Abstract
Palm-leaf manuscripts (PLMs) represent a significant component of cultural heritage as a medium for information recording. However, the inherent fragility of these organic materials presents a major challenge for their long-term preservation. Therefore, enhancing the durability of PLMs to ensure longevity has become a critical issue in conservation research. This study includes an examination of the potential of two polymers, cationic polyacrylamide (CPAM) and chitosan quaternary ammonium salt (CQAS), for PLM encapsulation. The encapsulation effects of these materials were assessed through artificial moist heat-accelerated aging tests, enabling a comprehensive evaluation of structural and mechanical properties at both the macroscopic and microscopic levels. The results indicated that CPAM provided superior performance in terms of gloss, color stability, hydrophobicity, pH value, and tensile properties, whereas CQAS demonstrated notable antifungal efficacy. Both CPAM and CQAS possess distinct advantages and can significantly contribute to the preservation of PLMs. This work offers valuable insights for developing effective conservation strategies, emphasizing the potential of CPAM and CQAS as viable encapsulation materials in the face of conservation requirements.
1. Introduction
Palm-leaf manuscripts (PLMs), the ancient and essential written artifacts of South, Southeast, and Central Asia, are now acknowledged as invaluable components of global cultural heritage [1,2]. Significant collections of these manuscripts are also preserved in regions of China, such as in Yunnan and Xizang [1,2]. Beyond their role in the dissemination and preservation of knowledge across disciplines, including history, literature, philosophy, art, and science, they also have profound cultural and religious significance in Buddhism [3,4,5,6]. Around the 7th century AD, PLMs were introduced to the Yunnan region of China via Myanmar and Thailand, following the southern transmission of Theravada Buddhism from Sri Lanka [5,7]. With its tropical climate, Xishuangbanna is the only region on the Chinese mainland where the talipot palm, regarded as the principal medium used for these manuscripts, can grow. Consequently, Xishuangbanna has become the sole region in China that preserves traditional techniques and cultural practices associated with the creation and application of PLMs [8]. The region is home to many manuscripts that are rich in content, often referred to as the “Encyclopedia of Dai Culture”.
PLMs are composed primarily of organic materials, such as cellulose, hemicellulose, and lignin, making them highly susceptible to degradation from environmental factors, including elevated temperature, humidity, and biological erosion [2]. Typical forms of damage include insect infestation, staining, missing fragments, fiber fraying, tearing, and perforation. Such deterioration affects both the structural integrity of the manuscripts and the completeness of the recorded information, underscoring the need for conservation and restoration [9,10]. With a history extending back at least to the 2nd/3rd centuries CE and continuing to the present, PLMs have endured for centuries despite severe deterioration and increasing fragility [2,11,12]. Given their rarity and immense cultural significance, the application of effective materials and techniques to reinforce these manuscripts and secure their long-term preservation is imperative.
To extend PLMs’ lifespan, various conservation strategies have been employed. Traditional toughening methods have relied on both natural substances (such as plant essential oils and water) and synthetic chemical agents [6,13,14]. In the early 1990s, plant essential oils were investigated for their enhancement of PLM flexibility [15]. Oils such as clove, camphor, and eucalyptus are readily absorbed by palm-leaf cells and significantly improve flexibility [15]. In contrast, certain viscous and sticky oils, including mustard, cedarwood, and castor oils, fail to maintain leaf pliability. Other approaches include treating leaves with spices such as turmeric and calamus to repel insects, applying organic oils such as lemongrass oil to retain leaf flexibility and deter pests, and fumigating with thymol vapors to restrict fungal growth [6]. Conversely, essential oils can act as consolidants to seal insect perforations. Nevertheless, their application often causes discoloration and adhesion between leaves [9]. Regarding synthetic reagents, substances such as glycerol have been used to toughen PLMs [16]. However, their volatility limits the durability of their strengthening effect. Recently, Zhu et al. [17] investigated the influence of ionic liquids on the toughness of PLMs. The ionic liquid ([BMIm][BF4]) improved tensile strength and fracture toughness without altering its appearance. It penetrated the manuscripts, filled internal pores, and adsorbed strongly to cellulose, thereby enhancing load transfer, reducing stress concentration, and increasing toughness. Remarkably, [BMIm][BF4] remained effective under harsh conditions, including low humidity, elevated temperatures, alkaline environments, and UV exposure [17].
Despite these efforts, there are two major problems associated with current studies. First, there is permeability and volatility. Both natural materials, such as essential oils, and synthetic materials, such as glycerol or ionic liquids, permeate into the PLMs, creating structural gradients that damage the manuscript [18]. Moreover, the volatility of these materials limits the durability of their strengthening effects [17]. Second, the durability evaluation method is flawed. Previous studies have typically focused on ambient conditions (23 ± 1 °C and 50 ± 2% RH) [17] or dry-heat accelerated aging at 105 °C [15]. However, evaluating the aging conditions in humid environments, such as the climate of Yunnan, China, and Southeast Asia, is more significant for the long-term protection of palm-leaf manuscripts.
Given these challenges, the conservation of PLMs remains a pressing issue. Research on effective protective materials remains in its infancy. Although numerous studies have examined materials for preserving organic cultural artifacts, the unique structure and performance characteristics of PLMs, unlike those of paper, wood, and other organic substrates, may significantly complicate the evaluation of these protective methods. This complexity highlights the urgent need for systematic exploration and evaluation of suitable materials specifically tailored to impart durable protective effects to PLMs.
To address these challenges, this study applied a novel approach of directly encapsulating PLMs with polymers. In this method, a polymer solution was applied to the manuscript surface to form a continuous protective coating that encapsulates the entire leaf. The research objective was to enhance durability by providing a barrier to environmental degradation. The present work employed moist heat accelerated aging (80 °C and 65% RH) to more effectively simulate degradation in high-temperature and humid environments. Two polymers, chitosan quaternary ammonium salt (CQAS) and cationic polyacrylamide (CPAM), were selected for investigation. CQAS can exhibit strong antibacterial activity and high water solubility owing to the presence of quaternary ammonium groups [19,20]. Hence, CQAS is considered among the most promising candidates for antibacterial coatings for the preservation of organic heritage materials. PAM, as a synthetic polymer widely applied in wastewater treatment, papermaking, enhanced oil recovery, soil conditioning, erosion control, and medical devices, acquires antibacterial properties through cationization [21]. In this study, both materials, CPAM and CQAS, were comprehensively evaluated with respect to their surface morphology, color stability, lightness, mechanical behavior, infrared spectral characteristics, thermal degradation, and antibacterial performance. In this study, through a systematic assessment, we offer valuable insights and alternative references for improving the durability of PLMs. These findings are expected to support the development of more effective conservation strategies while advancing the practical application of polymer-based preservation techniques to safeguard these cultural artifacts.
2. Materials and Methods
2.1. Materials
PLMs were provided by Dai Autonomous Prefecture Library of Xishuangbanna. They were obtained from the leaves of the talipot palm (Corypha umbraculifera), a tall and robust arborescent plant characterized by its long, wide, and densely textured foliage [1,7,22,23]. The preparation of PLMs involved a series of meticulous steps, including leaf collection, cutting, steaming, polishing, flattening, air-drying, engraving, inking, and binding [3,24,25,26,27].
CQAS: Chitosan quaternary ammonium salt (degree of quaternary ammonium was 98%, purchased from Shanghai Macklin Biochemical Co., Ltd., Shanghai, China) was prepared as a 5% (w/w) aqueous solution.
CPAM: Cationic polyacrylamides (degree of ionization was 30–35%, purchased from Shanghai Macklin Biochemical Co., Ltd., Shanghai, China) were prepared as a 2% (w/w) aqueous solution.
The PLMs were cut into strips measuring 15 cm × 2 cm, immersed in CQAS or CPAM solutions for 5 s, and passed through a slit to remove excess liquid from the surface. The treated samples were air-dried at 25 °C for 48 h. The PLM samples encapsulated with CPAM and CQAS are designated as PLM-CPAM and PLM-CQAS, respectively. The schematic diagram depicting the procedure for test specimen preparation and moist heat-aging treatment is presented in Figure 1.
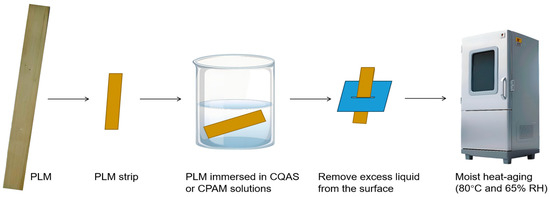
Figure 1.
Schematic diagram of the procedure for test specimen preparation and moist heat-aging treatment.
2.2. Aging Treatment
A moist heat-aging method was used to assess the tensile strength. The samples were exposed to a constant-temperature and humidity chamber (Model JW-2004, Shanghai Juwei Instrument Equipment Co., Ltd., Shanghai, China) in accordance with the GB/T 22894-2008 standard [28] for accelerated aging of paper and paperboard (moist heat method). The experimental conditions were set at 80 °C and 65% relative humidity, with aging durations of 7, 14, 21, and 28 days.
2.3. Microscope Observation
The surface and cross-sectional morphologies of control PLMs and those encapsulated in CPAM and CQAS were recorded using a Nikon SMZ18 microscope (Nikon Corporation, Tokyo, Japan).
2.4. Color Measurements
Colorimetric analysis was performed using a 3nh colorimeter (Model NR10CQAS, Shenzhen Sanen Shi Technology Co., Ltd., Shenzhen, Guangdong, China) based on the CIE 1976 (CIELAB) L*, a*, and b* color space system established by the International Commission on Illumination. In this system, the L-axis represents lightness, the a-axis represents the green–red range, and the b-axis represents the blue–yellow range. The color difference (ΔE*) was calculated according to Equation (1). Each sample test was in triplicate.
2.5. Gloss Testing
Gloss measurements were conducted using a gloss meter (HST268, Xiangbang Automation Instrument and Equipment Co., Ltd., Shenzhen, Guangdong, China) at incident light angles of 20° and 60°. Each sample was repeated in triplicate.
2.6. Contact Angle Test
The static contact angles of the samples were measured using an SL200 contact-angle system (KINO Scientific Instrument Inc., Somerville, MA, USA) with water at room temperature. The volume of each drop was set as 2 µL for the contact angle investigation. Each sample test was repeated twice.
2.7. Determination of the pH Value
The pH value was measured according to the GB/T 13528–2015 standard [29] for paper and paperboard. A HANNA pH meter (Model HI99171, Hanna Instruments (Shanghai) Co., Ltd., Shanghai, China) was used for this purpose. Each sample test was repeated thrice.
2.8. Scanning Electron Microscopy (SEM)
The surface and cross-section morphology of all samples was observed using an SEM (5 kV) system (Model ZEISS sigma 360, Carl Zeiss Microscopy GmbH, Jena, Germany), and all samples were gold-sputtered before observation. The fracture surface morphology of the selected specimens was observed after they were fractured in liquid nitrogen.
2.9. Tensile Strength Testing
The tensile strength of PLM samples under different aging conditions was evaluated using a universal testing machine (WDW-100, Changzhou Sanfeng Instrument Technology Co., Ltd., Changzhou, Guangdong, China) at a test speed of 0.5 mm/min. The gauge marks (initial length) used for the tensile test were 5 cm. Each sample test was repeated thrice.
2.10. Fourier Transform Infrared Spectra (FTIR)
FTIR of PLM samples was recorded using a spectrometer (Model Nicolet iS20, Thermo Fisher Scientific, Waltham, MA, USA) over a wavenumber range of 4000–400 cm−1, with a spectral resolution of 4 cm−1. The measurements were conducted in Attenuated Total Reflectance (ATR) mode, and the type of crystal used is diamond. Each sample test was repeated twice.
2.11. Thermogravimetric Analysis (TGA)
The thermal stability of PAM samples was analyzed using a TGA analyzer (Q500, TA Instruments Inc., New Castle, DE, USA). Tests were performed over a temperature range of 30–800 °C with a heating rate of 10 °C/min under a nitrogen flow rate of 40 mL/min.
2.12. Strains
Five fungal strains were tested in this study, including Aspergillus niger, Trichoderma sp., Chaetomium globosum, Penicillium chrysogenum, and Cladosporium cladosporioides. All strains were isolated and purified in the laboratory from the surface of palm-leaf manuscripts in the lab. Subsequent taxonomic identification of these isolates was performed using polymerase chain reaction (PCR) technology. All strains were stored on PDA plates at 4 °C.
2.13. Antifungal Assay
A small amount of mycelium of the test strains preserved on PDA plates was selected under aseptic conditions and transferred onto fresh PDA plates, respectively. The inoculated plates were incubated at 28 °C for 3 days to revitalize the strains until vigorous growth was observed. The antifungal effect of coated palm-leaf disks was evaluated using a PDA plate assay, wherein both coated (experimental) and uncoated (control) disks were placed on agar medium, inoculated with pre-activated test strains near the disk edge, and incubated at 28 °C for 3 days. After incubation, fungal growth on the disks was assessed using the cross-cross method by comparing mycelial coverage, density, and sporulation against the control group to determine antibacterial efficacy. Each sample test was repeated thrice.
3. Results and Discussion
3.1. Surface Properties Analysis
Improving the durability of PLMs is critical for their long-term preservation. Encapsulation with protective agents is an effective mitigator of embrittlement. After cutting and processing, the internal structure of PLMs was exposed at the edges. While painting the edges of certain PLMs is considered a form of encapsulation, engraved PLMs contain surface notches that not only reduce the mechanical strength but also permit moisture ingress, thereby accelerating hydrolysis and aging. In such cases, surface changes may not be readily visible, whereas side encapsulation alone cannot prevent performance degradation caused by notch defects. Therefore, a full encapsulation strategy is required to provide comprehensive protection.
In this study, CPAM and CQAS were applied to encapsulate PLM surfaces, and their effects on structural and property changes under humid and hot aging conditions were compared. Although cellulose, hemicellulose, and lignin are primary components, the structure of PLMs differs from that of wood, paper, and other lignocellulosic materials. This difference arises because PLMs retain the intact structure of the original leaf rather than the extracted fibers, although they can be subjected to certain cooking treatments. Leaf structure has been extensively reported in the studies [11]. Figure 2 illustrates the optical microscopy images of the untreated PLMs and those encapsulated with CPAM and CQAS. Both CPAM and CQAS formed coatings on the PLM surfaces. The unaged PLM-CPAM sample appeared similar to the control, whereas PLM-CQAS produced noticeable differences in color and gloss. With the progression of moist heat aging, these differences became increasingly pronounced. By day 14, the PLM-CQAS displayed mottling (Figure 2f), which deepened in color by day 28 (Figure 2i). In contrast, the PLM-CPAM remained closer in appearance to the control, demonstrating a trend further confirmed by subsequent color difference and gloss analyses.
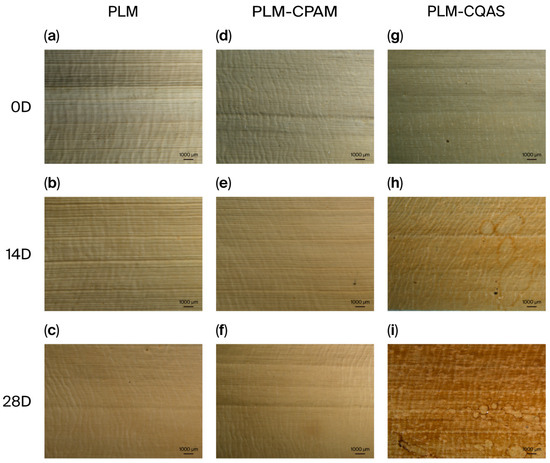
Figure 2.
Surfaces of microscopic images of (a–c) PLMs, (d–f) PLM-CPAM, and (g–i) CQAS, as well as the changes after moist heat aging treatment (D—day).
Encapsulation with CPAM and CQAS provided complete coverage, including the cut ends (Figure S1). The cut surface of the untreated PLM revealed visible internal fibers (Figure S1a). In contrast, the samples encapsulated with CPAM and CQAS exhibited filled internal pores, forming a continuously sealed surface (Figure S1d,g). The seal remained intact even after prolonged moist heat aging.
To evaluate the influence of different encapsulation agents on PLM color, the colorimetry of the unaged control was selected as the baseline, enabling comparisons between the encapsulated and unencapsulated samples at identical time points during moist heat aging. Figure 3a shows the changes in color difference before and after encapsulation, with specific LAB values provided in Table S1. The results showed a progressive increase in the color difference in the control PLM during moist heat aging. On day 0, the LAB values of the control samples corresponded well with those previously reported in the literature [30]. After aging, the color difference rose to 4.39 within the first 7 days, followed by a slower increase of approximately 1.0–2.0 every 7 days. As a colorless and transparent solution, PLM-CPAM exhibited a trend in color difference similar to that of the control, though consistently slightly higher. In contrast, CQAS, a slightly yellow solution, produced a marked color difference immediately upon application, visible to the naked eye. The color difference between PLM-CPAM and the control samples remained within 3.0 (Figure 3a, red bars). In contrast, the PLM-CQAS exhibited significantly higher discrepancies than the untreated palm leaves, with values exceeding 5.0 (Figure 3a, blue bars). This divergence became increasingly pronounced in the later stages of aging, showing a sharp upward trend and reaching 9.2.
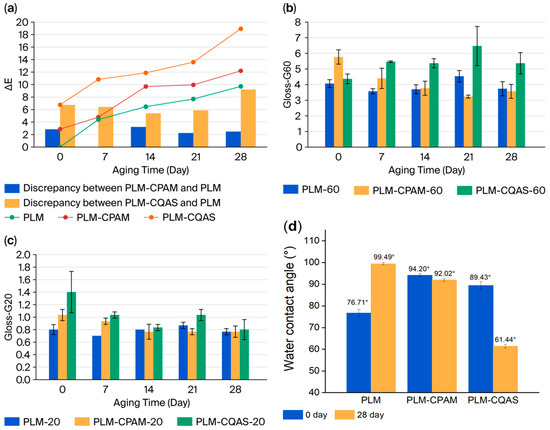
Figure 3.
Changes in the (a) color difference, (b) gloss G60, (c) gloss G20, and (d) static contact angle of PLMs, PLM-CPAM, and PLM-CQAS.
The gloss values G60 and G20 of the control samples fluctuated around 4.0 and 0.8, respectively (Figure 3b,c). For PLM-CQAS, the average G60 value initially increased from 4.0 to 6.5 by day 21, then declined to 5.2, while G20 gradually decreased. Although PLM-CPAM showed a slightly higher initial gloss than the control, the gloss values steadily decreased during aging, converging towards those of the control samples. Overall, both the G20 and G60 values of PLM-CPAM remained closer to those of the control than CQAS, demonstrating that CPAM provided superior retention of optical properties. These results clearly indicated that with respect to both color stability and gloss, CPAM outperformed CQAS.
Figure 3d provides a detailed depiction of the static water contact angle measurements for the three materials under investigation: PLM, PLM-CPAM, and PLM-CQAS. Upon subjecting these materials to conditions of moist heat aging, a notable shift in the water contact angle of PLM was observed, escalating from an initial 75.06° to a subsequent 98.96°. This increase underscores a significant enhancement in the material’s hydrophobic characteristics. In the case of PLM-CPAM, a subtle decrease in the water contact angle was recorded, suggesting a marginal change in its hydrophilic or hydrophobic properties. This subtlety indicates that the CPAM coating imparts a degree of stability to the PLM surface, resisting substantial alterations in wettability. Conversely, PLM-CQAS demonstrated a marked increase in hydrophilicity post-exposure to moist heat aging. The water contact angle for this treatment decreased significantly from 91.31° to 62.32°, highlighting a pronounced shift towards wettability. Although CPAM and CQAS are both hydrophilic polymers, their application as surface coatings on PLM surfaces results in contrasting hydrophilic or hydrophobic behaviors under conditions of moist heat aging. This divergence in surface behavior may be attributed to the inherent differences in the acidic or basic properties of CPAM and CQAS, which could precipitate a series of changes at the material surface (Figure S2). CPAM, with its basic nature, appears to have a minimal impact on the degradation of the underlying PLM, leading to minimal surface changes. In stark contrast, CQAS, characterized by its acidity, may cause more severe degradation. This degradation could potentially expose the internal cellulosic components of the PLM surface after an extended period, such as 30 days, thereby increasing its hydrophilicity. The hypothesis regarding the degradation and subsequent exposure of cellulosic components is further corroborated by the findings from subsequent scanning electron microscopy (SEM) imaging and infrared spectroscopy analyses. These analyses provide a more nuanced understanding of the material’s surface chemistry and morphology, reinforcing the observed changes in hydrophilicity.
Figure 4 shows the SEM images. After 28 days of moist heat aging, the control PLM displayed more pronounced surface pores and an evident collapse of the internal structure. In contrast, the PLM-CQAS appeared to be covered by a thicker coating, while the cross-sectional images obtained through liquid nitrogen fracturing revealed a slight internal collapse. In comparison, the PLM-CPAM retained visible pores and clear contours of internal fiber bundles, with the overall internal structure remaining intact. This preservation of structural integrity indicated superior mechanical performance, as fewer structural defects were observed.
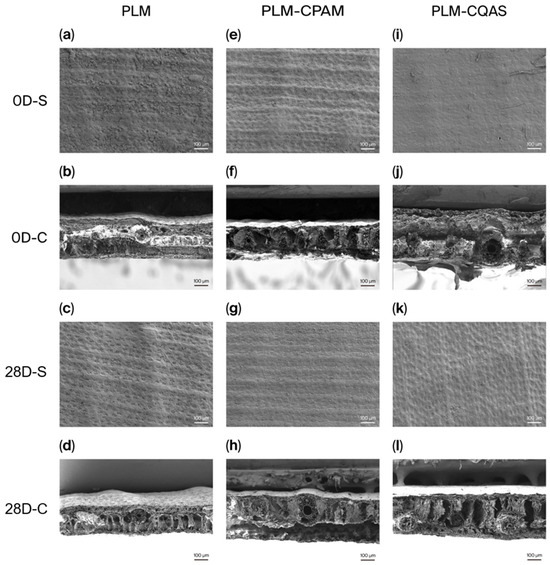
Figure 4.
SEM images of the surfaces and cross-sections of (a–d) PLMs, (e–h) PLM-CPAM, and (i–l) CQAS after moist heat aging treatment. (D—day; S—surface; C—cross-section).
The thickness of the coatings on both PLM-CPAM and PLM-CQAS diminished progressively throughout the moist heat aging process (Figure S3). Notably, the initial phase of aging significantly reduced coating thickness, which subsequently tapered off. Specifically, the thickness of the PLM-CPAM coating, which initially measured 1.64 ± 0.15 μm, decreased to 0.52 ± 0.06 μm following 14 days of exposure to moist heat conditions, and ultimately reached 0.41 ± 0.15 μm after 28 days. In contrast, the PLM-CQAS coating, with an initial thickness of 2.94 ± 0.20 μm, reduced to 0.38 ± 0.06 μm by the 14th day. By the 28th day, only a minimal amount of CQAS likely remained on the PLM surface, with the majority of the underlying surface being exposed.
3.2. Tensile Properties
To assess the mechanical properties, this study employed moist heat-accelerated aging at 80 °C and 65% RH, adapted from paper testing methods, to evaluate the effectiveness of encapsulation under high-temperature and humid conditions. Figure 5 shows the longitudinal tensile curves of the control PLM and its CPAM- and CQAS-encapsulated counterparts. The results showed that the tensile strength of the control PLM was approximately 17 MPa, which was significantly lower than the 40 MPa reported in the literature [30]. This discrepancy may reflect the differences in preparation years and batches, although it remains consistent with other reported values converted to MPa [11]. During aging, the tensile stress of the control PLM initially increased before gradually declining, likely owing to the compaction of the internal structure during early aging, which temporarily improved the strength. As the collapse progressed and internal defects accumulated, strength decreased. By day 28, the tensile strength decreased to 5 MPa, while the strain decreased from 4.4% to 0.5%. Fractographic observations confirmed that moist heat aging caused varying degrees of contraction and delamination between surface and internal fibers (Figure S4). Encapsulation with CPAM and CQAS significantly enhanced the tensile strength, increasing it from 17 to 32 and 28 MPa, respectively, although the strain values remained similar across all three groups (~4.4%). With continued aging, both encapsulated samples exhibited a downward trend. By day 28, the tensile stress of the encapsulated samples reached 8 MPa at a strain of 1%, which was superior to that of the unencapsulated control.
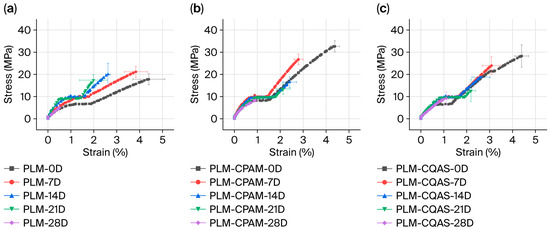
Figure 5.
Tensile properties of (a) PLMs, (b) PLM-CPAM, and (c) PLM-CQAS after moist heat aging treatment. (D—day).
Notably, the PLM samples displayed stress yielding during tensile testing, indicating plastic deformation or permanent structural changes in the palm-leaf fibers. This deformation appeared relatively uniform within a specific strain range, resulting in stress plateaus. The tensile behavior observed here corresponds well with literature reports [11]. The yield strength of the control increased from 5 to 10 MPa after aging. In the PLM-CQAS group, the yield strength increased from 7 to 10 MPa, whereas PLM-CPAM maintained a stable yield strength of 10 MPa. Overall, CPAM encapsulation provided more effective tensile enhancement for PLMs than both PLM-CQAS and the control PLM.
CQAS and CPAM both serve as functional modifiers for paper. In coating technology, CQAS and ZnO was successfully synthesized a functional nanocomposites [31], which provided excellent support. Although only a 72 h dry heat accelerated aging process was employed, the results demonstrated significant improvements in the mechanical strength, alkalinity, and pH of the coated paper. A CQAS/PVA coating was formulated by blending CQAS with polyvinyl alcohol (PVA) [32]. Investigations revealed that the porosity of the coating diminished with increasing CQAS concentration. CQAS effectively filled the interstitial spaces between PVA fibers, thereby reducing the pore area in the base paper. Upon the addition of higher concentrations of CQAS to PVA, aggregation and network formation between CQAS and PVA occurred, which further decreased the porosity. As a dry strength resin, CPAM was applied to the surface of dried paper via an external coating method [33]. Studies have demonstrated that paper with double-sided coatings exhibited superior strength compared to that with single-sided coatings, which in turn outperformed paper treated with the impregnation method. This indicates that the external coating method is significantly better at enhancing paper strength. In the realm of paper fillers, cationic polyacrylamide and cationic corn starch were incorporated into PCC (precipitated calcium carbonate) particles to produce a modified filler with a cationic structure [34]. This filler enhanced the hydrogen bonding capability with cellulose fibers, thereby augmenting the performance of the paper.
PLM exhibits substantial structural disparities from paper, lacking the extensive porosity and textural architecture characteristic of paper. Consequently, CQAS and CPAM did not infiltrate the PLM surface and form a concentration gradient inward as ionic liquids would [17]. Instead, they merely adhered to and combined with the PLM surface. The enhancement of PLM performance relied on the adhesion properties of the coating to the substrate and the intrinsic strength of the coating. Although the CPAM coating was thinner than the CQAS coating, it likely possessed superior adhesion and a more neutral pH value. As a result, during the aging process, PLM-CPAM maintained better tensile strength. In contrast, CQAS acidified and degraded more rapidly during moist heat aging. However, its thicker coating and superior strength similarly mitigated the decline in tensile strength of PLM compared to that of pure PLM.
3.3. FT-IR Analysis
Figure 6 shows the ATR-FTIR of PLMs and their CPAM- and CQAS-encapsulated samples. Figure 6a–c present the spectral peak changes in unencapsulated PLMs under moist heat aging. The major components of PLMs are similar to those of wood, consisting primarily of cellulose, hemicellulose, and lignin, along with other substances such as fats and proteins [35,36]. Infrared analysis revealed that the broad band at 3000–3700 cm−1 corresponded to the −OH stretching vibrations of the hydrogen bonding network in cellulose and hemicellulose [37]. The peaks at 2916 and 2848 cm−1 represented asymmetric and symmetric CH2 stretching, respectively. Although often attributed to cellulose and hemicellulose [38], these peaks were strongly associated with lipids in PLMs [11,39,40,41], as the leaf structure was retained. The degradation of these lipid and wax layers under external stress weakened their absorption intensity [11,42]. The absorption peak near 1732 cm−1 was assigned to the C=O stretching in acetyl and carboxyl groups, a key marker for distinguishing hemicellulose from cellulose and lignin [36,42,43,44]. The peaks around 1655 cm−1 were linked to the aromatic skeletal vibrations of lignin [45,46], whereas the 1464 cm−1 peak resulted from the C=C stretching and aromatic ring vibrations of lignin [47]. The 1370 cm−1 peak represented cellulose, which was attributed to aliphatic C–H bending in CH3 [48,49]. An additional peak at 1161 cm−1 corresponded to the C–O–C stretching in cellulose and hemicellulose [42]. The peak at 1505 cm−1 related to butyl propane backbone vibration in butyl lignin was selected as the characteristic lignin marker [4,50].
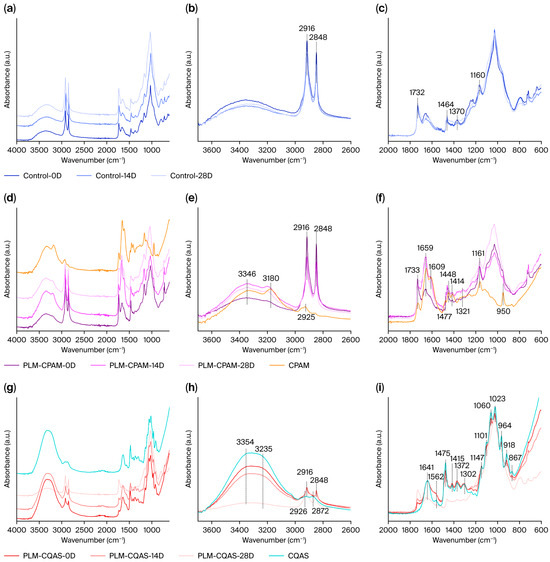
Figure 6.
Infrared spectra of (a–c) PLMs, (d–f) PLM-CPAM, and (g–i) PLM-CQAS after moist heat aging treatment (D—day).
Previous studies included the use of I1730/I1505 and I1370/I1505 ratios to evaluate the relative changes in hemicellulose, cellulose, and lignin [3]. The results indicated that the relative intensities of the cellulose- and hemicellulose-associated peaks decreased to varying extents during moist heat aging, with greater changes observed as aging progressed. Notably, the cellulose peaks exhibited a faster and more pronounced decline than those of hemicellulose. Although some studies have suggested that hemicellulose undergoes more rapid degradation [38], our findings indicate that cellulose degrades slightly faster than hemicellulose (Figure S5). The infrared ratios provided only indicative trends, and further studies are required to validate these results.
Figure 6d–f illustrate the FTIR of PLM-CPAM after moist heat aging. As shown by the orange curves, PAM exhibited a strong and broad peak at 3346 cm−1, attributed to N–H stretching in amide groups, indicating the presence of a primary amine with symmetric stretching. The –OH peak was observed at 3180 cm−1, whereas the asymmetric CH2 stretching of –N+(CH3)2C2H5 appeared at 2925 cm−1. The characteristic peaks of CPAM were also recorded at 1609 cm−1 (bending of imino groups) and 1659 cm−1 (C=O stretching in –CONH2) [51,52,53]. The absorption peaks at 1477 and 950 cm−1 were assigned to CH2 vibrations in –CH2-N+(CH3)2C2H5 and to the characteristic quaternary ammonium group, respectively. The peak at 1448 cm−1 corresponded to CH3 deformation in –N+(CH3)3 [54]. The peak at 1321 cm−1 was attributed to -CO2- groups, whereas the absorption at 1161 cm−1 corresponded to the bending vibration of acyl oxygen groups. These results indicated that the CPAM molecules containing positively charged –N+(CH3)3 groups exhibited a cationic structure [55].
Figure 6g–i present the ATR-FTIR of PLM-CQAS at different aging times. Across all spectra, the broad band near 3354 cm−1 was attributed to N–H stretching vibrations overlapped with O–H stretching [56]. The weak peaks at 2926 and 2872 cm−1 were attributed to CH stretching of methyl and methylene groups [57,58]. Characteristic N–H stretching bands appeared at both the 1641 and 1562 cm−1 positions [20,59]. The band at 1475 cm−1 corresponded to the CH bending of trimethyl groups [60], whereas the 1415 cm−1 peak was attributed to CH2 deformation in CH2OH groups. The peak at 1372 cm−1 reflected the symmetrical angular deformation of CH3 in NHCOCH3 groups [61]. Additional peaks at 1147, 1060, and 1023 cm−1 were associated with C–O stretching vibrations [62]. As seen in Figure 6d–i, absorption peaks of PLM-CPAM and PLM-CQAS overlapped substantially with those of the control, preventing a clear evaluation of relative content changes via the I1730/I1505 and I1370/I1505 ratios. While more cellulose, hemicellulose, and lignin may be exposed due to surface lipid degradation, the CPAM absorption peaks overlapped extensively with those of PLMs, making it difficult to distinguish compositional variations. The absorption peaks of CPAM overlapped with the major peaks of PLMs in the range between 1100 and 1800 cm−1. Although not fully consistent, all characteristic peaks were enhanced (Figure 6f). In contrast, CQAS exerted a more pronounced influence on the infrared spectra of PLMs, with the coating-related signals remaining clearly visible between 600 and 1800 cm−1. Under moist heat aging, these characteristic peaks within 600–1900 cm−1 gradually shifted towards the intrinsic PLM peaks, indicating the progressive degradation of CQAS on the surface. Notably, the peak at 1732 cm−1, characteristic of hemicellulose, was absent in CQAS. Its suppression in encapsulated samples may result from surface coverage by CQAS, which weakens hemicellulose absorption. As the coating degraded, the palm-leaf surface was re-exposed, leading to an increase in hemicellulose absorption intensity at this position.
It is worth noting that the infrared absorption spectra depicted in the figures reveal distinct degradation patterns for CPAM and CQAS. For CPAM, a slight attenuation in absorption intensity is observed at 1609 cm−1 (attributable to the bending vibration of imino groups) and 1659 cm−1 (corresponding to the C=O stretching vibration within the -CONH2 moiety) [51,52,53]. Additionally, the disappearance of the absorption peak at 950 cm−1, which is characteristic of the quaternary ammonium group in -CH2-N+(CH3)2C2H5, indicates that the primary site of molecular chain scission in CPAM is within the side-chain groups. Conversely, the characteristic N–H stretching bands of CQAS, located at 1641 and 1562 cm−1 [20,59], exhibit no significant change in intensity. However, a notable reduction in intensity is observed for the additional peaks at 1147 cm−1, 1060 cm−1, and 1023 cm−1, which are associated with C–O stretching vibrations [62]. This suggests that the degradation of CQAS primarily occurs within the main chain. Additionally, the degradation spectra of PLM-CPAM and PLM-CQAS further illustrate the disparities in degradation extent following moist heat aging. At the 28-day mark, PLM-CPAM continues to exhibit characteristic peaks attributable to both PLM and CPAM, signifying the retention of the CPAM coating on the PLM surface. Conversely, the infrared spectrum of PLM-CQAS at 28 days reveals only the characteristic peaks of PLM, indicative of the degradation of the CQAS coating. These observations concur with the findings depicted in Figure S3, which delineate the alterations in the CPAM and CQAS coatings.
3.4. Thermal Stability Analysis
Figure 7 shows the TGA curves and derivative weight profiles of the PLM, PLM-CPAM, and PLM-CQAS samples. Their data results are listed in Table S2. All samples exhibited three distinct stages: low-temperature decomposition (<200 °C), rapid decomposition (200–500 °C), and equilibrium (>500 °C). In the low-temperature stage, approximately 10% of the sample mass was lost because of moisture evaporation and the release of volatile small molecules. In the main degradation stage, the DTG curves of aged samples indicated three distinct sub-stages: 200–330 °C, 330–370 °C, and 370–500 °C. These degradation patterns differed from those reported in the literature [30], primarily owing to the different heating rates employed in TG tests. A heating rate of 10 °C/min was adopted for the study, whereas previous reports adopted 20 °C/min.
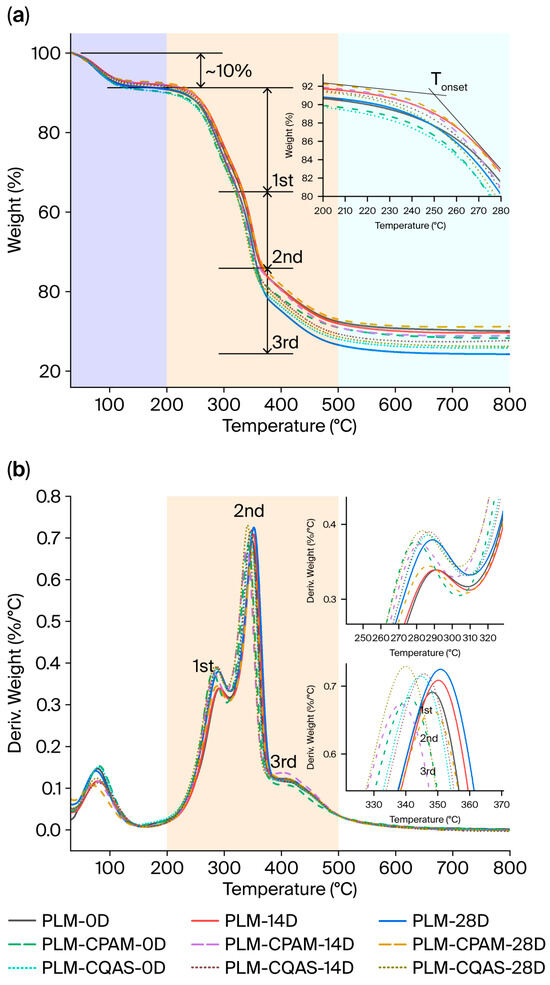
Figure 7.
(a) TGA and (b) DTG results for PLMs, PLM-CPAM, and PLM-CQAS after moist heat-aging treatment (D—day).
In general, cellulose and hemicellulose decompose almost completely within 200–500 °C, resulting in low carbon yields. Lignin, with its thermally stable aromatic rings, decomposed over a broader temperature range at higher temperatures, leading to higher carbon yields [63]. Thus, biomass with higher lignin content typically produces greater carbon residue, whereas higher cellulose content results in lower carbon yields. The preparation of PLMs, involving soaking and boiling talipot leaves in acidic solutions, such as sour horn and lemon in rice-washing water, introduced significant amounts of residual acidic substances. During the moist heat treatment, the pH of all samples steadily decreased (Figure S2), influencing the thermal degradation behavior.
The surface coating further affected thermal stability. In the control group, the initial degradation temperature decreased after moist heat aging owing to acidification, accelerating the degradation of cellulose, hemicellulose, and even lignin. The carbon yield decreased to 24.21%, suggesting either low lignin content or pre-aging of the samples. In contrast, PLM-CPAM encapsulation provided protection, as evidenced by a slightly higher initial degradation temperature after 28 d of moist heat aging and a higher carbon yield, confirming the protective function of the coating. The PLM-CQAS group exhibited a lower overall pH, likely resulting from acid-induced degradation of internal PLM components, which in turn reduced the thermal stability.
3.5. Antifungal Performance Analysis
Figure 8 illustrates a comparative analysis of the antifungal efficacy between control, PLM-CPAM, and PLM-CQAS against Aspergillus niger, Trichoderma sp., Chaetomium globosum, Penicillium chrysogenum, and Cladosporium cladosporioides. Macroscopically, with the exception of Cladosporium cladosporioides, the remaining four fungal species produced distinct colonies or areas of corrosion on the surface of the blank samples. Notably, Trichoderma sp. nearly covered the entire blank area, highlighting its pronounced invasive capabilities. This observation also implies that the base material is devoid of inherent antimicrobial properties.
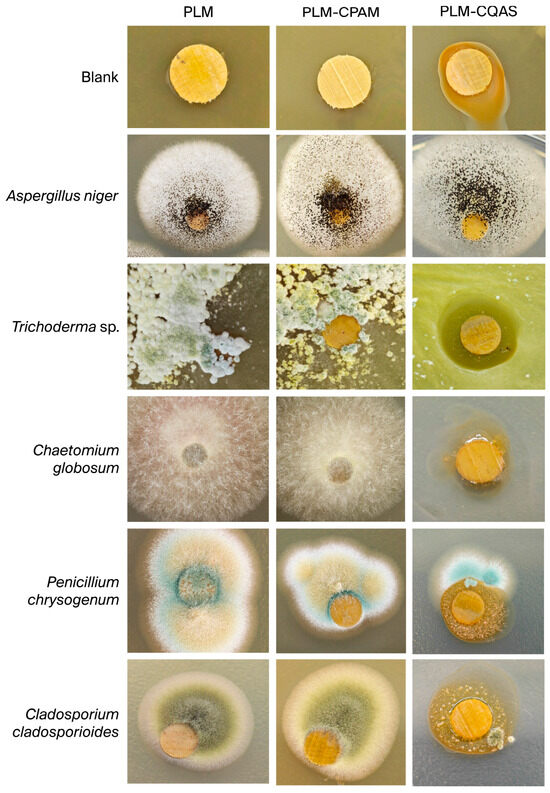
Figure 8.
Antibacterial properties of PLMs, PLM-CPAM, and PLM-CQAS.
The PLM-CPAM samples exhibited significant inhibitory effects against Trichoderma sp. and Penicillium chrysogenum, evidenced by a marked decrease in mycelial thickness. However, the inhibitory boundary against Aspergillus niger was challenging to distinguish due to its dense and dark mycelium, which compromised its effectiveness. It is also noteworthy that Chaetomium globosum continued to thrive on the PLM-CPAM surface, suggesting that CPAM is largely ineffective against this particular strain.
Conversely, the PLM-CQAS samples demonstrated the most remarkable broad-spectrum antimicrobial activity, completely inhibiting all four fungi, with the exception of A. niger. Even the robustly growing Aspergillus niger was unable to cover the PLM-CQAS surface, with only minor colonies forming at the periphery. In conclusion, CQAS effectively disrupts fungal cell membranes and inhibits spore germination through the incorporation of quaternary ammonium cations, thereby offering comprehensive protection to palm-leaf manuscripts. Although CPAM is effective against certain fungi, its inefficacy against Chaetomium species renders its overall protective capability inferior to that of CQAS. Further refinement of the long-term weather resistance performance can be anticipated by modulating the density of quaternary ammonium cations and the thickness of the CQAS film.
CQAS is a highly promising antimicrobial agent for the protection of paper [64] and cotton fabric [65]. A combination, such as clotrimazole (CTZ) and CQAS, was combined to prepare a microemulsion [66]. The synergistic effects of CTZ and CQAS can enhance the antifungal effect and reduce the required concentration of a single drug. This composite emulsion can effectively improve the retention of drugs on the surface of paper cultural relics and enhance the solubility of hydrophobic drugs. Additionally, the incorporation of nano-zinc oxide composites can also effectively improve antimicrobial properties [31]. Although the antimicrobial efficacy of CPAM is not as pronounced as that of CQAS, the future development trajectory may be inclined towards the development of novel composite materials that synergistically integrate optical properties, mechanical properties, and antimicrobial performance.
4. Conclusions
This study included a comprehensive evaluation of the effects of CPAM and CQAS encapsulation on the preservation of PLMs under moist heat aging. The results demonstrated that both materials enhanced the durability and mechanical performance of the manuscript. Encapsulation materials notably influenced the color difference, gloss, and hydrophilicity of PLMs. PLM-CPAM maintained a closer match to the original manuscript and exhibited minimal changes even after accelerated aging, whereas PLM-CQAS caused more pronounced color shifts, gloss variations, and hydrophilicity as aging progressed. These results indicate that CPAM provides stronger protection against color degradation and loss of gloss. The tensile strength of PLMs was significantly improved by both CPAM and CQAS encapsulation, and PLM-CPAM produced a greater increase and better-preserved mechanical integrity during aging. SEM analysis confirmed that CPAM more effectively maintained the internal structural integrity, with fewer defects and superior mechanical stability, whereas the PLM-CQAS samples showed greater internal collapse and degradation after extended aging. The TGA and ATR-FTIR analyses further demonstrated that CPAM encapsulation resulted in the improved preservation of cellulose- and hemicellulose-associated peaks, indicating reduced chemical degradation compared with CQAS encapsulation. Overall, these findings highlight that CPAM showed superior physical preservation, whereas CQAS showed strong antifungal protection. Future work should focus on optimizing the encapsulation process and investigating additional materials, including organic–inorganic composites, to further improve performance and strengthen conservation strategies for this important cultural heritage.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/coatings15101178/s1. Figure S1: Microscope images of the side ends of (a, b, c) PLMs, (d, e, f) PLM-CPAM and (g, h, i) PLM-CQAS after moist heat aging treatment; Figure S2: pH changes of PLMs, PLM-CPAM and PLM-CQAS; Figure S3: SEM images of the surfaces and cross-sections of (a–d) PLMs, (e–h) PLM-CPAM and (i–l) CQAS after moist heat aging treatment; Figure S4: Microscope images of the tensile fracture surfaces of (a, b, c) PLMs, (d, e, f) PLM-CPAM and (g, h, i) PLM-CQAS after moist heat aging treatment; Figure S5: Changes in the ratios I1730/I1505 and I1370/I1505 of the infrared spectra of control PLMs with moist heat aging treatment; Table S1: LAB values of PLMs, PLM-CPAM and PLM-CQAS; Table S2: TGA data of PLMs, PLM-CPAM and PLM-CQAS.
Author Contributions
Conceptualization, P.L. and Y.H.; methodology, S.W., Y.L., H.Y. (Hanwei Yu), H.Y. (Hui Yu) and P.L.; validation, S.W., Y.L. and Y.H.; formal analysis, S.W., Y.L., P.L. and Y.H.; investigation, Y.L., J.Z., H.Y. (Hanwei Yu), R.L., H.Y. (Hui Yu), P.L. and Y.H.; resources, S.W., H.Y. (Hanwei Yu) and P.L.; data curation, S.W., Y.L., J.Z., H.Y. (Hanwei Yu) and R.L.; writing—original draft preparation, S.W., Y.L. and R.L.; writing—review and editing, P.L. and Y.H.; supervision, P.L. and Y.H.; funding acquisition, P.L., Y.L. and Y.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Palm Leaf Manuscript Conservation Foundation of Yunnan Academician Workstation (YAW23002) and the National Ethnic Affairs Commission Ethnic Research Project of China (2024-GMI-036).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wiland, J.; Brown, R.; Fuller, L.; Havelock, L.; Johnson, J.; Kenn, D.; Kralka, P.; Muzart, M.; Pollard, J.; Snowdon, J. A literature review of palm leaf manuscript conservation—Part 1: A historic overview, leaf preparation, materials and media, palm leaf manuscripts at the British Library and the common types of damage. J. Inst. Conserv. 2022, 45, 236–259. [Google Scholar] [CrossRef]
- Voges, L.F.; Horn, N.; Ciotti, G.; Seifert, S. Differentiation of Species and Provenance of Palm-Leaf Manuscripts Using Fourier Transform Infrared Spectroscopy and Chemometrics. Chemosensors 2025, 13, 196. [Google Scholar] [CrossRef]
- Yu, D.; Li, X.; Sun, S.a.; Guo, H.; Luo, H.; Zhu, J.; Li, L.; Wang, S.; Han, L. The effect of traditional processing craft on the hygroscopicity of palm leaf manuscripts. Herit. Sci. 2024, 12, 280. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, S.; Guo, H. Study on the Effects of Temperature and Relative Humidity on the Hygroscopic Properties of Palm Leaf Manuscripts. Forests 2024, 15, 1816. [Google Scholar] [CrossRef]
- Yu, C.; Zhang, M.; Song, X. Analysis of Two Different Inks and Application Techniques on Palm Leaf Manuscripts Through Non-Invasive Analysis. Restaurator. Int. J. Preserv. Libr. Arch. Mater. 2024, 45, 237–256. [Google Scholar] [CrossRef]
- Sharma, D.; Singh, M.R.; Dighe, B. Chromatographic Study on Traditional Natural Preservatives Used for Palm Leaf Manuscripts in India. Restaurator. Int. J. Preserv. Libr. Arch. Mater. 2018, 39, 249–264. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, H.; Li, Y.; Liu, P.; Huang, Y. Advances in Palm Leaf Manuscripts from a Biological Perspective. J. Fudan Univ. (Nat. Sci.) 2024, 63, 658–666. (In Chinese) [Google Scholar]
- Li, Y.; Zhang, J.; Qin, Y.; Li, Y.; Wu, S.; Yan, B.; Yu, H.; Xu, Q.; Liu, P.; Huang, Y.; et al. Scientific restoration of engraved palm leaf manuscripts. NPJ Herit. Sci. 2025, 13, 380. [Google Scholar] [CrossRef]
- Agrawal, O.P. Conservation of manuscripts and paintings of South-East Asia; Butterworth-Heinemann: London, 1984. [Google Scholar]
- Dyke, Y.V. Sacred leaves: The conservation and exhibition of early Buddhist manuscripts on palm leaves. Book Pap. Group Annu. 2009, 28, 83–97. [Google Scholar]
- Chu, S.; Lin, L.; Tian, X. Evaluation of the Deterioration State of Historical Palm Leaf Manuscripts from Burma. Forests 2023, 14, 1775. [Google Scholar] [CrossRef]
- Wiland, J.; Brown, R.; Fuller, L.; Havelock, L.; Johnson, J.; Kenn, D.; Kralka, P.; Muzart, M.; Pollard, J.; Snowdon, J. A literature review of palm leaf manuscript conservation—Part 2: Historic and current conservation treatments, boxing and storage, religious and ethical issues, recommendations for best practice. J. Inst. Conserv. 2023, 46, 64–91. [Google Scholar] [CrossRef]
- Nishanthi, M.; Wijayasundara, N.D. Preservation and Conservation of Palm Leaf Manuscripts at the Library of University of Sri Jayewardenepura. Vidyodaya J. Humanit. Soc. Sci. 2022, 7, 81–96. [Google Scholar] [CrossRef]
- Rachman, Y.B. The Use of Traditional Conservation Methods in the Preservation of Ancient Manuscripts: A Case Study from Indonesia. Preserv. Digit. Technol. Cult. 2017, 46, 109–115. [Google Scholar] [CrossRef]
- Suryawanshi, D.G.; Nair, M.V.; Sinha, P.M. Improving the Flexibility of Palm Leaf. Restaurator. Int. J. Preserv. Libr. Arch. Mater. 1992, 13, 37–46. [Google Scholar] [CrossRef]
- Joshi, B.R. Preservation of Palm Leaf Manuscripts. Conserv. Cult. Prop. 1989, 22, 120–127. [Google Scholar]
- Ding, J.; Yu, J.; Zhu, J.; Han, L.; Guo, H.; Feng, R.; Dong, W.; Zhao, X.; Wang, S.; Li, L.; et al. Exploring the Effects of Ionic Liquid on the Toughness of Palm Leaf Manuscripts. Langmuir 2025, 41, 1625–1638. [Google Scholar] [CrossRef]
- Sah, A. Palm Leaf manuscripts of the world: Material, technology and conservation. Stud. Conserv. 2002, 47, 15–24. [Google Scholar] [CrossRef]
- Chen, Y.; Li, J.; Li, Q.; Shen, Y.; Ge, Z.; Zhang, W.; Chen, S. Enhanced water-solubility, antibacterial activity and biocompatibility upon introducing sulfobetaine and quaternary ammonium to chitosan. Carbohydr. Polym. 2016, 143, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xie, B.; Xia, K.; Zhao, C.; Li, Y.; Li, D.; Han, J. Facile synthesis and characterization of cross-linked chitosan quaternary ammonium salt membrane for antibacterial coating of piezoelectric sensors. Int. J. Biol. Macromol. 2018, 120, 745–752. [Google Scholar] [CrossRef]
- Xue, Y.; Pan, Y.; Xiao, H.; Zhao, Y. Novel quaternary phosphonium-type cationic polyacrylamide and elucidation of dual-functional antibacterial/antiviral activity. RSC Adv. 2014, 4, 46887–46895. [Google Scholar] [CrossRef]
- Freeman, R. Turning Over Old Leaves: Palm Leaves Used in South Asian Manuscripts. Book Pap. Group Annu. 2005, 24, 99–102. [Google Scholar]
- Sharma, D.; Singh, M.; Krist, G.; Velayudhan, N.M. Structural Characterisation of 18TH Century Indian Palm Leaf Manuscripts of India. Int. J. Conserv. Sci. 2018, 9, 257–264. [Google Scholar]
- Panigrahi, A.K.; Litt, D. Odia script in palm-leaf manuscripts. IOSR J. Humanit. Soc. Sci. 2018, 23, 13–19. [Google Scholar]
- Ghosh, S.; Mahajan, A.; Banerjee, S. Palm leaf manuscript conservation, the process of seasoning with special reference to Saraswati Mahal library, Tamilnadu in India: Some techniques. Int. J. Inf. Mov. 2017, 122–128. [Google Scholar]
- Sahoo, J. A Selective Review of Scholarly Communications on Palm Leaf Manuscripts. Libr. Philos. Pract. 2016. [Google Scholar]
- Kumar, D.U.; Sreekumar, G.; Athvankar, U.A. Traditional Writing System in Southern India—Palm Leaf Manuscripts. Des. Thoughts 2009, 7, 2–7. [Google Scholar]
- GB/T 22894-2008; Paper and Board—Accelerated Ageing—Moist Heat Treatment at 80 Celsius and 65% Relative Humidity. National Standards of People’s Republic of China: Beijing, China, 2008.
- GB/T 13528–2015; Paper and Board—Determination of Surface pH. National Standards of People’s Republic of China: Beijing, China, 2015.
- Zhang, W.; Wang, S.; Han, L.; Guo, H. Aging effects of relative humidity on palm leaf manuscripts and optimal humidity conditions for preservation. NPJ Herit. Sci. 2025, 13, 218. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, P.; Huang, Y.; Zhang, H. Nanocomposites composed of modified natural polymer and inorganic nanomaterial for safe, high-efficiency, multifunctional protection of paper-based relics. Sci. China Technol. Sci. 2023, 66, 2225–2236. [Google Scholar] [CrossRef]
- Cheng, Z.; Li, J.; He, G.; Su, M.; Xiao, N.; Zhang, X.; Zhong, L.; Wang, H.; Zhong, Y.; Chen, Q.; et al. Biodegradable packaging paper derived from chitosan-based composite barrier coating for agricultural products preservation. Int. J. Biol. Macromol. 2024, 280, 136112. [Google Scholar] [CrossRef]
- Takushi, S.; Tatsuo, Y. Strength properties of paper containing polyacrylamide-based dry strength resin-effect of its Z-directional distribution. APPITA J. 2011, 64, 331–337. [Google Scholar]
- Nikkhah Dafchahi, M.; Resalati, H.; Zabihzadeh, S.M.; Nazarnezhad, N.; Asadpour, G.; Pirayesh, H. Novel calcium carbonate filler for cellulose industry. Nord. Pulp Pap. Res. J. 2021, 36, 536–547. [Google Scholar] [CrossRef]
- Tang, Z.; Yang, M.; Qiang, M.; Li, X.; Morrell, J.J.; Yao, Y.; Su, Y. Preparation of Cellulose Nanoparticles from Foliage by Bio-Enzyme Methods. Materials 2021, 14, 4557. [Google Scholar] [CrossRef]
- Fang, S.; Lyu, X.; Tong, T.; Lim, A.I.; Li, T.; Bao, J.; Hu, Y.H. Turning dead leaves into an active multifunctional material as evaporator, photocatalyst, and bioplastic. Nat. Commun. 2023, 14, 1203. [Google Scholar] [CrossRef]
- Jayaramudu, J.; Jeevan Prasad Reddy, D.; Guduri, B.R.; Varada Rajulu, A. Properties of natural fabric polyalthia cerasoides. Fibers Polym. 2009, 10, 338–342. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, S.; Guo, H. Study on the Aging Effects of Relative Humidity on the Primary Chemical Components of Palm Leaf Manuscripts. Polymers 2025, 17, 83. [Google Scholar] [CrossRef] [PubMed]
- AlMaadeed, M.A.; Kahraman, R.; Noorunnisa Khanam, P.; Al-Maadeed, S. Characterization of untreated and treated male and female date palm leaves. Mater. Des. 2013, 43, 526–531. [Google Scholar] [CrossRef]
- Kayabas, A.; Yildirim, E. Biochemical Fingerprints of Some Endemic Plants Growing in Gypsum Soils: Attenuated Total Reflection-Fourier Transform Infrared (ATR-FTIR) Spectroscopic Study. Eur. J. Biol. 2021, 80, 97–106. [Google Scholar] [CrossRef]
- Mahdi, A.S.; Abd, A.M.; Awad, K.M. The Role of Nano-selenium in Alleviating the Effects of Salt Stress in Date Palm Trees (Phoenix dactylifera L.): A Fourier Transform Infrared (FTIR) Spectroscopy Study. BioNanoScience 2023, 13, 74–80. [Google Scholar] [CrossRef]
- Chu, S.; Lin, L.; Tian, X. Analysis of Aspergillus niger isolated from ancient palm leaf manuscripts and its deterioration mechanisms. Herit. Sci. 2024, 12, 199. [Google Scholar] [CrossRef]
- Traoré, M.; Kaal, J.; Martínez Cortizas, A. Application of FTIR spectroscopy to the characterization of archeological wood. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 153, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Lazić, B.D.; Pejić, B.M.; Kramar, A.D.; Vukčević, M.M.; Mihajlovski, K.R.; Rusmirović, J.D.; Kostić, M.M. Influence of hemicelluloses and lignin content on structure and sorption properties of flax fibers (Linum usitatissimum L.). Cellulose 2018, 25, 697–709. [Google Scholar] [CrossRef]
- Wang, P.; Pang, J.; Zhang, H.; Liao, H.; Xiong, D.; Zhu, L.; Xue, D.; You, M.; Liu, Y.; Li, J.; et al. Composition, structural, and thermal analysis of cellulose, hemicellulose, and lignin of reconstituted cut stems in tobacco. Ind. Crops Prod. 2024, 222, 119614. [Google Scholar] [CrossRef]
- Ivanova, D.G.; Singh, B.R. Nondestructive FTIR monitoring of leaf senescence and elicitin-induced changes in plant leaves. Biopolymers 2003, 72, 79–85. [Google Scholar] [CrossRef]
- Zhang, T.; Guo, M.; Cheng, L.; Li, X. Investigations on the structure and properties of palm leaf sheath fiber. Cellulose 2015, 22, 1039–1051. [Google Scholar] [CrossRef]
- Schwanninger, M.; Rodrigues, J.C.; Pereira, H.; Hinterstoisser, B. Effects of short-time vibratory ball milling on the shape of FT-IR spectra of wood and cellulose. Vib. Spectrosc. 2004, 36, 23–40. [Google Scholar] [CrossRef]
- He, Z.; Qian, J.; Qu, L.; Yan, N.; Yi, S. Effects of Tung oil treatment on wood hygroscopicity, dimensional stability and thermostability. Ind. Crops Prod. 2019, 140, 111647. [Google Scholar] [CrossRef]
- Marchessault, R.H. Application of infra-red spectroscopy to cellulose and wood polysaccharides. Pure Appl. Chem. 1962, 5, 107–130. [Google Scholar] [CrossRef]
- Feng, L.; Zheng, H.; Gao, B.; Zhao, C.; Zhang, S.; Chen, N. Enhancement of textile-dyeing sludge dewaterability using a novel cationic polyacrylamide: Role of cationic block structures. RSC Adv. 2017, 7, 11626–11635. [Google Scholar] [CrossRef]
- Sun, J.; Ma, X.; Li, X.; Fan, J.; Chen, Q.; Liu, X.; Pan, J. Preparation of a cationic polyacrylamide (CPAM) and its flocculation performance in the environmental estrogen removal and separation. Desalination Water Treat. 2017, 100, 231–242. [Google Scholar] [CrossRef]
- Sun, W.; Zhang, G.; Pan, L.; Li, H.; Shi, A. Synthesis, Characterization, and Flocculation Properties of Branched Cationic Polyacrylamide. Int. J. Polym. Sci. 2013, 2013, 397027. [Google Scholar] [CrossRef]
- Zhao, C.; Zheng, H.; Sun, Y.; Zhang, S.; Liang, J.; Liu, Y.; An, Y. Evaluation of a novel dextran-based flocculant on treatment of dye wastewater: Effect of kaolin particles. Sci. Total Environ. 2018, 640–641, 243–254. [Google Scholar] [CrossRef]
- Gao, M.; Yang, Y.L.; Xu, Z.Q. Preparation of Cationic Polyacrylamide Flocculant and its Application in the Treatment of Coal Slurry Wastewater. Adv. Mater. Res. 2012, 560–561, 591–594. [Google Scholar] [CrossRef]
- Yue, L.; Li, J.; Chen, W.; Liu, X.; Jiang, Q.; Xia, W. Geraniol grafted chitosan oligosaccharide as a potential antibacterial agent. Carbohydr. Polym. 2017, 176, 356–364. [Google Scholar] [CrossRef]
- Zhang, J.; Tan, W.; Li, Q.; Liu, X.; Guo, Z. Preparation of Cross-linked Chitosan Quaternary Ammonium Salt Hydrogel Films Loading Drug of Gentamicin Sulfate for Antibacterial Wound Dressing. Mar. Drugs 2021, 19, 479. [Google Scholar] [CrossRef]
- Rodríguez-Núñez, J.R.; Madera-Santana, T.J.; Sánchez-Machado, D.I.; López-Cervantes, J.; Soto Valdez, H. Chitosan/Hydrophilic Plasticizer-Based Films: Preparation, Physicochemical and Antimicrobial Properties. J. Polym. Environ. 2014, 22, 41–51. [Google Scholar] [CrossRef]
- Barbosa, H.F.G.; Francisco, D.S.; Ferreira, A.P.G.; Cavalheiro, É.T.G. A new look towards the thermal decomposition of chitins and chitosans with different degrees of deacetylation by coupled TG-FTIR. Carbohydr. Polym. 2019, 225, 115232. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.-X.; Wang, L.; Du, L.; Guo, S.-R.; Wang, X.-Q.; Tang, T.-T. Adjustment of the antibacterial activity and biocompatibility of hydroxypropyltrimethyl ammonium chloride chitosan by varying the degree of substitution of quaternary ammonium. Carbohydr. Polym. 2010, 81, 275–283. [Google Scholar] [CrossRef]
- Mauricio-Sánchez, R.A.; Salazar, R.; Luna-Bárcenas, J.G.; Mendoza-Galván, A. FTIR spectroscopy studies on the spontaneous neutralization of chitosan acetate films by moisture conditioning. Vib. Spectrosc. 2018, 94, 1–6. [Google Scholar] [CrossRef]
- Xing, H.; Wang, J.; Ma, O.; Chao, X.; Zhou, Y.; Li, Y.; Jia, Z. Hydroxypropyltrimethyl Ammonium Chloride Chitosan Nanoparticles Coatings for Reinforcement and Concomitant Inhibition of Anionic Water-Sensitive Dyes Migration on Fragile Paper Documents. Polymers 2022, 14, 3717. [Google Scholar] [CrossRef]
- Apaydın Varol, E.; Mutlu, Ü. TGA-FTIR Analysis of Biomass Samples Based on the Thermal Decomposition Behavior of Hemicellulose, Cellulose, and Lignin. Energies 2023, 16, 3674. [Google Scholar] [CrossRef]
- Nechita, P.; Bobu, E.; Parfene, G.; Dinică, R.M.; Bălan, T. Antimicrobial coatings based on chitosan derivatives and quaternary ammonium salts for packaging paper applications. Cellul. Chem. Technol. 2015, 49, 625–632. [Google Scholar]
- Zhang, W.; Zhou, J.; Dai, X. Preparation and characterization of reactive chitosan quaternary ammonium salt and its application in antibacterial finishing of cotton fabric. Text. Res. J. 2017, 87, 759–765. [Google Scholar] [CrossRef]
- Liu, N.; Chu, D.; Chen, X.; Fu, P.; Xing, H.; Chao, X.; Luo, Y.; Mai, B.; Li, Y. A Spray-On Microemulsion with Mold-Proof Effect on Paper. Coatings 2023, 13, 745. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).