Abstract
Micro-arc oxidation (MAO) technology demonstrates remarkable advantages in fabricating ceramic coatings on lightweight alloys. For aluminum alloys, MAO rapidly forms dense, pore-free ceramic layers within minutes, significantly enhancing corrosion and wear resistance at low processing costs. In magnesium alloys, optimized electrolyte compositions and process parameters enable composite coatings with a combination of high hardness and self-lubrication properties, while post-treatments like laser melting or corrosion inhibitors extend salt spray corrosion resistance. Titanium alloys benefit from MAO coatings with exceptional interfacial bonding strength and mechanical performance, making them ideal for biomedical implants and aerospace components. Notably, dense ceramic oxide films grown in situ via MAO on high-entropy alloys (HEAs) triple surface hardness and enhance wear/corrosion resistance. However, MAO applications on steel require pretreatments like aluminizing, thermal spraying, or ion plating. Current challenges include coating uniformity control, efficiency for complex geometries, and long-term stability. Future research focuses on multifunctional coatings (self-healing, antibacterial) and eco-friendly electrolyte systems to expand engineering applications.
1. Introduction
Micro-arc oxidation (MAO), also known as Plasma Electrolytic Oxidation (PEO), is an electrochemical surface modification technique that generates in situ ceramic coatings on metal substrates through high voltage-induced micro-discharges [1]. During MAO, localized plasma discharges at the metal–electrolyte interface facilitate the oxidation of metal ions, forming dense oxide or composite ceramic layers. This process is characterized by its simplicity, environmental friendliness, and strong coating–substrate adhesion. Currently, MAO has achieved industrial-scale applications in lightweight alloys (e.g., aluminum, magnesium, and titanium alloys) for corrosion protection and functionalization [2,3,4].
Existing reviews on MAO have predominantly focused on light alloys, with dedicated analyses of aluminum (Al), magnesium (Mg), and titanium (Ti) alloys. For Al alloys, reviews have thoroughly explored electrolyte compositions, electrical parameters, and the resultant coating microstructures, emphasizing enhancements in wear resistance and corrosion protection through dense α-Al2O3/γ-Al2O3 layers [4,5,6]. Mg alloy reviews have centered on functionalization strategies, such as incorporating antibacterial elements (Ag, Cu, Zn) and sealing porous structures with biopolymers or calcium phosphate to mitigate rapid corrosion, while highlighting biocompatibility for biomedical applications [3,7]. Ti alloy-focused reviews have detailed the formation of bioactive TiO2 coatings, tuning of porous topographies for osseointegration, and improvements in interfacial bonding strength for orthopedic and dental implants [4,8]. These studies collectively establish MAO as a mature technology for light alloys, with optimized processes for specific properties like osteoconductivity or tribological performance. Beyond traditional light alloys, recent advancements have expanded MAO applications to high-entropy alloys (HEAs) and steel, addressing emerging engineering demands [9,10,11,12].
Upon conducting a comprehensive search using the specific keywords “Micro-arc oxidation” within the Web of Science database, as depicted in Figure 1, it has been revealed that over the course of the past 22 years, there have been more than 3195 scholarly articles published that are pertinent to the subject of MAO. Furthermore, the citation count for MAO literature has been on a steady rise, with the cumulative number of citations approaching the impressive figure of 90,000 times. This review not only discusses the applications of MAO technology on the surface of light alloys but also explores its applications on the surface of high-entropy alloys (HEAs) and steel substrates in recent years, focusing on coating processes and key functional properties (corrosion resistance, wear resistance, mechanical properties, and biocompatibility) improvements to highlight research achievements.
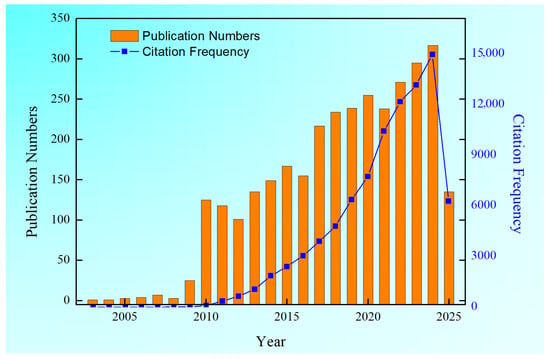
Figure 1.
The histogram of publication numbers and citation frequency with the keywords “Micro-arc oxidation” retrieved from Web of Science database.
This review summarizes the current knowledge of MAO coating processes, strategies for enhancing property, and technical challenges, emphasizing electrolyte composition, process control, and hybrid methods. The goal is to provide a comprehensive overview to promote MAO’s use in multifunctional and eco-friendly applications, and to address key issues such as coating consistency, scalability for complex shapes, and long-term durability, aiming to encourage wider industrial use in various engineering fields.
2. MAO Treatment Technology for Various Metallic Materials
The MAO processing techniques diverge considerably across different metallic materials, with material-specific optimization protocols and prioritized performance metrics. The following sections will provide detailed elucidations on these aspects.
2.1. MAO Treatment Technology for Al-Based Alloys
MAO of Al-based alloys is an advanced surface treatment technology that in situ synthesizes a ceramic oxide film on the aluminum alloy surface [13,14,15]. Its principle involves applying high voltage and high current to break through the Faraday region of conventional anodization, generating micro-arc plasma discharge on the metal surface [16,17,18]. This causes complex reactions, including melting, sintering, and phase transformation of the aluminum substrate, ultimately forming a ceramic layer. This layer is primarily composed of α-Al2O3 (corundum phase), characterized by a dense structure, high hardness, and strong adhesion strength [19,20,21].
Current density is a critical factor influencing the MAO treatment of aluminum alloys [22,23,24]. Current density significantly influences the quality of MAO coatings on 7075 aluminum alloy prepared in silicate-based electrolyte. The α-Al2O3 phase content first increases then decreases with rising current density, consistent with micro-hardness and wear test results. The coating prepared at 12 A/dm2 exhibits the highest adhesion force, while that at 10 A/dm2 shows the best corrosion resistance, directly related to coating morphology, which was conducted by Cao et al. [25]. Wang et al. [26] investigated MAO treatment on 7E04 aluminum drill pipe material in an electrolyte containing SiC particles under current densities of 1–20 A/dm2; the specific electrolyte composition is detailed in Table 1. Key results include the following: Increased current density raises oxidation voltage and coating thickness (2.66 μm at 1 A/dm2 vs. 14.81 μm at 20 A/dm2). High current density induces rougher surfaces and larger pore sizes (1–10 μm), reducing micro-hardness (peak hardness at 1 A/dm2 decreases at 20 A/dm2). SiC oxidation generates SiO2, enhancing corrosion resistance, but increased porosity partially counteracts this benefit.

Table 1.
The specific electrolyte composition of MAO treatment on 7E04 aluminum drill pipe material [26].
Scholars have systematically investigated the effects of cathodic-to-anodic current ratio [27], hybrid voltage (DC + AC) [28], and electrolyte composition on coating properties [26]. The findings reveal that elevated cathodic current density promotes densification layer thickening and porosity reduction [27]. Hybrid voltage coatings exhibit superior corrosion resistance compared to pure DC counterparts, with more uniform pore distribution (<1 μm) [28]. When implementing MAO in Na2SiO3-NaOH electrolyte containing 3 g/L SiC particles, SiC oxidation to SiO2 was observed to enhance corrosion resistance, though excessive current density induced porosity increment which compromised performance. Incorporation of Na2WO4 in the electrolyte effectively reduces breakdown voltage and improves coating densification.
In their mechanistic investigation of MAO on 6061 aluminum alloy utilizing a Na2SiO3-CH3COONa-Na5P3O10 electrolyte system, Li et al. [29] demonstrated that micro-arc discharges are sequentially initiated at the coating–substrate interface, thereby forming spark clusters. The ejection of molten metal results in the generation of characteristic molten pools, whose diameters exhibit a linear correlation with coating thickness. Structural analyses revealed the following findings: (1) The coating is composed of α-Al2O3 and γ-Al2O3 phases, with the α-phase content progressively increasing from the surface toward the substrate interface; (2) Trumpet-shaped molten pools interconnect to form three-dimensional pore networks; (3) Discharge-induced micropores located at the coating base have a diameter range of 150–200 nm.
Researchers have explored the incorporation of various additives into electrolytes to enhance MAO coating performance. For instance, Jin et al. [30] introduced Fe micrograins (several μm in diameter) into a Na2SiO3-NaOH electrolyte to fabricate Fe-Al2O3 composite coatings on LY2 aluminum alloy. Results demonstrated that Fe micrograins optimized discharge channel reactions (e.g., diffusion and electrophoresis), reducing porosity and improving mechanical properties. Li et al. [31] found two-step oxidation of 2A70 Al MAO coatings: silicate (dumbbell channels, island growth) vs. phosphate (trumpet channels, layered growth), highlighting electrolyte composition’s role in discharge and morphology. Chen et al. [32] incorporated graphene particles (0–0.2 g/L) into a Na2SiO3-based electrolyte to prepare graphene-Al2O3 composite coatings.
The phase composition of the MAO coating consists of a dense, high-hardness inner α-Al2O3 layer and a porous, low-density outer γ-Al2O3 layer [6]. Performance investigations encompass friction wear [33], impact wear [34], corrosion (including stress corrosion) [35], fatigue [2], and high-temperature behavior [36]. Arslan et al. [33] demonstrated that polished MAO coatings exhibit low wear rates (3.00–5.00 × 10−6 mm3/N·m) and stable friction coefficients (0.45–0.6) under ceramic-on-abrasion conditions, with further friction reduction observed at 200 °C. The MAO coating significantly reduces wear volume by absorbing impact energy (with energy absorption ratios exceeding those of the substrate) [34]. While increased coating thickness enhances wear resistance, excessive thickness may induce fatigue cracks due to residual stresses. MAO coatings improve impedance values by 1–2 orders of magnitude in 3.5% NaCl solution, effectively suppressing localized corrosion (Venugopal et al.) [35]. Pretreatments like thermal oxidation further enhance corrosion resistance through structural optimization. The coating notably delays stress corrosion failure in aluminum alloys: uncoated specimens failed within 30 days under 80% yield strength, while coated specimens maintained high elongation (10.5%) [35]. Microdefects (pores, cracks) and residual stress constitute primary fatigue failure mechanisms. Process optimizations such as gradient current and post-treatment polishing can extend fatigue life [2]. MAO coatings maintain integrity under 873 K thermal shock, though thermal expansion coefficient mismatch (7.38 × 10−6 K−1) induces thermal stress without observable cracking [36].
The performance of MAO is synergistically influenced by multiple parameters (current mode, ultrasonic power, electrolyte flow rate), with optimized parameter combinations being crucial for enhancing coating properties. Recent studies include the work by Ma et al. [37] on 17% SiC particle-reinforced aluminum matrix composites (17% SiCp/2009 AMC), which combined experimental analysis with density functional theory (DFT) simulations to elucidate the growth mechanisms and product formation processes of MAO coatings. Scholars have summarized the MAO-oxidized ceramic film’s growth mechanism diagram. New phenomena were discovered through mechanism analysis: (1) SiC oxidation induced porosity formation; (2) Ce3+-mediated composite oxide stabilization; (3) interface preference of Al matrix; and (4) phase evolution of Al2O3 and mullite. Thus, several new questions are raised: the formation mechanism of Ce2O3, the kinetic relationship of SiC melting acceleration, the coating mechanism of amorphous SiO2, the long-term stability of CeO2·SiO2, and the tunability of interfacial energy, which require further research [37].
Liu et al. [38] investigated the effects of ultrasonic power (0–90 W)-assisted MAO on the microstructure and properties of 6061 aluminum alloy coatings, revealing that at 50 W ultrasonic power (abbreviated as U-50), the cross-sectional micromorphology and EDS spectra are shown in Figure 2a–e, the coating achieved maximum hardness (approximately 240 HV), minimum porosity (1.88%), optimal wear resistance, and corrosion resistance. Figure 2f shows the equivalent circuit diagram, and the sample U-50 has the best-fitting degree. Figure 2g illustrates the growth mechanism of the MAO coating under ultrasonic assistance. In the pre-growth phase of the coating, microflow and emission waves generated by ultrasound increase the number of charged particles in the solution and accelerate their migration, thereby enhancing the coating’s growth rate. However, how ultrasonic energy precisely regulates the dispersion of oxide melt and the homogeneity of elements is an issue worthy of subsequent discussion. Scanning micro-arc oxidation (SMAO) technology overcomes traditional size limitations through dynamic parameter control (e.g., robotic arm manipulation), enabling efficient processing of complex-shaped components [39,40].
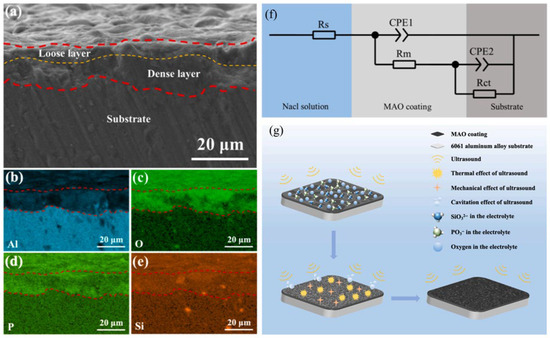
Figure 2.
Schematic diagrams illustrating the microstructure, elemental analysis, electrochemical modeling, and growth mechanism of the MAO coating on sample U-50: (a) Cross-sectional morphology of sample U-50; Energy-dispersive X-ray spectroscopy (EDS) elemental distribution maps of sample U-50: (b) aluminum (Al), (c) oxygen (O), (d) phosphorus (P), and (e) silicon (Si). (f) Equivalent circuit model of the coated specimen, where Rs represents solution resistance, Rm denotes coating resistance, Rct refers to charge transfer resistance, and two constant phase elements (CPEs) correspond to capacitive components at different interfaces. (g) Growth mechanism of the MAO coating under ultrasonic assistance [38].
2.2. MAO Treatment Technology for Mg-Based Alloys
Similarly, MAO of Mg-based alloys, based on the principle of plasma electrolytic oxidation, is an advanced technology for directly synthesizing ceramic coatings on the surface of magnesium alloys [41,42,43]. This process occurs under high voltage and strong current conditions, surpassing the limitations of conventional anodization and inducing intense micro-discharge phenomena on the magnesium alloy substrate [44,45,46]. During this process, intense plasma chemical reactions occur, causing the magnesium substrate to undergo melting, oxidation, and sintering, ultimately resulting in the in situ formation of a ceramic layer. This layer is primarily composed of MgO (periclase) and complex spinel-type composite oxides [47,48,49]. The resulting dense ceramic structure significantly enhances the key properties of magnesium alloys, particularly highlighting its unique advantage in the biomedical field—biocompatibility [50,51,52].
The performance of MAO coatings on Mg alloys is synergistically regulated by electrolyte composition (e.g., silicates, Hydroxyapatite (HA) precursors), current mode (bipolar pulses), and voltage. Parameter optimization is critical for enhancing coating densification and functionality [53,54,55,56], and the MAO electrolyte compositions are listed in Table 2 [53,54,55]. Zhang et al. [55] studied the dynamic coating formation on dual-phase AZ91HP Mg alloy (α-Mg + β-Mg17Al12) in a neutral electrolyte. The coatings preferentially nucleated at α-phase edges before expanding to β-phase, eventually covering the entire surface. This work elucidated the heterogeneous distribution of microarc discharges in dual-phase alloys, providing theoretical insights for surface treatment of multiphase materials.

Table 2.
The MAO electrolyte compositions for Mg-based alloys of Refs. [53,54,55].
Tang et al. [56] synthesized HA-containing MAO coatings in a calcium glycerophosphate-based electrolyte by adjusting voltages (250–500 V). Figure 3a shows that MAO coating thickness and surface roughness increase with applied voltage. Below 400 V, thickness grows slowly and linearly; above 400 V, it rises sharply. Roughness exhibits a similar voltage dependent trend. Higher instantaneous energy accelerates MAO coating growth, consistent with previous studies. At 400 V, the coatings achieved superior hydrophobicity (contact angle 128°), as shown in Figure 3b. As shown in Figure 3c, the corrosion current of the maintained oxide film layer prepared under 400 V is presented, with the corrosion current density of 1.06 × 10−7 A/cm2 in SBF solution. As illustrated in Figure 3d, this kinetic difference leads to the sequential adsorption of ions on the Mg anode surface: OH− first encapsulates the anode, followed by PO43− and calcium ions. The preferential participation of OH− in micro-discharge reactions, succeeded by PO43− and calcium ions, aligns with the elemental distribution observed in the MAO coatings. This raises new questions: how to balance voltage to optimize HA content, coating thickness, and defect quantity for better performance; what impact does the migration kinetics of hydroxide ions (OH−) and calcium ions (Ca2+)/phosphate ions (PO43−) have on coating structure; and how does hydrophobicity affect long-term biocompatibility in physiological environments.
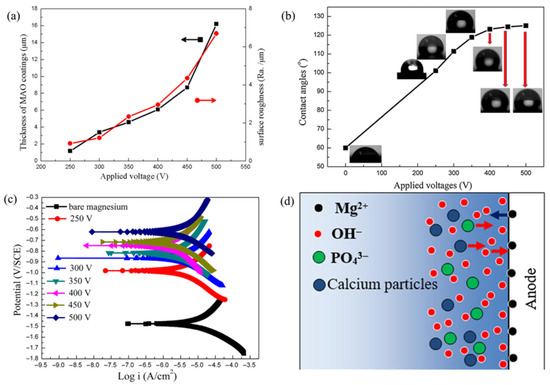
Figure 3.
The characterizations of MAO coatings on AZ31 magnesium alloy: (a) changes in thickness and surface roughness of MAO coatings fabricated under different applied voltages; (b) surface contact angles of bare AZ31 Mg alloy and MAO coatings; (c) polarization curves comparing bare magnesium and MAO coatings; and (d) schematic diagram showing the ion distribution on the Mg anode surface during the MAO process [56].
The performance of MAO technology in surface treatment of magnesium alloys includes corrosion resistance [57,58,59,60], mechanical properties [61,62,63], biocompatibility and degradation behavior [64,65], and high-temperature oxidation resistance [66]. The solution systems used for its corrosion resistance include aluminate–fluoride system [57], silicate system [58,59], and phosphate system [60]. In the aluminate–fluoride system, an MgAl2O4 spinel phase coating formed on AZ91D magnesium alloy significantly improved corrosion resistance, reducing the corrosion current density (jcorr) from 43.52 μA/cm2 to 0.351 μA/cm2 [57]. In the phosphate system, Mg3(PO4)2 (farringtonite) was generated, but the higher porosity of the coating resulted in inferior corrosion resistance compared to the silicate system [58,59,60].
Durdu et al. [61] investigated the hardness and wear resistance of MAO coatings on magnesium alloys. Figure 4a shows that the coatings prepared in silicate-based electrolytes exhibited significantly higher hardness (260–470 HV) compared to those formed in phosphate-based electrolytes (175–260 HV). Regarding adhesion strength, the critical load (Lc) of silicate coatings reached 83 N, while phosphate coatings reached 72–85 N, with optimal adhesion achieved under high current density (0.14 A/cm2), see Figure 4b,c. The wear resistance improved by 2–3 times (wear rate as low as 5.6 × 10−5 mm3/N·m for the current density of 0.140 A/cm2), as illustrated in Figure 4d. Through comparison, it was found that silicate solution coatings are harder than phosphate solution coatings due to the formation of Mg2SiO4. The wear rate is not directly determined by hardness; microstructural compactness, adhesion strength, and phase composition are also critical factors. Silicate coatings exhibit a lower wear rate due to their higher hardness, denser structure, and stronger adhesion to the substrate. However, MAO coatings synthesized in aluminate-based electrolytes demonstrated inferior wear resistance performance on AZ91 magnesium alloy [60].
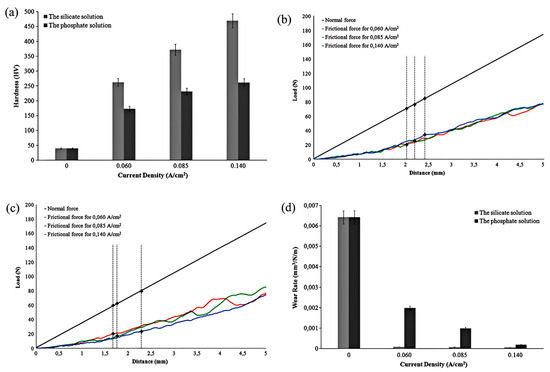
Figure 4.
Mechanical properties of MAO coatings on magnesium substrates are as follows: (a) Mean hardness of pure magnesium and MAO coatings prepared at different current densities for 30 min: light gray bars represent the silicate electrolyte, and dark gray bars represent the phosphate electrolyte. (b) Load–displacement curve for coatings fabricated in the silicate solution. (c) Load–displacement curve for coatings fabricated in the phosphate solution. (d) Wear rates of commercial pure magnesium and MAO coatings synthesized at various current densities over 30 min: light gray bars correspond to the silicate electrolyte, and dark gray bars correspond to the phosphate electrolyte [61].
The ceramic coatings formed on magnesium alloys via MAO technology exhibit excellent biocompatibility. ZK60 magnesium alloy was used to prepare ceramic coatings through MAO in electrolytes containing calcium (Ca) and phosphorus (P) with Ca/P ratios of 1:1, 3:1, and 5:1. After 30 days of immersion in simulated body fluid (SBF), hydroxyapatite (HA) and calcium pyrophosphate (CPP) formed on the coating surface. In vitro cytotoxicity tests (MTT assay) demonstrated a cell survival rate exceeding 95% [62]. Pan et al. [63] found higher Ca/P electrolytes promoted more HA/CPP on MAO-prepared CaP coatings on ZK60 Mg alloy; XRD showed main phases (MgO, β-Ca3(PO4)2, etc.), SBF immersion formed HA with β-Ca3(PO4)2 dissolution.
In vivo experiments (rabbit femoral implantation), MAO-coated samples showed no abnormalities in serum biochemical indicators such as magnesium, creatinine, and blood urea nitrogen (BUN). Micro-CT imaging indicated greater new bone formation around MAO-coated samples compared to uncoated ones [64]. Studies on the degradation mechanism of MAO coatings on magnesium alloys found that degradation rates correlate with protein type and concentration. In protein-rich physiological environments (e.g., plasma), the degradation behavior of coatings differs from that in pure SBF. Proteins like albumin accelerate localized corrosion by chelating Mg2+, yet they facilitate the long-term formation of protective HA layers [65].
Shao et al. [66] addressed the insufficient corrosion resistance of MAO films on magnesium AZ31 alloys in physiological environments by proposing surface structure and performance optimization through high-intensity pulsed ion beam (HIPIB) irradiation. Combining thermodynamic numerical simulations with experimental validation, the study systematically investigated the influence mechanisms of HIPIB irradiation energy density (1–5 J/cm2) on the microstructure and corrosion resistance of MAO films. But how the radial temperature gradient of HIPIB-irradiated MAO films (with the peak temperature at the beam center and gradually decreasing radially) affects the uniformity of surface densification and corrosion resistance of the coating is a question worthy of consideration [66].
2.3. MAO Treatment Technology for Ti-Based Alloys
Titanium and its alloys are widely utilized in biomedical fields such as orthopedic implants and dental prostheses due to their excellent mechanical properties, corrosion resistance, and biocompatibility [67,68,69]. However, the inherent bioinertness of titanium alloy surfaces limits their direct bonding ability with surrounding bone tissue, and corrosion behavior in physiological environments may lead to metal ion release, triggering long-term implant failure risks. MAO treatment of titanium-based alloys is a surface modification technique developed from anodic oxidation, which triggers micro-arc discharge via applying high voltage to in situ grow ceramic oxide films on the alloy surface [70]. The oxide film produced by this technique exhibits strong adhesion to the substrate, high hardness, wear resistance, corrosion resistance, and insulation properties [71,72,73].
For instance, Han et al. [74] systematically investigated the formation mechanism of HA coatings under calcium–phosphate electrolyte systems, revealing the regulatory effect of high voltage (>450 V) induced melting recrystallization on coating densification. The MAO process parameters used by Chen et al. are shown in Table 3 [75]. To further optimize performance, researchers have introduced functional additives: Song et al. [76] endowed coatings with long-term antibacterial activity (bacterial inhibition rate > 95% against Staphylococcus aureus) by doping with Ag or Cu ions; the incorporation of ZrO2 particles enhanced coating hardness to over 12 GPa via dispersion strengthening, significantly improving wear resistance [77]. Additionally, studies on the synergistic effect of MAO with ultrafine grained titanium substrates demonstrated that nanostructured substrates can accelerate coating growth and enhance interfacial bonding strength (>50 MPa), providing new insights for the design of drug-loaded sustained-release coatings [78,79].

Table 3.
Processing parameters of the micro-arc oxidized [75].
MAO, as an efficient surface modification technique, significantly enhances the bioactivity, corrosion resistance, and functional performance of titanium and its alloys by in situ forming porous ceramic coatings. Below is a summary of key findings and technological advancements from multiple related studies.
Nanocrystalline anatase TiO2 coatings prepared by MAO [80], when subjected to UV irradiation, generate abundant Ti3+ and oxygen vacancies on the surface. The MAO coating remained porous without precipitate after 25-day SBF immersion, showing no bonelike apatite induction. UV-0.5 h coatings developed sphere-like particles at 17 days (full surface coverage by 19 days) but none at 15 days as shown in Figure 5a. UV-2 h coatings exhibited such particles as early as 13 days, with a dense precipitate layer fully covering the surface by 17 days, as Figure 5b shows. Figure 5c shows the surface morphology and XRD pattern of the dark-stored UV-2 h coating after 17-day SBF immersion, indicating apatite induction within a short SBF immersion period. XPS analysis further reveals that the UV-irradiated coating exhibits long-term stability of Ti-OH groups and sustains bioactivity. Figure 5d shows ALP activity of cells cultured on MAO and UV-2 h coatings over 1–3 days: no statistical difference before day 2, but UV-2 h coatings exhibited significantly higher ALP activity than MAO coatings at day 3. However, methods to maintain long-term UV-induced hydroxyl stability require further investigation.
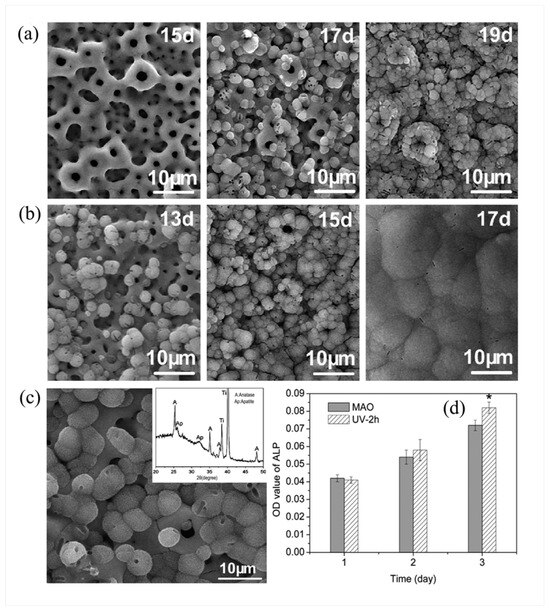
Figure 5.
Impact of UV irradiation duration on MAO coatings after SBF immersion: (a,b) Surface microstructures of coatings with 0.5 h (a) and 2 h (b) UV irradiation after SBF soaking for different periods (time marked in images); (c) SEM micrograph and XRD pattern of UV-2 h irradiated coating (stored in the dark) after 17-day SBF immersion; (d) Alkaline phosphatase (ALP) activity of cells cultured on MAO and UV-2 h coatings over one, two, and three days (* p < 0.05 vs. MAO coatings) [80].
Kung et al. [81] studied the MAO coatings on titanium substrates, which were prepared in Sr-containing calcium phosphate electrolytes that exhibit a porous structure incorporating Sr, Ca, and P. Apatite formation initiates after one day of SBF immersion and fully covers the surface within seven days. The release of Sr ions promotes bone formation. Electrochemical tests reveal that these coatings display a more noble corrosion potential (Ecorr) and lower passive current density compared to pure titanium, indicating superior corrosion resistance. The biological response of Ti6Al4V and Ti6Al7Nb alloys [82], after MAO treatment, forms ~10 μm thick TiO2 coatings (anatase + rutile). The Ti6Al4V coating is more porous and contains HA, whereas the Ti6Al7Nb coating exhibits a granular structure with calcium titanate (CaTiO3). Both coatings induce apatite formation in SBF. Cell experiments show that the Ti6Al4V oxide layer, due to its more microporous structure, supports better SAOS-2 cell adhesion than the Ti6Al7Nb coating [82].
Li et al. [83] researched the MAO treatment that forms a porous and dense oxide layer on titanium surfaces; MAO-prepared layers consisted of rutile and Ti, while sandblasted surfaces contained anatase and Ti. Compared to sandblasted surfaces, MAO-treated surfaces exhibit a smaller contact angle (55.4° vs. 74.4°). The titanium–porcelain bond strength increases from 33.28 MPa to 46.46 MPa (39.6% improvement), which is primarily attributed to the following: (1) the porous structure enhancing mechanical interlocking; (2) the stable rutile phase inhibiting high-temperature oxidation; and (3) improved hydrophilicity promoting porcelain wetting. For TC4 alloys fabricated by selective laser melting (SLM), the growth characteristics (e.g., thickness, porosity) of MAO coatings are dependent on oxidation time.
Optimized MAO coatings on SLM-TC4 alloy exhibit bioactivity and mechanical stability (Yao et al. [84]). Adhesion strength increases from 15.22 N (5 min) to 20.86 N (15 min), then decreases to 17.73 N (20 min), indicating an optimal oxidation time range (Figure 6a). Potentiodynamic polarization in 36.5 °C SBF shows that MAO coatings have lower Icorr than substrate; 15 min coating performs best (Icorr about 1 order lower, Ecorr +444 mV vs. substrate), with Icorr increasing at 20 min despite greater thickness (Figure 6(b1)). Bode plots reveal higher LF/HF impedance for MAO coatings, peaking at 15 min, then decreasing at 20 min (Figure 6(b2)). Figure 6c outlines the four-stage MAO process for Ca-P-titania coatings: (I) Initial anodic oxidation forms primary layer with spark discharge/channel formation. (II) Spark propagation enables oxide melting and nucleation. (III) Steady growth under constant current increases thickness/impedance, with Ca/P incorporation enhancing properties via rough porosity. (IV) Prolonged oxidation increases porosity/defects, reducing compactness and corrosion resistance/bioactivity due to SBF penetration and lower surface Ca/P. A key question arises as to how SLM-induced grain boundary characteristics regulate MAO discharge channel formation and energy distribution during growth, requiring further study.
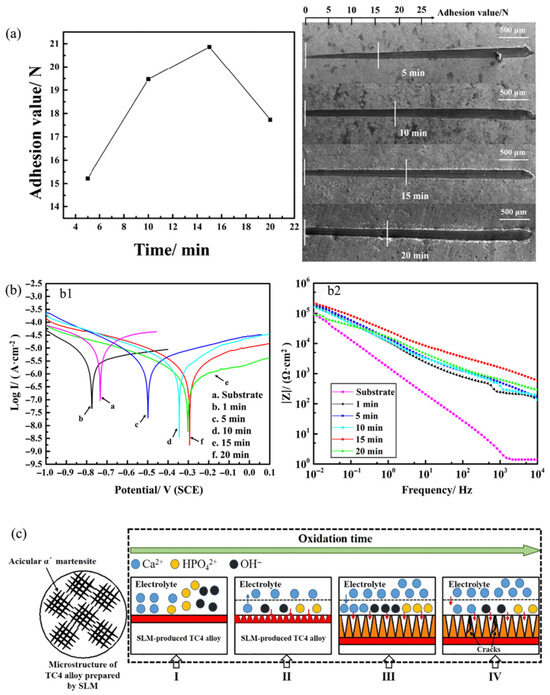
Figure 6.
The adhesion, electrochemical characteristics, and formation mechanism of MAO coatings on SLM-TC4 alloy under different oxidation durations: (a) Adhesion values (left) and scratch morphologies (right) at varying oxidation times. (b) Electrochemical plots (substrate vs. MAO coatings) in SBF. (b1) Potentiodynamic polarization curves and (b2) experimental Bode diagrams. (c) Schematic of MAO process on SLM-TC4 alloy [84].
Ceramic coatings prepared by MAO in aluminate electrolytes on titanium alloys form dense Al-Ti-O composite oxide layers, significantly enhancing the high temperature oxidation resistance by inhibiting oxygen diffusion into the substrate [85]. Meanwhile, MAO can also enhance the high-temperature oxidation resistance of additively manufactured Ti2AlNb alloys [86].
MAO technology, through the regulation of electrolyte composition, electrical parameters, and post-treatments, enables the construction of multifunctional coatings on titanium and its alloys: (1) Bioactivity: induces bone-like apatite formation and promotes osseointegration. (2) Corrosion Resistance: reduces corrosion current and enhances long-term stability. (3) Functional integration: promotes bone formation via strontium-containing coatings, regulates hydrophilicity through UV irradiation, and provides high-temperature oxidation protection.
2.4. MAO Treatment Technology for High-Entropy Alloys
High-entropy alloys (HEAs), particularly refractory high-entropy alloys (RHEAs), are considered candidates for next-generation high-temperature structural materials due to their excellent high-temperature mechanical properties (e.g., high strength and resistance to softening) [87,88,89]. However, RHEAs often contain volatile elements such as V, Mo, and W, whose high-temperature oxidation products (e.g., V2O5, MoO3) are prone to volatilization or form loose oxide films, leading to poor high-temperature oxidation resistance and limiting their practical applications. MAO, also known as an environmentally friendly and efficient surface treatment technology, has emerged as a critical method to enhance the high-temperature oxidation resistance of RHEAs by in situ growing ceramic coatings [90]. The development is summarized below from the perspectives of background, process optimization, coating characteristics, performance enhancement, challenges, and future directions.
RHEAs are composed of refractory elements such as Ti, V, Cr, Zr, Nb, and Mo, and maintain high strength even above 1000 °C (e.g., the yield strength of NbMoTaW exceeds 400 MPa at 1600 °C). However, their high-temperature oxidation often results in volatile oxides (e.g., V2O5, MoO3) or loose oxide films (e.g., Nb2O5), causing significant mass gain and protective failure [91,92,93]. For instance, the oxidation layer thickness of AlTiNbVZr0.25 can reach 800 μm due to V volatilization at high temperatures [94]; the mass gain of uncoated AlTiNbMo0.5Ta0.5Zr reaches 12.91 mg/cm2 [91]. MAO forms ceramic coatings on alloy surfaces via arc discharge, with strong adhesion to the substrate and controllable composition. It effectively blocks O2 diffusion and suppresses the oxidation of volatile elements, making it a key technology to address the high-temperature oxidation issues of RHEAs [95,96,97].
MAO process parameters (e.g., electrolyte composition, voltage) directly affect coating quality. Key optimization directions include (1) electrolyte composition optimization. Basic electrolytes often use silicon- and phosphorus-containing solutions (e.g., Na2SiO3, (NaPO3)6) to promote the formation of stable oxides such as SiO2 and phosphates, enhancing coating stability [91,94]. Additives like Na2B4O7 and KF can suppress uneven discharge and reduce microcracks and pores. The corresponding MAO electrolyte compositions and process parameters are shown in Table 4.

Table 4.
Processing parameters of the micro-arc oxidized HEAs [91,94].
Shi et al. [94] showed that adding Na2B4O7 and KF to Na2SiO3+(NaPO3)6+NaOH reduced AlTiCrVZr MAO coating surface roughness (16.507~7.241 μm), decreased V content (inhibiting V2O5 volatilization), and improved densification. (2) Voltage regulation: Voltage affects thickness and densification. Wang et al. [95] studied AlTiCrVZr RHEAs MAO coatings, and found that voltage increase (360–450 V) raised thickness (40–50 μm), as presented in Figure 7a–d. But excessive voltage (e.g., 450 V) caused more cracks due to higher thermal stress; optimal voltage (420 V) balanced thickness and densification, showing the best long-term oxidation performance [95]. The research raises questions, such as how voltage-induced microcracks and thickness synergistically affect long-term high-temperature oxidation resistance of RHEAs MAO coatings (which needs in-depth investigation). Yang et al. [9] studied AlCoCrFeNi HEAs-MAO coating growth mechanism, finding that it forms inner/outer oxide layers via micro-arc discharge (mainly Al2O3 with Co3O4, enhancing wear/corrosion resistance, see Figure 7e), but how sintering temperature affects Co3O4 distribution in coatings is highly worthy of further study.
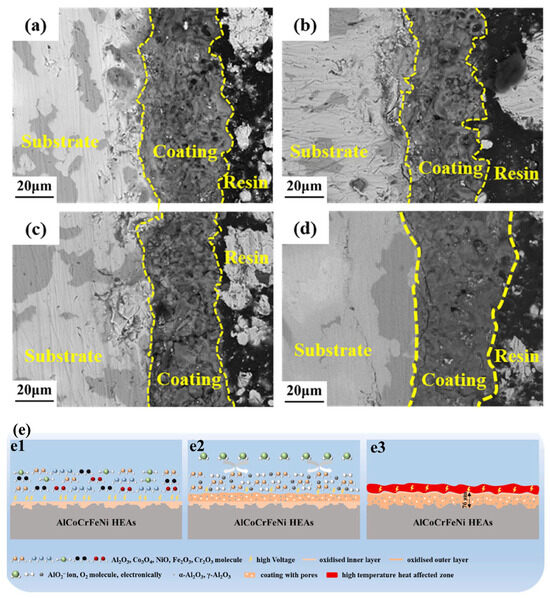
Figure 7.
Cross-sectional microstructures of coatings fabricated under varying voltages: (a) 360 V, (b) 390 V, (c) 420 V, and (d) 450 V [95]. (e) Growth mechanism of MAO coating on AlCoCrFeNi high-entropy alloys [10].
The core advantage of MAO coatings lies in their composite protective layers composed of multiple high-temperature stable oxides: (1) Composition: Coatings primarily contain oxides such as Al2O3, Cr2O3, SiO2, ZrO2, and TiO2 (confirmed by XPS analysis), with trace V2O5 (content controlled by electrolytes) [91,94,95]. For example, MAO coatings on AlTiNbMo0.5Ta0.5Zr are dominated by SiO2 and Ta2O5 [91]; those on AlTiCrVZr contain Al2O3, Cr2O3 and SiO2 [94]. (2) Microstructure: Typical “volcanic crater” morphology with micropores (residual discharge channels) and minor microcracks. Optimized coatings (e.g., with Na2B4O7 + KF or 420 V voltage) exhibit significantly improved densification, reduced porosity, and tighter interfacial bonding [94,95].
MAO coatings enhance RHEAs’ high-temperature oxidation resistance through dual mechanisms: (1) Physical Barrier: Dense coatings directly block O2 contact with the substrate and inhibit O diffusion. For example, the diffusion layer thickness of uncoated AlTiCrVZr is 800 μm, which decreases to below 600 μm after MAO treatment [60]; the mass gain of AlTiNbMo0.5Ta0.5Zr is reduced from 12.91 mg/cm2 to 8.67 mg/cm2 with MAO [91]. (2) Chemical Inhibition: Oxides such as Al2O3 and Cr2O3 in coatings suppress the oxidation and volatilization of volatile elements (e.g., V, Mo). For instance, in AlxCoCrFeMnNi alloys, increasing Al content leads to the formation of a dense Cr2O3/Al2O3 film at the coating bottom, reducing oxidation rate and oxide layer thickness [93]. Additionally, MAO coatings can synergistically improve wear and corrosion resistance. For example, the hardness of AlxCoCrFeMnNi coatings increases with Al content (from 154.5 HV to 631.1 HV), but excessive Al (x ≥ 1.0) reduces corrosion resistance due to increased BCC phase [93].
2.5. MAO Treatment Technology for Steel
MAO is a technique that uses plasma discharge to in situ grow ceramic coatings on valve metals (e.g., Al, Mg, Ti). However, steel, as a non-valve metal (without self-passivation properties), cannot directly undergo MAO. In recent years, researchers have constructed composite structures of “interlayer + MAO ceramic layer” on steel surfaces by combining pretreatment techniques (e.g., high-energy micro-arc alloying, wire arc spraying, hot-dip aluminizing, laser cladding), significantly enhancing the high-temperature oxidation resistance, corrosion resistance, wear resistance, and mechanical properties of steel [96].
The core of MAO on steel lies in constructing an interlayer suitable for MAO (e.g., Al-based alloy layers, FeAl, or LaCrO3 functional layers) through pretreatment. Methods are categorized into indirect (pretreatment + MAO) and direct (direct MAO) approaches:
(1) Pretreatment + MAO composite processes. Pretreatments form valve metal (e.g., Al, Mg, Ti) or functional alloy layers on steel, followed by MAO. This is the mainstream technique for MAO on steel.
(1) High-energy micro-arc alloying (HEMAA) coatings [97,98,99], a short-pulse high-current micro-welding technique, form dense coatings via metallurgical bonding between electrode materials and substrates, suitable for high-temperature functional coatings (e.g., solid oxide fuel cell (SOFC) interconnects). On ferritic stainless steel (430 SS), a Cr-alloyed layer is first deposited by HEMAA (to enhance adhesion) [97,98], followed by a LaCrO3–20 wt.% Ni electrode, forming a three-layer structure: “NiFe2O4 outer layer + LaCrO3 middle layer + Cr2O3 inner layer.” On 316 stainless steel, a 50 μm thick FeAl coating (38.26 at.% Al) is prepared by HEMAA [99], with significantly refined grains (electrode grains: 50–150 μm; coating grains: finer).
(2) Wire arc spraying + MAO composite process [11]. Al-based alloy layers (Al-Mg6, Al-Si 12, or pure Al) are first deposited on low-carbon steel (AISI 1010) by wire arc spraying, followed by MAO. Spraying parameters (current: 100–300 A; voltage: 28–32 V; distance: 15–20 cm; pressure: 3–4 Bar) affect coating thickness. MAO converts Al-based layers into Al2O3-based ceramic layers [11].
(3) Hot-dip aluminizing + MAO. Low-carbon steel (Q235) [100] or high-strength steel (AerMet100) [101] is hot-dip aluminized (710 °C, 2 min) to form a “FeAl alloy layer + pure Al layer” structure, followed by MAO. The MAO process includes three stages: common anodization (thin oxide film), stable MAO (ceramic layer growth), and coating destruction (outer layer ablation by large arcs). Current density (0.5–2.5 A/dm2) and time (0–14 min) affect total ceramic thickness (max ~47.5 μm) and inner/outer layer ratio (higher current promotes outer layer growth).
(4) Laser cladding + MAO [12]. Al-based alloy layers (e.g., Al-Si) are first laser-clad on S355 offshore steel, followed by MAO. Laser cladding adjusts interlayer composition and density, resulting in Al2O3-based ceramic layers with higher adhesion strength (>50 MPa) than wire arc spraying + MAO (~30 MPa) [12].
(5) Electro-spark deposition (ESD) + MAO [102,103]. Al- or Ti-based alloy layers (5–10 μm thick) are first deposited on steel by ESD, followed by MAO. ESD enables precise interlayer composition control, forming α-Al2O3 or TiO2 containing ceramic layers suitable for bioactive coatings on medical steel (e.g., Ti-alloyed steel).
The composition of electrolytes and electrical parameters for several typical MAO treatment methods is shown in Table 5. Figure 8a is the schematic setup for area-specific contact resistance measurements [97], while Figure 8b to 8d shows schematic diagrams of typical setups for different methods of MAO treatment on steel surfaces [11,101,102].

Table 5.
The composition of electrolytes and electrical parameters for several typical micro-arc oxidation treatment methods [11,12,100,102].
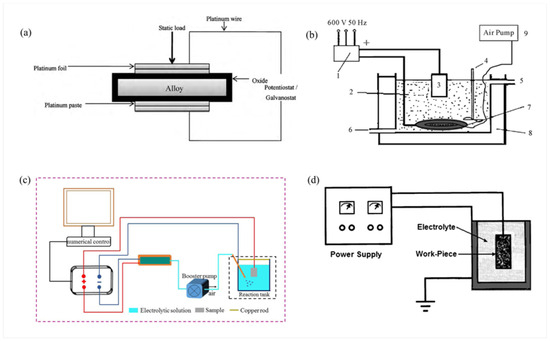
Figure 8.
Schematic diagrams of measurement setups and MAO systems tailored for different steel substrates. (a) Schematic diagram of the experimental setup for area-specific contact resistance measurements [97]. (b) Schematic illustration of the MAO deposition system applied to alloy steel, with components labeled: 1: power supply and control system, 2: electrolyte, 3: workpiece, 4: mixer, 5: cooling water inlet, 6: cooling water outlet, 7: stainless steel anode plate, 8: plexiglass, and 9: air pump [11]. (c) Schematic representation of the MAO process on AerMet100 steel [101]. (d) Schematic diagram of the MAO coating system configured for St35 steel [102].
(2) Direct Methods. Direct MAO on steel is attempted via electrolyte optimization (e.g., adding high-activity metal ions) or pretreatment (e.g., passivation layers) [104,105,106]. (1) Passivation Layer-Assisted MAO [104]: A Cr-based passivation film (1–2 μm thick) is first formed on low-carbon steel, followed by MAO. The self-repairing passivation film suppresses steel dissolution, resulting in Fe3O4-Cr2O3 composite ceramic layers (~20 μm thick). (2) Electrolyte-controlled MAO [105,106]: using strongly alkaline electrolytes (pH > 13) with Na2SiO3 and KOH, α-Al2O3 (Fe-doped) ceramic layers (~15 μm thick) are formed on low-carbon steel (10B21) via Fe oxidation-dissolution-redeposition, though with lower density (porosity > 10%), and the composition of electrolytes and electrical parameters are shown in Table 6.

Table 6.
The composition of electrolytes and electrical parameters of electrolyte-controlled MAO [106].
Sun et al. [104] illustrate, in Figure 9A, the preparation steps and mechanism of PASS + MAO coating: low-carbon steel surface undergoes passivation to form a rough passivation layer, followed by MAO treatment. During MAO, alternating current pulse causes micro-arc discharge on the anode, generating localized high temperatures (>1000 °C), vaporizing water to form bubbles as channels for charge transfer and arc discharge, resulting in a composite coating. Li et al. [106] present, in Figure 9B, the formation mechanism of MAO coatings in three electrolytes. M1 (NaAlO2-NaH2PO4) forms Al2O3 with Fe2O3/FePO4 via arc discharge and Fe oxidation. M2 adds Na2CO3, enhancing arc discharge and Al2O3 growth. M3 with Na2CO3-Na2B4O7 inhibits Fe compounds, promotes α-Al2O3 via B2O3 dissolving Fe2O3, forming dense, corrosion-resistant coatings. Both mechanisms involve the MAO process, using aluminate and phosphate electrolyte systems to generate Al2O3-based coatings. Borate reduces impurities by reacting with iron oxides and promotes α-Al2O3 crystallization. High-temperature micro-arc discharge plays a role in both, and both aim to reduce coating defects (micropores, cracks), enhance densification by regulating electrolyte reactions, and improve corrosion and wear resistance. However, they exhibit significant differences in aspects such as pretreatment process, key electrolyte additives, coating growth basis, and iron oxide regulation methods.
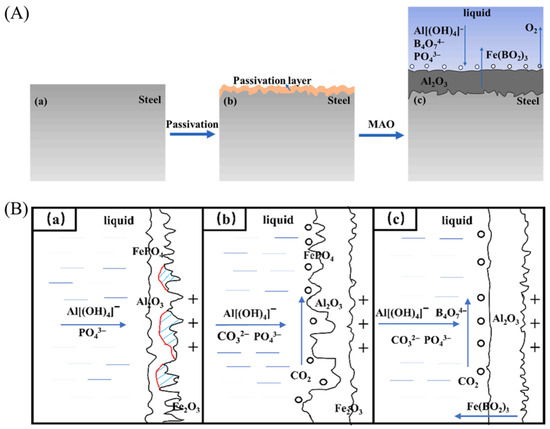
Figure 9.
Studies on MAO applied to steel surfaces. (A) Investigations into MAO methods for non-valve metal low-carbon steel surfaces utilizing a passivation layer as the foundation, including flowcharts (a–c), along with a mechanism diagram (c) [104]. (B) Preparation, characterization, and corrosion performance of α-Al2O3 coatings on 10B21 carbon steel via MAO, illustrating the formation mechanisms of MAO coatings produced with different electrolytes: (a) M1, (b) M2, and (c) M3 [106].
Performance varies significantly among different pretreatment + MAO composite coatings, with a focus on high-temperature oxidation resistance, corrosion resistance, wear resistance, area-specific resistance (for SOFC applications), and hardness:
(1) High-temperature oxidation resistance. Feng et al. [97,98] researched HEMAA-LaCrO3 coatings after oxidation at 850 °C for 200 h in air. The three-layer structure (NiFe2O4 outer layer inhibiting Cr volatilization, conductive LaCrO3 middle layer, Cr2O3 inner layer resisting oxidation) exhibits a significantly lower oxidation rate than uncoated steel (mass gain: ~0.5 mg/cm2 vs. 2.0 mg/cm2 for uncoated steel), with ASR of 0.035–0.055 Ω·cm2 (uncoated steel: 0.1 Ω·cm2) [97]. For HEMAA-FeAl coatings, after oxidation at 800–1000 °C for 100 h in air, Al diffusion forms dense Al2O3 protective films, outperforming FeAl electrodes [99].
(2) Corrosion and wear resistance. Kumruoglu et al. [11] explored the potential of wire arc spraying technology to prepare Al-Mg6, Al-Si12, and pure Al coatings on low-carbon steel surfaces, and further enhanced the corrosion and wear resistance of the coatings through MAO to address the issues of steel materials being prone to corrosion or wear. Hardness tests indicated that the Al-Si12 coating (140 Hk) exhibited the highest hardness, followed by Al-Mg6 (80 Hk), and pure Al (45 Hk) the lowest, with MAO significantly enhancing the coating hardness. High-performance composite coatings were successfully prepared on the surface of S355 offshore steel by combining laser cladding with MAO [12]. When the MAO current density was 5 A/dm2, the coating exhibits the optimal overall performance: high hardness (1424.3 HV 0.2), good bonding strength (28.4 N), and remarkable corrosion resistance (corrosion current density of 1.21 × 10−8 A/cm2). This effectively inhibits the synergistic damage of corrosion–wear in the marine environment, providing a new technical solution for the protection of offshore steel [12].
(3) Area-specific resistance (ASR). After oxidation to form LaCrO3 coatings, ASR remains < 40 mΩ·cm2 for 500 h, far lower than uncoated steel (288 mΩ·cm2), meeting SOFC requirements (≤0.1 Ω·cm2) [97].
(4) Hardness and mechanical properties. The average thickness of the ESD coating is 17.5 ± 3 μm, while that of the duplex coating is 32.3 ± 5 μm (significantly thickened by MAO) [102]. Hardness values follow the order: St35 steel (270 HV) < ESD coating (510 HV) < duplex coating (940 HV). The enhanced hardness of the duplex coating is primarily attributed to the formation of α-Al2O3 (with a theoretical hardness of 2500 HV). Scratch test results show that the critical loads of the duplex coating (Lc1 = 29.530 N, Lc3 = 50.932 N) are significantly higher than those of the ESD coating (Lc1 = 15.199 N, Lc3 = 36.123 N), due to its greater thickness and hardness, which improve load-bearing capacity [102]. For uncoated steel, the friction coefficient rapidly increases in the initial stage and then stabilizes; for both ESD and duplex coatings, the friction coefficient is higher in the initial stage (attributed to their porous and rough surfaces) but stabilizes to a lower level than that of uncoated steel, demonstrating superior wear resistance [102].
3. Summary and Outlook
3.1. Summary
MAO, also known as plasma electrolytic oxidation, has emerged as a versatile electrochemical surface modification technique for fabricating in situ ceramic coatings on diverse metallic materials, including lightweight alloys (Al, Mg, Ti), high-entropy alloys (HEAs), and steel (via hybrid processes). This review systematically synthesizes recent advancements in MAO technology, focusing on material-specific processing strategies, coating performance optimization, and critical challenges, and outlines future research directions to expand its industrial applications [1,2,3].
Aluminum alloys: MAO enables rapid formation of dense α-Al2O3/γ-Al2O3 coatings (10–100 μm thick) with hardness up to 2500 HV, significantly enhancing wear resistance (wear rate: 3.00–5.00 × 10−6 mm3/N·m) and corrosion protection [6,25,33]. Electrolyte additives (e.g., SiC, graphene) and process controls (current density, ultrasonic assistance) further optimize densification, reducing porosity to <2% and extending salt spray resistance (e.g., 530 h with 1.5 mg/cm2 mass loss) [26,32].
Magnesium alloys: MAO addresses Mg’s poor corrosion and wear resistance by forming bilayer coatings (inner dense MgO/Mg2SiO4, outer porous layer). Electrolyte systems (silicate, aluminate, phosphate) and post-treatments (laser melting, sealing) enhance corrosion resistance (e.g., reducing jcorr from 43.52 μA/cm2 to 0.351 μA/cm2 in aluminate–fluoride electrolytes) [53,57]. Bioactive MAO coatings (Ca/P-doped) on Mg alloys exhibit excellent biocompatibility (cell survival > 95%) and promote bone integration, making them promising for biodegradable implants [62,64].
Titanium alloys: MAO generates porous TiO2-based coatings (anatase/rutile) with tailored bioactivity. Calcium phosphate electrolytes enable hydroxyapatite (HA) formation, while Ag/Cu doping confers antibacterial properties (inhibition > 95% against S. aureus) [76]. UV-irradiated MAO coatings achieve superhydrophilicity (contact angle 0°), accelerating apatite nucleation in simulated body fluid (SBF) within 13–17 days [80]. These coatings also improve titanium–porcelain bonding strength (39.6% increase) and high-temperature oxidation resistance (stable at 800 °C) [83,85].
High-entropy alloys (HEAs): MAO mitigates HEAs’ poor high-temperature oxidation by forming composite oxide layers (Al2O3, Cr2O3, SiO2) that suppress volatile oxide (V2O5, MoO3) formation. Optimized electrolytes (Na2SiO3, Na2B4O7) and voltage controls (420 V) reduce porosity and surface roughness (from 16.5 μm to 7.2 μm), lowering oxidation mass gain (e.g., from 12.91 mg/cm2 to 8.67 mg/cm2 for AlTiNbMo0.5Ta0.5Zr) [91,94].
Steel: Direct MAO is hindered by steel’s high conductivity, but hybrid processes (pretreatment + MAO) enable ceramic coating growth. Techniques like hot-dip aluminizing, laser cladding, and high-energy micro-arc alloying (HEMAA) form valve metal interlayers (Al, FeAl), followed by MAO to produce Al2O3-based coatings. These hybrid coatings enhance high-temperature oxidation resistance (mass gain < 0.5 mg/cm2 vs. 2.0 mg/cm2 for uncoated steel), corrosion resistance (jcorr = 1.21 × 10−8 A/cm2), and hardness (up to 1424 HV) [97,102].
3.2. Outlook
Despite progress, critical challenges persist: (1) Coating uniformity: complex geometries (e.g., aerospace components) suffer from uneven thickness and porosity due to localized discharge dynamics [39]. (2) Efficiency and scalability: traditional MAO struggles with large-area or intricate parts; emerging scanning MAO (SMAO) and ultrasonic assistance processes show promise but require further optimization [37,38]. (3) Long-term stability: residual stresses and microdefects (pores, cracks) compromise fatigue life and high-temperature durability [2]. (4) Environmental sustainability: current electrolytes often contain hazardous components (e.g., strong alkalis); eco-friendly systems (biodegradable additives, low-toxicity salts) demand development [26,32].
Future research directions are as follows: (1) Multifunctional coatings: efforts should focus on integrating self-healing (via encapsulated corrosion inhibitors), antibacterial (Ag/Cu doping), and tribological (graphene/SiC reinforcement) properties to meet demands in biomedical, aerospace, and energy sectors. (2) Eco-friendly electrolytes: key strategies include designing low-pH, non-toxic electrolytes (e.g., phosphate-free, bio-derived stabilizers) to reduce environmental impact without sacrificing coating quality. (3) Mechanistic understanding: critical steps include advanced characterization (e.g., operando XRD, in situ Raman spectroscopy) and computational modeling (DFT, phase-field simulations) to clarify discharge dynamics, oxide growth mechanisms, and interface bonding. (4) Process innovation: scalable techniques such as robotic SMAO and hybrid energy fields (ultrasonic, magnetic fields) aim to enhance uniformity and efficiency for large/complex components.
In summary, MAO technology has transformed the surface engineering of metals, offering tailored solutions for corrosion, wear, and functionalization. By addressing current challenges and advancing interdisciplinary research, MAO is poised to unlock new applications in next-generation materials, ranging from biodegradable medical implants to high-temperature aerospace components.
Author Contributions
Writing—original draft preparation, N.L.; conceptualization, N.L., H.W., Z.H., D.X., L.X. and X.C.; methodology, N.L., H.W., Z.H., Q.L., X.C. and Y.F.; writing—review and editing, L.X. and Y.F.; investigation, N.L., H.W., Z.H., D.X., Q.L., D.C. and Y.F.; formal analysis, N.L., H.W., Z.H., D.X., Q.L., D.C., X.C. and L.X.; data curation, H.W., Z.H., D.X., Q.L., D.C. and L.X.; resources, H.W., Z.H., D.X., Q.L. and Y.F.; supervision L.X. and Y.F. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Key R&D and Promotion Project of the Henan Province (Science and Technology Research) (No. 252102220078), the “Deputy General Manager of Technology” of Henan Province (HNFZ 20240193).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
Author Ningning Li was employed by Luoyang Aijia Mold Manufacturing Co., Ltd. Author Qiuzhen Liu was employed by Zhengzhou Jintai Can Manufacturing Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Golubkov, P.E.; Pecherskaya, E.A.; Artamonov, D.V.; Zinchenko, T.O.; Gerasimova, Y.E.; Rozenberg, N.V. Electrophysical model of the micro-arc oxidation process. Russ. Phys. J. 2020, 62, 2137–2144. [Google Scholar] [CrossRef]
- Dai, W.B.; Zhang, C.; Yue, H.T.; Li, Q.; Guo, C.G.; Zhang, J.Z.; Zhao, G.C.; Yang, X.L. A review on the fatigue performance of micro-arc oxidation coated Al alloys with micro-defects and residual stress. J. Mater. Res. Technol. 2023, 25, 4554–4581. [Google Scholar] [CrossRef]
- Lin, Z.; Wang, T.; Yu, X.; Sun, X.; Yang, H. Functionalization treatment of micro-arc oxidation coatings on magnesium alloys: A review. J. Alloys Compd. 2021, 879, 160453. [Google Scholar] [CrossRef]
- Ming, X.; Wu, Y.; Zhang, Z.; Li, Y. Micro-arc oxidation in titanium and its alloys: Development and potential of implants. Coatings 2023, 13, 2064. [Google Scholar] [CrossRef]
- Liu, X.D.; Wang, C.X.; Lei, J.L.; Song, P.; Huang, T.H.; Zhang, X.W.; Yang, T.F.; Ji, V. Micro-arc oxidation of aluminum alloys: Mechanism, defects, and corrosion resistance. Adv. Eng. Mater. 2025, 27, 2402748. [Google Scholar] [CrossRef]
- Rodriguez, L.; Paris, J.Y.; Denape, J.; Delbé, K. Micro-arcs oxidation layer formation on aluminium and coatings tribological properties—A review. Coatings 2023, 13, 373. [Google Scholar] [CrossRef]
- Chi, J.W.; Zhang, H.L.; Song, S.Y.; Zhang, W.S.; He, X.Y.; Nong, Z.S.; Cui, X.; Liu, T.; Man, T.N. The impact of pre-and post-treatment processes on corrosion resistance of micro-arc oxidation coatings on Mg alloys: A systematic review. Materials 2025, 18, 723. [Google Scholar] [CrossRef]
- Wang, S.P.; Zhou, L.; Li, C.J.; Li, Z.X.; Li, H.Z.; Yang, L.J. Micrographic properties of composite coatings prepared on TA2 substrate by hot-dipping in Al-Si alloy and using micro-arc oxidation technologies (MAO). Coatings 2020, 10, 374. [Google Scholar] [CrossRef]
- dos Santos, R.F.; Kuroda, P.A.; de Almeida, G.S.; Zambuzzi, W.F.; Grandini, C.R.; Afonso, C.R. New MAO coatings on multiprincipal equimassic β TiNbTaZr and TiNbTaZrMo alloys. BME Adv. 2025, 9, 100139. [Google Scholar] [CrossRef]
- Yang, F.; Du, C.; Tao, S.Y.; Chang, Y.Q.; Nie, Z.H.; Wang, Z.; Lu, H.L. Study on the effect and growth mechanism of micro-arc oxidation coating on AlCoCrFeNi high entropy alloy. J. Alloys Compd. 2025, 1020, 179469. [Google Scholar] [CrossRef]
- Kumruoglu, L.C.; Ustel, F.; Ozel, A.; Mimaroglu, A. Micro arc oxidation of wire arc sprayed Al-Mg6, Al-Si12, Al coatings on low alloyed steel. Engineering 2011, 3, 680–690. [Google Scholar] [CrossRef]
- He, X.; Song, R.G.; Kong, D.J. Microstructure and corrosion behaviours of composite coatings on S355 offshore steel prepared by laser cladding combined with micro-arc oxidation. Appl. Surf. Sci. 2019, 497, 143703. [Google Scholar] [CrossRef]
- Yang, C.; Sun, Z.M.; Wang, C.Y.; Huang, A.H.; Ye, Z.S.; Ying, T.; Zhou, L.P.; Xiao, S.; Chu, P.K.; Zeng, X.Q. A self-sealing and self-healing MAO corrosion-resistant coating on aluminum alloy by in situ growth of CePO4/Al2O3. Corros. Sci. 2025, 245, 112706. [Google Scholar] [CrossRef]
- Wang, S.; Gu, Y.; Geng, Y.; Liang, J.; Zhao, J.; Kang, J. Investigating local corrosion behavior and mechanism of MAO coated 7075 aluminum alloy. J. Alloys Compd. 2020, 826, 153976. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, L.; Liu, H.; Li, W. Investigation of MAO coating growth mechanism on aluminum alloy by two-step oxidation method. Appl. Surf. Sci. 2014, 293, 12–17. [Google Scholar] [CrossRef]
- Tseng, C.C.; Lee, J.L.; Kuo, T.H.; Kuo, S.N.; Tseng, K.H. The influence of sodium tungstate concentration and anodizing conditions on microarc oxidation (MAO) coatings for aluminum alloy. Surf. Coat. Technol. 2012, 206, 3437–3443. [Google Scholar] [CrossRef]
- Wang, J.; Huang, S.; Huang, H.; He, M.; Wangyang, P.; Gu, L. Effect of micro-groove on microstructure and performance of MAO ceramic coating fabricated on the surface of aluminum alloy. J. Alloys Compd. 2019, 777, 94–101. [Google Scholar] [CrossRef]
- Huang, H.; Wei, X.; Yang, J.; Wang, J. Influence of surface micro grooving pretreatment on MAO process of aluminum alloy. Appl. Surf. Sci. 2016, 389, 1175–1181. [Google Scholar] [CrossRef]
- Shehadeh, L.; Mohamed, K.; Al-Qawabeha, U.; Abu-Jdayil, B. The Role of Copper Incorporation in Improving the Electrical Insulation Properties of Microarc Oxidation Coatings on Aluminum Alloys. Eng. Sci. 2025, 33, 1380. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, Y.; Li, J.; Zhao, J.; Ma, C.; Zhao, G. Analysis of frictional performance of microarc oxidized aluminum alloys in various media: Integration of experimental and MD simulation approaches. Mater. Today. Commun. 2025, 46, 112528. [Google Scholar] [CrossRef]
- Yin, J.H.; Cui, Y.W.; Shang, Y.Y.; Lu, L.H.; Du, R.W.; Zhu, M. Investigation of third harmonic laser-induced damage on micro-arc oxidized and composite-coated aluminum alloy 7075. J. Alloys Compd. 2025, 1032, 181087. [Google Scholar] [CrossRef]
- Yu, D.L.; Jiang, B.; Qi, X.; Wang, C.; Song, R.G. Effect of current density on microstructure, mechanical behavior and corrosion resistance of black MAO coating on 6063 aluminum alloy. Mater. Chem. Phys. 2024, 326, 129800. [Google Scholar] [CrossRef]
- Long, B.H.; Wu, H.H.; Long, B.Y.; Wang, J.B.; Wang, N.D.; Lü, X.Y.; Jin, Z.S.; Bai, Y.Z. Characteristics of electric parameters in aluminium alloy MAO coating process. J. Phys. D. Appl. Phys. 2005, 38, 3491. [Google Scholar] [CrossRef]
- Li, H.X.; Li, W.J.; Song, R.G.; Ji, Z.G. Effects of different current densities on properties of MAO coatings embedded with and without α-Al2O3 nanoadditives. Mater. Sci. Technol. 2012, 28, 565–568. [Google Scholar] [CrossRef]
- Cao, G.P.; Song, R.G. Microstructure and properties of ceramic coatings prepared by micro-arc oxidation on 7075 aluminum alloy. Mater. Res. Express 2018, 5, 026407. [Google Scholar] [CrossRef]
- Wang, P.; Wu, T.; Xiao, Y.T.; Zhang, L.; Pu, J.; Cao, W.J.; Zhong, X.M. Characterization of micro-arc oxidation coatings on aluminum drillpipes at different current density. Vacuum 2017, 142, 21–28. [Google Scholar] [CrossRef]
- Wang, J.H.; Du, M.H.; Han, F.Z.; Yang, J. Effects of the ratio of anodic and cathodic currents on the characteristics of micro-arc oxidation ceramic coatings on Al alloys. Appl. Surf. Sci. 2014, 292, 658–664. [Google Scholar] [CrossRef]
- Tran, Q.P.; Kuo, Y.C.; Sun, J.K.; He, J.L.; Chin, T.S. High quality oxide-layers on Al-alloy by micro-arc oxidation using hybrid voltages. Surf. Coat. Technol. 2016, 303, 61–67. [Google Scholar] [CrossRef]
- Li, X.Y.; Li, X.G.; Li, Y.; Dong, C.F.; Tian, H.P.; Wang, S.X.; Zhao, Q. Growth mechanism of micro-arc oxidation film on 6061 aluminum alloy. Mater. Res. Express 2019, 6, 066404. [Google Scholar] [CrossRef]
- Jin, F.Y.; Chu, P.K.; Tong, H.H.; Zhao, J. Improvement of surface porosity and properties of alumina films by incorporation of Fe micrograins in micro-arc oxidation. Appl. Surf. Sci. 2006, 253, 863–868. [Google Scholar] [CrossRef]
- Li, W.P.; Qian, Z.Y.; Liu, X.H.; Zhu, L.Q.; Liu, H.C. Investigation of micro-arc oxidation coating growth patterns of aluminum alloy by two-step oxidation method. Appl. Surf. Sci. 2015, 356, 581–586. [Google Scholar] [CrossRef]
- Chen, Q.Z.; Jiang, Z.Q.; Tang, S.G.; Dong, W.B.; Tong, Q.; Li, W.Z. Influence of graphene particles on the micro-arc oxidation behaviors of 6063 aluminum alloy and the coating properties. Appl. Surf. Sci. 2017, 423, 939–950. [Google Scholar] [CrossRef]
- Arslan, E.; Totik, Y.; Demirci, E.E.; Vangolu, Y.; Alsaran, A.; Efeoglu, I. High temperature wear behavior of aluminum oxide layers produced by AC micro arc oxidation. Surf. Coat. Technol. 2009, 204, 829–833. [Google Scholar] [CrossRef]
- Li, Z.Y.; Cai, Z.B.; Cui, Y.; Liu, J.H.; Zhu, M.H. Effect of oxidation time on the impact wear of micro-arc oxidation coating on aluminum alloy. Wear 2019, 426, 285–295. [Google Scholar] [CrossRef]
- Venugopal, A.; Panda, R.; Manwatkar, S.; Sreekumar, K.; Krishna, L.R.; Sundararajan, G. Effect of micro arc oxidation treatment on localized corrosion behaviour of AA7075 aluminum alloy in 3.5% NaCl solution. Trans. Nonferrous Metal. Soc. 2012, 22, 700–710. [Google Scholar] [CrossRef]
- Xin, S.G.; Song, L.X.; Zhao, R.G.; Hu, X.F. Properties of aluminium oxide coating on aluminium alloy produced by micro-arc oxidation. Surf. Coat. Technol. 2005, 199, 184–188. [Google Scholar] [CrossRef]
- Ma, G.F.; Li, Z.P.; Zhao, X.R.; Wang, Z.Y.; Sun, S.N.; Yang, Y.H.; Sun, Y.; Wang, S.Y.; Ren, S.T.; Kou, R.H. Growth mechanism and product formation of Micro-arc oxide film layers on aluminum matrix composites: An analytical experimental and computational simulation study. Appl. Surf. Sci. 2025, 684, 161968. [Google Scholar] [CrossRef]
- Liu, Z.H.; Lu, H.L.; Zhao, Z.Y.; Zhu, Z.Q.; Li, S.B. Influence of ultrasonic power modulation on the optimisation of aluminium alloy micro-arc oxidation coating properties. Appl. Surf. Sci. 2025, 679, 161067. [Google Scholar] [CrossRef]
- Zhu, Z.Q.; Lu, H.L.; Shen, T.J.; Wang, Z.Z.; Xu, G.S.; Liu, Z.H.; Yang, H. Performance study of scanning micro-arc oxidation ceramic coatings on aluminum alloys based on different electrolyte flow rates. Surf. Coat. Technol. 2025, 496, 131686. [Google Scholar] [CrossRef]
- Zhu, Z.Q.; Li, S.B.; Xue, Z.C.; Liu, Z.H.; Tu, N.; Lu, H.L. Performance evaluation of scanning micro-arc oxidation ceramic coating on aluminum alloy under different current working modes. Mater. Chem. Phys. 2025, 336, 130538. [Google Scholar] [CrossRef]
- Narayanan, T.S.; Park, I.S.; Lee, M.H. Strategies to improve the corrosion resistance of microarc oxidation (MAO) coated magnesium alloys for degradable implants: Prospects and challenges. Prog. Mater. Sci. 2014, 60, 1–71. [Google Scholar] [CrossRef]
- Zhang, R.F.; Zhang, S.F.; Duo, S.W. Influence of phytic acid concentration on coating properties obtained by MAO treatment on magnesium alloys. Appl. Surf. Sci. 2009, 255, 7893–7897. [Google Scholar] [CrossRef]
- Li, Z.; Yu, Q.; Zhang, C.; Liu, Y.; Liang, J.; Wang, D.; Zhou, F. Synergistic effect of hydrophobic film and porous MAO membrane containing alkynol inhibitor for enhanced corrosion resistance of magnesium alloy. Surf. Coat. Technol. 2019, 357, 515–525. [Google Scholar] [CrossRef]
- Ezhilselvi, V.; Nithin, J.; Balaraju, J.N.; Subramanian, S. The influence of current density on the morphology and corrosion properties of MAO coatings on AZ31B magnesium alloy. Surf. Coat. Technol. 2016, 288, 221–229. [Google Scholar] [CrossRef]
- Guo, C.; Li, Y.; Qi, C.; Sun, H.; Zhang, D.; Wan, Y. Effect of solvent acids on the microstructure and corrosion resistance of chitosan films on MAO-treated AZ31B magnesium alloy. Int. J. Biol. Macromol. 2024, 277, 134349. [Google Scholar] [CrossRef]
- Dou, J.; Yu, H.; Chen, C.; Ma, R.L.W.; Yuen, M.M.F. Preparation and microstructure of MAO/CS composite coatings on Mg alloy. Mater. Lett. 2020, 271, 127729. [Google Scholar] [CrossRef]
- Ungan, G.; Cakir, A. Investigation of MgO effect on bioactivity of coatings produced by MAO. Surf. Coat. Technol. 2015, 282, 52–60. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, W.; Xu, T.; Li, H.; Jiang, B.; Miao, X. Preparation and corrosion resistance of a self-sealing hydroxyapatite-MgO coating on magnesium alloy by microarc oxidation. Ceram. Int. 2022, 48, 13676–13683. [Google Scholar] [CrossRef]
- Vladimirov, B.V.; Krit, B.L.; Lyudin, V.B.; Morozova, N.V.; Rossiiskaya, A.D.; Suminov, I.V.; Epel’Feld, A.V. Microarc oxidation of magnesium alloys: A review. Surf. Eng. Appl. Electrochem. 2014, 50, 195–232. [Google Scholar] [CrossRef]
- Pesode, P.; Barve, S.; Dayane, S. Antibacterial coating on magnesium alloys by MAO for biomedical applications. Res. Biomed. Eng. 2024, 40, 409–433. [Google Scholar] [CrossRef]
- Xiong, Y.; Lu, C.; Wang, C.; Song, R. Degradation behavior of n-MAO/EPD bio-ceramic composite coatings on magnesium alloy in simulated body fluid. J. Alloys Compd. 2015, 625, 258–265. [Google Scholar] [CrossRef]
- Razavi, M.; Fathi, M.; Savabi, O.; Vashaee, D.; Tayebi, L. Biodegradable magnesium alloy coated by fluoridated hydroxyapatite using MAO/EPD technique. Surf. Eng. 2014, 30, 545–551. [Google Scholar] [CrossRef]
- Guo, H.F.; An, M.Z.; Xu, S.; Huo, H.B. Formation of oxygen bubbles and its influence on current efficiency in micro-arc oxidation process of AZ91D magnesium alloy. Thin Solid Films 2005, 485, 53–58. [Google Scholar] [CrossRef]
- Guo, H.F.; An, M.Z.; Huo, H.B.; Xu, S.; Wu, L.J. Microstructure characteristic of ceramic coatings fabricated on magnesium alloys by micro-arc oxidation in alkaline silicate solutions. Appl. Surf. Sci. 2006, 252, 7911–7916. [Google Scholar] [CrossRef]
- Zhang, R.F.; Zhang, S.F. Formation of micro-arc oxidation coatings on AZ91HP magnesium alloys. Corros. Sci. 2009, 51, 2820–2825. [Google Scholar] [CrossRef]
- Tang, H.; Han, Y.; Wu, T.; Tao, W.; Jian, X.; Wu, Y.; Xu, F. Synthesis and properties of hydroxyapatite-containing coating on AZ31 magnesium alloy by micro-arc oxidation. Appl. Surf. Sci. 2017, 400, 391–404. [Google Scholar] [CrossRef]
- Guo, H.F.; An, M.Z. Growth of ceramic coatings on AZ91D magnesium alloys by micro-arc oxidation in aluminate–fluoride solutions and evaluation of corrosion resistance. Appl. Surf. Sci 2005, 246, 229–238. [Google Scholar] [CrossRef]
- Zhao, L.C.; Cui, C.X.; Wang, Q.Z.; Bu, S.J. Growth characteristics and corrosion resistance of micro-arc oxidation coating on pure magnesium for biomedical applications. Corros. Sci. 2010, 52, 2228–2234. [Google Scholar] [CrossRef]
- Durdu, S.; Aytac, A.; Usta, M. Characterization and corrosion behavior of ceramic coating on magnesium by micro-arc oxidation. J. Alloys Compd. 2011, 509, 8601–8606. [Google Scholar] [CrossRef]
- Muhaffel, F.; Cimenoglu, H. Development of corrosion and wear resistant micro-arc oxidation coating on a magnesium alloy. Surf. Coat. Tech. 2019, 357, 822–832. [Google Scholar] [CrossRef]
- Durdu, S.; Usta, M. Characterization and mechanical properties of coatings on magnesium by micro arc oxidation. Appl. Surf. Sci. 2012, 261, 774–782. [Google Scholar] [CrossRef]
- Pan, Y.K.; Chen, C.Z.; Wang, D.G.; Yu, X. Microstructure and biological properties of micro-arc oxidation coatings on ZK60 magnesium alloy. J. Biomed. Mater. Res. B 2012, 100, 1574–1586. [Google Scholar] [CrossRef]
- Pan, Y.K.; Chen, C.Z.; Wang, D.G.; Lin, Z.Q. Preparation and bioactivity of micro-arc oxidized calcium phosphate coatings. Mater. Chem. Phys. 2013, 141, 842–849. [Google Scholar] [CrossRef]
- Ma, W.H.; Liu, Y.J.; Wang, W.; Zhang, Y.Z. Improved biological performance of magnesium by micro-arc oxidation. Braz. J. Med. Biol. Res. 2015, 48, 214–225. [Google Scholar] [CrossRef]
- Zhang, Z.Q.; Wang, L.; Zeng, M.Q.; Zeng, R.C.; Kannan, M.B.; Lin, C.G.; Zheng, Y.F. Biodegradation behavior of micro-arc oxidation coating on magnesium alloy-from a protein perspective. Bioact. Mater. 2020, 5, 398–409. [Google Scholar] [CrossRef]
- Shao, Y.; Han, X.; Ma, C.; Wei, Y.; Zhu, X.; Xu, J. The thermal dynamic simulation and microstructure characterization of micro-arc oxidation (MAO) films on magnesium AZ31 irradiated by high-intensity pulsed ion beam. Appl. Surf. Sci. 2025, 682, 161705. [Google Scholar] [CrossRef]
- Li, Y.; Yang, C.; Zhao, H.; Qu, S.; Li, X.; Li, Y. New developments of Ti-based alloys for biomedical applications. Materials 2014, 7, 1709–1800. [Google Scholar] [CrossRef]
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Zhu, S.L.; Wang, X.M.; Qin, F.X.; Inoue, A. A new Ti-based bulk glassy alloy with potential for biomedical application. Mater. Sci. Eng. A 2007, 459, 233–237. [Google Scholar] [CrossRef]
- Liao, S.C.; Chang, C.T.; Chen, C.Y.; Lee, C.H.; Lin, W.L. Functionalization of pure titanium MAO coatings by surface modifications for biomedical applications. Surf. Coat. Technol. 2020, 394, 125812. [Google Scholar] [CrossRef]
- Xi, F.Q.; Zhang, X.W.; Kang, Y.Y.; Wen, X.Y.; Liu, Y. Mechanistic analysis of improving the corrosion performance of MAO coatings on Ti-6Al-4V alloys by annealing. Surf. Coat. Technol. 2024, 476, 130264. [Google Scholar] [CrossRef]
- Kuroda, P.A.B.; Rossi, M.C.; Grandini, C.R.; Afonso, C.R.M. Assessment of applied voltage on the structure, pore size, hardness, elastic modulus, and adhesion of anodic coatings in Ca-, P-, and Mg-rich produced by MAO in Ti-25Ta-Zr alloys. J. Mater. Res. Technol. 2023, 26, 4656–4669. [Google Scholar] [CrossRef]
- Maj, Ł.; Muhaffel, F.; Jarzębska, A.; Trelka, A.; Trembecka-Wójciga, K.; Kawałko, J.; Kulczyk, M.; Bieda, M.; Çimenoğlu, H. Enhancing the tribological performance of MAO coatings through hydrostatic extrusion of cp-Ti. J. Alloys Compd. 2025, 1010, 178246. [Google Scholar] [CrossRef]
- Han, Y.; Sun, J.; Huang, X. Formation mechanism of HA-based coatings by micro-arc oxidation. Electrochem. Commun. 2008, 10, 510–513. [Google Scholar] [CrossRef]
- Chen, J.Z.; Shi, Y.L.; Wang, L.; Yan, F.Y.; Zhang, F.Q. Preparation and properties of hydroxyapatite-containing titania coating by micro-arc oxidation. Mater. Lett. 2006, 60, 2538–2543. [Google Scholar] [CrossRef]
- Song, W.H.; Ryu, H.S.; Hong, S.H. Antibacterial properties of Ag (or Pt)-containing calcium phosphate coatings formed by micro-arc oxidation. J. Biomed. Mater. Res. A 2009, 88, 246–254. [Google Scholar] [CrossRef]
- Li, H.; Sun, Y.; Zhang, J. Effect of ZrO2 particle on the performance of micro-arc oxidation coatings on Ti6Al4V. Appl. Surf. Sci. 2015, 342, 183–190. [Google Scholar] [CrossRef]
- Yao, Z.Q.; Ivanisenko, Y.; Diemant, T.; Caron, A.; Chuvilin, A.; Jiang, J.Z.; Valiev, R.Z.; Qi, M.; Fecht, H.J. Synthesis and properties of hydroxyapatite-containing porous titania coating on ultrafine-grained titanium by micro-arc oxidation. Acta Biomater. 2010, 6, 2816–2825. [Google Scholar] [CrossRef]
- Xu, L.; Wu, C.; Lei, X.; Zhang, K.; Liu, C.; Ding, J.; Shi, X. Effect of oxidation time on cytocompatibility of ultrafine-grained pure Ti in micro-arc oxidation treatment. Surf. Coat. Technol. 2018, 342, 12–22. [Google Scholar] [CrossRef]
- Han, Y.; Chen, D.; Sun, J.; Zhang, Y.; Xu, K. UV-enhanced bioactivity and cell response of micro-arc oxidized titania coatings. Acta Biomater. 2008, 4, 1518–1529. [Google Scholar] [CrossRef]
- Kung, K.C.; Lee, T.M.; Lui, T.S. Bioactivity and corrosion properties of novel coatings containing strontium by micro-arc oxidation. J. Alloys Compd. 2010, 508, 384–390. [Google Scholar] [CrossRef]
- Cimenoglu, H.; Gunyuz, M.; Kose, G.T.; Baydogan, M.; Uğurlu, F.; Sener, C. Micro-arc oxidation of Ti6Al4V and Ti6Al7Nb alloys for biomedical applications. Mater. Charact. 2011, 62, 304–311. [Google Scholar] [CrossRef]
- Li, J.X.; Zhang, Y.M.; Han, Y.; Zhao, Y.M. Effects of micro-arc oxidation on bond strength of titanium to porcelain. Surf. Coat. Technol. 2010, 204, 1252–1258. [Google Scholar] [CrossRef]
- Yao, J.H.; Wang, Y.; Wu, G.L.; Sun, M.; Wang, M.; Zhang, Q.L. Growth characteristics and properties of micro-arc oxidation coating on SLM-produced TC4 alloy for biomedical applications. Appl. Surf. Sci. 2019, 479, 727–737. [Google Scholar] [CrossRef]
- Hao, G.D.; Zhang, D.Y.; Lou, L.Y.; Yin, L.C. High-temperature oxidation resistance of ceramic coatings on titanium alloy by micro-arc oxidation in aluminate solution. Prog. Nat. Sci. 2022, 32, 401–406. [Google Scholar] [CrossRef]
- Zhou, Y.H.; Chen, P.H.; Huang, D.N.; Wu, Z.Z.; Yang, T.; Kai, J.J.; Yan, M. Micro-arc oxidation for improving high-temperature oxidation resistance of additively manufacturing Ti2AlNb. Surf. Coat. Technol. 2022, 445, 128719. [Google Scholar] [CrossRef]
- George, E.P.; Raabe, D.; Ritchie, R.O. High-entropy alloys. Nat. Rev. Mater. 2019, 4, 515–534. [Google Scholar] [CrossRef]
- Miracle, D.B.; Senkov, O.N. A critical review of high entropy alloys and related concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef]
- Li, W.D.; Xie, D.; Li, D.Y.; Zhang, Y.; Gao, Y.F.; Liaw, P.K. Mechanical behavior of high-entropy alloys. Prog. Mater. Sci. 2021, 118, 100777. [Google Scholar] [CrossRef]
- Cheng, Z.; Yang, W.; Xu, D.; Shao, W.; Chen, J. In situ formation of micro arc oxidation ceramic coating on refractory high entropy alloy. Int. J. Refract. Met. Hard Mater. 2024, 120, 106563. [Google Scholar] [CrossRef]
- Cheng, Z.H.; Yang, W.; Xu, D.P.; Wu, S.K.; Yao, X.F.; Lv, Y.K.; Chen, J. Improvement of high temperature oxidation resistance of micro arc oxidation coated AlTiNbMo0.5Ta0.5Zr high entropy alloy. Mater. Lett. 2020, 262, 127192. [Google Scholar] [CrossRef]
- Senkov, O.N.; Wilks, G.B.; Miracle, D.B.; Chuang, C.P.; Liaw, P.K. Refractory high-entropy alloys. Intermetallics 2010, 18, 1758–1765. [Google Scholar] [CrossRef]
- Ye, F.X.; Jiao, Z.P.; Yan, S.; Guo, L.; Feng, L.Z.; Yu, J.X. Microbeam plasma arc remanufacturing: Effects of Al on microstructure, wear resistance, corrosion resistance and high temperature oxidation resistance of AlxCoCrFeMnNi high-entropy alloy cladding layer. Vacuum 2020, 174, 109178. [Google Scholar] [CrossRef]
- Shi, X.Q.; Yang, W.; Cheng, Z.H.; Shao, W.T.; Xu, D.P.; Zhang, Y.; Chen, J. Influence of micro arc oxidation on high temperature oxidation resistance of AlTiCrVZr refractory high entropy alloy. Int. J. Refract. Met. Hard Mater. 2021, 98, 105562. [Google Scholar] [CrossRef]
- Wang, Z.; Cheng, Z.H.; Zhang, Y.; Shi, X.Q.; Rao, M.; Wu, S.K. Effect of voltage on the microstructure and high-temperature oxidation resistance of micro-arc oxidation coatings on AlTiCrVZr refractory high-entropy alloy. Coatings 2023, 13, 14. [Google Scholar] [CrossRef]
- Zhao, Z.H.; Chen, M.F.; You, C.; Li, W.; Tie, D.; Liu, H.F. Effect of α-Al2O3 additive on the microstructure and properties of MAO coatings prepared on low carbon steel. J. Mater. Res. Technol. 2020, 9, 3875–3884. [Google Scholar] [CrossRef]
- Feng, Z.J.; Zeng, C.L. Oxidation behavior and electrical property of ferritic stainless steel interconnects with a Cr–La alloying layer by high-energy micro-arc alloying process. J. Power Sources 2010, 195, 7370–7374. [Google Scholar] [CrossRef]
- Feng, Z.J.; Zeng, C. LaCrO3-based coatings deposited by high-energy micro-arc alloying process on a ferritic stainless steel interconnect material. J. Power Sources 2010, 195, 4242–4246. [Google Scholar] [CrossRef]
- Guo, P.; Shao, Y.; Zeng, C.; Wu, M.; Li, W. Oxidation characterization of FeAl coated 316 stainless steel interconnects by high-energy micro-arc alloying technique for SOFC. Mater. Lett. 2011, 65, 3180–3183. [Google Scholar] [CrossRef]
- Lu, L.H.; Shen, D.J.; Zhang, J.W.; Song, J.; Li, L. Evolution of micro-arc oxidation behaviors of the hot-dipping aluminum coatings on Q235 steel substrate. Appl. Surf. Sci. 2011, 257, 4144–4150. [Google Scholar] [CrossRef]
- Wang, W.Z.; Feng, S.S.; Li, Z.M.; Chen, Z.G.; Zhao, T.Y. Microstructure and properties of micro-arc oxidation ceramic films on AerMet100 steel. J. Mater. Res. Technol. 2020, 9, 6014–6027. [Google Scholar] [CrossRef]
- Durdu, S.; Aktuğ, S.L.; Korkmaz, K. Characterization and mechanical properties of the duplex coatings produced on steel by electro-spark deposition and micro-arc oxidation. Surf. Coat. Technol. 2013, 236, 303–308. [Google Scholar] [CrossRef]
- Durdu, S.; Korkmaz, K.; Aktuğ, S.L.; Çakır, A. Characterization and bioactivity of hydroxyapatite-based coatings formed on steel by electro-spark deposition and micro-arc oxidation. Surf. Coat. Tech. 2017, 326, 111–120. [Google Scholar] [CrossRef]
- Sun, S.C.; Cheng, B.; Liu, A.; Liu, Z.H.; Xu, G.M.; Liu, P.; Lu, H.L. Research on micro-arc oxidation method based on passivation layer on non-valve metal low-carbon steel surface. Tribol. Int. 2024, 200, 110114. [Google Scholar] [CrossRef]
- Malinovschi, V.; Marin, A.; Moga, S.; Negrea, D. Preparation and characterization of anticorrosive layers deposited by micro-arc oxidation on low carbon steel. Surf. Coat. Technol. 2014, 253, 194–198. [Google Scholar] [CrossRef]
- Li, Y.; Chen, M.; Li, W.; Wang, Q.; Wang, Y.; You, C. Preparation, characteristics and corrosion properties of α-Al2O3 coatings on 10B21 carbon steel by micro-arc oxidation. Surf. Coat. Technol. 2019, 358, 637–645. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).