Influence of Annealing Temperatures on Raman and Optical Absorption Spectra of TiO2 Nanorod Thin Film Coatings
Abstract
1. Introduction
2. Experimental Section
2.1. Synthesis of TiO2 Nanorod Thin Film Coatings
2.2. Annealing Treatment
2.3. Characterization
3. Results and Discussion
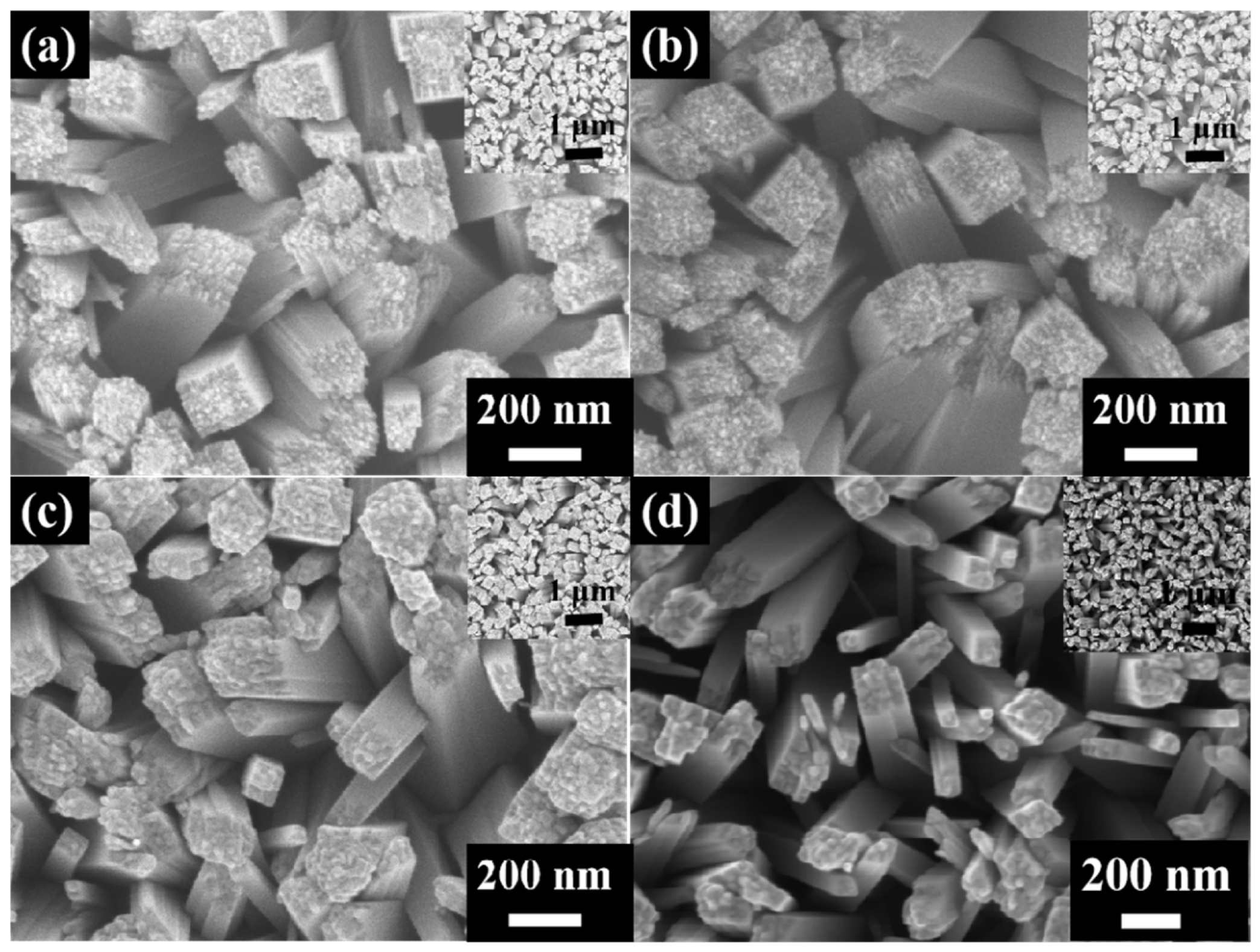
3.1. Surface Topography
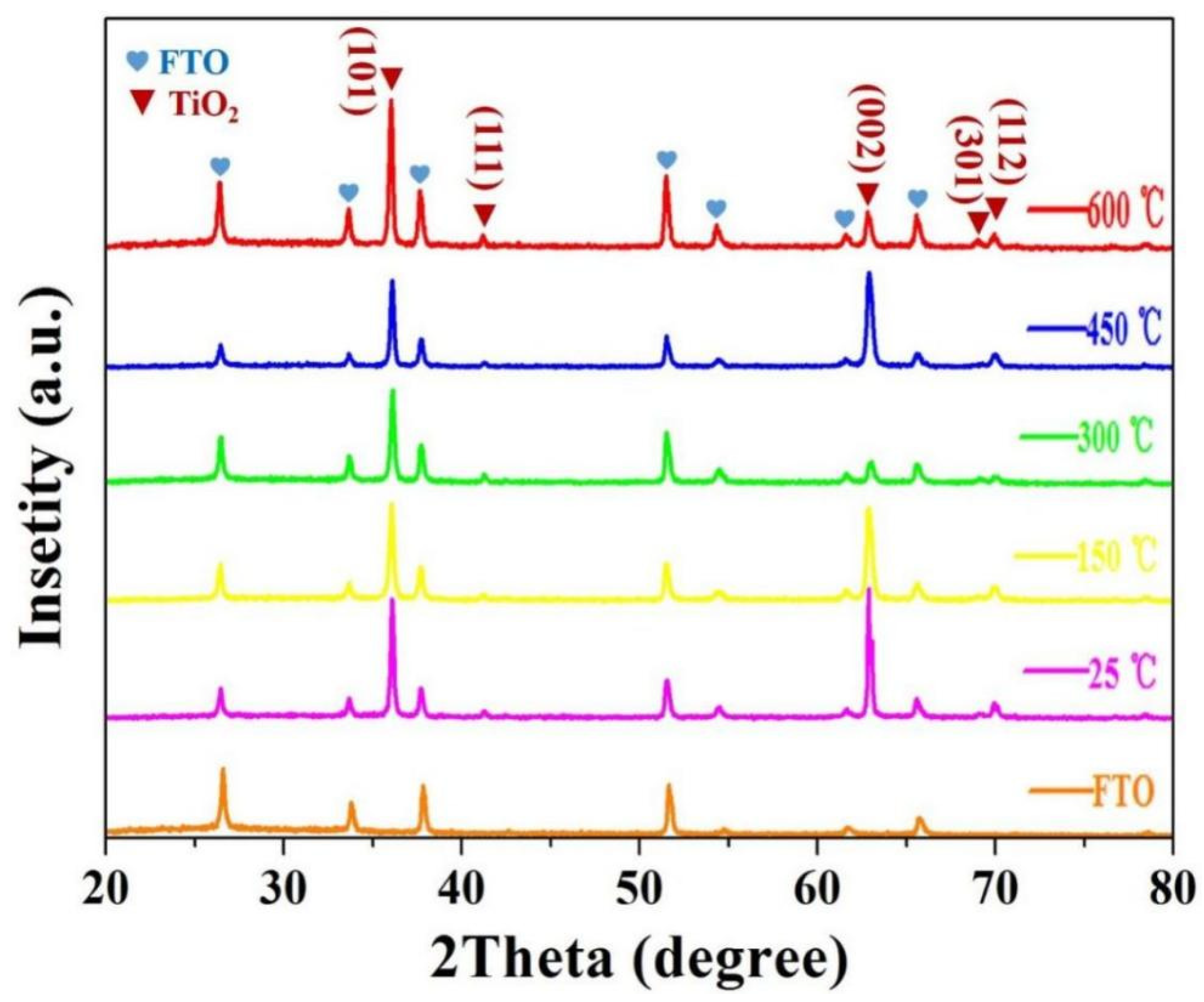
3.2. XRD Characterization
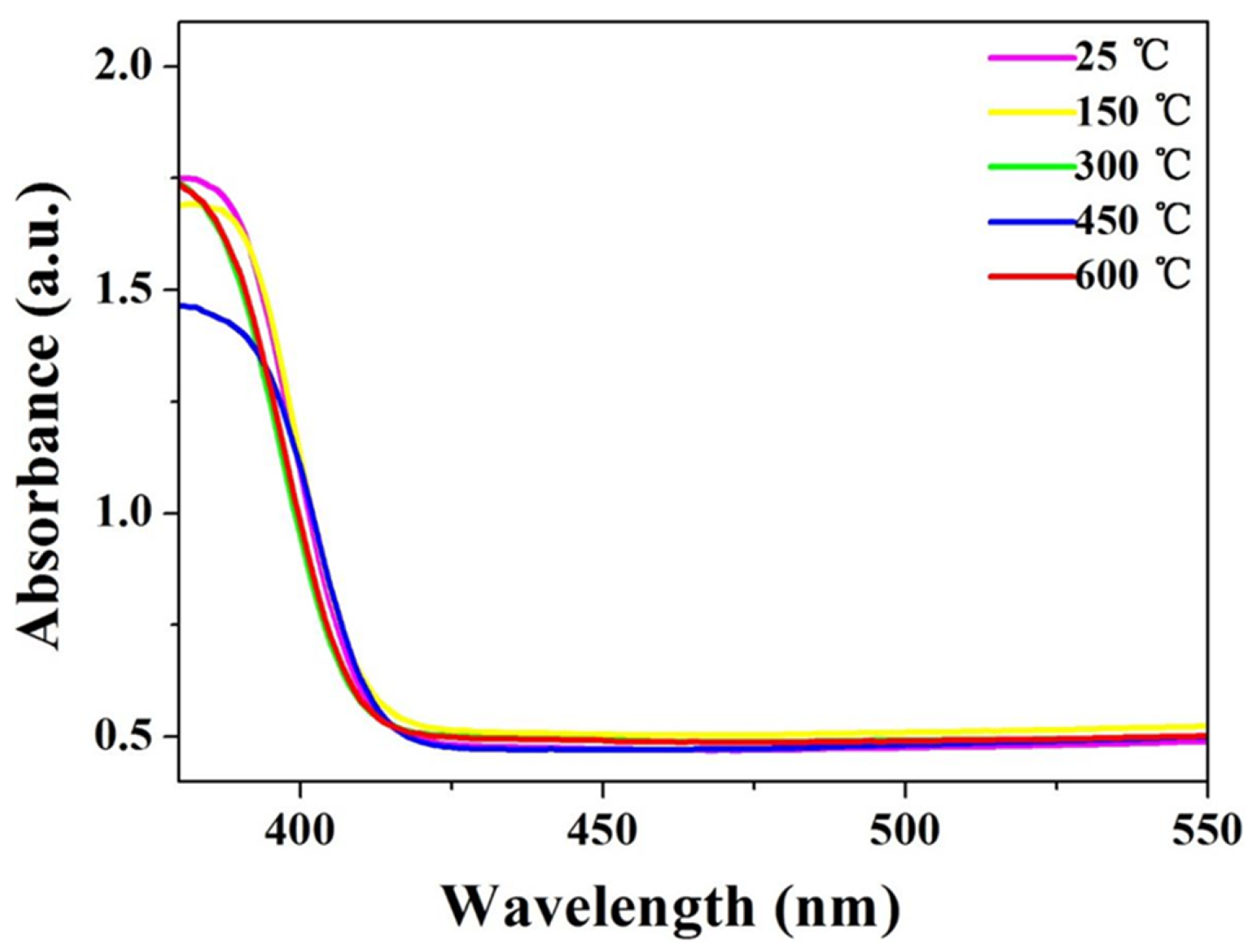
3.3. Optical Absorption Spectra
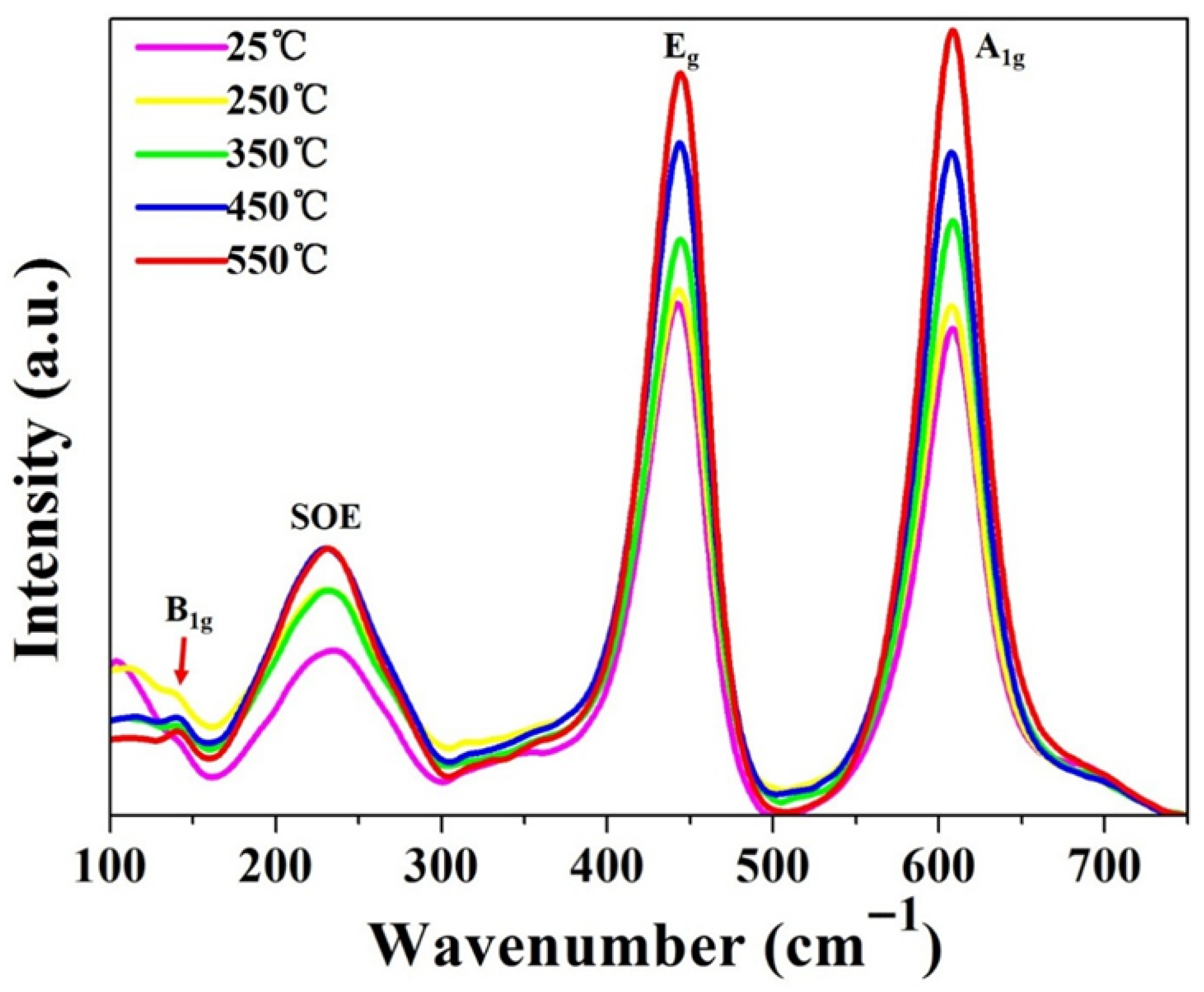
3.4. Raman Spectra
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Li, C.L.; Chen, S.R.; Gao, X.X.; Zhang, W.; Wang, Y.F. Fabrication, characterization and photoelectrochemical properties of CdS/CdSe nanofilm co-sensitized ZnO nanorod arrays on Zn foil substrate. J. Colloid Interface Sci. 2021, 588, 269–282. [Google Scholar] [CrossRef]
- Yao, Y.H.; Tang, Y.; Zhou, Y.L.; Cui, D.; Wang, P.P.; Luo, D.J.; Wang, Q.; Tian, B.; Pan, K.K.; Yu, F. Visible light-driven high efficient water hydrogen evolution using Ru-RuO2/TiO2 catalyst. Int. J. Hydrogen Energy 2025, 145, 473–484. [Google Scholar] [CrossRef]
- Murugesam, S.; Kuppusami, P.; Mohandas, E. Rietveld X-ray diffraction analysis of nanostructured rutile films of titania prepared by pulsed laser deposition. Mater. Res. Bull. 2010, 45, 6–9. [Google Scholar] [CrossRef]
- Chen, X.; Mao, S.S. Titanium dioxide nanomaterials: Synthesis, properties, modifications, and applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, L.; Yu, P.Y.; Mao, S.S. Increasing solar absorption for photocatalysis with black hydrogenated titanium dioxide nanocrystals. Science 2011, 331, 746–750. [Google Scholar] [CrossRef]
- Tuntithavornwat, S.; Saisawang, C.; Ratvijitvech, T.; Watthanaphanit, A.; Hunsom, M.; Kannan, M.A. Recent development of black TiO2 nanoparticles for photocatalytic H2 production: An extensive review. Int. J. Hydrogen Energy 2024, 55, 1559–1593. [Google Scholar] [CrossRef]
- Zhang, H.; Besteiro, L.V.; Liu, L.; Wang, C.; Selopal, G.S.; Chen, Z.; Barba, D.; Wang, Z.M.; Zhao, H.; Lopinski, G.P. Efficient and stable photoelectrochemical hydrogen generation using optimized colloidal heterostructured quantum dots. Nano Energy 2021, 79, 105416. [Google Scholar] [CrossRef]
- Cheng, X.F.; Leng, W.H.; Liu, D.H.; Zhan, J.Q.; Cao, C.N. Enhanced photoelectrocatalytic performance of Zn-doped WO3 photocatalysts for nitrite ions degradation under visible light. Chemosphere 2007, 68, 1976–1984. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.L.; Harris, C.X.; Wallenmeyer, P.; James, M.; Chen, X.B. Asymmetric lattice vibrational characteristics of rutile TiO2 as revealed by laser power dependent Raman spectroscopy. J. Phy. Chem. C 2013, 117, 24015–24022. [Google Scholar] [CrossRef]
- Ghorai, N.; Yang, Z.C.; Gebre, S.T.; Wu, S.X.; Zhao, F.Y.; Ivanov, I.N.; Lian, T.Q. Efficient size-dependent hot electron transfer from Au to TiO2 nanoparticles. Nano Lett. 2025, 25, 3253–3258. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.F.; Li, C.Y.; Campos, R.F.; Esplandiu, M.J.; Fraxedas, J.; Liguori, N.; Villa, K. Unraveling charge transport in heterostructured nanomotors for efficient photocatalytic motion. Nano Lett. 2025, 25, 10169–10177. [Google Scholar] [CrossRef] [PubMed]
- Wolcott, A.; Smith, W.A.; Kuykendall, T.R.; Zhao, Y.; Zhang, J.Z. Photoelectrochemical water splitting using dense and aligned TiO2 nanorod arrays. Small 2009, 5, 104–111. [Google Scholar] [CrossRef]
- Yao, H.; Wu, Y.F.; Yang, H.; Ma, J.; Sun, M.; Chen, Y.; Zhang, W.; Di, W.; Lv, P.; Li, M. Vertical growth of two-dimensional TiO2 nanosheets array films and enhanced photoelectrochemical properties sensitized by CdS quantum dots. Electrochim. Acta 2014, 125, 258–265. [Google Scholar] [CrossRef]
- Cho, I.S.; Choi, J.; Zhang, K.; Kim, S.J.; Jeong, M.J.; Cai, L.; Park, T.; Zheng, X.; Park, J.H. Highly efficient solar water splitting from transferred TiO2 nanotube arrays. Nano Lett. 2015, 15, 5709–5715. [Google Scholar] [CrossRef]
- Sreedhar, A.; Jung, H.; Kwon, J.H.; Yi, J.; Sohn, Y.; Gwag, J.S. Novel composite ZnO/TiO2 thin film photoanodes for enhanced visible-light-driven photoelectrochemical water splitting activity. J. Electroanal. Chem. 2017, 804, 92–98. [Google Scholar] [CrossRef]
- Liu, C.; Yang, Y.; Li, J.; Chen, S. Phase transformation synthesis of TiO2/CdS heterojunction film with high visible light photoelectrochemical activity. Nanotechnology 2018, 29, 265401. [Google Scholar] [CrossRef]
- Chen, S.R.; Li, C.L.; Hou, Z.Y. A novel in situ synthesis of TiO2/CdS heterojunction for improving photoelectrochemical water splitting. Int. J. Hydrogen Energy 2019, 44, 25473–25485. [Google Scholar] [CrossRef]
- Yu, H.J.; Zhao, Y.; Chao, Z.L.; Shang, Y.; Cao, Y.; Li, Z.W.; Tung, C.H.; Zhang, T. Carbon quantum dots/TiO2 composites for efficient photocatalytic hydrogen evolution. J. Mater. Chem. A 2014, 2, 3344–3351. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, D.; Qin, X.; Cheng, C. Three-dimensional CdS-sensitized sea urchin like TiO2-ordered arrays as efficient photoelectrochemical anodes. J. Phys. Chem. C 2015, 119, 27875–27881. [Google Scholar] [CrossRef]
- Mehta, M.; Singh, A.P.; Kumar, S.; Krishnamurthy, S.; Wickman, B.; Basu, S. Synthesis of MoS2-TiO2 nanocomposite for enhanced photocatalytic and photoelectrochemical performance under visible light irradiation. Vacuum 2018, 155, 675–681. [Google Scholar] [CrossRef]
- Naidu, P.Y.; Kumar, M.; Meena, B.; Challapalli, S. Synergistic integration of TiO2/Mn:CdS/CZTS ternary heterojunction photoanode for superior solar-driven hydrogen evolution. Int. J. Hydrogen Energy 2025, 166, 151000. [Google Scholar] [CrossRef]
- Yuan, J.; Chen, M.; Shi, J.; Shang, G.W. Preparations and photocatalytic hydrogen evolution of N-doped TiO2 from urea and titanium tetrachloride. Int. J. Hydrogen Energy 2006, 31, 1326–1331. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Hong, X.; Wang, Z.; Cai, W.; Feng, L.; Zhang, J.; Yang, Y.; Ma, N.; Liu, Y. Visible-light-activated nanoparticle photocatalyst of iodine-doped titanium dioxide. Chem. Mater. 2005, 17, 1548–1552. [Google Scholar] [CrossRef]
- Ming, X.; Da, P.; Hao, Y.W.; Zhao, D.; Zheng, G. Controlled Sn-doping in TiO2 nanowire photoanodes with enhanced photoelectrochemical conversion. Nano Lett. 2012, 12, 1503–1508. [Google Scholar]
- Cho, I.S.; Lee, C.H.; Feng, Y.; Logar, M.; Rao, P.M.; Cai, L.; Kim, D.R.; Sinclair, R.; Zheng, X. Co-doping titanium dioxide nanowires with tungsten and carbon for enhanced photoelectrochemical performance. Nat. Commun. 2013, 4, 1723. [Google Scholar] [CrossRef]
- Zhang, S.; Gu, X.; Zhao, Y.; Qiang, Y. Enhanced photoelectrochemical performance of TiO2 nanorod arrays by a 500 °C annealing in air: Insights into the mechanism. J. Electron. Mater. 2016, 45, 648–653. [Google Scholar] [CrossRef]
- Chen, S.R.; Li, C.L.; Hou, Z.Y. The novel behavior of photoelectrochemical property of annealing TiO2 nanorod arrays. J. Mater. Sci. 2020, 55, 5969–5981. [Google Scholar] [CrossRef]
- Mohan, C.M.; Anand, K.T.; Manish, K.S.; Gairola, S.P.; Pandey, S.N.; Arvind, A. Effect of annealing temperature on Raman spectra of TiO2 nanoparticles. Chem. Phys. Lett. 2013, 555, 182–186. [Google Scholar] [CrossRef]
- Khan, A.F.; Mehmood, M.; Durrani, S.K.; Ali, M.L.; Rahim, N.A. Structural and optoelectronic properties of nanostructured TiO2 thin films with annealing. Mat. Sci. Semicon. Proc. 2015, 29, 161–169. [Google Scholar] [CrossRef]
- Lan, T.; Tang, X.; Fultz, B. Phonon anharmonicity of rutile TiO2 studied by Raman spectrometry and molecular dynamics simulations. Phys. Rev. B 2012, 85, 094305. [Google Scholar] [CrossRef]
- Xie, Z.; Shuang, S.; Ma, L.W.; Zhu, F.; Liu, X.X.; Zhang, Z.J. Annealing effect on the photoelectrochemical and photocatalytic performance of TiO2 nanorod arrays. RSC Adv. 2017, 7, 51382–51390. [Google Scholar] [CrossRef]
- Feng, W.J.; Lin, L.Y.; Li, H.J.; Chi, B.; Pu, J.; Li, J. Hydrogenated TiO2/ZnO heterojunction nanorod arrays with enhanced performance for photoelectrochemical water splitting. Int. J. Hydrogen Energy 2017, 42, 3938–3946. [Google Scholar] [CrossRef]
- Jaramillo-Quintero, O.A.; Triana, M.A.; Rincon, M.E. Optimization of charge transfer and transport processes at the CdSe quantum dots/TiO2 nanorod interface by TiO2 interlayer passivation. J. Phys. D Appl. Phys. 2017, 50, 235305. [Google Scholar] [CrossRef]
- Fàbrega, C.; Andreu, T.; Tarancón, A.; Flox, C.; Morata, A.; Calvo-Barrio, L.; Morante, J.R. Optimization of surface charge transfer processes on rutile TiO2 nanorods photoanodes for water splitting. Int. J. Hydrogen Energy 2013, 38, 2979–2985. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, J.G.; Xu, Q.; Li, M.T.; Guo, L.J. Photoelectrochemical performance of CdS nanorods grafted vertically aligned TiO2 nanorods. Mater. Res. Bull. 2013, 48, 4548–4554. [Google Scholar] [CrossRef]
- Cheng, C.; Wang, H.S.; Li, J.C.; Yang, H.; Xie, A.J.; Chen, P.; Li, S.K.; Huang, F.Z.; Shen, Y.H. Ordered macroporous CdS-sensitized N-doped TiO2 inverse opals films with enhanced photoelectrochemical performance. Electrochim. Acta 2014, 146, 378–385. [Google Scholar] [CrossRef]
- Teng, W.; Wang, Y.M.; Huang, H.H.; Li, X.Y.; Tang, Y.B. Enhanced photoelectrochemical performance of MoS2 nanobelts-loaded TiO2 nanotube arrays by photo-assisted electrodeposition. Appl. Surf. Sci. 2017, 425, 507–517. [Google Scholar] [CrossRef]
- Liu, B.K.; Wang, D.J.; Wang, L.L.; Sun, Y.J.; Lin, Y.H.; Zhang, X.Q.; Xie, T.F. Glutathione-assisted hydrothermal synthesis of CdS-decorated TiO2 nanorod arrays for quantum dot-sensitized solar cells. Electrochim. Acta 2013, 113, 661–667. [Google Scholar] [CrossRef]
- Wu, P.C.; Song, X.Y.; Si, S.Y.; Ke, Z.J.; Cheng, L.; Li, W.Q.; Xiao, X.H.; Jiang, C.Z. Significantly enhanced visible light response in single TiO2 nanowire by nitrogen ion implantation. Nanotechnology 2018, 29, 184005. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.K.; Mokhtar, S.M.; Soon, C.F.; Nafarizal, N. Raman investigation of rutile-phased TiO2 nanorods/nanoflowers with various reaction times using one step hydrothermal method. J. Mater. Sci. Mater. Electron. 2016, 27, 7920–7926. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Li, H. Influence of Annealing Temperatures on Raman and Optical Absorption Spectra of TiO2 Nanorod Thin Film Coatings. Coatings 2025, 15, 1338. https://doi.org/10.3390/coatings15111338
Chen S, Li H. Influence of Annealing Temperatures on Raman and Optical Absorption Spectra of TiO2 Nanorod Thin Film Coatings. Coatings. 2025; 15(11):1338. https://doi.org/10.3390/coatings15111338
Chicago/Turabian StyleChen, Shangrong, and Hong Li. 2025. "Influence of Annealing Temperatures on Raman and Optical Absorption Spectra of TiO2 Nanorod Thin Film Coatings" Coatings 15, no. 11: 1338. https://doi.org/10.3390/coatings15111338
APA StyleChen, S., & Li, H. (2025). Influence of Annealing Temperatures on Raman and Optical Absorption Spectra of TiO2 Nanorod Thin Film Coatings. Coatings, 15(11), 1338. https://doi.org/10.3390/coatings15111338




