An SWIR-MIR Spectral Database of Organic Coatings Used on Historic Metals †
Abstract
1. Introduction
2. Materials and Methods
2.1. Coupon Preparation
| Linseed Oil-Based Mock-Ups (Figure 1) | Walnut Oil-Based Mock-Ups | Linseed–Walnut Oil-Based Mock-Ups | Other Mock-Ups |
|---|---|---|---|
| Linseed | Walnut | Linseed–walnut | Bitumen |
| Linseed–bitumen | Walnut–bitumen | Linseed–walnut– bitumen | Mastic |
| Linseed–mastic | Walnut–mastic | Linseed–walnut–mastic | Pine |
| Linseed–mastic– bitumen | Walnut–mastic– bitumen | Linseed–walnut–mastic–bitumen | |
| Linseed–pine | Walnut–pine | Linseed–walnut–pine | |
| Linseed–pine– bitumen | Walnut–pine– bitumen | Linseed–walnut–pine–bitumen | |
| Linseed–mastic–pine | Walnut–mastic–pine | Linseed–walnut–mastic–pine | |
| Linseed–mastic–pine–bitumen | Walnut–mastic–pine–bitumen | Linseed–walnut–mastic–pine–bitumen |

2.2. Accelerated Aging
2.3. ER-FTIR and FORS Analysis
3. Results and Discussion
3.1. ER-FTIR
3.2. FORS
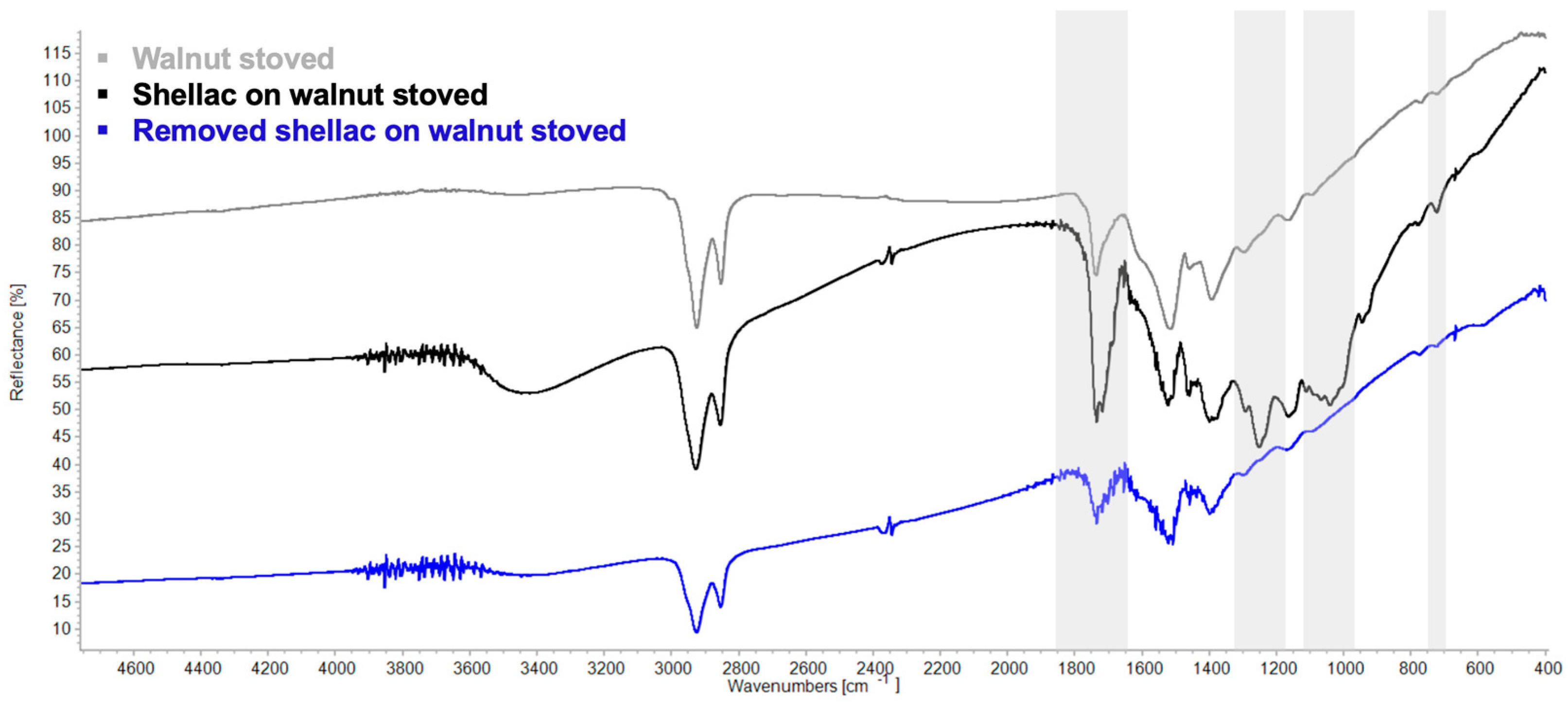
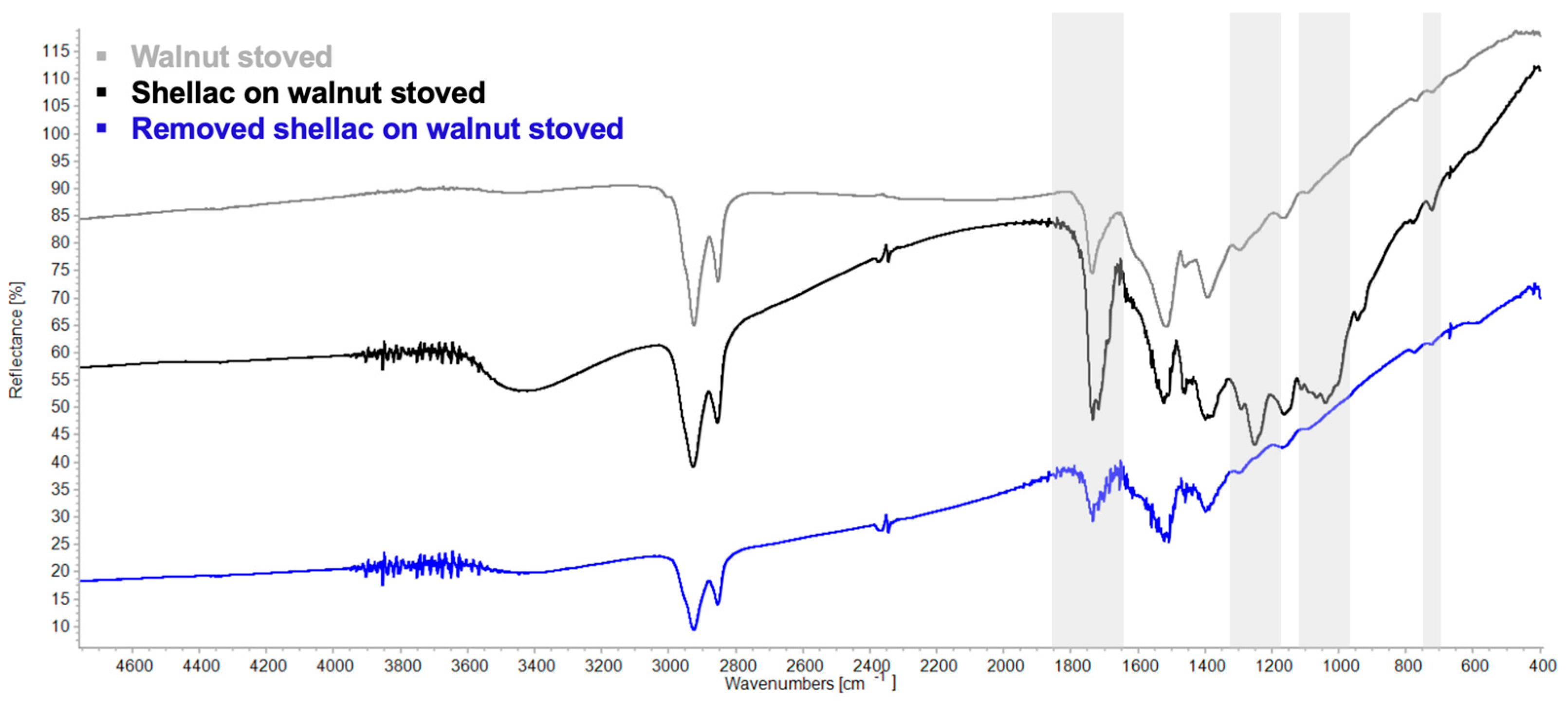
3.3. Re-Coating/Overcoating
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ER-FTIR | External reflectance Fourier transfer infrared spectroscopy |
| FORS | Fiber optic reflectance spectroscopy |
| GC-MS | Gas chromatography–mass spectroscopy |
| MIR | Mid-infrared |
| NIR | Near-infrared |
| PCA | Principle component analysis |
| SOC | Surface organic coating |
| SWIR | Short-wave infrared |
References
- Montagu, J. Bronzes; Octopus Books Ltd.: London, UK, 1972. [Google Scholar]
- Stone, R.E. Organic Patinas on Small Bronzes of the Italian Renaissance. Metrop. Mus. J. 2010, 45, 107–124. [Google Scholar] [CrossRef]
- Hughes, R.; Rowe, M. The Coloring, Bronzing and Patination of Metals; Thames and Hudson: London, UK, 1991. [Google Scholar]
- Allen, D.; Borsch, L.; Draper, J.D.; Fraiman, J.; Stone, R.E. (Eds.) Italian Renaissance and Baroque Bronzes in the Metropolitan Museum of Art; The Metropolitan Museum of Art: New York, NY, USA, 2022. [Google Scholar]
- Ciardi Dupre, M.G.; Ross, B., Translators; Small Renaissance Bronzes; Paul Hamlyn: London, UK, 1970. [Google Scholar]
- Galeotti, M.; Porcinai, S.; Cagnini, A.; Baruffetti, M.; Biondi, C.; Dal Fovo, A.; Fontana, R. Organic Patinas on Small Historical Bronzes: From Mock-Ups to Actual Artworks. Coatings 2024, 14, 212. [Google Scholar] [CrossRef]
- Leithe-Jasper, M. (Ed.) Renaissance Master Bronzes: From the Collection of the Kunsthistorisches Museum, Vienna; Scala Books: London, UK, 1986. [Google Scholar]
- Bewer, F.G. A Study of the Technology of Renaissance Bronze Statuettes. Ph.D. Thesis, University of London, London, UK, 1996. [Google Scholar]
- Pitthard, V.; Stanek, S.; Griesser, M.; Kryza-Gersch, C.; Hanzer, H. Comprehensive Investigation of the ‘organic Patina’ on Renaissance and Baroque Indoor Bronze Sculptures from the Collection of the Kunsthistorisches Museum, Vienna. Conserv. Sci. 2007, 12, 44–53. [Google Scholar]
- Pitthard, V.; Stone, R.; Stanek, S.; Griesser, M.; Kryza-Gersch, C.; Hanzer, H. Organic Patinas on Renaissance and Baroque Bronzes—Interpretation of Compositions of the Original Patination by Using a Set of Simulated Varnished Bronze Coupons. J. Cult. Herit. 2011, 12, 44–53. [Google Scholar] [CrossRef]
- Appolonia, L.; Vaudan, D.; Chatel, V.; Aceto, M.; Mirti, P. Combined Use of FORS, XRF and Raman Spectroscopy in the Study of Mural Paintings in the Aosta Valley (Italy). Anal. Bioanal. Chem. 2009, 395, 2005–2013. [Google Scholar] [CrossRef]
- Cheilakou, E.; Troullinos, M.; Koui, M. Identification of Pigments on Byzantine Wall Paintings from Crete (14th Century AD) Using Non-Invasive Fiber Optics Dif-fuse Reflectance Spectroscopy (FORS). J. Archaeol. Sci. 2014, 41, 541–555. [Google Scholar] [CrossRef]
- Garofano, I.; Perez-Rodriguez, J.L.; Robador, M.D.; Duran, A. An Innovative Combination of Non-Invasive UV-Visible-FORS, XRD and XRF Techniques to Study Roman Wall Paintings from Seville, Spain. J. Cult. Herit. 2016, 22, 1028–1039. [Google Scholar] [CrossRef]
- Nodari, L.; Ricciardi, P. Non-Invasive Identification of Paint Binders in Illuminated Manuscripts by ER-FTIR Spectroscopy: A Systematic Study of the Influence of Different Pigments on the Binders’ Characteristic Spectral Features. Herit. Sci. 2019, 7, 7. [Google Scholar] [CrossRef]
- Bell, J.; Nel, P.; Stuart, B. Non-Invasive Identification of Polymers in Cultural Heritage Collections: Evaluation, Optimisation and Application of Portable FTIR (ATR and External Reflectance) Spectroscopy to Three-Dimensional Polymer-Based Objects. Herit. Sci. 2019, 7, 95. [Google Scholar] [CrossRef]
- Grehk, T.M.; Berger, R.; Bexell, U. Investigation of the Drying Process of Linseed Oil Using FTIR and ToF-SIMS. J. Phys. Conf. Ser. 2008, 100, 12019. [Google Scholar] [CrossRef]
- Ricci, C.; Miliani, C.; Brunetti, B.G.; Sgamellotti, A. Non-Invasive Identification of Surface Materials on Marble Artifacts with Fiber Optic Mid-FTIR Reflectance Spectroscopy. Talanta 2006, 69, 1221–1226. [Google Scholar] [CrossRef]
- Catelli, E.; Sciutto, G.; Prati, S.; Chavez Lozano, M.V.; Gatti, L.; Lugli, F.; Silvestrini, S.; Benazzi, S.; Genorini, E.; Mazzeo, R. A New Miniaturised Short-Wave Infrared (SWIR) Spectrometer for on-Site Cultural Heritage Investigations. Talanta 2020, 218, 121112. [Google Scholar] [CrossRef]
- Catelli, E.; Sciutto, G.; Prati, S.; Jia, Y.; Mazzeo, R. Characterization of Outdoor Bronze Monument Patinas: The Potentialities of near-Infrared Spectroscopic Analysis. Environ. Sci. Pollut. Res. 2018, 25, 24379–24393. [Google Scholar] [CrossRef]
- Dooley, K.A.; Coddington, J.; Krueger, J.; Conover, D.M.; Loew, M.; Delaney, J.K. Standoff Chemical Imaging Finds Evidence for Jackson Pollock’s Selective Use of Alkyd and Oil Binding Media in a Famous ‘Drip’ Painting. Anal. Methods 2017, 9, 28–37. [Google Scholar] [CrossRef]
- Dooley, K.A.; Lomax, S.; Zeibel, J.G.; Miliani, C.; Ricciardi, P.; Hoenigswald, A.; Loewb, M.; Delaney, J.M. Mapping of Egg Yolk and Animal Skin Glue Paint Binders in Early Renaissance Paintings Using near Infrared Reflectance Imaging Spectroscopy. Analyst 2013, 138, 4838–4848. [Google Scholar] [CrossRef]
- Invernizzi, C.; Rovetta, T.; Licchelli, M.; Malagodi, M. Mid and Near-Infrared Reflection Spectral Database of Natural Organic Materials in the Cultural Heritage Field. Int. J. Anal. Chem. 2018, 2018, 7823248. [Google Scholar] [CrossRef] [PubMed]
- Ricciardi, P.; Delaney, J.K.; Zeibel, J.G.; Picollo, M.; Lomax, S.; Loew, M. Near Infrared Reflectance Imaging Spectroscopy to Map Paint Binders In Situ on Illuminated Manuscripts. Angew. Chem. Int. Ed. 2012, 51, 5607–5610. [Google Scholar] [CrossRef]
- Delaney, J.K.; Thoury, M.; Zeibel, J.G.; Ricciardi, P.; Morales, K.M.; Dooley, K.A. Visible and Infrared Imaging Spectroscopy of Paintings and Improved Reflectography. Herit. Sci. 2016, 4, 6. [Google Scholar] [CrossRef]
- Vagnini, M.; Miliani, C.; Cartechini, L.; Rocchi, P.; Brunetti, B.G.; Sgamellotti, A. FT-NIR Spectroscopy for Non-Invasive Identification of Natural Polymers and Resins in Easel Paintings. Anal. Bioanal. Chem. 2009, 395, 2107–2118. [Google Scholar] [CrossRef]
- Liu, W.; Li, M.; Wu, N.; Liu, S.; Chen, J. A New Application of Fiber Optics Reflection Spectroscopy (FORS): Identification of ‘Bronze Disease’ Induced Corrosion Products on Ancient Bronzes. J. Cult. Herit. 2021, 49, 19–27. [Google Scholar] [CrossRef]
- Liggins, F.; Vichi, A.; Liu, W.; Hogg, A.; Kogou, S.; Chen, J.; Liang, H. Hyperspectral Imaging Solutions for the Non-Invasive Detection and Automated Mapping of Copper Trihydroxychlorides in Ancient Bronze. Herit. Sci. 2022, 10, 142. [Google Scholar] [CrossRef]
- Hu, Q.; Liu, W.; Liu, S.; Chen, J. Detecting Copper Trihydroxychlorides with Reflectance Spectroscopy and Machine Learning Methods. J. Cult. Herit. 2023, 59, 49–56. [Google Scholar] [CrossRef]
- Badr Eldin, A. Near Infra Red Spectroscopy. In Wide Spectra of Quality Control; Akyar, I., Ed.; IntechOpen: Rijeka, Croatia, 2011; pp. 237–248. [Google Scholar]
- Basso, E.; Pozzi, F.; Day, J.; Borsch, L. Unmasking a Wild Man: Scientific Analysis of Bertoldo Di Giovanni’s Shield Bearer in The Frick Collection. Herit. Sci. 2020, 8, 109. [Google Scholar] [CrossRef]
- Boon, J.; van Langh, R. Comprehensive studies of patinas on Renaissance bronze statuettes with laboratory, synchrotron and neutron aided techniques. In Proceedings of the ICOM Committee for Conservation 17th Triennial Meeting, Melbourne, Australia, 19–23 September 2014; Bridgland, J., Ed.; International Council of Museums: Paris, France, 2014. [Google Scholar]
- Scott, D.A.; Swartz Dodd, L. Examination, Conservation and Analysis of a Gilded Egyptian Bronze Osiris. J. Cult. Herit. 2002, 3, 333–345. [Google Scholar] [CrossRef]
- Benzonelli, A.; Freestone, I.C.; Martinón-Torres, M. A Better Shade of Black: Effects of Manufacturing Parameters on the Development of Ancient Black Bronzes. Archaeometry 2017, 59, 1034–1049. [Google Scholar] [CrossRef]
- Pollini, J.; Giumlia-Mair, A. The Statue of Germanicus from Amelia: New Discoveries. Am. J. Archaeol. 2019, 123, 675–686. [Google Scholar] [CrossRef]
- Mass, J.L.; Shugar, A.; Finnefrock, A.C.; Tanimoto, S.; Little, M.; Stein, R.; Archie, E. Selenium-Based Black Bronze Treatment, as Compared to Other Patination Technologies of Ancient and Historic Black Bronzes. Technè 2023, 55, 64–74. [Google Scholar] [CrossRef]
- Ng, A.; Noelle, A.J.; Salomon, X.F. Bertoldo Di Giovanni: The Renaissance of Sculpture in Medici Florence; D Giles Ltd.: London, UK, 2019. [Google Scholar]
- Bourgarit, D. French Bronze Sculpture: Materials and Techniques 16th–18th Century; Archetype Publications Ltd.: London, UK, 2014. [Google Scholar]
- Valera-Medina, A.; Amer-Hatem, F.; Azad, A.K.; Dedoussi, I.C.; de Joannon, M.; Fernandes, R.X.; Glarborg, P.; Hashemi, H.; He, X.; Mashruk, S.; et al. Review on Ammonia as a Potential Fuel: From Synthesis to Economics. Energy Fuels 2021, 35, 6964–7029. [Google Scholar] [CrossRef]
- Siatou, A.; Argyropoulos, V.; Charalambous, D.; Polikreti, K.; Kaminari, A. Testing New Coating Systems for the Long-Term Protection of Copper and Iron Alloy Collections Exposed in Uncontrolled Museum Environment. In Proceedings of the CSSIM Conference, Cairo, Egypt, 25 February–1 March 2007. [Google Scholar]
- Leonor Brito do Nascimento e Oliveira, M. A Technical Investigation of an Oil Painting on Copper Support, Including a Study on Consolidants for Treatment. Master’s Thesis, Universidade Nova de Lisboa, Lisbon, Portugal, 2015. [Google Scholar]
- De Viguerie, L.; Payard, P.A.; Portero, E.; Walter, P.; Cotte, M. The Drying of Linseed Oil Investigated by Fourier Transform Infrared Spectroscopy: Historical Recipes and Influence of Lead Compounds. Prog. Org. Coat. 2016, 93, 46–60. [Google Scholar] [CrossRef]
- Favvas, E.P.; Kouvelos, E.P.; Papageorgiou, S.K.; Tsanaktsidis, C.G.; Mitropoulos, A.C. Characterization of Natural Resin Materials Using Water Adsorption and Various Advanced Techniques. Appl. Phys. A 2015, 119, 735–743. [Google Scholar] [CrossRef]
- Yut, I.; Zofka, A. Attenuated Total Reflection (ATR) Fourier Transform Infrared (FT-IR) Spectroscopy of Oxidized Polymer-Modified Bitumens. Appl. Spectrosc. 2011, 65, 765–770. [Google Scholar] [CrossRef]
- Derrick, M.R.; Stulik, D.; Landry, J.M. Infrared Spectroscopy in Conservation Science: Scientific Tools for Conservation; The Getty Conservation Institute: Los Angeles, CA, USA, 1999. [Google Scholar]
- Ma, L.; Varveri, A.; Jing, R.; Erkens, S. Chemical Characterisation of Bitumen Type and Ageing State Based on FTIR Spectroscopy and Discriminant Analysis Integrated with Variable Selection Methods. Road Mater. Pavement Des. 2023, 24, 506–520. [Google Scholar] [CrossRef]
- Herold, M.; Roberts, D. Spectral Characteristics of Asphalt Road Aging and Deterioration: Implications for Remote-Sensing Applications. Appl. Opt. 2005, 44, 7125. [Google Scholar] [CrossRef]
- Speta, M.A. Hyperspectral Imaging for the Characterization of Althabasca Oil Sands Core. Ph.D. Thesis, University of Alberta, Edmonton, AB, Canada, 2016. [Google Scholar]
- Amato, S.R.; Burnstock, A.; Michelin, A. A Preliminary Study on the Differentiation of Linseed and Poppy Oil Using Principal Component Analysis Methods Applied to Fiber Optics Reflectance Spectroscopy and Diffuse Reflectance Imaging Spectroscopy. Sensors 2020, 20, 7125. [Google Scholar] [CrossRef]
- Cloutis, E.A. Spectral Reflectance Properties of Hydrocarbons: Remote-Sensing Implications. Science 1989, 245, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Poli, T.; Chiantore, O.; Nervo, M.; Piccirillo, A. Mid-IR Fiber-Optic Reflectance Spectroscopy for Identifying the Finish on Wooden Furniture. Anal. Bioanal. Chem. 2011, 400, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Robertson, D.; Van Reenen, A.; Duveskog, H. A Comprehensive Investigation into the Structure-Property Relationship of Wax and How It Influences the Properties of Hot Melt Adhesives. Int. J. Adhes. Adhes. 2020, 99, 102559. [Google Scholar] [CrossRef]
- Chua, L.; Head, K.; Thomas, P.; Stuart, B. FTIR and Raman Microscopy of Organic Binders and Extraneous Organic Materials on Painted Ceremonial Objects from the Highlands of Papua New Guinea. Microchem. J. 2017, 134, 246–256. [Google Scholar] [CrossRef]
- Biribicchi, C.; Doutre, M.; Favero, G. Characterization and Assessment of Cleaning Systems Based on Fatty Acid Methyl Esters (FAMEs) for the Removal of Wax-Based Coatings from Cultural Heritage Objects. Mater. Adv. 2024, 5, 9359–9375. [Google Scholar] [CrossRef]
- Bucio, A.; Moreno-Tovar, R.; Bucio, L.; Espinosa-Dávila, J.; Anguebes-Franceschi, F. Characterization of Beeswax, Candelilla Wax and Paraffin Wax for Coating Cheeses. Coatings 2021, 11, 261. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, Z.; Shi, K.; Jiang, Z.; Guan, C.; Zhang, L.; Yang, T.; Xie, F. Shellac-Based Materials: Structures, Properties, and Applications. Int. J. Biol. Macromol. 2024, 279, 135102. [Google Scholar] [CrossRef] [PubMed]
- Truffa Giachet, M.; Schröter, J.; Brambilla, L. Characterization and Identification of Varnishes on Copper Alloys by Means of UV Imaging and FTIR. Coatings 2021, 11, 298. [Google Scholar] [CrossRef]
- Carlesi, S.; Ricci, M.; Cucci, C.; La Nasa, J.; Lofrumento, C.; Picollo, M.; Becucci, M. Multivariate Analysis of Combined Fourier Transform Near-Infrared Spectrometry (FT-NIR) and Raman Datasets for Improved Discrimination of Drying Oils. Appl. Spectrosc. 2015, 69, 865–876. [Google Scholar] [CrossRef] [PubMed]
- Provost, E.; Sugar, A. Surface Organic Coatings on Renaissance Bronzes at the Royal Ontario Museum: A Non-Destructive Spectroscopic Study, 2025. (Internal report).
- Provost, E.; Sugar, A. Non-destructive characterization of organic patinas on Renaissance bronzes using short-wave infrared and mid-infrared spectroscopy. In METAL 2025: Proceedings of the International Conference on Metals Conservation, Cardiff, UK, 1–5 September 2025; Emmerson, N., Thunberg, J., Watkinson, D., Eds.; Cardiff University: Cardiff, UK, 2025; pp. 231–237. [Google Scholar]


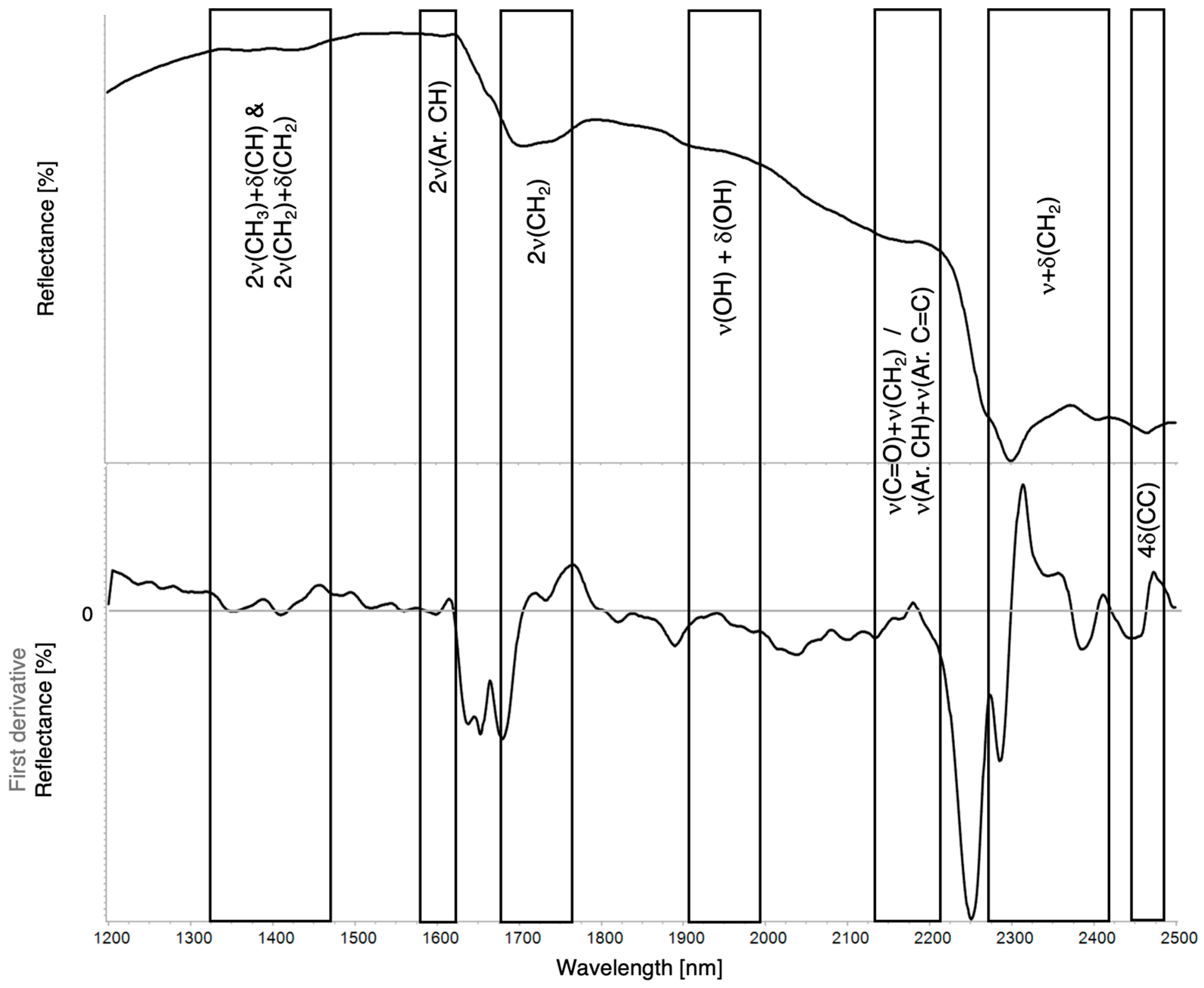
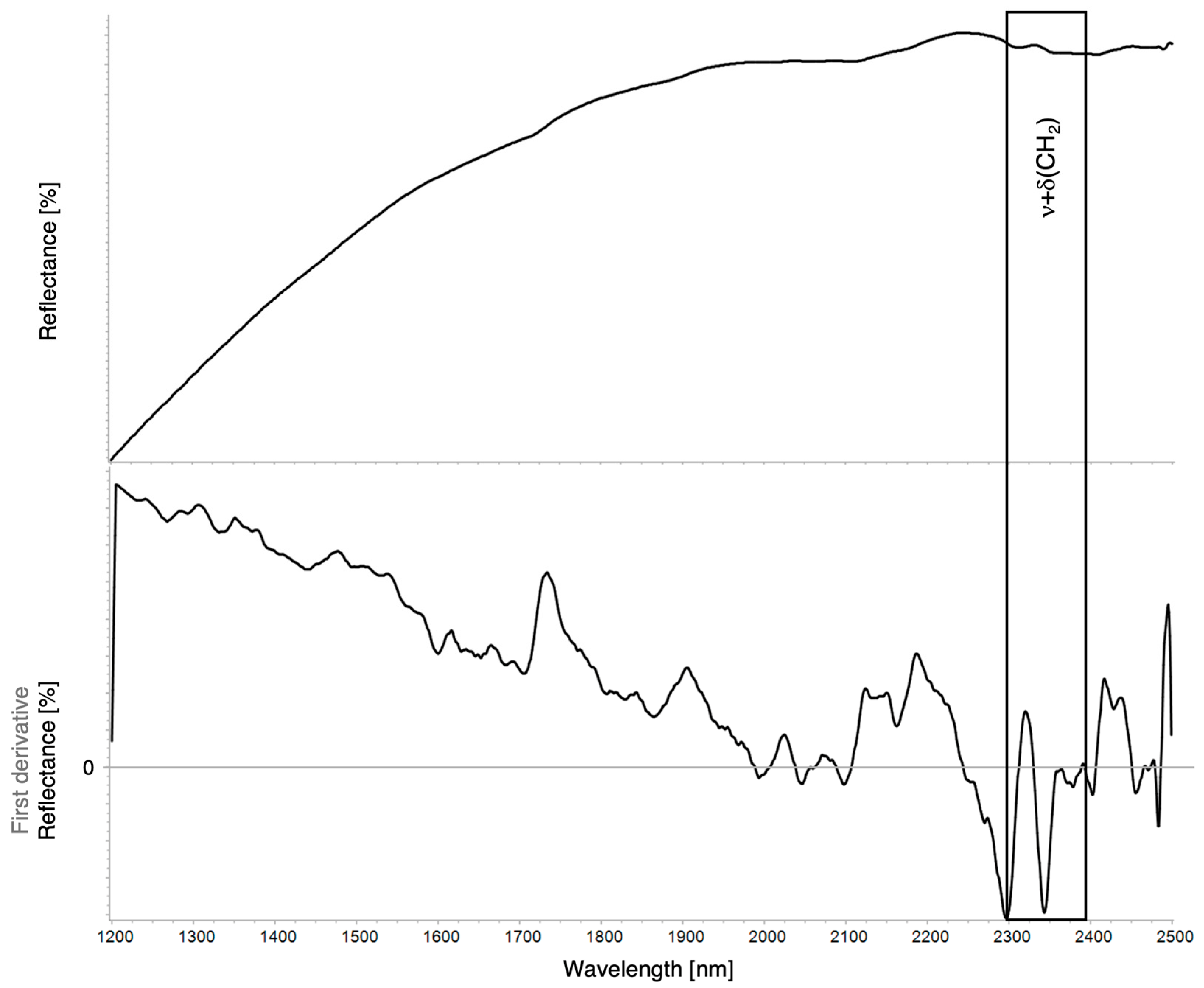
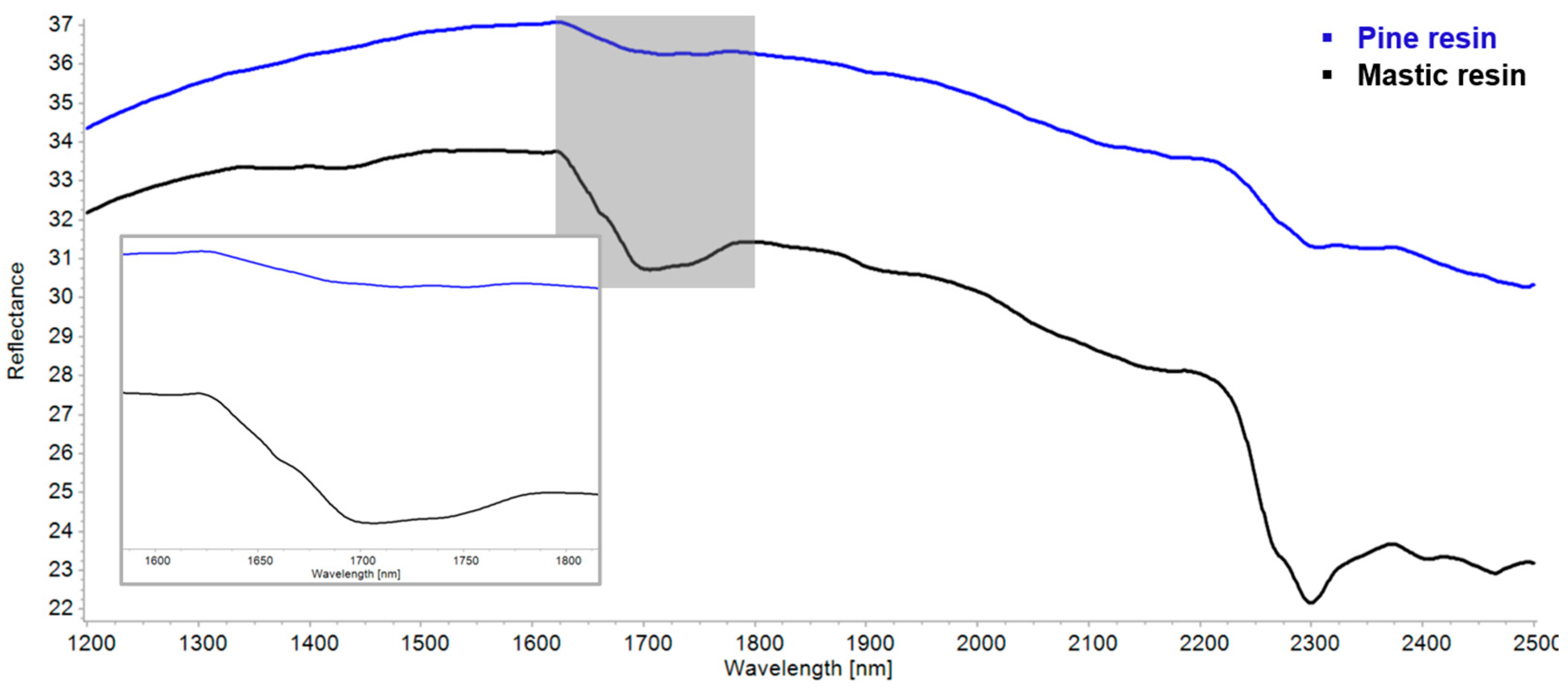
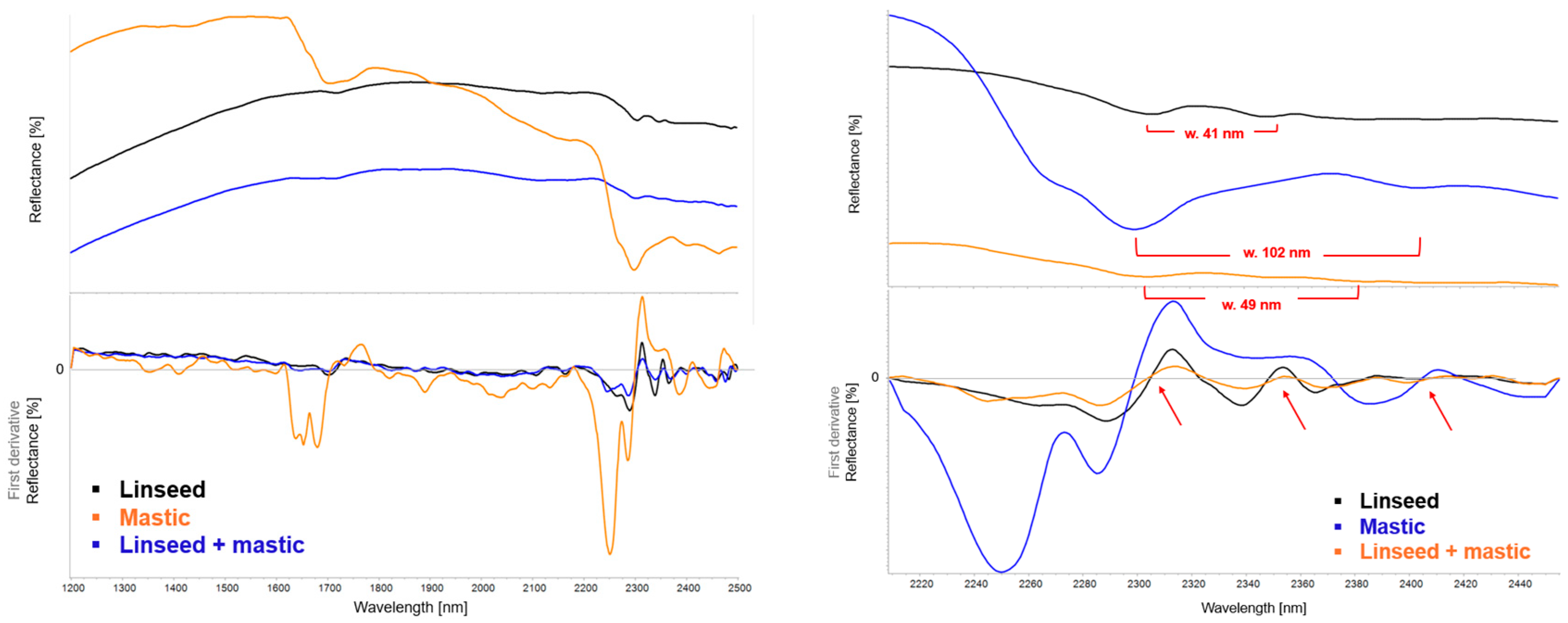
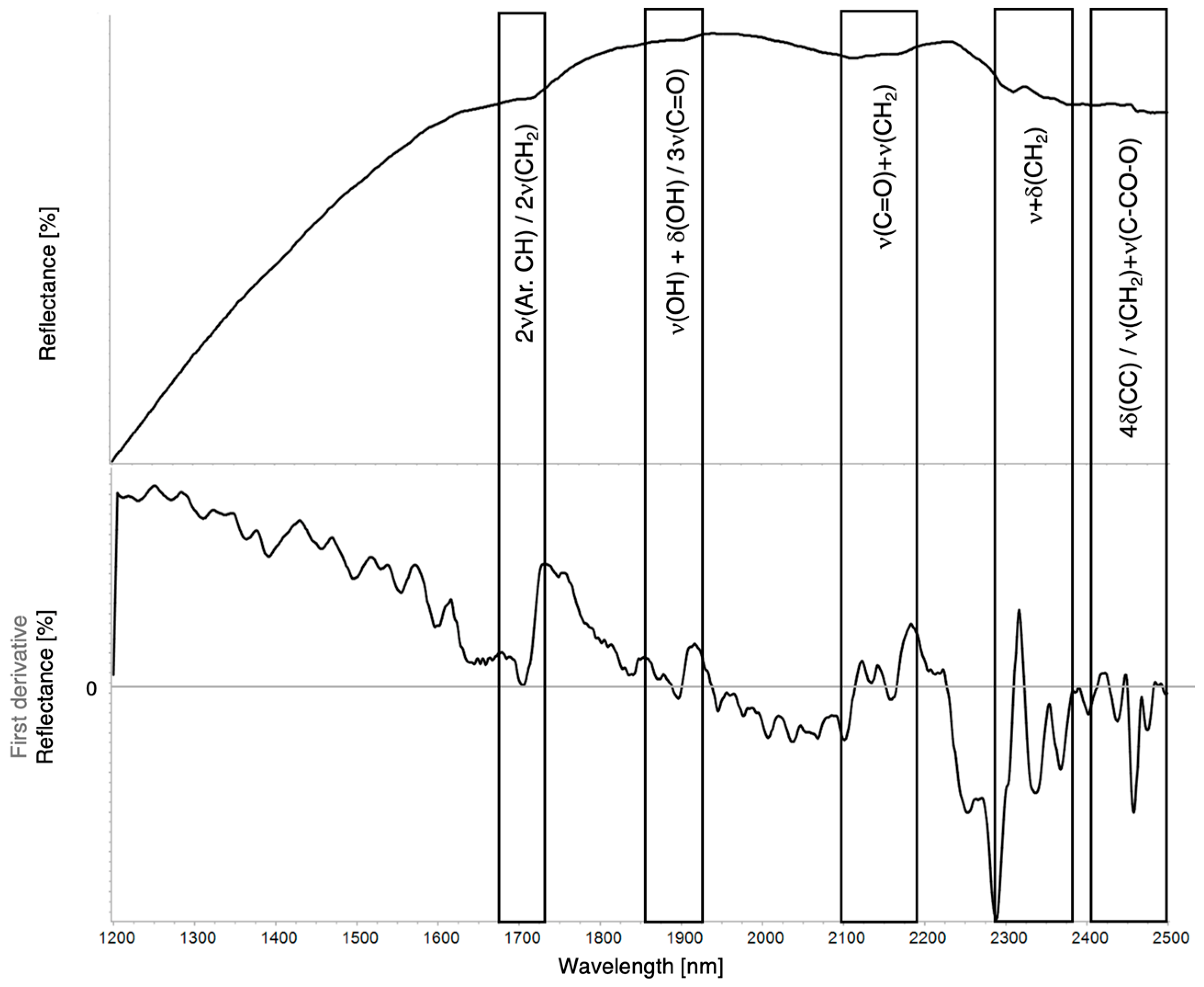
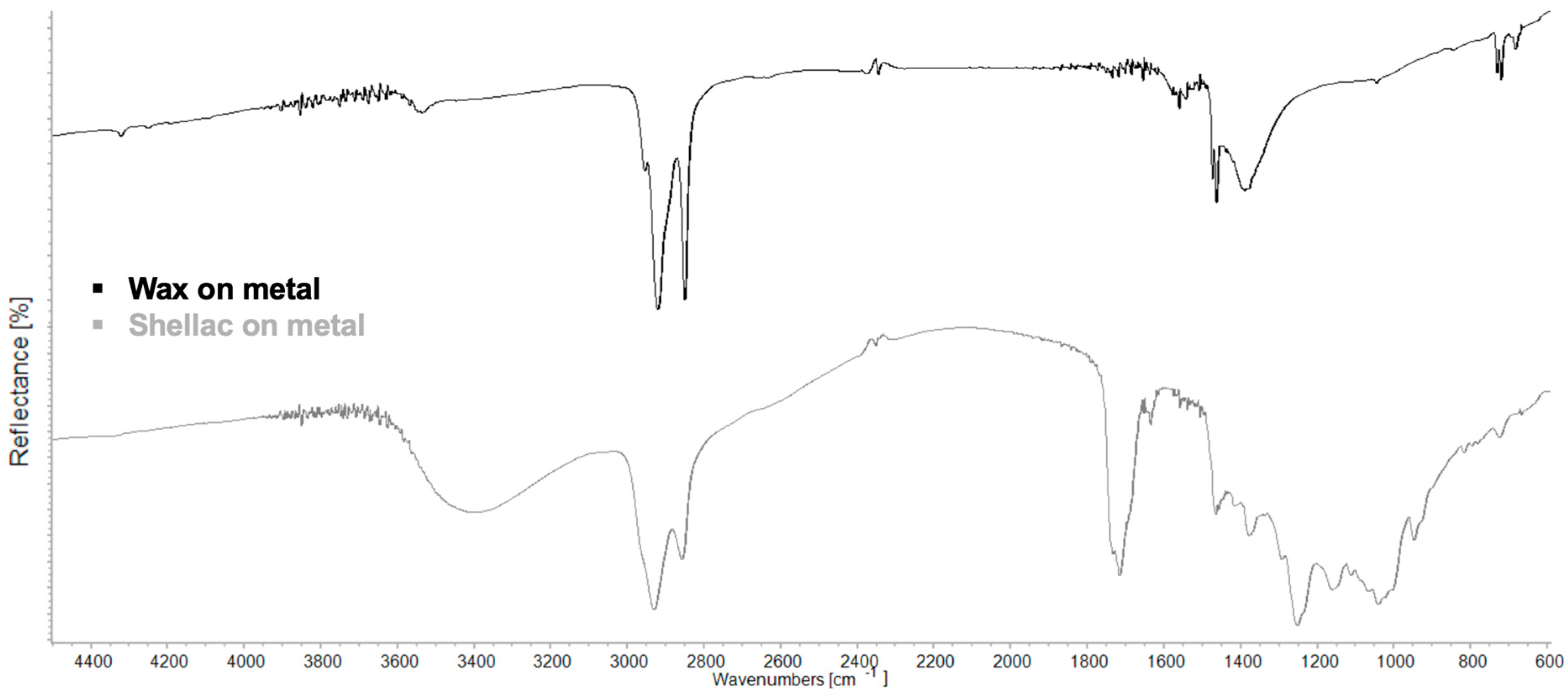
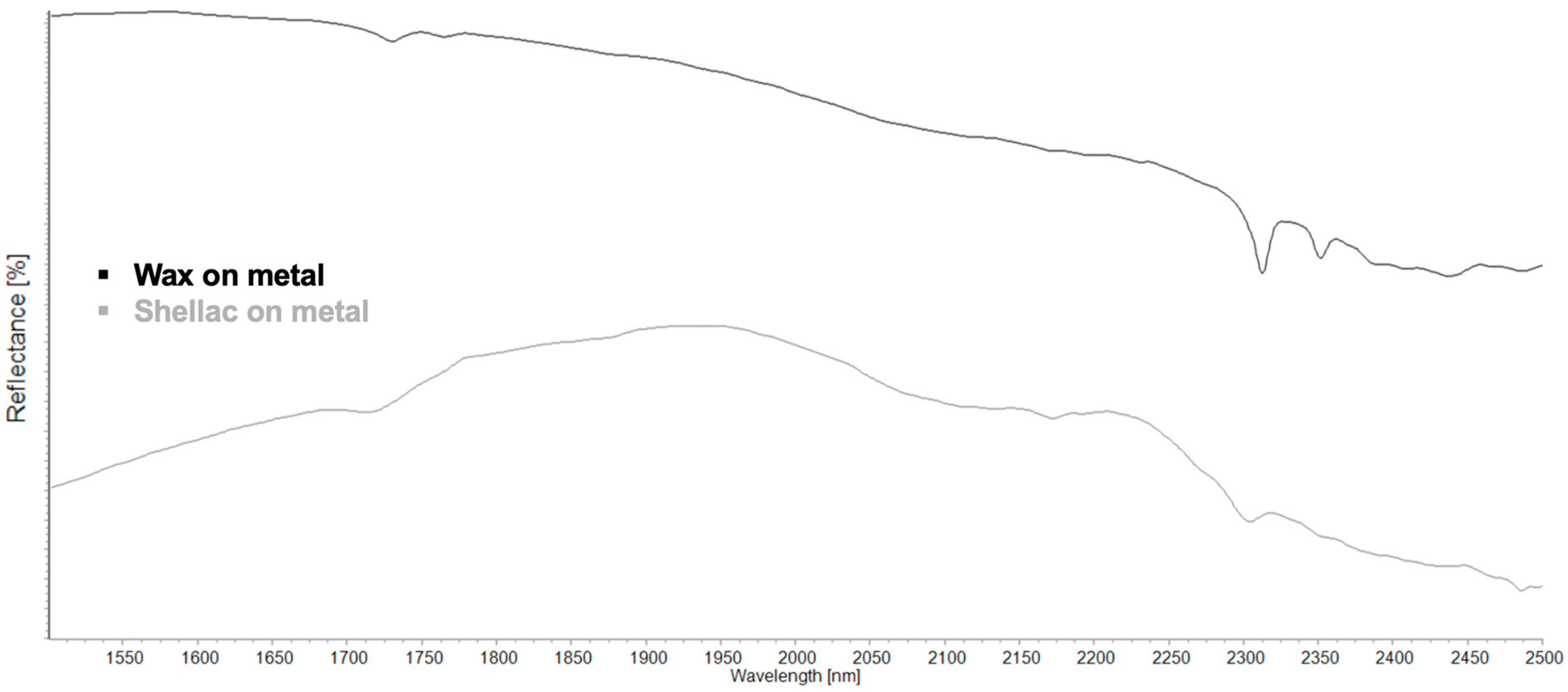
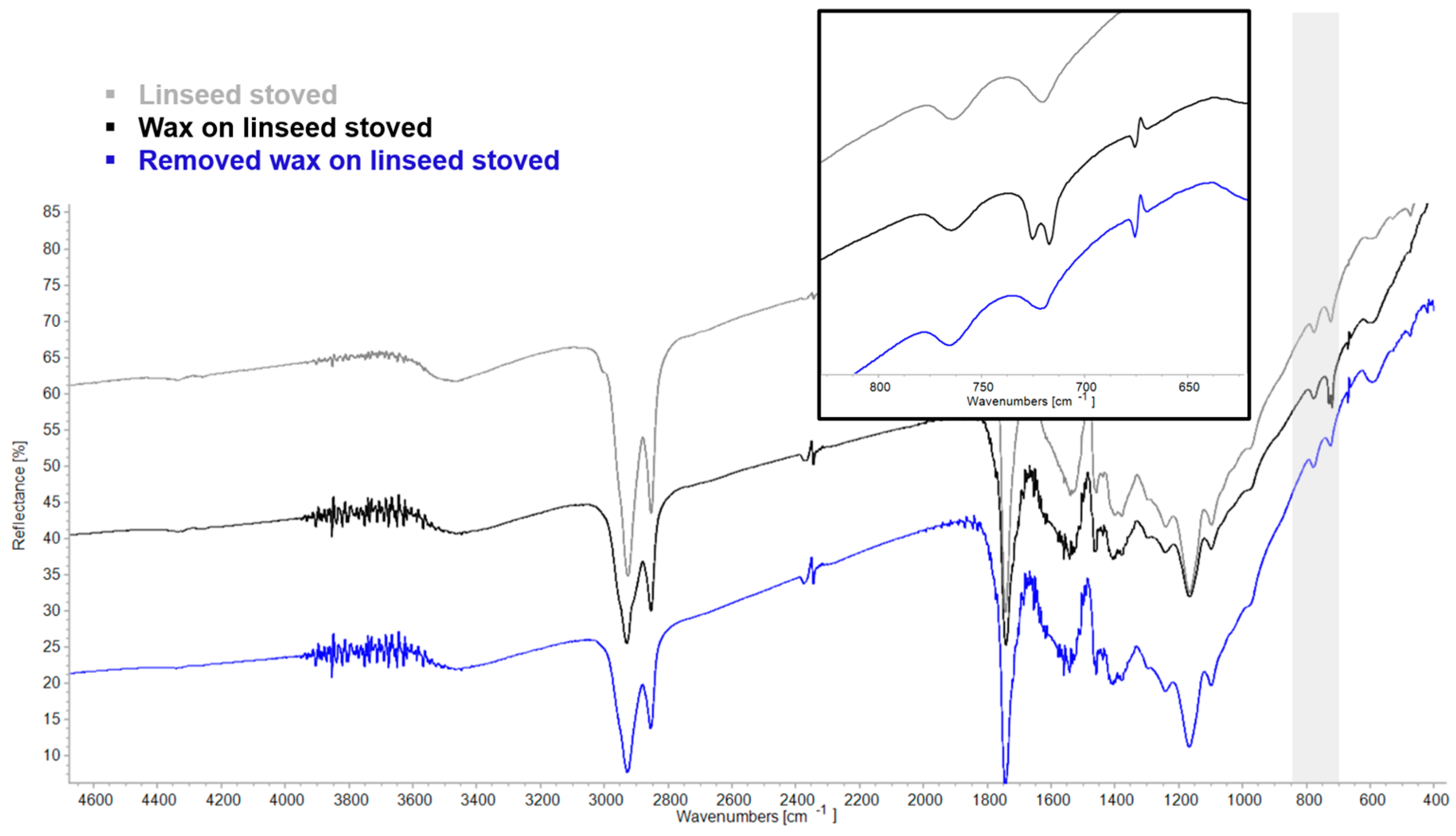

| Region | Instrument | Wavelength (nm) | Wavenumber (cm−1) | Features |
|---|---|---|---|---|
| NIR | FORS | 780–2500 | 12,820–4000 | Overtones/combination bands |
| SWIR | FORS | 1000–2500 | 10,000–4000 | Overtones/combination bands |
| MIR | ER-FTIR | 2500–25,000 | 4000–400 | Fundamental vibrations |
| Element | Min (%) | Max (%) | Average (%) | C83600 (%) |
|---|---|---|---|---|
| Copper | 59.25 | 96 | 85.59 | 84–86 |
| Tin | 0.12 | 14.36 | 4.27 | 4.0–6.0 |
| Lead | 0 | 41.64 | 4.09 | 4.0–6.0 |
| Zinc | 0 | 31.1 | 4.94 | 4.0–6.0 |
| Antimony | 0 | 3.29 | 0.42 | 0.25 |
| Iron | 0 | 5.5 | 0.70 | 0.30 |
| Nickel | 0 | 1.18 | 0.27 | 1.0 |
| Silver | 0 | 0.41 | 0.14 | - |
| Arsenic | 0 | 2 | 0.52 | - |
| Silicon | 0 | 0.16 | 0.15 | 0.005 |
| Phosphorus | - | - | - | 1.50 |
| Aluminum | - | - | - | 0.005 |
| Sulfur | - | - | - | 0.08 |
| Aging Cycle | Cycle Periods and Conditions | ||
|---|---|---|---|
| 1 cycle of 24 h | Normal conditions | time = 8 h | T = 23 °C RH = 55% |
| Extreme conditions | time = 16 h | T = 35 °C RH = 90% | |
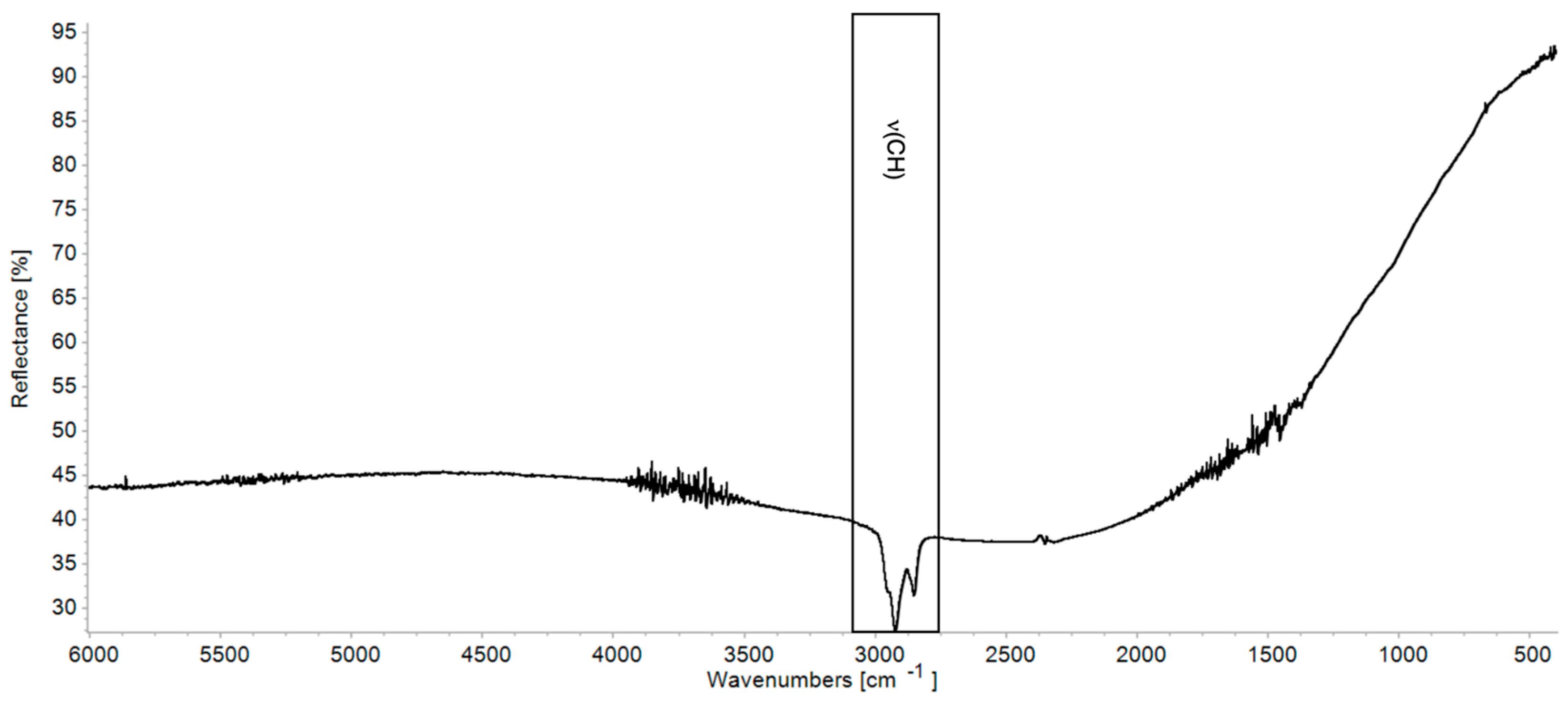
| νa(CH2) + δ(CH2) | νs(CH2) + δ(CH2) | νOH | νaCH | νsCH | νC=O | δCH | νC–O | |
|---|---|---|---|---|---|---|---|---|
| Linseed oil | 4339 | 4259 | 3468 | 3011 sh, 2926 | 2852 | 1743 | 1532–1387 | 1166 |
| Walnut oil | 4347 | 4259 | 3468 | 3015 sh, 2924 | 2852 | 1736 | 1523–1391 | 1164 |
| Mastic resin | 4412 | 4347 | 3473 | 3079 sh, 2949 | 2869 | 1707 | 1457, 1382 | 1247–1033 |
| Pine resin | ~4389 | 3434 | 3070 sh, 2943 | 2870 | 2638 sh, 2529 sh, 1717, 1696 | 1460–1387 | 1247–1032 | |
| Bitumen | 2952 sh, 2925 | 2852 |
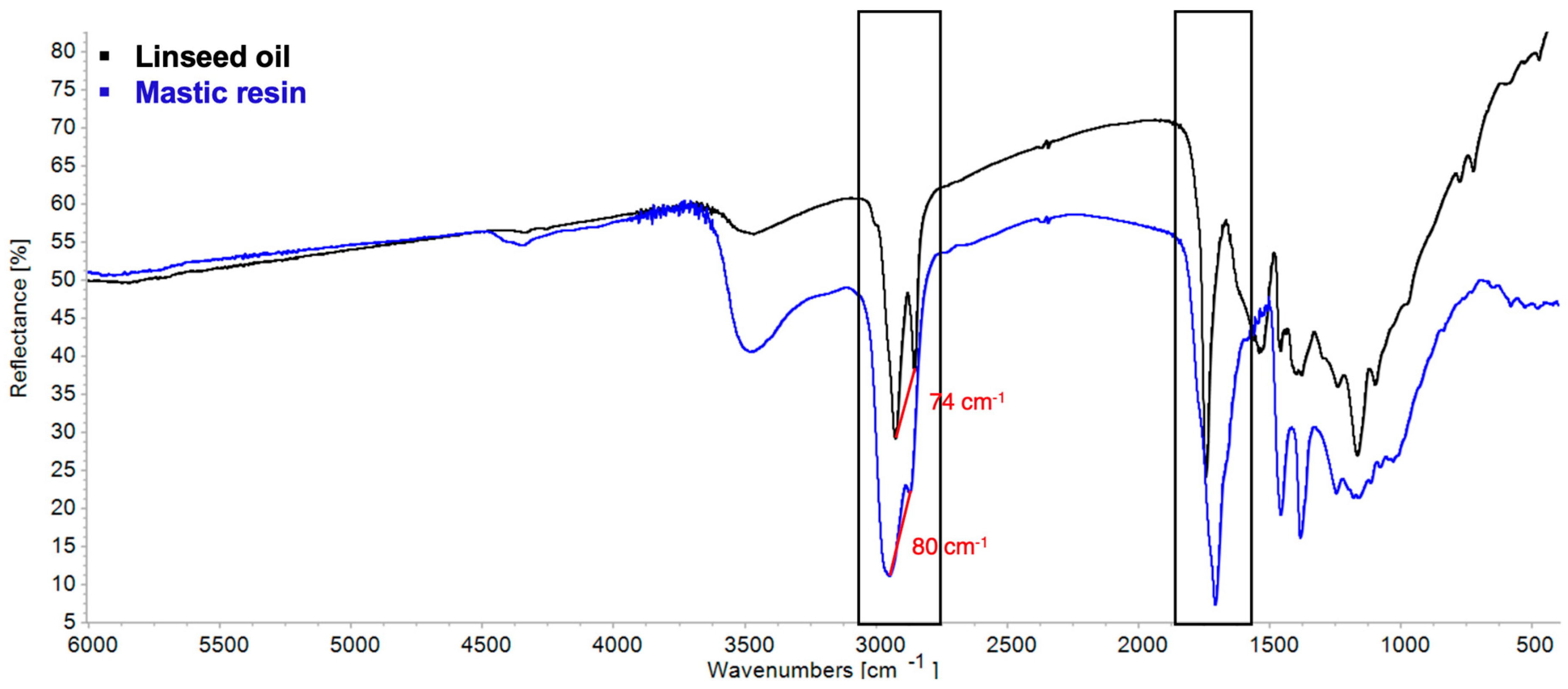
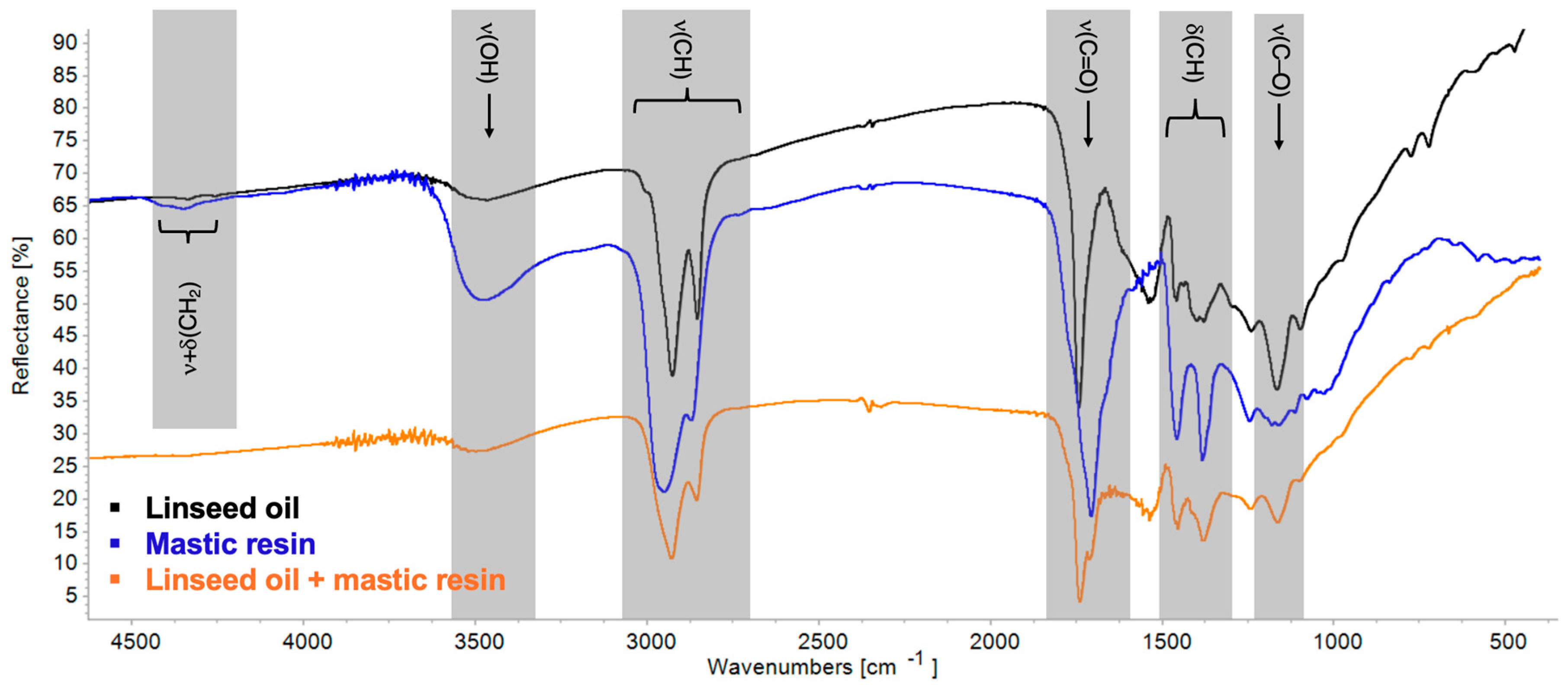
| Linseed Oil | Mastic Resin | Blend | Observed Change | |
|---|---|---|---|---|
| νa(CH2) + δ(CH2) | 4339 | 4412 | 4338 | Weakening |
| νs(CH2) + δ(CH2) | 4259 | 4347 | 4251 | Weakening |
| νOH | 3468 | 3473 | 3500 | Increased absorbance |
| νaCH | 3011 sh, 2926 | 3079 sh, 2943 | 2928 | Shift and broadening |
| νsCH | 2852 | 2870 | 2855 | Shift and broadening |
| νC=O | 1743 | 2638 sh, 2529 sh, 1717, 1696 | 1739, 1711 | Splitting, shift, and broadening |
| δCH | 1532–1387 | 1460–1387 | 1453, 1378 | Shift, appearance of shoulders |
| νC–O | 1166 | 1247–1032 | 1162 | Increased absorbance |
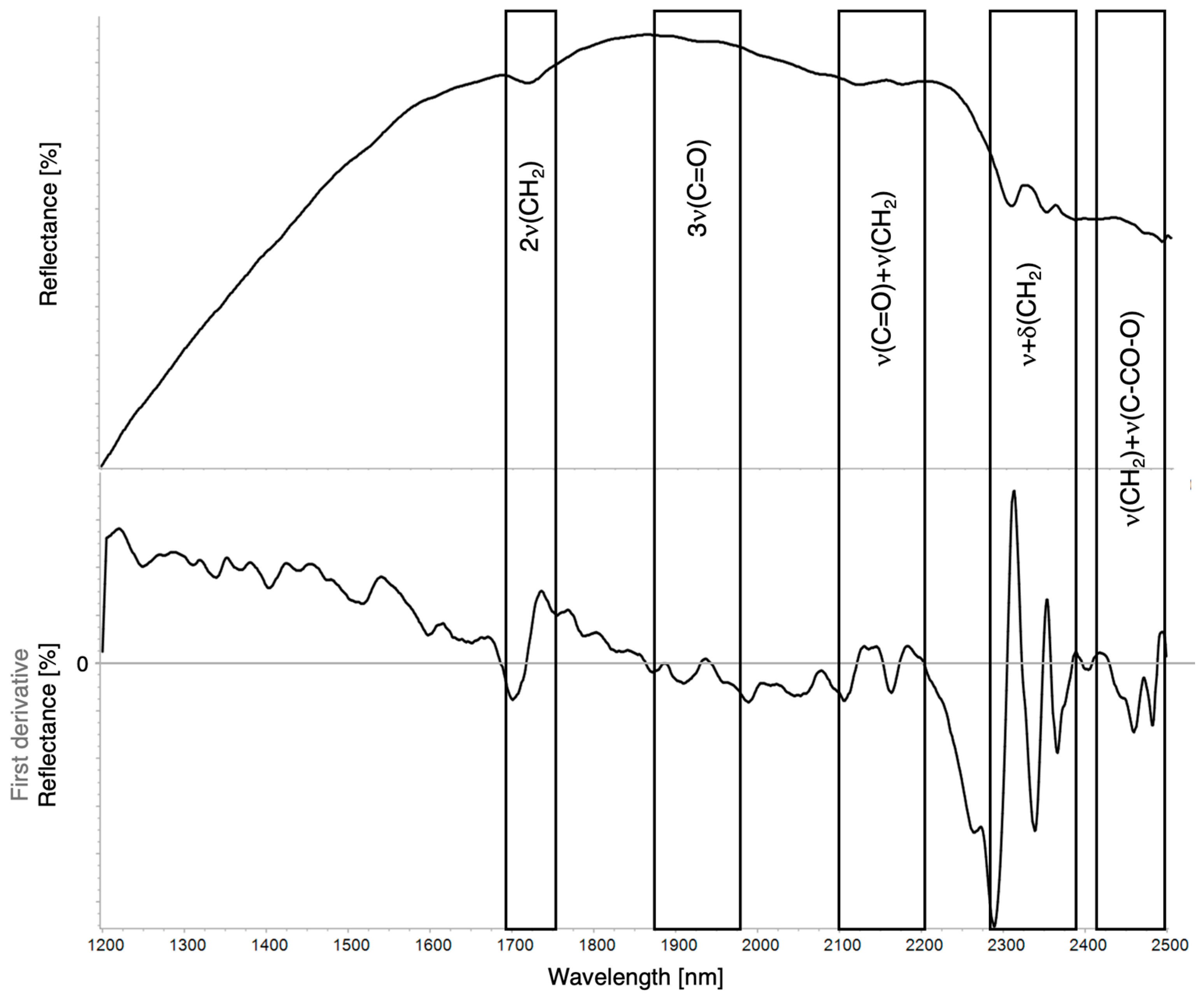
| 2ν(CH3) + δ(CH) | 2ν(CH2) + δ(CH2) | 2ν(Ar.CH) | 2νa+s(CH2) | 3ν(C=O) | ν(OH) + δ(OH) | ν(C=O) + δ(CH2) | ν(Ar.CH) + ν(Ar.C=C) | νa(CH2) + δ(CH2) | νs(CH2) + δ(CH2) | 4δ(CC) | ν(CH2) + ν(C–CO–O)aliphatic/aromatic | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Linseed oil | 1717 | 1929 | 2120–2165 | 2306 | 2347 | 2460–2480 | ||||||
| Walnut oil | 1716 | 2118–2169 | 2308 | 2355 | 2460–2486 | |||||||
| Mastic resin | 1371 | 1426 | ~1615 sh | 1706–1738 | ~1940 | 2175, 2265 sh | 2299 | 2401 | 2463 | |||
| Pine resin | 1600–1623 sh | 1717–1774 | 2180 | 2305 | 2354 | 2493 | ||||||
| Bitumen | 2311 | 2359–2408 | ||||||||||
| νa(CH2) + δ(CH2) | νs(CH2) + δ(CH2) | νOH | νaCH | νsCH | νC=O | δaCH | δsCH | δOH | νC–O | ρCH2 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Wax | 4320 | 4250 | 3539 | 2952 sh, 2920 | 2849 | ~1719 | 1472 | 1462, 1383 | 729, 718 | ||
| Shellac | ~4338 | 3406 | 2927 | 2856 | 1732 sh, 1715, 1634 sh | 1463 | 1455, 1377 | 1291, 1252, 1157 | 1038 | 725 | |
| 2νa(CH2) | 2νs(CH2) | ν(C=O) + ν(CH2) | νa(CH2) + δ(CH2) | νs(CH2) + δ(CH2) | ? | ν(CH) + ν(CO)/ OH vib. | |
|---|---|---|---|---|---|---|---|
| Wax | 1730 | 1764 | 2312 | 1251 | 2386 | ||
| Shellac | 1710 | ~2135–2172 | 2305 | 2434, 2488 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Provost, E.; Shugar, A. An SWIR-MIR Spectral Database of Organic Coatings Used on Historic Metals. Coatings 2025, 15, 1226. https://doi.org/10.3390/coatings15101226
Provost E, Shugar A. An SWIR-MIR Spectral Database of Organic Coatings Used on Historic Metals. Coatings. 2025; 15(10):1226. https://doi.org/10.3390/coatings15101226
Chicago/Turabian StyleProvost, Elizabeth, and Aaron Shugar. 2025. "An SWIR-MIR Spectral Database of Organic Coatings Used on Historic Metals" Coatings 15, no. 10: 1226. https://doi.org/10.3390/coatings15101226
APA StyleProvost, E., & Shugar, A. (2025). An SWIR-MIR Spectral Database of Organic Coatings Used on Historic Metals. Coatings, 15(10), 1226. https://doi.org/10.3390/coatings15101226







