Morphological, Compositional and Optical Properties of CuxS Films on FTO Glass
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
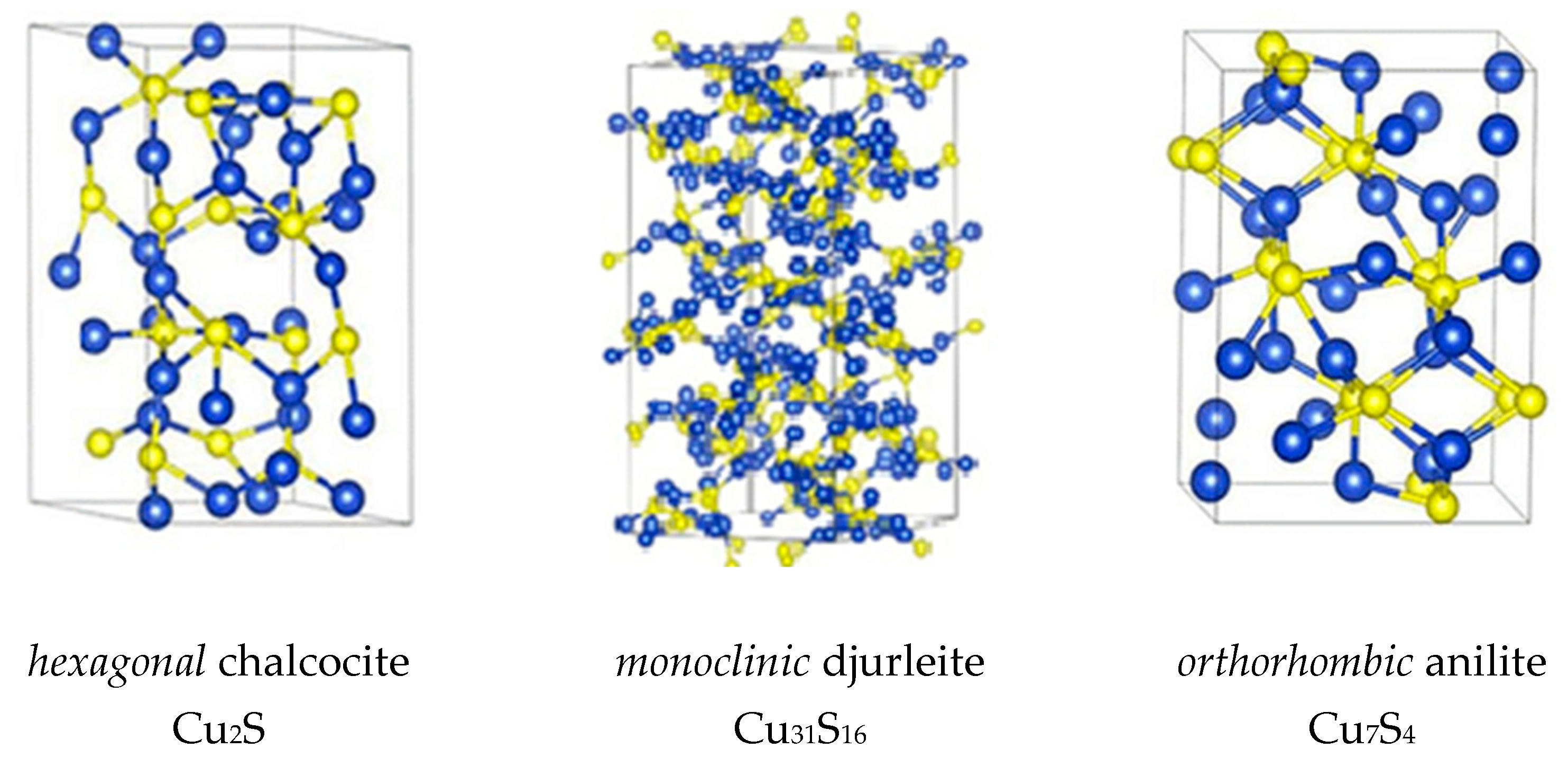
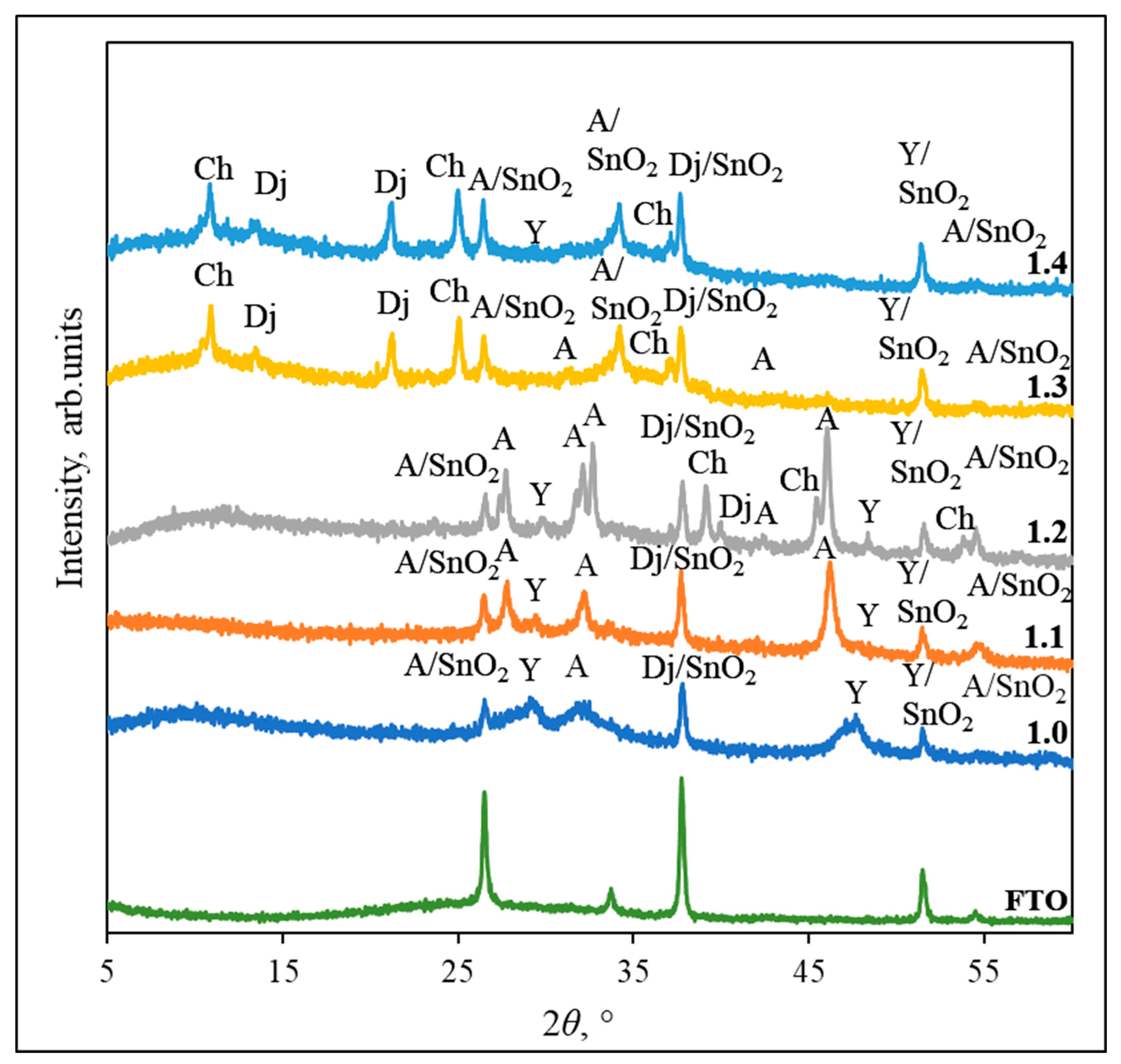
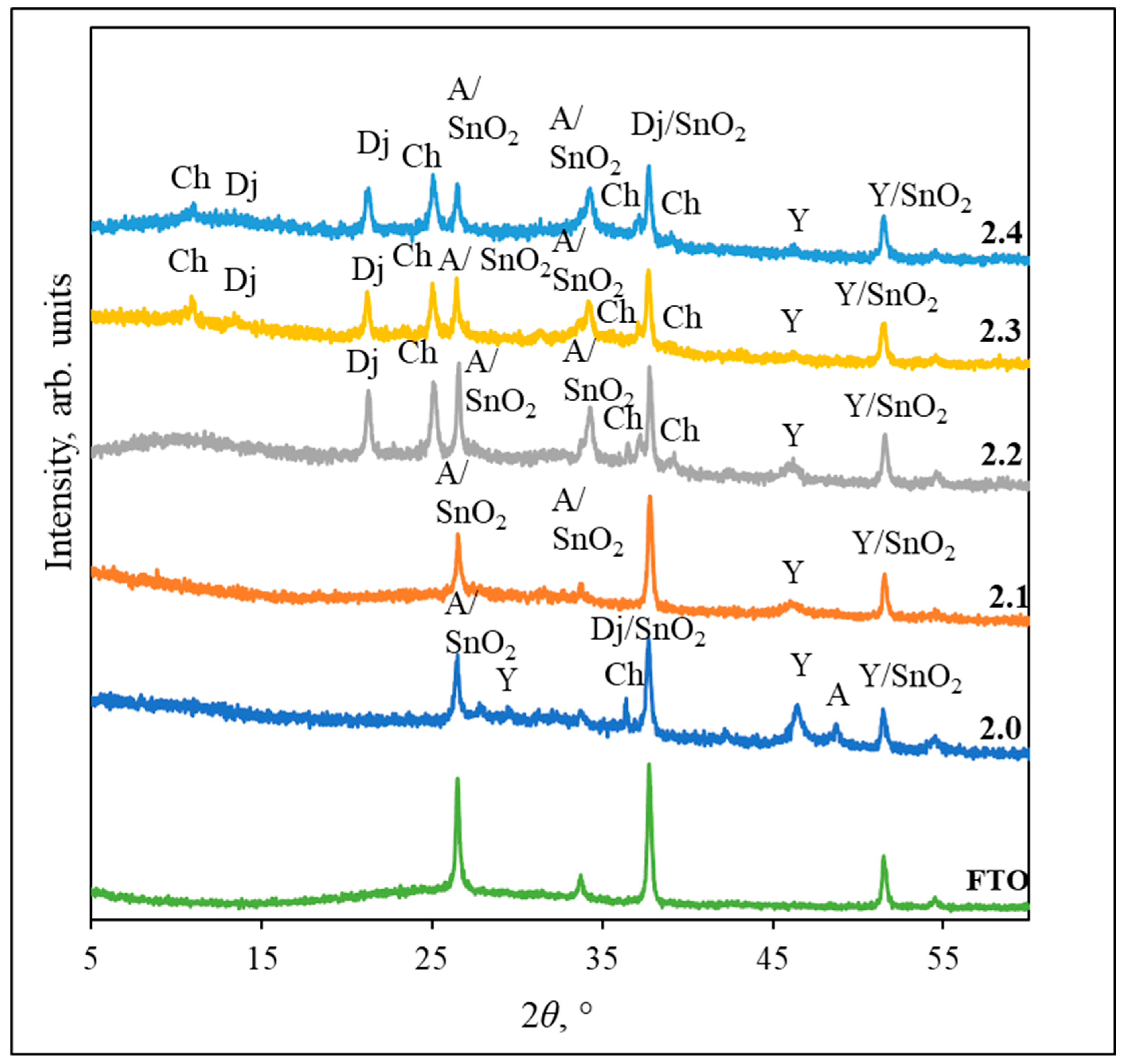
3.1. XRD Analysis
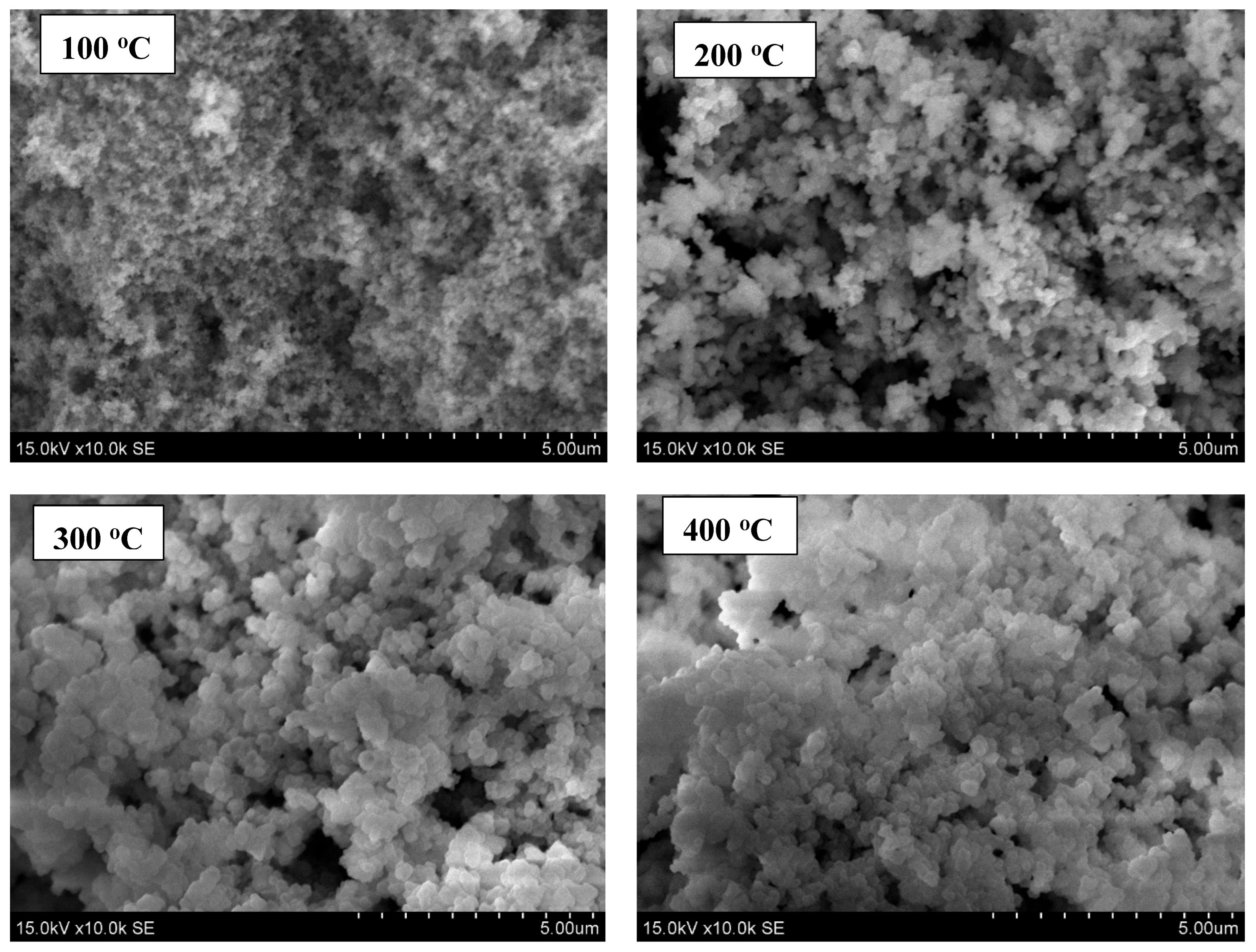
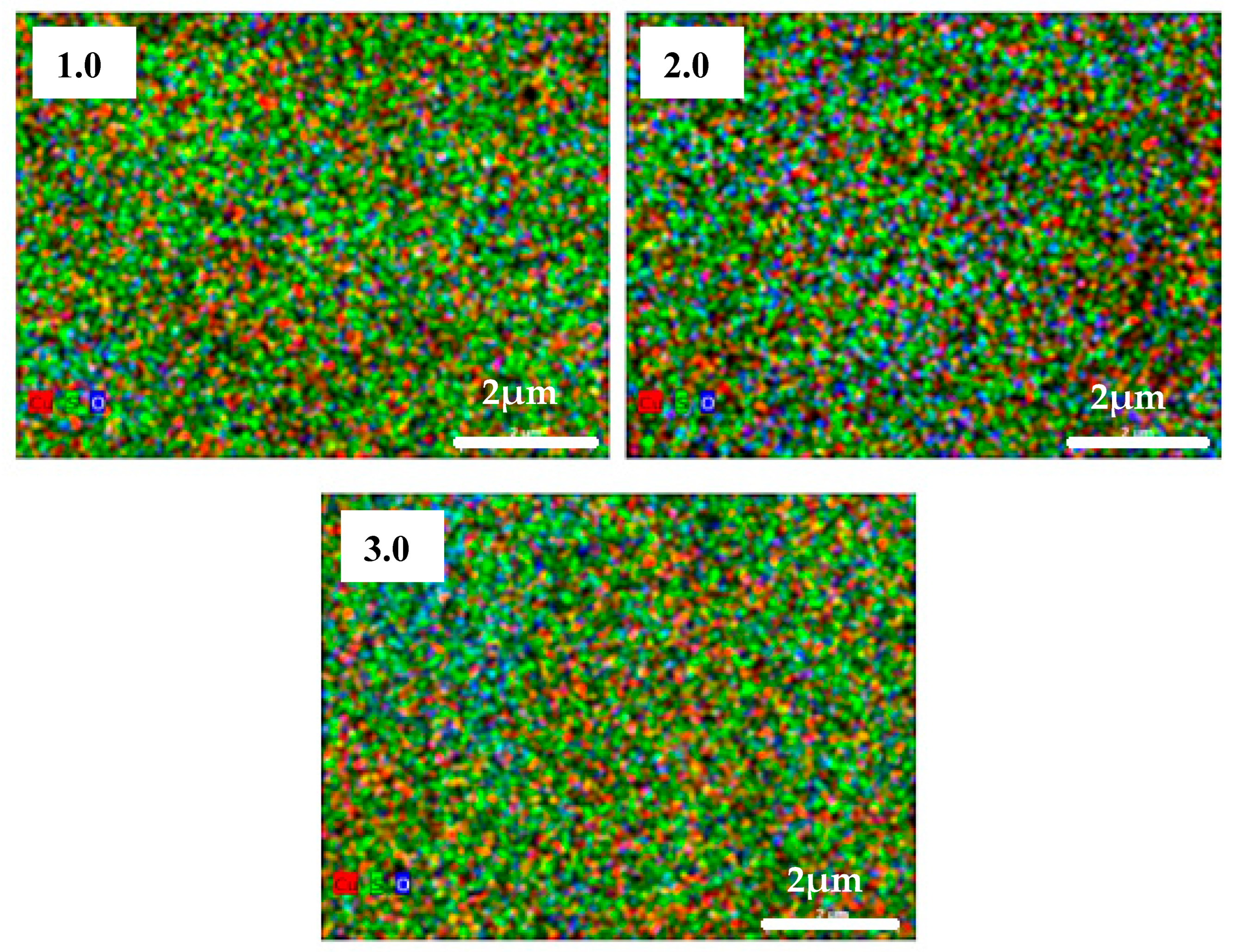
3.2. SEM and EDS Analysis
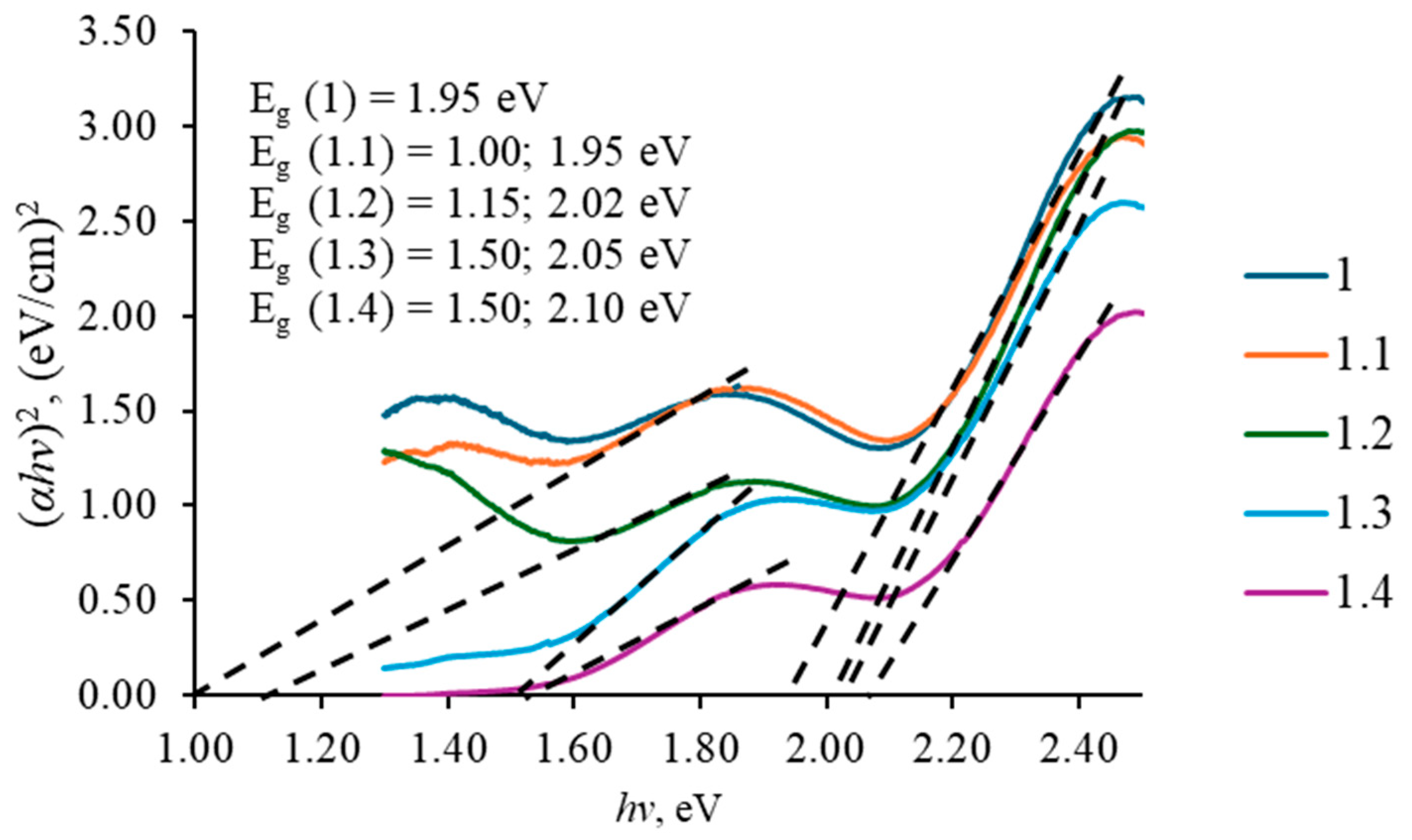
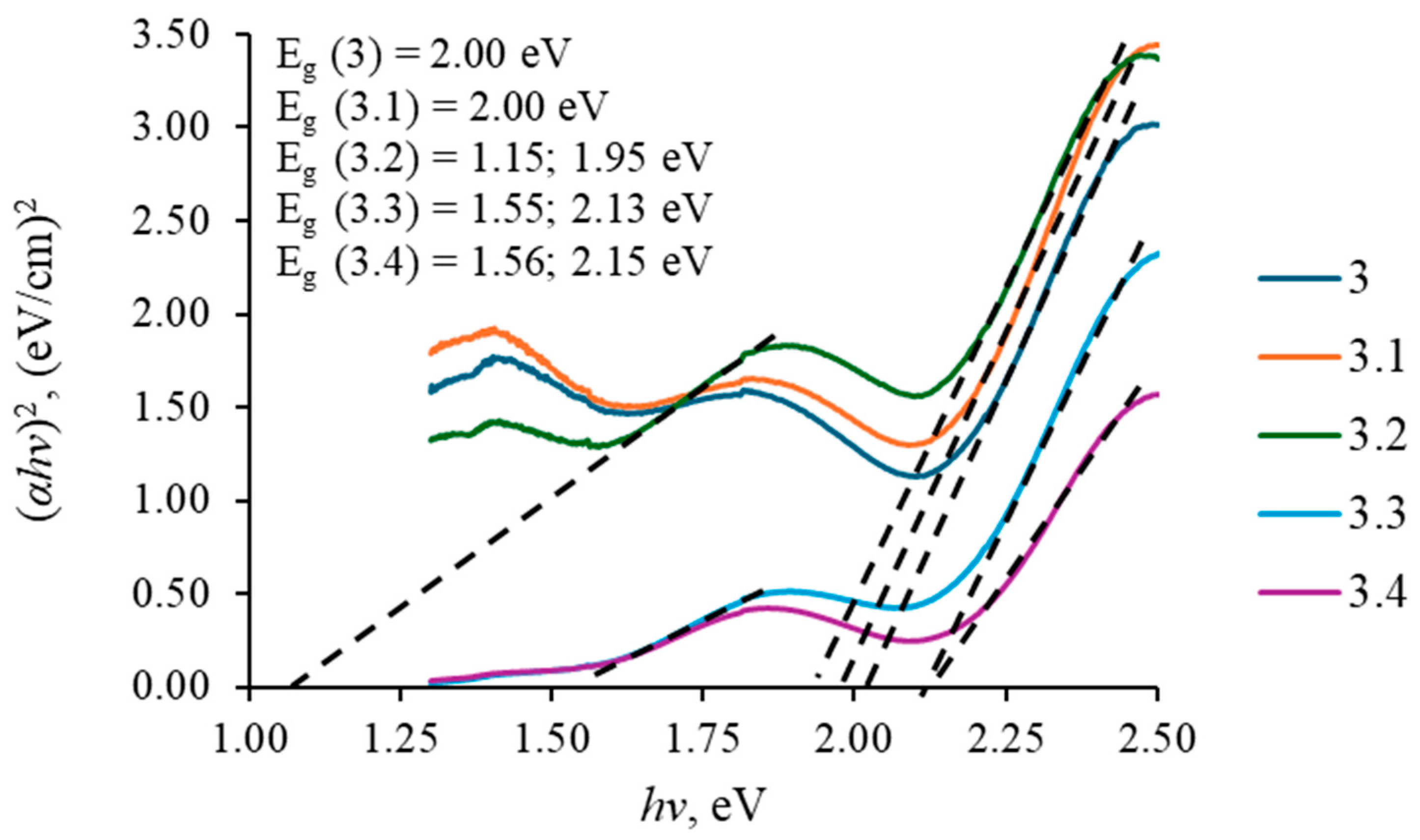
3.3. Optical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Samdhyan, K.; Chand, P.; Anand, H. Effective Doping of Phosphorus in Copper Sulfide for High Performance Energy Storage Devices. J. Alloys Compd. 2023, 936, 168322. [Google Scholar] [CrossRef]
- Chiu, Y.H.; Chung, R.J.; Yougbaré, S.; Lin, L.Y. Novel Synthesis of Copper Sulfide Plate-Assembled Hollow Cages as Electrocapacitive Material of Energy Storage Device. J. Solid State Chem. 2023, 324, 124096. [Google Scholar] [CrossRef]
- Meng, X.; Riha, S.C.; Libera, J.A.; Wu, Q.; Wang, H.H.; Martinson, A.B.F.; Elam, J.W. Tunable Core-Shell Single-Walled Carbon Nanotube-Cu2S Networked Nanocomposites as High-Performance Cathodes for Lithium-Ion Batteries. J. Power Sources 2015, 280, 621–629. [Google Scholar] [CrossRef]
- Kim, W.J.; Cho, S.; Hong, J.; Hong, J.P. Hierarchically Nanostructured 1D-2D Flowerlike Copper Sulfide Electrode for High-Performance Supercapacitor Application by One-Pot Synthetic Procedure. Appl. Surf. Sci. 2022, 578, 152086. [Google Scholar] [CrossRef]
- Geng, P.; Zheng, S.; Tang, H.; Zhu, R.; Zhang, L.; Cao, S.; Xue, H.; Pang, H. Transition Metal Sulfides Based on Graphene for Electrochemical Energy Storage. Adv. Energy Mater. 2018, 8, 1703259. [Google Scholar] [CrossRef]
- Xiong, F.; Yuan, K.; Aftab, W.; Jiang, H.; Shi, J.; Liang, Z.; Gao, S.; Zhong, R.; Wang, H.; Zou, R. Copper Sulfide Nanodisk-Doped Solid-Solid Phase Change Materials for Full Spectrum Solar-Thermal Energy Harvesting and Storage. ACS Appl. Mater. Interfaces 2021, 13, 1377–1385. [Google Scholar] [CrossRef]
- Majumdar, D. Recent Progress in Copper Sulfide Based Nanomaterials for High Energy Supercapacitor Applications. J. Electroanal. Chem. 2021, 880, 114825. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Yao, L.; Deng, L.; Bowen, C.; Zhang, Y.; Chen, S.; Lin, Z.; Peng, F.; Zhang, P. Recent Advances in Metal Sulfides: From Controlled Fabrication to Electrocatalytic, Photocatalytic and Photoelectrochemical Water Splitting and Beyond. Chem. Soc. Rev. 2019, 48, 4178–4280. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, Y.; Iqbal, A.; Li, B.; Gong, D.; Liu, W.; Iqbal, K.; Liu, W.; Qin, W. One-Step Synthesis of the 3D Flower-like Heterostructure MoS2/CuS Nanohybrid for Electrocatalytic Hydrogen Evolution. Int. J. Hydrogen Energy 2018, 43, 1251–1260. [Google Scholar] [CrossRef]
- Shi, Y.; Yang, B.; Guo, X.; Wu, X.; Pang, H. Copper Sulfides and Their Composites for High-Performance Rechargeable Batteries. Mater. Today Chem. 2022, 23, 100675. [Google Scholar] [CrossRef]
- Liu, M.; Liu, Y.; Gu, B.; Wei, X.; Xu, G.; Wang, X.; Swihart, M.T.; Yong, K.T. Recent Advances in Copper Sulphide-Based Nanoheterostructures. Chem. Soc. Rev. 2019, 48, 4950–4965. [Google Scholar] [CrossRef]
- Tezuka, K.; Sheets, W.C.; Kurihara, R.; Shan, Y.J.; Imoto, H.; Marks, T.J.; Poeppelmeier, K.R. Synthesis of Covellite (CuS) from the Elements. Solid State Sci. 2007, 9, 95–99. [Google Scholar] [CrossRef]
- Roy, P.; Srivastava, S.K. Nanostructured Copper Sulfides: Synthesis, Properties and Applications. CrystEngComm 2015, 17, 7801–7815. [Google Scholar] [CrossRef]
- Podder, J.; Kobayashi, R.; Ichimura, M. Photochemical Deposition of CuxS Thin Films from Aqueous Solutions. Thin. Solid. Films 2005, 472, 71–75. [Google Scholar] [CrossRef]
- Ratnayake, S.P.; Ren, J.; Colusso, E.; Guglielmi, M.; Martucci, A.; Della Gaspera, E. SILAR Deposition of Metal Oxide Nanostructured Films. Small 2021, 17, 2101666. [Google Scholar] [CrossRef]
- Rickard, D.T. Copper Sulphide Formation Chemistry at Low Temperatures; Springer: Berlin/Heidelberg, Germany, 1972. [Google Scholar]
- Wei, S.-H.; Xu, Q.; Huang, B.; Zhao, Y.; Yan, Y.; Noufi, R. Stability and electronic structures of CuxS solar cell absorbers. In Proceedings of the 2012 38th IEEE Photovoltaic Specialists Conference, Austin, TX, USA, 3–8 June 2012. [Google Scholar]
- Chen, M.; Li, K.; Luo, Y.; Shi, J.; Weng, C.; Gao, L.; Duan, G. Improved SERS Activity of Non-Stoichiometric Copper Sulfide Nanostructures Related to Charge-Transfer Resonance. Phys. Chem. Chem. Phys. 2020, 22, 5145–5153. [Google Scholar] [CrossRef] [PubMed]
- Dunn, J.G.; Ginting, A.R.; O’connor, B. A Thermoanalytical Study of the Oxidation of Chalcocite. J. Therm. Anal. Calorim. 1994, 41, 671–686. [Google Scholar] [CrossRef]
- Rawat, P.; Kala, S. Effect of Annealing on Structure and Morphology of Copper Sulfide Nanoparticles Prepared by Green Methodology. Mater. Today Proc. 2022, 67, 719–725. [Google Scholar] [CrossRef]
- Xu, Q.; Huang, B.; Zhao, Y.; Yan, Y.; Noufi, R.; Wei, S.H. Crystal and Electronic Structures of Cu XS Solar Cell Absorbers. Appl. Phys. Lett. 2012, 100, 3682503. [Google Scholar] [CrossRef]
- Zhao, Y.; Pan, H.; Lou, Y.; Qiu, X.; Zhu, J.; Burda, C. Plasmonic Cu 2-XS Nanocrystals: Optical and Structural Properties of Copper-Deficient Copper(I) Sulfides. J. Am. Chem. Soc. 2009, 131, 4253–4261. [Google Scholar] [CrossRef]
- Senthilkumar, M.; Mary, C.I.; Manobalaji, G.; Babu, S.M. Ligand Assisted Tunability of Morphological and Optical Properties of Copper Sulfide Nanocrystals. Mater. Sci. Semicond. Process 2019, 104, 104685. [Google Scholar] [CrossRef]
- Vikraman, D.; Thiagarajan, S.; Karuppasamy, K.; Sanmugam, A.; Choi, J.H.; Prasanna, K.; Maiyalagan, T.; Thaiyan, M.; Kim, H.S. Shape- and Size-Tunable Synthesis of Tin Sulfide Thin Films for Energy Applications by Electrodeposition. Appl. Surf. Sci. 2019, 479, 167–176. [Google Scholar] [CrossRef]
- Bronusiene, A.; Popov, A.; Barauskiene, I.; Ancutiene, I. Effect of Ascorbic Acid on the Properties of Tin Sulfide Films for Supercapacitor Application. Surf. Interfaces 2021, 25, 101275. [Google Scholar] [CrossRef]
- Bronusiene, A.; Kleinauskas, R.; Ancutiene, I. Facile Synthesis and Characterization of TiO2/SnS Nanocomposites by Eco-Friendly Methods. Coatings 2024, 14, 88. [Google Scholar] [CrossRef]




| Cationic and Anionic Precursors | Sample No | ||||
|---|---|---|---|---|---|
| Annealing Temperature, °C | |||||
| Unannealed | 100 °C | 200 °C | 300 °C | 400 °C | |
| 0.2 M CuSO4 solution with 0.2 M hydroquinone and 0.2 M Na2S solution | 1.0 | 1.1 | 1.2 | 1.3 | 1.4 |
| 0.2 M CuSO4 solution with 0.2 M hydroquinone and 0.1 M Na2S solution | 2.0 | 2.1 | 2.2 | 2.3 | 2.4 |
| 0.2 M CuSO4 solution with 0.1 M hydroquinone and 0.2 M Na2S solution | 3.0 | 3.1 | 3.2 | 3.3 | 3.4 |
| Sample No. | Cu (at. %) | S (at. %) | O (at. %) | Na (at. %) | x in CuxS |
|---|---|---|---|---|---|
| 1.0 | 53.6 ± 2.7 | 38.0 ± 1.0 | 7.7 ± 0.6 | - | 1.4 |
| 2.0 | 61.0 ± 3.0 | 27.9 ± 0.8 | 9.6 ± 0.7 | - | 2.2 |
| 3.0 | 49.4 ± 2.7 | 32.8 ± 1.0 | 10.3 ± 0.8 | 6.3 ± 0.8 | 1.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zubrickaite, D.; Bronusiene, A.; Ancutiene, I. Morphological, Compositional and Optical Properties of CuxS Films on FTO Glass. Coatings 2025, 15, 1221. https://doi.org/10.3390/coatings15101221
Zubrickaite D, Bronusiene A, Ancutiene I. Morphological, Compositional and Optical Properties of CuxS Films on FTO Glass. Coatings. 2025; 15(10):1221. https://doi.org/10.3390/coatings15101221
Chicago/Turabian StyleZubrickaite, Dominyka, Asta Bronusiene, and Ingrida Ancutiene. 2025. "Morphological, Compositional and Optical Properties of CuxS Films on FTO Glass" Coatings 15, no. 10: 1221. https://doi.org/10.3390/coatings15101221
APA StyleZubrickaite, D., Bronusiene, A., & Ancutiene, I. (2025). Morphological, Compositional and Optical Properties of CuxS Films on FTO Glass. Coatings, 15(10), 1221. https://doi.org/10.3390/coatings15101221





