Slurry Aluminizing of Nickel Electroless Coated Nickel-Based Superalloy
Abstract
1. Introduction
2. Materials and Methods
3. Results
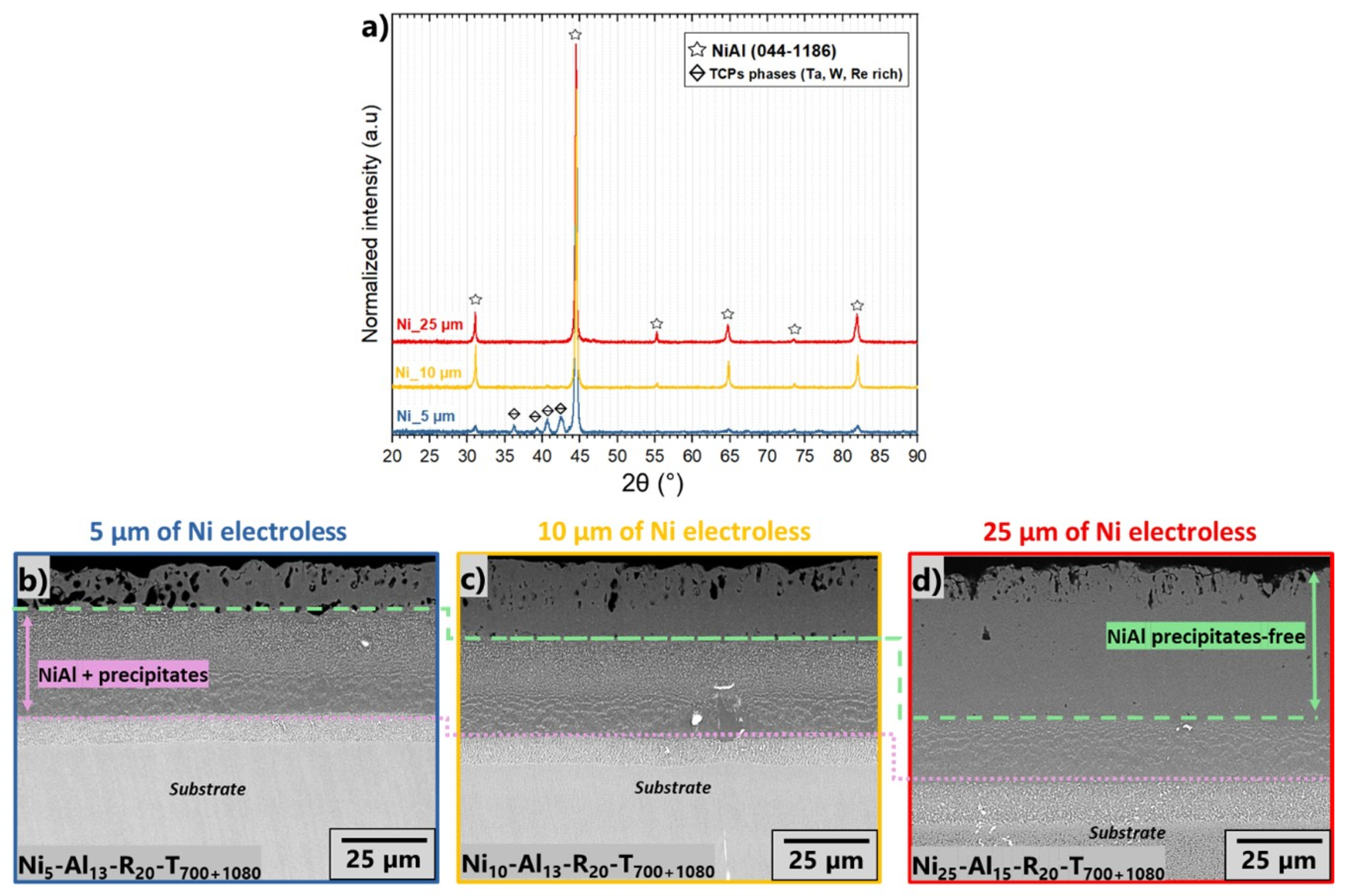
3.1. Nickel Electroless Layer
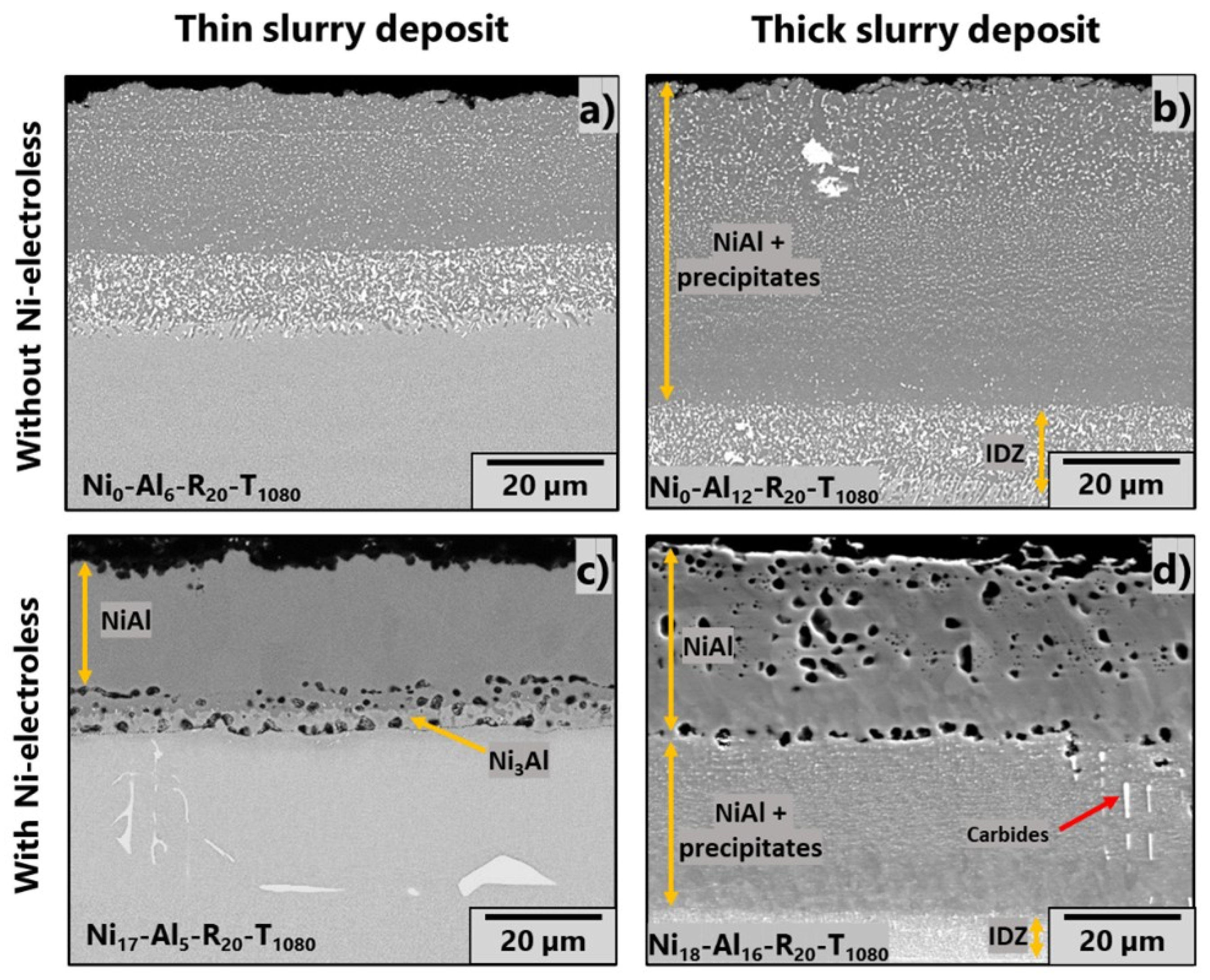
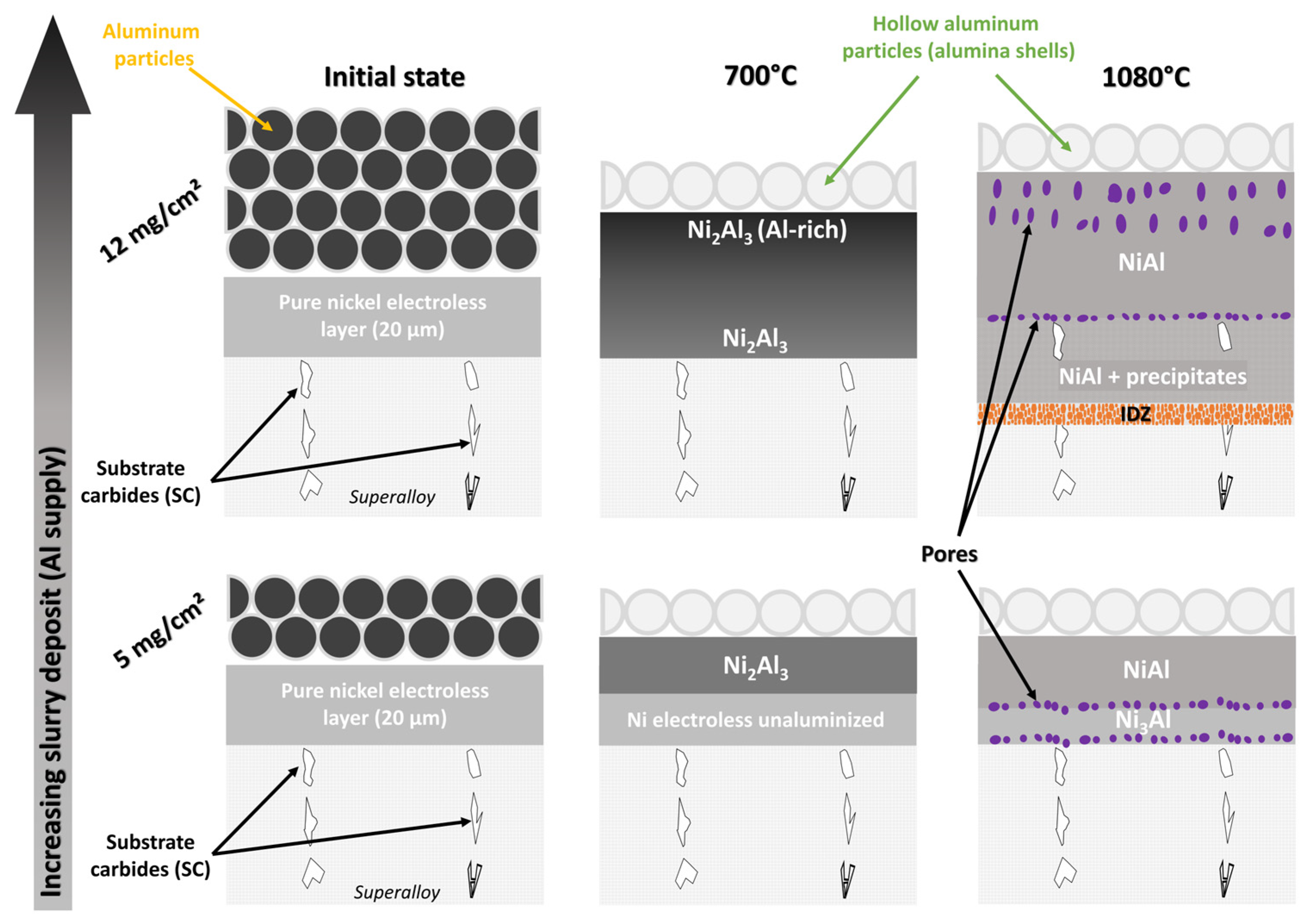
3.2. Effect of Slurry Amount
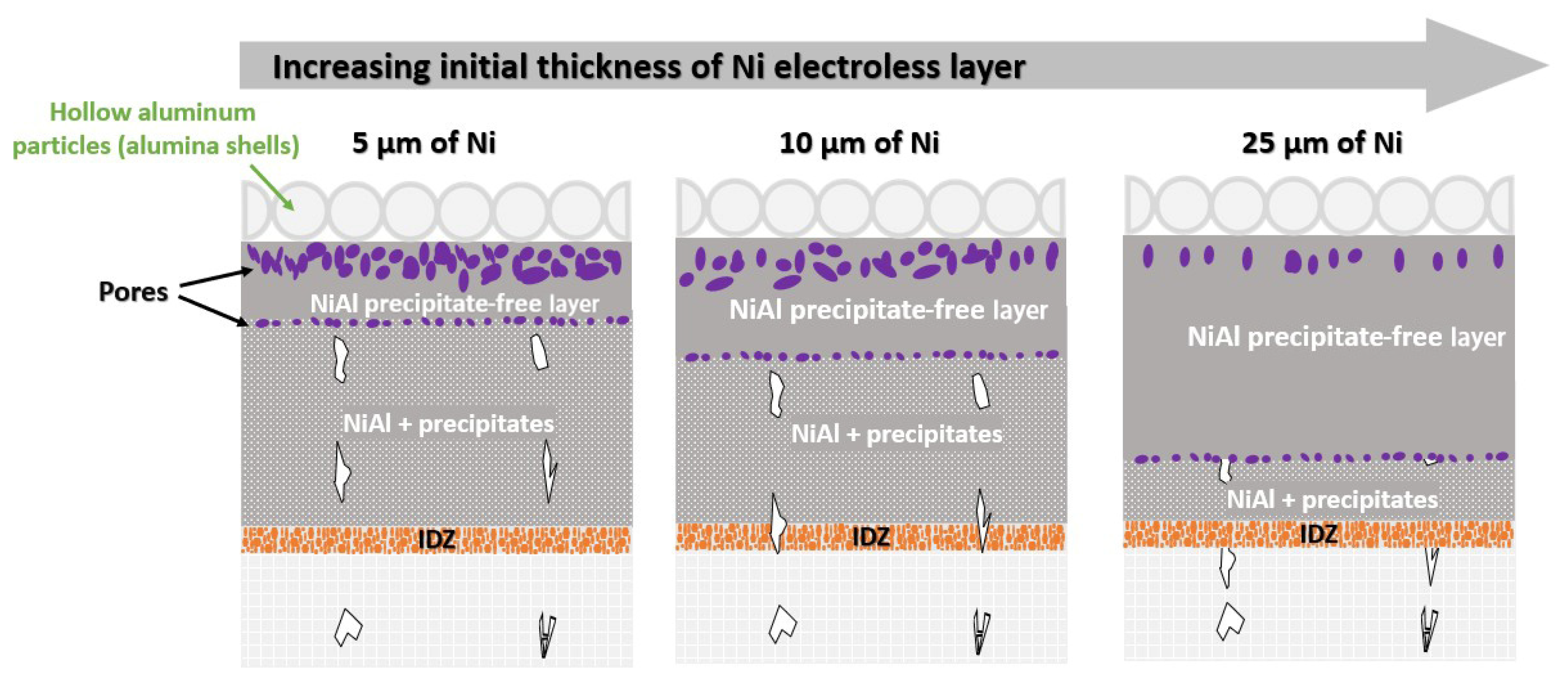
3.3. Effect of Initial Thickness of the Nickel Electroless Layer
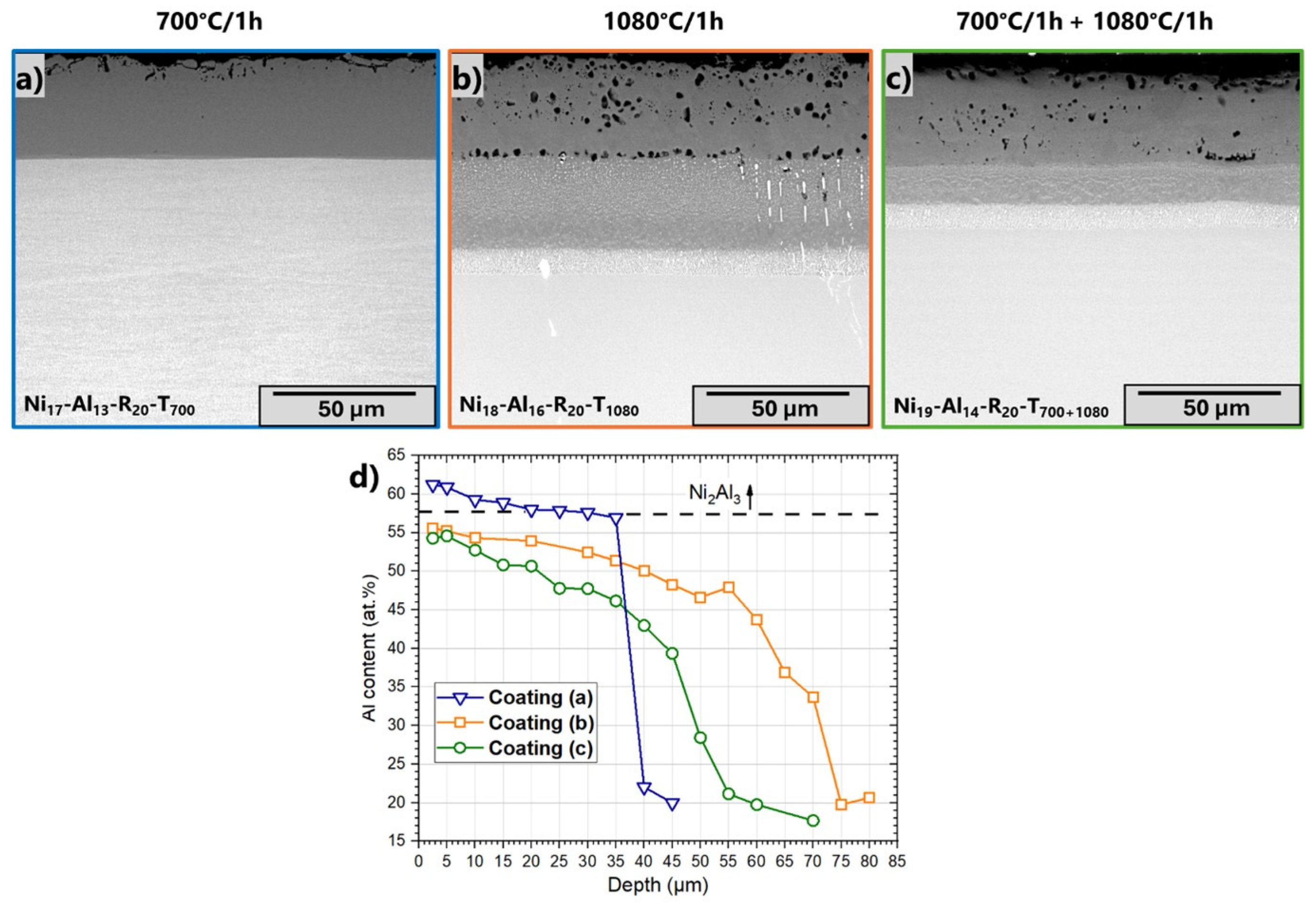
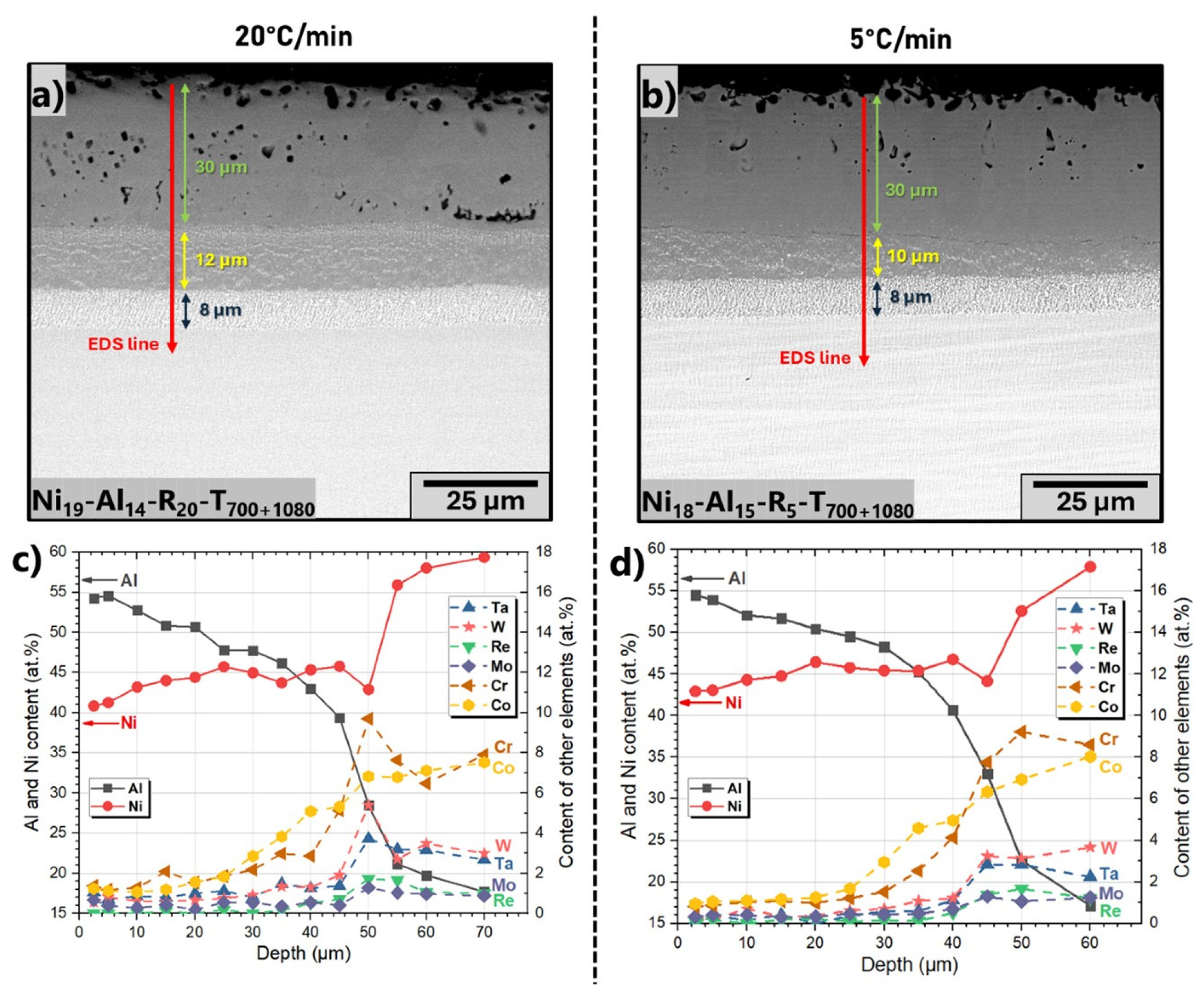
3.4. Influence of Heat Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CVD | Chemical vapor deposition |
| IDZ | Interdiffusion zone |
| TCP | Topologically close-packed phases |
References
- Sims, C.T.; Stoloff, N.S.; Hagel, W.C. Superalloys II; John Wiley & Sons: New York, NY, USA, 1987. [Google Scholar]
- Reed, R.C. The Superalloys: Fundamentals and Applications; Cambridge University Press: New York, NY, USA, 2006. [Google Scholar]
- Cailletaud, G.; Eggeler, G.; Nazé, L.; Cormier, J.; Maurel, V. Nickel Base Single Crystals Across Length Scales; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Mevrel, R.; Pichoir, R. Les revêtements par diffusion. Mater. Sci. Eng. 1987, 88, 1–9. [Google Scholar] [CrossRef]
- Patnaik, P.C. Intermetallic coatings for high temperature applications—A review. Mater. Manuf. Proc. 1989, 4, 133–152. [Google Scholar] [CrossRef]
- Goward, G.W. Progress in coatings for gas turbine airfoils. Surf. Coat. Technol. 1998, 108–109, 73–79. [Google Scholar] [CrossRef]
- Caron, P.; Khan, T. Evolution of Ni-based superalloys for single crystal gas turbine blade applications. Aerosp. Sci. Technol. 1999, 3, 513–523. [Google Scholar] [CrossRef]
- Galetz, M.C. Coatings for Superalloys. In Superalloys; Aliofkhazraei, M., Ed.; InTech: London, UK, 2015. [Google Scholar] [CrossRef]
- Goward, G.W.; Boone, D.H. Mechanisms of formation of diffusion aluminide coatings on nickel-base superalloys. Oxid. Met. 1971, 3, 475–495. [Google Scholar] [CrossRef]
- Bonnet, G.; Mollard, M.; Rannou, B.; Balmain, J.; Pedraza, F.; Montero, X.; Galetz, M.; Schütze, M. Initial aluminizing steps of pure nickel from Al micro-particles. Defect Diff. Forum 2012, 323–325, 381–386. [Google Scholar] [CrossRef]
- Montero, X.; Demler, I.; Kuznetsov, V.; Galetz, M.C. Factors governing slurry aluminization of steels. Surf. Coat. Technol. 2017, 309, 179–186. [Google Scholar] [CrossRef]
- Nicholls, J.R. Designing oxidation-resistant coatings. JOM 2000, 52, 28–35. [Google Scholar] [CrossRef]
- Angenete, J.; Stiller, K. Comparison of inward and outward grown Pt modified aluminide diffusion coatings on a Ni based single crystal superalloy. Surf. Coat. Technol. 2002, 150, 107–118. [Google Scholar] [CrossRef]
- Montero, X.; Galetz, M.C.; Schütze, M. Low-activity aluminide coatings for superalloys using a slurry process free of halide activators and chromates. Surf. Coat. Technol. 2013, 222, 9–14. [Google Scholar] [CrossRef]
- Grégoire, B.; Bonnet, G.; Pedraza, F. Reactivity of Al-Cr microparticles for aluminizing purposes. Intermetallics 2017, 81, 80–89. [Google Scholar] [CrossRef]
- Grégoire, B.; Bonnet, G.; Pedraza, F. Mechanisms of formation of slurry aluminide coatings from Al and Cr microparticles. Surf. Coat. Technol. 2019, 359, 323–333. [Google Scholar] [CrossRef]
- Montero, X.; Galetz, M.C.; Schütze, M. Slurry coated Ni-plated Fe-base alloys: Investigation of the influence of powder and substrate composition on interdiffusional and structural degradation of aluminides. Surf. Coat. Technol. 2013, 236, 465–475. [Google Scholar] [CrossRef]
- Cheng, W.-J.; Liao, Y.-J.; Wang, C.-J. Effect of nickel pre-plating on high-temperature oxidation behavior of hot-dipped aluminide mild steel. Mater. Charact. 2013, 82, 58–65. [Google Scholar] [CrossRef]
- Safari, M.; Shahriari Nogorani, F. Formation mechanism of high activity aluminide coating on Ni-CeO2 coated Rene 80 alloy. Surf. Coat. Technol. 2017, 329, 218–223. [Google Scholar] [CrossRef]
- Masoumi Balashadehi, M.; Nourpour, P.; Sabour Rouh Aghdam, A.; Allahyarzadeh, M.H.; Heydarzadeh, A.; Hamdi, M. The formation, microstructure and hot corrosion behaviour of slurry aluminide coating modified by Ni/Ni-Co electrodeposited layer on Ni-base superalloy. Surf. Coat. Technol. 2020, 402, 126283. [Google Scholar] [CrossRef]
- Zakeri, A.; Masoumi Balashadehi, M.R.; Sabour Rouh Aghdam, A. Development of hybrid electrodeposition/slurry diffusion aluminide coatings on Ni-based superalloy with enhanced hot corrosion, resistance. J. Compos. Cmpd. 2021, 3, 14115–14143. [Google Scholar] [CrossRef]
- Sarraf, S.; Rastegari, S.; Soltanieh, M. Deposition of a low-activity type cobalt-modified aluminide coating by slurry aluminizing of a pre-Co-electroplated Ni-based superalloy, (IN738LC). J. Mater. Res. Technol. 2024, 30, 183–1193. [Google Scholar] [CrossRef]
- Delaunois, F.; Vitry, V.; Bonin, L. (Eds.) Electroless Nickel Plating: Fundamentals to Applications; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar] [CrossRef]
- Genova, V.; Pedrizzetti, G.; Paglia, L.; Marra, F.; Bartuli, C.; Pulci, G. Diffusion aluminide coating modified via electroless nickel plating for Ni-based superalloy protection. Surf. Coat. Technol. 2022, 439, 128452. [Google Scholar] [CrossRef]
- Genova, V.; Paglia, L.; Marra, F.; Bartuli, C.; Pulci, G. Pure thick nickel coating obtained by electroless plating: Surface characterization and wetting properties. Surf. Coat. Technol. 2019, 357, 595–603. [Google Scholar] [CrossRef]
- Rannou, B.; Mollard, M.; Bouchaud, B.; Balmain, J.; Bonnet, G.; Kolarik, V.; Pedraza, F. On the influence of a heat treat for an aluminizing process based on Al microparticles slurry for model Ni and Ni20Cr. Experimental and theoretical approaches. Defect Diff. Forum 2012, 323–325, 373–379. [Google Scholar] [CrossRef]
- Bermejo Sanz, J.; Roussel García, R.; Kolarik, V.; Juez Lorenzo, M.M. Influence of the slurry thickness and heat treatment parameters on the formation of aluminium diffusion coating. Oxid. Met. 2017, 88, 179–190. [Google Scholar] [CrossRef]
- Li, Z.; Wang, C.; Ding, X.; Li, X.; Yu, J.; Li, Q.; Qu, Y. Effect of slurry thickness on the quality of aluminized coatings. Materials 2022, 15, 6758. [Google Scholar] [CrossRef] [PubMed]
- Rae, C.M.F.; Hook, M.S.; Reed, R.C. The effect of TCP morphology on the development of aluminide coated superalloys. Mater. Sci. Eng. A 2005, 396, 231–239. [Google Scholar] [CrossRef]
- Matuszewski, K.; Rettig, R.; Rasiński, M.; Kurzydłowski, K.J.; Singer, R.F. The three-dimensional morphology of topologically close packed phases in a high rhenium containing nickel-based superalloy. Adv. Eng. Mater. 2014, 16, 171–175. [Google Scholar] [CrossRef]
- Wilson, A.S.; Christofidou, K.A.; Evans, A.; Hardy, M.C.; Stone, H.J. Comparison of methods for quantification of topologically close-packed phases in Ni-based superalloys. Metall. Mater. Trans. A 2019, 50, 5925–5934. [Google Scholar] [CrossRef]
- Mehrer, H.; Divinski, S. Diffusion in Metallic Elements and Intermetallics. Defect Diff. Forum 2009, 289–292, 15–38. [Google Scholar] [CrossRef]
- Rasmussen, A.J.; Agüero, A.; Gutierrez, M.; Østergård, M.J.L. Microstructures of thin and thick slurry aluminide coatings on Inconel 690. Surf. Coat. Technol. 2008, 202, 1479–1485. [Google Scholar] [CrossRef]
- Kepa, T.; Pedraza, F.; Rouillard, F. Intermetallic formation of Al-Fe and Al-Ni phases by ultrafast slurry aluminization (flash aluminizing). Surf. Coat. Technol. 2020, 397, 126011. [Google Scholar] [CrossRef]
- Deevi, S.C.; Sikka, V.K. Nickel and iron aluminides: An overview on properties, processing, and applications. Intermetallics 1996, 4, 357–375. [Google Scholar] [CrossRef]
- Gedevanishvili, S.; Deevi, S.C. Processing of iron aluminides by pressureless sintering through Fe+Al elemental route. Mater. Sci. Eng. A 2002, 325, 163–176. [Google Scholar] [CrossRef]
- Siemiaszko, D.; Mościcki, R. Kinetics study on the SHSreaction in massive samples with high heating rate in the Fe–Al, system. J. Alloys Compd. 2015, 632, 335–342. [Google Scholar] [CrossRef]
- Okamoto, H. Al-Ni (aluminium-nickel). J. Phase Equilib Diffus. 2004, 25, 394. [Google Scholar] [CrossRef]
- Brossard, J.M.; Panicaud, B.; Balmain, J.; Bonnet, G. Modelling of aluminized coating growth on nickel. Acta Mater. 2007, 55, 6586–6595. [Google Scholar] [CrossRef]
- Seitz, F. On the porosity observed in the Kirkendall effect. Acta Metall. 1953, 1, 355–369. [Google Scholar] [CrossRef]
- Paul, A. The Kirkendall Effect in Solid State Diffusion. Ph.D. Thesis, Technische Universiteit Eindhoven, Eindhoven, The Netherlands, 2004. [Google Scholar] [CrossRef]
- Mehrer, H. Diffusion in Solids; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar] [CrossRef]
- Pieraggi, B. Diffusion and solid state reactions. In Developments in High Temperature Corrosion and Protection of Materials; Series in Metals and Surface Engineering; Woodhead Publishing: London, UK, 2008; pp. 9–35. [Google Scholar] [CrossRef]
- Janssen, M.M.P. Diffusion in the nickel-rich part of the Ni-Al system at 1000 °C to 1300 °C; Ni3Al layer growth; diffusion coefficients, and interface concentrations. Metall. Trans. 1973, 4, 1623–1633. [Google Scholar] [CrossRef]
- Thevand, A.; Poize, S.; Crousier, J.-P.; Streiff, R. Aluminization of nickel-formation of intermetallic phases Ni2Al3, coatings. J. Mater. Sci. 1981, 16, 2467–2479. [Google Scholar] [CrossRef]
- Shiomi, S.; Miyake, M.; Hirato, T.; Sato, A. Aluminide coatings fabricated on nickel by aluminium electrodeposition from DMSO2 based electrolyte and subsequent annealing. Mater. Trans. 2011, 52, 1216–1221. [Google Scholar] [CrossRef]
- Kogachi, M.; Haraguchi, T. Point defects in B2-type intermetallic compounds. Mater. Sci. Eng. A 2001, 312, 189–195. [Google Scholar] [CrossRef]
- Frank, S.; Divinski, S.V.; Södervall, U.; Herzig, C. Ni tracer diffusion in the B2-compound NiAl: Influence of temperature and composition. Acta Mater. 2001, 49, 1399–1411. [Google Scholar] [CrossRef]
- Divinski, S.V.; Herzig, C. Ni tracer self-diffusion, interdiffusion and diffusion mechanisms in NiAl. Defect Diff. Forum 2002, 203–205, 177–192. [Google Scholar] [CrossRef]
- Paul, A.; Kodentsov, A.A.; van Loo, F.J.J. On diffusion in the β-NiAl, phase. J. Alloys Compd. 2005, 403, 147–153. [Google Scholar] [CrossRef]
- Chang, Y.A.; Neumann, J.P. Thermodynamics and defect structure of intermetallic phases with the B2 (CsCl) structure. Prog. Solid State Chem. 1982, 14, 221–301. [Google Scholar] [CrossRef]
- Tan, X.; Peng, X.; Wang, F. The mechanism for self-formation of a CeO2 diffusion barrier layer in an aluminide coating at high temperature. Surf. Coat. Technol. 2013, 224, 62–70. [Google Scholar] [CrossRef]


| Ni | Cr | Co | Mo | W | Ta | Al | Hf | Re | |
|---|---|---|---|---|---|---|---|---|---|
| wt. % | 61.6 | 7 | 8 | 2 | 5 | 7 | 6.2 | 0.2 | 3 |
| at. % | 63.5 | 8.1 | 8.2 | 1.3 | 1.6 | 2.3 | 13.9 | 0.06 | 1 |
| Name of Sample | Thickness of Electroless Layer (µm) | Slurry Sprayed (mg/cm2) | Heating Rate (°C/min) | Dwell |
|---|---|---|---|---|
| Influence of slurry amount | ||||
| Ni0-Al6-R20-T1080 | None | 6 ± 1 | 20 | 1080 °C/1.5 h |
| Ni0-Al12-R20-T1080 | 12 ± 1 | |||
| Ni17-Al5-R20-T1080 | 18 ± 2 | 5 ± 1 | 1080 °C/1 h | |
| Ni20-Al16-R20-T1080 | 16 ± 1 | |||
| Influence of Ni electroless thickness | ||||
| Ni5-Al13-R20-T700+1080 | 5 ± 1 | 14 ± 1 | 20 | 700 °C/1 h +1080 °C/1 h |
| Ni10-Al13-R20-T700+1080 | 10 ± 1 | |||
| Ni25-Al15-R20-T700+1080 | 25 ± 1 | |||
| Influence of heating rate for Kirkendall porosity | ||||
| Ni17-Al13-R20-T700 | 18 ± 1 | 15 ± 2 | 20 | 700 °C/1 h |
| Ni18-Al16-R20-T1080 | 1080 °C/1 h | |||
| Ni19-Al14-R20-T700+1080 | 700 °C/1 h +1080 °C/1 h | |||
| Ni18-Al15-R5-T700+1080 | 5 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kepa, T.; Bonnet, G.; Pedrizzetti, G.; Genova, V.; Pulci, G.; Bartuli, C.; Pedraza, F. Slurry Aluminizing of Nickel Electroless Coated Nickel-Based Superalloy. Coatings 2025, 15, 1337. https://doi.org/10.3390/coatings15111337
Kepa T, Bonnet G, Pedrizzetti G, Genova V, Pulci G, Bartuli C, Pedraza F. Slurry Aluminizing of Nickel Electroless Coated Nickel-Based Superalloy. Coatings. 2025; 15(11):1337. https://doi.org/10.3390/coatings15111337
Chicago/Turabian StyleKepa, Thomas, Gilles Bonnet, Giulia Pedrizzetti, Virgilio Genova, Giovanni Pulci, Cecilia Bartuli, and Fernando Pedraza. 2025. "Slurry Aluminizing of Nickel Electroless Coated Nickel-Based Superalloy" Coatings 15, no. 11: 1337. https://doi.org/10.3390/coatings15111337
APA StyleKepa, T., Bonnet, G., Pedrizzetti, G., Genova, V., Pulci, G., Bartuli, C., & Pedraza, F. (2025). Slurry Aluminizing of Nickel Electroless Coated Nickel-Based Superalloy. Coatings, 15(11), 1337. https://doi.org/10.3390/coatings15111337









