1. Introduction
Superhydrophobic (SHP) materials are typically defined by static contact angles (SCA) greater than 150°, contact angle hysteresis (H) below 10° and roll-off angles (RO) under 5° [
1]. These properties are a result of a combination of hierarchical micro/nanoscale roughness and low surface energy coatings.
Due to their complete water repellency, such surfaces demonstrate advanced functionalities, including self-cleaning, anti-corrosion and also icephobicity [
2,
3,
4,
5,
6]. In particular, the application of SHP coatings represents an intriguing passive method for facing environmental icing issues on metal surfaces, such as aluminum alloys, without energy consumption.
Although good results are evidenced in many works [
3,
7,
8], several studies highlight the limit of SHP surfaces for icephobic applications [
9,
10,
11]. Their failure can be attributed to several factors, among them the deterioration of wettability properties, linked to the transition from the Cassie–Baxter to the Wenzel state [
12], particularly under low temperature conditions [
10].
An optimized design of SHP surfaces obtained with sophisticated processes such as laser treatment and nanolithography can hinder or even prevent this transition, as reported in some works [
13]. However, these processes are complex, expensive and difficult to scale.
Chemical etching is a cost-effective and straightforward method for fabricating superhydrophobic surfaces (SHPs) from metal substrates, and its application has been widely reported in the literature, including studies addressing icing-related issues. For example, Yang and Li [
14] fabricated superhydrophobic surfaces on aluminum substrates by immersion in hydrochloric acid as an etching solution, followed by coating with a fluoroalkylsilane (FAS), obtaining SCAs up to 162° and a delay in the freezing time. A cupric chloride solution was used by Liao et al. [
15] to etch aluminum surface forming microstructures, while hydrochloric acid was adopted to impart nanoroughness and a long-chain siloxane as a low-energy coating. A similar approach was adopted by Zuo et al. [
16] but using hot water for the nanostructuration step: both studies reported SHP surfaces with high SCAs and an observed freezing delay. Barthwal et al. [
17,
18] developed both SHP and slippery coatings by etching of aluminum alloy in hydrochloric acid followed by anodization in sulfuric acid and subsequent coating/infusion with polydimethylsiloxane. An increase in freezing time (up to 700 s) and a reduced ice adhesion (down to 22 ± 5 kPa) were reported. A hydrochloric acid solution was also used as etchant by Lo et al. [
19], who after the subsequent hydrothermal treatment and coating with FAS or polydimethylsiloxane obtained SCAs up to 176° and ice adhesion lower than 50 kPa. Peng et al. [
20] treated aluminum plates in a hydrochloric acid/hydrogen peroxide solution with different molar ratios; then, using stearic acid for functionalization, they obtained a reduced ice accumulation under a specific test with water spray equipment in a climate chamber. More recently, Rodic et al. [
21] developed SHP coatings by means of a similar etching treatment followed by grafting the surface with n-octyltrimethoxysilane or with FAS. The resulting surfaces achieved SCAs higher than 150° and demonstrated early ice-melting properties.
The aforementioned studies typically focused on only one or a few preparation conditions, and even when morphology, wettability and anti-icing performance (mainly in terms of freezing delay and/or reduction in ice nucleation temperature) were investigated, the results were discussed separately. To the best of our knowledge, while comprehensive studies such as [
22] have systematically investigated the combined effects of different chemical etching conditions on the microroughness of aluminum surfaces, the relationship between this microroughness and key surface properties—such as wettability (including both static and dynamic contact angles) and ice adhesion—remains largely unexplored in the current literature. Furthermore, if a correlation between roughness, wettability and ice adhesion can be established, it becomes essential to identify the optimal surface characteristics that maximize icephobic performance.
This study aims to investigate how a simple chemical etching process can lead to hydrophobic and superhydrophobic surfaces characterized by different roughness and morphology, and which are the key features for icephobicity.
Several samples are fabricated through a simple and scalable process involving chemical etching, hydrothermal treatment and fluoroalkylsilane (FAS) functionalization. By varying parameters such as etchant concentration, immersion time and hydrothermal treatment duration, we obtained surfaces with different roughness.
All surfaces exhibited icephobic performances spanning in various degrees from poor to excellent, and a correlation with their wettability was observed. The most icephobic surface exhibited an up to 25-fold reduction in ice adhesion, representing a promising strategy to mitigate icing on aluminum substrates.
This study provides insights into the design of anti-icing surfaces and identifies the key features required for developing scalable, low-wettability and icephobic materials, contributing to the development of passive anti-icing strategies that are both effective and economically viable.
2. Materials and Methods
2.1. Materials
Flat plates (20 mm × 70 mm × 2 mm) and bars (12 mm diameter × 100 mm length) of aluminum alloy (6082) were used as substrates.
Dynasylan® SIVO CLEAR EC was purchased from EVONIK (Essen, Germany); the product is composed of fluoroalkylsilane (FAS) 2%, propan-2-ol 93% and dodecane 5%, and was used as received.
Acetone (>99.5%) was purchased from Sigma Aldrich (St. Louis, MI, USA). Ethanol (≥99.9%) and hydrochloric acid (36%) were purchased from Carlo Erba (Milan, Italy). All reagents were used without further treatments.
2.2. Characterization Methods
Surface morphologies were examined using a field emission scanning electron microscope (FE-SEM; Mira III, Tescan, Brno, Czech Republic) before the coating with FAS (step 3).
A Taylor Hobson stylus profilometer was used to measure surface roughness. Data were averaged over at least 5 runs for each sample.
The FT-IR characterizations were conducted on the prepared surfaces with the FT-IR Alpha 1 (Bruker, Ettlingen, Germany) spectrometer with ATR apparatus (with diamond crystal as the internal reflection element), before the coating with FAS (step 3).
The static water contact angles were measured with the DSA 30 Drop Shape Analyzer (Kruss, Hamburg, Germany) with the sessile drop method using 4 μL volume of water. The measurements of roll-off angle were performed with the tilting plate method using a 20 μL water volume, following the Standard ISO 19403-7:2017. This volume was used to ensure that droplets could detach from the needle and be placed on the sample, particularly for surfaces exhibiting high hydrophobicity. The droplet was placed on a stage parallel to the horizon, and the table was then tilted.
The measurements of advancing and receding contact angles were carried out with the needle-in method starting from a 4 μL volume of water droplet. The droplet volume was increased at 1 µL/s, and the advancing contact angle was measured when the contact point started to move outward. Then, water was withdrawn from the droplet and the receding contact angle was measured when the contact point started to retract. The contact angle hysteresis was calculated as the difference between the advancing and receding contact angles. All wettability measurements were carried out at 23 ± 2 °C and at least 5 measurements in different points for each sample were executed.
The ice adhesion strength was evaluated by means of the shear force needed to extract a sample pole from an ice block, with a method described in a previous work [
23] (see also
Figure S1). The ice block was formed by pouring 50 mL of deionized water into a rigid vessel together with the pole and cooling the system at −19 °C for 8 h. After this period the extraction of the pole from the ice was carried out by means of a home-made apparatus equipped with an electromechanical testing system (INSTRON 4507). All tests were conducted at 23 ± 2 °C with relative humidity ranging from 40% to 60%. Each measurement was completed in under one minute to ensure that the low temperature at the surface/ice interface was preserved throughout testing.
The ice adhesion strength (
τ) in shear can be calculated as
where
F is the peak force needed to extract the sample pole from the ice and
A is the surface of the bar in contact with the ice. The shear stresses were measured on 10 different specimens for each treatment.
The ice adhesion of bare Al alloy was also measured as a reference, and an adhesion reduction factor (ARF) was calculated as
Generative artificial intelligence (GenAI) has been used in this paper to enhance the quality of the English language.
2.3. Preparation of Samples
All the aluminum alloy specimens were cleaned with basic soap, rinsed in an ultrasonic bath for 10 min with acetone and dried under nitrogen flux.
The etched samples were prepared through a three-step process: (1) microroughness formation; (2) nanoroughness growth; (3) surface functionalization with FAS.
In the first step different aluminum alloy plates were etched in HCl solutions with three different concentrations (0.5, 1 or 3 M) for different time frames (15, 30 or 60 min). After the immersion in HCl, the samples were accurately rinsed with deionized water in the ultrasonic bath.
In the second step some samples were immersed in deionized water at 100 °C for different times (5 or 30 min) and then dried at 100 °C for 1 h in a laboratory oven. Samples without further treatment in boiling water were also prepared.
In the third step, all surfaces were dip-coated in the FAS solution with the following conditions: immersion speed 0.7 mm/s, permanence time 2 min, extraction speed 0.7 mm/s. Samples were then heated at 120 °C for 1 h.
From this point onward, samples are labeled based on their etching and boiling treatments using an abbreviation system. The first letter indicates the HCl concentration (L = 0.5 M, I = 1 M, H = 3 M, where L = low, I = intermediate, H = high), the second letter denotes the immersion time in HCl (S = 15 min, M = 30 min, L = 60 min, where S = short, M = medium, L = long) and the number represents the immersion time in boiling water (0, 5 or 30 min).
As reference samples, ‘Pristine’ was prepared as described above but omitting steps 1 and 2, while ‘Nano-5’ was prepared without step 1.
3. Results and Discussion
3.1. Morphological and Chemical Evaluation of the Texturized Surfaces
HCl can be used to tune the roughness and morphology of metal surfaces and lattices with different methods such as chemical etching or electrochemical leaching, giving rise to different grooves [
24].
In the chemical etching process HCl reacts with aluminum surfaces, first removing the native oxide layer and then oxidizing the metal and producing AlCl3 and H2. HCl initiates the etching process from surface defects (e.g., grain boundary and surface dislocations) and after a certain amount of time, cavities on the surface are formed. The duration of the etching influences the final groove: prolonging the immersion time, the surfaces become more uniform and texturized.
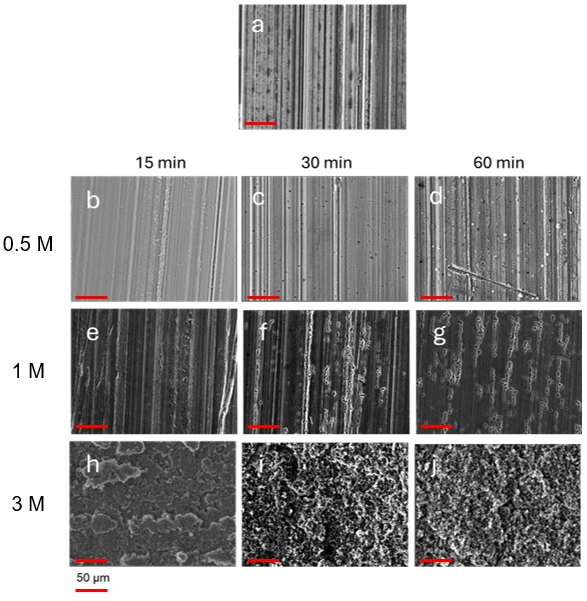
SEM analysis shows that depending on the concentration of the etchant and the immersion time, different microroughed textures were obtained with respect to the starting Pristine surface, as shown in
Figure 1.
The concentration of the acid also influences the final texture of the surface. More diluted HCl primarily etches areas of the surface where native roughness, originating from the manufacturing process, is present, deepening the longitudinal grooves proportionally to treatment duration.
By increasing the concentration of HCl, rougher surfaces with well-marked grain boundaries and cavities are obtained.
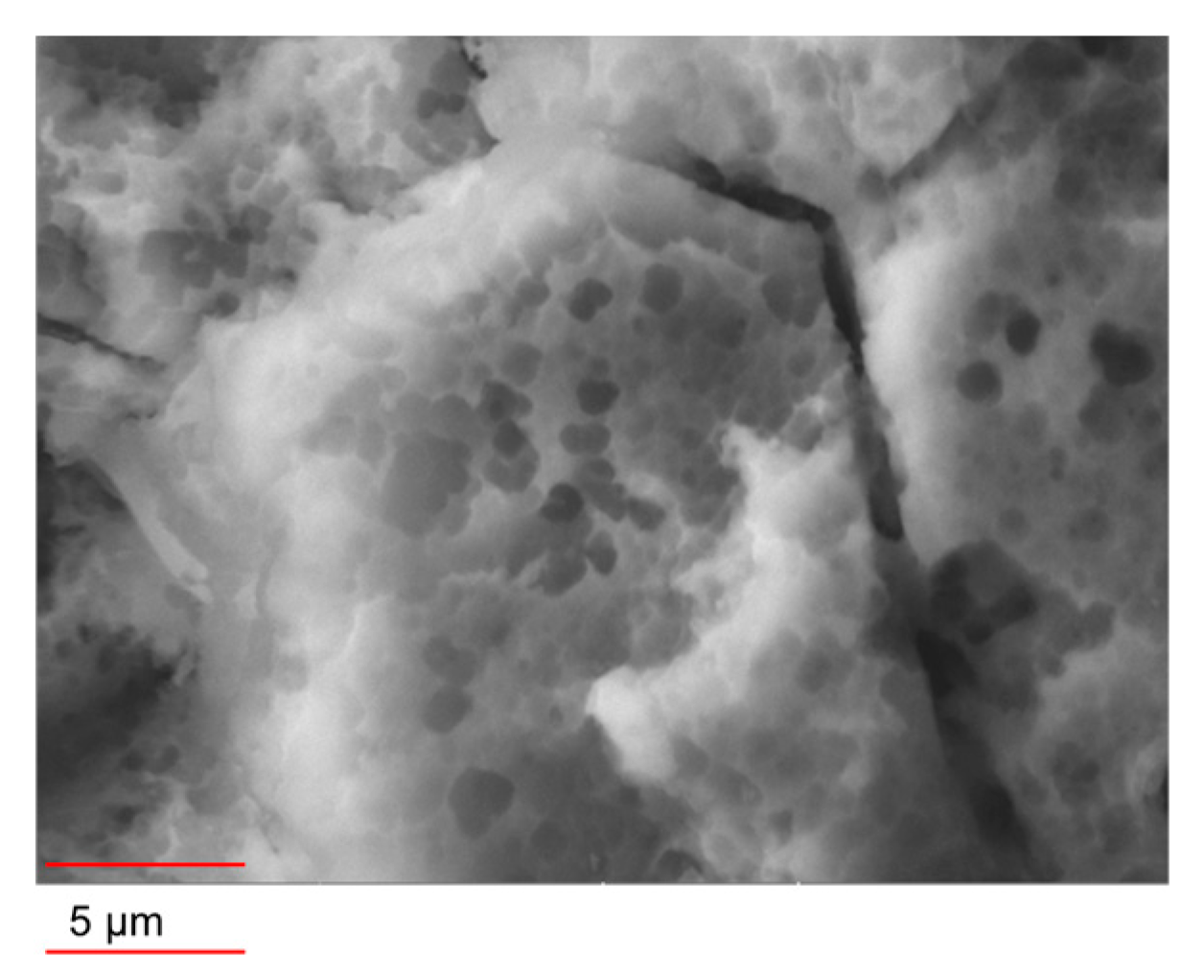
A porous, textured surface featuring honeycomb-like structures, many of which are sub-micrometric in size, is formed after prolonged exposure to HCl 3 M, as displayed in
Figure 2.
Nanoroughness formation, induced by the hydrothermal treatment of aluminum in boiling water [
25], involves a chemical reaction driven by the negative standard reduction potential of Al (Al
3+ + 3e
−→Al(s), E
0 = −1.66 eV), giving rise to nanostructured oxide-hydroxide aluminum species (AlOOH-boehmite) with the release of hydrogen gas (H
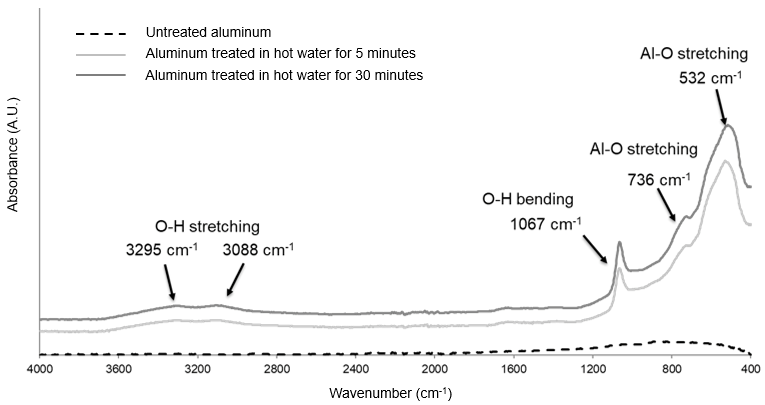
2). All samples evidenced the typical signals due to the presence of AlOOH on the surface, as shown in the FT-IR spectrum (see
Figure 3 for samples LS5 and LS30).
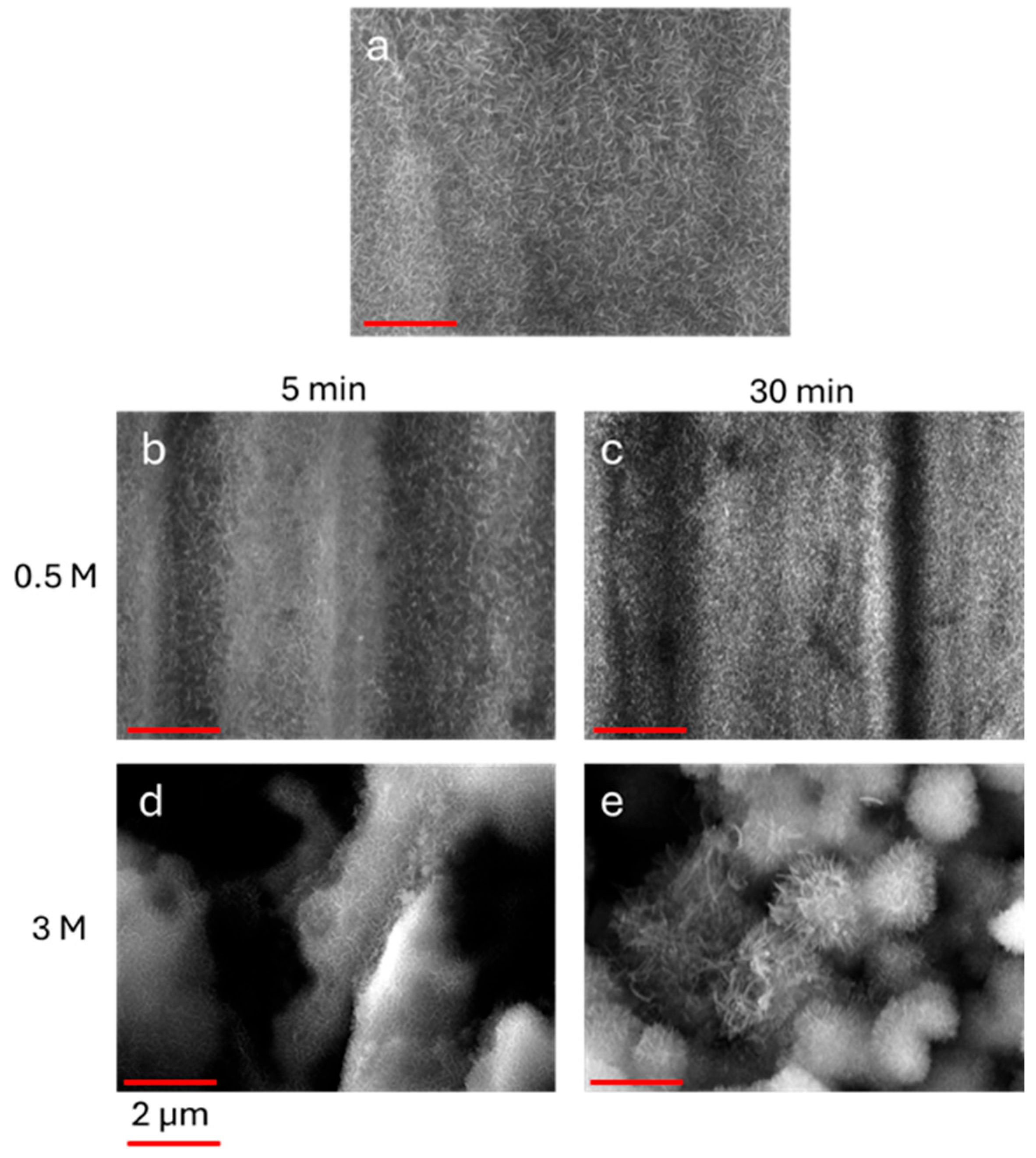
The nanostructures exhibit a needle-like shape with some differences linked to the duration of the process and to the underlying microroughness. In
Figure 4, the SEM images of the AlOOH on Nano-5 and on samples LL5, LL30, HL5 and HL30 are shown.
The duration of the hydrothermal treatment induces some differences in the morphology and density of the oxidized species. As shown in
Figure 4, a 5 min long immersion time in boiling water is sufficient to induce the formation of acicular nanostructures on the surface. As expected [
26], by prolonging the treatment up to 30 min, the formation of denser structures that ubiquitously cover the aluminum specimens, is evidenced.
For samples treated in HCl 3 M, the acicular nanostructures homogeneously cover the microrough surfaces, giving rise to hierarchical aggregates. Moreover, the dimensions of AlOOH grown on sample HL30 are the largest; this can be attributable both to the prolonged immersion time in water and the higher surface area obtained after the etching process.
3.2. Roughness
Micrometric-scale roughness was measured on the prepared samples and the results of roughness parameters, for Pristine and for samples that did not undergo hydrothermal treatment (xx0 series), are reported in
Figure 5. The dimensions of the resulting nanostructures were too small to be properly resolved with the employed profilometer and the microroughness of the nanostructured samples was found to be comparable to that of the corresponding untreated samples (see
Table S1 and Figure S2 for full dataset).
All surfaces treated with 0.5 M and 1 M HCl (Lxx and Ixx series) have roughness parameters comparable to each other, exhibiting low values even if immersed for 60 min. In comparison with Pristine, these samples exhibit only a slight increase in roughness parameters although, as shown in
Figure 1, the SEM images reveal that in certain areas of these surfaces the etching treatment has altered the surface morphology.
Surfaces treated with 3 M HCl for 15 min (HS0), despite no significant increase in the average roughness (Ra), exhibit higher Rv values compared to Rp. This suggests that aluminum ablation under these etching conditions primarily deepens the valleys rather than increasing peak height.
Conversely, samples treated in HCl 3 M for at least 30 min show higher values across all roughness parameters as expected. Both peak height (Rp) and valley depths (Rv) reach up to 10 µm, while Rz increases up to 25 µm and Ra to 4 µm, indicating a uniformly roughened surface. It is worth noting that, in the case of treatment with 3 M HCl, surface roughness parameters increase sharply when the immersion time is extended from 15 to 30 min, while only a slight further increase is observed at 60 min.
3.3. Wettability of Etched Surfaces
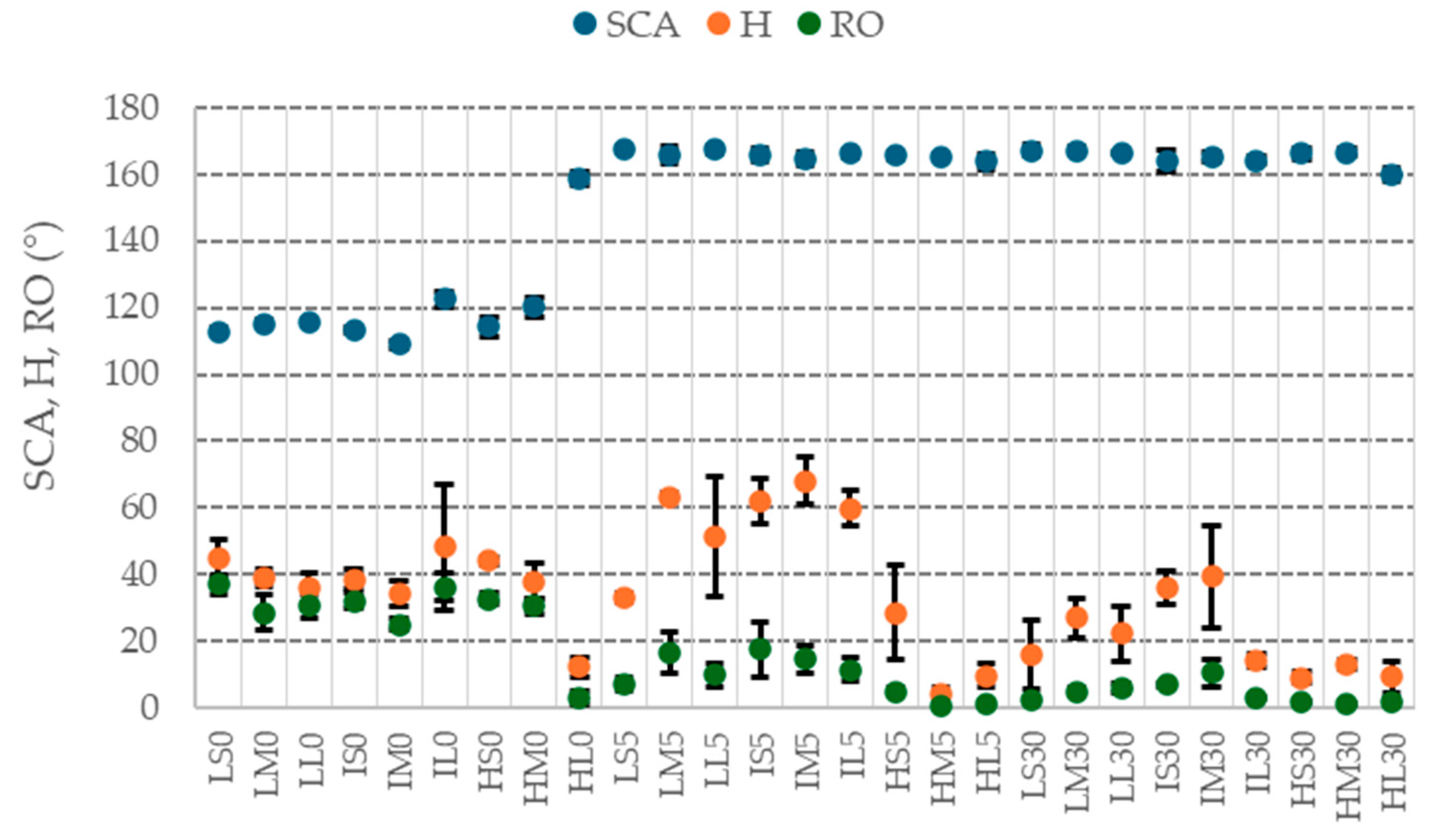
The results of wettability measurements on coated samples are reported in
Figure 6.
All the surfaces evidenced a pronounced hydrophobic behavior. The boiled samples, which have an acicular nanostructure, exhibit an SCA of about 160°, while the un-boiled ones, with the exception of HL0, have an SCA ranging from 110° to 120°. The wettability of HL0 can indeed be compared to those of the boiled samples. Although the HL0 does not exhibit the AlOOH nanostructure, the peculiar honeycomb-like morphology formed after the prolonged treatment in strong acid is sufficient to impart a pronounced hydrophobic property (
Figure 2).
Similarly, roll-off angles are lower for boiled samples and the longer treatment in hot water leads to slightly lower RO values than the 5 min treatment. Samples treated in boiling water for 30 min evidence, in some cases, RO values lower than 5°. By comparing the chemical etching, it is shown that samples etched with HCl 3 M (Hxx), resulting in more pronounced roughness, generally evidence lower RO angles, while the different immersion times in etching solution seem to be not relevant.
The H results do not show a clear trend among samples with different hydrothermal treatments. It is possible to observe, for instance, that the 5 min treatment in boiling water gives rise to high H values, even higher than the samples that did not undergo the water treatment. The immersion time of 30 min leads to a decrease in hysteresis. Similarly to RO, the treatment in HCl 3 M gives rise to surfaces with low H values independently from the immersion time in the etching solution.
For many samples, especially among those with an SCA higher than 160°, hysteresis angles are very high even if roll-off angles are very low (see also
Figure S3). As stated above, the advancing and receding angles were measured, for all the surfaces, by increasing and decreasing the volume of the water droplets (needle-in method). This method was chosen in advance as it allows for the measurement of both advancing and receding contact angles even on surfaces lacking water mobility (no roll-off). It is also unaffected by the difference in droplet volumes, which can lead to different results when measuring with the tilting plate method. Furthermore, it provides practical benefits by also ensuring consistent measurements on superhydrophobic surfaces exhibiting high water mobility. However, an increase in volume inherently corresponds to an increase in mass, and during this process, additional pressure is exerted on the droplet. As a result, the droplet may infiltrate the porous structure, leading to a transition from the Cassie–Baxter to the Wenzel state [
12], with consequent generally lower receding contact angles. At the same time, low receding angles are also indications of defects on the surface, and the very high dispersion of some H measures confirm this.
3.4. Icephobicity of Etched Surfaces
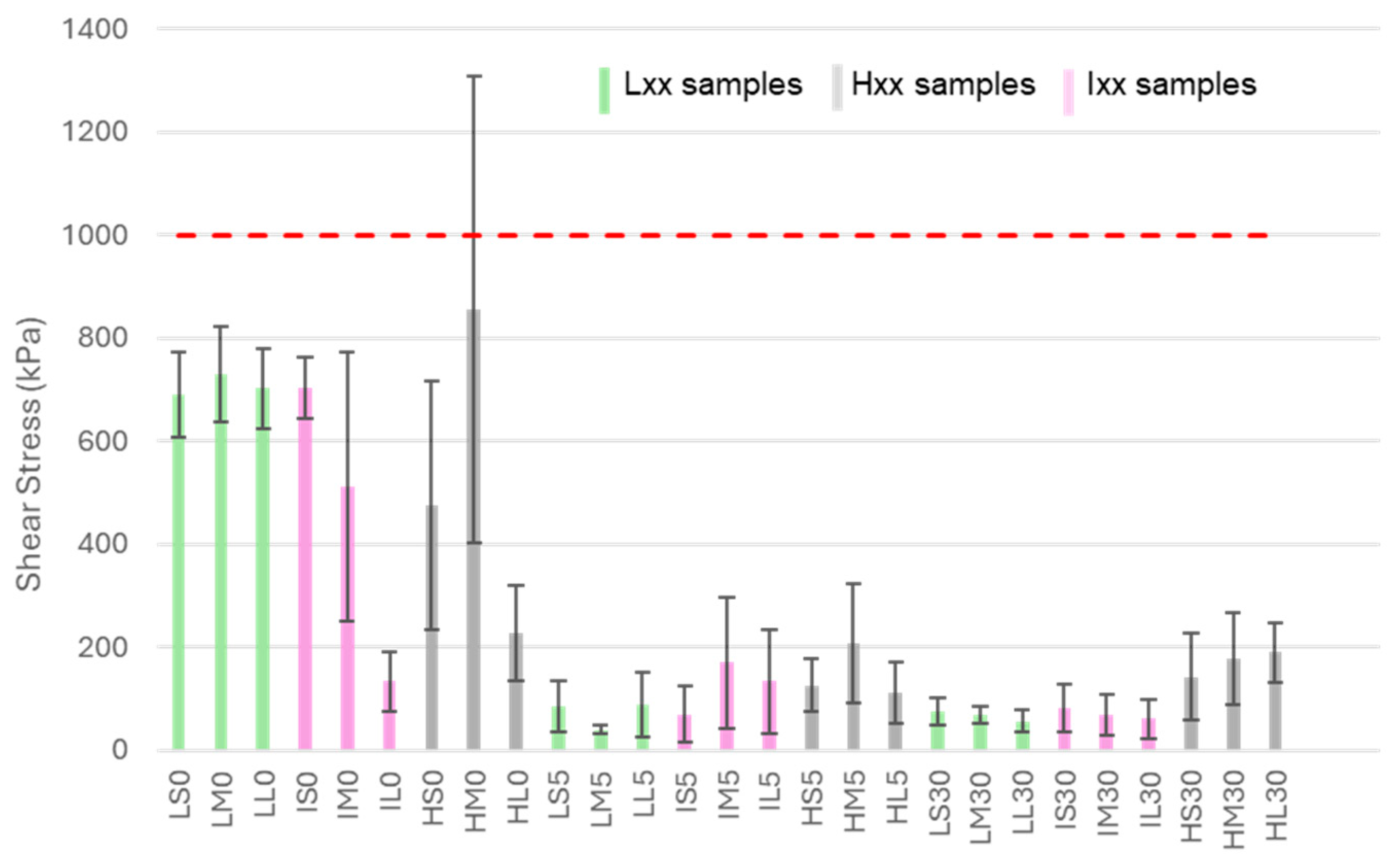
The ice adhesion results are reported in
Figure 7.
All samples decrease ice adhesion with respect to bare untreated aluminum on which an ice adhesion of about 1000 kPa in shear was measured. The ARFs exhibit a range from 1.2 to 25 across samples, with only a partially discernible pattern. The values are listed in
Table 1; samples are grouped based on their values.
All data clearly indicate that the principal key feature responsible for ice reduction is the nanometric structure, and the hydrothermal treatment is the primary factor affecting icephobicity. All samples treated in boiling water exhibit high or mild icephobicity, while all the un-boiled ones, with the exception of HL0 and IL0, showed poor anti-icing properties.
In the case of HL0, the presence of a nanometric honeycomb-like structure not only reduces water adhesion, as previously discussed, but may also contribute to lowering ice adhesion by mimicking the AlOOH layer and forming a nanostructured surface. For IL0, since no nanostructures were observed on its surface, the low shear stress measured is somewhat unexpected, and further investigations will be necessary to clarify this behavior.
Although it is difficult to observe a clear trend related to the conditions applied, especially considering the high data dispersion of some samples, some correlation between the etching process and icephobicity can be identified. In particular, among nanostructured samples, surfaces treated with HCl 3 M (Hxx samples) generally exhibit higher ice adhesion than those treated with HCl 0.5 M (Lxx samples) or 1 M (Ixx samples). This is likely due to the increased microroughness, which leads to a larger surface area and consequently provides more sites where ice can mechanically interlock with the surface. It is also important to note that there is not a significative difference between samples Lxx and samples Ixx, which is consistent with the observed similarities in their morphology and surface roughness.
As already evidenced for wettability, the different etching time does not influence the icephobic performances.
3.5. Features for Icephobicity
The design of micro/nanostructured icephobic surfaces requires the combined consideration of roughness and morphology, and the resulting hydrophobicity can yield important indications of the expected ice adhesion.
Icephobic performances among etched surfaces are thus achieved on substrates with minimal microroughness and acicular nanostructures. This is also confirmed by the surfaces Pristine and Nano-5, whose ice adhesion results are listed in
Table 2, together with their wettability results.
Once again, the presence of nanoroughness emerges as the key factor influencing ice adhesion, as the shear stress measured for Nano-5 is comparable to that of the best-performing etched surfaces. Conversely, the un-boiled Pristine sample—despite being the smoothest among all the samples—does not exhibit significant icephobicity.
Overall, the data suggest that while a hierarchical micro/nanostructure is necessary to achieve low water adhesion, a purely nanometric texture is sufficient to reduce ice adhesion. This morphology yields highly hydrophobic surfaces that facilitate the easy movement and detachment of water droplets, while minimal microroughness hinders ice anchoring.
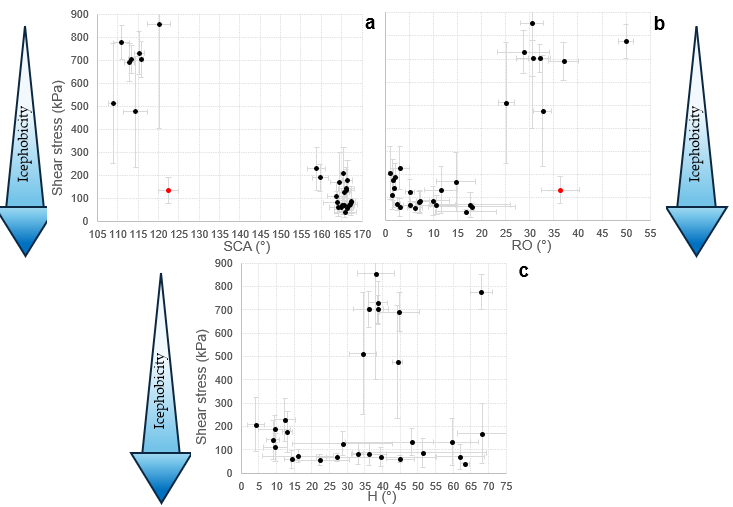
It is worth asking if a relationship can be found for these samples between hydrophobicity and icephobicity and if an effect in reducing ice adhesion can be inferred from the wettability data. In
Figure 8 shear stress data are plotted against SCA, RO and H.
With only one exception (corresponding to sample IL0, discussed in
Section 3.3) high SCA (≃160°) and low RO (<20°) values are consistently associated with low ice adhesion. In contrast to some findings reported in the literature [
27,
28], no clear correlation between H and ice adhesion can be established for our samples, as low ice adhesion is observed across a wide range of H values, from less than 5° to more than 65°.
Despite these correlations, the results show that SHP samples are not the most icephobic among the studied surfaces.
During the freezing phase of the ice adhesion test conducted in this study, a transition from the Cassie–Baxter to the Wenzel state is likely to occur. This transition is associated with a temporary increase in water pressure on the sample surface [
29], caused by the water-to-ice phase change occurring in the partially confined space of the mold.
As the surface asperities become partially wetted, the resulting regime promotes stronger mechanical interlocking between the ice and the surface. This effect is particularly pronounced for surfaces with higher roughness, such as SHPs, which become more susceptible to increased contact area and interlocking phenomena. This wetting transition may thus explain the suboptimal performance observed in the SHP samples, a finding that is also supported by other studies [
30,
31,
32].
4. Conclusions
Starting from aluminum plates and bars, several hydrophobic and superhydrophobic samples were prepared and their icephobicity was studied. The synthesis was carried out with a very simple process that includes chemical etching, immersion in hot water and coating with a fluorinated molecule. Changing the concentration of the etchant and the treatment time allows the tuning of surface roughness and, consequently, both the hydrophobic and icephobic properties.
All samples exhibited hydrophobic behavior, and the wettability results indicate that the nanometric roughness generated by hydrothermal treatment plays a key role in increasing SCA and reducing RO values. Superhydrophobicity is achieved in samples subjected to harsher etching conditions, which produce the highest levels of microroughness.
All samples were found to reduce ice adhesion compared to the uncoated aluminum alloy, although the extent of reduction varies significantly among them. Analogously to wettability, the presence of the nanometric layer is the key factor in lowering ice adhesion. The etching treatment has a less pronounced effect on icephobicity; however, excessive microroughness can lead to a certain increase in ice adhesion, likely due to mechanical interlocking between the ice and surface asperities. Thus, superhydrophobic surfaces are not the most icephobic, due to an excessive microroughness that causes the anchoring of the ice to the surface. However, all the superhydrophobic samples displayed a mild icephobicity, reaching ARF values of about 5.
The best icephobic sample is LM5, treated in HCl 0.5 M for 30 min and then treated in hot water for 5 min. Notably, Nano-5, obtained by only a simple immersion in hot water, also demonstrated significant icephobicity, with an ARF value of about 17.
The relationship between ice adhesion and wettability shows that surfaces with ARF higher than 10 are characterized by SCA > 150° and RO angle < 20°, while contact angle hysteresis was found to be substantially unrelated. However, these wettability properties constitute a necessary but not sufficient condition to achieve high icephobicity, as several samples exhibiting such features display an ARF value between 9 and 4. Since wettability is in turn governed by morphology and roughness, both must be carefully engineered to ensure a high SCA angle and low RO angle while simultaneously avoiding excessive surface asperities.
A fabrication process involving short chemical etching in diluted HCl, instead of harsher and prolonged treatment in more concentrated acid, is thus preferable to achieve icephobicity.
This study demonstrates that a balanced surface design is essential for the highest icephobic performances, and some key features to obtain them are proposed.