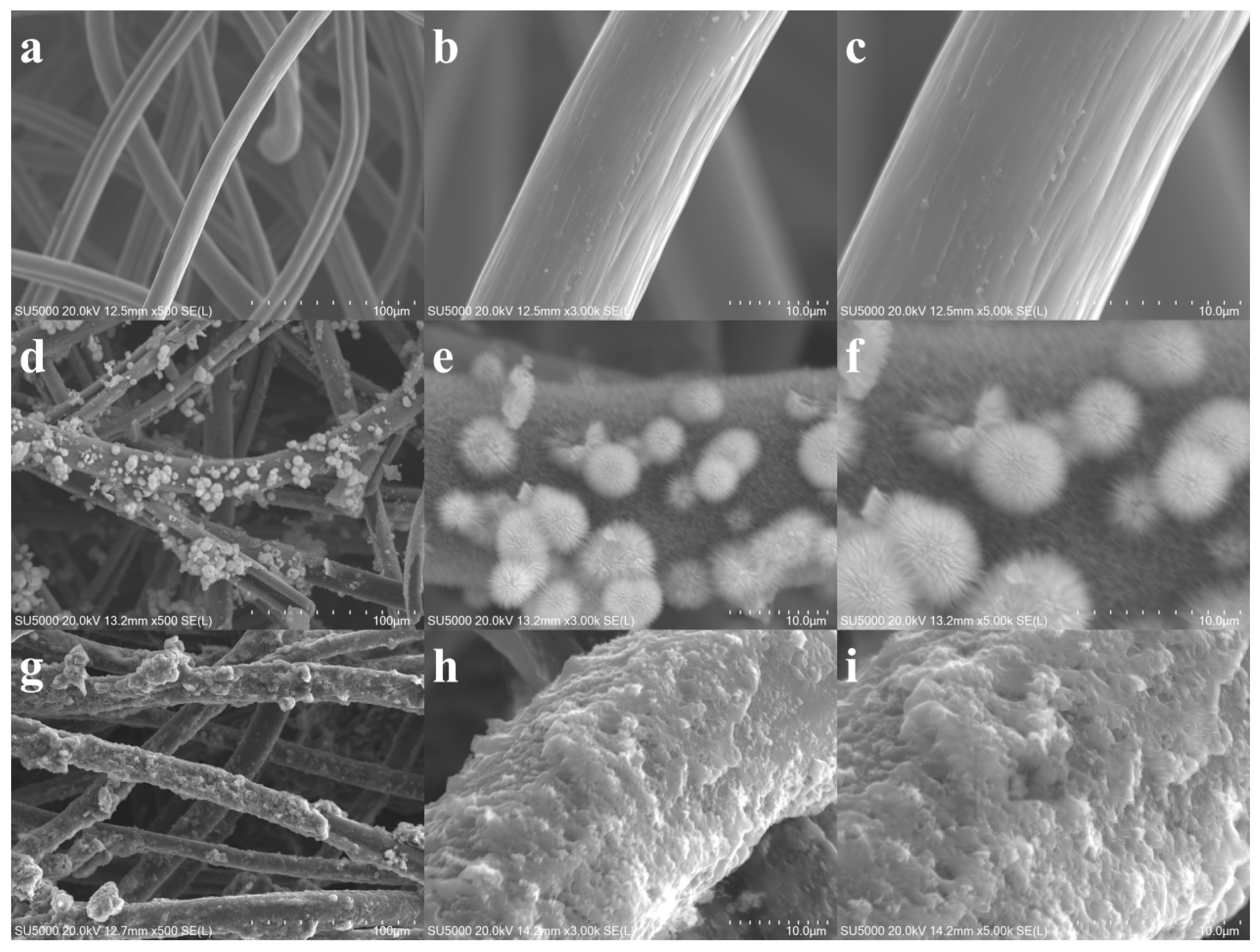
3.1. Morphological and Structural Characterization of the Composite Electrodes
The three-dimensional reticulated framework of the bare CF electrode (
Figure 1a–c) exhibits a highly interconnected porous skeleton. This open topology provides continuous pathways for electron conduction; however, the intrinsically smooth surface of the carbon fibers, while possessing fundamental conductivity, offers limited active sites, constraining the electrochemical response. Following hydrothermal modification, the CF/NiCo
2O
4 electrode (
Figure 1d–f) undergoes a significant morphological evolution: NiCo
2O
4 microspheres serve as core units, uniformly decorated with radially aligned nano-needle arrays, forming a characteristic urchin-like hierarchical structure [
25,
26]. These sharp nano-needles are densely anchored perpendicularly onto the carbon fiber surfaces. This architecture not only multiplies the specific surface area compared to pristine CF but also substantially enhances charge collection capability. The intimate electronic pathways formed between the nano-needles and the carbon fiber substrate, coupled with the micron-scale pores between needle clusters facilitating electrolyte infiltration, collectively optimize interfacial ion/electron transfer kinetics.
The ternary CF/NiCo
2O
4/PPy electrode (
Figure 1g–i), constructed subsequently, achieves further functional integration: PPy is selectively deposited as sub-micron spherical particles within the interstices of the NiCo
2O
4 nano-needles [
27], forming a unique “needle-sphere interlocked” composite. The PPy spheres enhance electrode performance through two primary mechanisms: (i) Their nitrogen-rich molecular chains provide abundant hydrophilic functional groups, significantly improving electrode biocompatibility to promote electroactive microbial adhesion and biofilm formation; (ii) the intrinsic high conductivity of PPy synergizes with the NiCo
2O
4 nano-needles to establish a hierarchical “electron highway” in three dimensions. The nano-needles act as vertical charge transport backbones, while the PPy spheres bridge adjacent needle clusters laterally, creating a multi-dimensional conductive network. This configuration significantly shortens electron transfer pathways, while the amplified surface roughness creates an ideal microenvironment for microbial colonization.
The SEM images reveal the hierarchical architecture of the composite electrode. As shown in
Figure 1a–c, the bare carbon felt exhibits a macroporous and interconnected network of fibers, which serves as a conductive scaffold. Upon modification, the NiCo
2O
4 nano-needles create an urchin-like morphology that densely anchors onto the carbon fibers (
Figure 1d–f). Subsequently, the PPy is deposited as sub-micron spherical particles within the interstices of the nano-needles, forming a “needle-sphere interlocked” composite structure (
Figure 1g–i). This multi-level architecture, observed across the images, significantly increases the specific surface area and is expected to facilitate mass transport and provide abundant sites for microbial colonization and electron transfer.
Ultimately, the synergistic effect between the 3D conductive skeleton of carbon felt and the multi-level functional modifications achieves a breakthrough in electrode performance: The open channels ensure efficient mass transport, the NiCo2O4 urchin structure expands the reactive interface, and the PPy composite layer, optimized for dual conductivity–biofunctionality, constructs an efficient microbe–electrode electron transfer bridge.
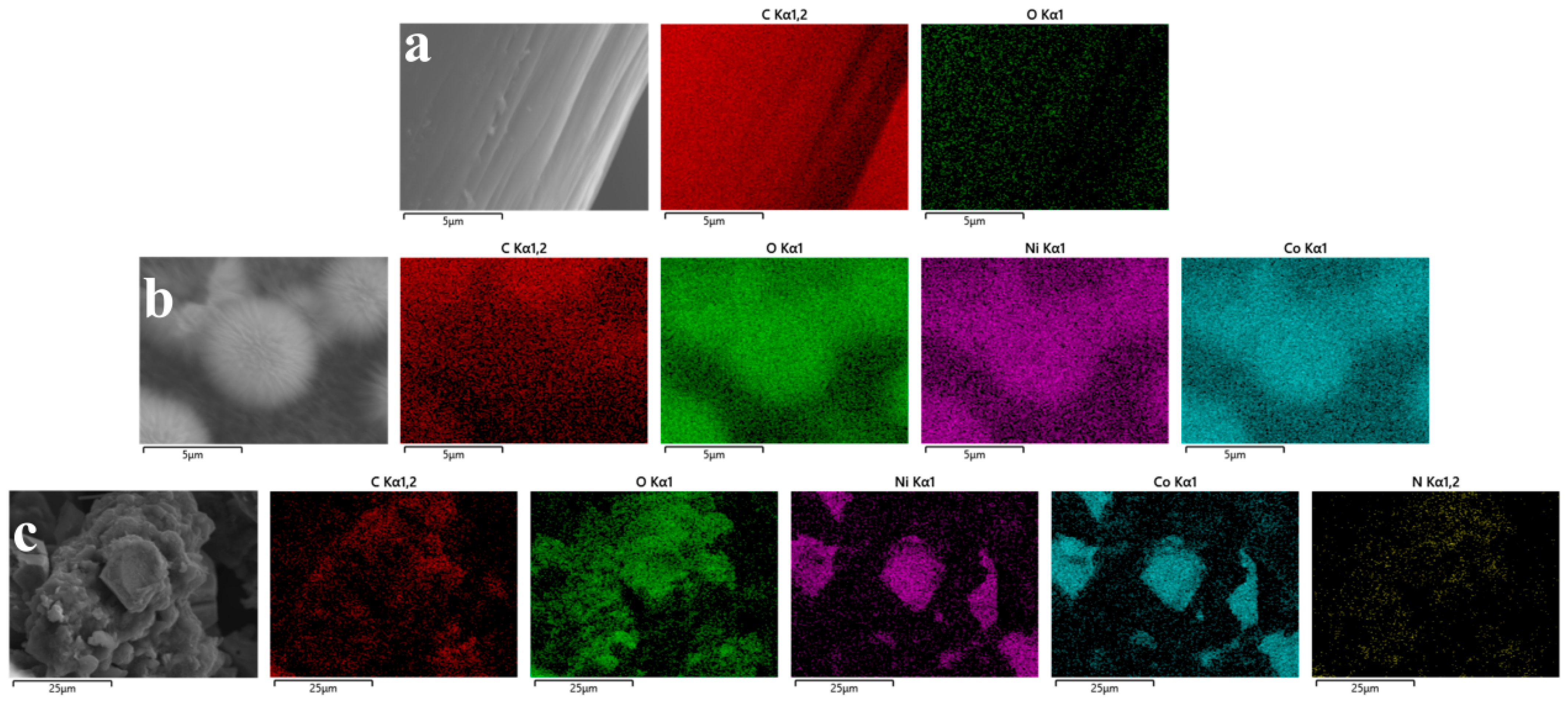
To validate the compositional distribution and modification effects of the composite electrodes, selected-area energy-dispersive X-ray spectroscopy (EDS) analysis was performed (
Figure 2). The SEM image of blank carbon felt (CF) exhibited clean and smooth fiber surfaces (
Figure 2a), with corresponding elemental mapping revealing a uniform and continuous distribution of C (
Figure 2b) and a sparse, scattered presence of O (
Figure 2c), consistent with intrinsic oxygen-containing groups in carbon felt. Upon NiCo
2O
4 modification, EDS analysis of the CF/NiCo
2O
4 composite electrode revealed compositional evolution: Within the selected region (
Figure 2b), the C signal maintained skeletal continuity but demonstrated significant attenuation in intensity. Conversely, the O signal density substantially increased with expanded distribution coverage. Highly overlapping punctate distributions of Co and Ni elements directly confirmed the formation of a dense NiCo
2O
4 nanoneedle coating on the fiber surfaces [
22]. Upon introducing polypyrrole to form the ternary composite electrode, the EDS mapping displayed new characteristics (
Figure 2c): The uniform and continuous distribution of N confirmed the complete encapsulation of NiCo
2O
4 nanostructures by the PPy coating. The C signal intensity recovered and exhibited edge blurring, reflecting the secondary coating of carbon fibers by the PPy layer. While the Co and Ni elements maintained their co-distribution pattern, their signal intensity attenuated due to absorption of low-energy X-rays (Co-Kα: 6.93 keV, Ni-Kα: 7.47 keV) by the PPy layer. The O distribution further expanded and demonstrated gradient variations, corresponding to the combined contributions of NiCo
2O
4 lattice oxygen and oxygen-containing functional groups on the PPy surface [
28].
Elemental composition evolution of the electrodes was quantitatively analyzed by EDS (
Figure 3). The pristine CF electrode (
Figure 3a) exhibited carbon-dominated characteristics with C and O contents of 94.55 wt% and 5.45 wt%, respectively, yielding a C/O mass ratio of 17.35. Upon NiCo
2O
4 modification (
Figure 3b), significant elemental redistribution occurred: characteristic Co (26.46 wt%) and Ni (12.85 wt%) signals emerged alongside elevated O content (31.66 wt%, representing a 480.9% increase relative to CF), while carbon content decreased to 31.66 wt%. The atomic ratio of Ni/Co was calculated as 1:2.05, deviating merely 2.5% from the theoretical stoichiometry of NiCo
2O
4 (1:2), confirming successful oxide loading [
29]. Further PPy deposition (
Figure 3c) introduced distinct nitrogen signatures (5.64 wt%) attributable to pyrrolic rings (C
4H
4NH), with concurrent carbon content adjustment to 30.31 wt% (reflecting polymeric carbon backbone contributions) and oxygen reduction to 29.78 wt%. The observed elemental progression—from carbon–oxygen dominance to transition metal–oxygen coexistence and finally nitrogen emergence—systematically validates the hierarchical electrode architecture: carbon felt substrate to NiCo
2O
4 decoration to conductive polymer encapsulation.
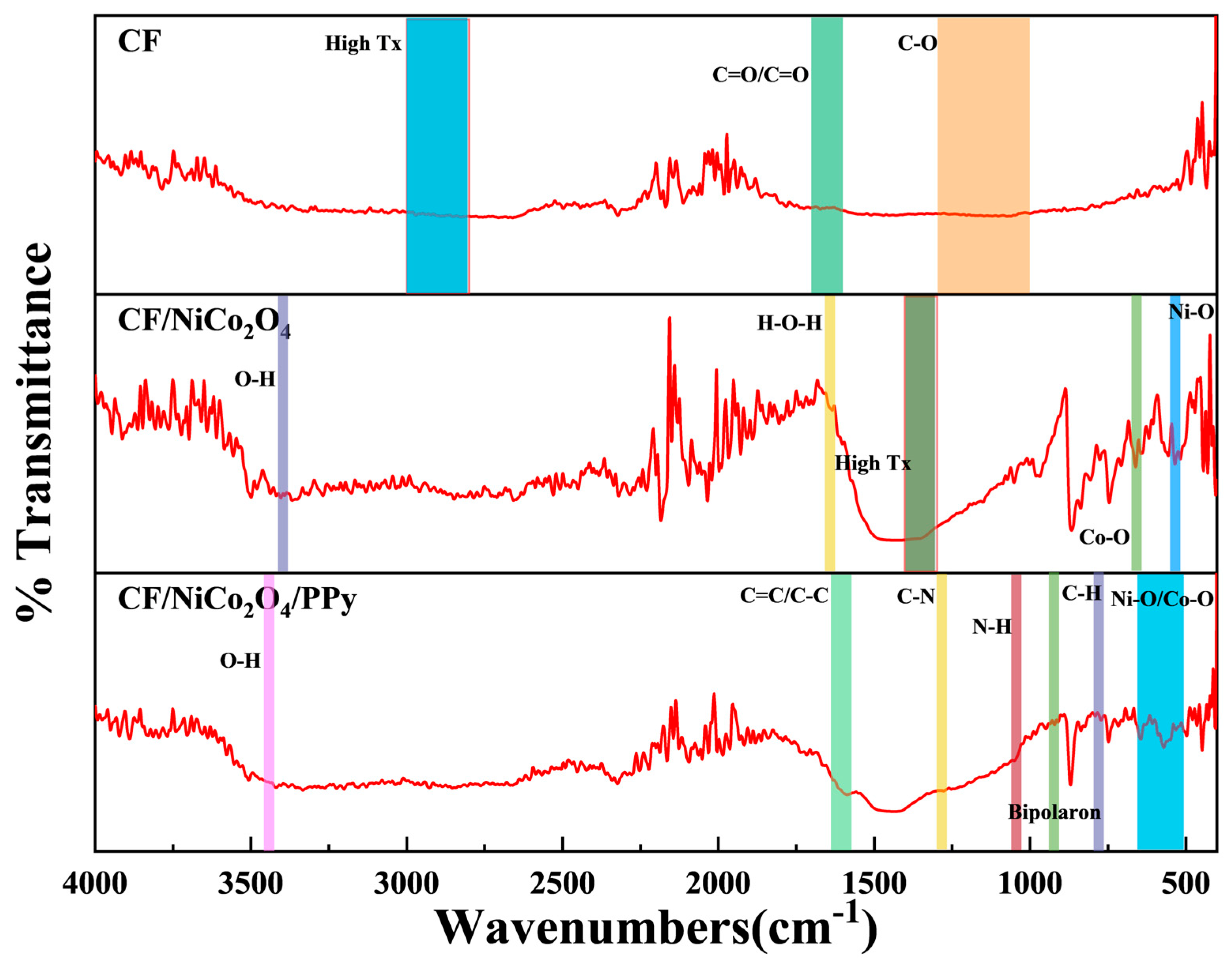
The FTIR spectrum (
Figure 4) of pristine CF exhibits distinct features reflecting surface chemistry evolution during pretreatment. The absence of C-H stretching vibrations (2800–3000 cm
−1, transmittance >99%) confirms efficient removal of aliphatic contaminants through alcohol cleaning. Characteristic C-O stretching bands (1000–1300 cm
−1, transmittance 40%–60%) indicate successful introduction of oxygen-containing groups (e.g., hydroxyl, carboxyl) via H
2O
2 oxidation, which enhances hydrophilicity. Deposition of spinel-type NiCo
2O
4 onto CF is validated by metal–oxygen vibrational modes below 800 cm
−1. Characteristic dips in transmittance at 560 cm
−1 (Ni
2+-O) and 655 cm
−1 (Co
3+-O) confirm the formation of a crystalline spinel structure. The absence of nitrate precursor residues (no absorption at 1380 cm
−1, transmittance >85%) indicates complete decomposition during hydrothermal-annealing synthesis [
30]. Broad O-H stretching at 3400 cm
−1 (transmittance drop: 60%→40%) and H-O-H bending at 1630 cm
−1 (transmittance: 85%→80%) reveal adsorbed water, attributable to the hygroscopic nature of the high-surface-area nanostructure. These features collectively affirm successful NiCo
2O
4 growth on CF.
In situ polymerization of PPy on CF/NiCo
2O
4 yields a ternary composite with identifiable interfacial interactions. Signature PPy peaks include C=C/C-C skeletal vibrations at 1600 cm
−1 (transmittance ~15%), C-N stretching at 1290 cm
−1 (~16.5%), N-H deformation at 1040 cm
−1 (~19.7%), and C-H bending at 790 cm
−1 (~23.6%). Critically, the bipolaron band at 920 cm
−1 (~22.5% transmittance) confirms PPy in a conductive oxidized state, favorable for charge transfer [
31]. The persistent sub-650 cm
−1 absorption (near 0% transmittance) verifies retained NiCo
2O
4, while a shifted C-N peak (vs. pure PPy) suggests potential Co/Ni-N coordination at the interface. The intensified O-H stretch at 3430 cm
−1 (near 0% transmittance) implies enhanced hydrophilicity from PPy incorporation. Broadened PPy peaks indicate polymer chain disorder, possibly arising from kinetically limited polymerization in mixed solvents [
32].
The FTIR results demonstrate a progressive optimization of electrode interfaces: CF provides a functionalized substrate, NiCo2O4 contributes spinel-phase conductivity, and PPy coating establishes a conductive polymer network with synergistic metal–polymer interactions. Next, electrochemical analysis (CV/EIS) will be carried out for verification, so as to correlate the spectral characteristics with the charge transfer efficiency.
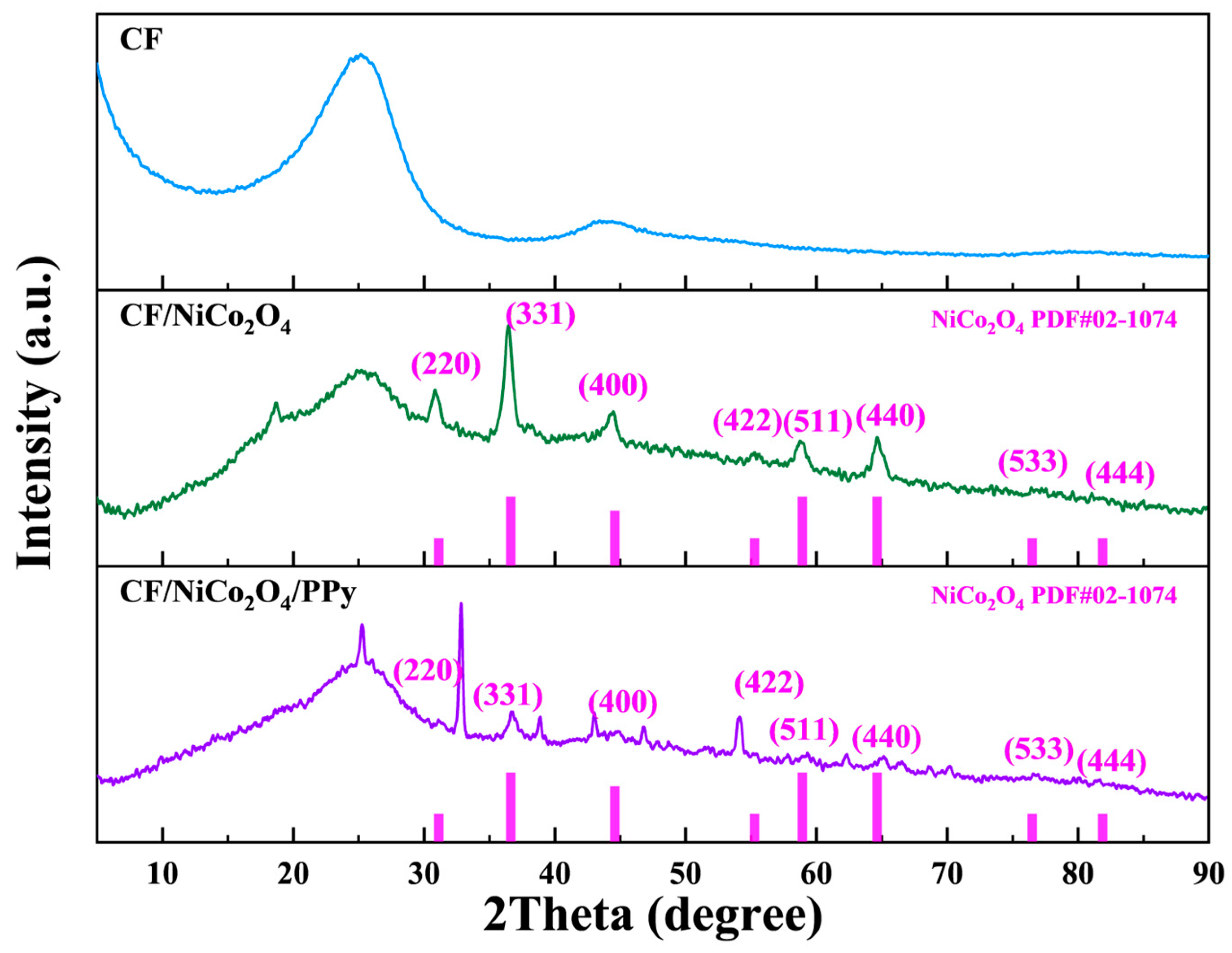
X-ray diffraction (XRD) analysis confirmed the crystallographic evolution and interfacial interactions within the composite electrodes (
Figure 5). For the CF/NiCo
2O
4 composite, distinct diffraction peaks observed at 31.1°, 36.6°, 44.6°, 58.9°, and 64.7° 2θ were unambiguously indexed to the (220), (311), (400), (511), and (440) planes of cubic spinel NiCo
2O
4 (PDF#02-1074, space group Fd-3m), verifying phase-pure synthesis without detectable impurities [
33]. The calculated lattice parameter (a = 8.128 Å) aligned closely with theoretical values (8.11 Å), indicating structural integrity essential for Faradaic redox reactions. Upon introducing polypyrrole (PPy) to form the CF/NiCo
2O
4/PPy ternary composite, characteristic NiCo
2O
4 peaks persisted at 32.8°, 36.7°, 46.7°, 59.3°, and 65.2° 2θ with marginal peak shifts (<0.5°), confirming that PPy deposition preserved the spinel framework and crystallinity of NiCo
2O
4. Notably, a broad scattering hump emerged within 10–30° 2θ exclusively in the ternary system, attributable to the amorphous nature of conductive PPy chains [
34]. This feature signifies successful PPy encapsulation and suggests interfacial interactions (e.g., π–π stacking or H-bonding) between PPy and the CF/NiCo
2O
4 substrate, which facilitates charge transfer across the hybrid interface.
The synergistic configuration endows the electrode with dual functionality: (1) The well-maintained NiCo2O4 spinel structure provides abundant pseudocapacitive sites for rapid ion adsorption/desorption, while (2) the conformal PPy coating enhances electrode conductivity, prevents active material dissolution, and crucially improves biocompatibility for microbial colonization in MFCs. This unique integration enables simultaneous energy harvesting (via bioelectrochemical reactions) and in situ energy storage (through pseudocapacitance), collectively boosting the power density and operational stability of MFC systems.
3.2. Electrochemical Performance Analysis
Cyclic voltammetry (CV) measurements (scan rate: 5 mV/s, potential window: 1 V to −0.5 V to 1 V;
Figure 6a) were performed to evaluate the electrochemical properties of CF-based electrodes. Analysis of the CV data revealed that the pristine CF electrode exhibited behavior characteristic of electric double-layer capacitance (EDLC), evidenced by a quasi-rectangular CV curve devoid of distinct redox peaks and a specific capacitance of approximately 5.5 F/g. This confirmed minimal Faradaic activity. Upon modification with NiCo
2O
4, the CF/NiCo
2O
4 electrode displayed pronounced redox peaks at approximately 0.98 V (anodic) and 0.20 V (cathodic). These peaks were attributed to the reversible redox reactions of Co
2+/Co
3+ and Ni
2+/Ni
3+ within the NiCo
2O
4, signifying a substantial pseudocapacitive contribution [
35,
36]. Consequently, the specific capacitance increased to 15.0 F/g. However, the peak potential separation (ΔEp = 0.78 V) indicated limited reaction reversibility.
Further modification with PPy to form the CF/NiCo
2O
4/PPy composite electrode resulted in a significant performance enhancement. This electrode exhibited markedly higher redox peak current densities of 55.73 A/m
2 (anodic) and −95.85 A/m
2 (cathodic), representing an 85% increase compared to the CF/NiCo
2O
4 electrode. Concurrently, a positive shift in the cathodic peak potential to 0.25 V and a reduced ΔEp of 0.73 V were observed. These changes confirmed that the incorporation of PPy improved reaction reversibility by enhancing electrode conductivity and accelerating ion diffusion kinetics. The specific capacitance of the CF/NiCo
2O
4/PPy electrode reached 28.0 F/g, which is five times greater than that of the pristine CF. This substantial improvement highlights a pronounced synergistic effect between PPy and NiCo
2O
4. The PPy layer not only expanded the active electrode/electrolyte interfacial area but also significantly reduced charge transfer resistance [
37].
In summary, the PPy modification constructed a conductive network and optimized the interface, rendering the CF/NiCo2O4/PPy composite a high-performance pseudocapacitive electrode.
Figure 6b presents the CP curves acquired through potentiostatic analysis of the blank CF, CF/NiCo
2O
4, and CF/NiCo
2O
4/PPy anodes. The specific capacitance (
Csp), expressed in F/cm
2, was determined using Equation (1):
where
is the discharge current (A);
is the discharge time (s);
is the working voltage window during discharge (V); and
is the electrode geometric area (in cm
2).
The Csp value of the CF/NiCo2O4 electrode was 0.206 F/cm2. This value signifies an enhancement in capacitance performance by 6.06 times compared to the blank CF electrode (Csp = 0.034F/cm2) under the identical current density of 2.5 mA/cm2. The Csp value of the CF/NiCo2O4/PPy electrode reached 1.115 F/cm2. This represents a remarkable improvement of 32.79 times over the blank CF electrode and 5.41 times over the CF/NiCo2O4 electrode at the same current density. Furthermore, the CP curve recorded at a current density of 2.5 mA/cm2 exhibited a characteristic profile that strongly aligns with the high capacitive behavior typically associated with double-layer capacitors, indicating excellent charge storage capability primarily through electrostatic processes.
Figure 7a shows that the interfacial kinetics of the composite electrode gradually increases, as revealed by EIS analysis. The solution resistance (R
s) derived from the high-frequency intercept decreases from 3.56 Ω for pristine CF to 2.36 Ω after NiCo
2O
4 modification, with PPy incorporation (CF/NiCo
2O
4/PPy) further reducing R
s to 2.14 Ω (39.9% reduction versus CF). This trend indicates optimized electronic pathways through conductive polymer integration. Concomitantly, the charge transfer resistance (R
ct) determined from semicircle diameters exhibits a dramatic decline: 10.4 Ω (CF)→6.1 Ω (CF/NiCo
2O
4)→1.9 Ω (CF/NiCo
2O
4/PPy; 68.9% decrease versus CF/NiCo
2O
4). Such reduction signifies accelerated Faradaic processes at the electrode–electrolyte interface due to PPy’s charge-transfer mediation. Furthermore, low-frequency diffusion behavior evolves from a restricted Warburg characteristic (~45° slope for CF) to improved ion accessibility (~60° for CF/NiCo
2O
4), culminating in near-ideal capacitive behavior (~75° slope for CF/NiCo
2O
4/PPy). These collective improvements—facilitated electron conduction, enhanced charge transfer kinetics, and optimized ion diffusion pathways—demonstrate PPy’s critical role in elevating the electrochemical performance. This conclusion aligns with earlier galvanostatic charge–discharge results where CF/NiCo
2O
4/PPy delivered 29-fold higher capacitance than CF/NiCo
2O
4 [
38,
39].
3.3. Charge Storage Capacity of MFCs
Figure 8 displays the discharge profiles of MFCs equipped with three different electrodes under an external resistance of 100 Ω, at discharge cycles of 250 s, 500 s, 750 s, and 1000 s, respectively. As shown in
Figure 6a–d, the discharge curves of all three electrodes exhibited a peak current density, followed by a gradual decrease over time, eventually reaching a relatively stable state. Based on the available data,
Table 1 was derived, which summarizes the peak current density (
ih), steady-state current density (
is), charge storage capacity (
Qs), and total charge amount (
Qt) for each electrode at different cycling periods. According to
Table 1, during the 1000 s charge–discharge cycle, the MFC with the CF/NiCo
2O
4/PPy electrode achieved an
ih of 96.0 A/m
2, which was significantly higher than those of the bare CF electrode (19.8 A/m
2) and the CF/NiCo
2O
4 electrode (22.1 A/m
2). Similarly, the i
s of the CF/NiCo
2O
4/PPy cell reached 28.9 A/m
2, markedly superior to those of the bare CF (4.9 A/m
2) and CF/NiCo
2O
4 (6.0 A/m
2) cells. Notably, the Q
t and Q
s for the CF/NiCo
2O
4/PPy electrode were calculated to be 32,509.0 C/m
2 and 3609.0 C/m
2, respectively, significantly exceeding those of the bare CF electrode (Q
t = 6189.9 C/m
2, Q
s = 1327.45 C/m
2) and the CF/NiCo
2O
4 electrode (Q
t = 8470.6 C/m
2, Q
s = 2460.6 C/m
2). The total and stored charge values of the CF/NNiCo
2O
4/PPy electrode were 5.3 times and 2.7 times those of the bare CF, and 3.8 times and 1.5 times that of the CF/NiCo
2O
4 electrode, respectively.
Chronoamperometry (CA) revealed that the MFC with the CF/NiCo2O4/PPy electrode exhibited superior peak and steady-state current densities, along with the highest Qt and Qs, values that were 5.3 and 2.7 times greater, respectively, than those of the bare CF electrode. This enhancement is attributed to the NiCo2O4 modification, which significantly improves energy storage and power output. The uniform deposition of NiCo2O4 on carbon fibers increased the specific surface area, promoting microbial colonization and providing additional active sites, thereby enhancing electrochemical activity. As an efficient catalyst, NiCo2O4 accelerated reaction kinetics, yielding higher current density and improved output at equal potential. Furthermore, it enhanced the electrode’s electric double-layer capacitance, leading to greater charge storage without compromising conductivity.
The elevated surface area from NiCo2O4 deposition facilitated higher PPy loading. PPy, as a conductive polymer, improved overall electrode conductivity and charge transfer efficiency. Both NiCo2O4 and PPy contributed electrocatalytic activity, synergistically boosting reaction kinetics and current density. Their combination provided additional active sites and improved reactant accessibility. The modified surface also strengthened microbe–electrode interactions, enhancing bio-catalytic activity and energy conversion efficiency. During energy deficits, the capacitive anode stores microbial metabolic energy; upon demand, the MFC delivers increased current through combined newly produced and stored charge, thereby improving overall power performance.
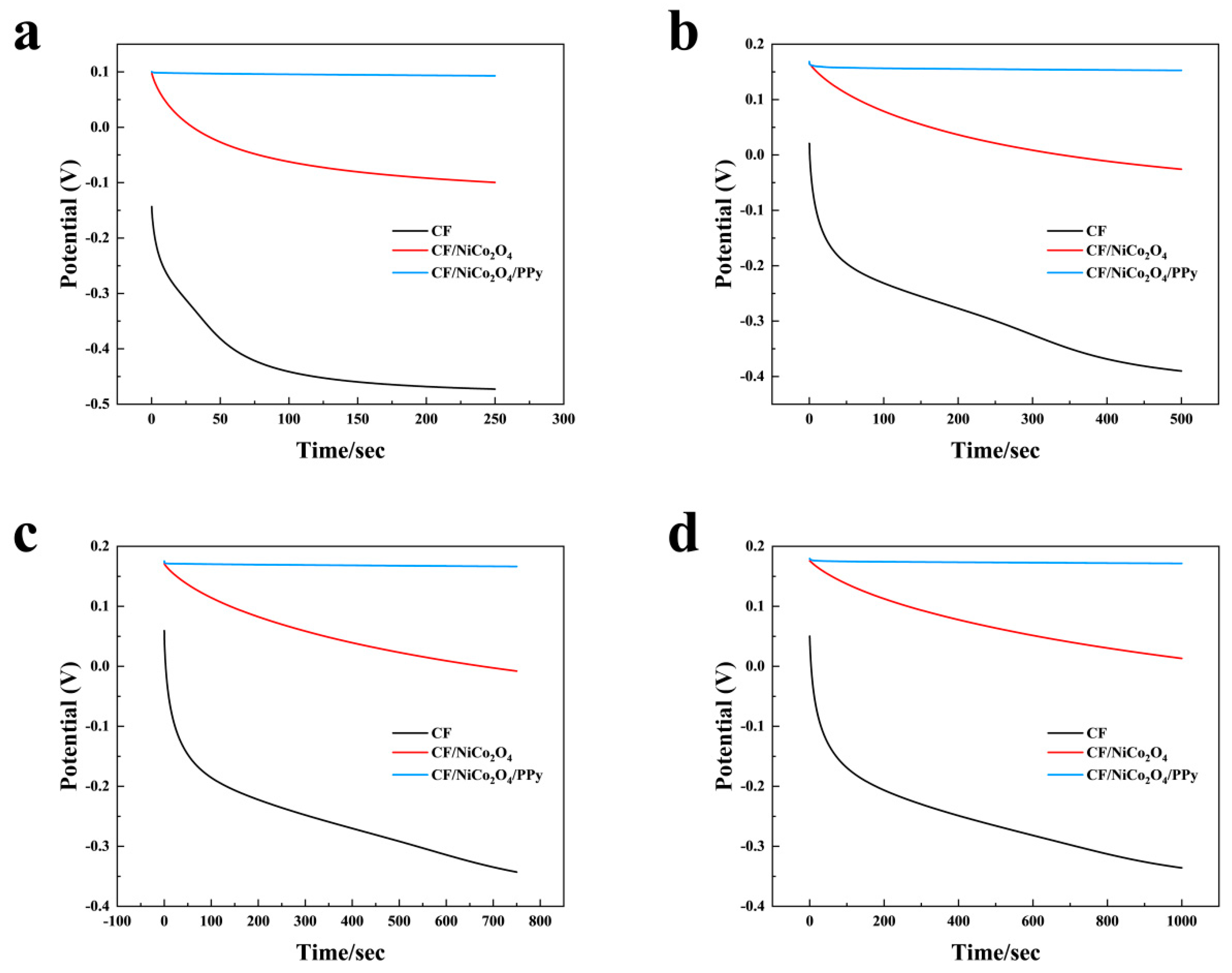
3.4. Open-Circuit Potential and Stability Analysis
Figure 9a–d present the open-circuit potential-time profiles of MFCs employing CF, CF/NiCo
2O
4, and CF/NiCo
2O
4/PPy electrodes, measured at intervals of 250 s, 500 s, 750 s, and 1000 s. Notably, the open-circuit potentials of all three anodes exhibited a similar downward trajectory across the 250 s, 500 s, 750 s, and 1000 s datasets. However, distinct disparities in the decay rates were evident among the anodes in each measurement period. Among them, the bare CF anode displayed the most pronounced potential decline, followed by the CF/NiCo
2O
4 anode, while the CF/NiCo
2O
4/PPy composite anode demonstrated the weakest downward trend. In
Figure 9d, the potential of the bare CF anode plummeted from 0.05 V to −0.34 V. In contrast, the potential decay of the CF/NiCo
2O
4 anode was relatively slower, decreasing from an initial potential of 0.17 V to 0.01 V over the 1000 s period. Compared to the bare CF anode, this modified electrode exhibited a higher initial potential and suppressed potential decay. This behavior suggests that NiCo
2O
4’s inherent electrochemical activity enhanced charge transfer kinetics, while its uniform nanostructure coating (as evidenced in
Figure 1) reduced interfacial resistance. These combined effects enabled the CF/NiCo
2O
4 anode to sustain a higher initial potential with mitigated current loss, thereby prolonging its potential retention. Most significantly, the initial potential of the CF/NiCo
2O
4/PPy anode decreased from 0.18 V to 0.17 V after 1000 s. This decay of
V 0.1 V was substantially smaller than the losses of
V 0.39 V and
V 0.16 V observed for the bare CF and CF/NiCo
2O
4 anodes, respectively. The incorporation of NiCo
2O
4 provided a significantly larger specific surface area and active sites on the CF fibers, facilitating the effective deposition of the PPy layer. Concurrently, the pseudocapacitive nature of both NiCo
2O
4 and PPy enabled rapid Faradaic redox reactions (involving cation doping/dedoping for PPy and reversible Co
2+/Co
3+ and Ni
2+/Ni
3+ transitions for NiCo
2O
4), ultimately leading to enhanced interfacial charge storage capacity. Consequently, the CF/NiCo
2O
4/PPy composite electrode stored a greater amount of charge compared to the CF/NiCo
2O
4 and bare CF electrodes, achieved a higher initial potential, exhibited superior electrical conductivity and interfacial stability, thereby augmenting the energy storage capability within the MFC system and significantly retarding the decrease in anode potential [
40,
41,
42].
3.5. Power Output and Polarization Behavior
The power density profiles of the CF, CF/NiCo
2O
4, and CF/NiCo
2O
4/PPy anodes under varying external resistances (10–9000 Ω) are systematically compared in
Figure 10a. All three electrodes exhibited characteristic parabolic trends, with power densities initially rising and subsequently declining as resistance decreased. Critically, the CF/NiCo
2O
4/PPy ternary composite achieved a maximum power density of 1901.25 mW/m
2 at 200 Ω, surpassing the peak values of the CF/NiCo
2O
4 binary electrode (1020.10 mW/m
2 at 100 Ω) and the bare CF anode (319.70 mW/m
2 at 600 Ω) by 86.4% and 495%, respectively. This superiority was consistently maintained across both high- and low-resistance regimes: at 9000 Ω, the ternary electrode generated 157.92 mW/m
2, exceeding the binary (96.04 mW/m
2) and CF (42.47 mW/m
2) outputs; at 1000 Ω, it delivered 1074.68 mW/m
2, nearly double that of the binary electrode (567.51 mW/m
2) and quadruple the CF baseline (275.63 mW/m
2). Notably, the ternary composite demonstrated exceptional stability under high-current conditions (100–300 Ω), where its power density retained >87% of peak performance (1742.40 mW/m
2 at 100 Ω), contrasting sharply with the binary electrode’s 18.3% decline and the CF anode’s 32% loss in the same resistance range. Such enhancements are attributed to the synergistic interplay between NiCo
2O
4 and PPy: the spinel oxide provides a high-surface-area conductive scaffold facilitating rapid charge transfer, while the pseudocapacitive PPy layer augments interfacial Faradaic reactions and charge storage capacity, collectively optimizing energy extraction efficiency across diverse operational loads [
43].
The polarization curves of MFCs equipped with CF, CF/NiCo
2O
4, and CF/NiCo
2O
4/PPy anodes reveal significant improvements in electrochemical performance through electrode modification (
Figure 10b). The ternary CF/NiCo
2O
4/PPy electrode exhibited the highest open-circuit voltage of 754 mV, substantially exceeding the CF/NiCo
2O
4 (588 mV) and bare CF (391 mV) anodes, indicating enhanced thermodynamic driving force for electron transfer. Under operational loads, the composite electrode demonstrated superior polarization resistance mitigation: at a current density of 2 A/m
2, it maintained a voltage of 584 mV, while the binary and bare anodes dropped to 421 mV and 84 mV, respectively. This advantage amplified at higher current densities (>5 A/m
2), where the ternary electrode sustained 219 mV at 6.84 A/m
2—2.2× and 3.2× higher than the current densities achievable by CF/NiCo
2O
4 (3.18 A/m
2 at 219 mV) and CF (0.95 A/m
2 at 304 mV) at equivalent voltage. Notably, the voltage decay slope for the ternary anode was −70.2 mV·m
2/A (754→40 mV over 0.21→10 A/m
2), markedly shallower than the binary (−69.5 mV·m
2/A) and bare CF (−116.3 mV·m
2/A) electrodes, confirming reduced activation and concentration polarization.
The anodic polarization profiles in
Figure 10c demonstrate markedly enhanced reaction kinetics for modified electrodes across operational current densities (0.2–10 A/m
2). The CF/NiCo
2O
4/PPy ternary anode exhibited the most negative onset potential (−489 mV at 0.21 A/m
2), significantly lower than CF/NiCo
2O
4 (−378 mV) and bare CF (−136 mV), confirming superior bioelectrocatalytic activity for substrate oxidation. This kinetic advantage persisted under high-current regimes: at 2 A/m
2, the ternary electrode maintained −394 mV, while the binary and bare anodes operated at −256 mV and −81 mV, respectively, indicating 54% and 79% reductions in activation overpotential. Crucially, the composite electrode sustained stable performance at ultrahigh current densities (>5 A/m
2), delivering −147 mV at 7.1 A/m
2—a voltage 2× more negative than the binary anode (−73 mV) at equivalent current density. The polarization resistance, quantified by the voltage decay slope (ΔV/ΔJ), was minimized for the ternary electrode (−39.1 mV·m
2/A from 0.21 to 10 A/m
2), compared to −48.8 mV·m
2/A (binary) and −42.3 mV·m
2/A (CF), reflecting synergistic mitigation of charge transfer limitations. These improvements originate from the dual-functional modification: NiCo
2O
4 nanowires enhance interfacial electron collection efficiency, while the conductive PPy matrix facilitates rapid extracellular electron transfer through reversible quinone/hydroquinone redox mediation, collectively suppressing anode polarization across the microbial electrochemical activity spectrum.
3.6. Microbial Community Structure and Biodiversity Analysis
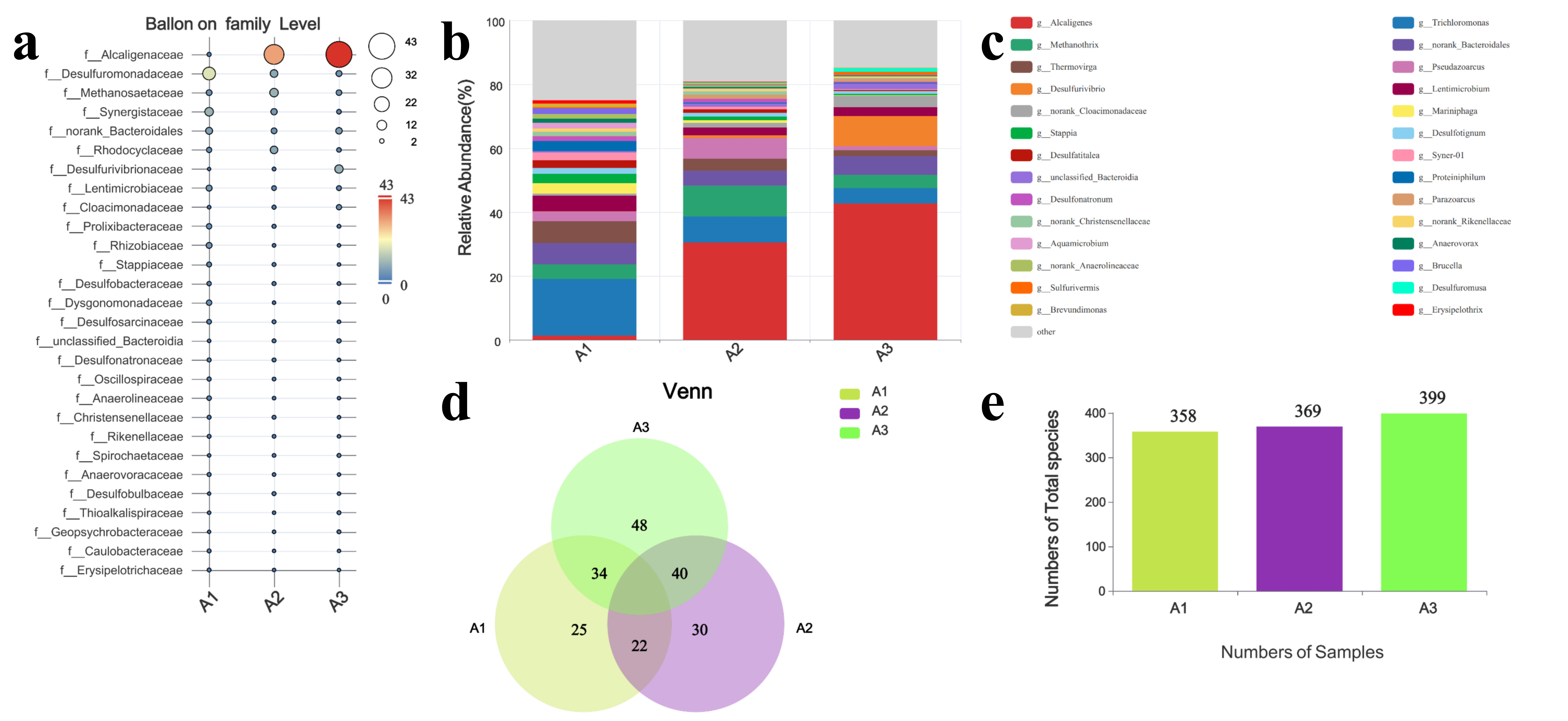
High-throughput sequencing at the family level (
Figure 11a) revealed significant restructuring of electroactive communities driven by anode modifications. The ternary CF/NiCo
2O
4/PPy electrode (A3) exhibited dominant enrichment of Geopsychrobacteraceae (43% relative abundance), a known exoelectrogen family capable of direct electron transfer via cytochrome c pathways. This represented a 2.7-fold increase compared to the binary CF/NiCo
2O
4 anode (A2, 16%) and near absence in bare CF (A1, <1%). Critically, synergistic selection for secondary electroactive taxa was observed: Desulfuromonadaceae (32% in A3 vs. 12% in A2) and Desulfobulbaceae (22% in A3 vs. 8% in A2)—both sulfate-reducing bacteria with extracellular electron transfer competence—collectively constituted 54% of the ternary biofilm community. Conversely, non-electrogenic families (e.g., Methanosacetaceae and Christensenellaceae) were suppressed to <5% in A3, contrasting with their proliferation in CF (15–28%) [
44]. This selective enrichment of highly efficient exoelectrogens provides a direct biological explanation for the superior charge transfer kinetics, evidenced by the lowest charge transfer resistance (R
ct,
Figure 7a) and highest current outputs (
Table 1) achieved with the CF/NiCo
2O
4/PPy anode. This microbial selection correlates with the hierarchical electrode architecture: the NiCo
2O
4 nanowire substrate provides high surface area for biofilm anchoring, while the conductive PPy matrix enhances interfacial redox kinetics through quinone-mediated electron shuttling, preferentially enriching electroactive consortia that maximize current generation efficiency.
During different electrode modification processes, the anode microbial community was observed to reorganize, as shown in
Figure 11b,c. The unmodified CF was dominated by Trichloromonas (17.69%), Thermovirga (6.74%), and unclassified Bacteroidales (6.73%). Coating with cobalt–nickel oxide (CF/NiCo
2O
4) induced a significant shift, enriching Alcaligenes from 1.41% (CF) to 30.51% (CF/NiCo
2O
4) and Methanothrix to 9.71%. Further functionalization with polypyrrole (CF/NiCo
2O
4/PPy) intensified this selection: Alcaligenes became overwhelmingly dominant (42.70%), while the known electrogenic genus Desulfurivibrio exhibited substantial enrichment (0.03%→0.83%→9.34%). This microbial restructuring is directly correlated with the superior electrochemical performance observed in the polarization and anodic polarization curves (
Figure 10b,c). The progressively enriched electroactive consortia synergistically enhance the bioelectrocatalytic activity, serving as the fundamental driver for the enhanced power output and stability.
Based on the high-throughput sequencing data of anodic microbial communities (
Figure 11d,e), the CF/NiCo
2O
4/PPy composite electrode (A3) exhibited significantly enhanced biodiversity compared to the CF/NiCo
2O
4 (A2) and bare CF (A1) anodes. Specifically, the total number of observed species increased progressively from 358 (A1) to 369 (A2) and further to 399 (A3), indicating superior bio-affinity of the NiCo
2O
4/PPy-modified surface for electroactive consortia. Venn analysis revealed that A3 possessed the highest number of unique species (48), substantially exceeding those of A1 (25) and A2 (30). The expanded biodiversity and unique species suggest the formation of a more robust and functionally resilient biofilm, which underpins the outstanding long-term operational stability and charge storage performance (Q
t,
Table 1) of the MFC. Furthermore, the increased species overlap between A2 and A3 (40 unique shared species) compared to A1–A2 (22) or A1–A3 (34) pairs suggests a synergistic effect of the NiCo
2O
4/PPy composite in enriching specialized electrogens. This expansion in microbial diversity and abundance directly correlates with the improved electrogenic activity, as evidenced by the concurrent enhancement in power density (
Figure 10a). The results validate the efficacy of the hierarchical NiCo
2O
4/PPy coating in optimizing the bioelectrochemical interface for microbial fuel cells.