Preparation and Performance Study of Self-Repairing External Anticorrosion Coating for Submarine Crude Oil Pipeline Based on Organic Corrosion Inhibitor
Abstract
1. Introduction
2. Experimental
2.1. Raw Materials
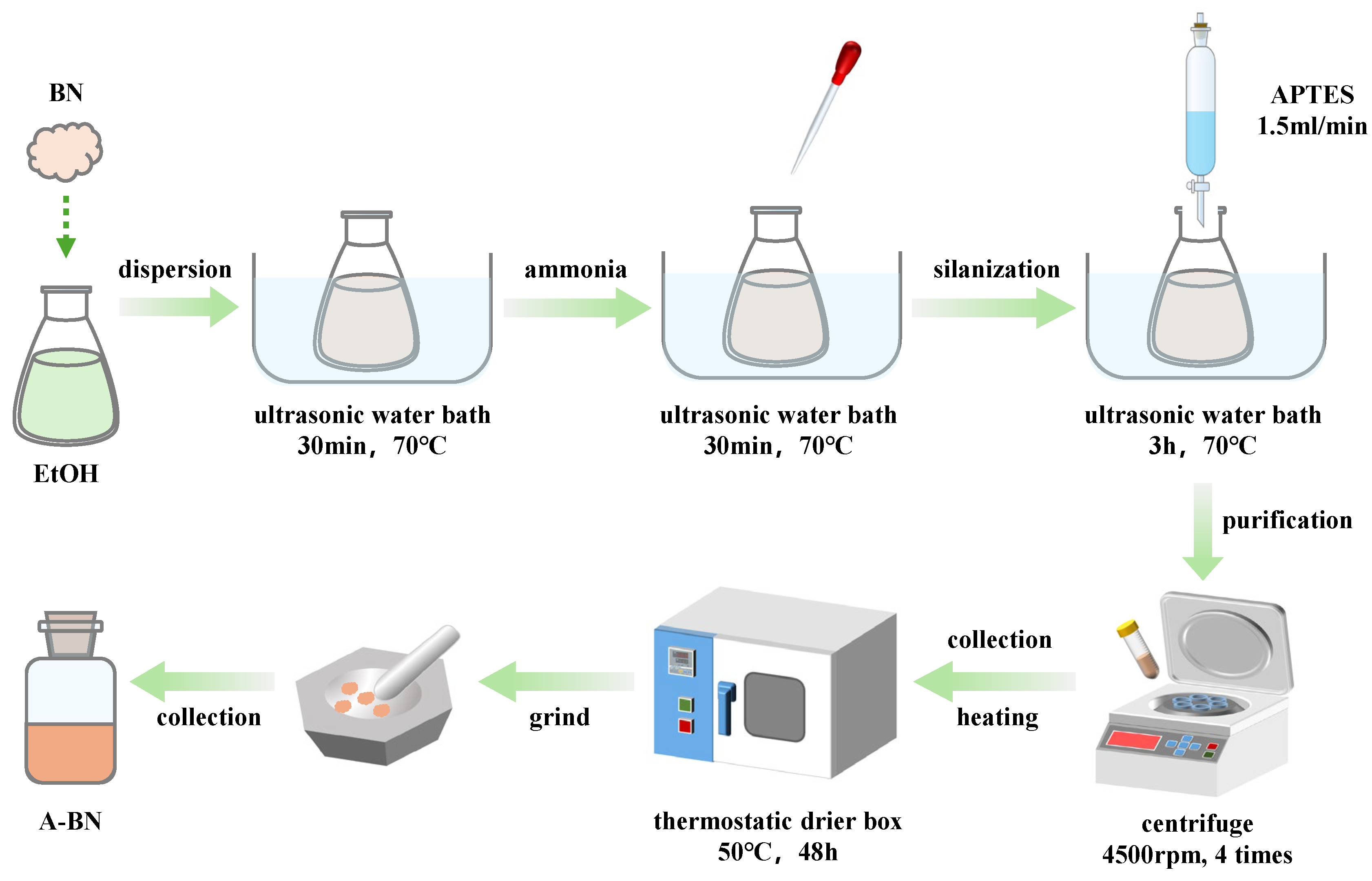
2.2. Preparation of A-BN
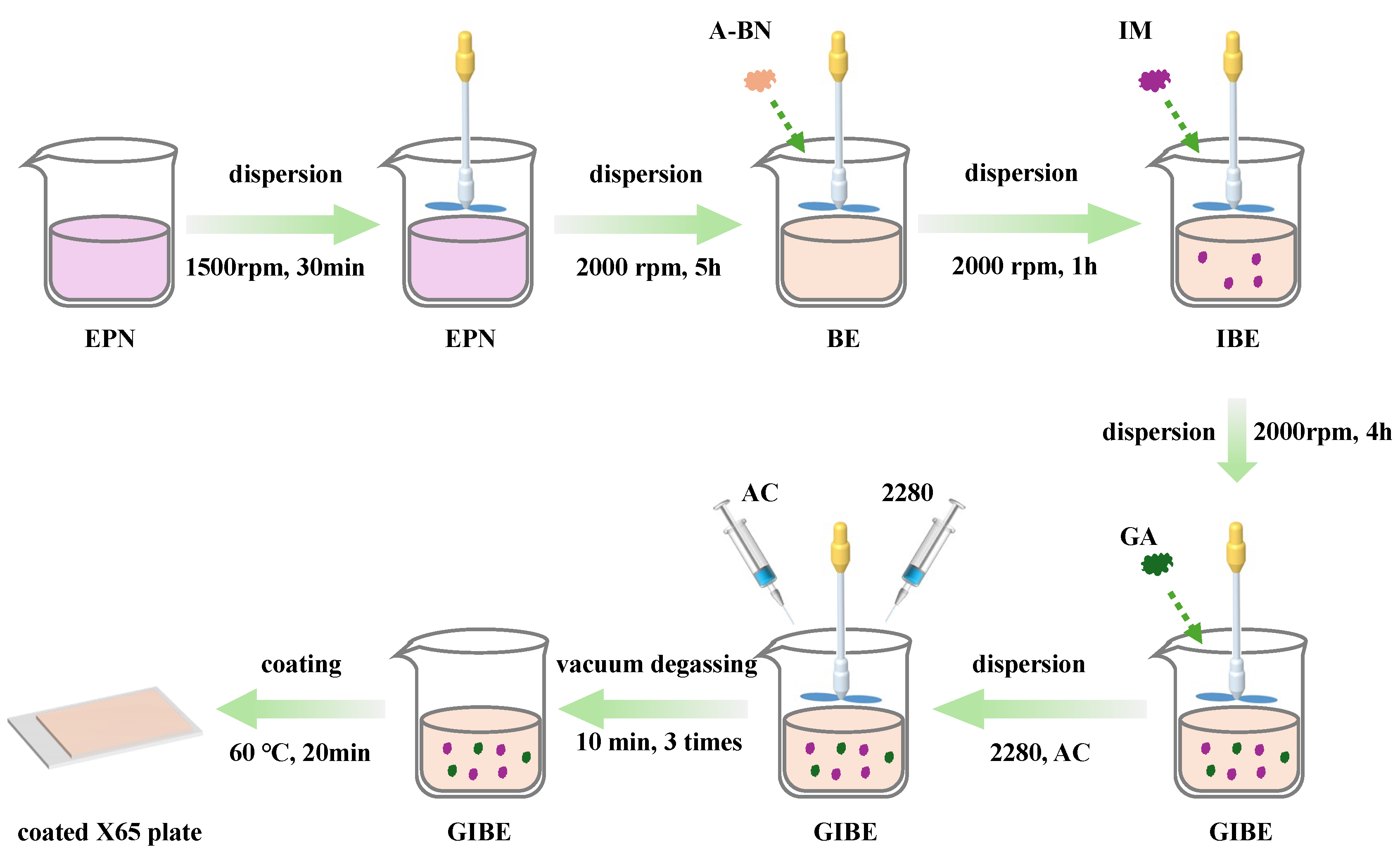
2.3. Preparation of Coatings
2.4. Preparation of Coating Characterization Sample
2.5. Material Characterization and Performance Testing
3. Result and Discussion
3.1. Corrosion Resistance Properties of Coatings
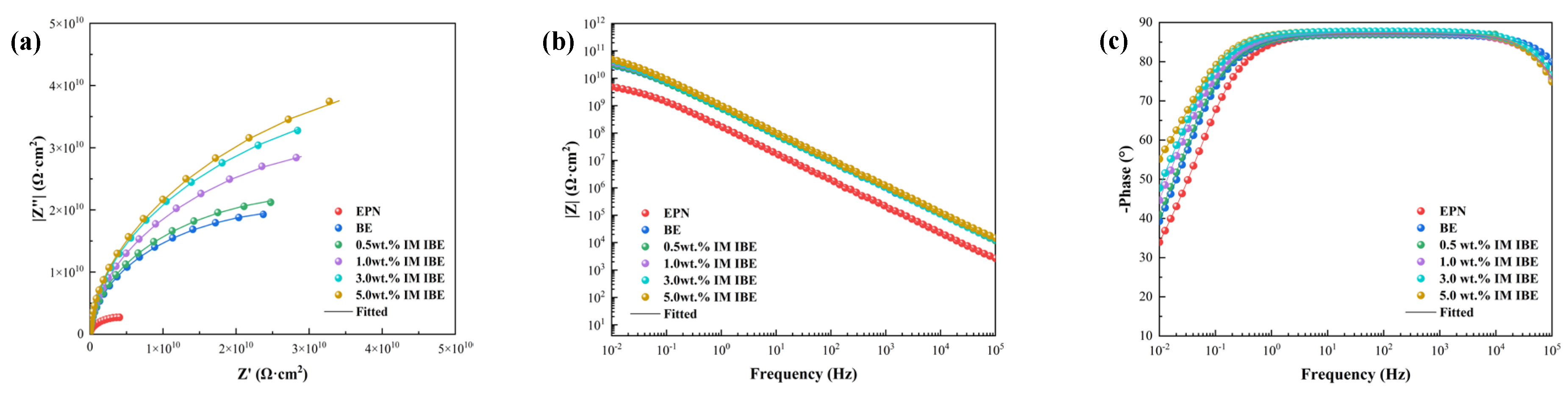
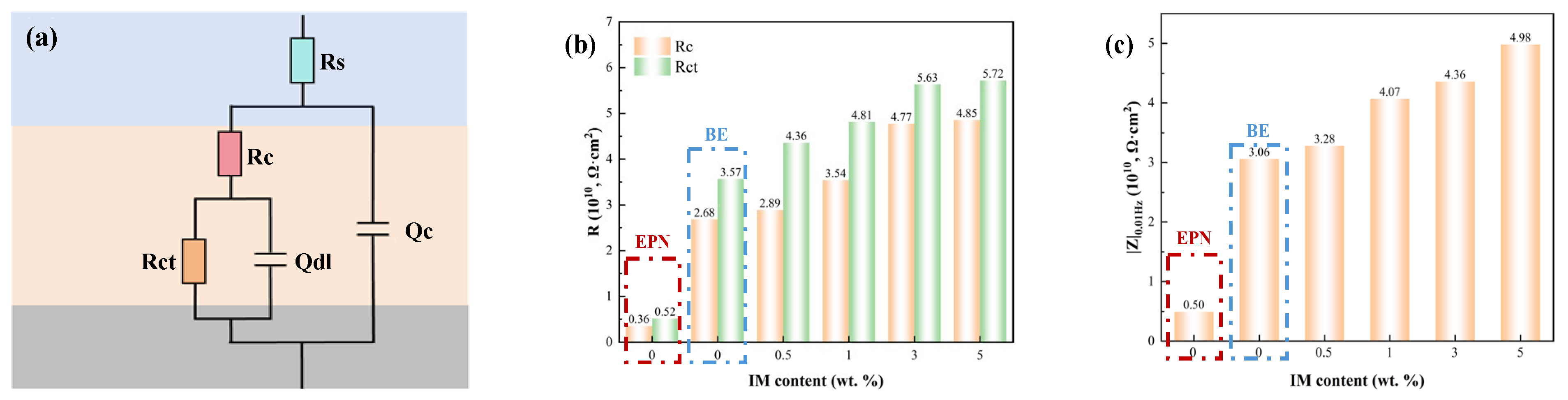
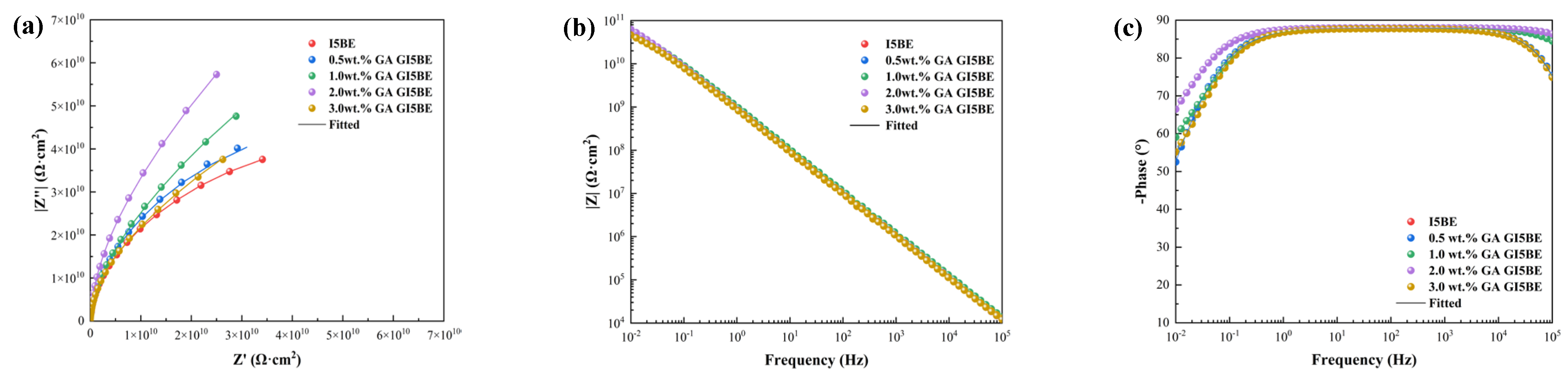
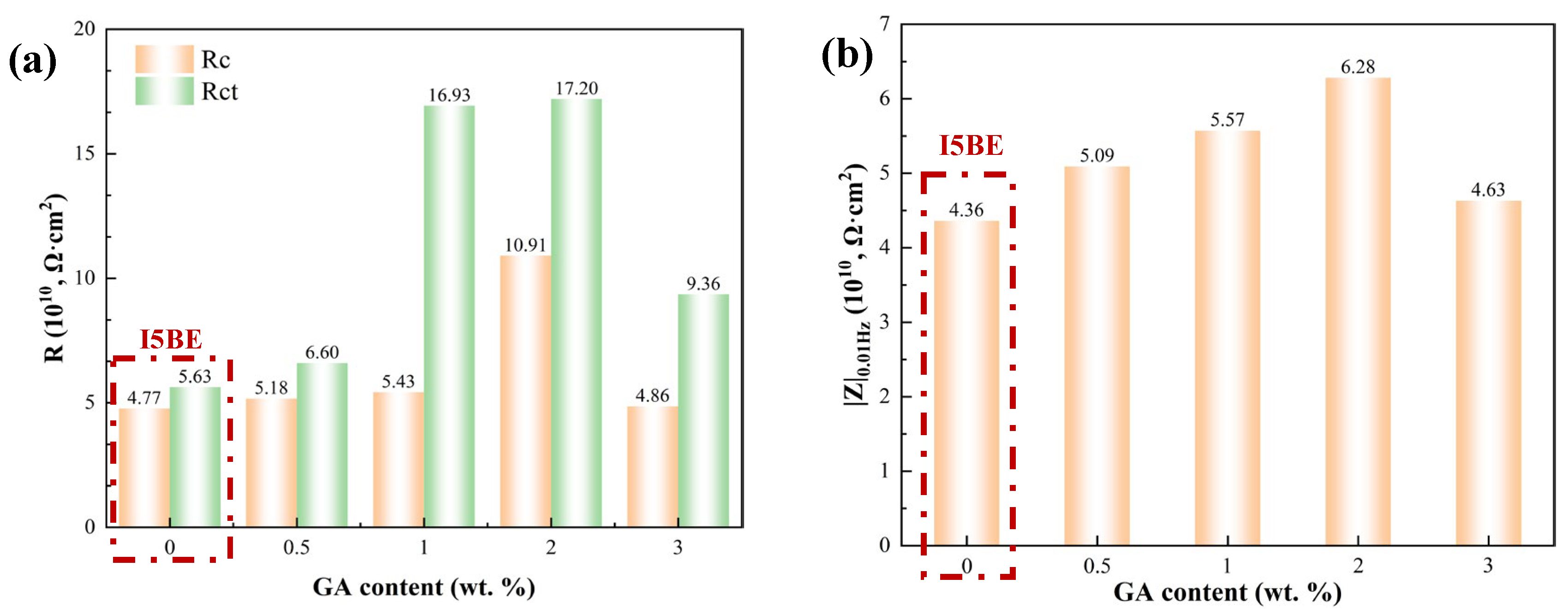
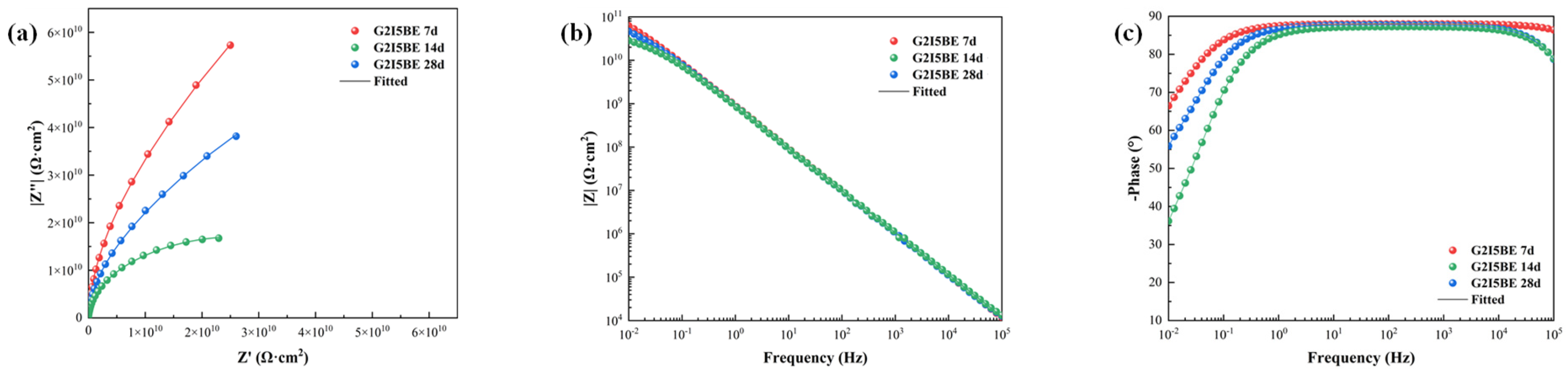
3.1.1. EIS Tests of the Intact Coatings
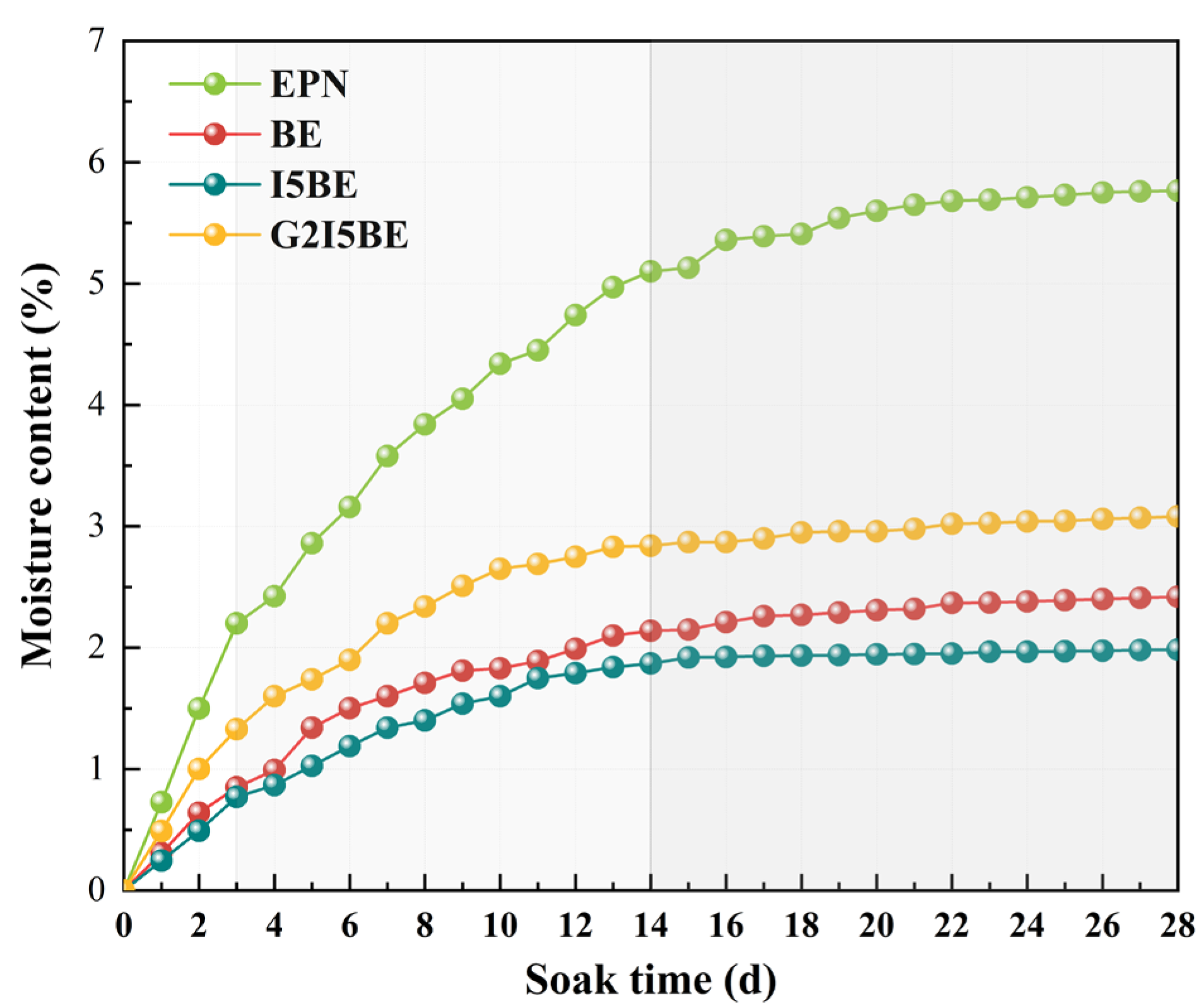
3.1.2. Water Absorption Tests of Intact Coatings
3.2. Self-Healing Properties of Coatings
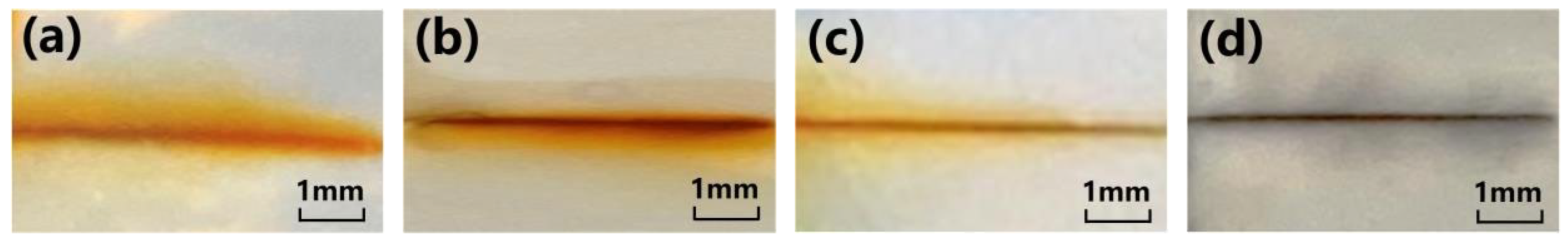
3.2.1. Macroscopic Morphology of Coating Damage
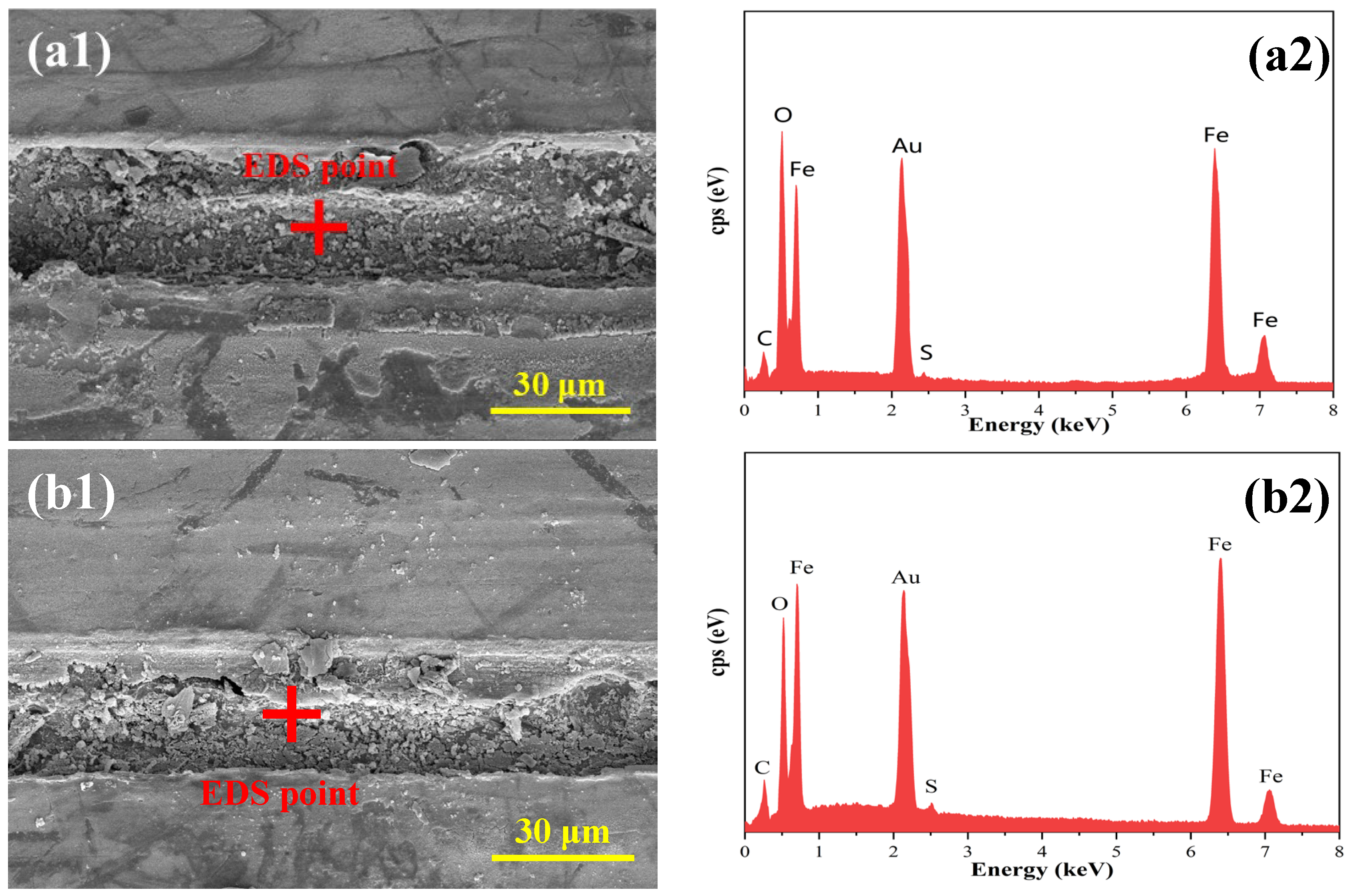
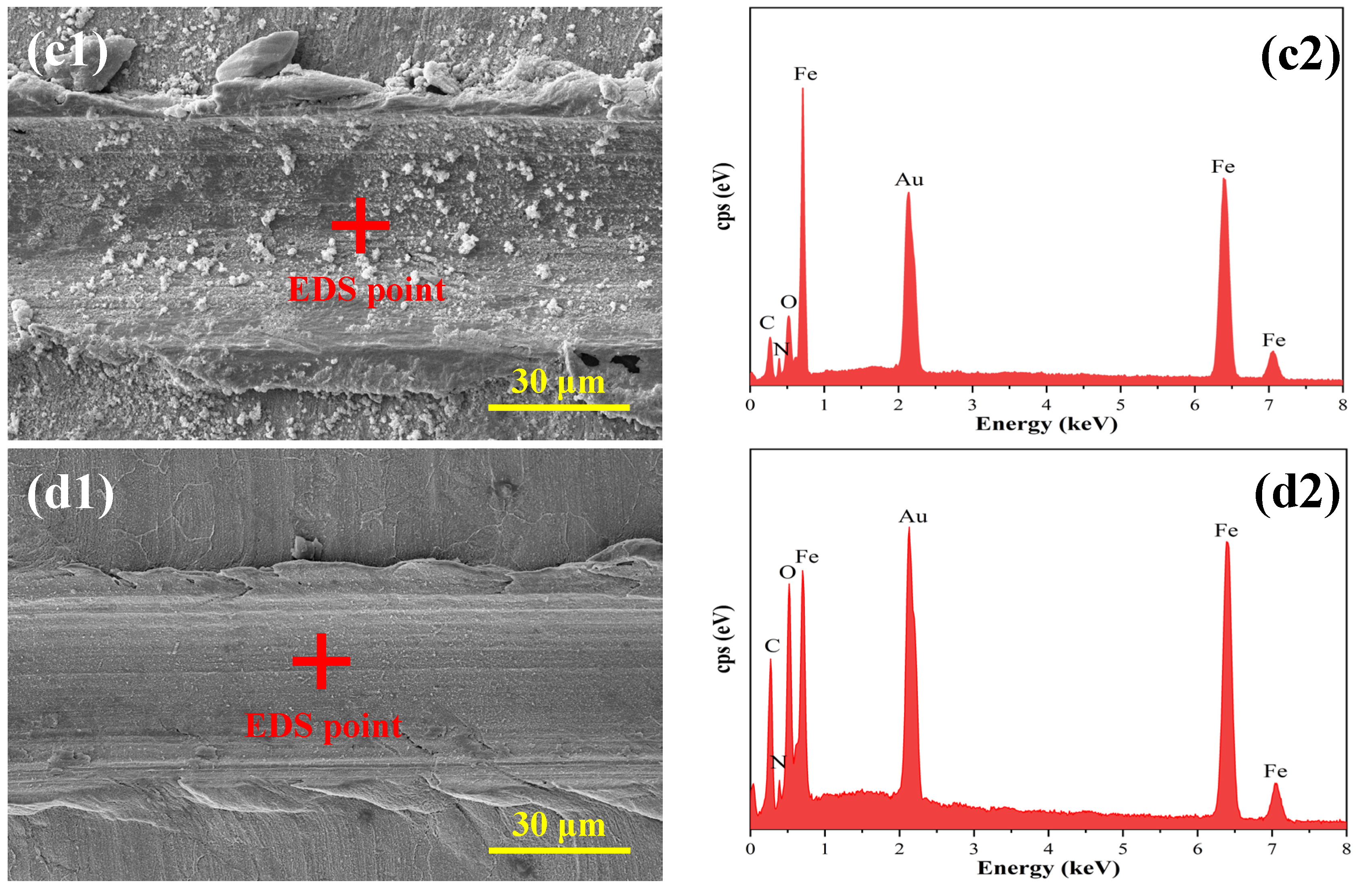
3.2.2. Microscopic Morphology of the Base Metal at the Damage Site
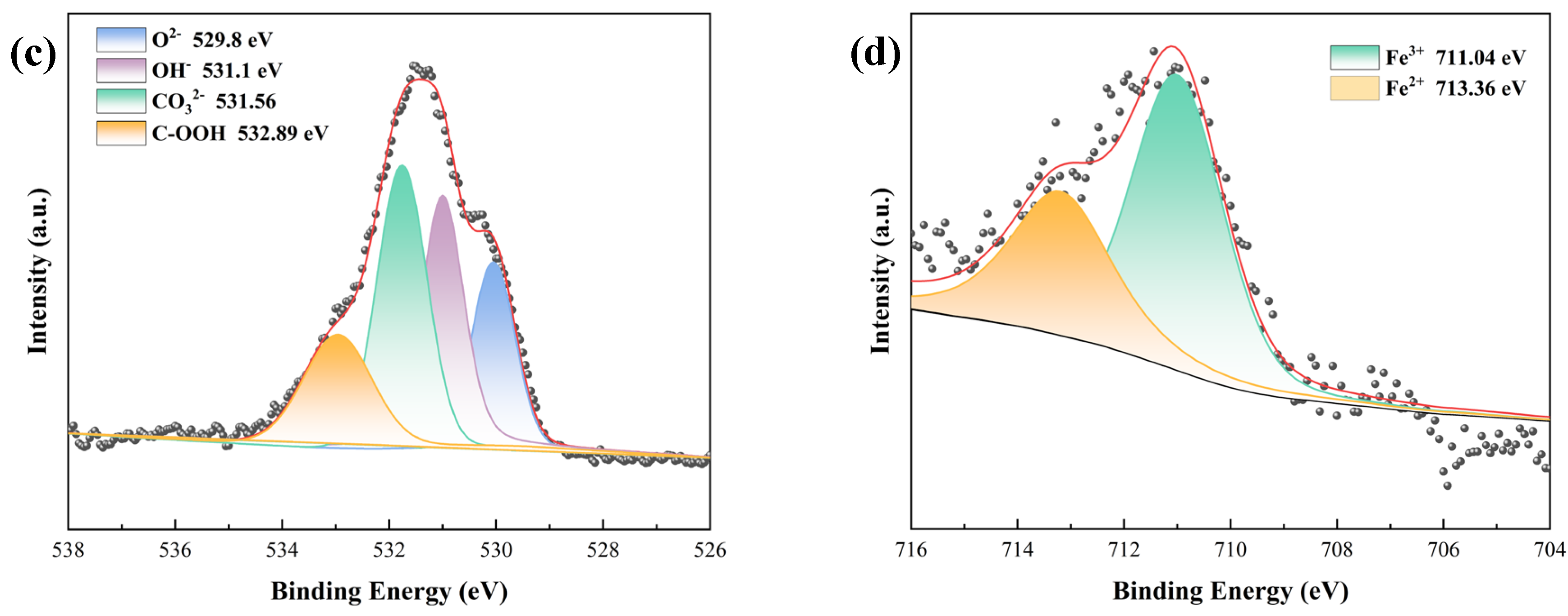
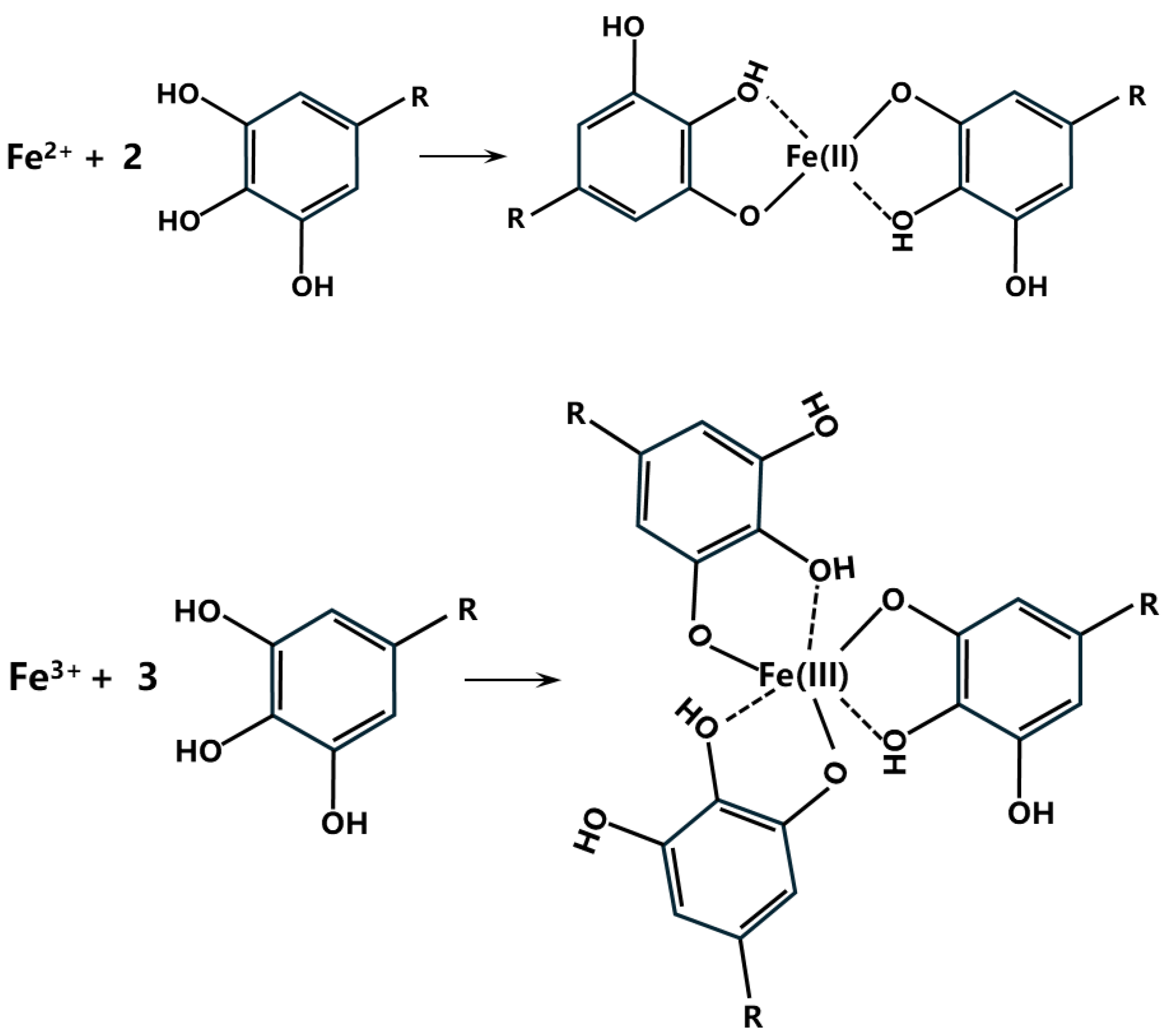
3.2.3. Compositional Analyses of Substrate Metal Surface Products at Breaks
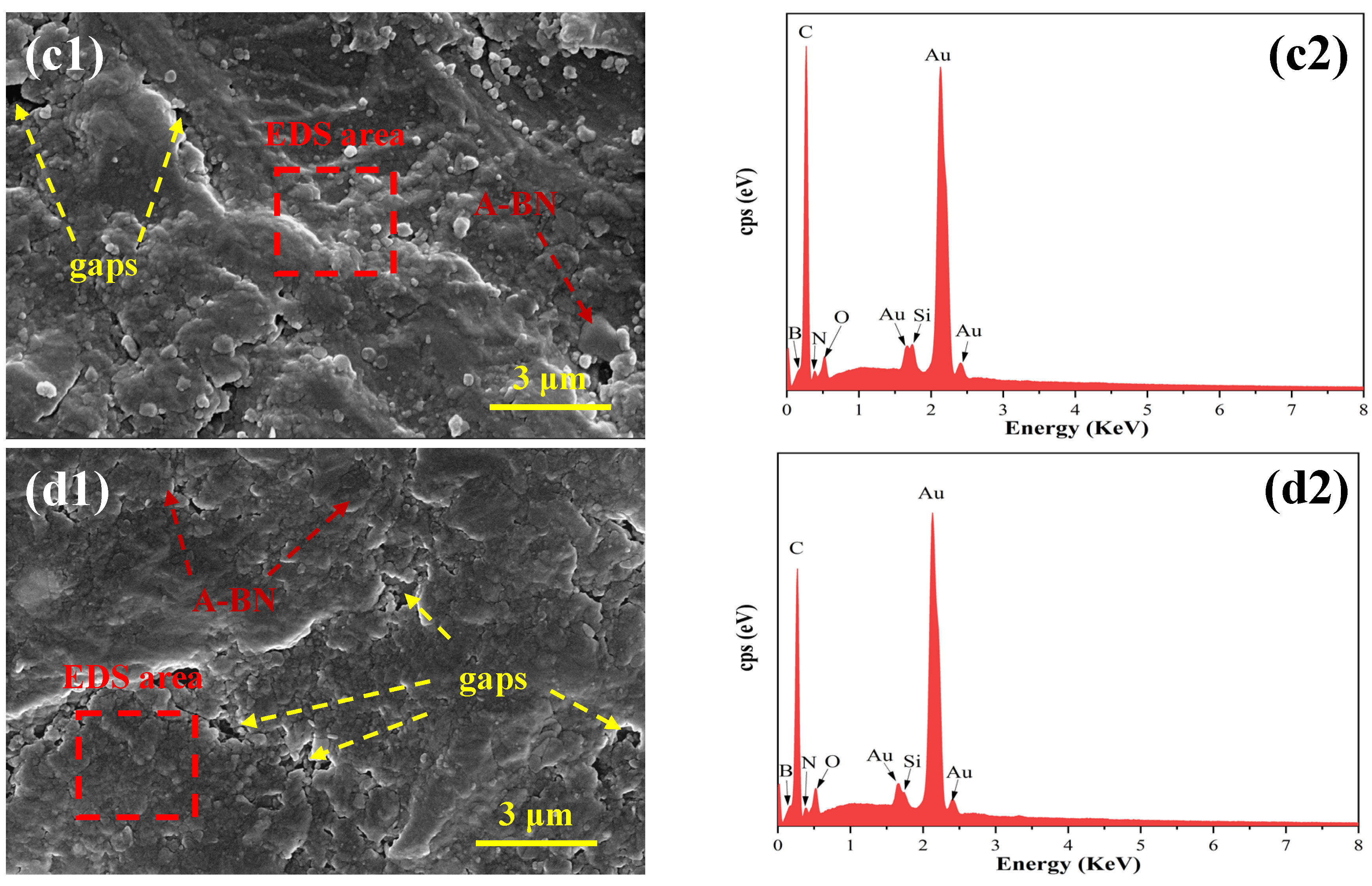
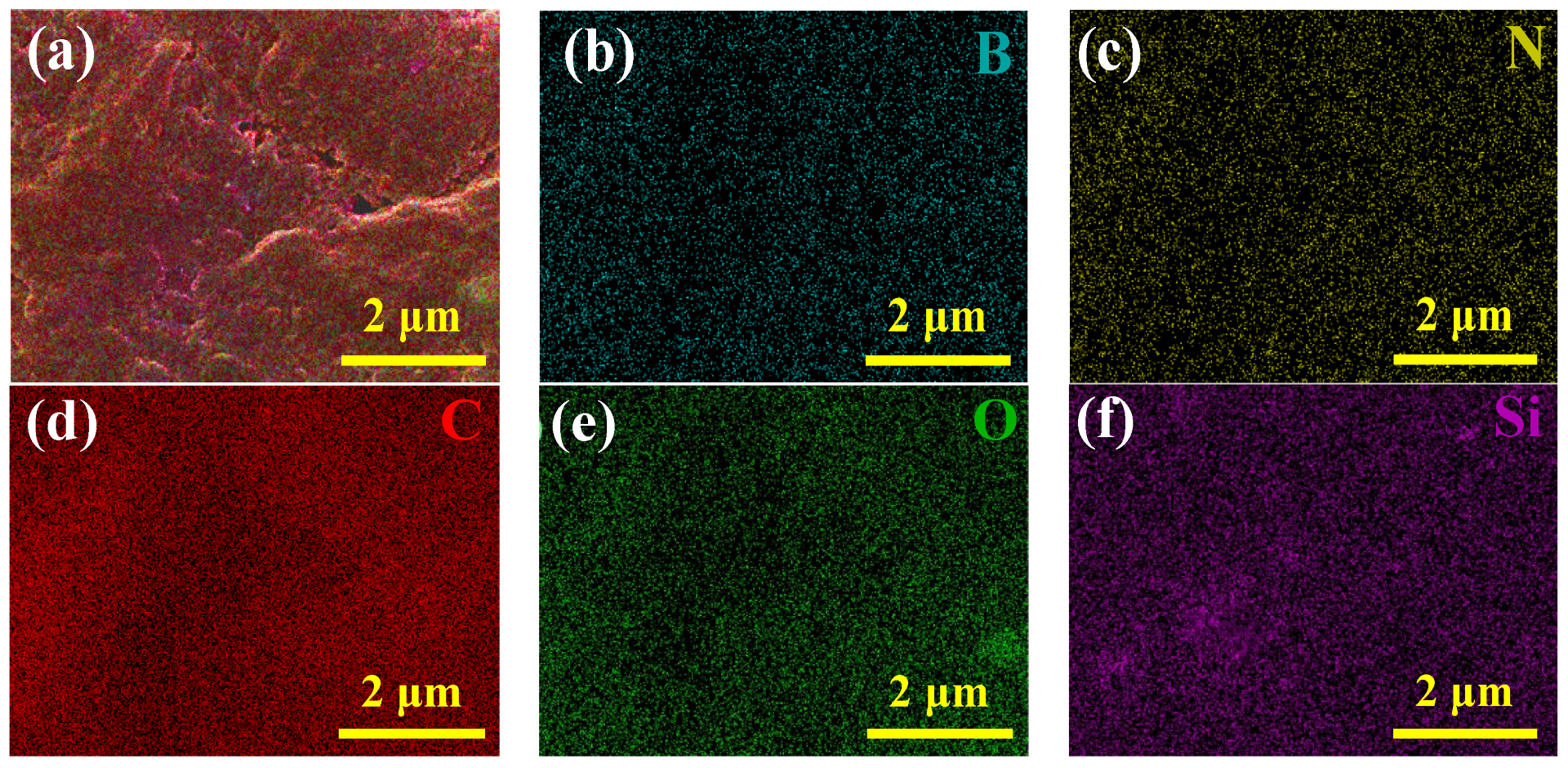
3.3. Morphologies and Elementary Composition of Coatings
4. The Influence of IM and GA Content on Coating Properties
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Z.; Li, Q. Balancing Submarine landslides and the Marine Economy for Sustainable Development: A review and future prospects. Sustainability 2024, 16, 6490. [Google Scholar] [CrossRef]
- Tarakhtiyeva, G.K. Methods for transporting oil and gas. Acad. Res. Educ. Sci. 2022, 3, 1004–1008. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, Y.; Zhang, R.; Guan, F.; Hou, B.; Duan, J. Microbiologically influenced corrosion of marine steels within the interaction between steel and biofilms: A brief view. Appl. Microbiol. Biotechnol. 2020, 104, 515–525. [Google Scholar] [CrossRef] [PubMed]
- De Vargas, V.H.; Marczak, L.D.F.; Flôres, S.H.; Mercali, G.D. Advanced technologies applied to enhance properties and structure of films and coatings: A review. Food Bioprocess Technol. 2022, 15, 1224–1247. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, R.; Pu, J.; He, Z.; Xiong, L. 2D graphene and h-BN layers application in protective coatings. Corros. Rev. 2021, 39, 93–107. [Google Scholar] [CrossRef]
- Wu, Y.; He, Y.; Zhou, T.; Chen, C.; Zhong, F.; Xia, Y.; Xie, P.; Zhang, C. Synergistic functionalization of h-BN by mechanical exfoliation and PEI chemical modification for enhancing the corrosion resistance of waterborne epoxy coating. Prog. Org. Coat. 2020, 142, 105541. [Google Scholar] [CrossRef]
- Zhao, H.; Ding, J.; Liu, P.; Yu, H. Boron nitride-epoxy inverse “nacre-like” nanocomposite coatings with superior anticorrosion performance. Corros. Sci. 2021, 183, 109333. [Google Scholar] [CrossRef]
- Zhao, X.; Jiang, D.; Ma, L.; Zeng, X.; Li, Z.; Huang, G. Corrosion effects and smart coatings of corrosion protection. Coatings 2022, 12, 1378. [Google Scholar] [CrossRef]
- Diraki, A.; Omanovic, S. Smart PANI/epoxy anti-corrosive coating for protection of carbon steel in sea water. Prog. Org. Coat. 2022, 168, 106835. [Google Scholar] [CrossRef]
- Liu, X.; Liu, R.; Li, T.; Liu, Y.; Liu, L.; Lyu, K.; Shah, S.P. Research on the anticorrosion properties of CeO2-GO/EP nanocomposite coating in simulated sea water. Polymers 2021, 13, 2072. [Google Scholar] [CrossRef]
- Al-Amiery, A.A.; Isahak, W.N.R.W.; Al-Azzawi, W.K. Corrosion inhibitors: Natural and synthetic organic inhibitors. Lubricants 2023, 11, 174. [Google Scholar] [CrossRef]
- Lin, B.; Wang, J.; Zhang, H.; Wang, Y.; Zhang, H.; Tang, J.; Hou, J.; Zhang, H.; Sun, M. Self-healing performance of ethyl-cellulose based supramolecular gel coating highly loaded with different carbon chain length imidazoline inhibitors in NaCl corrosion medium. Corros. Sci. 2022, 197, 110084. [Google Scholar] [CrossRef]
- Lei, Y.; Xiao, W.; Peng, H.; Yu, P.; Cai, X.; Luan, Z.; Deng, S.; Wang, S. An integrated epoxy rust conversion coating: Its anticorrosion properties and rust conversion mechanism. J. Alloys Compd. 2021, 853, 157005. [Google Scholar] [CrossRef]
- Fang, S.; Chen, K.; Yao, H.; Cao, Y.; Guo, S.; Wang, L.; Wang, Y.; Yu, S.; Wang, N. Preparation of gallic acid intercalated layered double hydroxide for enhanced corrosion protection of epoxy coatings. Coatings 2023, 13, 128. [Google Scholar] [CrossRef]
- Cao, L.; Wang, W.; Cheng, J.; Wang, T.; Zhang, Y.; Wang, L.; Li, W.; Chen, S. Synergetic inhibition and corrosion-diagnosing nanofiber networks for self-healing protective coatings. ACS Appl. Mater. Interfaces 2023, 15, 48645–48659. [Google Scholar] [CrossRef]
- Liu, J.; Wu, Y.; Yan, Y.; Wang, F.; Zhang, G.; Zeng, L.; Ma, Y.; Li, Y. Preparation and Performance Study of Graphene Oxide Doped Gallate Epoxy Coatings. Materials 2025, 18, 3536. [Google Scholar] [CrossRef]
- Shi, H.; Liu, W.; Liu, C.; Yang, M.; Xie, Y.; Wang, S.; Zhang, F.; Liang, L.; Pi, K. Polyethylenimine-assisted exfoliation of h-BN in aqueous media for anticorrosive reinforcement of waterborne epoxy coating. Prog. Org. Coat. 2020, 142, 105591. [Google Scholar] [CrossRef]
- Yan, Y.; Liao, K.; Hu, J.; Qin, M.; He, T.; Ou, T.; Fan, Y.; Leng, J.; He, G. Effects of h-BN content and silane functionalization on thermal conductivity and corrosion resistance of h-BN/EPN coating. Surf. Coat. Technol. 2024, 476, 130185. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, J.; Su, F.; Hu, L.; Wu, Q.; Qi, W.; Zhang, T.; Wang, F. Hydrogen embrittlement susceptibility of Ti-6Al-4V alloys fabricated by electron beam melting in simulated deep-sea environment. Corrosion 2024, 80, 24–40. [Google Scholar] [CrossRef]
- Charis, G.; Patel, B.; Rashama, C.; Nyamunda, B. Progress towards the standardization of test parameters and protocols for evaluating densified fuel products. Paliva 2024, 16, 16–29. [Google Scholar] [CrossRef]
- Pistone, A.; Scolaro, C.; Visco, A. Mechanical properties of protective coatings against marine fouling: A review. Polymers 2021, 13, 173. [Google Scholar] [CrossRef]
- Dementjev, A.P.; De Graaf, A.; Van de Sanden, M.C.M.; Maslakov, K.; Naumkin, A.; Serov, A. X-Ray photoelectron spectroscopy reference data for identification of the C3N4 phase in carbon–nitrogen films. Diam. Relat. Mater. 2000, 9, 1904–1907. [Google Scholar] [CrossRef]
- Lin, Q.; Shang, C.; Chen, Z.; Wang, X.; Zhou, G. Boron-doped molybdenum carbide as a pH-independent electrocatalyst for the hydrogen evolution reaction. Int. J. Hydrog. Energy 2020, 45, 30659–30665. [Google Scholar] [CrossRef]
- Feng, C.; Bo, T.; Maity, P.; Zuo, S.; Zhou, W.; Huang, K.; Mohammed, O.F.; Zhang, H. Regulating photocatalytic CO2 reduction kinetics through modification of surface coordination sphere. Adv. Funct. Mater. 2024, 34, 2309761. [Google Scholar] [CrossRef]
- Payne, B.P.; Biesinger, M.C.; McIntyre, N.S. Use of oxygen/nickel ratios in the XPS characterisation of oxide phases on nickel metal and nickel alloy surfaces. J. Electron Spectrosc. Relat. Phenom. 2012, 185, 159–166. [Google Scholar] [CrossRef]
- Zeng, D.; Han, X.; Yu, C.; Zheng, C.; Su, R.; Sun, J.; Li, Y.; Chen, J. Analysis of typical cases of corrosion failure of tubing in heavy oil fire-flooding production wells. Eng. Fail. Anal. 2025, 172, 109391. [Google Scholar] [CrossRef]
- Li, H.; Yin, C.; Li, D.; Xu, F. Oxygen Vacancy-Engineered FeP/BiOBr Heterojunction for Enhanced Photo-Fenton Degradation of Tetracycline Hydrochloride: Synergistic Catalysis and Mechanistic Insights. Langmuir 2025, 41, 19455–19471. [Google Scholar] [CrossRef]
- Zhao, J.; Liao, N.; Luo, J. Transforming NiFe layered double hydroxide into NiFeP x for efficient alkaline water splitting. J. Mater. Chem. A 2023, 11, 9682–9690. [Google Scholar] [CrossRef]
- Li, D.; Jia, S.; Fodjo, E.K.; Xu, H.; Wang, Y.; Deng, W. In situ SERS and X-ray photoelectron spectroscopy studies on the pH-dependant adsorption of anthraquinone-2-carboxylic acid on silver electrode. Appl. Surf. Sci. 2016, 367, 153–159. [Google Scholar] [CrossRef]





| No. | Name | Specification | Model | Supplier |
|---|---|---|---|---|
| 1 | h-BN | Industrial-grade | 100 nm | Shanghai Mitsuda Nano New Material Co., Shanghai, China |
| 2 | EPN | Industrial-grade | NPPN-630L | Shanghai Mitsuda Nano New Material Co., Shanghai, China |
| 3 | solidify agent | Industrial-grade | Ancamine 2280 | Shanghai Mitsuda Nano New Material Co., Shanghai, China |
| 4 | GA | Industrial-grade | 99% | Shanghai McLean Biochemical Technology Co., Shanghai, China |
| 5 | IM | Industrial-grade | oil solubility, 98% | Shanghai McLean Biochemical Technology Co., Shanghai, China |
| 6 | APTES | AR | 99% | Shanghai McLean Biochemical Technology Co., Shanghai, China |
| 7 | Et-OH | AR | 99.9% | Chengdu Kelong Chemical Co., Chengdu, China |
| 8 | ammonia water | AR | 99.9% | Chengdu Kelong Chemical Co., Chengdu, China |
| 9 | AC | AR | 99.9% | Chengdu Kelong Chemical Co., Chengdu, China |
| 10 | NaCl | AR | 99.99% | Shanghai McLean Biochemical Technology Co., Shanghai, China |
| 11 | MgCl2 | AR | 99.99% | Shanghai McLean Biochemical Technology Co., Shanghai, China |
| 12 | Na2SO4 | AR | 99% | Shanghai McLean Biochemical Technology Co., Shanghai, China |
| 13 | CaCl2 | AR | 99.99% | Shanghai McLean Biochemical Technology Co., Shanghai, China |
| 14 | KCl | AR | 99.5% | Shanghai McLean Biochemical Technology Co., Shanghai, China |
| 15 | NaHCO3 | AR | 99.5% | Shanghai McLean Biochemical Technology Co., Shanghai, China |
| 16 | KBr | AR | 99% | Shanghai McLean Biochemical Technology Co., Shanghai, China |
| 17 | H3BO3 | AR | 99.5% | Shanghai McLean Biochemical Technology Co., Shanghai, China |
| 18 | SrCl2 | AR | 99.99% | Shanghai McLean Biochemical Technology Co., Shanghai, China |
| 19 | NaF | AR | 99.99% | Shanghai McLean Biochemical Technology Co., Shanghai, China |
| Coatings | BE | IBE | |||
|---|---|---|---|---|---|
| IM content (wt.%) | 0 | 0.5 | 1 | 3 | 5 |
| Coatings | IBE | GIBE | |||
|---|---|---|---|---|---|
| GA content (wt.%) | 0 | 0.5 | 1 | 2 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Yan, Y.; Leng, J.; Liu, X.; Wu, J.; Meng, Q.; Xiang, P.; Li, J.; Wang, S.; Feng, D.; et al. Preparation and Performance Study of Self-Repairing External Anticorrosion Coating for Submarine Crude Oil Pipeline Based on Organic Corrosion Inhibitor. Coatings 2025, 15, 1281. https://doi.org/10.3390/coatings15111281
Zhou J, Yan Y, Leng J, Liu X, Wu J, Meng Q, Xiang P, Li J, Wang S, Feng D, et al. Preparation and Performance Study of Self-Repairing External Anticorrosion Coating for Submarine Crude Oil Pipeline Based on Organic Corrosion Inhibitor. Coatings. 2025; 15(11):1281. https://doi.org/10.3390/coatings15111281
Chicago/Turabian StyleZhou, Jing, Yongbo Yan, Jihui Leng, Xin Liu, Jirong Wu, Qinghua Meng, Peng Xiang, Jun Li, Shutao Wang, Danyang Feng, and et al. 2025. "Preparation and Performance Study of Self-Repairing External Anticorrosion Coating for Submarine Crude Oil Pipeline Based on Organic Corrosion Inhibitor" Coatings 15, no. 11: 1281. https://doi.org/10.3390/coatings15111281
APA StyleZhou, J., Yan, Y., Leng, J., Liu, X., Wu, J., Meng, Q., Xiang, P., Li, J., Wang, S., Feng, D., Liu, M., Yuan, Y., Jia, P., Ren, J., Liu, L., & Shi, X. (2025). Preparation and Performance Study of Self-Repairing External Anticorrosion Coating for Submarine Crude Oil Pipeline Based on Organic Corrosion Inhibitor. Coatings, 15(11), 1281. https://doi.org/10.3390/coatings15111281





