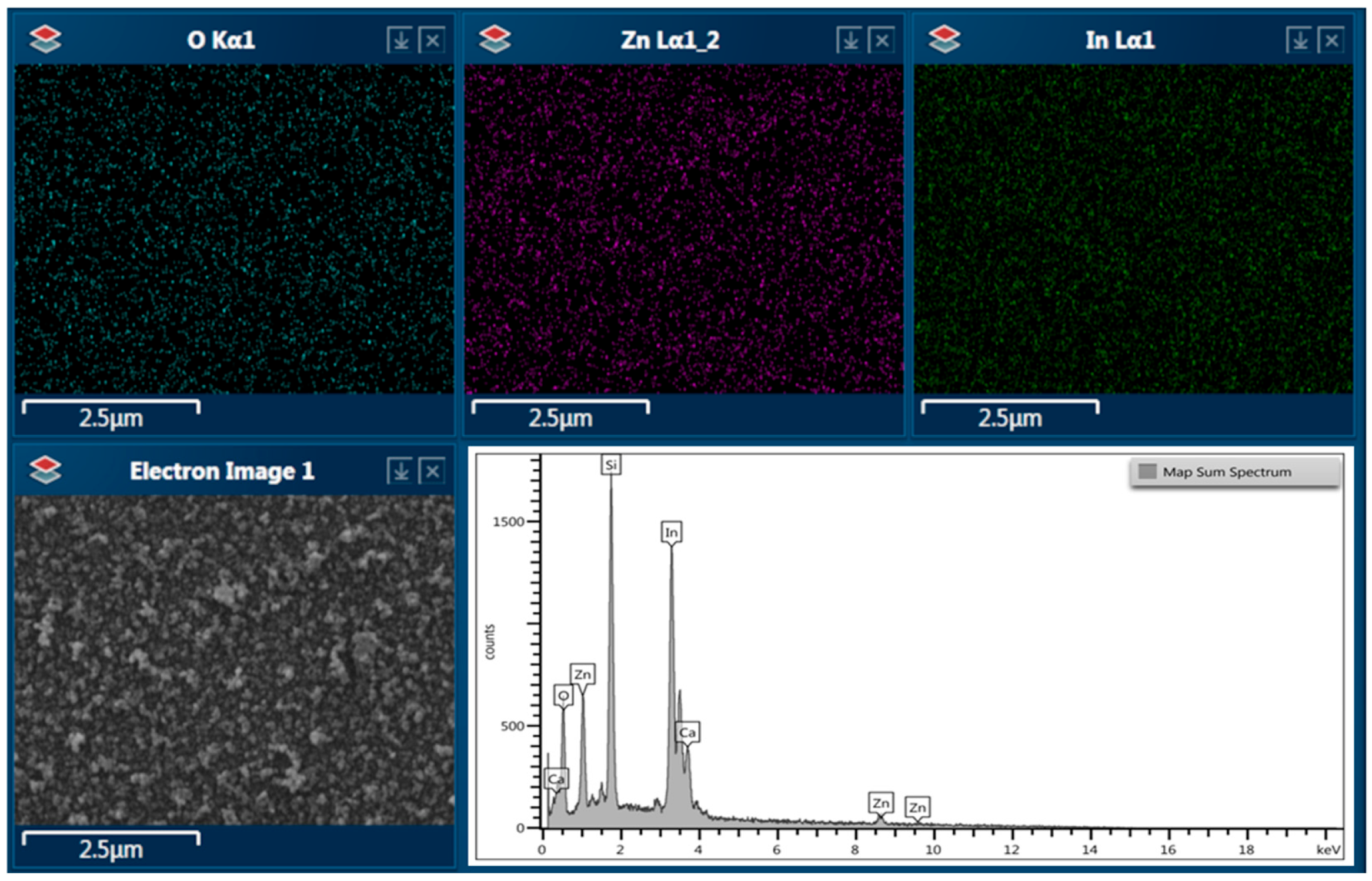
3.1. SEM
The analysis of SEM micrographs of the samples shows that the surface of all films is formed by rod-shaped particles of micro or nanometric dimensions, which is consistent with the results obtained by other authors [
23], as well as notable differences in said dimensions, according to the deposition conditions listed in
Table 1.
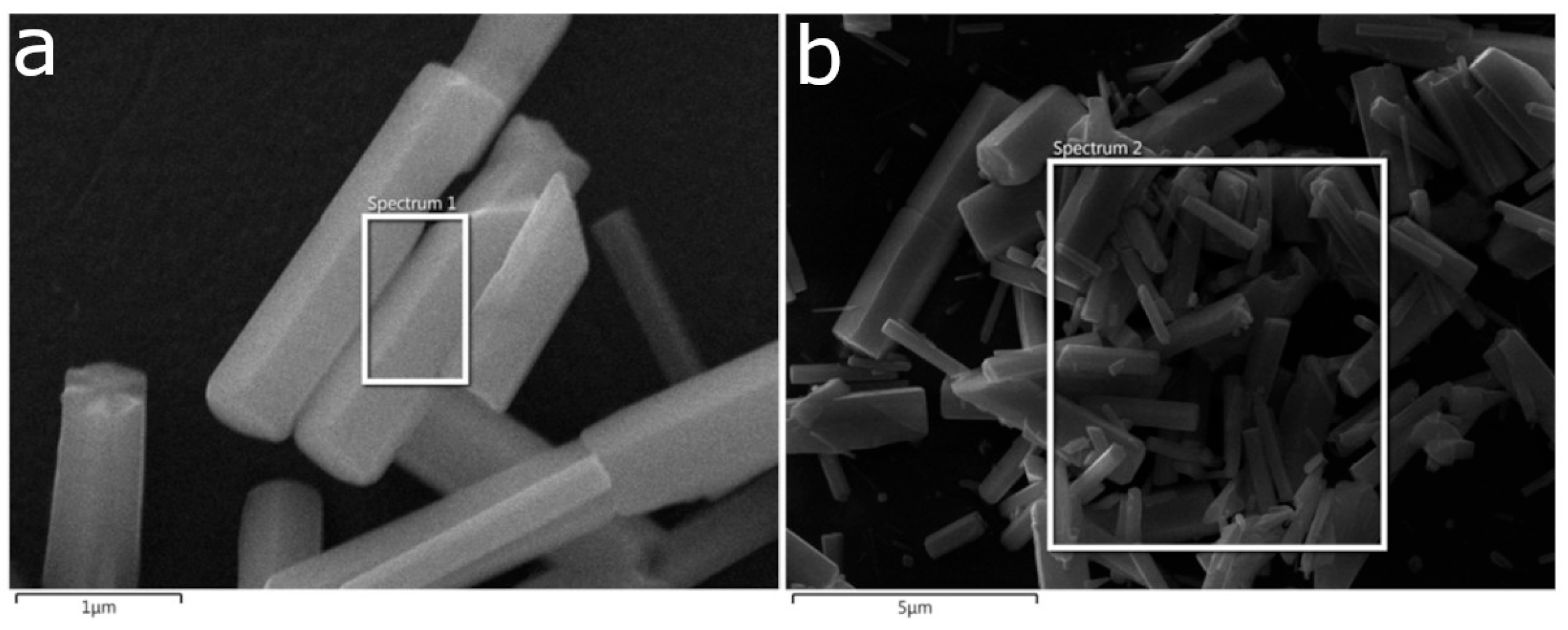
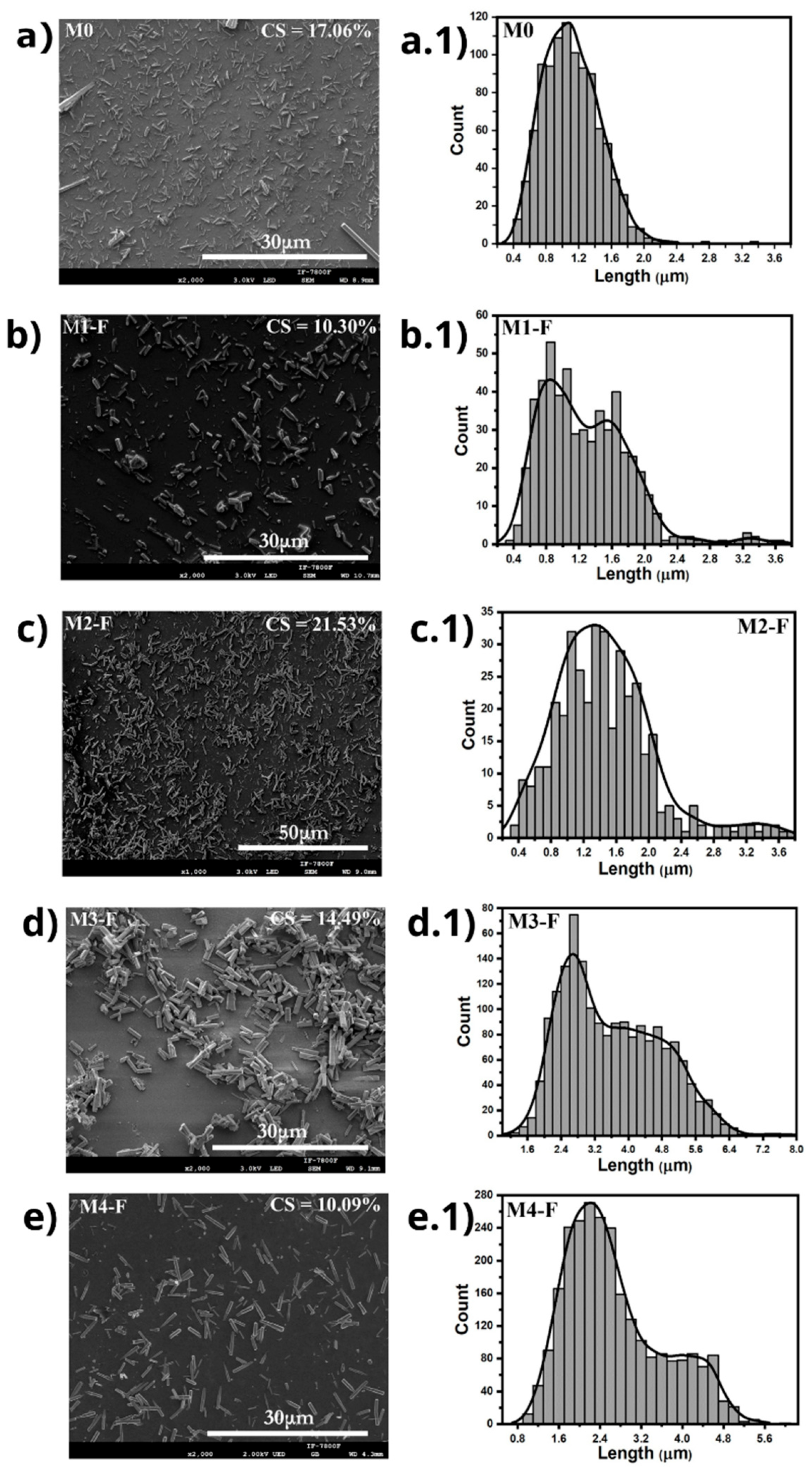
Figure 3 presents SEM micrographs of the samples synthesized by CBD.HVEF-A; these micrographs show that the ZnO coatings have rod-shaped microparticles in their composition. The sizes of the rods in the samples were measured and their corresponding size distribution functions are shown in
Figure 3. In order to measure the percentual quantity of rod-shaped microparticles present on the ZnO coating (CS), a program designed in Mathematica Software version 11.3 [
24] was employed, and the corresponding obtained values are presented in each micrograph.
Table 2 presents the descriptive statistics that summarize the information from the size distribution functions for each sample synthesized by CBD-HVEF-A shown in
Figure 3. The distribution function along with the cumulative curves for these samples (
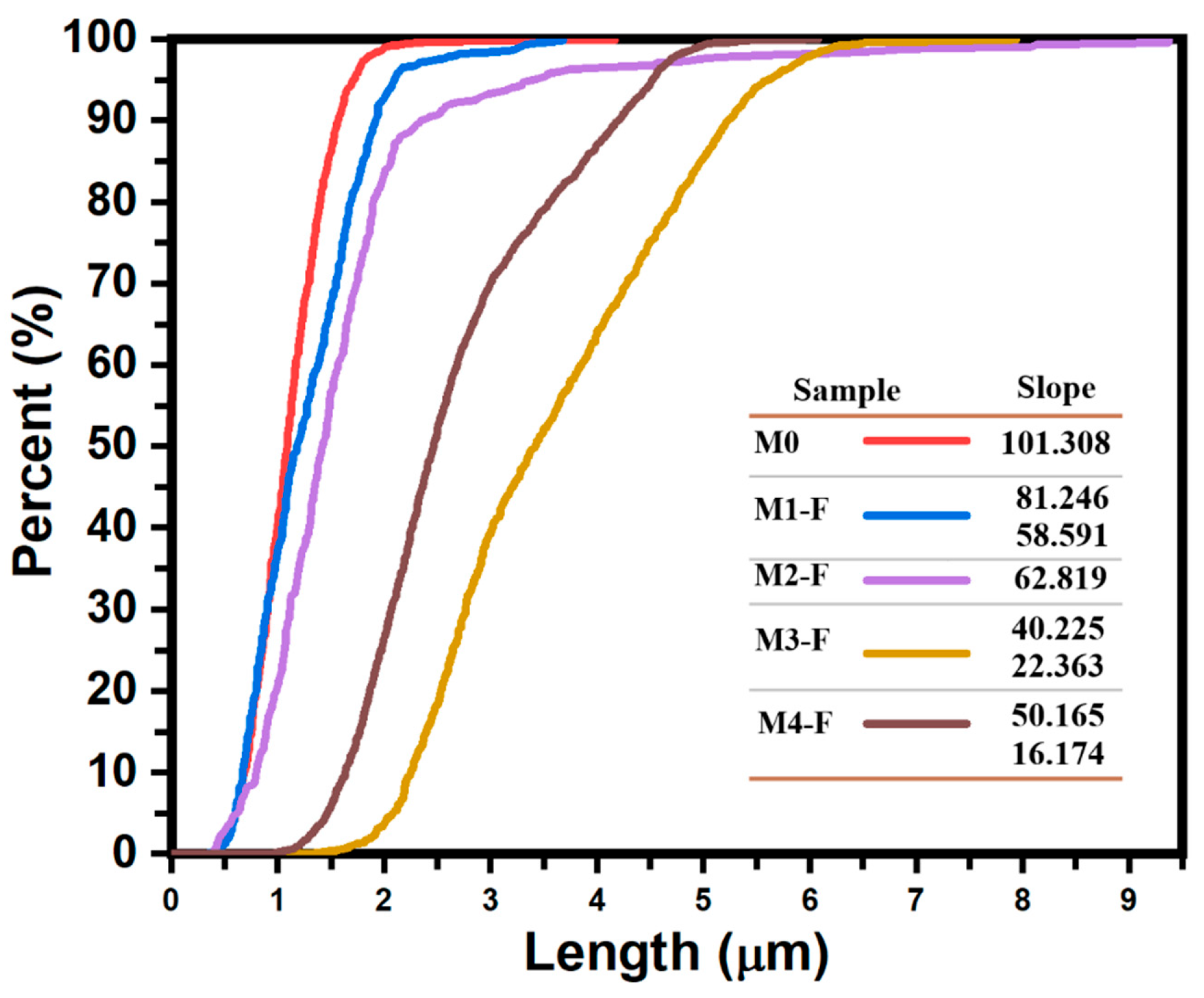
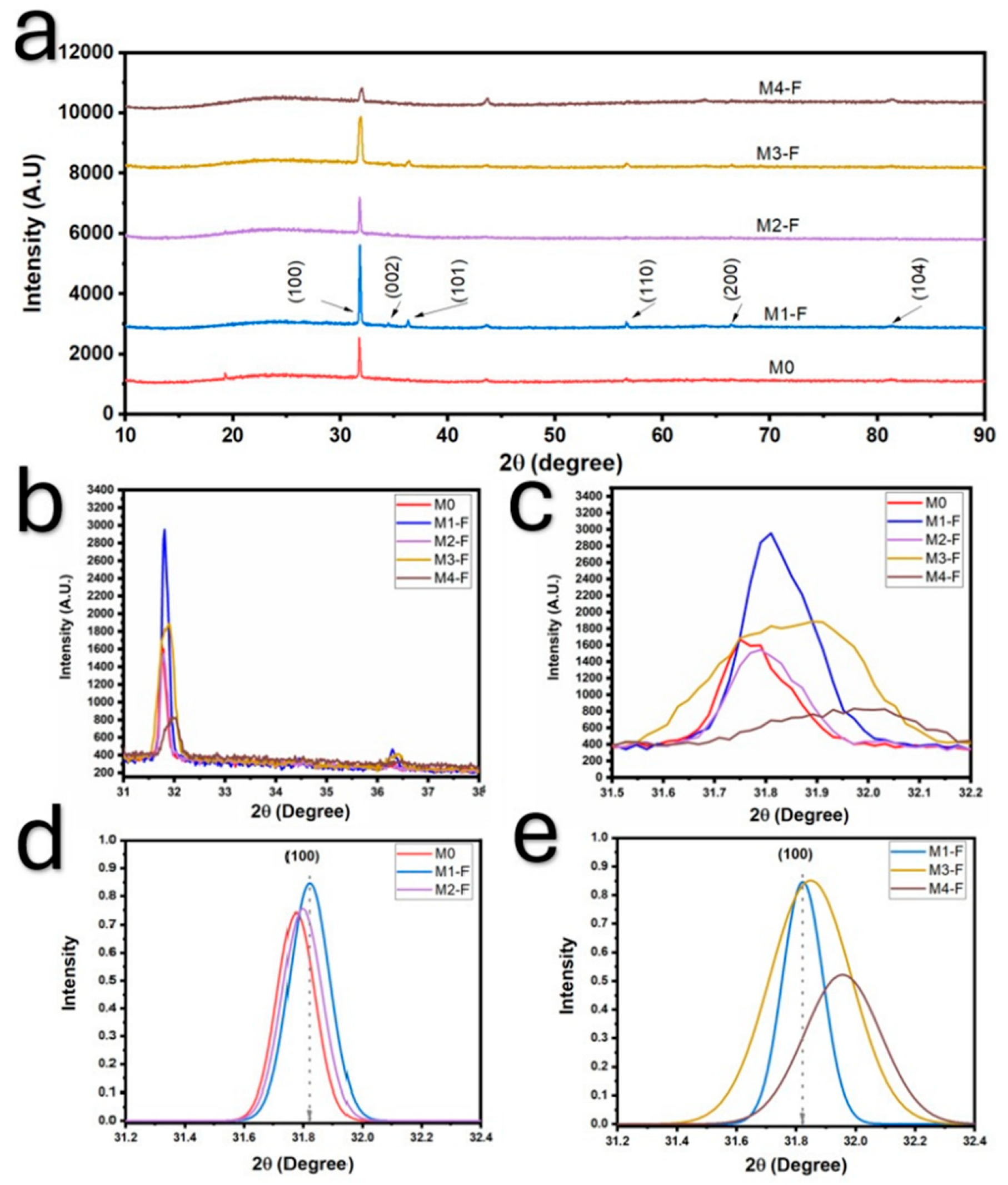
Figure 4) shows that the application of an electric field results in a dispersion and increase in the rod sizes, as the mean size went from 1.092 μm in sample M0, with no electric field applied, to 1.271 μm and 1.631 μm for samples M1-F (36 kV) and M2-F (24 kV), respectively, and interquartile range went from 0.486 μm for sample M0 to ≈0.76 μm in both M1-F and M2-F samples in which an electric field was applied, meaning that although dispersion in the rod size increased with the electric field, the value looks to be independent of the intensity of the electric field. When looking at SEM images for samples M3-F and M4-F, it is evident that the resulting particles maintain the same rod structure as other samples, although a quick glance at the average particle size confirms that in both cases, these rods are considerably larger when comparing them to M1-F, where no inversion of the electric field occurred, and being larger than M3-F modes in a ratio of 3.17:1 and 2.36:1, as well as 2.70:1 and 2.61:1 for M4-F. Furthermore, both samples M3-F and M4-F maintain the bimodal distribution function of sample M1-F, which is a good indicator of the consistency in the synthesis process that occurs when an electric field of 36 kV is applied, regardless of whether polarity inversion is applied or not. The interquartile range allows us to see that the dispersion in the rod size increases further with the polarity inversion in the electric field, increasing in M3-F, which has a higher inversion frequency (shorter period), which agrees with the fact that longer periods should behave as if no inversion occurs.
Figure 4 presents the cumulative curves of the length of the ZnO rods in samples synthesized by CBD-HVEF-A, where a higher slope indicates a lower dispersion of the rod lengths, the apparition of more than one “linear” segment is a consequence of multi-modal distribution functions, and the number of segments indicates the number of modes. This being said, the application of an electric field during the synthesis of ZnO results in higher dispersion rates and bi-modal functions. The slope values of each cumulative curve are inserted in the figure to show a quantitative measure of length dispersion. These curves make even more evident what was stated above: the application of an electric field results in an increase in particle length in all cases; to a greater degree, for samples with inversion of polarity, sample M3-F, synthesized under a 36 kV electric field with electrical polarity reversal every 2.5 min, is the one with the largest particles and the greatest dispersion in size.
Figure 5 presents micrographs for samples obtained using the CBD-HVEF-B system, each labeled according to the corresponding sample listed in
Table 1. These micrographs showed homogeneity in the ZnO deposit as well as the fact that all the coatings were composed of nanometric-sized particles, except for M0a-S, which is the sample deposited by a conventional CBD technique and was composed of submicrometric particles. Each micrograph has an insert with the percentage value of the substrate area coated by these submicrometric ZnO particles, obtained using software developed by our research team and employed in previous reports [
24]. The characteristic shape of the particles and the descriptive statistics of their sizes are presented in the table inserted in
Figure 6. The same figure also shows the particle size distribution functions for all the coatings, in correspondence with the micrographs in
Figure 5. There, next to each of the size distribution functions, their corresponding cumulative size curves are presented. The comparative analysis between the coatings, in terms of the shape and size of the particles that compose them, was carried out based on whether or not an electric field was used during the synthesis and whether or not the solution was stirred by injecting filtered air into it, in addition to the variation in its pH, thus resulting in eight comparative analyses between the coatings.
Figure 7 shows the different cumulative size curves of the coatings according to the comparative analysis in question. Likewise,
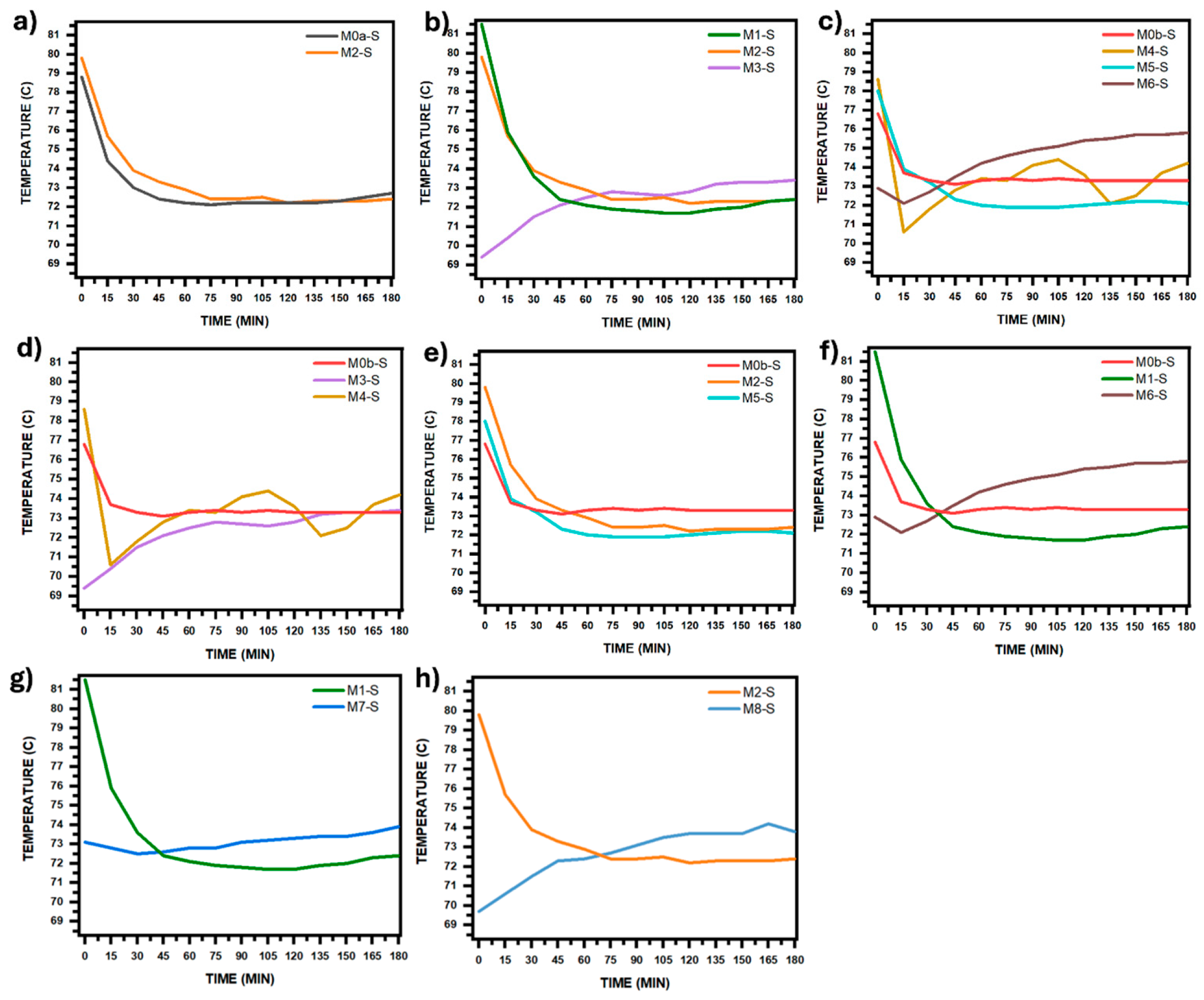
Figure 8 and
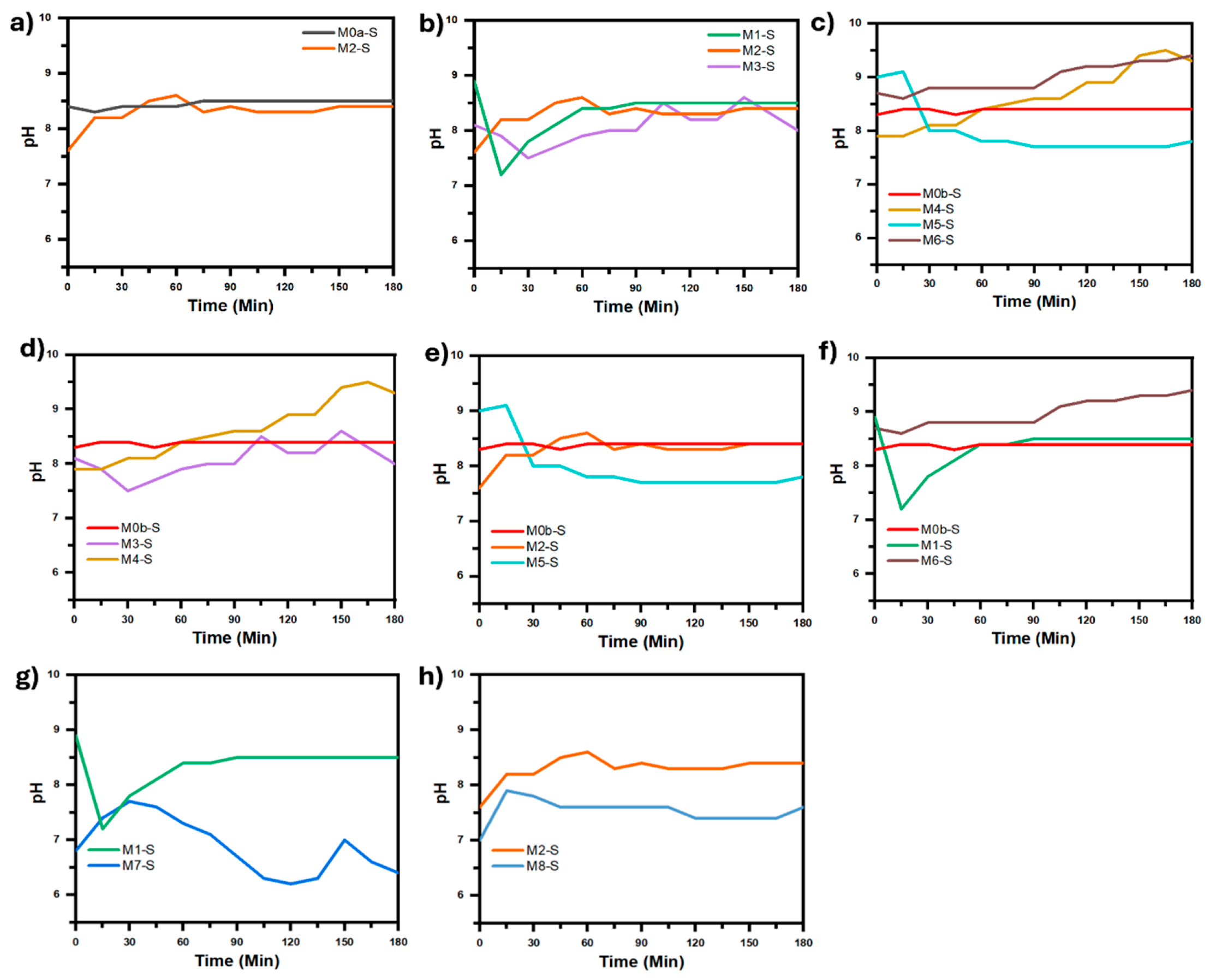
Figure 9 show the temperature and pH curves of the solution during synthesis process, corresponding to each comparative analysis in
Figure 7, this is in order to assess whether or not there are significant differences between the temperature and pH curves that should be considered for the corresponding analysis.
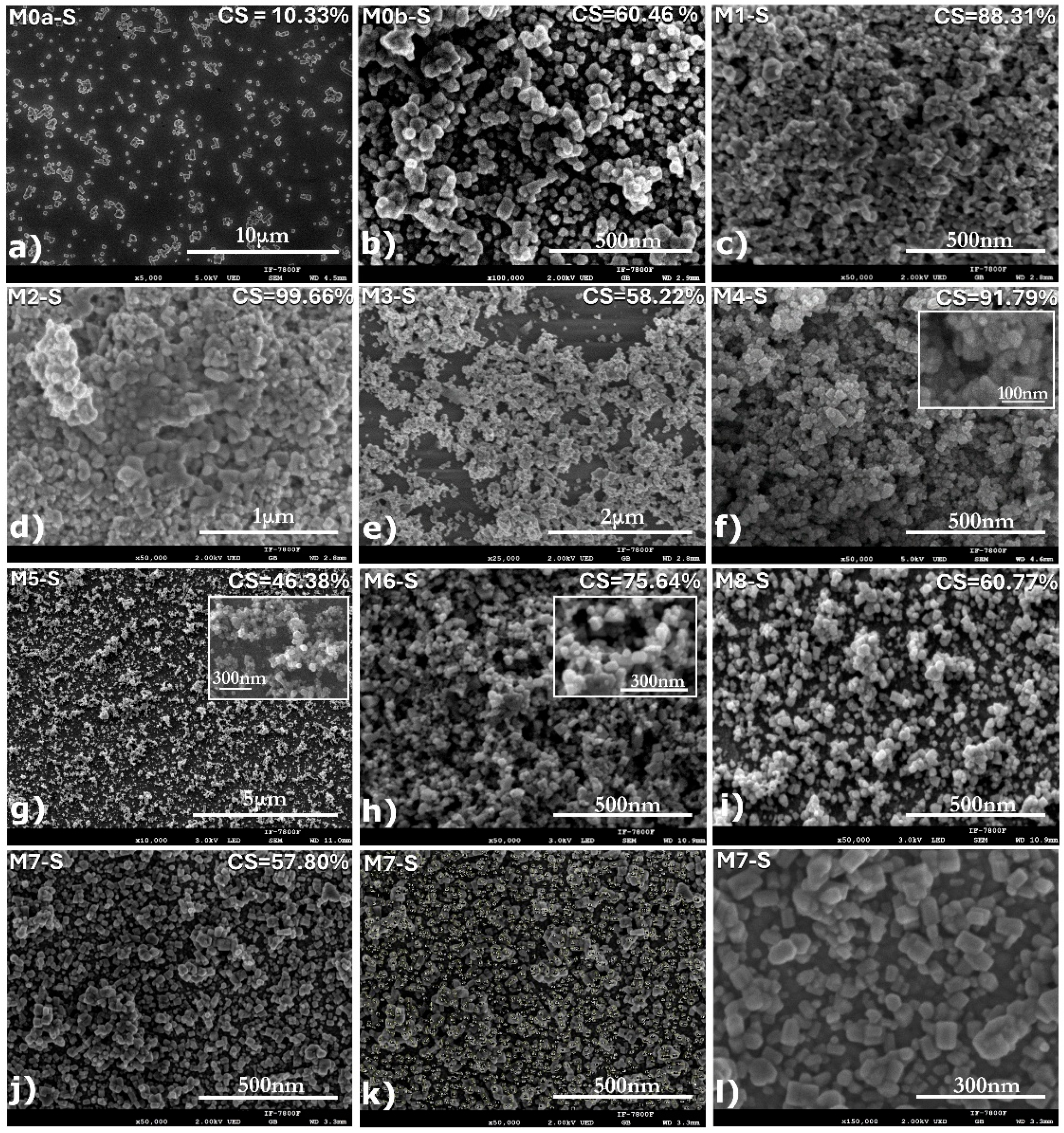
Figure 5.
SEM micrographs of the films obtained using CBD-HVEF-B (
a–
i); each image is labeled to its corresponding sample in the upper right corner. The film synthesis parameters are shown in
Table 1. The percentage of the substrate area covered by submicron ZnO particles is indicated in the upper right corner of each micrograph. Additional micrographs for sample M7-S ((
j–
l), also labeled in the upper left corner) show how size analysis was performed using Image J software as well as the shape of the ZnO nanocrystals with hexagonal-prismatic morphology.
Figure 5.
SEM micrographs of the films obtained using CBD-HVEF-B (
a–
i); each image is labeled to its corresponding sample in the upper right corner. The film synthesis parameters are shown in
Table 1. The percentage of the substrate area covered by submicron ZnO particles is indicated in the upper right corner of each micrograph. Additional micrographs for sample M7-S ((
j–
l), also labeled in the upper left corner) show how size analysis was performed using Image J software as well as the shape of the ZnO nanocrystals with hexagonal-prismatic morphology.
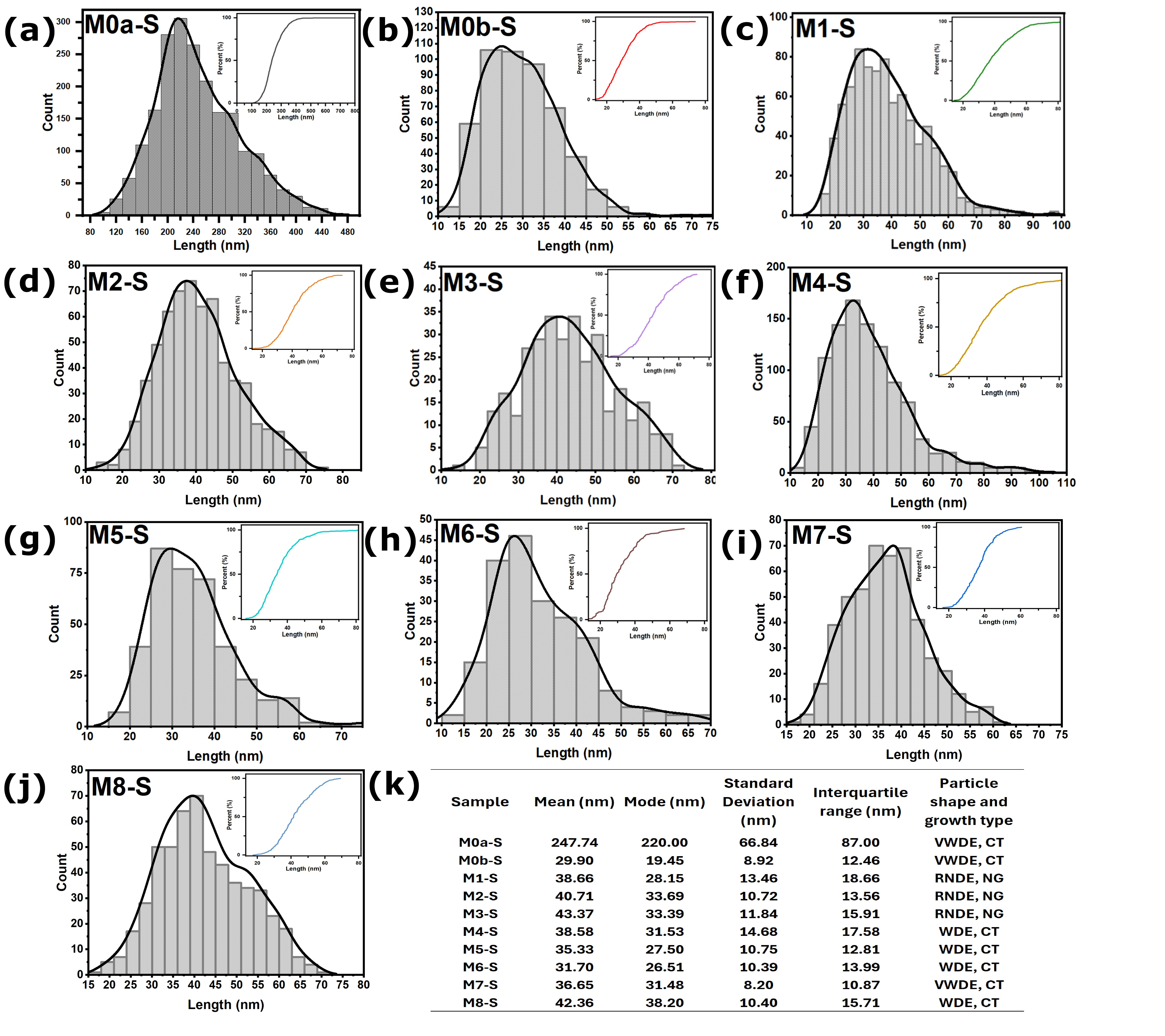
Figure 6.
Particle size histograms of the films obtained with CBD-HVEF-B ((a–j), each histogram is labeled to its corresponding sample on the upper left corner). Its corresponding cumulative percentage curve is inserted in the upper right corner. The table (k) shows the descriptive statistics for the samples and the characteristic shape of their submicron ZnO particles—Very-Well-Defined Edges (VWDE), Well-Defined Edges (WDE) and Rounded, Not-Defined Edges (RNDE)—and the type of growth: nodular (NG) or columnar type (CT).
Figure 6.
Particle size histograms of the films obtained with CBD-HVEF-B ((a–j), each histogram is labeled to its corresponding sample on the upper left corner). Its corresponding cumulative percentage curve is inserted in the upper right corner. The table (k) shows the descriptive statistics for the samples and the characteristic shape of their submicron ZnO particles—Very-Well-Defined Edges (VWDE), Well-Defined Edges (WDE) and Rounded, Not-Defined Edges (RNDE)—and the type of growth: nodular (NG) or columnar type (CT).
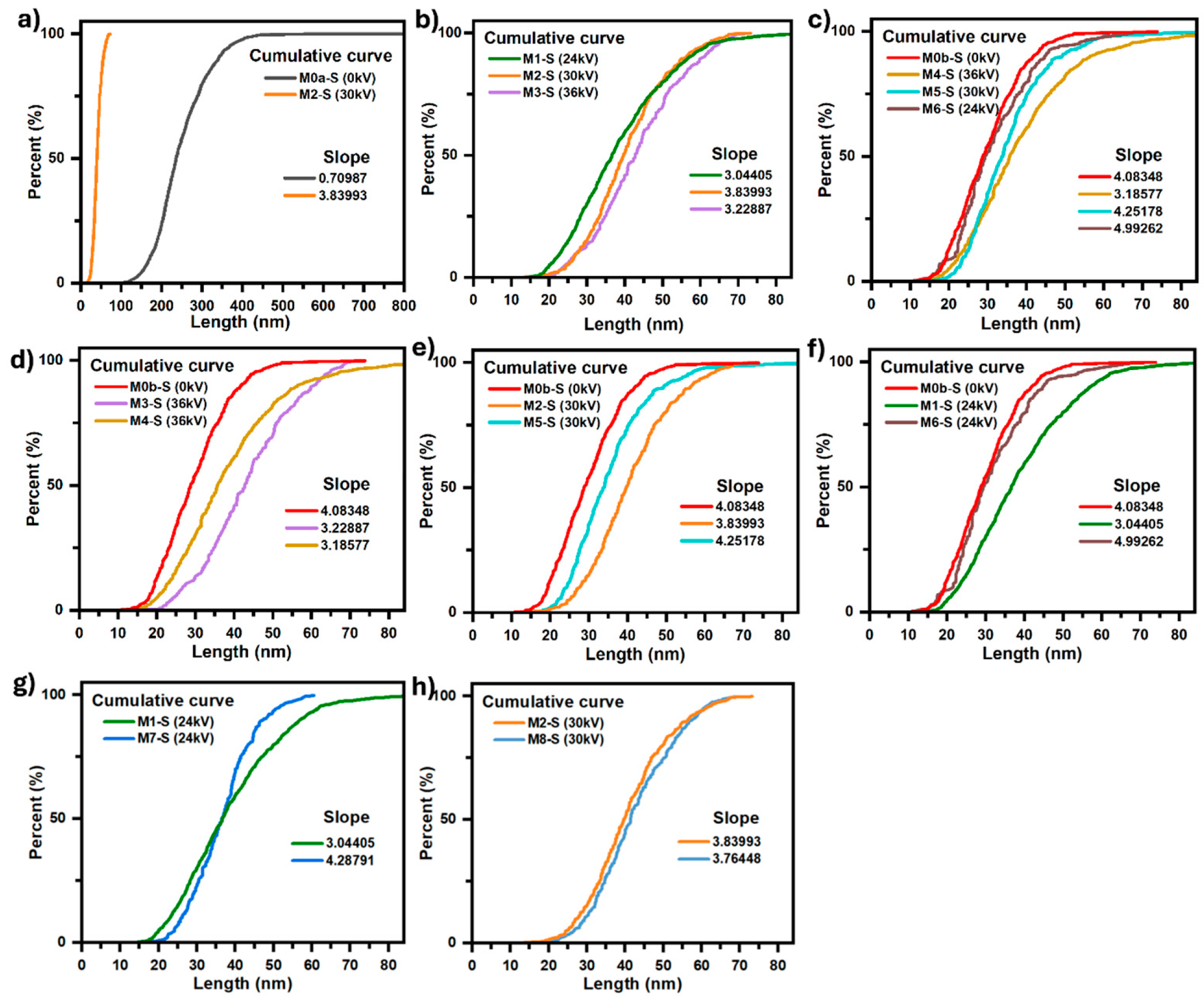
Figure 7.
Comparative analysis between the length and size dispersion of the particles that make up the samples, by comparing their corresponding cumulative curves. (a) Effect of electric field. (b) Effect of electric field intensity. (c) Effect of both: stirring with filtered air and electric field intensity. (d–f) Effect of stirring with filtered air. (g,h) Effect of pH change.
Figure 7.
Comparative analysis between the length and size dispersion of the particles that make up the samples, by comparing their corresponding cumulative curves. (a) Effect of electric field. (b) Effect of electric field intensity. (c) Effect of both: stirring with filtered air and electric field intensity. (d–f) Effect of stirring with filtered air. (g,h) Effect of pH change.
Figure 8.
Temperature curves of the precursor solution during the synthesis process. The curves were grouped according to each comparative analysis in
Figure 7: (
a) Effect of electric field. (
b) Effect of electric field intensity. (
c) Effect of both: stirring with filtered air and electric field intensity. (
d–
f) Effect of stirring with filtered air. (
g,
h) Effect of pH change.
Figure 8.
Temperature curves of the precursor solution during the synthesis process. The curves were grouped according to each comparative analysis in
Figure 7: (
a) Effect of electric field. (
b) Effect of electric field intensity. (
c) Effect of both: stirring with filtered air and electric field intensity. (
d–
f) Effect of stirring with filtered air. (
g,
h) Effect of pH change.
Figure 9.
pH curves of the precursor solution during the synthesis process. The curves were grouped according to each comparative analysis in
Figure 7: (
a) Effect of electric field. (
b) Effect of electric field intensity. (
c) Effect of both: stirring with filtered air and electric field intensity. (
d–
f) Effect of stirring with filtered air. (
g,
h) Effect of pH change.
Figure 9.
pH curves of the precursor solution during the synthesis process. The curves were grouped according to each comparative analysis in
Figure 7: (
a) Effect of electric field. (
b) Effect of electric field intensity. (
c) Effect of both: stirring with filtered air and electric field intensity. (
d–
f) Effect of stirring with filtered air. (
g,
h) Effect of pH change.
The comparison between micrographs of samples M0a-S and M2-S,
Figure 5a and
Figure 5d, respectively, clearly shows the influence of the electric field during the CBD synthesis process. It can be observed that the conventional CBD coating M0a-S covers only 10.33% of the ITO substrate surface, while the coating whose synthesis was assisted by the electric field (M2-S) covers 99.66% of the surface, and the particles that make up the coating went from being nanocrystals with hexagonal-prismatic morphology of dimensions in the order of 247 nm with very-well-defined edges in M0a-S to particles with rounded edges and practically no defined corners; the average dimension for M2-S was 40.71 nm with a mode of 33.69 nm, thus reducing its size by six times (see table inserted in
Figure 6). This reduction in particle size is even better appreciated by comparing the cumulative size curves for both coatings, see
Figure 7a, as the electric field can enhance the nucleation rate; a higher number of nuclei implies smaller average particle size and consequently that particle size distribution shifted towards smaller diameters. A comparative analysis of the micrographs corresponding to the M0a-S and M0b-S coatings (
Figure 5a and
Figure 5b, respectively), both synthesized in the absence of an electric field, reveals that stirring the precursor solution with filtered air injection markedly decreases the particle size. This effect, analogous to that induced by an electric field, results in a greater size reduction (up to twelvefold), yielding an average of 29.90 nm and a mode of 19.45 nm (see table in
Figure 6). The coatings maintain a hexagonal-prismatic morphology with well-defined edges and predominantly perpendicular orientation to the substrate, although ZnO coverage decreases to approximately 60.46%. In summary, applying an electric field or stirring the solution through the injection of filtered air led to a higher growth rate of ZnO and a reduction in the size of the particles that make up the coating, in addition to the fact that the electric field promoted the aggregation of the nanoparticles, which resulted in a much more compact coating.
Micrographs associated with coatings M1-S, M2-S, and M3-S (
Figure 5c–e) were compared to study the effect of the electric field intensity on the coating morphology, this for electric fields produced by potential differences between electrodes of 24, 30, and 36 kV, respectively. It was observed that the morphology of the coatings was similar, independent of the field intensity, observing a nodular-type growth followed by a coalescence process between particles. The degree of aggregation of the particles, as well as the percentage of the covered substrate area, were greater for 30 kV, covering up to 99.66% of the substrate surface, while for 24 and 36 kV it was 88.31 and 58.22%, resulting in a coating with greater compactness for 30 kV. When comparing the cumulative particle size curves,
Figure 7b, it is observed that the particle size grows slightly by 16.9% when the potential difference goes from 24 to 36 kV, as well as a variation in the dispersion of their sizes; the latter is noticeable by the relative change in the slope of the curves (see values in the same figure), with the dispersion being lower for 30 kV where the slope of the curve is greater, which is consistent with its interquartile range of 13.56 nm (see table in
Figure 7), while for the coatings obtained at 24 and 36 kV, the interquartile range was 18.66 and 15.91 nm, respectively.
To study the effect that the intensity of the electric field in combination with the injection of filtered air has on the coating morphology, micrographs for M4-S, M5-S, and M6-S were compared for 36, 30, and 24 kV, respectively (see
Figure 5). It is observed that the coating that now presents greater compactness is the one obtained at 36 kV and not at 30 kV, as it was when the solution was not stirred with filtered air, and that the substrate coverage reached up to 91.79% for 36 kV, while for 30 and 24 kV it was 46.36 and 75.64%, respectively.
Figure 7c presents the cumulative size curves for the three coatings, as well as the curve for the one in which only air injection was applied (M0b-S); as can be seen, applying an electric field in addition to air injection has the effect of increasing the average particle size in such a way that when its intensity is increased, this effect is even greater, going from an average size of 26.5 nm for a field produced by 24 kV to 31.5 nm for that produced by 36 kV. The same also occurs with the size dispersion, as can be seen through the decrease in the slope of the curve as the field intensity increases (values are in the same figure), going from 4.99 to 3.19 for 24 and 36 kV, respectively.
To further highlight the effect that applying an electric field and air injection have together on the average particle size that constitute the coating and the dispersion of their sizes,
Figure 7e presents the cumulative size curves for the M0b-S, M2-S, and M5-S coatings, the synthesis of which was assisted by air injection for M0b-S, an electric field produced by a voltage of 30 kV between electrodes for M2-S, and an electric field of 30 kV in addition to air injection for M5-S. These cumulative curves show how the effects of the electric field and the air injection on the size of the particles that make up the M5-S coating overlap, giving rise to a film made up of nanoparticles with an average size of 35.33 nm and a mode of 27.50 nm, which are intermediate values between the average sizes and modes values for when only air injection is applied, (M0b-S) 29.90 nm and 19.45 nm, and when only electric field is applied, (M2-S) 40.71 nm and 33.79 nm. Likewise,
Figure 7d–f show how the effect of the electric field on the size of the particles is shielded by the effect that air injection has on them. It is observed that as the intensity of the electric field is reduced, the size of the particles becomes increasingly similar to that obtained by only injecting filtered air into the precursor solution. Now, regarding the morphology of the coating, it is observed that applying an electric field, in addition to air injection, does not affect the columnar-type growth that ZnO presents with only air injection; it is even also possible to observe ZnO nanocrystals with hexagonal faces parallel to the substrate surface in the M5-S coating (30 kV), for which the coverage of the substrate surface was lower, around 46.38%. In the M6-S (24 kV) and M4-S (36 kV) coatings, the amount of material deposited on the substrate is such that the growth of ZnO nanocrystals with hexagonal faces parallel to the substrate surface cannot be seen.
In order to determine the effect of the pH of the precursor solution on the coating morphology and particle sizes that comprise it, a comparison was made between micrographs of M1-S vs. M7-S and micrographs of M2-S vs. M8-S that were synthesized only under the influence of an electric field, and with the same synthesis conditions between them, except for the pH of the solution, which between the coatings M1-S and M7-S, synthesized at 24 kV, had a pH difference of up to 2.5 (
Figure 7g), while between M2-S and M8-S, synthesized at 30 kV, the pH difference was never greater than 1 (
Figure 7h). In both comparisons it is observed that when the pH decreases the coalescence between the particles that make up the coating decreases notably to such an extent that it is practically imperceptible, in addition to the fact that the average size of the particles that make up the coating practically does not change; however, the dispersion of the particle sizes decreases notably when the pH decreases to such an extent that the solution goes from alkaline to acidic, which can be seen in
Figure 7g, with the significant increase in the slope of the cumulative size curve for M7-S with respect to M1-S.
3.4. Absorbance
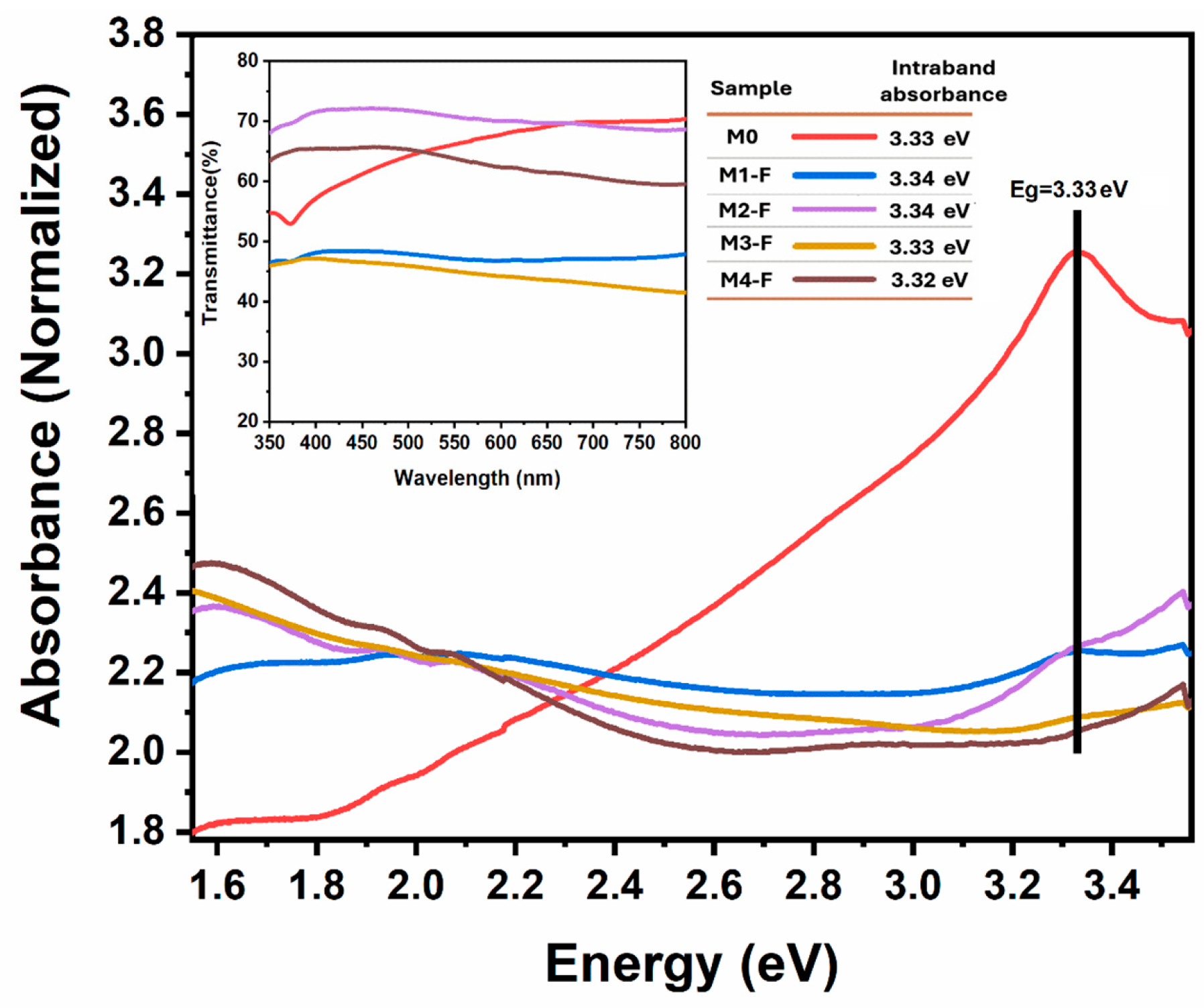
Figure 12 presents the absorbance spectra for samples synthesized by CBD-HVEF-A obtained by using a Cary 5000 UV-Vis-spectrometer (Agilent, Santa Clara, CA, USA); the values of their respective intraband absorption energies are summarized in the table inserted in the same figure. The insert of
Figure 12 shows the corresponding transmittance spectra. The intraband absorption energy values were obtained through the first and second derivatives of the absorbance curves. There is an evident blue-shift of ~5 nm in all samples as an effect of the applied electric field during synthesis, with the exception of M4-F. This blue shift of ~5 nm indicates a constant increase in the band gap of the material as a result of the electric field applied independently of its intensity or if its polarity was inverted. This is inconsistent with the diffraction angle shift to larger values observed in the XRD patterns, since a lower interplanar distance translates into a higher crystalline field, and shorter Zn–O bond in-plane distances strengthen the electrostatic crystal field interaction, enhancing Zn 3d–O 2p hybridization and causing a greater upward shift in the valence band maximum (VBM). In contrast, the Zn 4s orbitals forming the conduction band minimum (CBM) are less sensitive to bond shortening than the O 2p/Zn 3d states. As a result, the valence band rises more significantly than the conduction band shifts, leading to a reduction in the bandgap. More studies are needed to resolve this discrepancy, which should consider the non-homogeneous (100) interplanar reduction observed in XRD patterns.
Regarding the absorption of ZnO in the visible spectrum, commonly associated with the presence of intrinsic defects in its crystalline structure, it is observed that while sample M0 deposited by conventional CBD presents an absorption that begins at 550 nm and becomes more intense as it approaches the blue, none of the samples grown by CBD HVEF-A present this absorption (see
Figure 12). This absorption is commonly associated with oxygen defects and/or interstitial zinc [
25], so in principle it can be observed that deposition by CBD-HVEF-A inhibits the formation of these types of intrinsic defects. When comparing the transmittance spectra of the samples grown by CBD HVEF-A, it is clear that sample M1F is the one that in principle presents the fewest intrinsic crystalline defects, since it only presents a slight absorption centered at 2.05 eV, which is commonly associated with the presence of hydroxyl (OH) groups as an emission center, particularly present in synthesis methods involving aqueous solutions [
25], while M2-F, M3-F, and M4-F presented, in addition to this absorption, an absorption centered at 1.59 eV, commonly associated with hydrogen impurities [
26,
27,
28].
Figure 13 presents the absorbance spectra for samples synthesized by CBD-HVEF-A obtained using a Shimadzu UV-Vis spectrophotometer as well as their corresponding Tauc plots. At first glance, in the Tauc plots it can be seen that all samples appear to have the same energy gap (Eg) value of 3.10 eV; however, once you zoom in on the region between 3.00 and 3.30 eV, inserted in
Figure 13b, you can see that there is a slight but noticeable difference between the Eg values of the samples as a result of the electric field applied, independent of its intensity or if its polarity was inverted. These Eg values are listed in the table inserted in
Figure 13b.
Regarding the absorption of ZnO in the visible spectrum, it is observed that samples with absorption at 449 nm would correspond to an intraband level situated 2.76 eV above the valence band, and has been widely associated by several authors with zinc interstitial (Zn
i) defects and their extended energy levels (ex-Zn
i) that act as shallow donors [
25,
29,
30]. Additionally, the absorption situated at 556 nm has also been previously reported by Lizarraga, et al., who determined it could be linked to Zn
i. As for the peak present at 704 nm (about 1.76 eV) it is not commonly documented in the literature as being linked to a particular intrinsic defect, although a few authors do report that luminescence in this particular energy field could be linked to oxygen interstitials (O
i) [
31], but further studies would need to be carried out to associate this absorption to a particular defect, which constitutes interesting future work. The fact that the relative intensities of the 449, 556, and 704 nm bands in the samples remain practically constant means that the relative concentrations of intrinsic Zn
i and O
i defects in all samples are practically the same. This result is atypical since the relative concentration of intrinsic defects is very sensitive to variations in the synthesis parameters. Other studies such as FTIR and Raman spectroscopy are needed to resolve the relative concentrations of intrinsic defects to elucidate this atypical result.
3.5. General Discussion
The application of a high-intensity electric field during the CBD of ZnO thin films introduces significant electrostatic and electrochemical effects that strongly influence nucleation kinetics, ion transport, and film morphology. In conventional CBD, film growth proceeds through ion-by-ion and hydroxide cluster mechanisms, where Zn2+ and OH− species either react directly on the substrate surface or form Zn(OH)2 colloids that subsequently dehydrate into ZnO. In the absence of an electric field, ion mobility is primarily governed by diffusion and convection, allowing for homogeneous nucleation in the bulk solution. The application of an external electric field, however, promotes directional ionic migration, enhancing heterogeneous nucleation and improving film compactness and uniformity.
Additionally, the electric field induces water electrolysis, generating OH− ions and shifting the precursor solution toward alkaline conditions that favor heterogeneous over homogeneous nucleation. This effect was confirmed by the reduced compactness observed in films synthesized from acidic precursors compared with those obtained under alkaline conditions. Increasing field intensity further enhanced film compactness, reaching an optimum at 30 kV, consistent with the preferential promotion of heterogeneous nucleation.
At the substrate–solution interface, the electric field compresses the electric double layer, thereby reducing the nucleation energy barrier and facilitating uniform film formation. The combined effects of field-induced ion migration and electrochemical modification of the bath yield ZnO films with improved coalescence, crystallinity, and surface coverage. In particular, the CBD-HVEF-B configuration at 30 kV produced highly compact ZnO layers with minimal particle size dispersion, confirming the beneficial role of electric field-assisted CBD in tailoring ZnO film microstructure and growth behavior.