Effects of Relaxation and Nanocrystallization on Wear and Corrosion Behaviors of Fe-Based Amorphous Coating
Abstract
1. Introduction
2. Experimental
2.1. Coating Preparation
2.2. Microstructure and Microhardness Characterization
2.3. Wear Measurements
2.4. Electrochemical Measurements
3. Results and Discussion
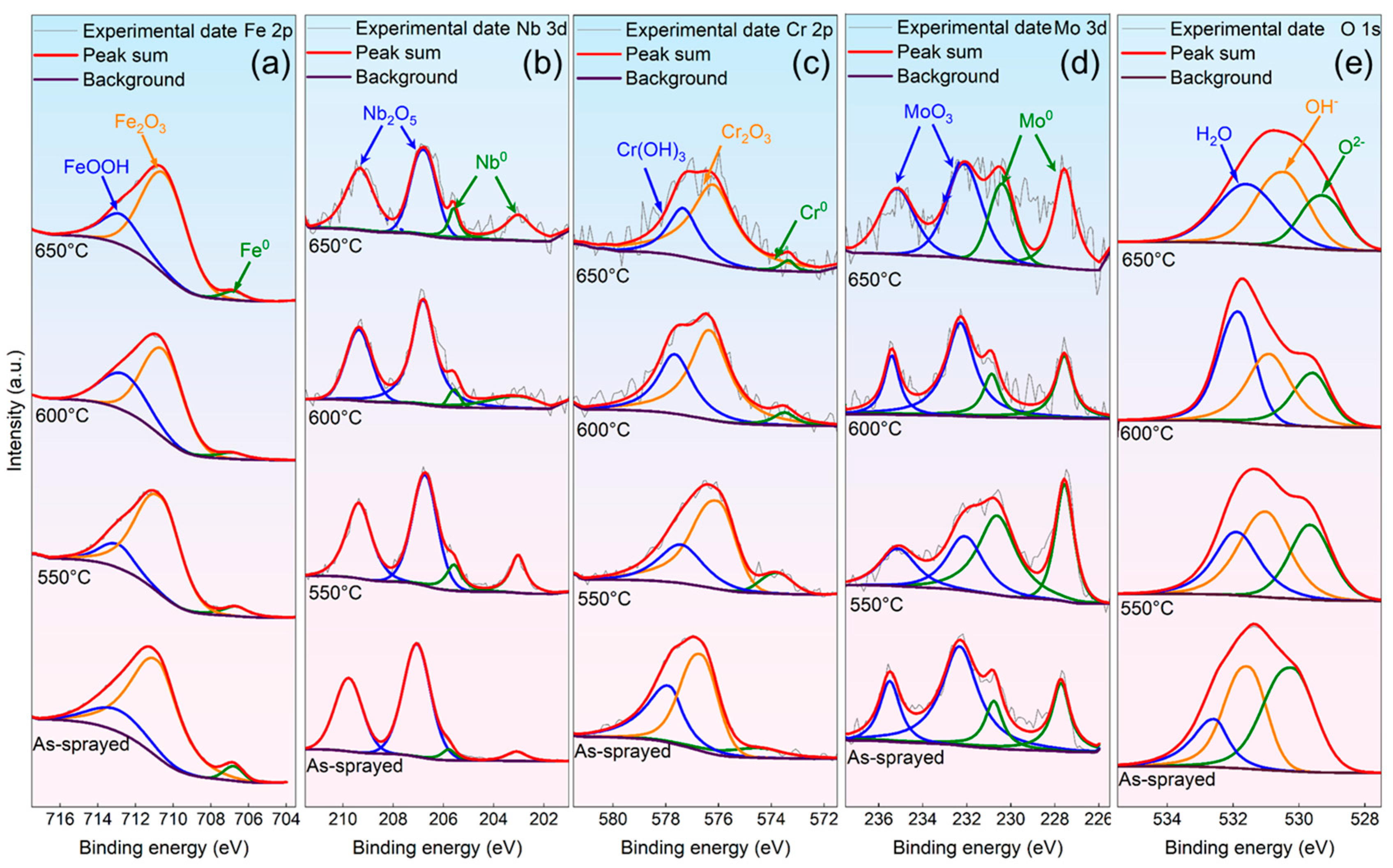
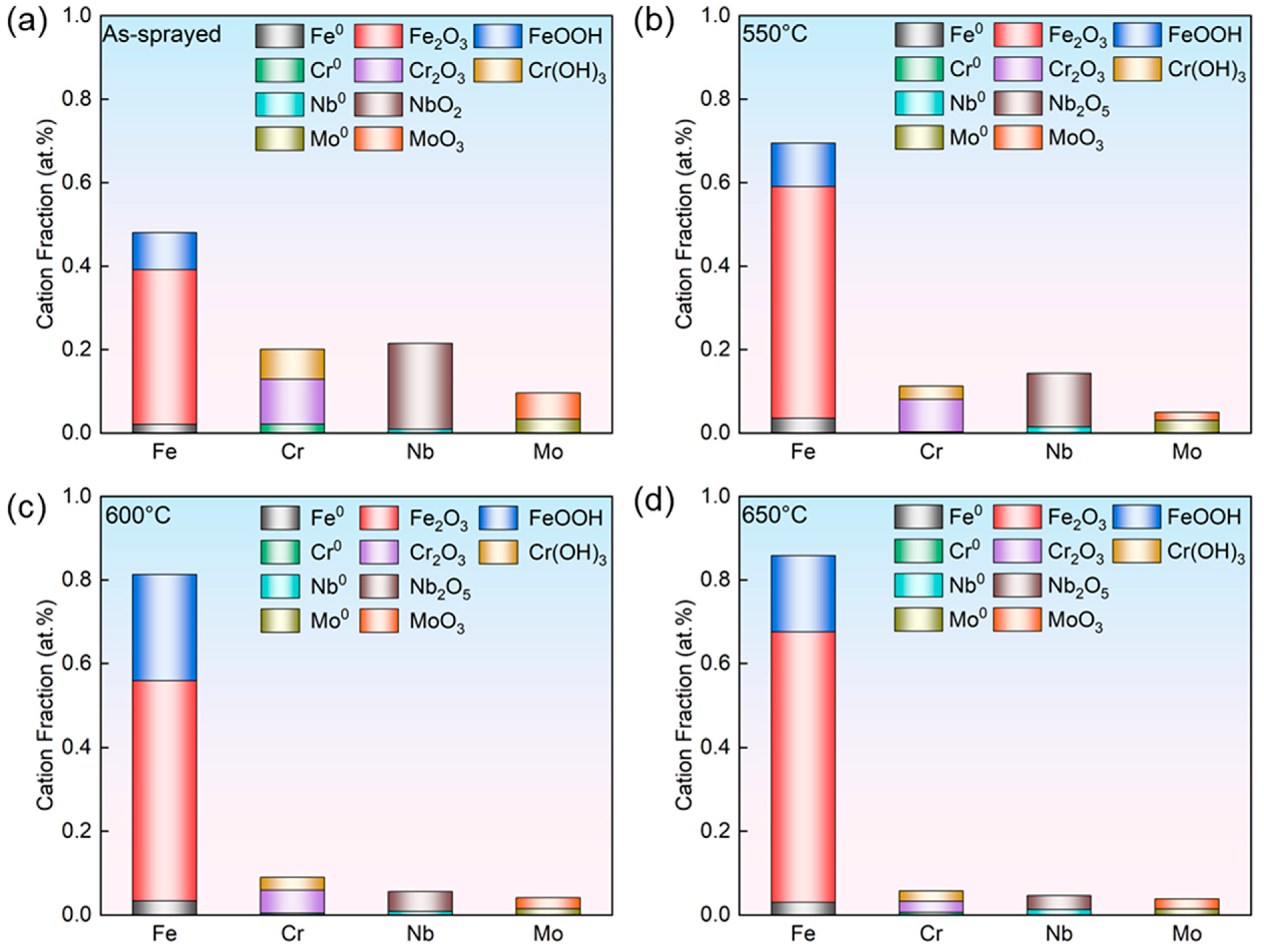
3.1. Phase Compositions and Microstructures
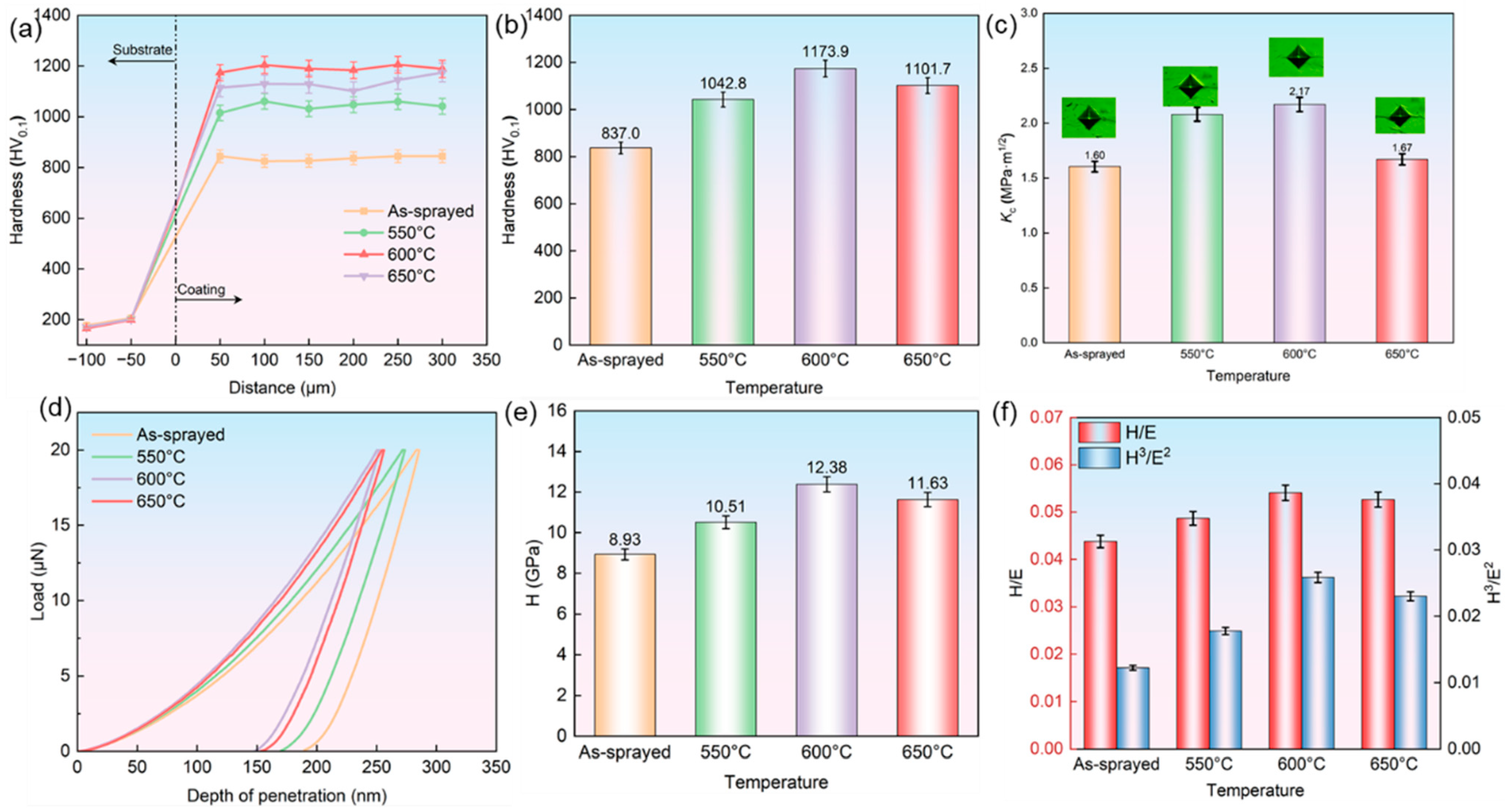
3.2. Mechanical and Tribological Properties
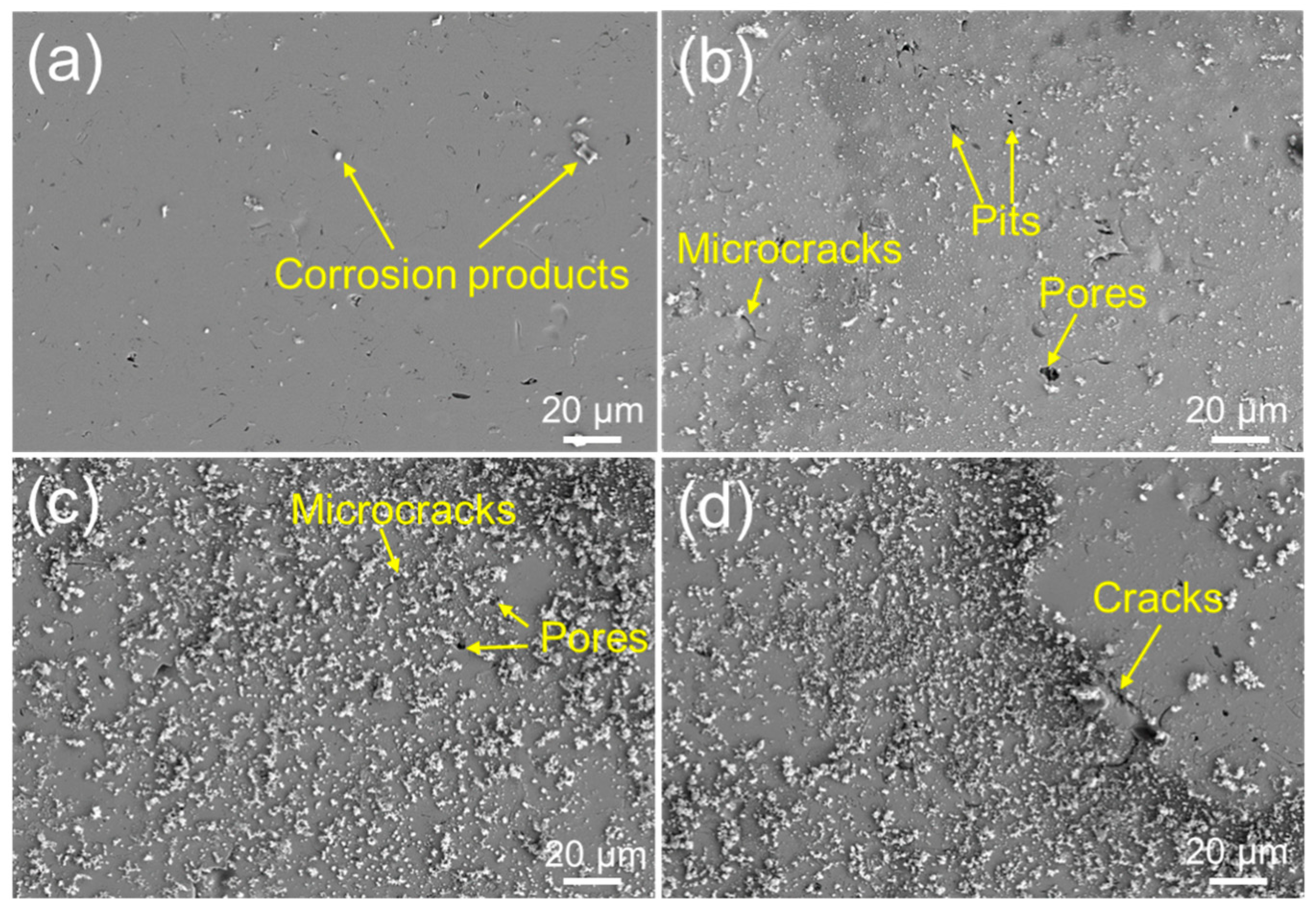
3.3. Corrosion Resistance
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, H.L.; Yang, L.X.; Wang, Q.; Wu, J.T.; Zhang, S.D.; Zeng, C.L. Hot corrosion behaviors of 921A alloy and HVAF-sprayed Fe-based amorphous coating covered with KCl-ZnCl2 salts. J. Iron Steel Res. Int. 2023, 30, 1537–1549. [Google Scholar] [CrossRef]
- Bobzin, K.; Ote, M.; Knoch, M.A.; Sommer, J. Influence of Powder Size on the Corrosion and Wear Behavior of HVAF-Sprayed Fe-Based Coatings. J. Therm. Spray Technol. 2019, 28, 63–75. [Google Scholar] [CrossRef]
- Tsai, P.H.; Xiao, A.C.; Li, J.B.; Jiang, J.S.C.; Chu, J.P.; Huang, J.P. Prominent Fe-based bulk amorphous steel alloy with large supercooled liquid region and superior corrosion resistance. J. Alloys Compd. 2014, 586, 94–98. [Google Scholar] [CrossRef]
- Cai, M.J.; Wang, J.J.; Wang, Q.Q.; Li, J.; Dong, W.Y.; Guo, Z.J.; Shen, B.L. Improvement of soft-magnetic properties for Fe-based amorphous alloys with high saturation polarization by stress annealing. Mater. Res. Lett. 2023, 11, 595–603. [Google Scholar] [CrossRef]
- Farmer, J.; Choi, J.S.; Saw, C.; Haslam, J.; Day, D.; Hailey, P.; Lian, T.; Rebak, R.; Perepezko, J.; Payer, J.; et al. Iron-Based Amorphous Metals: High-Performance Corrosion-Resistant Material Development. Metall. Mater. Trans. A-Phys. Metall. Mater. Sci. 2009, 40, 1289–1305. [Google Scholar] [CrossRef]
- Shi, L.X.; Qin, X.L.; Yao, K.F. Tailoring soft magnetic properties of Fe-based amorphous alloys through C addition. Prog. Nat. Sci.-Mater. Int. 2020, 30, 208–212. [Google Scholar] [CrossRef]
- Huang, F.; Kang, J.J.; Yue, W.; Fu, Z.Q.; Zhu, L.N.; She, D.S.; Liang, J.; Wang, C.B. Corrosion Behavior of FeCrMoCBY Amorphous Coating Fabricated by High-Velocity Air Fuel Spraying. J. Therm. Spray Technol. 2019, 28, 842–850. [Google Scholar] [CrossRef]
- Shao, L.L.; Luo, Q.; Zhang, M.J.; Xue, L.; Cui, J.X.; Yang, Q.Z.; Ke, H.B.; Zhang, Y.; Shen, B.L.; Wang, W.H. Dual-phase nano-glass-hydrides overcome the strength-ductility trade-off and magnetocaloric bottlenecks of rare earth based amorphous alloys. Nat. Commun. 2024, 15, 4159. [Google Scholar] [CrossRef]
- Lu, K.J.; Aktaa, J. Short-term tensile creep behavior of CoCrNi-based multi-principal element alloys. Intermetallics 2024, 175, 108500. [Google Scholar] [CrossRef]
- Wang, G.; Huang, Z.J.; Xiao, P.; Zhu, X.B. Spraying of Fe-based amorphous coating with high corrosion resistance by HVAF. J. Manuf. Process. 2016, 22, 34–38. [Google Scholar] [CrossRef]
- Li, M.C.; Guan, H.M. Unified upper temperature for cryogenic thermal cycling treatment in Fe- based bulk metallic glasses. J. Alloys Compd. 2023, 931, 167263. [Google Scholar] [CrossRef]
- Li, G.; Gan, Y.Y.; Liu, C.H.; Shi, Y.; Zhao, Y.C.; Kou, S.Z. Corrosion and Wear Resistance of Fe-Based Amorphous Coatings. Coatings 2020, 10, 73. [Google Scholar] [CrossRef]
- Liu, X.Q.; Qiu, Z.G.; Zeng, D.C. Corrosion and Reciprocating Sliding Behavior of AC-HVAF Sprayed FeCrMoCBSi Amorphous Coating on Stainless Steel. J. Therm. Spray Technol. 2022, 31, 2207–2218. [Google Scholar] [CrossRef]
- Wang, J.W.; Yang, R.; Tian, Y.; Zhou, P.; Huang, J.; Li, H.; Chen, X.R. Effect of Annealing on the Cavitation Erosion Resistance of HVOF-Sprayed Fe-Based Amorphous Composite Coatings. J. Therm. Spray Technol. 2023, 32, 1758–1771. [Google Scholar] [CrossRef]
- Liu, L.C.; Shao, L.L.; Ma, Y.; Wen, Z.L.; Zhou, J.; Yan, Y.Q.; Yang, W.M.; Ke, H.B.; Wang, W.H. Sparse Nanocrystals Enable Ultra-Low Coercivity and Remarkable Mechanical Robustness in High-Entropy Amorphous Alloy. Adv. Sci. 2025, 12, e03546. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.L.; Bai, R.; Wu, Y.; Zhou, J.; Tong, X.; Peng, H.L.; Liang, T.; Li, Z.Z.; Zeng, Q.S.; Zhang, B. Critical state-induced emergence of superior magnetic performances in an iron-based amorphous soft magnetic composite. Mater. Futures 2024, 3, 025301. [Google Scholar] [CrossRef]
- Bai, R.; Li, J.; Shao, L.L.; Zhou, J.; Lin, X.H.; Xue, Z.Y.; Lin, H.J.; Ke, H.B.; Wang, W.H. Ultrahigh saturation magnetization and optimized magnetic softness in a soft magnetic composite through nanoscale iron nitride. J. Mater. Sci. Technol. 2026, 248, 119–125. [Google Scholar] [CrossRef]
- Li, C.Y.; Wang, H.B.; Ding, J.Q.; Wang, S.P.; Li, J.L.; Kou, S.Z. Effects of heat treatment on HVOF-sprayed Fe-based amorphous coatings. Surf. Eng. 2021, 37, 590–598. [Google Scholar] [CrossRef]
- Lee, C.Y.; Sheu, H.H.; Tsay, L.W.; Hsiao, P.S.; Lin, T.J.; Lee, H.B. The Effect of Heat Treatment on the Corrosion Resistance of Fe-Based Amorphous Alloy Coating Prepared by High Velocity Oxygen Fuel Method. Materials 2021, 14, 7818. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, C.; Peng, Y.; Yu, Y.; Liu, L. Effects of crystallization on the corrosion resistance of Fe-based amorphous coatings. Corros. Sci. 2012, 59, 10–19. [Google Scholar] [CrossRef]
- Song, P.S.; Jing, Z.Y.; Zhang, Z.B.; Zhang, B.B.; Ge, Y.Y.; Xue, L.; Liang, X.B.; Cheng, J.B. Effect of Minor Mo Addition on Microstructure and Corrosion Resistance of High-Velocity Air Fuel-Sprayed Fe-Based Amorphous Coatings. Coatings 2023, 13, 2089. [Google Scholar] [CrossRef]
- Liu, W.H.; Shieu, F.S.; Hsiao, W.T. Enhancement of wear and corrosion resistance of iron-based hard coatings deposited by high-velocity oxygen fuel (HVOF) thermal spraying. Surf. Coat. Technol. 2014, 249, 24–41. [Google Scholar] [CrossRef]
- Liang, S.Y.; Zhu, F.; Wang, Y.J.; Pineda, E.; Wada, T.; Kato, H.; Qiao, J.C. On the kinetics of structural evolution in metallic glasses. Int. J. Eng. Sci. 2024, 205, 104146. [Google Scholar] [CrossRef]
- Mansfeld, F.; Lin, S.; Kim, S.; Shih, H. Corrosion Protection of Al Alloys and Al-Based Metal Matrix Composites by Chemical Passivation. Corrosion 1989, 45, 615–630. [Google Scholar] [CrossRef]
- Wang, D.P.; Wang, S.L.; Wang, J.Q. Relationship between amorphous structure and corrosion behaviour in a Zr-Ni metallic glass. Corros. Sci. 2012, 59, 88–95. [Google Scholar] [CrossRef]
- Cheng, J.B.; Wang, Z.H.; Xu, B.S. Wear and Corrosion Behaviors of FeCrBSiNbW Amorphous/Nanocrystalline Coating Prepared by Arc Spraying Process. J. Therm. Spray Technol. 2012, 21, 1025–1031. [Google Scholar] [CrossRef]
- Liu, X.; Wu, M.P.; Lu, P.P.; Ye, X.; Mxiao, X.J. Corrosion behavior of GO-reinforced TC4 nanocomposites manufactured by selective laser melting. Mater. Corros.-Werkst. Und Korros. 2020, 71, 628–636. [Google Scholar] [CrossRef]
- Liu, X.Y.; Chen, S.Y.; Zhang, J.J.; Yang, G.; Zhang, Y.; Wang, T.; Lei, J.B. Enhancement of the electrochemical corrosion resistance of Ti6Al4V alloy reinforced by nano- and micro-TiC particles through directed energy deposition. Corros. Sci. 2023, 221, 111343. [Google Scholar] [CrossRef]
- Navarro Laboulais, J.J.; Amigó Mata, A.; Amigó Borrás, V.; Igual Muñoz, A.N. Electrochemical characterization and passivation behaviour of new beta-titanium alloys (Ti35NblOTa-xFe). Electrochim. Acta 2017, 227, 410–418. [Google Scholar] [CrossRef]
- Hirschorn, B.; Orazem, M.E.; Tribollet, B.; Munoz, I.A. Determination of effective capacitance and film thickness from constant-phase-element parameters. Electrochim. Acta 2010, 55, 6218–6227. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, C.; Liu, L. Wear behavior and corrosion properties of Fe-based thin film metallic glasses. J. Alloys Compd. 2015, 650, 127–135. [Google Scholar] [CrossRef]
- Qin, W.; Man, C.; Zhang, H.; Zhang, H.W.; Cui, Z.Y.; Wang, L.; Kong, D.C.; Dong, C.F.; Cui, H.Z. Corrosion behavior of L-PBF Ti6Al4V with heat treatments in the F--containing environments. Corros. Sci. 2023, 210, 110811. [Google Scholar] [CrossRef]
- Ge, T.; Chen, L.; Gu, P.F.; Ren, X.D.; Chen, X.M. Microstructure and corrosion resistance of TiC/Inconel 625 composite coatings by extreme high speed laser cladding. Opt. Laser Technol. 2022, 150, 107919. [Google Scholar] [CrossRef]
- Gong, P.; Wang, D.L.; Zhang, C.; Wang, Y.; Jamili-Shirvan, Z.; Yao, K.F.; Wang, X.Y. Corrosion behavior of TiZrHfBeCu(Ni) high-entropy bulk metallic glasses in 3.5 wt.% NaCl. npj Mater. Degrad. 2022, 6, 77. [Google Scholar] [CrossRef]
- Zhang, M.D.; Shi, X.L.; Li, Z.Y.; Xu, H.; Li, G. Corrosion behaviors and mechanism of CrFeNi2 based high-entropy alloys. Corros. Sci. 2022, 207, 110562. [Google Scholar] [CrossRef]
- Fattah-alhosseini, A.; Soltani, F.; Shirsalimi, F.; Xu, H.Q.; Li, G. The semiconducting properties of passive films formed on AISI 316 L and AISI 321 stainless steels: A test of the point defect model (PDM). Corros. Sci. 2011, 53, 3186–3192. [Google Scholar] [CrossRef]
- Wang, L.; Yu, H.Y.; Wang, K.; Xu, H.S.; Wang, S.Y.; Sun, D.H. Local Fine Structural Insight into Mechanism of Electrochemical Passivation of Titanium. ACS Appl. Mater. Interfaces 2016, 8, 18608–18619. [Google Scholar] [CrossRef]
- Zhao, Q.C.; Pan, Z.M.; Wang, X.F.; Luo, H.; Liu, Y.; Li, X.G. Corrosion and passive behavior of AlxCrFeNi3-x (x=0.6, 0.8, 1.0) eutectic high entropy alloys in chloride environment. Corros. Sci. 2022, 208, 110666. [Google Scholar] [CrossRef]
- Sun, M.H.; Pang, Y.J.; Du, C.W.; Li, X.G.; Wu, Y.M. Optimization of Mo on the corrosion resistance of Cr-advanced weathering steel designed for tropical marine atmosphere. Constr. Build. Mater. 2021, 302, 124346. [Google Scholar] [CrossRef]
- Nayak, S.K.; Kumar, A.; Laha, T. Fe-based metallic glass coatings by thermal spraying: A focused review on corrosion properties and related degradation mechanisms. Int. Mater. Rev. 2023, 68, 404–485. [Google Scholar] [CrossRef]
- Song, P.S.; Cheng, J.B.; Wang, X.Y.; Zhang, Z.B.; Shao, L.L.; Xue, L.; Jing, Z.Y.; Zhang, B.S.; Wu, Y.P.; Hong, S.; et al. Corrosion and corrosion-wear behavior of the (FeCoCrNi)75B15Si10 high-entropy amorphous alloy coatings prepared by plasma spraying. Corros. Sci. 2025, 253, 113013. [Google Scholar] [CrossRef]
- Ge, Y.Y.; Cheng, J.B.; Xue, L.; Zhang, B.S.; Hong, S.; Liang, X.B.; Wang, S.G.; Zhang, X.C. Pore defect and corrosion behavior of HVAF-sprayed Co21Fe14Ni8Cr16Mo16C15B10 high entropy metallic glass coatings. Corros. Sci. 2025, 242, 112564. [Google Scholar] [CrossRef]




| Samples | AS | HT-550 | HT-600 | HT-650 |
|---|---|---|---|---|
| Ecorr (mV) | −491 | −505 | −518 | −732 |
| Icorr (μA·cm−2) | 3.78 | 6.43 | 7.26 | 27.42 |
| Rp (Ω) | 14,334 | 10,199 | 8726 | 1079 |
| Parameter | AS | HT-550 | HT-600 | HT-650 |
|---|---|---|---|---|
| Rs (Ω·cm2) | 11.67 | 10.17 | 12.77 | 11.07 |
| Qc × 10−5 (S·cm−2·sn) | 7.092 | 9.19 | 16.21 | 20.52 |
| nc | 0.7556 | 0.7986 | 0.7507 | 0.7366 |
| Rc (Ω·cm2) | 8714 | 2564 | 720.8 | 99.66 |
| Qdl × 10−5 (S·cm−2·sn) | 2.164 | 6.767 | 11.41 | 54.91 |
| ndl | 0.6026 | 0.6387 | 0.6380 | 0.5929 |
| Rct × 104 (Ω·cm2) | 7.736 | 5.364 | 2.029 | 0.967 |
| χ2 × 10−4 | 3.58 | 3.63 | 2.79 | 3.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weng, S.; Zhang, Z.; Fu, Y.; Xue, L.; Song, P.; Shao, L.; Liang, X.; Cheng, J.; Zhang, B. Effects of Relaxation and Nanocrystallization on Wear and Corrosion Behaviors of Fe-Based Amorphous Coating. Coatings 2025, 15, 1497. https://doi.org/10.3390/coatings15121497
Weng S, Zhang Z, Fu Y, Xue L, Song P, Shao L, Liang X, Cheng J, Zhang B. Effects of Relaxation and Nanocrystallization on Wear and Corrosion Behaviors of Fe-Based Amorphous Coating. Coatings. 2025; 15(12):1497. https://doi.org/10.3390/coatings15121497
Chicago/Turabian StyleWeng, Shenghai, Zhibin Zhang, Yuxi Fu, Lin Xue, Peisong Song, Liliang Shao, Xiubing Liang, Jiangbo Cheng, and Binbin Zhang. 2025. "Effects of Relaxation and Nanocrystallization on Wear and Corrosion Behaviors of Fe-Based Amorphous Coating" Coatings 15, no. 12: 1497. https://doi.org/10.3390/coatings15121497
APA StyleWeng, S., Zhang, Z., Fu, Y., Xue, L., Song, P., Shao, L., Liang, X., Cheng, J., & Zhang, B. (2025). Effects of Relaxation and Nanocrystallization on Wear and Corrosion Behaviors of Fe-Based Amorphous Coating. Coatings, 15(12), 1497. https://doi.org/10.3390/coatings15121497






