Oxidation-Resistant Ni-AlSi12 Composite Coating with Strong Adhesion on Ti-6Al-4V Alloy Substrate via Mechanical Alloying and Subsequent Laser Cladding
Abstract
1. Introduction
2. Experimental Details
2.1. Sample Preparation
2.2. Microstructural Characterization
2.3. Properties Testing
3. Results and Discussion
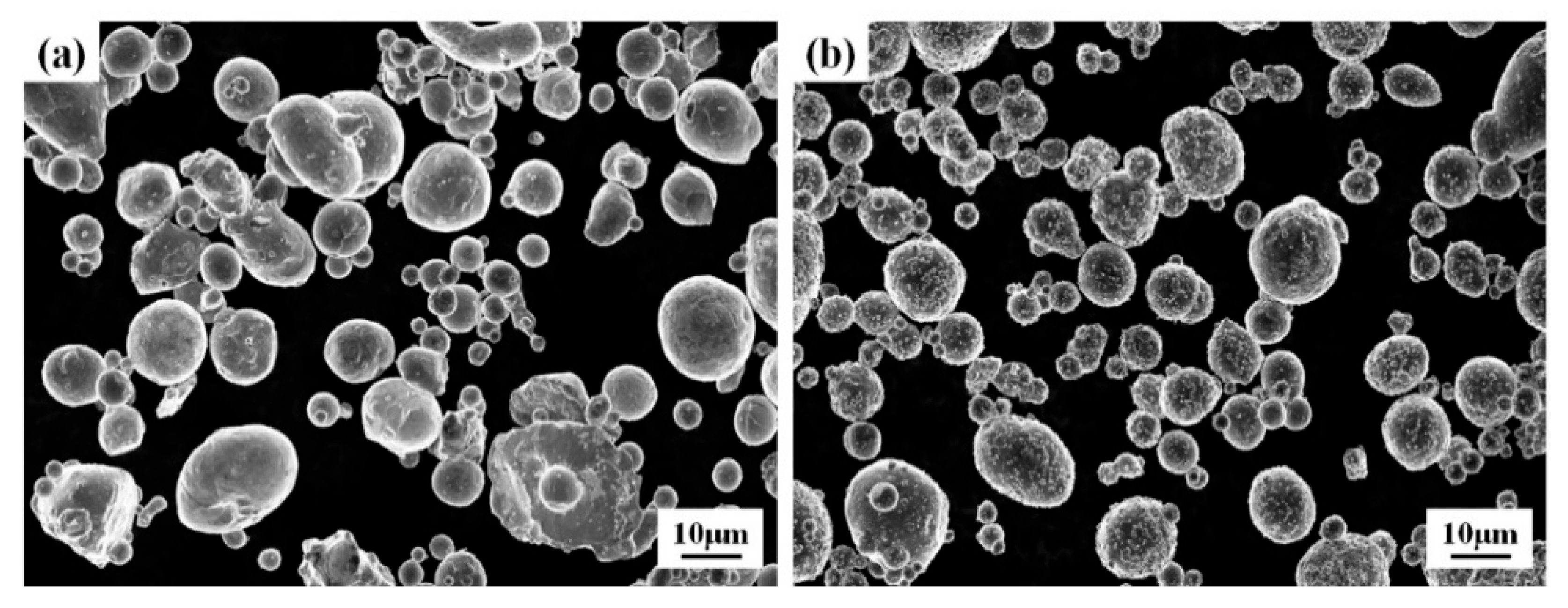
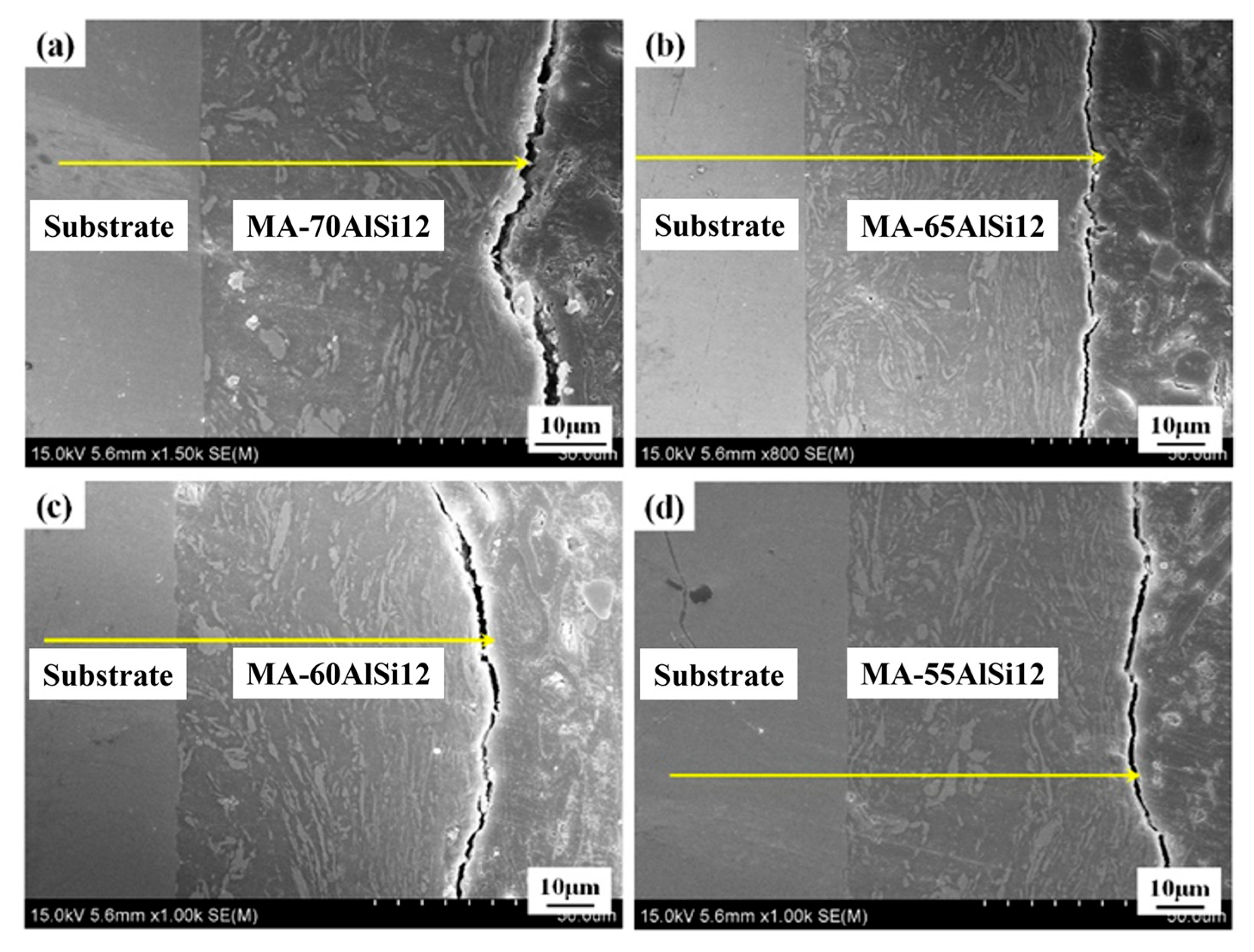
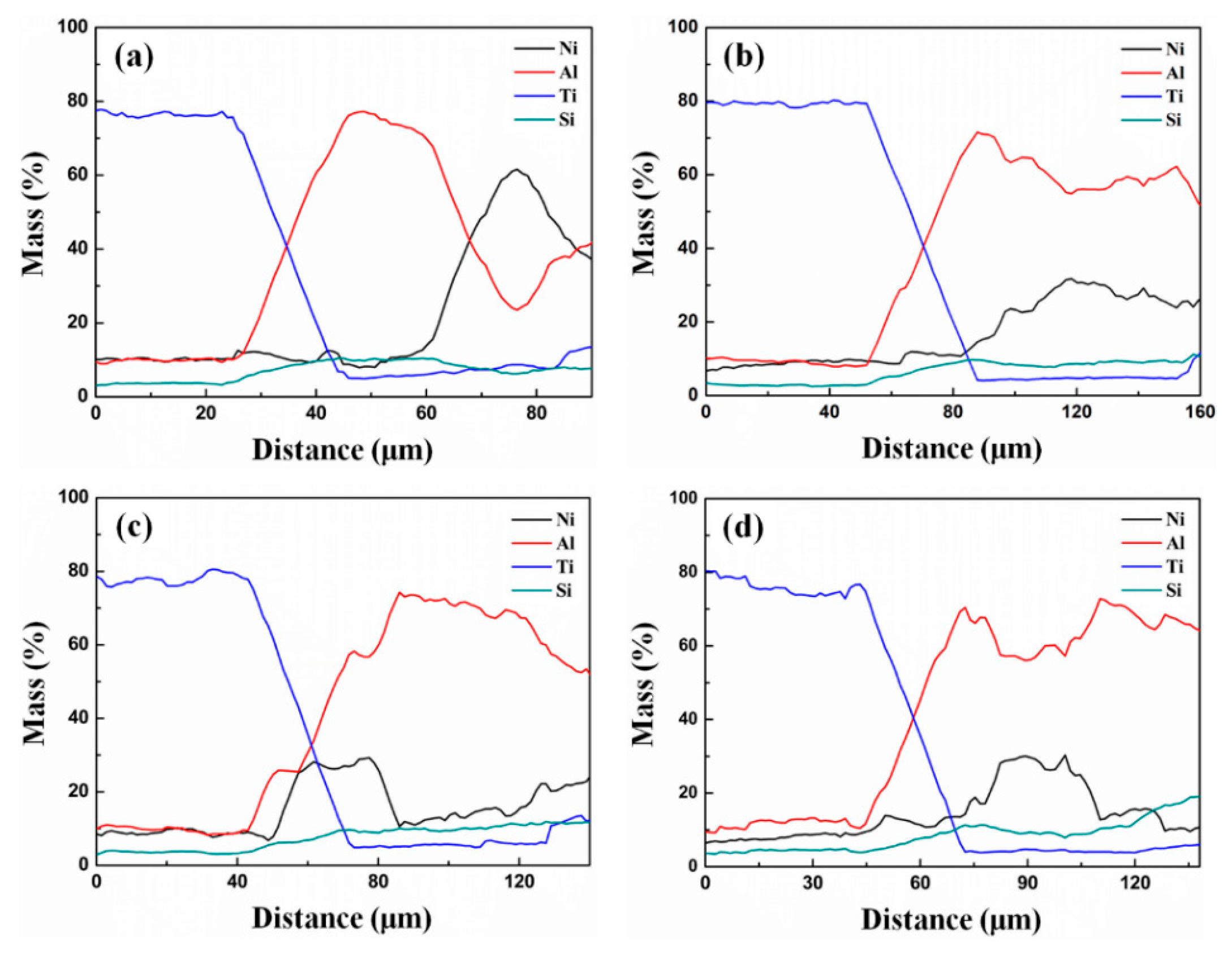
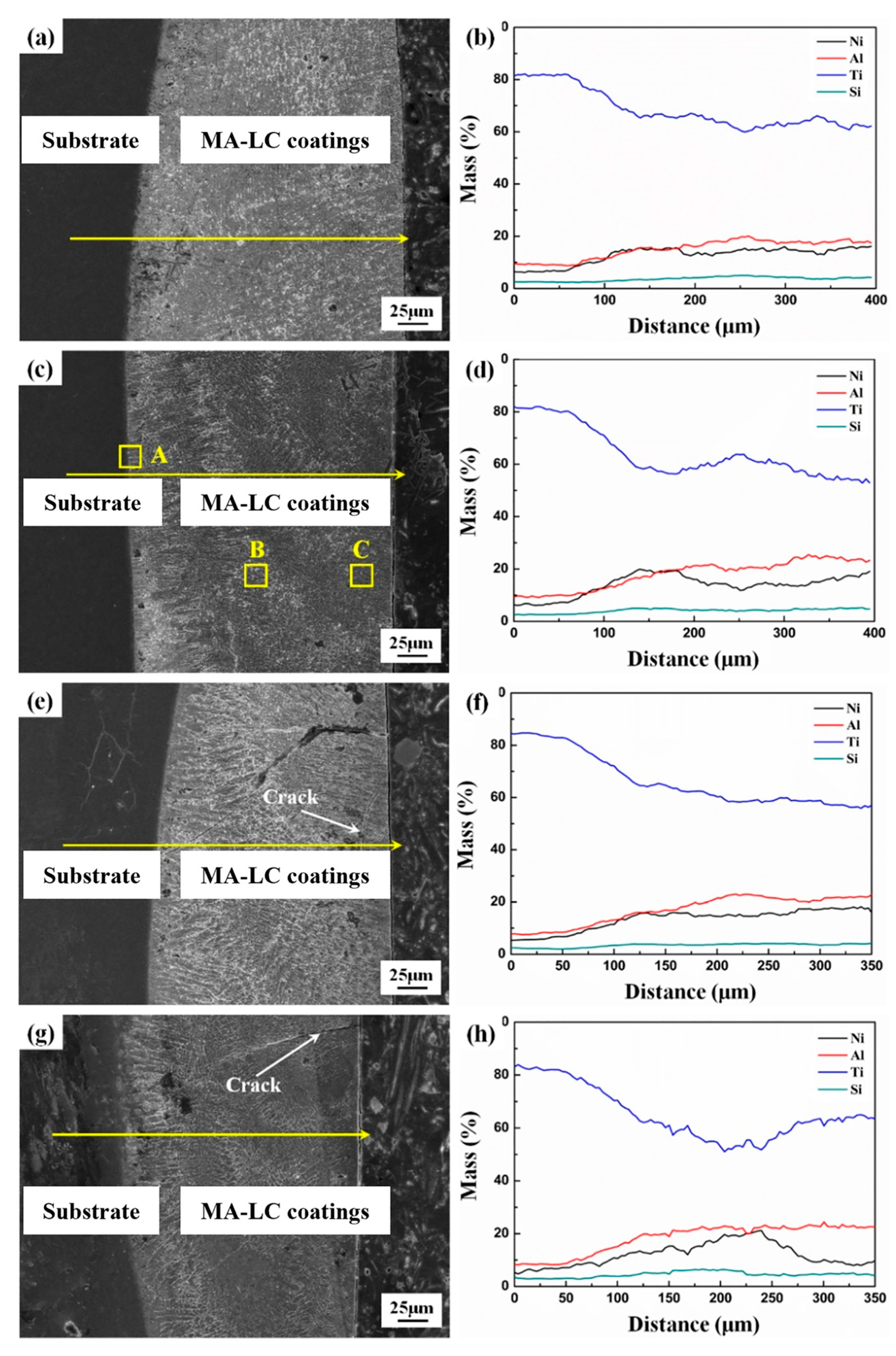
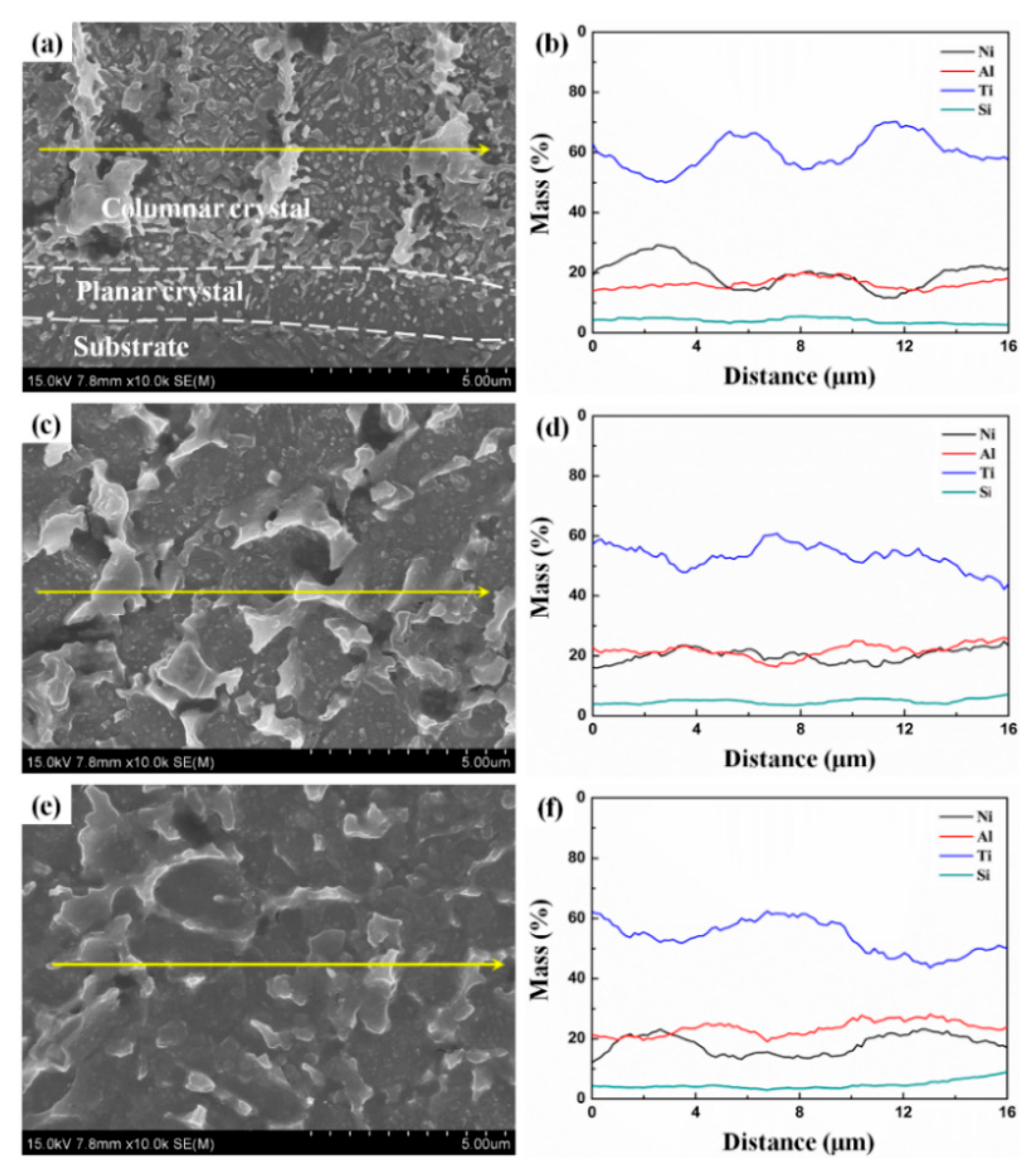
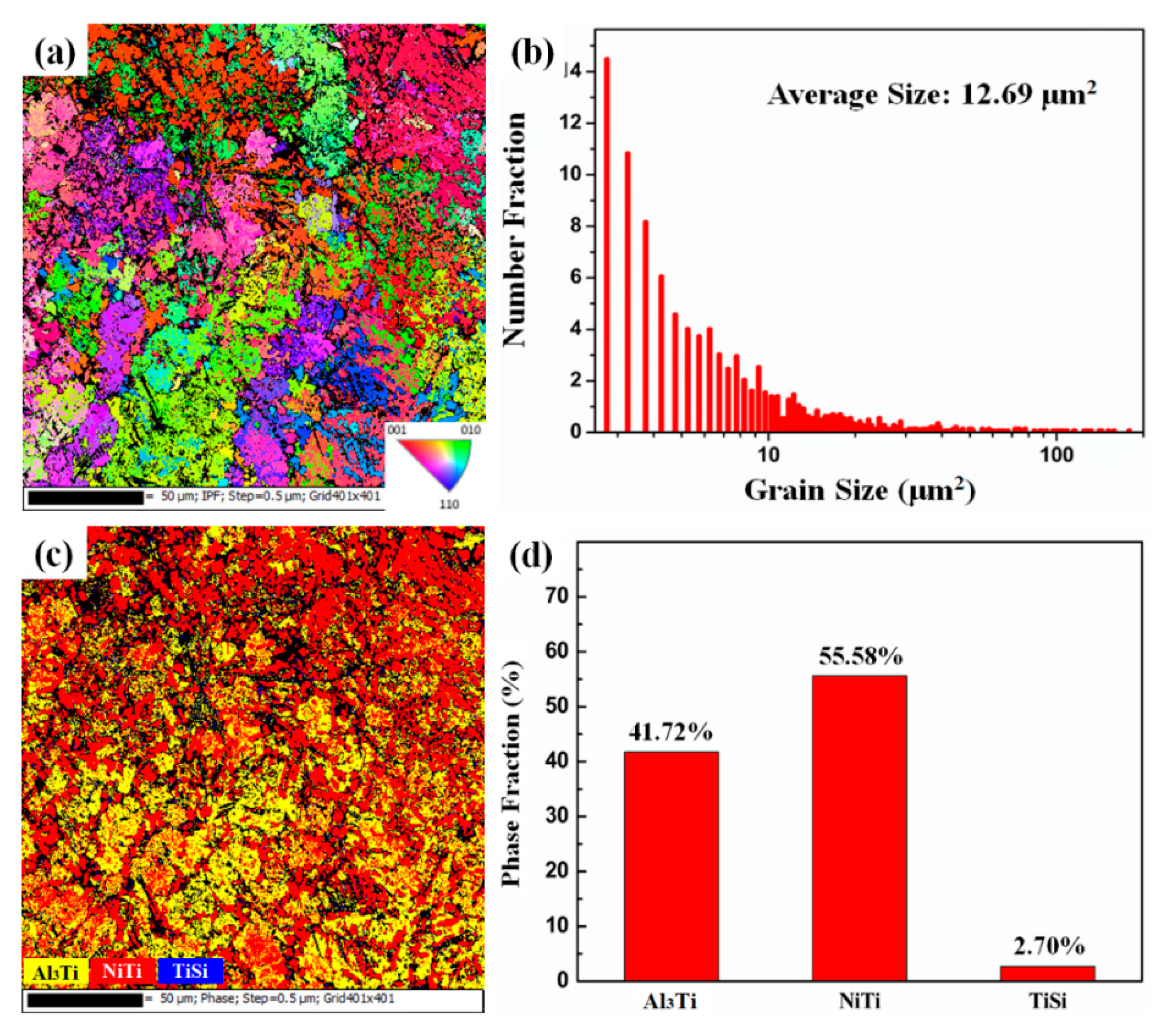
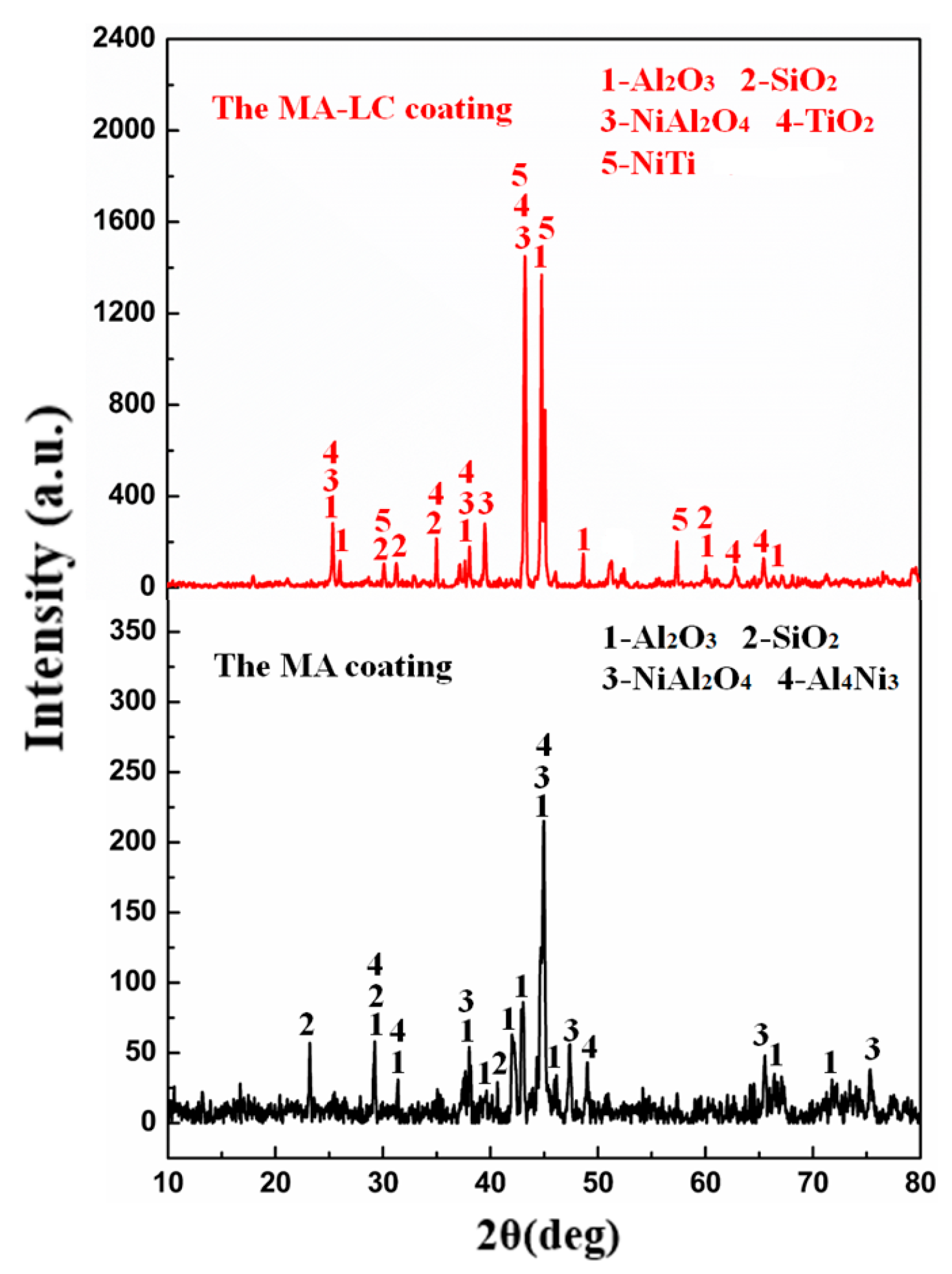
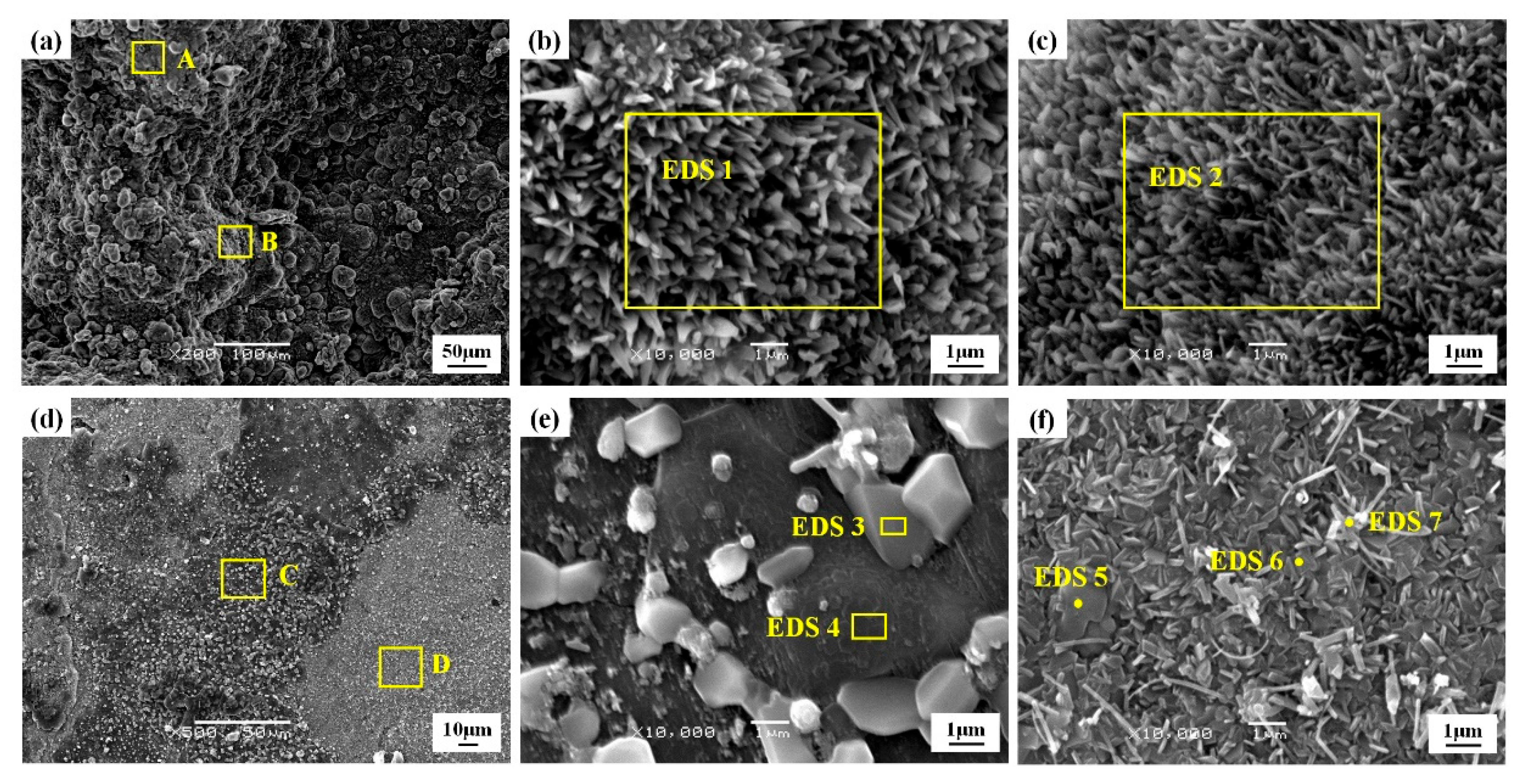
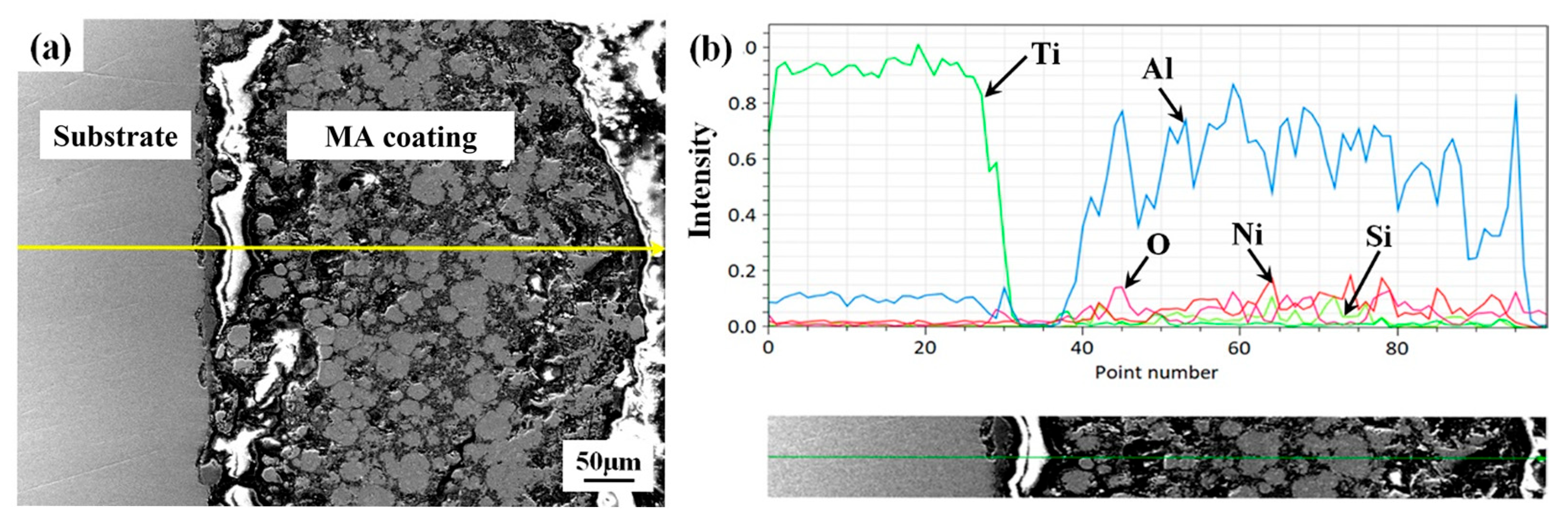
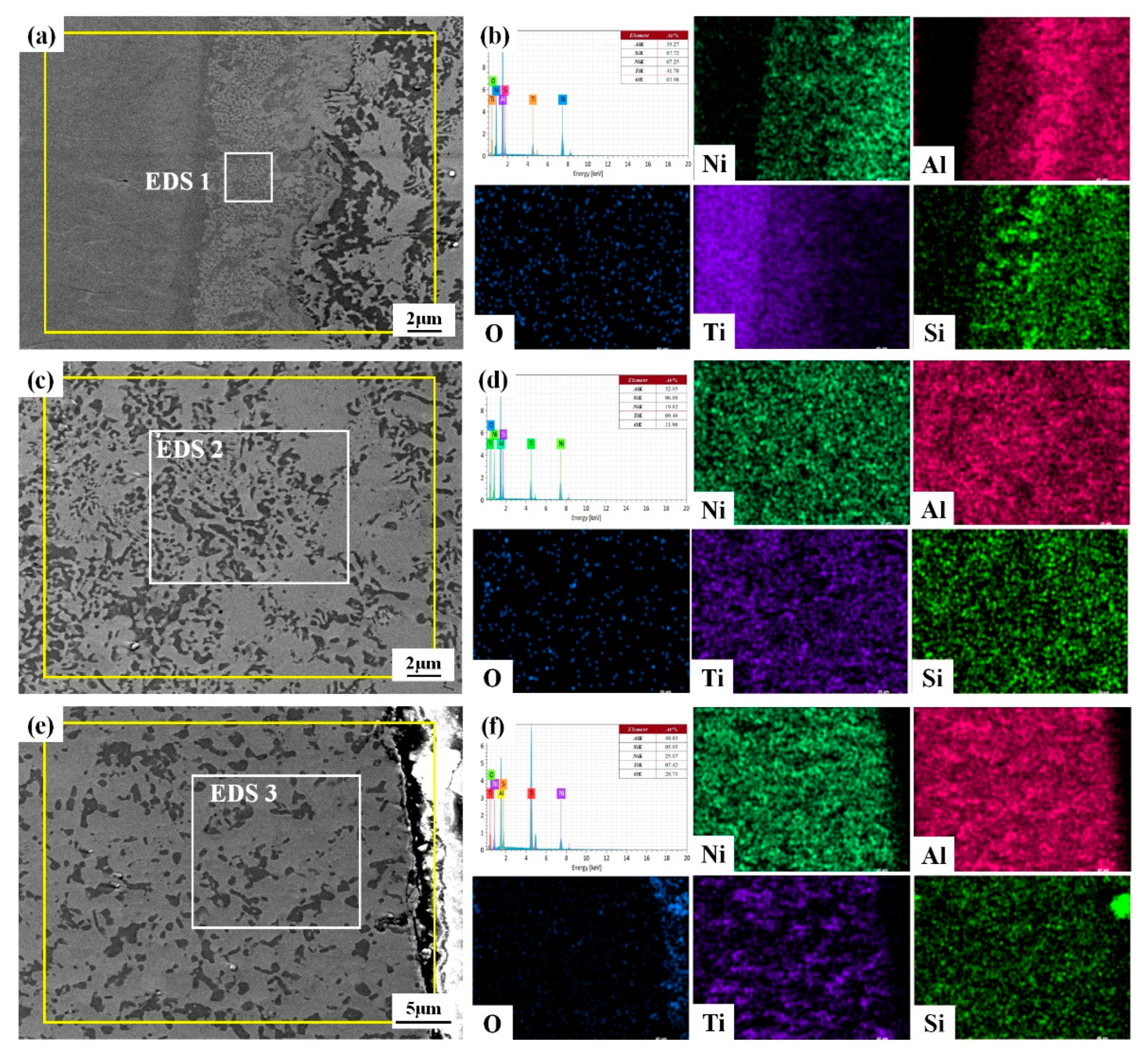
3.1. Microstructure and Phases
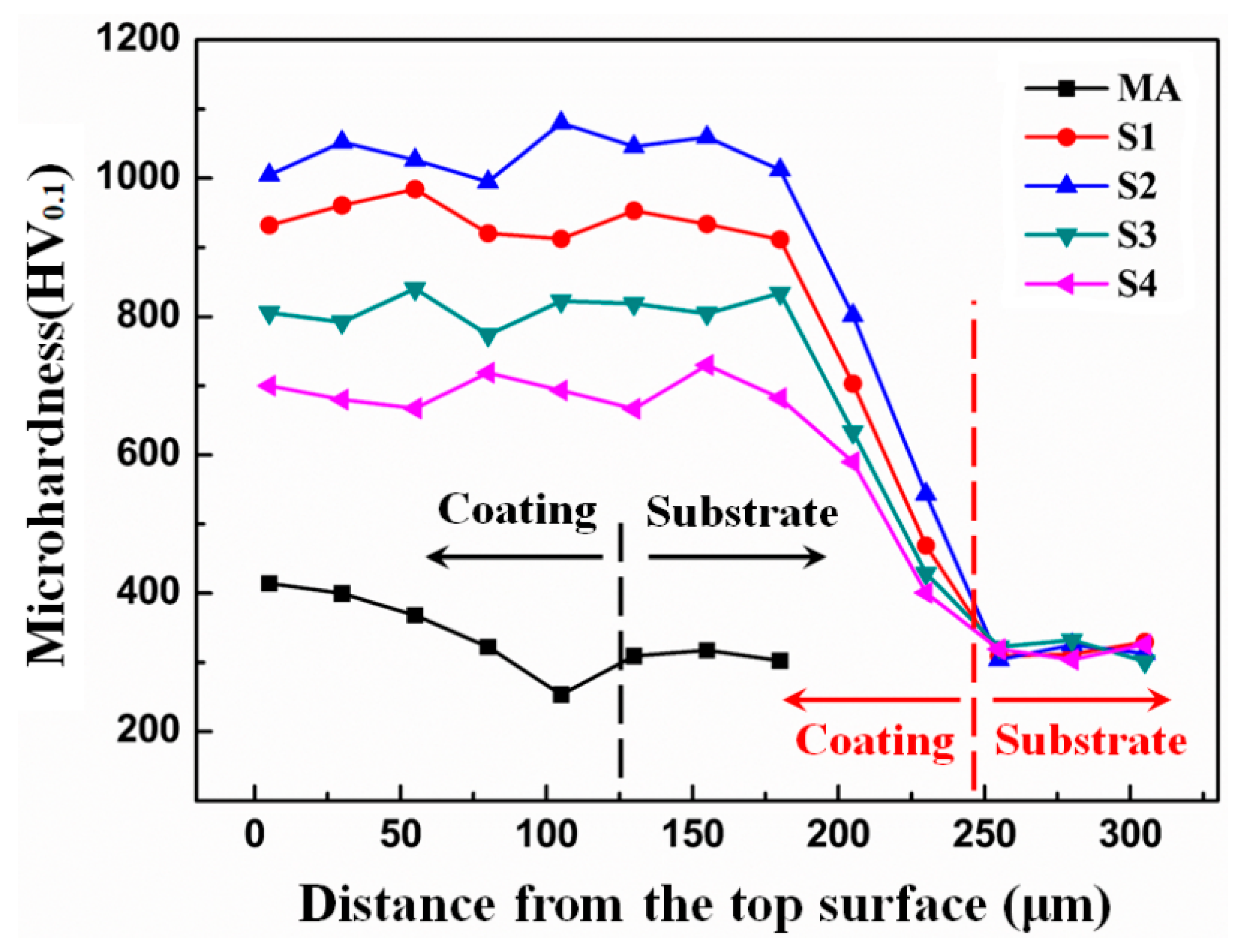
3.2. Mechanical Properties
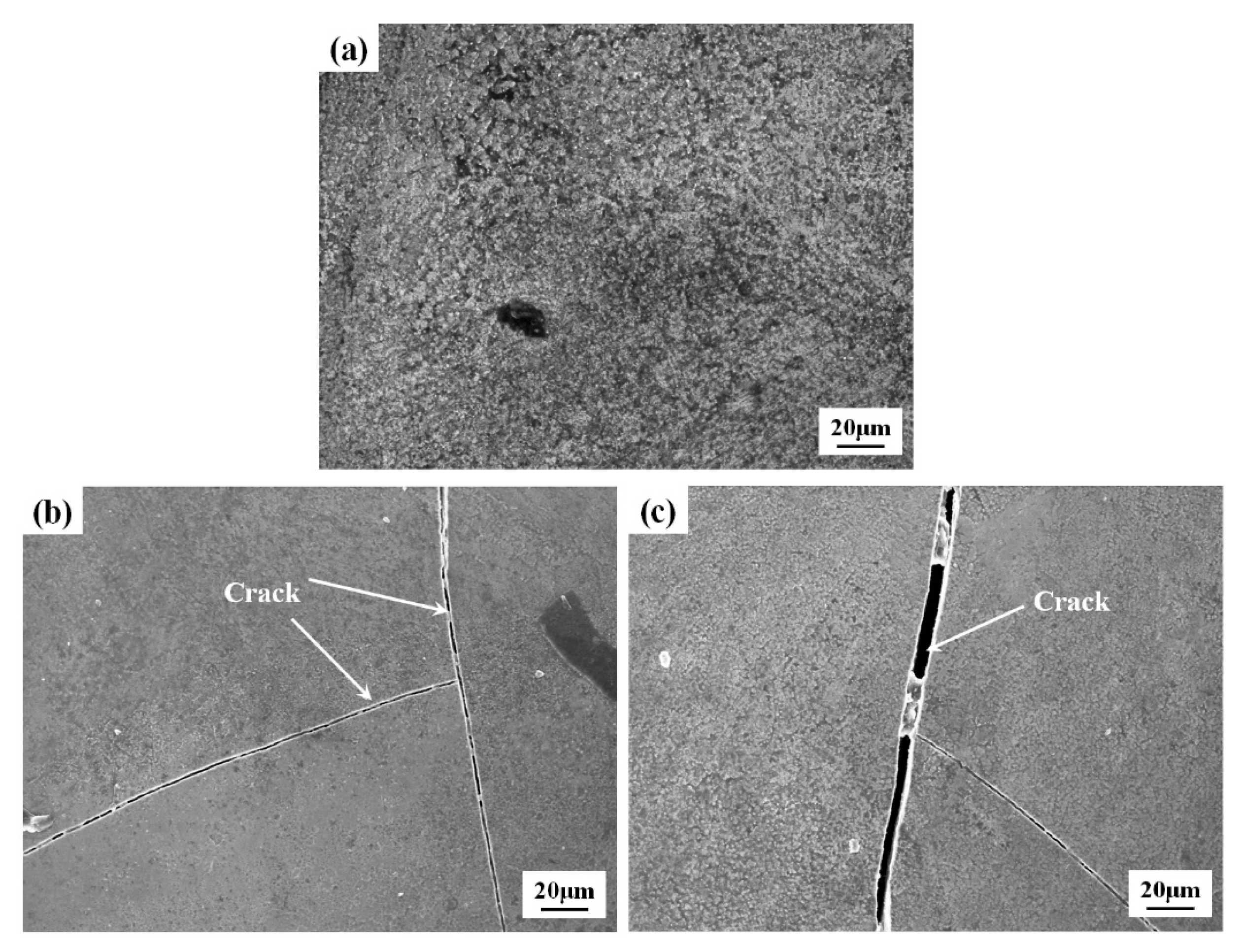
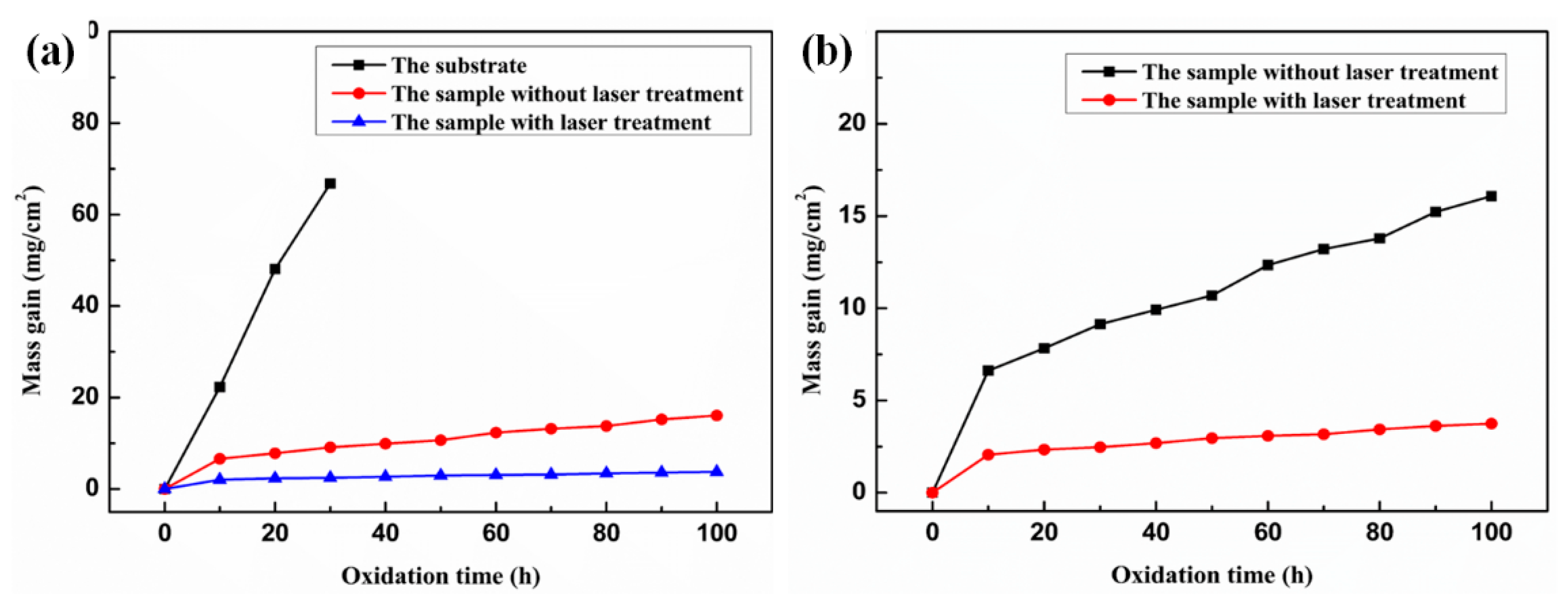
3.3. High-Temperature Oxidation Resistance
4. Conclusions
- (1)
- The coating–substrate interface achieves metallurgical bonding after laser cladding treatment. Also, the MA-LC coating is highly dense and crack-free. It mainly contains Al3Ti and NiTi phases. The average grain size is significantly refined, only 12.69 μm2. The coating exhibits a refined and homogeneous microstructure.
- (2)
- Owing to the subsequent laser cladding process, the MA-LC coating displays superior mechanical performance in comparison with the MA coating. In comparison to its MA counterpart, the MA-LC coating shows a marked enhancement in microhardness. The nano-hardness of MA-LC coating is 9.79 GPa, exhibiting that it is 6.84 times the nano-hardness of the MA sample. Due to metallurgical bonding, the MA-LC coating exhibits excellent scratch bonding properties.
- (3)
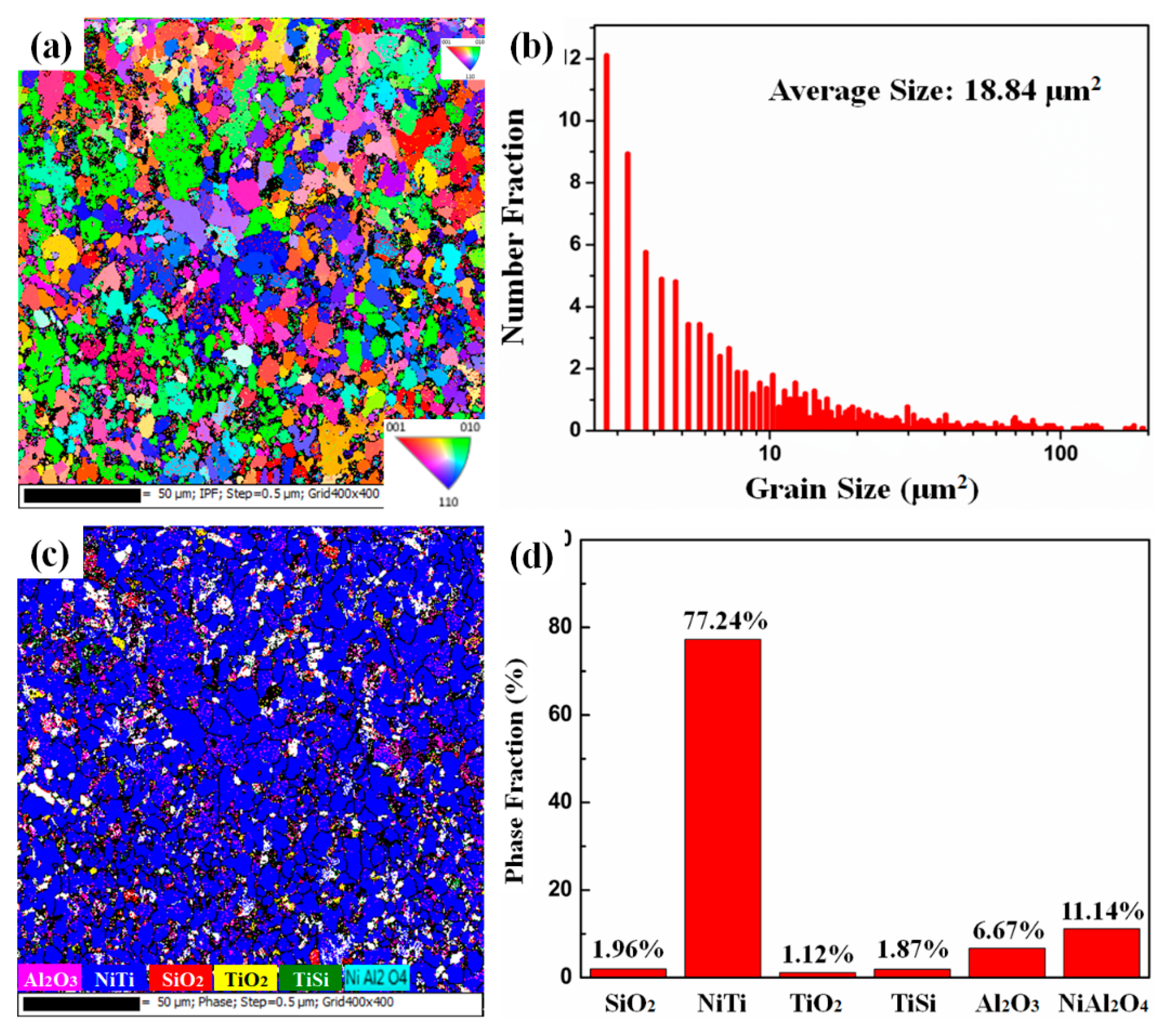
- During the oxidation process, the MA-LC coating shows desirable oxidation behavior. In the initial oxidation stage, the post-laser-cladding coating shows an elevated oxidation rate, which later declines as a dense Al2O3 oxide layer develops. Post-oxidation analysis reveals a grain coarsening phenomenon in the coating, with the average size increasing from 12.69 μm2 to 18.84 μm2. This reduces the grain boundaries that serve as oxygen diffusion channels. Consequently, the coating demonstrates improved resistance to oxidation. Simultaneously, the corresponding kernel average misorientation (KAM) map of the coating reveals a significant release of internal stress in the coating following 100 h oxidation, which can improve the spallation resistance of the coating.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ezugwu, E.O.; Wang, Z.M. Titanium alloys and their machinability—A review. J. Mater. Process. Technol. 1997, 68, 262–274. [Google Scholar] [CrossRef]
- Okulov, I.V.; Volegov, A.S.; Attar, H.; Bönisch, M.; Ehtemam-Haghighi, S.; Calin, M.; Eckert, J. Composition optimization of low modulus and high-strength TiNbbased alloys for biomedical applications. J. Mech. Behav. Biomed. 2017, 65, 866–871. [Google Scholar] [CrossRef]
- Okulov, I.V.; Wendrock, H.; Volegov, A.S.; Attar, H.; Kühn, U.; Skrotzki, W.; Eckert, J. High strength beta titanium alloys: New design approach. Mater. Sci. Eng. A 2015, 628, 297–302. [Google Scholar] [CrossRef]
- Tian, Y.; Chen, C.; Chen, L.; Huo, Q. Wear and oxidation resistance of composite coatings fabricated on Ti-6Al-4V by laser alloying with nitrogen and silicon. J. Phys. D 2005, 38, 4217–4221. [Google Scholar] [CrossRef]
- Zhou, W.; Zhao, Y.; Li, W.; Qin, Q.; Tian, B.; Hu, S. Al-Si coating fused by Al + Si powders formed on Ti–6Al–4V alloy and its oxidation resistance. Mater. Sci. Eng. A 2006, 430, 142–150. [Google Scholar] [CrossRef]
- Zhou, Q.; Ren, Y.; Du, Y.; Han, W.; Hua, D.; Zhai, H.; Huang, P.; Wang, F.; Wang, H. Identifying the significance of Sn addition on the tribological performance of Ti-based bulk metallic glass composites. J. Alloys Compd. 2019, 780, 671–679. [Google Scholar] [CrossRef]
- Xiao, C.; Zhang, H.; Davoodi, D.; Miri, R.; Tayebi, M. Microstructure evolutions of Ti-Al-Nb alloys with different Ta addition, produced by mechanical alloying and spark plasma sintering. Mater. Lett. 2022, 323, 132568. [Google Scholar] [CrossRef]
- Duan, Q.; Luan, Q.; Liu, J.; Peng, L. Microstructure and mechanical properties of directionally solidified high-Nb containing Ti–Al alloys. Mater. Des. 2010, 31, 3499–3503. [Google Scholar] [CrossRef]
- Maestro, C.A.R.; Moreto, J.A.; Chiaramonte, T.; Gelamo, R.V.; Oliveira, C.F.; Santos, M.M.; Silva, M.V.; Bueno, A.H.S.; Balestra, R.M.; Malafaia, A.M.S. Corrosion behavior and biological responses of a double coating formed on the Ti-6Al-4V alloy surface by using thermal oxidation and biomimetic deposition of bismuth-doped CaP. Surf. Coat. Technol. 2021, 425, 127717. [Google Scholar] [CrossRef]
- Xu, J.; Ding, G.; Li, J.; Yang, S.; Fang, B.; Sun, H.; Zhou, Y. Zinc-ion implanted and deposited titanium surfaces reduce adhesion of Streptococccus mutans. Appl. Surf. Sci. 2010, 256, 7540–7544. [Google Scholar] [CrossRef]
- Lei, J.; Shi, C.; Zhou, S. Enhanced corrosion and wear resistance properties of carbon fiber reinforced Ni-based composite coating by laser cladding. Surf. Coat. Technol. 2018, 334, 274–285. [Google Scholar] [CrossRef]
- Blanco, D.; Viesca, J.L.; Mallada, M.T. Wettability and corrosion of [NTf2] anionbased ionic liquids on steel and PVD (TiN, CrN, ZrN) coatings. Surf. Coat. Technol. 2016, 302, 13–21. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical alloying and milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Groza, J.R. Nanocrystalline powder consolidation method. In Nanostructured Materials-Processing, Properties and Potential Applications, 2nd ed.; Koch, C.C., Ed.; William Andrews Publication: New York, NY, USA, 2006. [Google Scholar]
- Chen, C.; Feng, X.; Shen, Y. Synthesis of Al–B4C composite coating on Ti–6Al–4V alloy substrate by mechanical alloying method. Surf. Coat. Technol. 2017, 321, 8–18. [Google Scholar] [CrossRef]
- Lu, C.; Tian, Y.; Shen, Y.; Feng, X.; Jiang, J. Thermal shock resistance and thermal conductivity of diamond-Cu composite coatings on Cu substrate via mechanical milling method. Surf. Coat. Technol. 2018, 352, 529–540. [Google Scholar] [CrossRef]
- Chen, C.; Feng, X.; Shen, Y. Effects of annealing treatment and pre-refinement of raw material on microstructures and properties of mechanically alloyed Cr–Al composite coatings on Ti–6Al–4V alloy. Mater. Character. 2016, 120, 97–108. [Google Scholar] [CrossRef]
- Wang, D.; Tian, Z.; Shen, L.; Huang, Y. Research status and development of laser cladding technology. Appl. Laser 2012, 32, 538–544. [Google Scholar]
- Liu, J.; Song, M.; Chen, C.; Liu, H. Research progress on laser cladding technology of titanium alloy surface. Heat Treat. Met. 2019, 44, 87–96. [Google Scholar]
- Chakraborty, R.; Raza, M.S.; Datta, S.; Saha, P. Synthesis and characterization of nickel free titanium–hydroxyapatite composite coating over nitinol surface through in-situ laser cladding and alloying. Surf. Coat. Technol. 2019, 358, 539–550. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Li, J.; Zhang, Y.Y.; Kang, D.N. Evolution in microstructure and high-temperature oxidation behaviors of the laser-cladding coatings with the Si addition contents. J. Alloys Compd. 2020, 827, 154131. [Google Scholar] [CrossRef]
- Carrullo, J.C.Z.; Falcon, J.C.P.; Borras, V.A. Influence of process parameters and initial microstructure on the oxidation resistance of Ti48Al2Cr2Nb coating obtained by laser metal deposition. Surf. Coating. Technol. 2019, 358, 114–124. [Google Scholar] [CrossRef]
- Liu, H.X.; Zhang, X.W.; Jiang, Y.H.; Zhou, R. Microstructure and high temperature oxidation resistance of in-situ synthesized TiN/Ti3Al intermetallic composite coatings on Ti6Al4V alloy by laser cladding process. J. Alloys Compd. 2016, 670, 268–274. [Google Scholar] [CrossRef]
- Diao, Y.H.; Zhang, K.M. Microstructure and corrosion resistance of TC2 Ti alloy by laser cladding with Ti/TiC/TiB2 powders. Appl. Surf. Sci. 2015, 352, 163–168. [Google Scholar] [CrossRef]
- Tan, H.; Luo, Z.; Li, Y.; Yan, F.; Duan, R.; Huang, Y. Effect of strengthening particles on the dry sliding wear behavior of Al2O3-M7C3/Fe metal matrix composite coatings produced by laser cladding. Wear 2015, 324, 36–44. [Google Scholar] [CrossRef]
- Akbari, M.K.; Baharvandi, H.R.; Shirvanimoghaddam, K. Tensile and fracture behavior of nano/micro TiB2, particle reinforced casting A356 aluminum alloy composites. Mater. Des. 2015, 66, 150–161. [Google Scholar] [CrossRef]
- Zhou, S.F.; Dai, X.Q. Laser induction hybrid rapid cladding of WC particles reinforced NiCrBSi composite coating. Appl. Surf. Sci. 2010, 256, 4708–4714. [Google Scholar] [CrossRef]
- Yu, T.; Deng, Q.L.; Zheng, J.F.; Dong, G.; Yang, J.G. Microstructure and wear behaviour of laser clad NiCrBSi + Ta composite coating. Surf. Eng. 2013, 28, 357–363. [Google Scholar] [CrossRef]
- Wang, X.H.; Zhang, M.; Du, B.S.; Li, S. Microstructure and wear properties of laser clad (TiB2 + TiC)/Fe composite coating. Surf. Rev. Lett. 2012, 19, 1250052. [Google Scholar] [CrossRef]
- Ma, Q.S.; Li, Y.J.; Wang, J.; Liu, K. Microstructure evolution and growth control of ceramic particles in wide-band laser clad Ni60/WC composite coatings. Mater. Des. 2016, 92, 897–905. [Google Scholar]
- Peng, Y.; Zhang, W.; Li, T.; Zhang, M.; Liu, B.; Liu, Y.; Wang, L.; Hu, S. Effect of WC content on microstructures and mechanical properties of FeCoCrNi high-entropy alloy/WC composite coatings by plasma cladding. Surf. Coat. Technol. 2020, 385, 125326. [Google Scholar] [CrossRef]
- Jiang, J.; Feng, X.; Shen, Y.; Lu, C.; Tian, Y. Oxidation behavior of Cr-AlSi12 composite coatings on Ti-6Al-4V alloy substrate fabricated via high-energy mechanical alloying method. Surf. Coat. Technol. 2019, 367, 212–224. [Google Scholar] [CrossRef]
- Sun, S.; Liu, J.-G.; Yan, C.-W.; Wang, F.-H. A novel process for electroless nickel plating on anodized magnesium alloy. Appl. Surf. Sci. 2008, 254, 5016–5022. [Google Scholar] [CrossRef]
- Wei, Y.K.; Li, Y.J.; Zhang, Y.; Luo, X.T.; Li, C.J. Corrosion resistant nickel coating with strong adhesion on AZ31B magnesium alloy prepared by an in-situ shot-peening-assisted cold spray. Corros. Sci. 2018, 138, 105–115. [Google Scholar] [CrossRef]
- Liu, J.; Shi, Y. Microstructure and wear behavior of laser-cladded Ni-based coatings decorated by graphite particles. Surf. Coat. Technol. 2021, 412, 127044. [Google Scholar] [CrossRef]
- Morawiec, M.; Rozanski, M.; Grajcar, A.; Stano, S. Effect of dual beam laser welding on microstructure–property relationships of hot-rolled complex phase steel sheets. Arch. Civ. Mech. Eng. 2017, 17, 145–153. [Google Scholar] [CrossRef]
- Dinda, G.P.; Dasgupta, A.K.; Mazumder, J. Laser aided direct metal deposition of Inconel 625 superalloy: Microstructural evolution and thermal stability. Mater. Sci. Eng. A 2009, 509, 98–104. [Google Scholar] [CrossRef]
- Torres, H.; Slawik, S.; Gachot, C.; Prakash, B.; Ripoll, M.R. Microstructural design of self-lubricating laser claddings for use in high temperature sliding applications. Surf. Coat. Technol. 2018, 337, 24–34. [Google Scholar] [CrossRef]
- Zheng, C.; Liu, Z.; Chen, S.; Liu, C. Corrosion behavior of a Ni-Cr-Mo alloy coating fabricated by laser cladding in a simulated sulfuric acid dew point corrosion environment. Coatings 2020, 10, 849. [Google Scholar] [CrossRef]
- Ren, L.; Ding, Y.; Cheng, Y.; Nong, J.; Wang, S.; Wang, S.; Zhang, D.; Qin, Q.; Si, J.; Shoji, T.; et al. The microstructure evolving and element transport of Ni-based laser-cladding layer in supercritical water. Corros. Sci. 2022, 198, 110112. [Google Scholar] [CrossRef]
- Luo, X.X.; Yao, Z.J.; Zhang, P.Z.; Gu, D.D.; Chen, Y. Laser cladding Fe-Al-Cr coating with enhanced mechanical properties. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2019, 34, 1197–1204. [Google Scholar] [CrossRef]
- Cao, S.; Liang, J.; Wang, L.; Zhou, J. Effects of NiCr intermediate layer on microstructure and tribological property of laser cladding Cr3C2 reinforced Ni60A-Ag composite coating on copper alloy. Opt. Laser Technol. 2021, 142, 106963. [Google Scholar] [CrossRef]
- Barin, I. Thermochemical Data of Pure Substance, 3rd ed.; Wiley-VCH: Weinheim, Germany, 1995. [Google Scholar]
- Tang, J.J.; Bai, Y.; Zhang, J.C.; Liu, K.; Liu, X.Y.; Zhang, P.; Wang, Y.; Zhang, L.; Liang, G.Y.; Gao, Y.; et al. Microstructural design and oxidation resistance of CoNiCrAlY alloy coatings in thermal barrier coating system. J. Alloys Compd. 2016, 688, 729–741. [Google Scholar] [CrossRef]
- Li, W.; Zhang, W.L.; Li, T.; Pen, X.; Sun, J.; Wang, T.G.; Jiang, S.M.; Gong, J.; Sun, C. Improvement of cyclic oxidation resistance of a β-(Ni,Pt)Al coating by the addition of Ni/Ni-Re layer at 1150 °C. Corros. Sci. 2022, 207, 110486. [Google Scholar] [CrossRef]
- Calcagnotto, M.; Ponge, D.; Demir, E.; Raabe, D. Orientation gradients and geometrically necessary dislocations in ultrafine grained dual-phase steels studied by 2D and 3D EBSD. Mater. Sci. Eng. A 2010, 527, 2738–2746. [Google Scholar] [CrossRef]






| Coatings | Laser Power P (W) | Scanning Speed v (mm/s) | Laser Beam Diameter D (mm) | Laser-Specific Energy Es (J/mm2) |
|---|---|---|---|---|
| S1 | 200 | 10 | 1.5 | 13.3 |
| S2 | 300 | 12 | 1.5 | 16.7 |
| S3 | 300 | 8 | 1.5 | 25.0 |
| S4 | 450 | 10 | 1.5 | 30.0 |
| Mechanical Properties | MA Coating | MA-LC Coating |
|---|---|---|
| Nanohardness H (GPa) | 1.51 | 9.79 |
| Elastic modulus E (GPa) | 96.51 | 159.76 |
| WEr (%) | 12.55 | 39.66 |
| H3/E2 | 0.00037 | 0.03676 |
| Coating | Cone Depth (µm) | Projected Cone Perimeter (µm) | Projected Cone Area (µm2) | Type of Failure |
|---|---|---|---|---|
| MA | 7.685 | 457.4 | 10,059 | Adhesive/Cohesive |
| MA-LC | 6.285 | 339.3 | 4951 | No failure |
| Element | Area 1 | Area 2 | Area 3 | Area 4 | Area 5 | Area 6 | Area 7 |
|---|---|---|---|---|---|---|---|
| OK | 48.18 | 42.39 | 43.91 | 53.96 | 31.11 | 23.86 | 14.34 |
| AlK | 36.04 | 41.10 | 41.04 | 44.00 | 36.08 | 49.25 | 56.58 |
| SiK | 3.55 | 1.88 | 7.32 | 0.16 | 7.61 | 6.38 | 4.40 |
| NiK | 12.24 | 14.63 | 5.29 | 1.48 | 3.55 | 6.99 | 18.40 |
| TiK | - | - | 2.44 | 0.40 | 21.65 | 13.53 | 6.28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, H.; Xu, L.; Jiang, J.; Shou, H.; Hao, H.; Feng, R. Oxidation-Resistant Ni-AlSi12 Composite Coating with Strong Adhesion on Ti-6Al-4V Alloy Substrate via Mechanical Alloying and Subsequent Laser Cladding. Coatings 2025, 15, 1329. https://doi.org/10.3390/coatings15111329
Xie H, Xu L, Jiang J, Shou H, Hao H, Feng R. Oxidation-Resistant Ni-AlSi12 Composite Coating with Strong Adhesion on Ti-6Al-4V Alloy Substrate via Mechanical Alloying and Subsequent Laser Cladding. Coatings. 2025; 15(11):1329. https://doi.org/10.3390/coatings15111329
Chicago/Turabian StyleXie, Huanjian, Luyan Xu, Jian Jiang, Haoge Shou, Hongzhang Hao, and Ruizhi Feng. 2025. "Oxidation-Resistant Ni-AlSi12 Composite Coating with Strong Adhesion on Ti-6Al-4V Alloy Substrate via Mechanical Alloying and Subsequent Laser Cladding" Coatings 15, no. 11: 1329. https://doi.org/10.3390/coatings15111329
APA StyleXie, H., Xu, L., Jiang, J., Shou, H., Hao, H., & Feng, R. (2025). Oxidation-Resistant Ni-AlSi12 Composite Coating with Strong Adhesion on Ti-6Al-4V Alloy Substrate via Mechanical Alloying and Subsequent Laser Cladding. Coatings, 15(11), 1329. https://doi.org/10.3390/coatings15111329







