Preparation and Corrosion Resistance of Hydrothermal Coatings on LZ91 Mg–Li Alloy
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
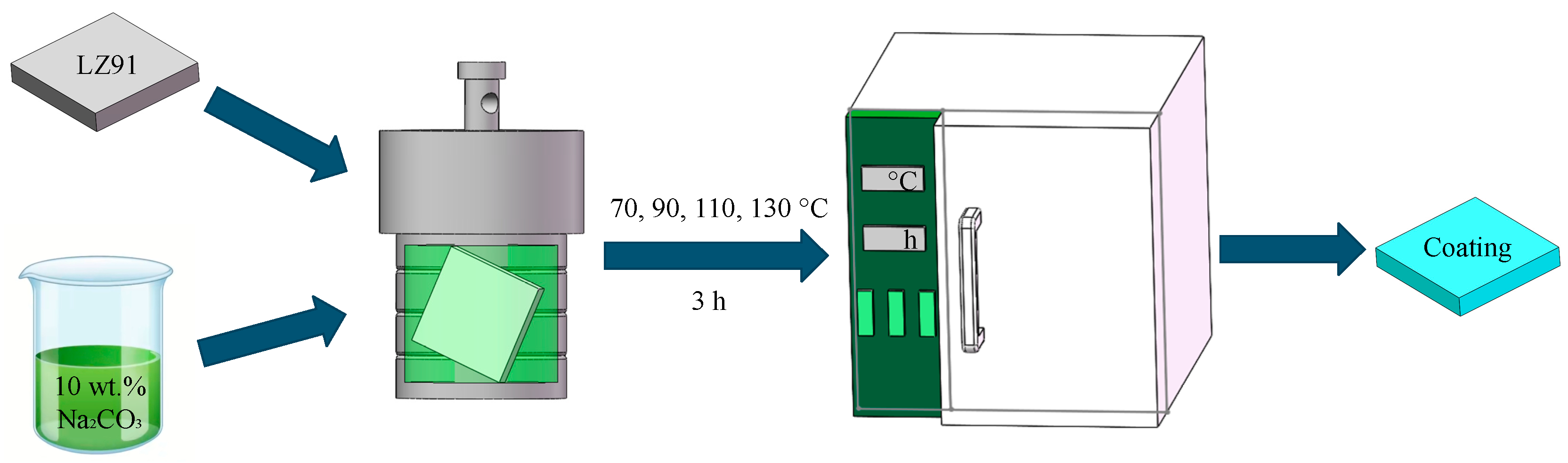
2.2. Hydrothermal Treatment
2.3. Characterization and Testing
3. Results and Discussion
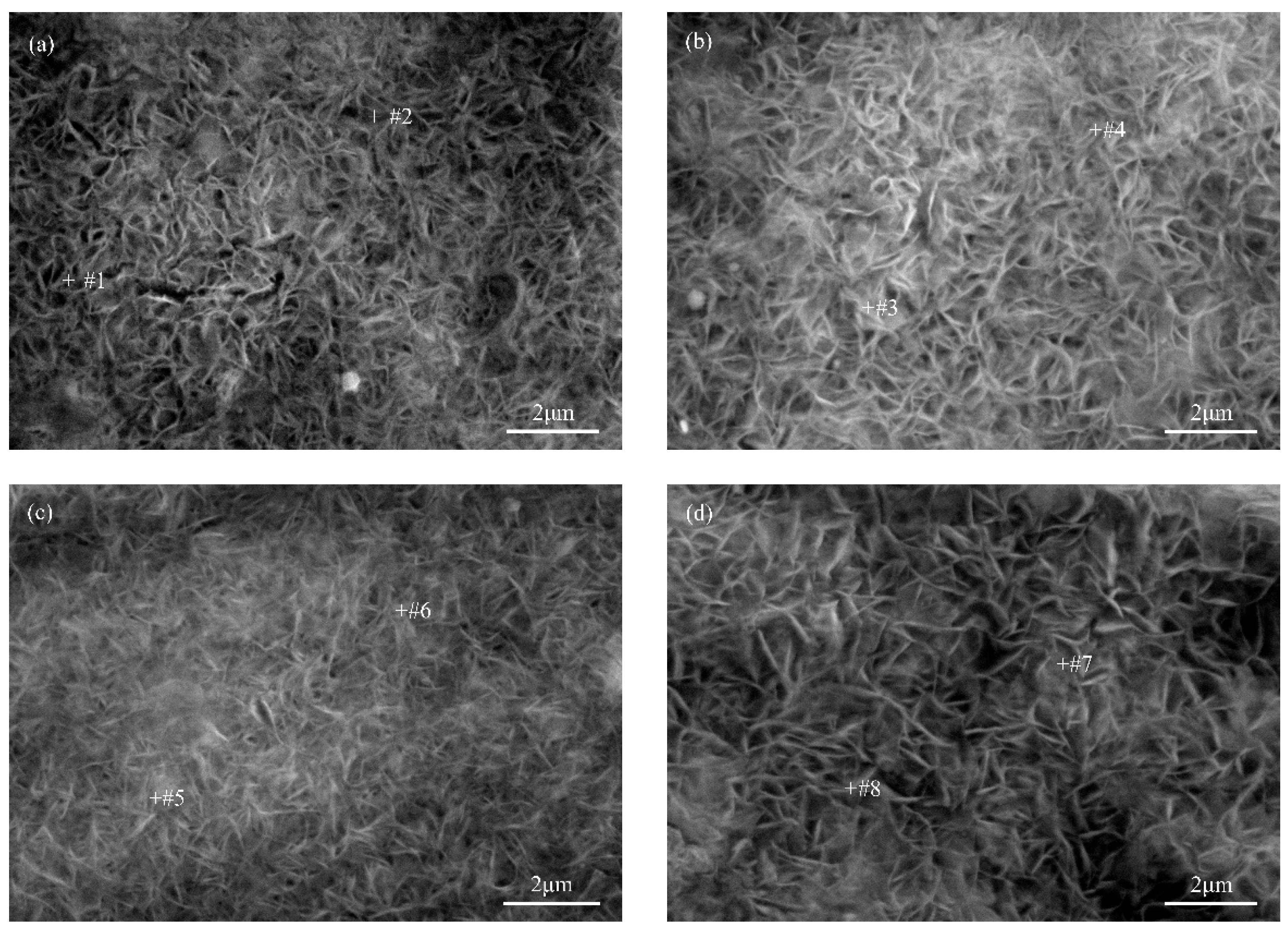
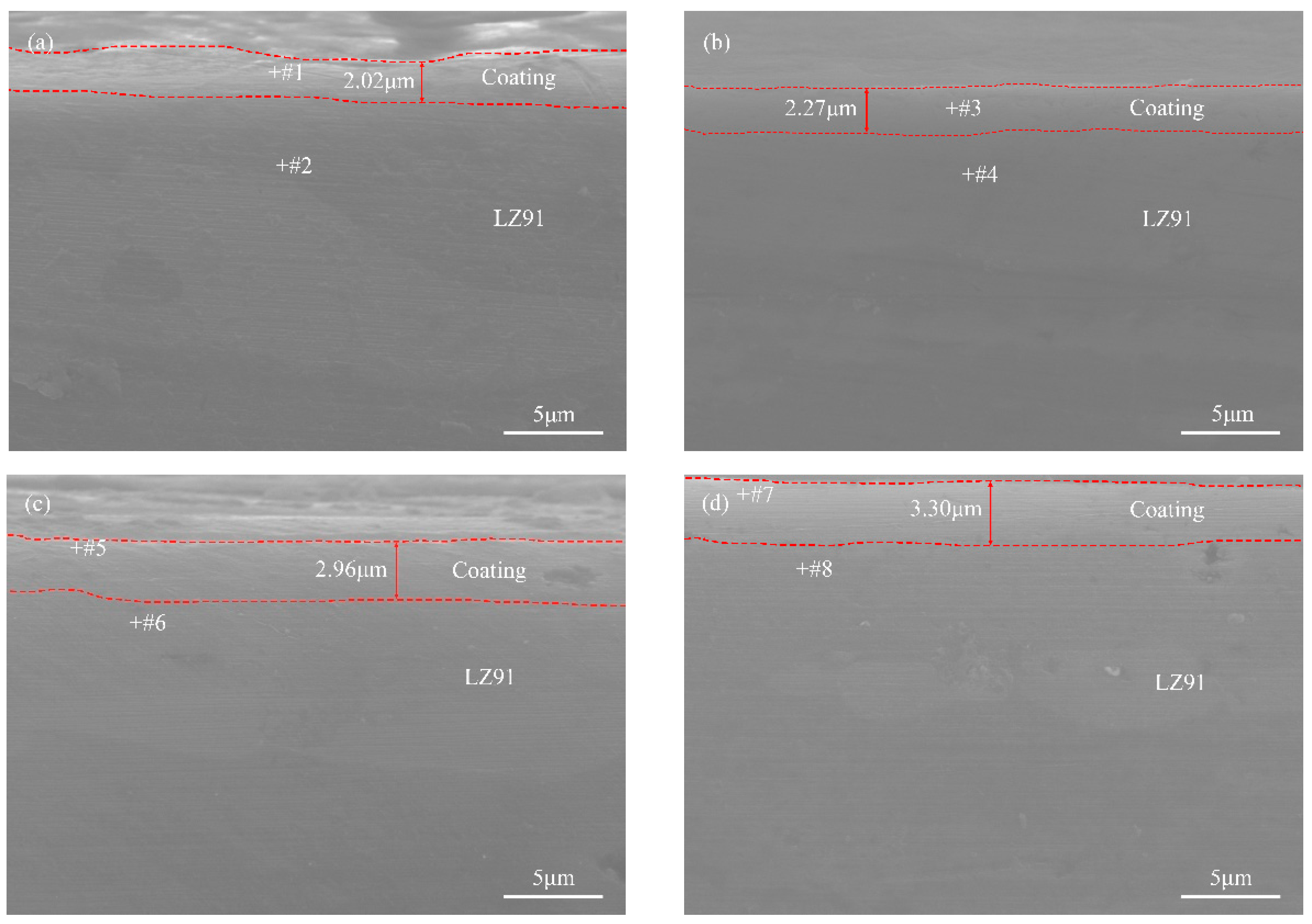
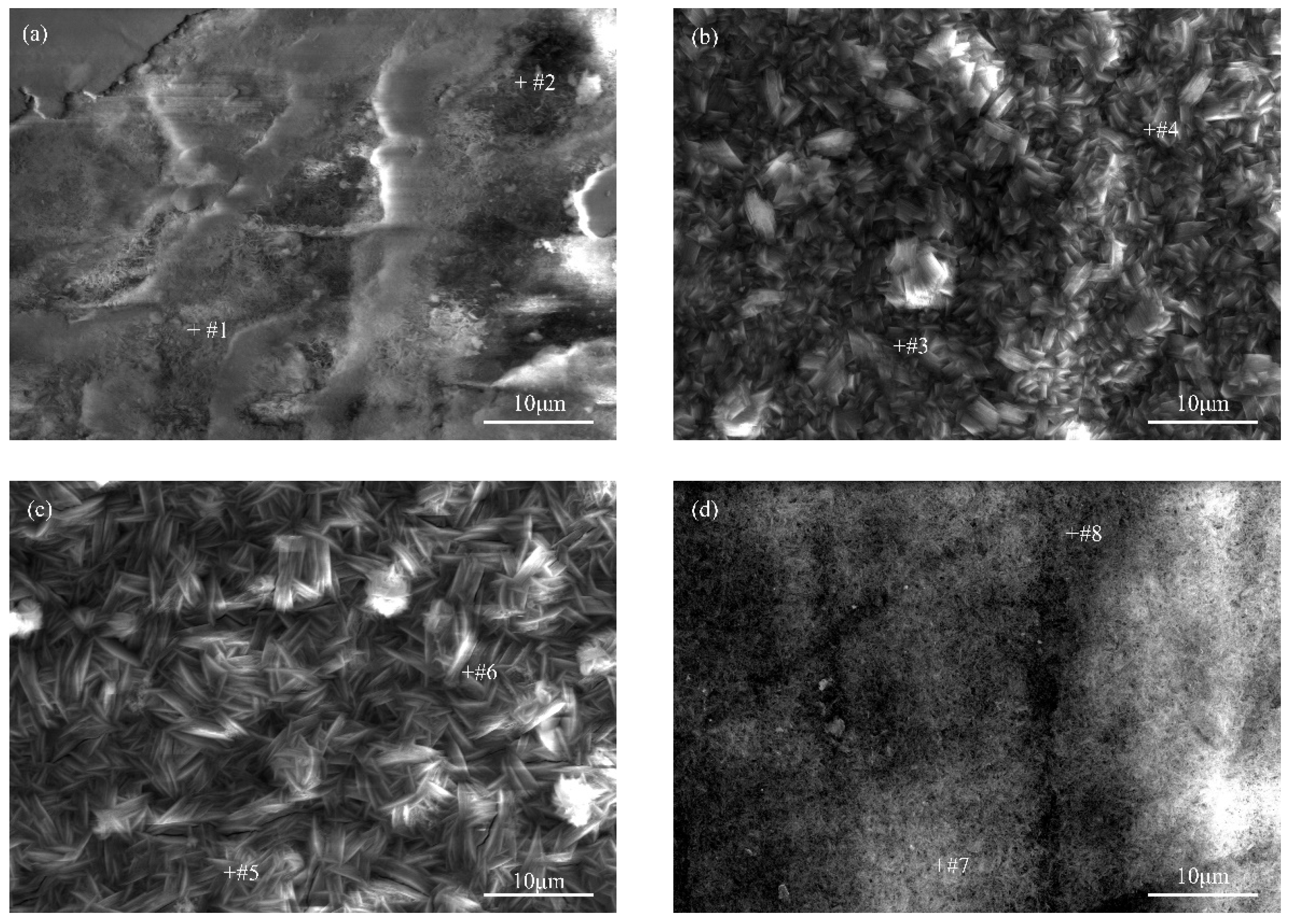
3.1. Coating Morphology Characterization
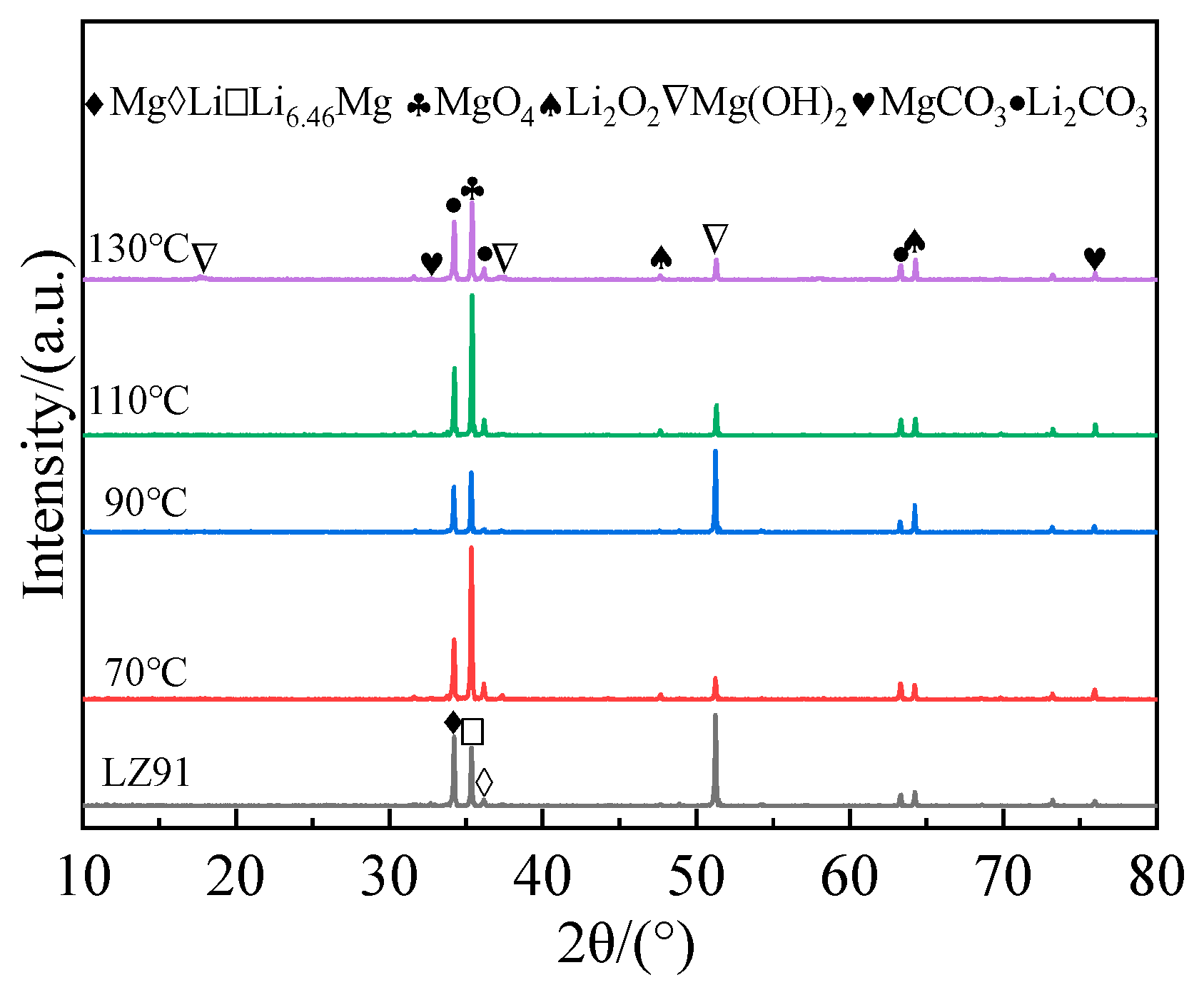
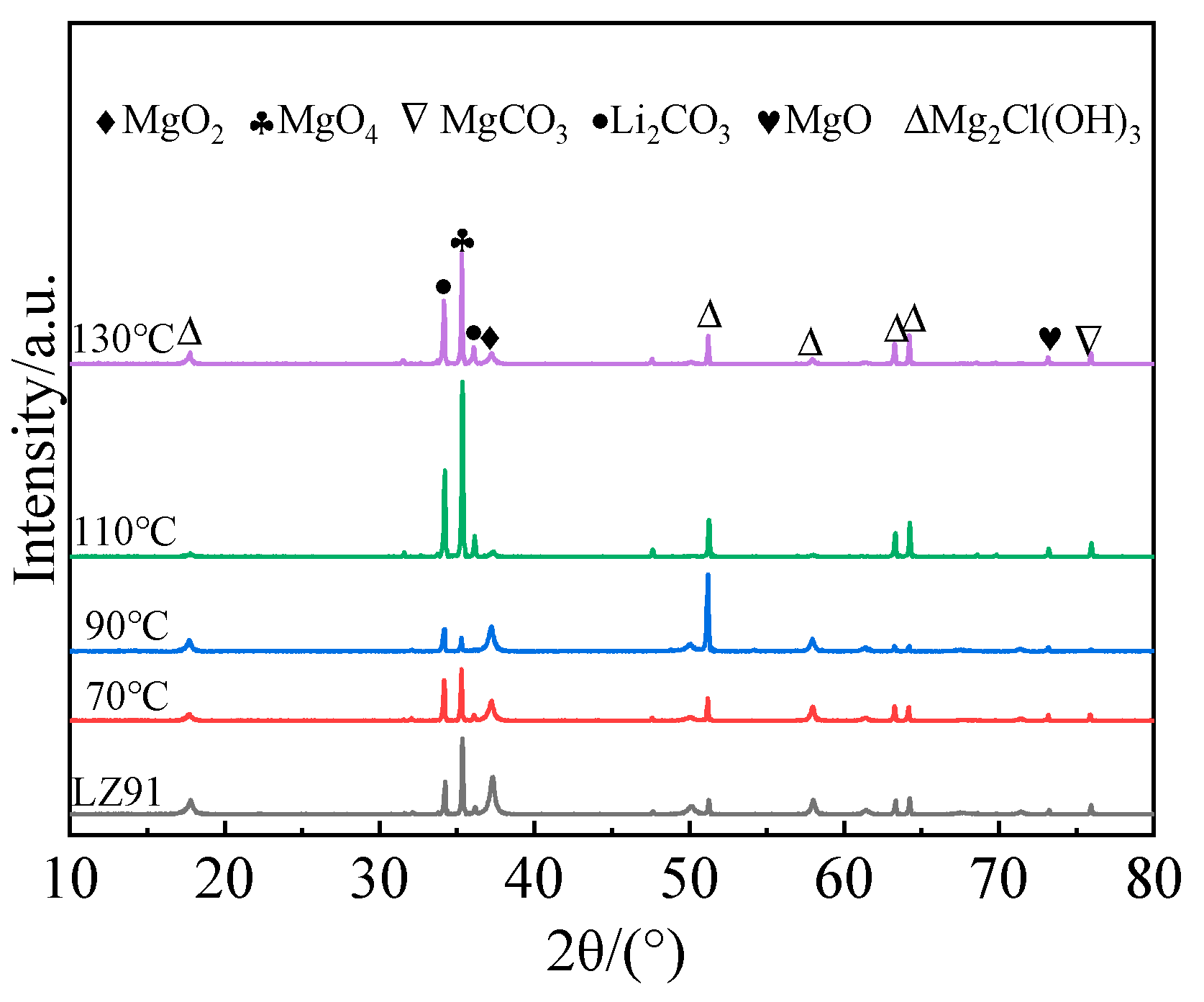
3.2. Phase Composition
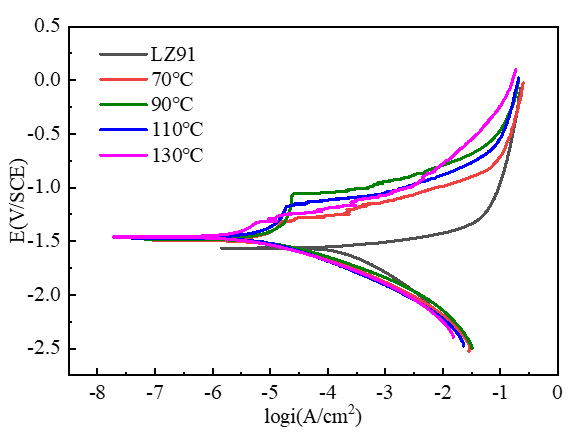
3.3. Corrosion Resistance of the Coating
3.3.1. EIS
3.3.2. Potentiodynamic Polarization Analysis
3.4. Formation and Degradation Mechanisms of Coatings
3.4.1. Coating Formation Mechanism
3.4.2. Coating Degradation Mechanism
4. Conclusions
- (1)
- A corrosion-resistant coating composed primarily of Mg(OH)2, MgCO3, and Li2CO3 was successfully prepared on an LZ91 Mg–Li alloy surface via a one-step hydrothermal method.
- (2)
- The hydrothermal temperature heavily influenced the surface morphology of the coating. As the reaction temperature increased, the coating thickness gradually increased in the following order: 70 °C < 90 °C < 110 °C < 130 °C.
- (3)
- The coatings markedly improved the corrosion resistance of the Mg–Li alloy substrate. The corrosion resistance of the coatings prepared at different hydrothermal temperatures decreased in the following order: 130 °C > 110 °C > 90 °C > 70 °C > bare LZ91.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, Y.; Qi, F.; Sun, J.; Xu, Y.; Guo, Y.; Yu, J.; Yi, L.; Tao, X.; Wu, G.; Liu, W. Strengthening dual-phase Mg-Li alloy through precipitation control. Mater. Res. Lett. 2025, 13, 17–23. [Google Scholar] [CrossRef]
- Yang, Y.; Xiong, X.; Chen, J.; Peng, X.; Chen, D.; Pan, F. Research advances in magnesium and magnesium alloys worldwide in 2020. J. Magnes. Alloy. 2021, 9, 705–747. [Google Scholar] [CrossRef]
- Cao, F.; Zhou, B.; Xu, P.; Xu, G. Quasi-superplasticity in an Mg-Li-Al-Y alloy processed by rolling. J. Mater. Res. Technol. 2025, 34, 2698–2714. [Google Scholar] [CrossRef]
- Su, H.; Wang, J.; Li, Y.; Xue, C.; Tian, G.; Wang, S.; Yang, X.; Li, Q.; Yang, Z.; Dou, R. Effective strategies for improving the mechanical stability of aged Mg-Li alloys. Mater. Des. 2024, 244, 113180. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Q.; Lu, X.; Zhang, T.; Wang, F. Effect of Process Parameters on Corrosion Resistance of MAO/LDH Composite Coatings. Surf. Technol. 2021, 50, 327–336. [Google Scholar]
- Wang, Z.; Zhang, J.; Li, Y.; Li, J.; Guo, J. Corrosion Resistance Enhancement of Micro-Arc Oxidation Ceramic Layer by Mg-Al-Co Layered Double Hydroxide Coating. Trans. Indian Ceram. Soc. 2020, 79, 59–66. [Google Scholar] [CrossRef]
- Tian, G.; Yan, C.; Yang, Z.Y.; Wang, J. Research Progress on Corrosion and Protection of Corrosion-resistant Mg-Li Alloys. J. Chin. Soc. Corros. Prot. 2023, 43, 1255–1263. [Google Scholar]
- Zheng, D.; Ma, H.; Fu, H.; Zeng, L.; Li, C.; Liu, Q.; Peng, F.; Lv, T.; Zhu, S.; Jiang, Y. Effect of solid solution treatment on biomedical Mg-Li alloy with high Zn content. Mater. Lett. 2024, 363, 136309. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, R.; Peng, C.; Cai, Z. Microstructure and corrosion behavior of as-extruded Mg-xLi-3Al-2Zn-0.2Zr alloys (x=5, 8, 11 wt.%). Corros. Sci. 2020, 167, 108487. [Google Scholar] [CrossRef]
- Zou, Y.; Qian, X.; Liu, S.; Xie, H.; Li, Y. Simultaneously improved mechanical properties and corrosion resistance of Mg-10.02Li-5.69Al-0.08Er alloy by solutionizing treatments. J. Alloys Compd. 2024, 1002, 175188. [Google Scholar] [CrossRef]
- He, H.; Du, J.; Sang, J.; Hirahara, H.; Aisawa, S.; Chen, D. Superhydrophobic coatings by electrodeposition on Mg-Li alloys: Attempt of armor-like Ni patterns to improve the robustness. Mater. Chem. Phys. 2023, 304, 127902. [Google Scholar] [CrossRef]
- Aktug, S.; Jian, S.; Chang, C.; Liu, Y. Novel Ce thin-film covering on a Mn-Ce-P conversion coating for excellent corrosion resistance of LZ91 alloy. Surf. Coat. Technol. 2024, 484, 130784. [Google Scholar] [CrossRef]
- Sukuroglu, S.; Sukuroglu, E.E.; Totik, Y.; Gulten, G.; Efeoglu, I.; Avc, S. Corrosion and adhesion properties of MAO-coated LA91 magnesium alloy. Mater. Sci. Technol. 2024, 40, 616–632. [Google Scholar] [CrossRef]
- Tan, J.; Liu, L.; Wang, H.; Luo, J. Advances in anti-corrosion coatings on magnesium alloys and their preparation methods. J. Coat. Technol. Res. 2024, 21, 811–825. [Google Scholar] [CrossRef]
- Ling, L.; Cai, S.; Li, Q.; Sun, J.; Bao, X.; Xu, G. Recent advances in hydrothermal modification of calcium phosphorus coating on magnesium alloy. J. Magnes. Alloy. 2022, 10, 62–80. [Google Scholar] [CrossRef]
- Guo, W.; Zhang, S.; Tian, M.; Wang, H.; Wang, D.; Liang, C. Formation of Mg-Al LDHs coating by hydrothermal treatment on laser remelting pre-treated AZ31 magnesium alloy for enhanced corrosion resistance. J. Mater. Sci. 2023, 58, 17080–17092. [Google Scholar] [CrossRef]
- Wang, G.; Song, D.; Qiao, Y.; Cheng, J.; Liu, H.; Jiang, J.; Ma, A.; Ma, X. Developing super-hydrophobic and corrosion-resistant coating on magnesium-lithium alloy via one-step hydrothermal processing. J. Magnes. Alloy. 2023, 11, 1422–1439. [Google Scholar] [CrossRef]
- Zhang, J.; Li, J.; Hou, A.; Duan, X.; Lian, D.; Zhang, M.; Zhang, T. Investigating the microstructures and properties of Mg-Al LDH film-coated Mg-Li alloy: Effect of hydrothermal temperature. Int. J. Appl. Ceram. Technol. 2022, 19, 3062–3071. [Google Scholar] [CrossRef]
- Mou, F.; Wang, Z.; Zeng, H.; Ma, Y.; Guo, F.; Chai, L.; She, Z.; Zhang, L. Fabricating a corrosion-protective Li2CO3/Mg(OH)2 composite film on LA141 magnesium-lithium alloy by hydrothermal method. Surf. Coat. Technol. 2023, 472, 129939. [Google Scholar] [CrossRef]
- Zhang, X.; Li, R.; Feng, X.; Pang, X.; He, X.; Jin, Z.; Ren, H.; Wang, D.; Li, K.; Dai, X.; et al. Influence of Li+/Al3+ on the corrosion behavior of Li-Al layered double hydroxides (LDHs) film on LA51 magnesium alloys. J. Magnes. Alloy. 2023, 11, 1083–1093. [Google Scholar] [CrossRef]
- Li, Y.; Kang, Z.; Zhang, X.; Pan, J.; Ren, Y.; Zhou, G. Fabricating an anti-corrosion carbonate coating on Mg-Li alloy by low-temperature plasma. Surf. Coat. Technol. 2022, 439, 128418. [Google Scholar] [CrossRef]
- Zhou, F.; Li, Y.; Kang, Z.; Shen, J.; Ren, Y.; Zhou, G. Effect of Li Content on Corrosion Resistance of Lithium Carbonate Coating on Mg-Li Alloys. Rare Met. Mater. Eng. 2023, 52, 2978–2984. [Google Scholar]
- Wang, Y.; Xiao, W.; Blawert, C.; Serdechnova, M.; Feng, L.; Wang, J.; Pan, F. Conception of corrosion converter for magnesium alloy surface: Replacing the corrosion product layer with a protective coating in high concentration corrosion medium. Corros. Sci. 2025, 244, 112639. [Google Scholar] [CrossRef]
- Chen, J.; Liang, S.; Fu, D.; Fan, W.; Lin, W.; Ren, W.; Zou, L.; Cui, X. Design and in situ prepare a novel composite coating on Mg alloy for active anti-corrosion protection. J. Alloys Compd. 2020, 831, 154580. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, R.; Peng, C.; Wang, X. Microstructure and corrosion behavior of as-homogenized Mg-xLi-3Al-2Zn-0.2Zr alloys (x=5, 8, 11 wt%). Mater. Charact. 2020, 159, 110031. [Google Scholar] [CrossRef]
- Wang, J.; Sun, X.; Song, L.; Kannan, M.B.; Zhang, F.; Cui, L.; Zou, Y.; Li, S.; Zeng, R. Corrosion resistance of Mg-Al-LDH steam coating on AZ80 Mg alloy: Effects of citric acid pretreatment and intermetallic compounds. J. Magnes. Alloy. 2023, 11, 2967–2979. [Google Scholar] [CrossRef]
- Feng, Z.; She, X.; Peng, J.; Qiang, Y.; Zhang, S. Robust corrosion protection of Ni-thiolate coordination polymer/Mg(OH)2 coating on magnesium alloy AZ31. J. Mater. Res. Technol. 2023, 26, 2407–2418. [Google Scholar] [CrossRef]
- Li, L.; Xie, Z.; Fernandez, C.; Wu, L.; Cheng, D.; Jiang, X.; Zhong, C. Development of a thiophene derivative modified LDH coating for Mg alloy corrosion protection. Electrochim. Acta 2020, 330, 135186. [Google Scholar] [CrossRef]
- Qiu, Z.; Zeng, R.; Zhang, F.; Song, L.; Li, S. Corrosion resistance of Mg-Al LDH/Mg(OH)2/silane-Ce hybrid coating on magnesium alloy AZ31. Trans. Nonferrous Met. Soc. China 2020, 30, 2967–2979. [Google Scholar] [CrossRef]
- Shi, F.; Zhao, J.; Tabish, M.; Wang, J.; Liu, P.; Chang, J. One-step hydrothermal synthesis of 2,5-PDCA-containing MgAl-LDHs three-layer composite coating with high corrosion resistance on AZ31. J. Magnes. Alloy. 2023, 11, 2541–2557. [Google Scholar] [CrossRef]


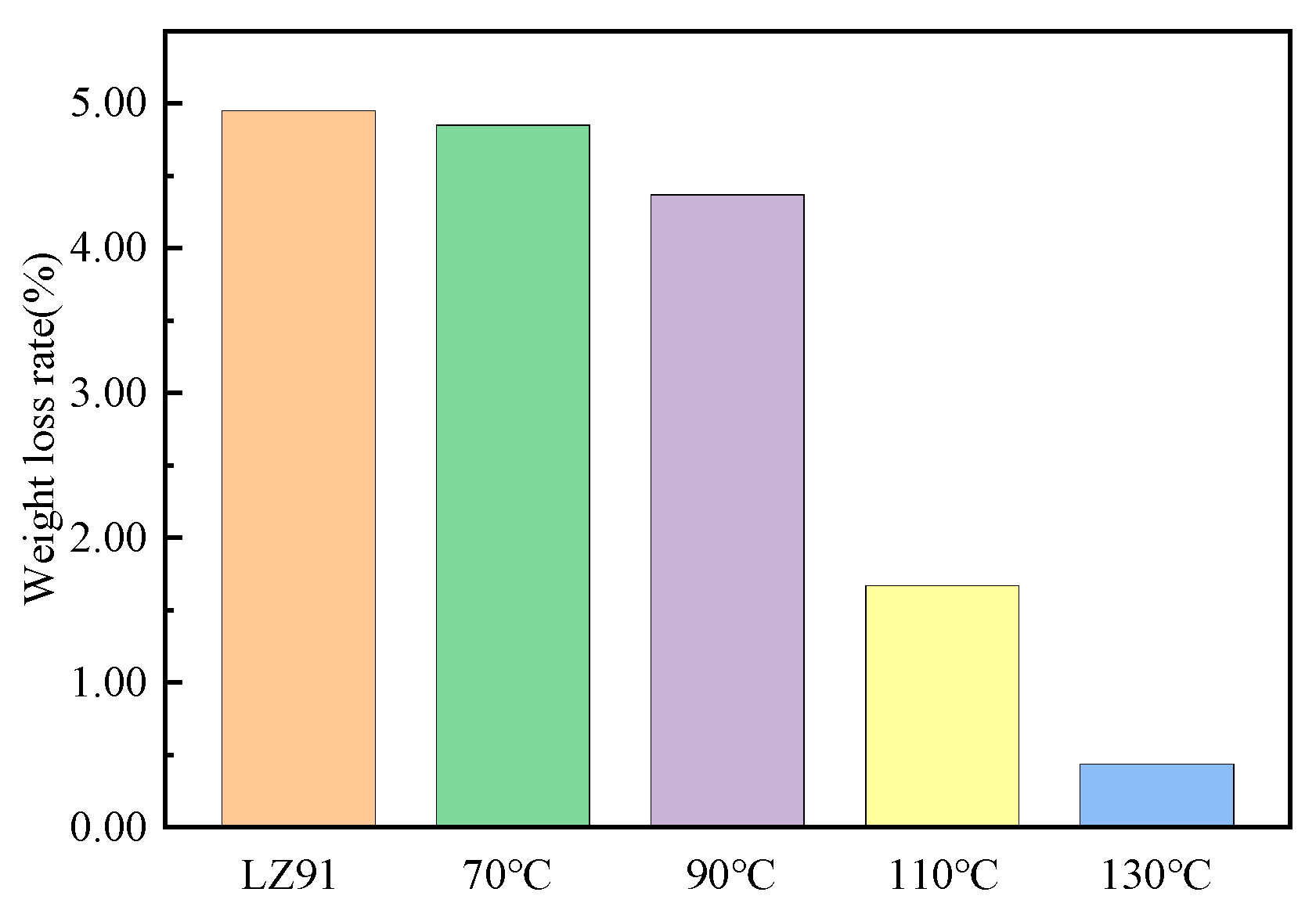

| Mg | Li | Zn | Mn | Fe | Cu | Ni | Si |
|---|---|---|---|---|---|---|---|
| Balance (wt.%) | 8.70 | 0.99 | 0.018 | 0.0034 | 0.0003 | 0.0003 | 0.0054 |
| Point | C | O | Zn | Mg |
|---|---|---|---|---|
| #1 | 1.02 | 22.52 | 0.85 | 75.61 |
| #2 | 1.24 | 22.88 | 0.96 | 74.91 |
| #3 | 0.36 | 26.24 | 1.45 | 71.95 |
| #4 | 0.63 | 24.35 | 1.75 | 73.27 |
| #5 | 0.61 | 16.70 | 1.31 | 81.38 |
| #6 | 0.37 | 14.45 | 0.99 | 84.19 |
| #7 | 0.00 | 27.36 | 1.34 | 71.30 |
| #8 | 0.34 | 28.77 | 1.19 | 69.70 |
| Point | C | O | Mg |
|---|---|---|---|
| #1 | 1.15 | 1.71 | 97.14 |
| #2 | 0.43 | 0.42 | 99.15 |
| #3 | 1.92 | 7.06 | 91.02 |
| #4 | 0.00 | 0.01 | 99.99 |
| #5 | 10.18 | 5.48 | 84.34 |
| #6 | 1.11 | 0.54 | 98.35 |
| #7 | 3.61 | 16.26 | 80.13 |
| #8 | 0.03 | 0.14 | 99.83 |
| Sample | Rs (Ω·cm2) | CPE × 10−5 (S·sn)/cm2 | n | Rct (Ω·cm2) | RL (Ω·cm2) | L (Ω·cm2) |
|---|---|---|---|---|---|---|
| LZ91 | 10.14 | 12.7 | 0.8964 | 75.9 | 27.02 | 689 |
| 70 °C | 8.206 | 3.337 | 0.8865 | 450.6 | 70.04 | 840.3 |
| 90 °C | 7.5 | 2.415 | 0.8988 | 726.1 | 129.5 | 214.1 |
| 110 °C | 7.652 | 2.427 | 0.7468 | 1057 | 223.5 | 4516 |
| 130 °C | 10.84 | 3.397 | 0.6806 | 1434 | 36.3 | 10,690 |
| Sample | Ecorr (V/SCE) | icorr (A/cm2) |
|---|---|---|
| LZ91 | −1.562 | 1.653 × 10−4 |
| 70 °C | −1.492 | 8.995 × 10−6 |
| 90 °C | −1.490 | 8.517 × 10−6 |
| 110 °C | −1.479 | 5.727 × 10−6 |
| 130 °C | −1.464 | 2.257 × 10−6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Li, S.; Peng, H.; Jiang, H.; Wang, Y.; Guan, Y.; Zhang, H. Preparation and Corrosion Resistance of Hydrothermal Coatings on LZ91 Mg–Li Alloy. Coatings 2025, 15, 1217. https://doi.org/10.3390/coatings15101217
Yang L, Li S, Peng H, Jiang H, Wang Y, Guan Y, Zhang H. Preparation and Corrosion Resistance of Hydrothermal Coatings on LZ91 Mg–Li Alloy. Coatings. 2025; 15(10):1217. https://doi.org/10.3390/coatings15101217
Chicago/Turabian StyleYang, Liu, Shiyuan Li, Hao Peng, Hao Jiang, Yong Wang, Yingping Guan, and Hongwang Zhang. 2025. "Preparation and Corrosion Resistance of Hydrothermal Coatings on LZ91 Mg–Li Alloy" Coatings 15, no. 10: 1217. https://doi.org/10.3390/coatings15101217
APA StyleYang, L., Li, S., Peng, H., Jiang, H., Wang, Y., Guan, Y., & Zhang, H. (2025). Preparation and Corrosion Resistance of Hydrothermal Coatings on LZ91 Mg–Li Alloy. Coatings, 15(10), 1217. https://doi.org/10.3390/coatings15101217






