Surface Engineering of Biodegradable Magnesium Alloys as Orthopedic Implant Materials: Recent Developments and Future Prospects
Abstract
1. Introduction
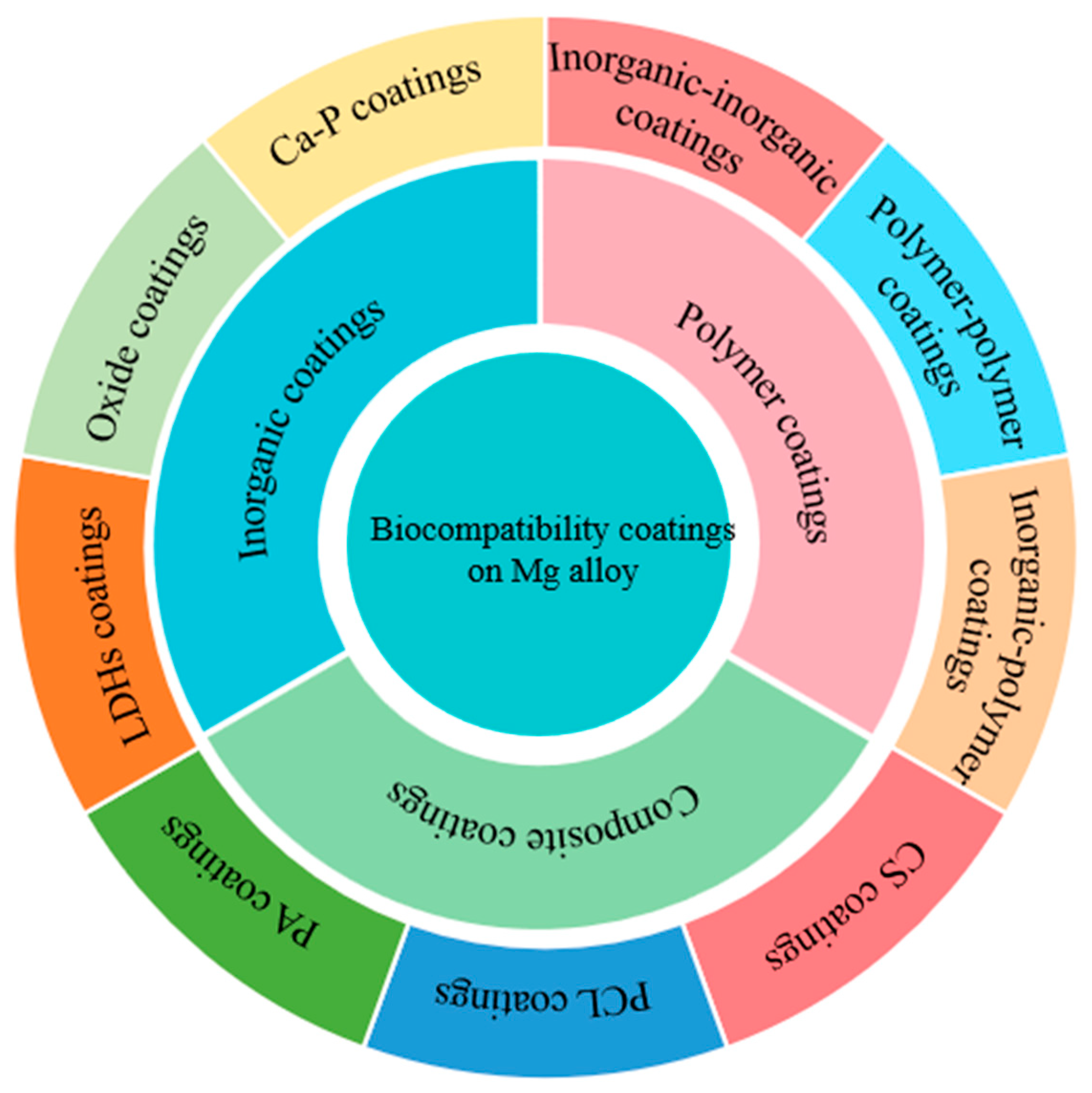
2. Inorganic Coatings
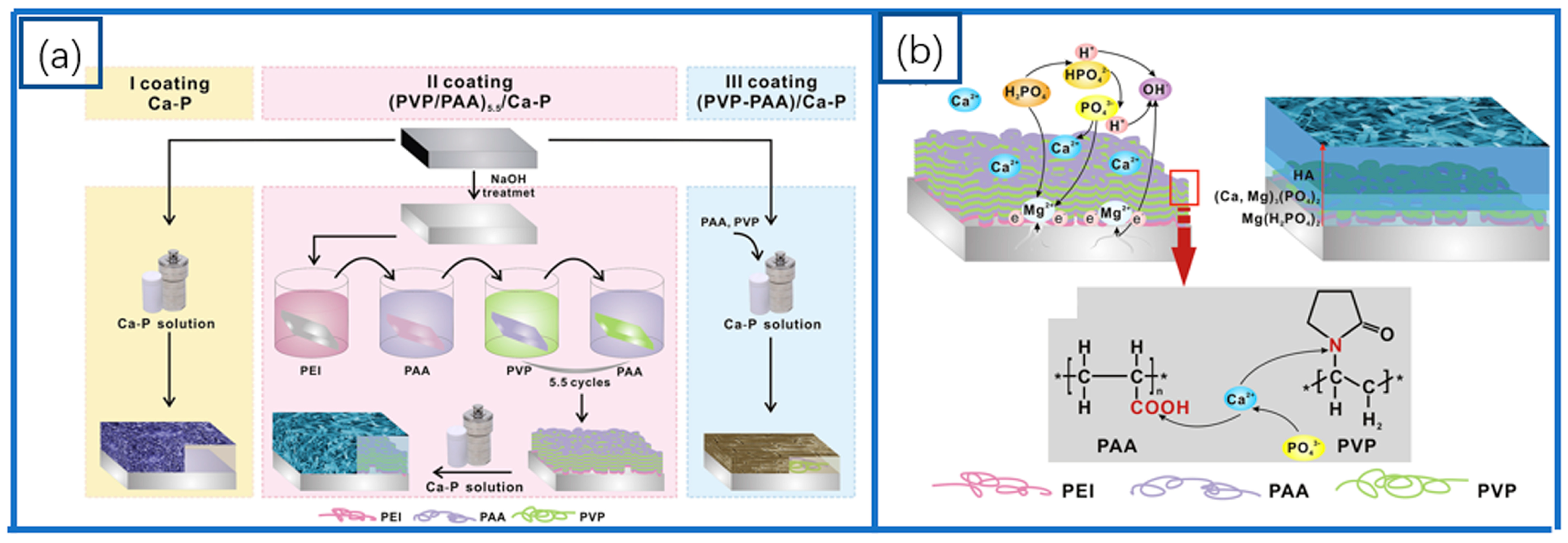
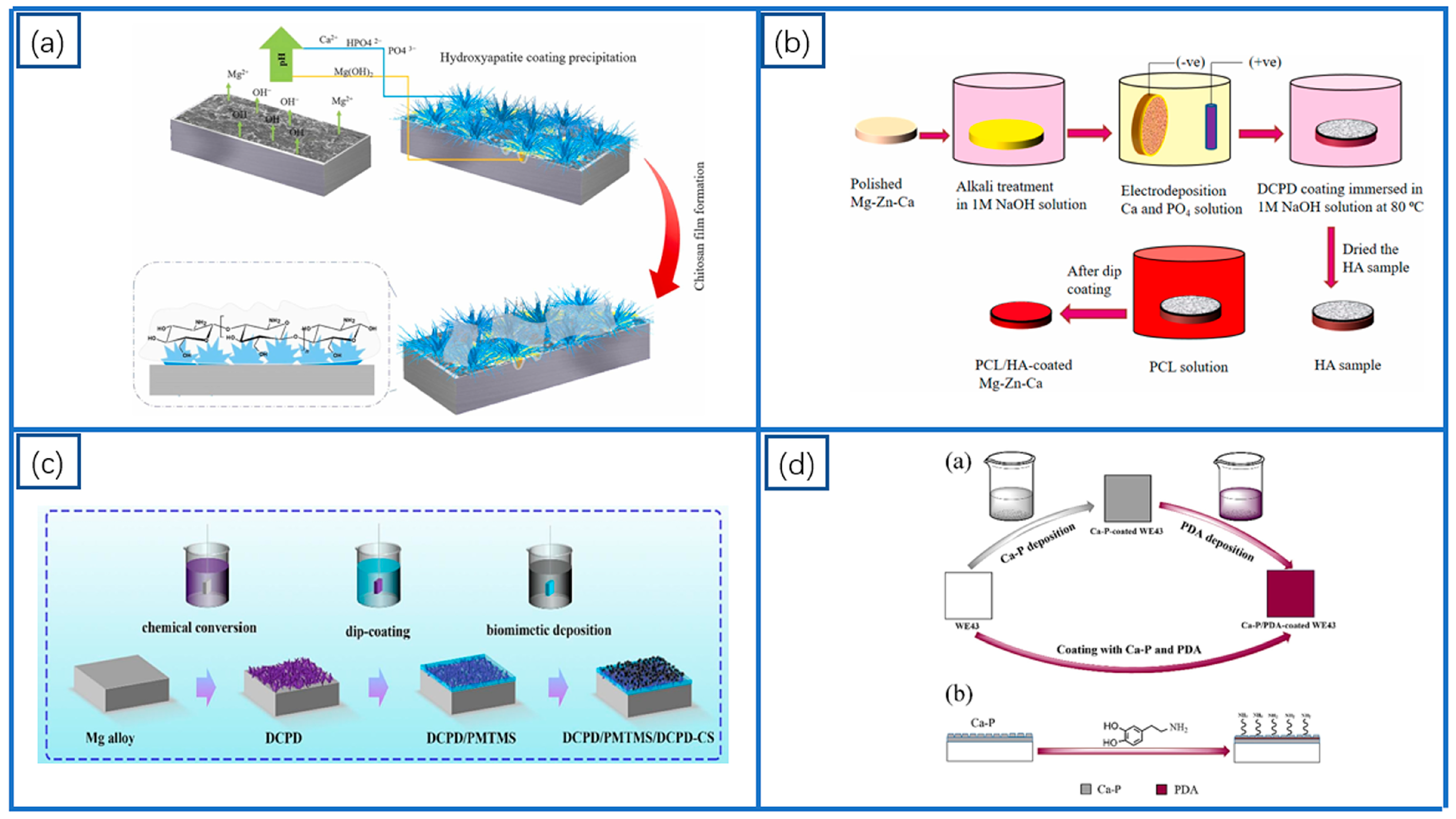
2.1. Ca-P-Based Coatings
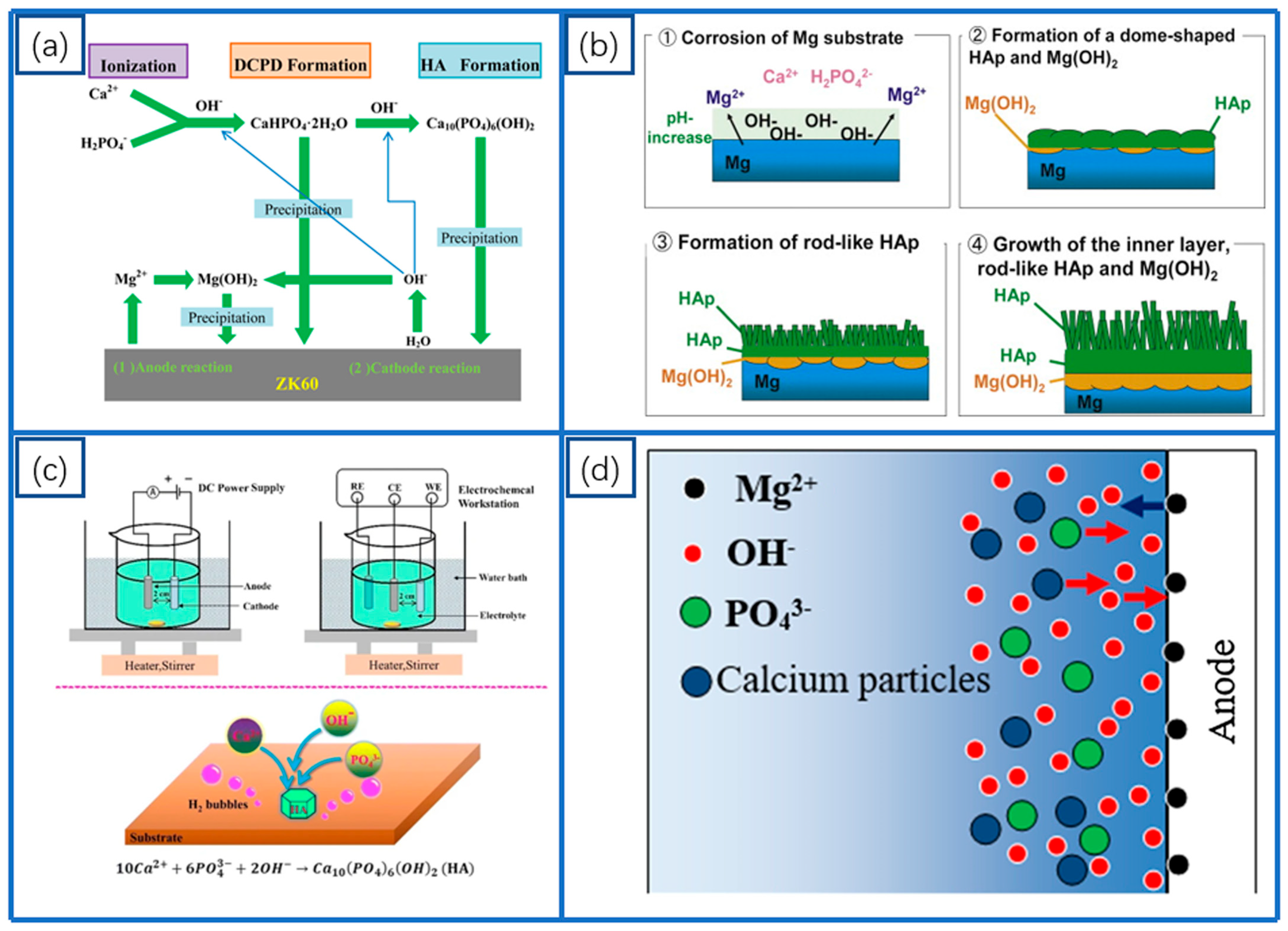
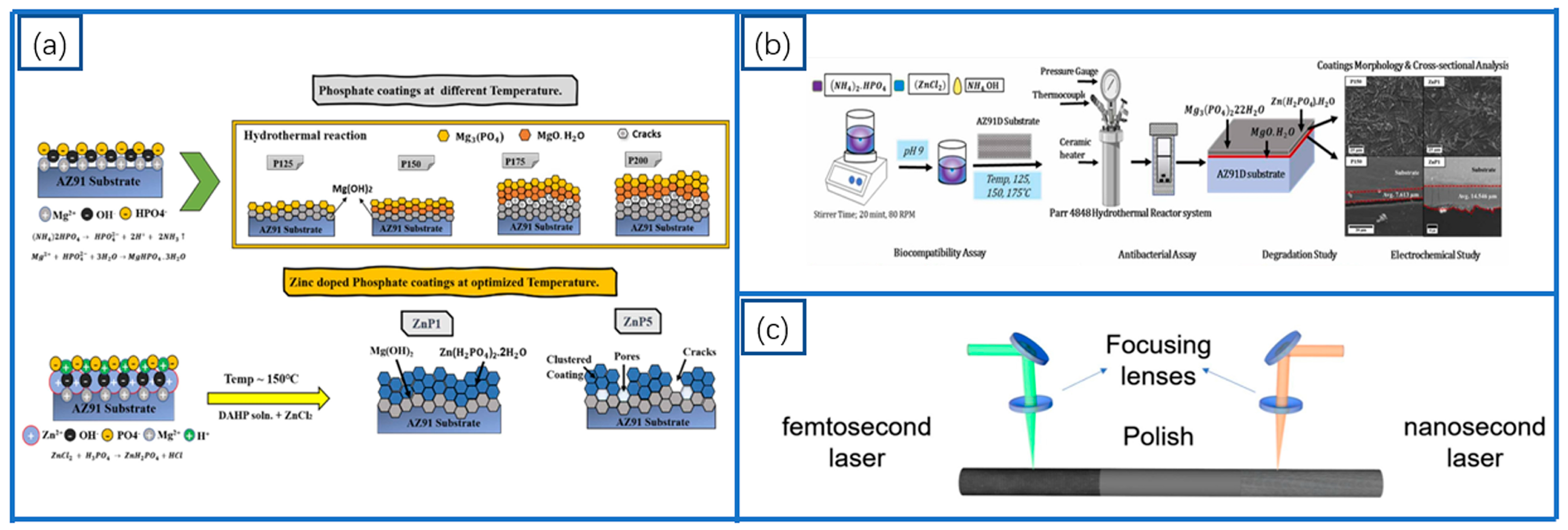
2.2. Oxide Coatings
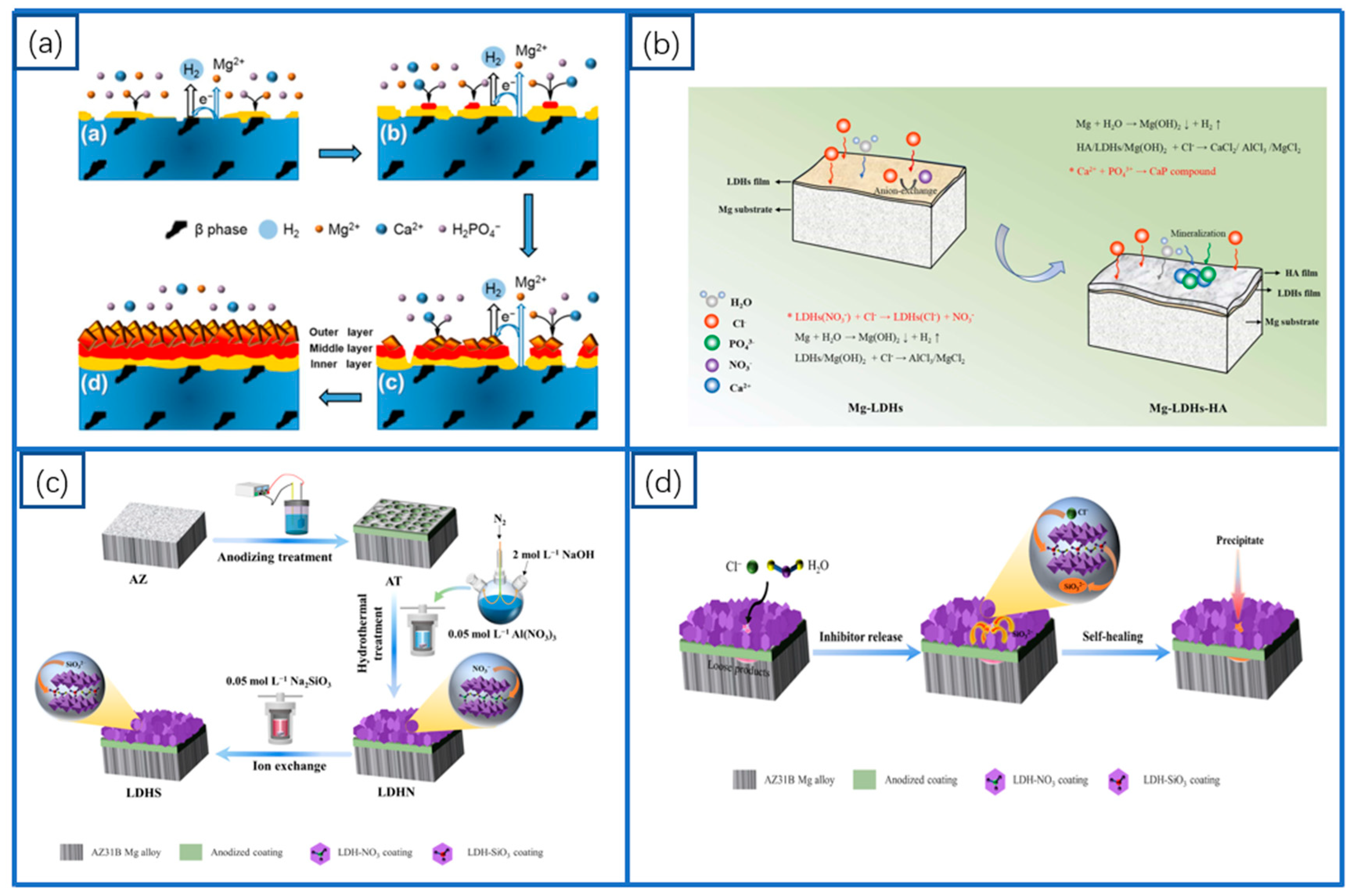
2.3. LDHs Coatings
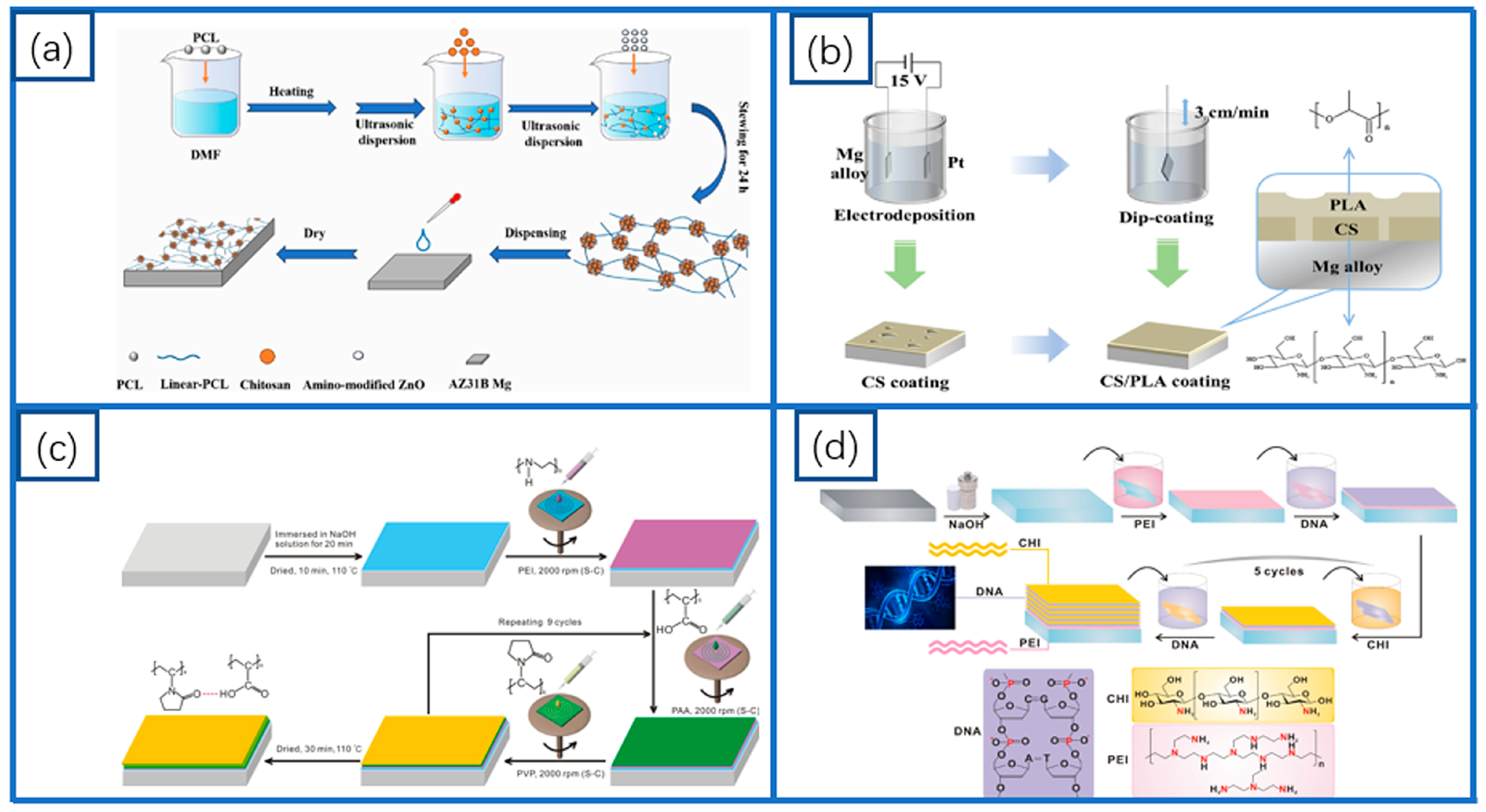
3. Polymer Coatings
3.1. Phytic Acid (PA) Coatings
3.2. Polycaprolactone (PCL) Coatings
3.3. Chitosan (CS) Coatings
4. Composite Coatings
4.1. Inorganic-Inorganic Composite Coatings
4.1.1. Ca-P-Based Composite Coatings
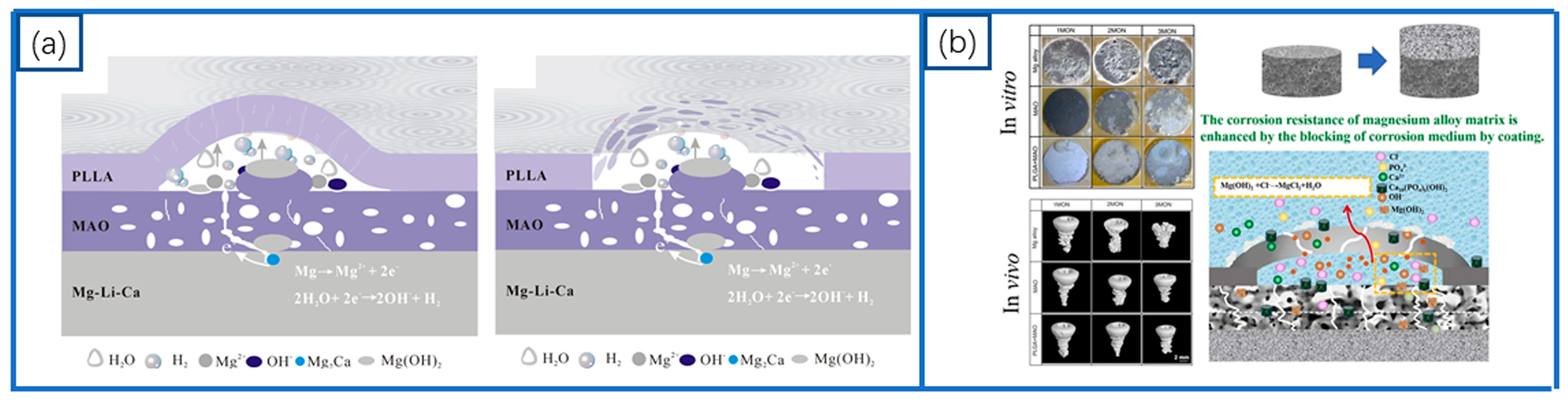
4.1.2. MAO-Based Composite Coatings

4.2. Polymer–Polymer Composite Coatings
4.3. Inorganic–Organic Composite Coatings
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vishnu, J.; Praveenkumar, K.; Kumar, A.A.; Nair, A.; Arjun, R.; Pillai, V.G.; Shankar, B.; Shankar, K.V. Multifunctional zinc oxide loaded stearic acid surfaces on biodegradable magnesium WE43 alloy with hydrophobic, self-cleaning and biocompatible attributes. Appl. Surf. Sci. 2025, 680, 161455. [Google Scholar] [CrossRef]
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and its alloys as orthopedic biomaterials: A review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef] [PubMed]
- Witte, F.; Fischer, J.; Nellesen, J.; Crostack, H.A.; Kaese, V.; Pisch, A.; Beckmann, F.; Windhagen, H. In vitro and in vivo corrosion measurements of magnesium alloys. Biomaterials 2006, 27, 1013–1018. [Google Scholar] [CrossRef] [PubMed]
- Taghipour, S.; Vakili-Tahami, F.; Chakherlou, T.N. Comparing the performance of a femoral shaft fracture fixation using implants with biodegradable and non-biodegradable materials. Biomed. Phys. Eng. Express 2024, 11, 015014. [Google Scholar] [CrossRef]
- Sousa, A.M.; Branco, R.V.; Morais, P.; Pereira, M.F.; Amaro, A.M.; Piedade, A.P. Evaluation of the interface of metallic-coated biodegradable polymeric stents with prokaryotic and eukaryotic cells. Bioact. Mater. 2025, 46, 55–81. [Google Scholar] [CrossRef]
- Waizy, H.; Weizbauer, A.; Maibaum, M.; Witte, F.; Windhagen, H.; Lucas, A.; Denkena, B.; Meyer-Lindenberg, A.; Thorey, F. Biomechanical characterisation of a degradable magnesium-based (MgCa0.8) screw. J. Mater. Sci. Mater. Med. 2012, 23, 649–655. [Google Scholar] [CrossRef]
- Wolters, L.; Besdo, S.; Angrisani, N.; Wriggers, P.; Hering, B.; Seitz, J.M.; Reifenrath, J. Degradation behaviour of LAE442-based plate-screw-systems in an in vitro bone model. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 49, 305–315. [Google Scholar] [CrossRef]
- On, S.W.; Cho, S.W.; Byun, S.H.; Yang, B.E. Bioabsorbable Osteofixation Materials for Maxillofacial Bone Surgery: A Review on Polymers and Magnesium-Based Materials. Biomedicines 2020, 8, 300. [Google Scholar] [CrossRef]
- Prasad, S.V.S.; Prasad, S.B.; Verma, K.; Mishra, R.K.; Kumar, V.; Singh, S. The role and significance of Magnesium in modern day research-A review. J. Magnes. Alloys 2022, 10, 1–61. [Google Scholar] [CrossRef]
- Zheng, Y.; Lu, J.; Liu, J.; Lu, Y.; Zhang, Z.; He, D.; Li, Y. Effect of glucose concentration on the corrosion behaviour of WE43 alloy in Hank’s balanced salt solution. Mater. Today Commun. 2025, 42, 111243. [Google Scholar] [CrossRef]
- Zhang, A.; Fan, X.; Yang, Z.; Xie, Y.; Wu, T.; Zhang, M.; Xue, Y.; Wang, Y.; Zhao, Y.; Wu, X.; et al. Optimized design and biomechanical evaluation of biodegradable magnesium alloy vascular stents. Acta Mech. Sin. 2025, 41, 624055. [Google Scholar] [CrossRef]
- Hashemi, T.S.; Jaiswal, S.; Celikin, M.; McCarthy, H.O.; Levingstone, T.J.; Dunne, N.J. Strategically designed bioactive dual-layer coating of octacalcium phosphate and dicalcium phosphate dihydrate for enhancement of the corrosion resistance of pure magnesium for orthopaedic applications. Surf. Coat. Technol. 2025, 495, 131556. [Google Scholar] [CrossRef]
- Ma, S.; Zhang, D.; Zhang, P.; Markert, B. Rapid prediction of the corrosion behaviour of coated biodegradable magnesium alloys using phase field simulation and machine learning. Comput. Mater. Sci. 2025, 247, 113546. [Google Scholar] [CrossRef]
- Ruggiero, R.; Marano, R.M.; Marrelli, B.; Facente, A.; Aiello, E.; Conte, R.; Serratore, G.; Ambrogio, G.; Paduano, F.; Tatullo, M. Enhancing magnesium-based materials for biomedical applications using an innovative strategy of combined single point incremental forming and bioactive coating. J. Mech. Behav. Biomed. Mater. 2025, 163, 106858. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Zhou, Y.; Zheng, X.; Cai, X.; Chen, Y.; Yang, W.; Wang, J.; Zhang, J.; Song, C. Structural optimization and in vitro corrosion analysis of biodegradable Mg-Nd-Zn-Zr alloy clip. J. Mech. Behav. Biomed. Mater. 2025, 161, 106790. [Google Scholar] [CrossRef]
- Vignesh, C.; Chockalingam, K.; Karthic, M.; Athithyan, K.C. Effect of β-TCP on the In Vitro Biocompatibility, Mechanical Properties, and Corrosion Resistance of Centrifugally Cast Mg-2Zn-1Mn Alloy for Orthopedic Implants. Int. J. Met. 2024; early access. [Google Scholar] [CrossRef]
- Xing, Y.; Tan, L.; Ma, Z.; Zhu, T.; Liang, G. Evaluating the Biodegradability and Cellular Compatibility of Mg-Zn-Nd Alloy for Vascular Stent Application. Adv. Biol. 2024, 8, 2400165. [Google Scholar] [CrossRef]
- Zhang, J.; Hou, S.; Zhang, M.; Zhang, S.; Li, W. Corrosion resistance and biocompatibility of silica coatings on AZ31 magnesium alloy via magnetron sputtering. Mater. Today Commun. 2024, 41, 110890. [Google Scholar] [CrossRef]
- On, S.-W.; An, H.-W.; Lee, S.M.; Choi, Y.I.; Woo, J.; Hong, S.O.; Choi, J.-Y. Safety and efficacy of Mg-Dy membrane with poly-L-lactic acid coating for guided bone regeneration. Sci. Rep. 2024, 14, 25522. [Google Scholar] [CrossRef]
- Meng, J.; Zhang, Y.; Yu, X.; Jiao, J.; Tan, L.; Yu, B. In vivo biodegradation and biological properties of a Mg-Zn-Ca amorphous alloy for bone defect repair. Mater. Technol. 2024, 39, 2307846. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, Y.; Zhao, Z.; Shi, X.; Zhang, R.; He, Y.; Zhang, H.; Wang, W. Magnesium-Doped Nano-Hydroxyapatite/Polyvinyl Alcohol/Chitosan Composite Hydrogel: Preparation and Characterization. Int. J. Nanomed. 2024, 19, 651–671. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, H.; Xu, X.; Chen, S.; Zhu, C.; Chen, K. Self-healing property and corrosion resistance of MgF2/polylactic acid/ amorphous calcium phosphate coating on biodegradable Mg alloy for medical implant. Ceram. Int. 2024, 50, 54835–54847. [Google Scholar] [CrossRef]
- Liu, J.; Yin, B.; Song, F.; Liu, B.; Peng, B.; Wen, P.; Tian, Y.; Zheng, Y.; Ma, X.; Wang, C. Improving corrosion resistance of additively manufactured WE43 magnesium alloy by high temperature oxidation for biodegradable applications. J. Magnes. Alloys 2024, 12, 940–953. [Google Scholar] [CrossRef]
- Kannan, M.B.; Walter, R.; Yamamoto, A. Biocompatibility and in Vitro Degradation Behavior of Magnesium-Calcium Alloy Coated with Calcium Phosphate Using an Unconventional Electrolyte. ACS Biomater. Sci. Eng. 2016, 2, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Cesarz-Andraczke, K.; Nowosielski, R.; Basiaga, M.; Babilas, R. Study of the Morphology and Properties of Biocompatible Ca-P Coatings on Mg Alloy. Materials 2019, 13, 2. [Google Scholar] [CrossRef]
- Trang, L.T.; Le, H.V.; Hiromoto, S.; Minho, O.; Kobayashi, E.; Nguyen, N.V.; Cao, N.Q. In vitro cellular biocompatibility and in vivo degradation behavior of calcium phosphate-coated ZK60 magnesium alloy. Biomed. Mater. 2023, 18, 035003. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, Z.; Xu, X.; He, Y.; Zhang, B. Improved corrosion resistance and biocompatibility of a calcium phosphate coating on a magnesium alloy for orthopedic applications. Eur. J. Inflamm. 2016, 14, 169–183. [Google Scholar] [CrossRef]
- Wei, J.; Cai, S.; Li, Q.; Xie, Y.; Bao, X.; Xu, G. Controllable Synthesis of Nanostructured Ca-P Coating on Magnesium Alloys via Sodium Citrate Template-Assisted Hydrothermal Method and Its Corrosion Resistance. Coatings 2020, 10, 1232. [Google Scholar] [CrossRef]
- Zaludin, M.A.F.; Jamal, Z.A.Z.; Derman, M.N.; Kasmuin, M.Z. Corrosion analysis of hydroxyapatite coated magnesium in simulated body fluid (SBF), phosphate buffered saline (PBS), and Ringer’s solution. Mater. Today Proc. 2019, 16, 1686–1691. [Google Scholar] [CrossRef]
- Yang, D.Y.; Gray-Munro, J.E. Influence of coating bath chemistry on biomimetic calcium phosphate coatings. Emerg. Mater. Res. 2013, 2, 263–273. [Google Scholar] [CrossRef]
- Yang, H.; Xia, K.; Wang, T.; Niu, J.; Song, Y.; Xiong, Z.; Zheng, K.; Wei, S.; Lu, W. Growth, in vitro biodegradation and cytocompatibility properties of nano-hydroxyapatite coatings on biodegradable magnesium alloys. J. Alloys Compd. 2016, 672, 366–373. [Google Scholar] [CrossRef]
- Tomozawa, M.; Hiromoto, S. Growth mechanism of hydroxyapatite-coatings formed on pure magnesium and corrosion behavior of the coated magnesium. Appl. Surf. Sci. 2011, 257, 8253–8257. [Google Scholar] [CrossRef]
- Tang, H.; Han, Y.; Wu, T.; Tao, W.; Jian, X.; Wu, Y.; Xu, F. Synthesis and properties of hydroxyapatite-containing coating on AZ31 magnesium alloy by micro-arc oxidation. Appl. Surf. Sci. 2017, 400, 391–404. [Google Scholar] [CrossRef]
- Singh, B.; Singh, G.; Sidhu, B.S.; Bhatia, N. In-vitro assessment of HA-Nb coating on Mg alloy ZK60 for biomedical applications. Mater. Chem. Phys. 2019, 231, 138–149. [Google Scholar] [CrossRef]
- Shen, S.; Cai, S.; Bao, X.; Xu, P.; Li, Y.; Jiang, S.; Xu, G. Biomimetic fluoridated hydroxyapatite coating with micron/nano-topography on magnesium alloy for orthopaedic application. Chem. Eng. J. 2018, 339, 7–13. [Google Scholar] [CrossRef]
- Su, Y.; Lu, C.; Hu, X.; Guo, Y.; Xun, X.; Zhang, Z.; Li, G.; Lian, J.; Ren, L. Improving the Degradation Resistance and Surface Biomineralization Ability of Calcium Phosphate Coatings on a Biodegradable Magnesium Alloy via a Sol-Gel Spin Coating Method. J. Electrochem. Soc. 2018, 165, C155–C161. [Google Scholar] [CrossRef]
- Wei, S.; Dapeng, Z.; Peng, S.; Hemin, N.; Yuan, Z.; Jincheng, T. Strontium-doped Hydroxyapatite Coatings Deposited on Mg-4Zn Alloy: Physical-chemical Properties and in vitro Cell Response. Rare Met. Mater. Eng. 2018, 47, 2371–2380. [Google Scholar] [CrossRef]
- Sedelnikova, M.B.; Sharkeev, Y.P.; Tolkacheva, T.V.; Khimich, M.A.; Bakina, O.V.; Fomenko, A.N.; Kazakbaeva, A.A.; Fadeeva, I.V.; Egorkin, V.S.; Gnedenkov, S.V.; et al. Comparative Study of the Structure, Properties, and Corrosion Behavior of Sr-Containing Biocoatings on Mg0.8Ca. Materials 2020, 13, 1942. [Google Scholar] [CrossRef]
- Lu, Y.; Tan, L.; Zhang, B.; Lin, J.; Yang, K. Synthesis and characterization of Ca–Sr–P coating on pure magnesium for biomedical application. Ceram. Int. 2014, 40, 4559–4565. [Google Scholar] [CrossRef]
- Dehghanian, C.; Aboudzadeh, N.; Shokrgozar, M.A. Characterization of silicon- substituted nano hydroxyapatite coating on magnesium alloy for biomaterial application. Mater. Chem. Phys. 2018, 203, 27–33. [Google Scholar] [CrossRef]
- Su, Y.; Su, Y.; Zai, W.; Li, G.; Wen, C. In Vitro Degradation Behaviors of Manganese-Calcium Phosphate Coatings on an Mg-Ca-Zn Alloy. Scanning 2018, 2018, 6268579. [Google Scholar] [CrossRef]
- Antoniac, I.V.; Filipescu, M.; Barbaro, K.; Bonciu, A.; Birjega, R.; Cotrut, C.M.; Galvano, E.; Fosca, M.; Fadeeva, I.V.; Vadalà, G.; et al. Iron Ion-Doped Tricalcium Phosphate Coatings Improve the Properties of Biodegradable Magnesium Alloys for Biomedical Implant Application. Adv. Mater. Interfaces 2020, 7, 2000531. [Google Scholar] [CrossRef]
- Singh, B.; Singh, G.; Sidhu, B.S. Analysis of corrosion behaviour and surface properties of plasma-sprayed composite coating of hydroxyapatite–tantalum on biodegradable Mg alloy ZK60. J. Compos. Mater. 2019, 53, 2661–2673. [Google Scholar] [CrossRef]
- Wang, X.; Guo, L.; Liu, X.; Dai, Y.; She, J.; Zhang, D.; Qi, F.; Wei, W.; Ouyang, X. Enhanced corrosion resistance and cell activity of magnesium alloy by DCPD/MgHPO4.3H2O coating via one-step chemical conversion. Surf. Coat. Technol. 2024, 476, 130228. [Google Scholar] [CrossRef]
- Pana, I.; Parau, A.C.; Cotrut, C.M.; Dinu, M.; Vranceanu, D.M.; Kiss, A.E.; Serratore, G.; Boehner, D.A.; Vitelaru, C.; Ambrogio, G.; et al. Influence of deposition temperature on the structure and functional properties of Mg doped hydroxyapatite coatings deposited on manufactured AZ31B alloy substrates by RF magnetron sputtering. Ceram. Int. 2023, 49, 22340–22354. [Google Scholar] [CrossRef]
- Kalaiyarasan, M.; Bharathi, S.M.; Rajendran, N.; Bargavi, P.; Ramya, R.; Saranya, K.; Kumar, B.S.; Pratibha, R. Synergetic effect of Fe/Ag ions incorporated HAp on AZ31 Mg alloy to improve corrosion resistance and osteogenic activity. J. Alloys Compd. 2023, 960, 170711. [Google Scholar] [CrossRef]
- Hernandez, L.; Gonzalez, J.E.; Barranco, V.; Veranes-Pantoja, Y.; Galvan, J.C.; Rodriguez Gattorno, G. Biomimetic hydroxyapatite (HAp) coatings on pure Mg and their physiological corrosion behavior. Ceram. Int. 2022, 48, 1208–1222. [Google Scholar] [CrossRef]
- Su, Y.; Li, K.; Zhu, X.; Wang, C.; Zhang, Y. Microwave-hydrothermal method post-treatment of sprayed Ca-P coating. Ceram. Int. 2019, 45, 874–884. [Google Scholar] [CrossRef]
- Gao, Y.E.; Zhao, L.F.; Yao, X.H.; Hang, R.Q.; Zhang, X.Y.; Tang, B. Corrosion behavior of porous ZrO2 ceramic coating on AZ31B magnesium alloy. Surf. Coat. Technol. 2018, 349, 434–441. [Google Scholar] [CrossRef]
- Amiri, H.; Mohammadi, I.; Afshar, A. Electrophoretic deposition of nano-zirconia coating on AZ91D magnesium alloy for bio-corrosion control purposes. Surf. Coat. Technol. 2017, 311, 182–190. [Google Scholar] [CrossRef]
- Gao, Y.L.; Jie, M.; Liu, Y. Mechanical properties of Al2O3 ceramic coatings prepared by plasma spraying on magnesium alloy. Surf. Coat. Technol. 2017, 315, 214–219. [Google Scholar] [CrossRef]
- Ji, R.N.; Ma, M.Y.; He, Y.T.; Liu, C.X.; Fang, T.; Zhang, Z.; Wang, Y.; He, Y.D.; Wu, J.S. Improved corrosion resistance of Al2O3 ceramic coatings on AZ31 magnesium alloy fabricated through cathode plasma electrolytic deposition combined with surface pore-sealing treatment. Ceram. Int. 2018, 44, 15192–15199. [Google Scholar] [CrossRef]
- Kong, J.; Kolooshani, A.; Kolahdouz, A.; Nejad, M.G.; Toghraie, D. Fabrication and characterization of magnesium implants coated with magnetic nanoparticles-wollastonite-hydroxyapatite for medical and sports injury applications: Finite element analysis. Ceram. Int. 2024, 50, 5755–5765. [Google Scholar] [CrossRef]
- Kotoka, R.; Yarmolenko, S.; Pai, D.; Sankar, J. Corrosion Behavior of Reactive Sputtered Al2O3 and ZrO2 Thin Films on Mg Disk Immersed in Saline Solution. J. Mater. Sci. Technol. 2015, 31, 873–880. [Google Scholar] [CrossRef]
- Yang, F.; Chang, R.; Webster, T.J. Atomic Layer Deposition Coating of TiO2 Nano-Thin Films on Magnesium-Zinc Alloys to Enhance Cytocompatibility for Bioresorbable Vascular Stents. Int. J. Nanomed. 2019, 14, 9955–9970. [Google Scholar] [CrossRef]
- Mujahid, K.; Iqbal, F.; Ali, A.; Butt, M.; Bukhari, N.; Nosheen, S.; Sharif, F.; Abbas, Z. Zinc-doped phosphate coatings for enhanced corrosion resistance, antibacterial properties, and biocompatibility of AZ91D Mg alloy. J. Alloys Compd. 2024, 1005, 176025. [Google Scholar] [CrossRef]
- Liu, S.; Chen, P.; Yang, T.; Xia, C.; Liang, C.; Liu, N. Laser-zoned treatment of magnesium surfaces with predictable degradation applications. Surf. Coat. Technol. 2024, 494, 131300. [Google Scholar] [CrossRef]
- Jiang, P.; Zeng, Z.; Hou, R.; Mei, D.; Zhu, S.; Wang, L.; Guan, S. Tunable corrosion protection of calcium carbonate (CaCO3) coating on biomedical Mg2Zn0.2Ca alloy. Mater. Des. 2022, 222, 111073. [Google Scholar] [CrossRef]
- Li, J.X.; Chen, J.X.; Siddiqui, M.A.; Kolawole, S.K.; Yang, Y.; Shen, Y.; Yang, J.P.; Wang, J.H.; Su, X.P. Enhancing Corrosion Resistance and Antibacterial Properties of ZK60 Magnesium Alloy Using Micro-Arc Oxidation Coating Containing Nano-Zinc Oxide. Acta Metall. Sin.-Engl. Lett. 2024; early access. [Google Scholar] [CrossRef]
- Gu, Z.; Atherton, J.J.; Xu, Z.P. Hierarchical layered double hydroxide nanocomposites: Structure, synthesis and applications. Chem. Commun. 2015, 51, 3024–3036. [Google Scholar] [CrossRef]
- Wu, F.X.; Liang, J.; Peng, Z.J.; Liu, B.X. Electrochemical deposition and characterization of Zn-Al layered double hydroxides (LDHs) films on magnesium alloy. Appl. Surf. Sci. 2014, 313, 834–840. [Google Scholar] [CrossRef]
- Peng, F.; Li, H.; Wang, D.; Tian, P.; Tian, Y.; Yuan, G.; Xu, D.; Liu, X. Enhanced Corrosion Resistance and Biocompatibility of Magnesium Alloy by Mg-Al-Layered Double Hydroxide. ACS Appl. Mater. Interfaces 2016, 8, 35033–35044. [Google Scholar] [CrossRef]
- Zheng, B.; Ou, J.; Li, H.; Zhou, Z.; Qin, A.; Zhang, K.; Wang, X. Preparation of phosphate ion-doped Zn-Fe-layered double hydroxide with corrosion resistance and inducing Ca-P deposition on AZ31 Mg alloy. J. Mater. Res. 2022, 37, 763–772. [Google Scholar] [CrossRef]
- Yang, H.M.; Liu, X.; Wang, J.H.; Hu, W.B. Preparation and Corrosion Resistance of ZIF-8-(5, 6-dimethylbenzimidazole)/LDHs Composite Film on Magnesium Alloy. Int. J. Electrochem. Sci. 2020, 15, 12203–12219. [Google Scholar] [CrossRef]
- Wu, L.; Ding, X.; Zheng, Z.C.; Ma, Y.L.; Atrens, A.; Chen, X.B.; Xie, Z.H.; Sun, D.E.; Pan, F.S. Fabrication and characterization of an actively protective Mg-Al LDHs/Al2O3 composite coating on magnesium alloy AZ31. Appl. Surf. Sci. 2019, 487, 558–568. [Google Scholar] [CrossRef]
- Li, J.; He, N.; Li, J.; Fu, Q.; Feng, M.; Jin, W.; Li, W.; Xiao, Y.; Yu, Z.; Chu, P.K. A silicate-loaded MgAl LDH self-healing coating on biomedical Mg alloys for corrosion retardation and cytocompatibility enhancement. Surf. Coat. Technol. 2022, 439, 128442. [Google Scholar] [CrossRef]
- Su, Y.; Su, Y.; Lu, Y.; Lian, J.; Li, G. Composite Microstructure and Formation Mechanism of Calcium Phosphate Conversion Coating on Magnesium Alloy. J. Electrochem. Soc. 2016, 163, G138–G143. [Google Scholar] [CrossRef]
- Zhu, D.; Wang, J.; Hu, W. Hydroxyapatite film prepared by hydrothermal method on layered double hydroxides coated Mg Alloy and its corrosion resistance. Colloids Surf. A Physicochem. Eng. Asp. 2022, 647, 129075. [Google Scholar] [CrossRef]
- Chen, J.; Wu, L.; Ding, X.X.; Liu, Q.; Dai, X.; Song, J.F.; Jiang, B.; Atrens, A.; Pan, F.S. Effects of deformation processes on morphology, microstructure and corrosion resistance of LDHs films on magnesium alloy AZ31. J. Mater. Sci. Technol. 2021, 64, 10–20. [Google Scholar] [CrossRef]
- Zhang, X.C.; Zhong, F.; Li, X.P.; Liu, B.; Zhang, C.Y.; Buhe, B.; Zhang, T.; Meng, G.Z.; Wang, F.H. The effect of hot extrusion on the microstructure and anti-corrosion performance of LDHs conversion coating on AZ91D magnesium alloy. J. Alloys Compd. 2019, 788, 756–767. [Google Scholar] [CrossRef]
- Zhang, X.C.; Wang, J.X.; Zhang, C.Y.; Liu, B.; Jiang, P.; Zhao, Y.; Buhe, B.; Zhang, T.; Meng, G.Z.; Wang, F.H. Formation Process of an LDHs Coating on Magnesium Alloy by a CO2 Pressurization Method. Coatings 2019, 9, 47. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, H.; Yuan, D.; Chen, S.; Zhu, C.; Chen, K. The effect of polylactic acid ordering on the long-term corrosion protection capacity of biodegradable magnesium alloys. Int. J. Biol. Macromol. 2024, 282, 135549. [Google Scholar] [CrossRef]
- Amani, S.; Faraji, G. Processing and Properties of Biodegradable Magnesium Microtubes for Using as Vascular Stents: A Brief Review. Met. Mater. Int. 2019, 25, 1341–1359. [Google Scholar] [CrossRef]
- Tabia, Z.; Akhtach, S.; Bricha, M.; El Mabrouk, K. Tailoring the biodegradability and bioactivity of green-electrospun polycaprolactone fibers by incorporation of bioactive glass nanoparticles for guided bone regeneration. Eur. Polym. J. 2021, 161, 110841. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, B.; Jia, Z.; Lu, X.; Fang, L.; Wang, K.; Ren, F. Polydopamine mediated assembly of hydroxyapatite nanoparticles and bone morphogenetic protein-2 on magnesium alloys for enhanced corrosion resistance and bone regeneration. J. Biomed. Mater. Res. Part A 2017, 105, 2750–2761. [Google Scholar] [CrossRef]
- He, P.; Zhao, Y.; Wang, B.; Liu, G.; Zhang, L.; Li, M.; Xu, B.; Cai, W.; Chu, C.; Cong, Y. A biodegradable magnesium phosphate cement incorporating chitosan and rhBMP-2 designed for bone defect repair. J. Orthop. Transl. 2024, 49, 167–180. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, L.; Yu, K.; Chen, C.; Dai, Y.; Qiao, X.; Yan, Y. Biodegradation performance of a chitosan coated magnesium-zinc-tricalcium phosphate composite as an implant. Biointerphases 2014, 9, 031004. [Google Scholar] [CrossRef]
- Kikuchi, M.; Ikoma, T.; Itoh, S.; Matsumoto, H.N.; Koyama, Y.; Takakuda, K.; Shinomiya, K.; Tanaka, J. Biomimetic synthesis of bone-like nanocomposites using the self-organization mechanism of hydroxyapatite and collagen. Compos. Sci. Technol. 2004, 64, 819–825. [Google Scholar] [CrossRef]
- Hernández-Alvarado, L.A.; Hernández, L.S.; Lomelí, M.A.; Miranda, J.M.; Narváez, L.; Diaz, I.; Escudero, M.L. Phytic acid coating on Mg-based materials for biodegradable temporary endoprosthetic applications. J. Alloys Compd. 2016, 664, 609–618. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, X.; Zhao, S.; Maitz, M.F.; Zhang, W.; Yang, S.; Mao, J.; Huang, N.; Wan, G. In situ incorporation of heparin/bivalirudin into a phytic acid coating on biodegradable magnesium with improved anticorrosion and biocompatible properties. J. Mater. Chem. B 2017, 5, 4162–4176. [Google Scholar] [CrossRef]
- Tang, X.; Zhang, X.; Chen, Y.Q.; Zhang, W.T.; Qian, J.Y.; Soliman, H.; Qu, A.; Liu, Q.J.; Pu, S.M.; Huang, N.; et al. Ultraviolet irradiation assisted liquid phase deposited titanium dioxide (TiO2)-incorporated into phytic acid coating on magnesium for slowing-down biodegradation and improving osteo-compatibility. Mat. Sci. Eng. C-Mater. 2020, 108, 110487. [Google Scholar] [CrossRef]
- Li, Y.; Cai, S.; Shen, S.B.; Xu, G.H.; Zhang, F.Y.; Wang, F.W. Self-healing hybrid coating of phytic acid/silane for improving the corrosion resistance of magnesium alloy. J. Coat. Technol. Res. 2018, 15, 571–581. [Google Scholar] [CrossRef]
- Degner, J.; Singer, F.; Cordero, L.; Boccaccini, A.R.; Virtanen, S. Electrochemical investigations of magnesium in DMEM with biodegradable polycaprolactone coating as corrosion barrier. Appl. Surf. Sci. 2013, 282, 264–270. [Google Scholar] [CrossRef]
- Palanisamy, M.S.; Kulandaivelu, R.; Nellaiappan, S.N.T.S. Improving the corrosion resistance and bioactivity of magnesium by a carbonate conversion-polycaprolactone duplex coating approach. New J. Chem 2020, 44, 4772–4785. [Google Scholar] [CrossRef]
- Niu, J.L.; Liu, H.Y.; Ping, X.; Xun, X.C.; Li, G.Y. Silane coupling agent (SCA) pretreatment and polycaprolactone (PCL) coating for enhanced corrosion resistance for magnesium. J. Coat. Technol. Res. 2019, 16, 125–133. [Google Scholar] [CrossRef]
- Carangelo, A.; Acquesta, A.; Monetta, T. In-vitro corrosion of AZ31 magnesium alloys by using a polydopamine coating. Bioact. Mater. 2019, 4, 71–78. [Google Scholar] [CrossRef]
- Yang, Y.; Zheng, K.; Liang, R.; Mainka, A.; Taccardi, N.; Roether, J.A.; Detsch, R.; Goldmann, W.H.; Virtanen, S.; Boccaccini, A.R. Cu-releasing bioactive glass/polycaprolactone coating on Mg with antibacterial and anticorrosive properties for bone tissue engineering. Biomed. Mater. 2017, 13, 015001. [Google Scholar] [CrossRef]
- Zomorodian, A.; Santos, C.; Carmezim, M.J.; Silva, T.M.E.; Fernandes, J.C.S.; Montemor, M.F. “In-vitro” corrosion behaviour of the magnesium alloy with Al and Zn (AZ31) protected with a biodegradable polycaprolactone coating loaded with hydroxyapatite and cephalexin. Electrochim. Acta 2015, 179, 431–440. [Google Scholar] [CrossRef]
- Liangjian, C.; Jun, Z.; Kun, Y.; Chang, C.; Yilong, D.; Xueyan, Q.; Zhiming, Y. Improving of in vitro Biodegradation Resistance in a Chitosan Coated Magnesium Bio-composite. Rare Met. Mater. Eng. 2015, 44, 1862–1865. [Google Scholar] [CrossRef]
- Dai, Y.L.; Yu, K.; Chen, L.J.; Chen, C.; Qiao, X.Y.; Yan, Y. In vitro corrosion behavior and cytotoxicity property of magnesium matrix composite with chitosan coating. J. Cent. South Univ. 2015, 22, 829–834. [Google Scholar] [CrossRef]
- Höhlinger, M.; Heise, S.; Wagener, V.; Boccaccini, A.R.; Virtanen, S. Developing surface pre-treatments for electrophoretic deposition of biofunctional chitosan-bioactive glass coatings on a WE43 magnesium alloy. Appl. Surf. Sci. 2017, 405, 441–448. [Google Scholar] [CrossRef]
- Francis, A.; Yang, Y.; Boccaccini, A.R. A new strategy for developing chitosan conversion coating on magnesium substrates for orthopedic implants. Appl. Surf. Sci. 2019, 466, 854–862. [Google Scholar] [CrossRef]
- Cui, L.Y.; Xu, J.; Lu, N.; Zeng, R.C.; Zou, Y.H.; Li, S.Q.; Zhang, F. In vitro corrosion resistance and antibacterial properties of layer-by-layer assembled chitosan/poly-L-glutamic acid coating on AZ31 magnesium alloys. Trans. Nonferrous Met. Soc. 2017, 27, 1081–1086. [Google Scholar] [CrossRef]
- Jia, R.; He, Y.; Liang, J.; Duan, L.; Ma, C.; Lu, T.; Liu, W.; Li, S.; Wu, H.; Cao, H.; et al. Preparation of biocompatibility coating on magnesium alloy surface by sodium alginate and carboxymethyl chitosan hydrogel. iScience 2024, 27, 109197. [Google Scholar] [CrossRef] [PubMed]
- Bakhsheshi-Rad, H.R.; Hamzah, E.; Daroonparvar, M.; Yajid, M.A.M.; Medraj, M. Fabrication and corrosion behavior of Si/HA nano-composite coatings on biodegradable Mg–Zn–Mn–Ca alloy. Surf. Coat. Technol. 2014, 258, 1090–1099. [Google Scholar] [CrossRef]
- Peng, F.; Zhang, D.; Wang, D.; Liu, L.; Zhang, Y.; Liu, X. Enhanced corrosion resistance and biocompatibility of magnesium alloy by hydroxyapatite/graphene oxide bilayer coating. Mater. Lett. 2020, 264, 127322. [Google Scholar] [CrossRef]
- Neupane, M.P.; Lee, S.J.; Kang, J.Y.; Park, I.S.; Bae, T.S.; Lee, M.H. Surface characterization and corrosion behavior of silanized magnesium coated with graphene for biomedical application. Mater. Chem. Phys. 2015, 163, 229–235. [Google Scholar] [CrossRef]
- Santos, C.; Piedade, C.; Uggowitzer, P.J.; Montemor, M.F.; Carmezim, M.J. Parallel nano-assembling of a multifunctional GO/HapNP coating on ultrahigh-purity magnesium for biodegradable implants. Appl. Surf. Sci. 2015, 345, 387–393. [Google Scholar] [CrossRef]
- Cao, L.; Wang, L.; Fan, L.; Xiao, W.; Lin, B.; Xu, Y.; Liang, J.; Cao, B. RGDC Peptide-Induced Biomimetic Calcium Phosphate Coating Formed on AZ31 Magnesium Alloy. Materials 2017, 10, 358. [Google Scholar] [CrossRef]
- Wang, M.-J.; Chao, S.-C.; Yen, S.-K. Electrolytic calcium phosphate/zirconia composite coating on AZ91D magnesium alloy for enhancing corrosion resistance and bioactivity. Corros. Sci. 2016, 104, 47–60. [Google Scholar] [CrossRef]
- Tan, C.; Zhang, X.; Li, Q. Fabrication of multifunctional CaP-TC composite coatings and the corrosion protection they provide for magnesium alloys. Biomed. Tech. 2017, 62, 375–381. [Google Scholar] [CrossRef]
- Bai, N.; Tan, C.; Li, Q.; Xi, Z. Study on the corrosion resistance and anti-infection of modified magnesium alloy. Biomed. Mater. Eng. 2017, 28, 339–345. [Google Scholar] [CrossRef]
- Tian, J.; Shen, S.; Zhou, C.; Dang, X.; Jiao, Y.; Li, L.; Ding, S.; Li, H. Investigation of the antimicrobial activity and biocompatibility of magnesium alloy coated with HA and antimicrobial peptide. J. Mater. Sci. Mater. Med. 2015, 26, 66. [Google Scholar] [CrossRef]
- Chen, D.; Mei, D.; Chen, L.; Wang, C.; Bai, J.; Xue, F.; Chu, C.; Wang, L.; Zhu, S.; Guan, S. A ceria/calcium-phosphate functional composite coating on magnesium alloy for enhanced adhesion strength, corrosion resistance, and biocompatibility. Appl. Surf. Sci. 2024, 672, 160790. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, J.Q.; Chen, C.F.; Gu, Y.H. Advances in microarc oxidation coated AZ31 Mg alloys for biomedical applications. Corros. Sci. 2015, 91, 7–28. [Google Scholar] [CrossRef]
- Seyfoori, A.; Mirdamadi, S.; Seyedraoufi, Z.S.; Khavandi, A.; Aliofkhazraei, M. Synthesis of biphasic calcium phosphate containing nanostructured films by micro arc oxidation on magnesium alloy. Mater. Chem. Phys. 2013, 142, 87–94. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Chen, M.; Zhao, Y.; You, C.; Li, Y.; Chen, G. In Vitro and in Vivo Degradation Behavior and Biocompatibility Evaluation of Microarc Oxidation-Fluoridated Hydroxyapatite-Coated Mg-Zn-Zr-Sr Alloy for Bone Application. ACS Biomater. Sci. Eng. 2019, 5, 2858–2876. [Google Scholar] [CrossRef]
- Wei, Z.; Tian, P.; Liu, X.; Zhou, B. In vitro degradation, hemolysis, and cytocompatibility of PEO/PLLA composite coating on biodegradable AZ31 alloy. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 103, 342–354. [Google Scholar] [CrossRef]
- Bai, K.; Zhang, Y.; Fu, Z.; Zhang, C.; Cui, X.; Meng, E.; Guan, S.; Hu, J. Fabrication of chitosan/magnesium phosphate composite coating and the in vitro degradation properties of coated magnesium alloy. Mater. Lett. 2012, 73, 59–61. [Google Scholar] [CrossRef]
- Feng, Y.; Ma, X.; Chang, L.; Zhu, S.; Guan, S. Characterization and cytocompatibility of polydopamine on MAO-HA coating supported on Mg-Zn-Ca alloy. Surf. Interface Anal. 2017, 49, 1115–1123. [Google Scholar] [CrossRef]
- Zhang, X.; Cui, S.-D.; Zhou, L.; Lian, J.-B.; He, J.; Li, X.-W. Preparation and characterization of calcium phosphate containing coating on plasma electrolytic oxidized magnesium and its corrosion behavior in simulated body fluids. J. Alloys Compd. 2022, 896, 163042. [Google Scholar] [CrossRef]
- Zhang, D.; Zhou, J.; Peng, F.; Tan, J.; Zhang, X.; Qian, S.; Qiao, Y.; Zhang, Y.; Liu, X. Mg-Fe LDH sealed PEO coating on magnesium for biodegradation control, antibacteria and osteogenesis. J. Mater. Sci. Technol. 2022, 105, 57–67. [Google Scholar] [CrossRef]
- Zarei, A.; Dehghanian, C.; Farhangi, H.; Jafari, Z. Improvement of Mg alloy corrosion resistance and apatite formation ability via PEO/Alginate/Akermanite hybrid coatings for biomedical purposes. Ceram. Int. 2024, 50, 20994–21007. [Google Scholar] [CrossRef]
- Xiong, Y.; Hu, X.; Song, R. Characteristics of CeO2/ZrO2-HA composite coating on ZK60 magnesium alloy. J. Mater. Res. 2017, 32, 1073–1082. [Google Scholar] [CrossRef]
- Li, C.Y.; Fan, X.L.; Zeng, R.C.; Cui, L.-Y.; Li, S.Q.; Zhang, F.; He, Q.K.; Kannan, M.B.; Jiang, H.W.; Chen, D.C.; et al. Corrosion resistance of in-situ growth of nano-sized Mg(OH)2 on micro-arc oxidized magnesium alloy AZ31-Influence of EDTA. J. Mater. Sci. Technol. 2019, 35, 1088–1098. [Google Scholar] [CrossRef]
- Bordbar-Khiabani, A.; Yarmand, B.; Mozafari, M. Functional PEO layers on magnesium alloys: Innovative polymer-free drug-eluting stents. Surf. Innov. 2018, 6, 237–243. [Google Scholar] [CrossRef]
- Xiong, Y.; Hu, Q.; Song, R.; Hu, X. LSP/MAO composite bio-coating on AZ80 magnesium alloy for biomedical application. Mater Sci. Eng. C Mater. Biol. Appl. 2017, 75, 1299–1304. [Google Scholar] [CrossRef]
- Liu, P.; Pan, X.; Yang, W.; Cai, K.; Chen, Y. Improved anticorrosion of magnesium alloy via layer-by-layer self-assembly technique combined with micro-arc oxidation. Mater. Lett. 2012, 75, 118–121. [Google Scholar] [CrossRef]
- Zhang, Y.; Liao, B.; Xu, L.; Wan, S.; Guo, X. A novel anti-corrosion and antibacterial integrated MAO/PCNZ composite coating on AZ31B Mg alloy. Surf. Coat. Technol. 2024, 483, 130794. [Google Scholar] [CrossRef]
- Wang, T.; Xu, Y.; Liu, Z.; Li, G.; Guo, Y.; Lian, J.; Zhang, Z.; Ren, L. A chitosan/polylactic acid composite coating enhancing the corrosion resistance of the bio-degradable magnesium alloy. Prog. Org. Coat. 2023, 178, 107469. [Google Scholar] [CrossRef]
- Zhao, Y.B.; Liu, H.P.; Li, C.Y.; Chen, Y.; Li, S.Q.; Zeng, R.C.; Wang, Z.L. Corrosion resistance and adhesion strength of a spin-assisted layer-by-layer assembled coating on AZ31 magnesium alloy. Appl. Surf. Sci. 2018, 434, 787–795. [Google Scholar] [CrossRef]
- Cui, L.Y.; Gao, L.; Zhang, J.C.; Tang, Z.; Fan, X.L.; Liu, J.C.; Chen, D.C.; Zeng, R.C.; Li, S.Q.; Zhi, K.Q. In vitro corrosion resistance, antibacterial activity and cytocompatibility of a layer-by-layer assembled DNA coating on magnesium alloy. J. Magnes. Alloys 2021, 9, 266–280. [Google Scholar] [CrossRef]
- Cui, L.Y.; Cheng, S.C.; Liang, L.X.; Zhang, J.C.; Li, S.Q.; Wang, Z.L.; Zeng, R.C. In vitro corrosion resistance of layer-by-layer assembled polyacrylic acid multilayers induced Ca–P coating on magnesium alloy AZ31. Bioact. Mater. 2020, 5, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.Y.; Fang, X.H.; Cao, W.; Zeng, R.C.; Li, S.Q.; Chen, X.B.; Zou, Y.H.; Guan, S.K.; Han, E.H. In vitro corrosion resistance of a layer-by-layer assembled DNA coating on magnesium alloy. Appl. Surf. Sci. 2018, 457, 49–58. [Google Scholar] [CrossRef]
- Monfared, A.; Ghaee, A.; Ebrahimi-Barough, S. Fabrication of tannic acid/poly(N-vinylpyrrolidone) layer-by-layer coating on Mg-based metallic glass for nerve tissue regeneration application. Colloids Surf. B-Biointerfaces 2018, 170, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; He, P.; Yao, J.; Li, M.; Wang, B.; Han, L.; Huang, Z.; Guo, C.; Bai, J.; Xue, F.; et al. pH/NIR-responsive and self-healing coatings with bacteria killing, osteogenesis, and angiogenesis performances on magnesium alloy. Biomaterials 2023, 301, 122237. [Google Scholar] [CrossRef]
- Asadi, H.; Ghalei, S.; Handa, H.; Ramasamy, R.P. Cellulose nanocrystal reinforced silk fibroin coating for enhanced corrosion protection and biocompatibility of Mg-based alloys for orthopedic implant applications. Prog. Org. Coat. 2021, 161, 106525. [Google Scholar] [CrossRef]
- Kannan, M.B.; Liyanaarachchi, S. Hybrid coating on a magnesium alloy for minimizing the localized degradation for load-bearing biodegradable mini-implant applications. Mater. Chem. Phys. 2013, 142, 350–354. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Q.; Li, L.; Zhang, P.; Wang, Z.; Chen, F. Fabrication of hydroxyapatite/stearic acid composite coating and corrosion behavior of coated magnesium alloy. Mater. Lett. 2012, 88, 76–78. [Google Scholar] [CrossRef]
- Fan, W.; Du, H.; An, Y.; Guo, C.; Wei, Y.; Hou, L. Fabrication and characterization of a hydroxyapatite–methylcellulose composite coating on the surface of AZ31 magnesium alloy. Mater. Lett. 2015, 152, 32–35. [Google Scholar] [CrossRef]
- Perumal, G.; Ramasamy, B.; Nandkumar, A.M.; Dhanasekaran, S.; Ramasamy, S.; Doble, M. Bilayer nanostructure coated AZ31 magnesium alloy implants: In vivo reconstruction of critical-sized rabbit femoral segmental bone defect. Nanomedicine 2020, 29, 102232. [Google Scholar] [CrossRef]
- Tian, Q.; Rivera-Castaneda, L.; Liu, H. Optimization of nano-hydroxyapatite/poly(lactic-co-glycolic acid) coatings on magnesium substrates using one-step electrophoretic deposition. Mater. Lett. 2017, 186, 12–16. [Google Scholar] [CrossRef]
- Zhang, J.; Wen, Z.; Zhao, M.; Li, G.; Dai, C. Effect of the addition CNTs on performance of CaP/chitosan/coating deposited on magnesium alloy by electrophoretic deposition. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 58, 992–1000. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Kang, M.H.; Kim, H.E.; Lim, H.K.; Byun, S.H.; Lee, J.H.; Lee, S.M. Innovative micro-textured hydroxyapatite and poly(l-lactic)-acid polymer composite film as a flexible, corrosion resistant, biocompatible, and bioactive coating for Mg implants. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 81, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Sun, M.; Huang, N.; Yang, P.; Jing, F.; Zhao, A.; Akhavan, B. Biodegradable PTMC-MAO composite coatings on AZ31 Mg-alloys for enhanced corrosion-resistance. J. Alloys Compd. 2024, 998, 175017–175025. [Google Scholar] [CrossRef]
- Lv, Y.; Zhang, Y.; Zheng, M.; Liu, H.; Dong, Z.; Zhang, X. NIR-induced corrosion regulation strategy for biodegradable Mg-based implants. Prog. Org. Coat. 2023, 185, 107921. [Google Scholar] [CrossRef]
- Monfared, M.M.; Aghdam, R.M.; Pishbin, F.; Rahmani, M. Enhancement of PEO-coated ZK60 Mg alloy: Curcumin-enriched mesoporous silica and PLA/bioglass for antibacterial properties, bioactivity and biocorrosion resistance. Surf. Coat. Technol. 2024, 493, 131237. [Google Scholar] [CrossRef]
- Zhao, Y.B.; Shi, L.Q.; Cui, C.Y.; Zhang, C.L.; Li, S.Q.; Zeng, R.C. Corrosion Resistance of Silane-Modified Hydroxyapatite Films on Degradable Magnesium Alloys. Acta Metall. Sin. -Engl. Lett. 2018, 31, 180–188. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Hamzah, E.; Daroonparvar, M.; Abdul Kadir, M.R.; Kasiri-Asgarani, M.; Staiger, M.P. Enhancement of corrosion resistance and mechanical properties of Mg–1.2Ca–2Bi via a hybrid silicon-biopolymer coating system. Surf. Coat. Technol. 2016, 301, 133–139. [Google Scholar] [CrossRef]
- Ren, Y.; Babaie, E.; Bhaduri, S.B. Nanostructured amorphous magnesium phosphate/poly (lactic acid) composite coating for enhanced corrosion resistance and bioactivity of biodegradable AZ31 magnesium alloy. Prog. Org. Coat. 2018, 118, 1–8. [Google Scholar] [CrossRef]
- Wang, C.; Shen, J.; Zhang, X.; Duan, B.; Sang, J. In vitro degradation and cytocompatibility of a silane/Mg(OH)2 composite coating on AZ31 alloy by spin coating. J. Alloys Compd. 2017, 714, 186–193. [Google Scholar] [CrossRef]
- Córdoba, L.C.; Marques, A.; Taryba, M.; Coradin, T.; Montemor, F. Hybrid coatings with collagen and chitosan for improved bioactivity of Mg alloys. Surf. Coat. Technol. 2018, 341, 103–113. [Google Scholar] [CrossRef]
- Chen, X.W.; Cai, L.P.; Zhang, D.F.; Ran, Y.; Ping, W. In Vitro Corrosion Behavior of Zr-Containing MAO-PLA Composite Coating on Magnesium Alloy. Trans. Indian Inst. Met. 2023, 76, 2123–2130. [Google Scholar] [CrossRef]
- Vafa, E.; Bazargan-lari, R.; Bahrololoom, M.E.; Amani, A.M. Effect of polyvinyl alcohol concentration on biomedical application of chitosan/bioactive glass composite coated on AZ91D magnesium alloy. Mater. Chem. Phys. 2022, 291, 126650. [Google Scholar] [CrossRef]
- Roshan, S.; Eivaz Mohammadloo, H.; Sarabi, A.A.; Afshari, M. Biocompatible hybrid chitosan/hydroxyapatite coating applied on the AZ31 Mg alloy substrate: In-vitro corrosion, surface and structure studies. Mater. Today Commun. 2022, 30, 103153–103168. [Google Scholar] [CrossRef]
- Rahman, M.; Chowdhury, M.A.; Mia, M.S.; Ali, M.R.; Rahman, A.; Ali, M.O.; Mahmud, S. Fabrication and characterization of hybrid coating on Mg-Zn-Ca Mg alloy for enhanced corrosion and degradation resistance as medical implant. Ceram. Int. 2022, 48, 23314–23324. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, T.; Xu, Y.; Liang, C.; Li, G.; Guo, Y.; Zhang, Z.; Lian, J.; Ren, L. Double-layer calcium phosphate sandwiched siloxane composite coating to enhance corrosion resistance and biocompatibility of magnesium alloys for bone tissue engineering. Prog. Org. Coat. 2023, 177, 107417. [Google Scholar] [CrossRef]
- Wang, X.; Liu, X.; Dai, Y.; She, J.; Zhang, D.; Qi, F.; Wei, W.; Ouyang, X. A novel Ca-Mg-P/PDA composite coating of Mg alloys to improve corrosion resistance for orthopedic implant materials. Surf. Coat. Technol. 2023, 471, 129920. [Google Scholar] [CrossRef]
- Zeng, R.C.; Cui, L.Y.; Jiang, K.; Liu, R.; Zhao, B.D.; Zheng, Y.F. In Vitro Corrosion and Cytocompatibility of a Microarc Oxidation Coating and Poly(L-lactic acid) Composite Coating on Mg-1Li-1Ca Alloy for Orthopedic Implants. ACS Appl. Mater. Interfaces 2016, 8, 10014–10028. [Google Scholar] [CrossRef]
- Li, X.; Hu, J.; Liu, M.; Xiao, X.; Yang, L.; Qin, G.; Zhang, E. Effect of PLGA+MAO composite coating on the degradation of magnesium alloy in vivo and in vitro. Mater. Today Commun. 2023, 34, 105197. [Google Scholar] [CrossRef]
- Abdal-Hay, A.; Hasan, A.; Kim, Y.K.; Lee, M.H.; Hamdy, A.S.; Khalil, K.A. Biocorrosion behavior of biodegradable nanocomposite fibers coated layer-by-layer on AM50 magnesium implant. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 58, 1232–1241. [Google Scholar] [CrossRef]
- Gao, F.; Hu, Y.; Gong, Z.; Liu, T.; Gong, T.; Liu, S.; Zhang, C.; Quan, L.; Kaveendran, B.; Pan, C. Fabrication of chitosan/heparinized graphene oxide multilayer coating to improve corrosion resistance and biocompatibility of magnesium alloys. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 104, 109947. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Wang, Y.; He, L.; Zhang, X.; Mei, Y.; Wu, T.; Wang, J.; Zheng, Y.; Tang, H. Surface Engineering of Biodegradable Magnesium Alloys as Orthopedic Implant Materials: Recent Developments and Future Prospects. Coatings 2025, 15, 191. https://doi.org/10.3390/coatings15020191
Chen H, Wang Y, He L, Zhang X, Mei Y, Wu T, Wang J, Zheng Y, Tang H. Surface Engineering of Biodegradable Magnesium Alloys as Orthopedic Implant Materials: Recent Developments and Future Prospects. Coatings. 2025; 15(2):191. https://doi.org/10.3390/coatings15020191
Chicago/Turabian StyleChen, Hualong, Yu Wang, Liang He, Xiaoyi Zhang, Yanan Mei, Tong Wu, Jia Wang, Yu Zheng, and Hui Tang. 2025. "Surface Engineering of Biodegradable Magnesium Alloys as Orthopedic Implant Materials: Recent Developments and Future Prospects" Coatings 15, no. 2: 191. https://doi.org/10.3390/coatings15020191
APA StyleChen, H., Wang, Y., He, L., Zhang, X., Mei, Y., Wu, T., Wang, J., Zheng, Y., & Tang, H. (2025). Surface Engineering of Biodegradable Magnesium Alloys as Orthopedic Implant Materials: Recent Developments and Future Prospects. Coatings, 15(2), 191. https://doi.org/10.3390/coatings15020191





