; biocompatibility; surface modifications 1. Introduction
Biomaterials have long been recognized for their biological and long-term medical applications in covering orthopedic prothesis and dental implants [1]. At the prothesis/implant–tissue interface, foreign-body issues are encountered, which biomaterial films can help overcome [1,2,3,4,5,6,7,8,9]. The composition of these films needs to be properly chosen to ensure osseointegration while also possessing biocompatible, antimicrobial, or antifungal properties. Testing of these materials for biological activity can be performed in media that simulate the body environment, such as simulated body fluids, RPMI media (Roswell Park Memorial Institute Medium), phosphate buffer solution, or Dulbecco’s Modified Eagle’s Medium (DMEM) [10].
The choice of DMEM (Dulbecco’s Modified Eagle’s Medium) solution for our studies comes from the fact that it is a biological medium, and together with SBF (Simulated Body Fluid), it has been proposed for the evaluation of bioactivity of various types of materials [11,12,13,14,15,16]. At the same time, DMEM offers a more complex environment and is better suited to simulate real biological conditions compared to SBF [11,12,13,14,15,16]. Previous studies have shown that immersing/exposing materials to DMEM or SBF for 1–28 days leads to the formation of a new apatite layer [11,12,13,14,15,16].
The calcium phosphate class of compounds is versatile and plays an important role in the biomaterials category, as their biological properties can be enhanced by incorporating chemical elements such as Mg, Zn, Ag, and Sr as cations that substitute for Ca2+ ions [2]. For example, antimicrobial activity is enhanced when Ag+, Sr2+, or Zn2+ ions are added. Mg has a significant contribution in the regeneration, mineralization, and growth of bones and increases antibacterial activity against E. coli, C. albicans, P. aeruginosa, and S. aureus [2]. Apatite compounds, either synthetic or natural, especially hydroxyapatite (HAp) and carbonated apatite, are key materials in biomedical applications. Both hydroxyapatite and carbonated apatite constitute the major components of bones and teeth. Hydroxyapatite has a hexagonal crystal structure formed from phosphate ions (PO43−) and calcium ions (Ca2+). Carbonated apatite is derived from hydroxyapatite when a part of phosphate ions is replaced by CO32− ions. In the human body, this substitution allows for better regeneration and resorption of bones [3].
Chitosan is a biocompatible polymer that supports the proliferation and mineralization of bone cells and is often used in bone tissue engineering [17].
Radiotherapy for cancer treatment involves the exposure of certain body parts to ionizing radiation, such as X-rays, gamma rays, electrons, protons, or neutron beams. Over the years, these ionizing radiations produced in medical linear accelerators have proven their importance in the treatment of bone cancers in the human body [18]. During such irradiations, cancer cells are damaged and subsequently destroyed [19]. In radiation therapy, radiation doses in the range from a few to tens of Gy are delivered to specific areas of the body in fractions, for well-defined time durations and as a function of cancer type and stage. For example, an 8 Gy single fraction radiotherapy is successfully used for the treatment of metastatic skeletal pain [20]. For painful bone metastases, a total radiation dose of 30 Gy has been used and reported [21]. These radiations not only affect the targeted tissue but also impact healthy tissue [22,23,24]. Therefore, biomedical devices, such as protheses or implants covered with biocompatible layers, need to be tested in such harsh environments.
In our earlier study, we assessed the effects of electron beams on magnesium-doped hydroxyapatite–chitosan layers when the absorbed radiation doses were 2 and 50 Gy [25]. The morphological features of the layer surfaces changed, but the apatite structure was not altered, although depletion of Ca, P, C, O and N was observed.
In the present study, magnesium-doped hydroxyapatite–chitosan layers exposed to electron beams at 8 and 30 Gy radiation doses were immersed in Dulbecco’s Modified Eagle’s Medium (DMEM), and their biological and physicochemical features before and after immersion in DMEM were evaluated.
2. Materials and Methods
2.1. Materials
For the preparation of magnesium-doped hydroxyapatite in chitosan matrix powder (MHA_Ch), the following reagents were used as precursors: calcium nitrate tetrahydrate (Ca(NO3)2·4H2O, ≥99.0%, Sigma Aldrich, St. Louis, MO, USA), magnesium nitrate hexahydrate (Mg(NO3)2·6H2O, 99.97%, Alfa Aesar, Kandel, Germany), chitosan (C6H11NO4; Sigma Aldrich, St. Louis, MO, USA), and diammonium hydrogen phosphate ((NH4)2HPO4, ≥99.0%, Sigma Aldrich, St. Louis, MO, USA).
2.1.1. Synthesis of MHA_Ch Powders
An adapted coprecipitation method was employed in order to obtain the MHA_Ch powders (xMg = 0.1), following the steps previously described [25,26]. In summary, during the synthesis of MHA_Ch powders, the [Ca + Mg]/P ratio was set to 1.67. The MHA_Ch synthesis involved dissolving the appropriate quantities of (NH4)2HPO4, Ca(NO3)2·4H2O, and Mg(NO3)2·6H2O in double-distilled water in order to obtain two solutions. The solution containing Ca and Mg was added to the P solution and stirred for 12 h at 80 °C. The mixture was then centrifuged and washed several times. Finally, the mixture was dispersed in the chitosan solution (2%) and dried at 80 °C in order to obtain MHA_Ch powders. The MHA_Ch powder was mechanically pressed into a disc with a 50 mm diameter and 4 mm thickness (sputtering target), which was then placed in the magnetron head.
2.1.2. Synthesis of Layers by Magnetron Sputtering Technique
The MHA_Ch powders were deposited as thin layers (~300 nm) on Si substrates by radio frequency magnetron sputtering in the following conditions: 6 h deposition time; 70 W rf power; 5 × 10−3 mbar pressure, 2 sln/min gas flow; 4 cm distance from the magnetron head. Supplementary details on the deposition procedure can be found in [9].
2.1.3. Exposure of Mg-HAp/Ch Layers to Electron Beams
A Siemens MEVATRON Primus clinical linear accelerator was used to produce an electron beam with 5 MeV energy. The samples were placed at a distance of 100 cm from the electron beam focal point and absorbed radiation doses of 8 and 30 Gy with a dose rate of 3 Gy/min. The radiation field was 20 × 20 cm2. The electron beam characteristics were assessed before sample exposure and calculated in accordance with radiation therapy protocols [18]. The unirradiated samples were labeled as MHA_Ch, the samples exposed to 8Gy were labeled as MHA_Ch-8Gy, and the samples exposed to 30 Gy were labeled as MHA_Ch-30Gy.
The MHA_Ch, MHA_Ch-8Gy, and MHA_Ch-30Gy layers were immersed in Dulbecco’s Modified Eagle Medium (DMEM, Sigma Aldrich, St. Louis, MO, USA) and kept in an incubator for 7 and 14 days at 37 ± 0.5 °C. The DMEM was renewed every day. After 7 and 14 days, all the samples were rinsed with bidistilled water and placed in a desiccator for storage. The layers immersed for 7 days were labeled as MHA_Ch-7D, MHA_Ch-8Gy-7D, and MHA_Ch-30Gy-7D, while those immersed for 14 days were labeled as MHA_Ch-14D, MHA_Ch-8Gy-14D, and MHA_Ch-30Gy-14D.
2.2. Physico-Chemical Characterization
The morphology of the surfaces of the unirradiated and irradiated MHA_Ch layers, before and after immersion in Dulbecco’s cell culture medium for 7 and 14 days, was investigated using an FEI Quanta Inspect F scanning electron microscope (FEI Company, Hillsboro, OR, USA) equipped with an EDAX detector.
The surface topography of unirradiated, 8Gy, and 30Gy irradiated MHA_Ch layers, both before and after immersion in DMEM (for 7 and 14 days), was evaluated by Atomic Force Microscopy (AFM) studies. The studies were conducted with an NT-MDT NTEGRA Probe Nano Laboratory instrument in semi-contact mode. For AFM image acquisition, a silicon NT-MDT NSG01 cantilever coated with a 35 nm gold layer (tetrahedral tip, 35 nm curvature radius, and a height of 14–16 µm) was used. AFM images were recorded for a 10 × 10 μm2 surface area, and the roughness parameter (RRMS) was estimated. The AFM images were analyzed using Gwidion 2.59 software [27]. Evaluations of the MHA_Ch layer surfaces, both before and after immersion in DMEM, were conducted using an inverted trinocular metallographic microscope (20× magnification objective, OX.2153-PLM, Euromex, Arnhem, Netherlands). The 3D representations of metallographic microscope images were obtained with the aid of Image J software (version 1.51j8) [28].
Data about the adherence of the MHA_Ch, MHA_Ch-8Gy, and MHA_Ch-30Gy layers to the Si substrate (before and after immersion in DMEM) were assessed using the tape-pull test. The adhesion tests were conducted with 3M Performance Flatback Tape 2525, which has a peel adhesion of 7.5 N/cm.
Attenuated total reflection IR spectra of irradiated and unirradiated MHA_Ch layers, before and after immersion in Dulbecco’s cell culture medium for 7 and 14 days, were recorded between 450 and 2000 cm−1 using a Perkin Elmer SP-100 spectrometer (Waltham, MS, USA). Furthermore, using the curve-fitting method outlined in [25,29], we conducted spectral deconvolutions in the 1250–750 cm⁻¹ spectral domain.
2.3. In Vitro Biological Assays
The biocompatibility of MHA_Ch layers, both unirradiated and irradiated with 8 and 30 Gy, was assessed before and after immersion in DMEM for 7 and 14 days using MG63 cells (ATCC CRL1427), following the experimental procedure detailed in [30]. For this purpose, the MG63 cells were cultured in DMEM with supplements and incubated at 37 °C in 5% CO2. The layers were placed in 24-well plates, seeded with 1 × 10⁵ cells/well, and cell viability was measured at 24 and 48 h using the MTT assay. After 48 h, the adhered cells were fixed, dehydrated, and analyzed by atomic force microscopy (AFM, NT-MDT NTEGRA Probe Nano Laboratory instrument, NT-MDT, Moscow, Russia) to evaluate cell adhesion and interactions with the layer surfaces. After 48 h of incubation, which allowed the cells to adhere and proliferate, the cells were gently rinsed with phosphate-buffered saline (PBS) to remove non-adherent cells and debris. The fixation was carried out using a 2.5% glutaraldehyde solution in PBS for 20 min at room temperature, followed by PBS washes. Afterwards, a 30% ethanol solution and air drying were used for the dehydration of the samples. This process ensured that the cells remained intact and attached to the MHA_Ch layers for further analysis.
3. Results
The surface features changes induced by the DMEM on the unirradiated and irradiated MHA_Ch layers were evaluated by SEM studies, and their results are presented in Figure 1, Figure 2 and Figure 3.
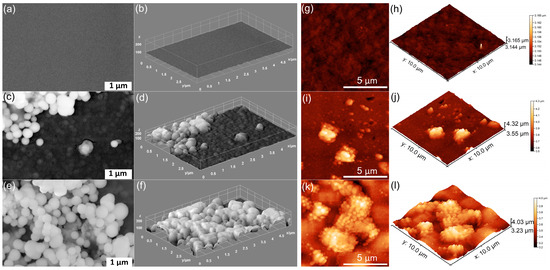
Figure 1.
SEM images of MHA_Ch (a), MHA_Ch-7D (c), and MHA_Ch-14D (e). The 3D representation of SEM images for MHA_Ch (b), MHA_Ch-7D (d), and MHA_Ch-14D (f). AFM 2D topography of MHA_Ch layers (g) and MHA_Ch layers immersed in DMEM for 7 days (i) and 14 days (k), as well as their 3D representations (h,j,l).
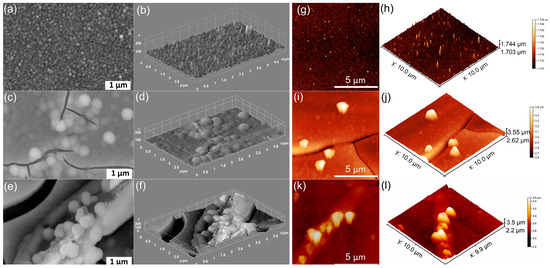
Figure 2.
SEM images of MHA_Ch-8Gy (a), MHA_Ch-8Gy-7D (c), and MHA_Ch-8Gy-14D (e). The 3D representation of SEM images for MHA_Ch-8Gy (b), MHA_Ch-8Gy-7D (d), and MHA_Ch-8Gy-14D (f). AFM 2D topography of MHA_Ch-8Gy layers (g) and MHA_Ch-8Gy layers immersed in DMEM for 7 days (i) and 14 days (k), as well as their 3D representations (h,j,l).
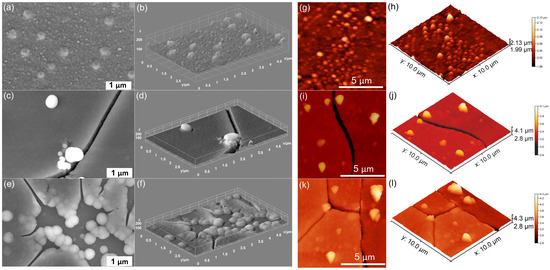
Figure 3.
SEM images of MHA_Ch-30Gy (a), MHA_Ch-30Gy-7D (c), and MHA_Ch-30Gy-14D (e). The 3D representation of SEM images for MHA_Ch-30Gy (b), MHA_Ch-30Gy-7D (d), and MHA_Ch-30Gy-14D (f). AFM 2D topography of MHA_Ch-30Gy layers (g) and MHA_Ch-30Gy layers immersed in DMEM for 7 days (i) and 14 days (k), as well as their 3D representations (h,j,l).
The surface of unirradiated MHA_Ch observed in Figure 1a,b reveals the presence of a smooth and uniform surface. In the case of unirradiated MHA_Ch layers kept for 7 days in DMEM, the appearance of nanospheres and partial surface coverage was observed (Figure 1c,d).
The layers kept for 14 days in DMEM exhibited a surface that was predominantly covered with nanospheres. The appearance of nanosphere on the unirradiated MHA_Ch layers’ surface could suggests the formation of an apatite-like layer induced by exposure to DMEM.
The SEM images of 8 Gy irradiated MHA_Ch layers reveal the presence of a nanostructured surface with no visible cracks (Figure 2a,b). By keeping the 8 Gy irradiated MHA_Ch layers in DMEM for 7 and 14 days, the occurrence of nanospheres along with the appearance of cracks was observed (Figure 2c–f). This behavior further supports the formation of an apatite-like layer on the 8 Gy irradiated MHA_Ch samples after exposure to DMEM.
The SEM micrographs of 30 Gy irradiated MHA_Ch layers, depicted in Figure 3a,b, show the presence of a continuous and nanostructured surface. After 7 and 14 days of exposure to DMEM, the presence of cracks and the formation of nanospheres indicating the formation of a new apatite-like layer were observed (Figure 3c–f). Furthermore, it could be observed that the apatite-like layer consists mainly of agglomerated nanospheres. The tendency of agglomeration and nanosphere formation increased with the exposure time of the MHA_Ch layers to DMEM. Thus, our results suggest that the new layers formed in all samples kept in DMEM are denser and much more compact, indicating a good biomineralization capacity. Similar results were reported by Cotrut, C.M. et al. [31] in their work entitled “Evaluation of the In Vitro Behavior of Electrochemically Deposited Plate-like Crystal Hydroxyapatite Coatings”.
Information about the surface properties of the MHA_Ch, MHA_Ch-8Gy, and MHA_Ch-30Gy layers before and after immersion in DMEM for 7 and 14 days was acquired by AFM studies. The 2D surface topographies and their 3D representation are presented in Figure 1, Figure 2 and Figure 3. The influence of Dulbecco’s Modified Eagle Medium (DMEM) on the surface morphology of MHA_Ch, MHA_Ch-8Gy, and MHA_Ch-30Gy layers was evidenced in the atomic force microscopy (AFM) topographies. The results showed that after 7 days of exposure, the MHA_Ch, MHA_Ch-8Gy, and MHA_Ch-30Gy surfaces exhibited significant changes in roughness. The roughness parameters (RRMS) calculated from the 2D AFM topographies are presented in Table 1.

Table 1.
Roughness parameter RRMS.
Furthermore, the 2D AFM topographies revealed that after DMEM exposure, on the surface of MHA_Ch, MHA_Ch-8Gy and MHA_Ch-30Gy was observed the development of nanoscale granules and distinct morphological features. These morphological changes could be attributed to the ion exchange and re-precipitation processes that occurred during DMEM immersion. The calcium and phosphate ions from DMEM interact with the MHA_Ch, MHA_Ch-8Gy, and MHA_Ch-30Gy layers, leading to the deposition of additional mineral phases. Thus, the AFM topographies depict a surface morphology that is relatively homogenous and uniform, showing a moderate increase in surface roughness, which is indicative of the initial stages of biomimetic mineralization.
The AFM images also highlighted that after 14 days of exposure to DMEM, the morphological changes of the surfaces of the MHA_Ch, MHA_Ch-8Gy, and MHA_Ch-30Gy layers became more pronounced. Moreover, further increases in surface roughness and the presence of more complex topographies were observed. The prolonged exposure to DMEM facilitated and enhanced biomineralization, leading to the appearance of denser clusters of nanoparticles on the layers’ surfaces. Compared to the 7-day exposure, the AFM images after 14 days depicted a higher density of particle conglomerates, an increased layer thickness, and the presence of interconnected nanostructures. This could be attributed to the deposition of calcium phosphate phases and the stabilizing influence of organic molecules in DMEM, which influenced the crystal growth process. The 2D AFM images, as well as their 3D reconstructions, clearly suggested that the DMEM exposure time influenced the layer’s surface morphology. While the 7-day exposure initiated surface modification and the development of a new bioactive layer, the 14-day exposure resulted in more significant structural changes, indicating the formation of a continuous and homogenous bioactive surface. These differences are critical for biomedical applications, as the increase in surface complexity and roughness observed after 14 days of DMEM exposure helps obtain layers that are more suited for promoting cell adhesion and proliferation. In contrast, the less developed surfaces after 7 days of exposure to DMEM might require some improvements. The changes induced by DMEM exposure reflect the dynamic interaction between the layers and the medium, emphasizing the role of exposure time in optimizing the surface morphology for specific biomedical applications, such as improving cell adhesion and promoting osteointegration. The increase in surface roughness that was observed in the AFM studies could be attributed to the formation of particles through ion exchange, precipitation, and organic adsorption. The immersion of the layers in DMEM and the release of ions, such as Ca2+ and PO43−, from the material can alter the local supersaturation conditions, leading to the nucleation and growth of microparticles, as well as the formation of nanoscale particles or nanoparticles conglomerates, which could be observed on the surfaces of the immersed layers in the AFM topographies. Additionally, DMEM contains proteins and organic molecules that could be adsorbed onto the surface, thus creating new nucleation sites for mineral deposition. Changes in the local pH and ionic strength during DMEM immersion further promote heterogeneous nucleation, leading to microstructural modifications and an increase in the surface roughness. The apatite formation by immersion in DMEM can occur through a biomineralization process, which is caused by the supersaturation of calcium and phosphate ions released in the medium. First, an amorphous calcium phosphate (ACP) phase forms, which may later crystallize into hydroxyapatite (HA), a key component of bone tissue. Additionally, in some cases, the presence of carbonate ions from DMEM could promote the formation of carbonated apatite, making the newly formed layer more biologically significant. Furthermore, the proteins found in DMEM help to stabilize intermediate mineral phases and may also influence the growth of apatite, improving the bioactivity of the material. This process helps to enhance the layers’ potential for biomedical applications.
Preliminary data on the adherence of MHA_Ch, MHA_Ch-8Gy, and MHA_Ch-30Gy layers on the Si substrate (before and after immersion in DMEM) were obtained using the tape-pull test method. This well-known test is a quick and simple way to assess layer adhesion. The results obtained for the studied layers revealed that the scotch tape came off almost clean, with an insignificant amount of material adhering to it. Consequently, these data suggests that a minimal amount of MHA_Ch, MHA_Ch-8Gy, and MHA_Ch-30Gy layers deposited on the Si substrate (before and after immersion in DMEM) were removed from the Si substrate. These results confirm that the MHA_Ch, MHA_Ch-8Gy, and MHA_Ch-30Gy layers deposited on the Si substrate (before and after immersion in DMEM) adhere well to the substrate. As can be seen, these results are in good agreement with those previously reported by Motelica-Heino, M. et al. [32].
Table 2 presents the layer thicknesses determined from SEM transversal cross-section studies. It can be observed that both processes (irradiation dose and immersion time) influence and modify the layer thickness.

Table 2.
Layer thickness determined from SEM transversal cross-section studies.
Through EDS studies, information on the chemical composition of unirradiated, 8 Gy, and 30 Gy irradiated MHA_Ch layers, kept for 7 and 14 days in DMEM, was obtained. The results of the EDS studies are presented in Figure 4, Figure 5 and Figure 6.
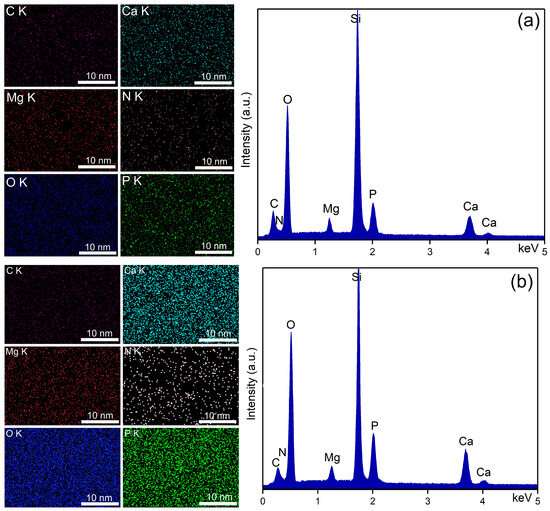
Figure 4.
Elemental distribution maps (left) and EDS spectra (right) characteristic for unirradiated MHA_Ch kept in DMEM for 7 days (a) and for 14 days (b).
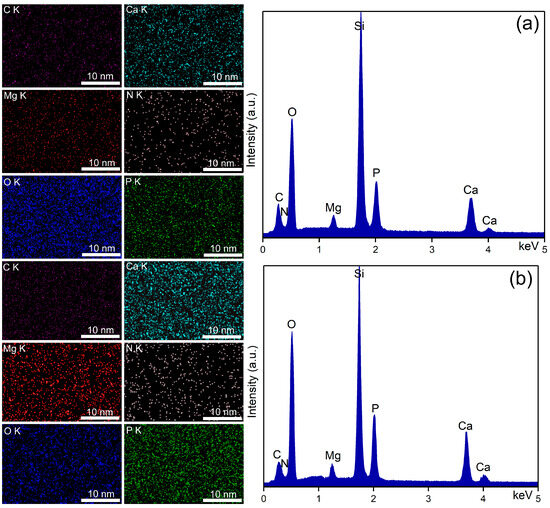
Figure 5.
Elemental distribution maps (left) and EDS spectra (right) characteristic for 8 Gy irradiated MHA_Ch kept in DMEM for 7 days (a) and for 14 days (b).
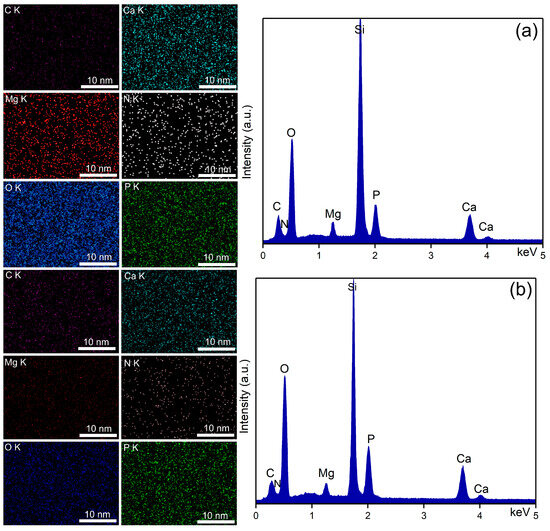
Figure 6.
Elemental distribution maps (left) and EDS spectra (right) characteristic for 30 Gy irradiated MHA_Ch kept in DMEM for 7 days (a) and for 14 days (b).
The EDS spectra of unirradiated and irradiated MHA_Ch layers kept for 7 and 14 days in DMEM confirmed the presence of calcium (Ca), phosphorus (P), nitrogen (N) oxygen (O), magnesium (Mg), and carbon (C), in the new apatite-like layer. No additional peaks were detected in any of the EDS spectra, confirming the purity of the analyzed thin films. Furthermore, an increase in the intensity of Ca and P lines was observed in the samples kept for 14 days in DMEM compared to the samples kept for 7 days.
Data on the compositional homogeneity across the surface of the studied samples were provided by elemental distribution maps. The elemental distribution maps of the unirradiated and irradiated MHA_Ch layers kept for 7 and 14 days in DMEM revealed that the chemical elements (Ca, P, N, O, Mg, and C) present in the surface composition of the newly formed apatite-like layer were uniformly distributed. These findings align with those reported in previous studies [33].
Data on the influence of DMEM exposure on the surface morphology of unirradiated MHA_Ch layers, 8 Gy irradiated MHA_Ch layers, and 30 Gy irradiated MHA_Ch layers were obtained by metallographic microscopy studies. The results of these studies, together with their 3D representations, are presented in Figure 7, Figure 8 and Figure 9. After 7 days of immersion in DMEM, the development of a new apatitic layer was noticeable for all the studied samples. Moreover, after 14 days of immersion in DMEM, a uniform distribution of the new apatitic layers across the MHA_Ch layers was observed. The metallographic microscopy images also revealed a change in the surface morphology aspects for the samples that were kept in DMEM compared to the deposited layers on the Si substrate. On the surface of the layers kept in DMEM, the presence of nano-agglomerates was observed. Another important feature revealed by the metallographic microscopy images was the absence of cracks on the surface of all the samples kept in DMEM for 7 and 14 days. Our data reveal that the irradiation process does not affect the formation of the new apatitic layer on the MHA_Ch layers. Therefore, the data obtained from metallographic microscopy studies support the findings from SEM and AFM measurements.
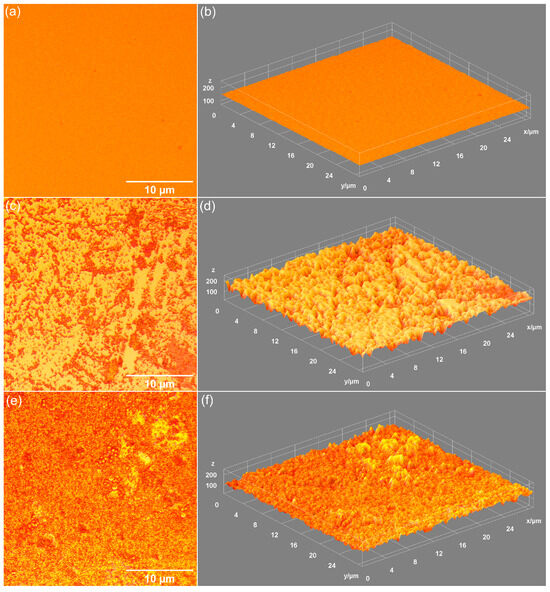
Figure 7.
Metallographic images of MHA_Ch (a), MHA_Ch-7D (c), and MHA_Ch-14D (e). 3D surface representation of metallographic images of unirradiated MHA_Ch before (b) and after immersion in DMEM for 7 days (d) and 14 days (f).
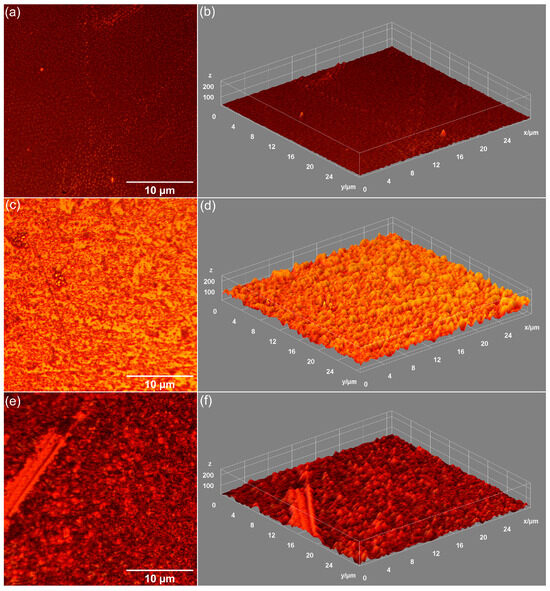
Figure 8.
Metallographic images of MHA_Ch-8Gy (a), MHA_Ch-8Gy-7D (c), and MHA_Ch-8Gy-14D (e). 3D surface representation of metallographic images of unirradiated MHA_Ch-8Gy before (b) and after immersion in DMEM for 7 days (d) and 14 days (f).
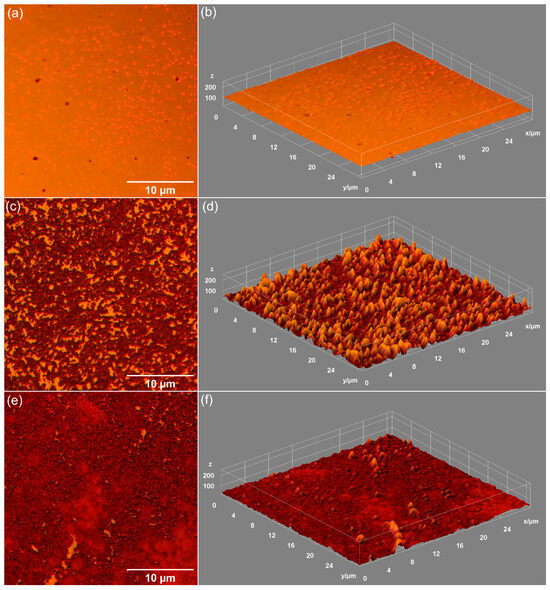
Figure 9.
Metallographic images of MHA_Ch-30Gy (a), MHA_Ch-30Gy-7D (c), and MHA_Ch-30Gy-14D (e). 3D surface representation of metallographic images of unirradiated MHA_Ch-30Gy before (b) and after immersion in DMEM for 7 days (d) and 14 days (f).
The FTIR data provide valuable information about the functional groups present in the studied samples. Additionally, the FTIR second derivative spectra improve the resolution of overlapping bands, enabling more accurate detection of subtle changes in chemical structures. Thus, Figure 10 reveals both the FTIR spectra (recorded between 450 and 2000 cm−1) and the FTIR second derivative spectra (obtained between 450 and 1250 cm−1).
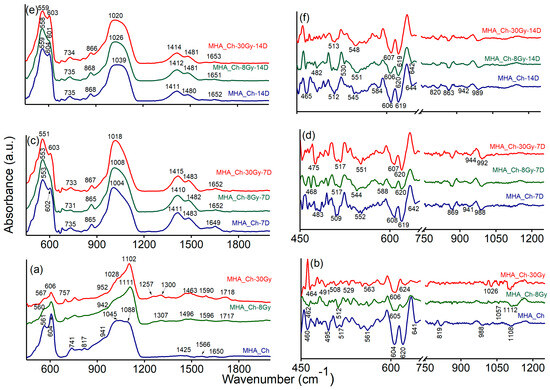
Figure 10.
FTIR spectra of: (a) MHA_Ch, MHA_Ch-8Gy, and MHA_Ch-30Gy; (c) MHA_Ch-7D, MHA_Ch-8Gy-7D, and MHA_Ch-30Gy-7D; (e) MHA_Ch-14D, MHA_Ch-8Gy-14D, and MHA_Ch-30Gy-14D; Second derivative spectra obtained for: (b) MHA_Ch, MHA_Ch-8Gy, and MHA_Ch-30Gy; (d) MHA_Ch-7D, MHA_Ch-8Gy-7D, and MHA_Ch-30Gy-7D; (f) MHA_Ch-14D, MHA_Ch-8Gy-14D, and MHA_Ch-30Gy-14D.
The active IR bands of MHA_Ch layers mainly appear in the 1200–500 cm−1 range, which can be assigned to the P-O vibrations in [PO4]3− groups. These groups have three characteristic frequencies, namely: 1200–900 cm−1 (ν3), 630–500 cm−1 (ν4), and ~470 cm−1 (ν2) [34,35,36].
The FTIR data obtained for the MHA_Ch, MHA_Ch-8Gy, and MHA_Ch-30Gy layers before immersion in DMEM are shown in Figure 10a. The general FTIR spectra of the three samples reveal the presence of bands primarily attributed to functional groups from HAp and chitosan structures. These results are in good agreement with the data previously reported by Bita, B., and collaborators [25], who indicated the absence of new bands in the FTIR spectra induced by irradiation of the samples with 2 and 50 Gy. The MHA_Ch exhibits an intense peak centered at 559 cm−1, which, together with the peak at 604 cm−1, can be assigned to the v4 degenerate state of phosphate groups. The band observed at around 868 cm−1 in the FTIR spectra of MHA_Ch, MHA_Ch-8Gy, and MHA_Ch-30Gy layers can be attributed to vibrational modes of carbonate (CO32−) groups. The large maxima observed at 1039 cm−1 in Figure 10 belongs to the asymmetric stretching vibration of phosphate (PO43−) groups. In the same spectra, three less intense maxima can be associated with the bending vibrations of carbonate groups (at 1411 cm−1 and 1480 cm−1) and the stretching vibration of C=O bonds (amide I) or N-H stretching of amide I from chitosan (at 1652 cm−1) [35,37].
Furthermore, the FTIR data obtained for the MHA_Ch layers irradiated with 8 Gy and 30 Gy (Figure 10) highlight the presence of the same vibrational bands (the position of the peaks is slightly shifted and accompanied by a narrowing of the bands as the radiation dose increases) observed in the FTIR spectra of the unirradiated layers. Hence, it can be concluded that increasing the irradiation dose results in the reorganization of phosphate groups in the apatite structure [25].
In Figure 10c,e, it can be observed that the IR spectra of unirradiated and irradiated MHA_Ch layers kept in DMEM exhibit broader bands than usual [25], mainly in the 1200–900 cm−1 and 630–500 cm−1 spectral range. This behavior can be attributed to the long period (7 and 14 days) of the layers in DMEM, as the ν3 and ν4 vibrational frequencies of P-O bonds in [PO4]3− groups are the most influenced by the surrounding medium [25]. Moreover, it is suggested that the broad band from 1100–900 cm−1 and the peaks at 1098 and 987 cm−1 result from the superposition of the following IR bands previously identified in the spectra of the MHA_Ch layer [25]: 1086 cm−1 (P-O asymmetric stretching vibrations of [PO4]3− groups), 1026 cm−1 (P-O symmetric stretching vibrations of [PO4]3− groups), 1004/991 cm−1 (C-O/C-H vibrations in chitosan), and C-O/C-H vibrations from DMEM.
In the FTIR spectra, between 2000 and 1300 cm−1, a weak IR band at 1652 cm−1 appears, characteristic of the stretching of the (-C=O-) bond in the amide I group or N-H stretching in amide I [34,37]. This IR band in the IR spectrum of the unirradiated sample kept for 7 days in Dulbecco’s medium (Figure 10c), shifts to 1649 cm−1 in the spectrum of the unirradiated sample kept for 14 days in Dulbecco’s medium (Figure 10e). The intense IR bands noticed around ~1400 cm−1 are characteristic of [CO3]2− group vibrations (ν3 vibrational mode) and are significantly decreased in the FTIR spectra of samples kept for 7 days in Dulbecco’s medium compared to those kept for 14 days. Furthermore, the IR bands observed around 1400 cm−1 are broader for samples kept for 7 days in DMEM compared to those kept for 14 days in the same medium.
The IR bands observed between 400 and 500 cm−1 can be attributed to the O-P-O bending mode of phosphate groups (ν2 vibration). On the other hand, the second derivative spectra obtained for all the analyzed samples are dominated by intense bands located at ~606, ~619, ~584, and ~552 cm−1, corresponding to P-O vibrations (bending) from phosphate groups (v4 degenerate state). Typically, the IR bands that belong to the ν3 asymmetric stretching mode of phosphate groups appear in the 1000–1100 cm−1 spectral range.
In both FTIR spectra and deconvoluted FTIR spectra obtained for all the samples, it is observed that the position of the IR bands of the samples kept for 14 days in the DMEM is slightly shifted compared to those kept for 7 days. Additionally, the IR bands are more intense for the samples kept in DMEM medium for 14 days compared to those kept for 7 days. These features likely occur due to the interaction of the MHA_Ch layers with Dulbecco’s medium and the formation of a new apatite layer. According to previous studies [32,38], the appearance of carbonate bands in the FTIR spectra highlights the substitution of phosphate groups with carbonate functional groups within the HAp structure, accompanied by the development of a new apatite layer.
Figure 11 presents the deconvoluted FTIR spectra obtained for unirradiated (Figure 11a–c), 8 Gy irradiated (Figure 11d–f), and 30 Gy (Figure 11g–i) irradiated MHA_Ch layers before and after immersion in DMEM for different times intervals (7 and 14 days). For the as-deposited MHA_Ch layers, eight subbands were required for a satisfactory fit (Figure 11a). For unirradiated MHA_Ch layers kept for 7 days in DMEM (Figure 11b), five subbands were needed to obtain a good fit, and for the samples kept for 14 days in DMEM, seven subbands were required.
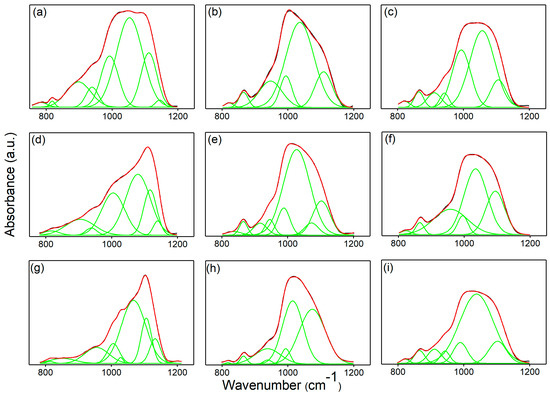
Figure 11.
FTIR deconvoluted spectra of MHA_Ch (a), MHA_Ch-7D (b), MHA_Ch-14D (c), MHA_Ch-8Gy (d) MHA_Ch-8Gy-7D (e), MHA_Ch-8Gy-14D (f), MHA_Ch-30Gy (g), MHA_Ch-30Gy-7D (h), and MHA_Ch-30Gy-14D (i).
For the 8 Gy irradiated MHA_Ch layers, the FTIR deconvolution analysis reveals that a satisfactory fit requires eight subbands for samples immersed in DMEM for 7 days and six subbands for samples immersed for 14 days (Figure 11e,f). Also, to achieve a satisfactory fit, seven subbands were needed for the as-deposited MHA_Ch layers irradiated at 8Gy.
For the 30 Gy irradiated MHA_Ch layers, the best fit was obtained using seven subbands for samples immersed in DMEM for 7 and 14 days (Figure 11h,i). On the other hand, for the as-deposited MHA_Ch layers irradiated at 30 Gy, the best fit was obtained with the aid of nine subbands.
For all studied layers, it can be observed that in the 750–1250 cm−1 spectral range, increasing the immersion period induces a decrease in the maxima intensity. Additionally, a broadening of the maxima is observed due to prolonged exposure to DMEM (14 days). Another notable effect is the slight shift in the maxima position, also induced by the immersion of MHA_Ch layers in DMEM. Moreover, in the same spectral region (750–1250 cm−1), it is observed that the vibrational bands in the MHA_Ch, MHA_Ch-8Gy, and MHA_Ch-30Gy layers become narrower with increasing irradiation dose, suggesting the recrystallization of the studied layers. These results are in agreement with previous reports [25,32].
In a study by Vlădescu, A. [38], it was shown that samples immersed in all biological solutions tested exhibited more or less visible carbonate bands. These bands were less intense for coatings immersed in SBF or PBS but more intense for those immersed in DMEM, likely due to the interaction between phosphate and carbonate ions. The authors also associated the decrease in peak intensity with a low degree of crystallinity of the new apatite phase formed on the coating’s surface [38]. According to the work conducted by Singh, G. and collaborators [39], the presence of carbonate groups in HAp (a functional group that naturally occurs in biomimetic hydroxyapatite) enhances the bioactivity of HAp coatings, promoting better cell attachment, proliferation, and differentiation [39]. Furthermore, the broadening or decrease in intensity of FTIR peaks suggests a reduction in the crystallinity of the samples, which could indicate the formation of more bioactive apatite phases [38,39]. The influence of surfaces with lower crystallinity on cell proliferation was studied by Smith, A. M. and coworkers [40], who found that surfaces with lower crystallinity could promote better cell attachment and higher proliferation rates [40].
The biological properties of MHA_Ch layers, unirradiated and irradiated with 8 and 30 Gy, were assessed before and after immersion in DMEM for 7 and 14 days with the aid of MG63 osteosarcoma cells. The in vitro assays were performed in triplicate, and the cell viability of MG63 cells after 24 and 48 h of incubation with the MHA_Ch, MHA_Ch-8Gy, and MHA_Ch-30Gy layers before and after immersion in DMEM for 7 and 14 days was evaluated via MTT assays. The results of the MTT studies are depicted in Figure 12a–c.
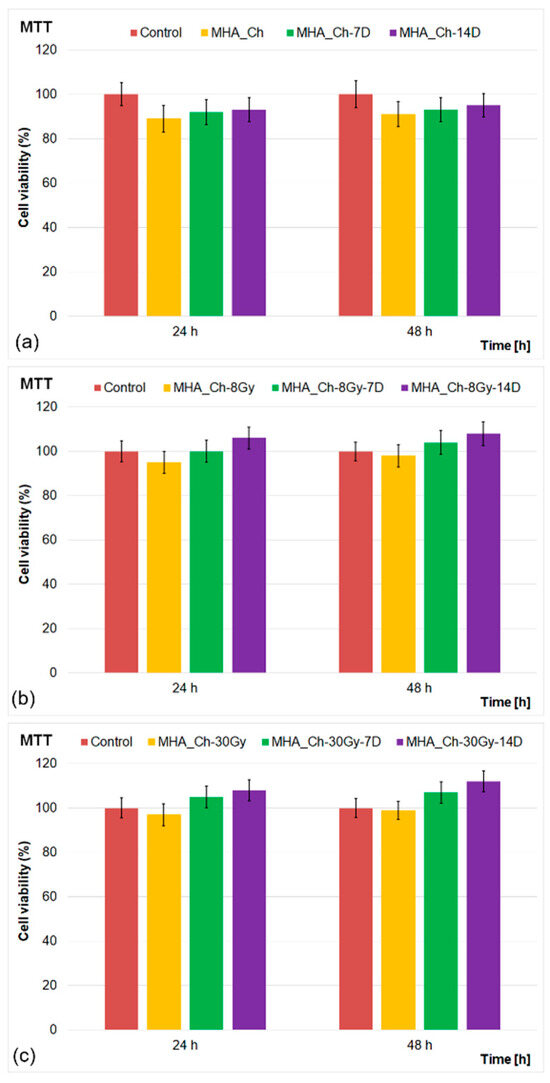
Figure 12.
MTT assay of MG63 cells incubated for 24 and 48 h with MHA_Ch (a), MHA_Ch-8Gy (b), and MHA_Ch-30Gy (c) layers before and after immersion in DMEM for 7 and 14 days. The results are represented as mean ± standard deviation (SD) and are expressed as percentages of control (100% viability).
The results of the MTT studies demonstrated excellent biocompatibility for all tested layers. The MG63 cell viability exceeded 89% for unirradiated layers after 24 h of incubation, increasing to 91% at 48 h. The data showed that the immersion in DMEM further enhanced cell viability, reaching 93% and 95% after 7 and 14 days, respectively. The irradiated layers displayed even higher biocompatibility, with cell viability exceeding 95% (8 Gy) and 97% (30 Gy) at 24 h, rising to 98% and 99% at 48 h. Notably, the irradiated layers immersed in DMEM exhibited the highest viability, reaching 104% (8 Gy) and 107% (30 Gy) after 48 h. The results of the cell viability assays highlighted the positive effects of DMEM immersion on the biological properties of the tested layers. This behavior could be attributed to the medium’s nutrient-rich composition, including essential amino acids, vitamins, and minerals, which promote cell proliferation and metabolic activity. The results of the MTT assays highlighted that no cytotoxic effects were observed for the tested samples, indicating the absence of toxic by-products from the layers. On the other hand, the findings suggested that the interaction between the layers and DMEM led to surface modifications that enhanced the MG63 cells’ attachment and growth on the layers’ surface. These results are attributed to the fact that the medium may have facilitated ion exchange at the material’s surface, potentially releasing bioactive ions (such as calcium and magnesium) into the medium. Calcium and magnesium ions are known to support osteogenic activity and enhance cell viability, contributing to the higher metabolic activity observed in the MTT assays [26,32,33,41,42,43,44]. Furthermore, the results of the cell viability assays revealed that immersion in DMEM for 7 and 14 days considerably improved the biological properties of the layers and modified the layer’s surface to create a favorable environment for cell attachment by promoting protein adsorption, a critical step for cellular adhesion and subsequent proliferation [26,32,33,41,42,43,44].
The results of the cell viability assays determined that the MgHA_Ch layers exhibited good biological activity toward MG63 cells. These results could be attributed to the release of bioactive magnesium (Mg2+) and calcium (Ca2+) ions through ion exchange when immersed in Dulbecco’s Modified Eagle Medium (DMEM), which promotes osteointegration. This process could help enhance bone regeneration by stimulating osteoblast proliferation, differentiation, and mineralization. Magnesium ions have the ability to improve hydroxyapatite deposition, while calcium ions help support bone matrix formation. Both of these processes are of great significance for implant stability. Additionally, it has been reported that Mg2+ aids in angiogenesis by fostering new blood vessel formation, which is needed for bone healing and tissue integration [45]. Beyond enhancing cell adhesion, the controlled release of these ions may also modulate inflammatory responses in surrounding tissues, leading to improved biological responses. Magnesium can further influence signaling mediated by the integrin protein, thus improving the cell’s cytoskeletal organization and cell adherence to the implant surface [46]. Therefore, the combined bioactivity of Mg2+ and Ca2+ makes MgHA_Ch layers highly effective for orthopedic and dental applications, ensuring improved bone bonding, faster healing, and enhanced implant longevity.
The increase in cell viability with longer immersion times suggests a cumulative effect of surface conditioning. Additionally, the MTT assays showed that gamma irradiation influenced the biological properties of the layers. Compared to unirradiated layers, those exposed to irradiation doses of 8 and 30 Gy exhibited an improvement in their structural and chemical stability, thus reducing the potential for material degradation and enhancing their interaction with the biological medium. This synergy between irradiation and DMEM immersion underscores the importance of these treatments in optimizing the biocompatibility of hydroxyapatite composite layers. Overall, the findings of the MTT assays indicate that immersion in DMEM combined with irradiation significantly enhances the biocompatibility of MHA_Ch layers. These results can be attributed to the synergistic effects of surface modifications, the release of bioactive ions, and the stabilization provided by the medium. These observations are consistent with previous studies demonstrating that such treatments effectively enhance the physico-chemical and biological properties of hydroxyapatite composite layers [26,32,33,41,42,43,44].
Complementary information on MG63 cell attachment and proliferation after 48 h of incubation with unirradiated and irradiated MHA_Ch layers (8 Gy and 30 Gy) immersed in DMEM for 7 and 14 days was obtained using atomic force microscopy (AFM). For this purpose, the layers were incubated with MG63 cell suspensions for 48 h. After 48 h of incubation, the cells were fixed on the surface of the layers and analyzed by AFM in non-contact mode under normal atmospheric conditions. AFM topographies were recorded over a 50 × 50 μm2 area, and the results of the scans are presented in Figure 13, Figure 14 and Figure 15.
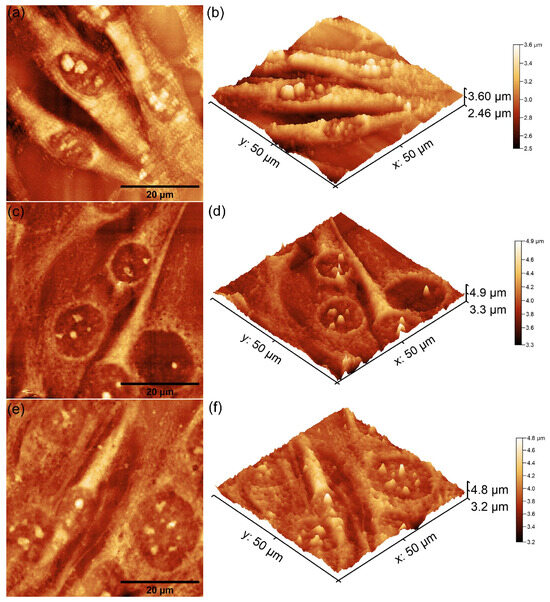
Figure 13.
Two-dimensional AFM topography of MG63 cells after 48 h of incubation with MHA_Ch unirradiated layers (a), those immersed in DMEM for 7 days (c), and those immersed for 14 days (e), along with their 3D representations (b,d,f).
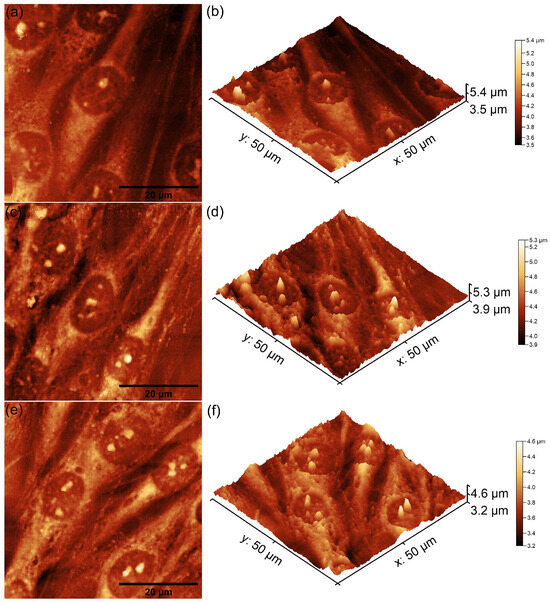
Figure 14.
Two-dimensional AFM topography of MG63 cells after 48 h of incubation with MHA_Ch layers irradiated with 8 Gy (a), those immersed in DMEM for 7 days (c), and those immersed for 14 days (e), along with their 3D representations (b,d,f).
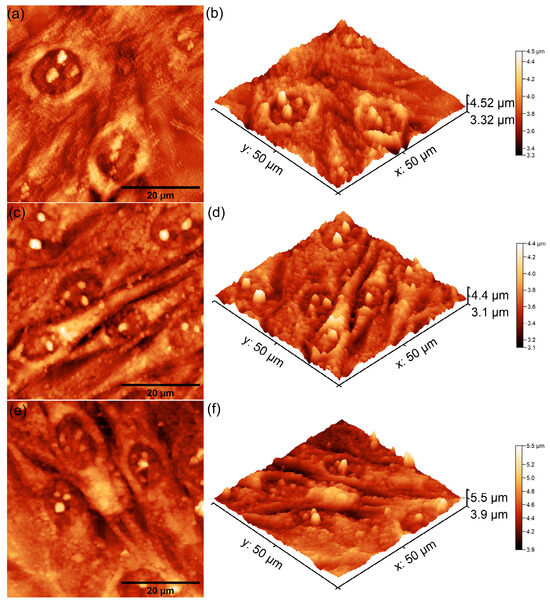
Figure 15.
Two-dimensional AFM topography of MG63 cells after 48 h of incubation with MHA_Ch layers irradiated with 30 Gy (a), those immersed in DMEM for 7 days (c), and those immersed for 14 days (e), along with their 3D representations (b,d,f).
The 2D AFM topographies revealed that MG63 cells adhered to all tested surfaces after 48 h of incubation. As reported by Knápek et al. [47], AFM studies conducted in non-contact mode provide reliable, non-destructive surface analysis, making it an effective technique for examining cell-surface interactions. Furthermore, the results of the AFM studies conducted on the unirradiated and 8 Gy-irradiated MHA_Ch layers revealed that the adhered cells exhibited an elongated and well-oriented fibroblastic morphology, which is typical of healthy cell attachment and proliferation (Figure 13 and Figure 14a–f). These observations suggest that the surface properties of the layers provided favorable conditions for MG63 cellular adherence and development. On the other hand, the 2D topographies of MG63 cells adhered on the surfaces of MHA_Ch layers irradiated at 30 Gy showed the appearance of some minor morphological alterations, including a rounder and more isolated appearance (Figure 15a–f), which deviates from the typical elongated morphology. These findings suggest that higher irradiation doses may influence surface biocompatibility, potentially altering cell attachment and proliferation. The 3D representations of the AFM topographies further confirmed these findings. Both 2D and 3D images highlighted that the unirradiated and 8 Gy-irradiated layers supported robust cell attachment and allowed the formation of well-aligned cell monolayers with fibroblastic morphology. In contrast, the results obtained for the layers irradiated with 30 Gy showed that the adhered cells exhibited minor changes in their cellular alignment, indicating a dose-dependent effect of gamma irradiation on biocompatibility. These findings align with previous studies on the impact of irradiation on the biological properties of magnesium-doped hydroxyapatite–chitosan composite layers [48,49]. Moreover, the AFM data emphasized that the immersion of MHA_Ch layers in DMEM for 7 and 14 days significantly influenced MG63 cell adherence and development. Due to its composition and enrichment with essential nutrients, amino acids, vitamins, and glucose, DMEM induced favorable modifications in the surface properties of MHA_Ch layers, promoting MG63 cell development. Biological media, including DMEM, are well known to facilitate ion exchange and also processes such as dissolution and reprecipitation on hydroxyapatite surfaces, leading to the formation of novel bioactive calcium phosphate phases. These modifications improve the surface properties of the layers and create a protein-rich coating that enhances cell adhesion and proliferation [32,33,43,44,50]. The AFM analysis showed that MG63 cells cultured on MHA_Ch layers immersed in DMEM for 7 and 14 days exhibited a well-organized, elongated fibroblastic morphology typical of healthy cells. Both the 2D AFM topographies as well as their 3D representations revealed the formation of cell monolayers on the layer surfaces. In contrast, cells adhered on MHA_Ch layers that were not immersed in DMEM displayed inconsistent attachment, fewer well-aligned cells, and also lower proliferation rates. These differences highlight the influence of surface modifications induced by DMEM immersion in improving the biocompatibility of the layers.
The results of the biological assays demonstrated that both irradiation and DMEM treatment of MHA_Ch layers significantly contributed to the enhancement of their biological activity. The results showed that unirradiated and 8 Gy-irradiated MHA_Ch layers exhibited better biological properties towards the development and proliferation of MG63 cells. This behavior could be attributed to the fact that irradiation has the ability to modify the structural and chemical properties of the layers, improving their surface characteristics to favor cell adhesion, proliferation, and differentiation, particularly for osteoblasts. Furthermore, irradiation can also induce controlled crosslinking of chitosan, enhancing the mechanical stability of the composite while promoting a more bioactive and osteoconductive surface. On the other hand, the use of irradiation may regulate the release of Mg2+ ions, which play an important role in stimulating bone growth and reducing inflammation [48,49]. These changes collectively improve the material’s overall biocompatibility and bioactivity under physiological conditions. Furthermore, DMEM exposure further enhanced the biological response of MHA_Ch layers by modifying surface morphology and chemistry, ensuring more effective interaction with the biological medium and cells [51,52,53,54,55]. DMEM exposure has the ability to regulate the degradation process of the layers, preventing excessive Mg2+ release while maintaining a stable and sustained ion exchange beneficial for bone tissue regeneration. Additionally, exposure to DMEM brings essential nutrients that support cellular activity and may reduce any initial cytotoxic effects, thereby improving the overall integration of the layer in a biological environment [51,52,53,54,55]. The synergistic effects of DMEM exposure and irradiation result in improved biological properties and an optimized microenvironment for bone healing, thus making MHA_Ch layers highly promising for use in orthopedic and dental applications.
Furthermore, the AFM analysis emphasized the influence of surface roughness on cell attachment and proliferation. The roughness of a surface affects the availability of anchorage points for cells, impacting their adhesion, morphology, and subsequent proliferation. The AFM analysis highlighted that changes in surface roughness induced by both irradiation as well as DMEM immersion facilitated strong cellular interaction between the surface and MG63 cells.
The roughness parameters determined from the AFM topographies offer important insights into the surface topography of biomaterials, as well as their surface physico-chemical and biological properties. An increase in surface roughness at the nanoscale and microscale has been reported to improve cell adhesion, proliferation, and differentiation [56,57,58]. This behavior can be attributed to the fact that a rougher surface has the ability to provide more surface area and topographical sites, thus promoting stronger focal adhesions via integrin proteins, which enhance cellular attachment onto the surface. This increased adhesion is particularly advantageous for biomaterials used in tissue engineering and implantable devices, as it offers a stable support for cell growth and development. Additionally, surface roughness influences cellular morphology and cytoskeletal organization of the adhered cells, playing a significant role in their proliferation and differentiation. Previous research conducted on this topic indicates that osteoblasts exhibit enhanced proliferation and increased expression of osteogenic markers when exposed to surfaces with micro- and nanoscale roughness [58,59,60,61,62]. For instance, in their study, Mendonça et al. [63] demonstrated that titanium surfaces with combined micro- and nanoscale features improved osteoblast differentiation and local factor production, suggesting potential benefits for implant osseointegration. Similarly, Wang et al. [64] reported that fibroblasts and endothelial cells adjust their morphology to align with the surface features of the samples, thus having the ability to improve wound healing and vascularization of surrounded tissue. Additionally, surface roughness can influence protein adsorption, which has a crucial role in mediating cell behavior. Increased roughness surface impacts the kinetics and structural conformation of extracellular matrix proteins such as fibronectin and vitronectin. These proteins have an important role in the mediation of cell adhesion by modulating integrin binding and activating signaling pathways [65]. These changes in their adsorption behavior and spatial arrangement can enhance integrin recognition, leading to improved cell attachment, proliferation, and differentiation. This helps create a more bioactive interface that enhances interactions between cells and the material’s surface, promoting better tissue integration, and reducing the occurrence of foreign body reactions. Consequently, the overall biocompatibility and functionality of the implanted material improve. In applications such as orthopedic and dental implants, an optimized roughness profile has been linked to faster osseointegration and improved long-term stability. Therefore, roughness parameters determined by AFM studies are essential for designing biomaterials with improved cell compatibility.
The literature indicates that increased roughness within a specific range enhances cell adhesion by providing micro-scale features for cell attachment, a crucial aspect of cell-surface interaction [32,33,43]. The AFM results, which depicted enhanced cell growth on immersed layers, are consistent with the MTT assay results, further emphasizing that the balanced composition of DMEM plays a crucial role in supporting cell adhesion and proliferation. These findings highlight the importance of combining surface roughness optimization with immersion in DMEM to maximize the biocompatibility of MHA_Ch layers for biomedical applications.
The results of the biological assays depict that the development of MHA_Ch layers using magnetron sputtering offers a highly precise and also environmentally cleaner alternative to the traditional wet chemical methods for obtaining thin layers with improved biological activity. This technique significantly reduces chemical waste, making it a sustainable option. However, it comes with notable challenges, such as the need for high energy consumption and the use of specialized equipment, which require initial investments and operational costs. These factors can limit large-scale production and also imply a careful consideration regarding its economic feasibility. Additionally, irradiation has a valuable complementary influence on the physico-chemical and biological properties of the layers by having the ability to enhance the material properties of MHA_Ch layers while enabling efficient batch processing. This capability improves its scalability, making it a promising approach for the future development of industrial applications. While the proposed process excels in precision and contamination control, its viability as part of an integrated manufacturing process depends on striking a balance between energy efficiency, capital investment, and production throughput. Optimizing these factors will have a crucial role in determining whether the combined use of magnetron sputtering and electron beam irradiation can be effectively scaled for industrial applications.
Layers based on hydroxyapatite have potential applications in cardiovascular stents, dental restorations, implantology, etc. According to studies reported in the literature, these types of layers could reduce thrombogenicity in stents [66,67] and support enamel remineralization and osseointegration in dental implants [68,69,70]. Previous studies reported that hydroxyapatite layers used on cardiovascular stents offer several advantages [66,67]. HAp is known for its excellent biocompatibility, which reduces the risk of adverse reactions when implanted in the body [66,67]. HAp-coated stents can be used for drug delivery, providing controlled release of therapeutic agents to prevent restenosis and promote healing [66,67]. Additionally, HAp layers can reduce the risk of blood clot formation, making them suitable for use in cardiovascular stents [66,67]. Hydroxyapatite coatings also have significant potential in dental applications [68,69,70]. HAp can be used to remineralize enamel, repair early-stage dental caries, and improve overall dental health [68,69,70]. According to previous studies, HAp layers used on dental implants can enhance osseointegration, promoting better integration with the surrounding bone and improving the longevity of the implants [68,69,70]. In conclusion, the irradiation of MgHAp layers and their immersion in DMEM can further enhance their properties. Irradiation can increase the surface roughness of HAp coatings, promoting better cell attachment and proliferation. Immersion in DMEM can lead to the formation of a bone-like apatite layer on the surface of MgHAp coatings, enhancing their bioactivity and making them more suitable for biomedical applications. However, before these layers can be widely used in such applications, further comprehensive studies, including in vivo assays, are required.
4. Conclusions
The MHA_Ch layers were developed using radio frequency magnetron sputtering technique and exposed to various irradiation doses. For the first time, unirradiated and irradiated MHA_Ch layers were immersed in DMEM for various time intervals. The results of SEM and MM studies revealed that both irradiation and immersion in DMEM significantly modified the layer’s surface topography. Moreover, the formation of a new apatite layer on the surface of the layers was confirmed by microscopy images. AFM studies provided significant insights into the influence of DMEM on MHA_Ch, MHA_Ch-8Gy, and MHA_Ch-30Gy. The results highlighted the medium’s role in modifying the layer’s surface morphology through ion exchange, biomimetic mineralization, and organic interactions. These findings emphasize the importance of understanding such interactions to optimize future materials for biomedical applications. The purity of the studied samples was confirmed by EDS results, while elemental mapping images demonstrated the homogeneous distribution of chemical elements across the layers. The results of the FTIR studies confirmed the presence of apatite in the studied layers. The results of biological assays highlighted that MHA_Ch, MHA_Ch-8Gy, and MHA_Ch-30Gy layers, both before and after immersion in DMEM for 7 and 14 days, were favorable to MG63 cell adhesion and proliferation. Furthermore, the results also demonstrated that the biological properties of the tested layers were strongly influenced by both immersion in DMEM and the applied irradiation doses. Preliminary data on the adhesion of the layers on the Si substrate highlighted their good adherence to the substrate. Atomic force microscopy (AFM) studies revealed that surface roughness of the layers also influenced cellular interactions. The unirradiated and 8 Gy-irradiated MHA_Ch layers exhibited surface textures favorable to MG63 cell attachment, leading to a well-organized, elongated fibroblastic morphology indicative of healthy cellular development. In contrast, layers exposed to higher irradiation doses, such as 30 Gy, exhibited altered surface roughness, resulting in minor morphological changes in the attached MG63 cells, indicating a dose-dependent modulation of the layers’ biocompatibility. These findings emphasize the influence of surface properties, particularly roughness, and the role of both irradiation parameters and DMEM exposure duration on cell development. The results highlight the potential of MHA_Ch layers as promising biomaterials for the development of future biomedical applications, particularly in the design of implantable devices where the tailored surface characteristics are essential for improving cell–material interactions. Overall, the synergistic effects of DMEM and irradiation on MHA_Ch layers represent a significant step forward in developing biomaterials for enhanced therapeutic applications, particularly in bone regeneration and implant coatings.
Author Contributions
C.B.: Conceptualization, validation, methodology, formal analysis, investigation, data curation, writing—original draft preparation, writing—review and editing, visualization, project administration; D.P.: resources, supervision, project administration, funding acquisition, visualization, validation, methodology, data curation, writing—original draft preparation, writing—review and editing, visualization; S.L.I.: Conceptualization, validation, software, methodology, formal analysis, investigation, data curation, writing—original draft preparation, writing—review and editing, visualization, project administration; C.S.C.: Conceptualization, validation, software, methodology, formal analysis, investigation, data curation, writing—original draft preparation, writing—review and editing, visualization, project administration; A.G.: Validation, formal analysis, investigation, data curation, writing—original draft preparation, writing—review and editing, visualization. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Core Program of the National Institute of Materials Physics, granted by the Romanian Ministry of Research, Innovation, and Digitalization through Project PC1-PN23080101.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author/s.
Acknowledgments
We thank Elena Stancu for performing sample irradiations with electron beams. This research was supported by the Romanian Ministry of Research and Innovation within the NUCLEU projects PN 19 15 01 01/2019 and Program LAPLAS VII—contract no. 30N/2023.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of this study; in the collection, analyses, or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results.
References
- Jeong, J.; Kim, J.H.; Shim, J.H.; Hwang, N.S.; Heo, C.Y. Bioactive Calcium Phosphate Materials and Applications in Bone Regeneration. Biomater. Res. 2019, 23, 4. [Google Scholar] [CrossRef] [PubMed]
- Filip, D.G.; Surdu, V.-A.; Paduraru, A.V.; Andronescu, E. Current Development in Biomaterials—Hydroxyapatite and Bioglass for Applications in Biomedical Field: A Review. J. Funct. Biomater. 2022, 13, 248. [Google Scholar] [CrossRef] [PubMed]
- Madupalli, H.; Pavan, B.; Tecklenburg, M.M.J. Carbonate substitution in the mineral component of bone: Discriminating the structural changes, simultaneously imposed by carbonate in A and B sites of apatite. J. Solid State Chem. 2017, 255, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Laptoiu, S.A.; Cojocaru, M.O.; Miculescu, M.; Branzei, M. Parameters Tailoring on the Deposition of Hydroxyapatite by Pulsed Electrical Discharge. Materials 2024, 17, 4583. [Google Scholar] [CrossRef]
- Gresita, A.; Raja, I.; Petcu, E.; Hadjiargyrou, M. Collagen-Coated Hyperelastic Bone Promotes Osteoblast Adhesion and Proliferation. Materials 2023, 16, 6996. [Google Scholar] [CrossRef]
- Veronesi, F.; Brogini, S.; De Luca, A.; Bellini, D.; Casagranda, V.; Fini, M.; Giavaresi, G. Cell Adhesion and Initial Bone Matrix Deposition on Titanium-Based Implants with Chitosan–Collagen Coatings: An In Vitro Study. Int. J. Mol. Sci. 2023, 24, 4810. [Google Scholar] [CrossRef] [PubMed]
- Parfenova, L.V.; Galimshina, Z.R.; Parfenov, E.V. Organic-Inorganic Biocompatible Coatings for Temporary and Permanent Metal Implants. Int. J. Mol. Sci. 2024, 25, 11623. [Google Scholar] [CrossRef]
- Krawiec, H.; Kozina, I.; Starowicz, M.; Lekka, M.; Zanella, C.; Fedrizzi, L.; Fedel, M.; Deflorian, F. Corrosion Rate and Mechanism of Degradation of Chitosan/TiO2 Coatings Deposited on MgZnCa Alloy in Hank’s Solution. Int. J. Mol. Sci. 2024, 25, 5313. [Google Scholar] [CrossRef]
- Kołodziejska, B.; Figat, R.; Kolmas, J. Biomimetic Apatite/Natural Polymer Composite Granules as Multifunctional Dental Tissue Regenerative Material. Int. J. Mol. Sci. 2023, 24, 16751. [Google Scholar] [CrossRef]
- McKee, T.J.; Komarova, S.V. Is it time to reinvent basic cell culture medium? Am. J. Physiol. Cell Physiol. 2017, 312, C624–C626. [Google Scholar] [CrossRef]
- Petrakova, N.V.; Teterina, A.Y.; Mikheeva, P.V.; Akhmedova, S.A.; Kuvshinova, E.A.; Sviridova, I.K.; Sergeeva, N.S.; Smirnov, I.V.; Fedotov, A.Y.; Kargin, Y.F.; et al. In Vitro Study of Octacalcium Phosphate Behavior in Different Model Solutions. ACS Omega 2021, 6, 7487–7498. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.T.; Leng, Y.; Chow, K.L.; Ren, F.; Ge, X.; Wang, K.; Lu, X. Cell culture medium as an alternative to conventional simulated body fluid. Acta Biomater. 2011, 7, 2615–2622. [Google Scholar] [CrossRef]
- Um, S.H.; Chung, Y.W.; Seo, Y.; Seo, H.; Ok, M.R.; Kim, Y.C.; Han, H.S.; Chung, J.J.; Edwards, J.R.; Jeon, H. Robust Hydroxyapatite Coating by Laser-Induced Hydrothermal Synthesis. Adv. Funct. Mater. 2020, 30, 2005233. [Google Scholar] [CrossRef]
- Faria, D.; Abreu, C.S.; Buciumeanu, M.; Dourado, N.; Carvalho, O.; Silva, F.S.; Miranda, G. Ti6Al4V laser surface preparation and functionalization using hydroxyapatite for biomedical applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 1534–1545. [Google Scholar] [CrossRef] [PubMed]
- Cojocaru, F.-D.; Gardikiotis, I.; Dodi, G.; Rotaru, A.; Balan, V.; Rezus, E.; Verestiuc, L. Polysaccharides-Calcium Phosphates Composite Beads as Bone Substitutes for Fractures Repair and Regeneration. Polymers 2023, 15, 1509. [Google Scholar] [CrossRef] [PubMed]
- Paul, B.; Lode, A.; Placht, A.M.; Voß, A.; Pilz, S.; Wolff, U.; Oswald, S.; Gebert, A.; Gelinsky, M.; Hufenbach, J. Cell-Material Interactions in Direct Contact Culture of Endothelial Cells on Biodegradable Iron-Based Stents Fabricated by Laser Powder Bed Fusion and Impact of Ion Release. ACS Appl. Mater. Interfaces 2022, 14, 439–451. [Google Scholar] [CrossRef]
- Islam, M.M.; Shahruzzaman, M.; Biswas, S.; Nurus Sakib, M.; Rashid, T.U. Chitosan Based Bioactive Materials in Tissue Engineering Applications—A Review. Bioact. Mater. 2020, 5, 164–183. [Google Scholar] [CrossRef]
- NCCN. Guidelines Version 2. 2020. Available online: https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf (accessed on 30 October 2024).
- Baskar, R.; Dai, J.; Wenlong, N.; Yeo, R.; Yeoh, K.-W. Biological response of cancer cells to radiation treatment. Front. Mol. Biosci. 2014, 1, 24. [Google Scholar] [CrossRef] [PubMed]
- Yarnold, J.R. 8 Gy Single Fraction Radiotherapy for the Treatment of Metastatic Skeletal Pain: Randomised Comparison with a Multifraction Schedule over 12 Months of Patient Follow-up. Bone Pain Trial Working Party. Radiother Oncol. 1999, 52, 111–121. [Google Scholar] [CrossRef]
- Foro Arnalot, P.; Fontanals, A.V.; Galcerán, J.C.; Lynd, F.; Latiesas, X.S.; de Dios, N.R.; Castillejo, A.R.; Bassols, M.L.; Galán, J.L.; Conejo, I.M.; et al. Randomized Clinical Trial with Two Palliative Radiotherapy Regimens in Painful Bone Metastases: 30 Gy in 10 Fractions Compared with 8 Gy in Single Fraction. Radiother. Oncol. 2008, 89, 150–155. [Google Scholar] [CrossRef]
- Debela, D.T.; Muzazu, S.G.Y.; Heraro, K.D.; Ndalama, M.T.; Mesele, B.W.; Haile, D.C.; Kitui, S.K.; Manyazewal, T. New Approaches and Procedures for Cancer Treatment: Current Perspectives. SAGE Open Med. 2021, 9, 20503121211034366. [Google Scholar] [CrossRef]
- Radiation Therapy to Treat Cancer, National Cancer Institute, Updated 9 January, 2019. Available online: https://www.cancer.gov/about-cancer/treatment/types/radiation-therapy/brachytherapy (accessed on 5 December 2024).
- Baskar, R.; Lee, K.A.; Yeo, R.; Yeoh, K.-W. Cancer and Radiation Therapy: Current Advances and Future Directions. Int. J. Med. Sci. 2012, 9, 193–199. [Google Scholar] [CrossRef]
- Bita, B.; Stancu, E.; Stroe, D.; Dumitrache, M.; Ciobanu, S.C.; Iconaru, S.L.; Predoi, D.; Groza, A. The Effects of Electron Beam Irradiation on the Morphological and Physicochemical Properties of Magnesium-Doped Hydroxyapatite/Chitosan Composite Coatings. Polymers 2022, 14, 582. [Google Scholar] [CrossRef] [PubMed]
- Predoi, D.; Ciobanu, C.S.; Iconaru, S.L.; Predoi, S.A.; Chifiriuc, M.C.; Raaen, S.; Badea, M.L.; Rokosz, K. Impact of Gamma Irradiation on the Properties of Magnesium-Doped Hydroxyapatite in Chitosan Matrix. Materials 2022, 15, 5372. [Google Scholar] [CrossRef] [PubMed]
- Gwyddion. Available online: http://gwyddion.net/ (accessed on 30 November 2022).
- ImageJ. Available online: http://imagej.nih.gov/ij (accessed on 20 November 2024).
- Iconaru, S.L.; Motelica-Heino, M.; Predoi, D. Study on europium-doped hydroxyapatite nanoparticles by Fourier transform infrared spectroscopy and their antimicrobial properties. J. Spectrosc. 2013, 2013, 284285. [Google Scholar] [CrossRef]
- Iconaru, S.L.; Predoi, D.; Ciobanu, C.S.; Motelica-Heino, M.; Guegan, R.; Bleotu, C. Development of Silver Doped Hydroxyapatite Thin Films for Biomedical Applications. Coatings 2022, 12, 341. [Google Scholar] [CrossRef]
- Cotrut, C.M.; Blidisel, A.; Vranceanu, D.M.; Vladescu, A.; Ungureanu, E.; Pana, I.; Dinu, M.; Vitelaru, C.; Parau, A.C.; Pruna, V.; et al. Evaluation of the In Vitro Behavior of Electrochemically Deposited Plate-like Crystal Hydroxyapatite Coatings. Biomimetics 2024, 9, 704. [Google Scholar] [CrossRef]
- Motelica-Heino, M.; Predoi, M.V.; Ciobanu, S.C.; Iconaru, S.L.; Predoi, D. Studies of New Layer Formation on the Surface of Zinc Doped Hydroxyapatite/Chitosan Composite Coatings in Biological Medium. Coatings 2023, 13, 472. [Google Scholar] [CrossRef]
- Predoi, D.; Ciobanu, S.C.; Iconaru, S.L.; Predoi, M.V. Influence of the Biological Medium on the Properties of Magnesium Doped Hydroxyapatite Composite Coatings. Coatings 2023, 13, 409. [Google Scholar] [CrossRef]
- Dreghici, D.B.; Butoi, B.; Predoi, D.; Iconaru, S.L.; Stoican, O.; Groza, A. Chitosan–Hydroxyapatite Composite Layers Generated in Radio Frequency Magnetron Sputtering Discharge: From Plasma to Structural and Mor-phological Analysis of Layers. Polymers 2020, 12, 3065. [Google Scholar] [CrossRef] [PubMed]
- Groza, A.; Dreghici, D.B.; Ganciu, M. Calcium Phosphate Layers Deposited on Thermal Sensitive Polymer Substrates in Radio Frequency Magnetron Plasma Discharge. Coatings 2019, 9, 709. [Google Scholar] [CrossRef]
- Berzina-Cimdina, L.; Borodajenko, N. Research of Calcium Phosphates Using Fourier Transform Infrared Spectroscopy. In Infrared Spectroscopy—Materials Science, Engineering and Technology; InTech: Rijeka, Croatia, 2012. [Google Scholar] [CrossRef]
- Zarif, M.E.; Bita, B.; Yehia-Alexe, S.A.; Negut, I.; Gradisteanu Pircalabioru, G.; Andronescu, E.; Groza, A. Biological and Physicochemical Analysis of Sr-Doped Hydroxyapatite/Chitosan Composite Layers. Polymers 2024, 16, 1922. [Google Scholar] [CrossRef] [PubMed]
- Vlădescu, A.; Pârâu, A.; Pană, I.; Cotruț, C.M.; Constantin, L.R.; Braic, V.; Vrânceanu, D.M. In Vitro Activity Assays of Sputtered HAp Coatings with SiC Addition in Various Simulated Biological Fluids. Coatings 2019, 9, 389. [Google Scholar] [CrossRef]
- Singh, G.; Singh, R.P.; Jolly, S.S. Customized hydroxyapatites for bone-tissue engineering and drug delivery applications: A review. J. Sol-Gel Sci. Technol. 2020, 94, 505–530. [Google Scholar] [CrossRef]
- Smith, A.M.; Paxton, J.Z.; Hung, Y.P.; Hadley, M.J.; Bowen, J.; Williams, R.L.; Grover, L.M. Nanoscale crystallinity modulates cell proliferation on plasma sprayed surfaces. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 48, 5–10. [Google Scholar] [CrossRef][Green Version]
- Lim, B.K.; Sun, F.; Ryu, S.C.; Koh, K.; Han, D.W.; Lee, J. Hydroxyapatite coating on damaged tooth surfaces by immersion. Biomed. Mater. 2009, 4, 025017. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Li, K.; Tielens, F.; Wang, J. Effect of sprayed techniques on the surface microstructure and in vitro behavior of nano-HAp coatings. Mater. Sci. Eng. C 2020, 117, 111318. [Google Scholar] [CrossRef] [PubMed]
- Janković, A.; Eraković, S.; Mitrić, M.; Matić, I.Z.; Juranić, Z.D.; Tsui, G.C.; Tang, C.Y.; Mišković-Stanković, V.; Rhee, K.Y.; Park, S.J. Bioactive hydroxyapatite/graphene composite coating and its corrosion stability in simulated body fluid. J. Alloys Compd. 2015, 624, 148–157. [Google Scholar] [CrossRef]
- Safavi, M.S.; Khalil-Allafi, J.; Restivo, E.; Ghalandarzadeh, A.; Hosseini, M.; Dacarro, G.; Malavasi, L.; Milella, A.; Listorti, A.; Visai, L. Enhanced in vitro immersion behavior and antibacterial activity of NiTi orthopedic biomaterial by HAp-Nb2O5 composite deposits. Sci. Rep. 2023, 13, 16045. [Google Scholar] [CrossRef] [PubMed]
- Hankenson, K.D.; Dishowitz, M.; Gray, C.; Schenker, M. Angiogenesis in bone regeneration. Injury 2011, 42, 556–561. [Google Scholar] [CrossRef]
- Cerqueira, A.; García-Arnáez, I.; Romero-Gavilán, F.; Azkargorta, M.; Elortza, F.; Martín de Llanos, J.J.; Carda, C.; Gurruchaga, M.; Goñi, I.; Sua, J. Complex effects of Mg-biomaterials on the osteoblast cell machinery: A proteomic study. Biomater. Adv. 2022, 137, 212826. [Google Scholar] [CrossRef] [PubMed]
- Knapek, A.; Sobola, D.; Tomanek, P.; Pokorna, Z.; Urbanek, M. Field emission from the surface of highly ordered pyrolytic graphite. Appl. Surf. Sci. 2017, 395, 157–161. [Google Scholar] [CrossRef]
- Predoi, D.; Ciobanu, C.S.; Iconaru, S.L.; Raaen, S.; Badea, M.L.; Rokosz, K. Physicochemical and Biological Evaluation of Chitosan-Coated Magnesium-Doped Hydroxyapatite Composite Layers Obtained by Vacuum Deposition. Coatings 2022, 12, 702. [Google Scholar] [CrossRef]
- Iconaru, S.L.; Ciobanu, C.S.; Predoi, G.; Rokosz, K.; Chifiriuc, M.C.; Bleotu, C.; Stanciu, G.; Hristu, R.; Raaen, S.; Raita, S.M.; et al. Biological and Physico-Chemical Properties of Composite Layers Based on Magnesium-Doped Hydroxyapatite in Chitosan Matrix. Micromachines 2022, 13, 1574. [Google Scholar] [CrossRef] [PubMed]
- El Hadad, A.A.G.; Barranco, V.; Jiménez-Morales, A.; Peón, E.; Hickman, G.J.; Perry, C.C.; Galván, J. Enhancing in vitro biocompatibility and corrosion protection of organic–inorganic hybrid sol–gel films with nanocrystalline hydroxyapatite. J. Mater. Chem. B 2014, 2, 3886–3896. [Google Scholar] [CrossRef]
- Shi, Y.; Pei, J.; Zhang, J.; Niu, J.; Zhang, H.; Guo, S.; Li, Z.; Yuan, G. Enhanced corrosion resistance and cytocompatibility of biodegradable Mg alloys by introduction of Mg(OH)2 particles into poly (L-lactic acid) coating. Sci. Rep. 2017, 7, 41796. [Google Scholar] [CrossRef] [PubMed]
- Posada, V.M.; Civantos, A.; Ramírez, J.; Fernández-Morales, P.; Allain, J.P. Tailoring adaptive bioresorbable Mg-based scaffolds with directed plasma nanosynthesis for enhanced osseointegration and tunable resorption. Appl. Surf. Sci. 2021, 550, 149388. [Google Scholar] [CrossRef]
- Zeller-Plumhoff, B.; Laipple, D.; Slominska, H.; Iskhakova, K.; Longo, E.; Hermann, A.; Flenner, S.; Greving, I.; Storm, M.; Willumeit-Römer, R. Evaluating the morphology of the degradation layer of pure magnesium via 3D imaging at resolutions below 40 nm. Bioact. Mater. 2021, 6, 4368–4376. [Google Scholar] [CrossRef] [PubMed]
- Agha, N.A.; Liu, Z.; Feyerabend, F.; Willumeit-Römer, R.; Gasharova, B.; Heidrich, S.; Mihailova, B. The effect of osteoblasts on the surface oxidation processes of biodegradable Mg and Mg-Ag alloys studied by synchrotron IR microspectroscopy. Mater. Sci. Eng. C 2018, 91, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.S.; Huang, H.H.; Tsai, Y.H.; Kuo, Y.L.; Lee, J.W.; Lee, Y.J.; Linn, T.Y.; Chen, P. Creating an extracellular matrix-like three-dimension structure to enhance the corrosion resistance and biological responses of titanium implants. J. Dent. Sci. 2024, 19 (Suppl. 1), S70–S80. [Google Scholar] [CrossRef]
- Zareidoost, A.; Yousefpour, M.; Ghaseme, B.; Amanzadeh, A. The relationship of surface roughness and cell response of chemical surface modification of titanium. J. Mater. Sci. Mater. Med. 2012, 23, 1479–1488. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.H.; Lee, H.H.; Lee, C.H. Substrate properties modulate cell membrane roughness by way of actin filaments. Sci. Rep. 2017, 7, 9068. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Xie, W.; Yu, L.; Camacho, L.C.; Nie, C.; Zhang, M.; Haag, R.; Wei, Q. Surface Roughness Gradients Reveal Topography-Specific Mechanosensitive Responses in Human Mesenchymal Stem Cells. Small 2020, 16, e1905422. [Google Scholar] [CrossRef] [PubMed]
- Gittens, R.A.; McLachlan, T.; Olivares-Navarrete, R.; Cai, Y.; Berner, S.; Tannenbaum, R.; Schwartz, Z.; Sandhage, K.H.; Boyan, B.D. The effects of combined micron-/submicron-scale surface roughness and nanoscale features on cell proliferation and differentiation. Biomaterials 2011, 32, 3395–3403. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, T.; Komatsu, K.; Cheng, J.; Park, G.; Ogawa, T. Beyond microroughness: Novel approaches to navigate osteoblast activity on implant surfaces. Int. J. Implant Dent. 2024, 10, 35. [Google Scholar] [CrossRef]
- Kieswetter, K.; Schwartz, Z.; Dean, D.D.; Boyan, B.D. The role of implant surface characteristics in the healing of bone. Crit. Rev. Oral Biol. Med. 1996, 7, 329–345. [Google Scholar] [CrossRef]
- Martin, J.Y.; Schwartz, Z.; Hummert, T.W.; Schraub, D.M.; Simpson, J.; Lankford, J., Jr.; Dean, D.D.; Cochran, D.L.; Boyan, B.D. Effect of titanium surface-roughness on proliferation, differentiation, and protein-synthesis of human osteoblast-like cells (MG63). J. Biomed. Mater. Res. 1995, 29, 389–401. [Google Scholar] [CrossRef]
- Mendonça, G.; Mendonça, D.B.S.; Simões, L.G.P.; Araújo, A.L.; Leite, E.R.; Duarte, W.R.; Aragão, F.J.L.; Cooper, L.F. The effects of implant surface nanoscale features on osteoblast-specific gene expression. Biomaterials 2009, 30, 4053–4062. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, M.; Zhang, W.; Liu, H.; Jin, F.; Mao, S.; Han, C.; Wang, X. Mechanical strategies to promote vascularization for tissue engineering and regenerative medicine. Burns Trauma 2024, 12, tkae039. [Google Scholar] [CrossRef]
- Degasne, I.; Baslé, M.F.; Demais, V.; Huré, G.; Lesourd, M.; Grolleau, B.; Mercier, L.; Chappard, D. Effects of roughness, fibronectin and vitronectin on attachment, spreading, and proliferation of human osteoblast-like cells (Saos-2) on titanium surfaces. Calcif. Tissue Int. 1999, 64, 499–507. [Google Scholar] [CrossRef]
- Rajtar, A.; Kaluza, G.L.; Yang, Q.; Hakimi, D.; Liu, D.; Tsui, M.; Lien, M.; Smith, D.; Clubb, F.J.; Troczynski, T. Hydroxyapatite-coated cardiovascular stents. EuroIntervention 2006, 2, 113–115. [Google Scholar] [PubMed]
- van der Giessen, W.J.; Sorop, O.; Serruys, P.W.; Peters-Krabbendam, I.; van Beusekom, H.M. Lowering the dose of sirolimus, released from a nonpolymeric hydroxyapatite coated coronary stent, reduces signs of delayed healing. JACC Cardiovasc. Interv. 2009, 2, 284–290. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Balhuc, S.; Campian, R.; Labunet, A.; Negucioiu, M.; Buduru, S.; Kui, A. Dental Applications of Systems Based on Hydroxyapatite Nanoparticles—An Evidence-Based Update. Crystals 2021, 11, 674. [Google Scholar] [CrossRef]
- Ittycheria, P.G.; George, T.; John, M.; Meenu, G.; Thomas, V.; Aswathy, S.; Kuriakose, R.; Thomas, J. Application of Hydroxyapatite in Regenerative Dentistry. In Novel Biomaterials for Tissue Engineering; IntechOpen: London, UK, 2024. [Google Scholar]
- Rau, J.V.; Cacciotti, I.; De Bonis, A.; Fosca, M.; Komlev, V.S.; Latini, A.; Santagata, A.; Teghil, R. Fe-doped hydroxyapatite coatings for orthopedic and dental implant applications. Appl. Surf. Sci. 2014, 307, 301–305. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).