Abstract
The durability of cement-based materials can be reduced by the invasion of water and ions from external environments. This can be alleviated by reducing the surface wettability. To evaluate the anti-wetting performances of different graphene-based materials, a molecular dynamics simulation was performed to investigate the wetting behaviors of water and NaCl droplets on a tobermorite surface coated with graphene and functionalized graphene (G-NH2 and G-CH3). The results demonstrate that functionalized graphene displays weak surface binding with water and ions, significantly weakening droplet wettability. Moreover, functionalized graphene surfaces exhibit reduced ion immobilization capacity compared with a pristine tobermorite surface. It obviously increases the number of free ionic hydration shells, thus amplifying the influence of ionic cage restriction. Specifically for the G-CH3 surface, the contact angle of the NaCl droplet reaches 94.8°, indicating significant hydrophobicity. Furthermore, the adhesion between functionalized graphene and tobermorite is attributed to the interlocking characteristics of these materials. Hopefully, this study can provide nanoscale insights for the design of functionalized graphene coatings to improve the durability of cement-based materials under harsh environments.
1. Introduction
Cement-based materials, as the most popular building materials, are widely used in concrete panels of highways, bridges and dams [1]. Water plays a critical role in industrial production [2]. It not only drives the hydration process of cement but also affects the durability of cement-based materials. The cement-based surface functions as a barrier between the internal structure and the external environment, significantly influencing water and ion interactions with the cement matrix. Surfaces with a contact angle less than 90° are defined as hydrophilic surfaces with high wettability, while those with a contact angle greater than 90° are defined as hydrophobic surfaces with low wettability [3]. The surface wettability of cement-based materials significantly influences the extent of water absorption into pores. On the one hand, hydrophilic surfaces facilitate the intrusion of water and harmful ions, accelerating the erosion process [4,5,6]. On the other hand, in cold regions, hydrophilic surfaces lead to the continuous accumulation of pore water, and when saturation is approached, freezing-induced volume expansion results in freeze-thaw damage to the structure [7]. Consequently, research on hydrophobic-surface cement-based materials is rapidly expanding, as reduced wettability enhances durability fundamentally [8,9].
Numerous experimental studies have been conducted to investigate the hydrophobic protection measures for cement-based materials. Currently, hydrophobic materials mainly include additives, polymers, nanomaterials, etc. [10,11]. For instance, Mundo et al. [12] incorporated tire rubber into cement paste and found that rubber could not only reduce paste porosity and block the continuous path of water transmission but also reduce the water contact angle to inhibit the permeation process. Qu et al. [13] investigated the water penetration and chloride corrosion of cement mortar blended with superhydrophobic fly ash, revealing that the modified mortar exhibited a 65.4% reduction in water absorption and 94.09% enhancement in chloride corrosion resistance. Zhang et al. [14] prepared the new organic–inorganic composite coating using a waterborne epoxy resin/silane coupling agent and the metakaolin-based geopolymer, and evaluated its mechanical performance and chloride permeability. To address the high degradability of epoxy coatings in aggressive environments, Yang et al. [15] fabricated waterborne epoxy composite coatings through multicomponent synergistic modification, with results demonstrating significant enhancements in both hydrophobicity and adhesion for the modified coatings.
With the progress of material science, nanomaterials have attracted great interests of researchers. Compared with polymer materials, nanomaterials have a smaller size and larger specific surface area, so they can exert toughening effects at the micro–nano scale [16]. Consequently, nanomaterials have been widely used in the field of cement in recent years [17,18,19]. Graphene-based materials, as an important member of the carbon nano family, exhibit a large specific surface area, high thermal conductivity, and excellent mechanical performance [20]. In addition, many studies have shown that functionalized graphene sheets (FGSs) can effectively enhance the resistance of cement-based materials to water and harmful ions [21,22,23,24,25], thereby mitigating durability issues associated with erosion. On the one hand, the addition of FGSs can provide the nucleation sites during the cement hydration process in order to form a denser microstructure, thus blocking the continuity of the pore structure. On the other hand, the continuous network structures of FGSs function as an “ionic sieve” [26] that intercepts the invasion of water and ions to a certain extent compared with other nanomaterials. Based on the above advantages, FGSs have been widely adopted for fabricating hydrophobic and corrosion-resistant coatings for high-performance concrete, with principal application methods encompassing both individual coating systems and composite formation incorporating functional modifiers [27,28,29,30,31]. In summary, FGS coatings suppress erosion by bridging cracks and promoting secondary hydration. When combined with other modifiers, they act as a physicochemical compatibilizer to enhance dispersion, interfacial bonding and barrier continuity.
However, the hydrophobic protection mechanisms of FGSs cannot be elucidated from the perspective of the interaction between water molecules, ions, and FGS functional groups by experimental methods, which hinders the design of a new generation of hydrophobic FGSs. Consequently, complementary computational approaches are required. Molecular dynamics (MD), as a powerful tool for characterizing the micro–nano scale of structure, has been widely used in the field of cement and concrete [32]. The primary hydration product of concrete is calcium silicate hydrate (C-S-H) gel, whose molecular structures have been basically clarified [33,34]. By the advantage of MD simulations, Zhu et al. [35] verified the hydrophilicity of the C-S-H surface using the ReaxFF force field and further revealed the wetting properties of nanodroplets from the perspective of surface free energy and adhesion. Zheng et al. [36] comparatively analyzed the wetting properties of nanodroplets on four distinct cement hydration product substrates, elucidating the wettability-driven mechanisms governed by localized structural variations at the substrate/droplet interface. Li et al. [37] employed MD simulations to investigate modification strategies for the wettability of C-S-H surfaces using fluoroalkylsilane superhydrophobic coatings, in which the operative mechanisms of these coatings were systematically evaluated with respect to calcium leaching and chloride penetration. Although a large number of studies have revealed the influences of graphene-based materials on the mechanical properties [38,39,40,41] and solution transport properties [23,42] of the cement-based system by MD simulations, the research on graphene-based materials directly coated on a cement-based surface for hydrophobic protection is still lacking at the atomic scale.
Compared with previous studies, this study provides the first atomic-level insight into the wetting inhibition mechanism of G-NH2 and G-CH3 nanosheets through MD simulations, including the hydrophobic and salt-blocking effects of -NH2 and -CH3 functional groups, ionic cage restriction effect, and FGS–tobermorite interfacial interlocking. Initially, the single-layer graphene nanosheet was functionalized with amino (-NH2) and methyl (-CH3) to form G-NH2 and G-CH3. It could be successfully synthesized for stable chemical structures by rich experimental methods [43,44,45,46]. Subsequently, various FGS types were coated on the tobermorite surface to investigate the influences of separated functional groups on the wetting behaviors. Furthermore, the contact angles of different composite interfaces under the water and NaCl nanodroplet wetting were calculated. The interactions among interfaces, water molecules and ions were analyzed in structure and dynamics, providing mechanistic insights into the hydrophobic protection offered by different graphene-based coatings. This study offers molecular-level insights for the design of cement/FGS waterproof coatings.
2. Methods
2.1. Model Construction
The realistic C-S-H structural model is constructed based on the tobermorite model, incorporating the presence of short silicate chains [33,47]. Compared to the realistic C-S-H model, the tobermorite model exhibits continuous long silicate chain structures, which better describe the growth state of C-S-H on the FGS surface. This is attributed to FGS nanosheets serving as nucleation sites during the cement hydration process, facilitating the formation of more ordered and less defective C-S-H gel on their surface [48]. Therefore, the 11 Å tobermorite crystal developed by Hamid [49] was employed as the C-S-H substrate structure to describe the interaction with FGS nanosheets. Figure 1a depicts the wetting schematic diagram of droplets on the tobermorite surface and the surface of coatings on tobermorite. Eight atomic models were established to describe the wetting processes of water and NaCl nanodroplets on the tobermorite surface (Figure 1b) and the surfaces coated with graphene-based materials (Figure 1c). The basic model includes two units: the tobermorite substrate and the nanodroplets. The graphene coating models consist of three units: the tobermorite substrate, the graphene-based coatings and the nanodroplets, respectively. First, the tobermorite structure was cleaved along the [001] direction of the interlayer, with exposed non-bridged oxygen atoms protonated. Surface Ca2+ ions were partially removed to achieve charge neutrality. Subsequently, the unit cell was expanded along the a and b directions. The final sizes of the tobermorite supercell are a = 134 Å, b = 133 Å, c = 23 Å and α = β = γ = 90°.
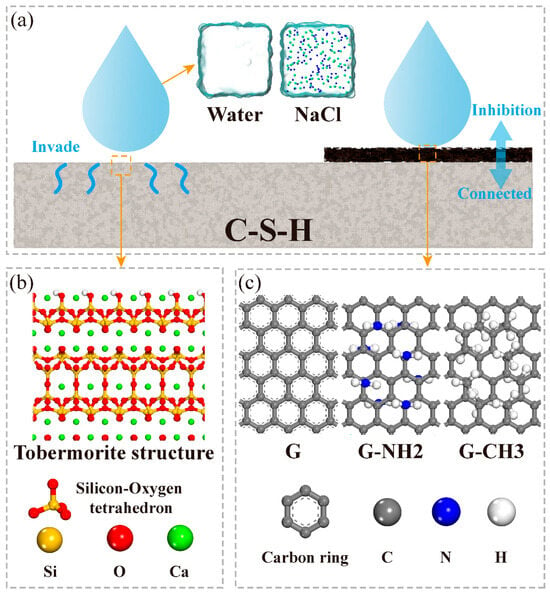
Figure 1.
Schematic diagrams of (a) wetting processes of water and NaCl solutions on the surfaces of tobermorite and its coatings, (b) tobermorite substrate and (c) graphene-based nanosheets.
The graphene-based coatings were expanded with the dimensions of a = 125 Å, b = 128 Å and α = β = γ = 90° to match the sizes of the tobermorite substrate. Next, as shown in Figure 1c, the amino (-NH2) and methyl (-CH3) groups were randomly grafted into the carbon ring with a coverage of about 20%, consistent with the typical coverage of FGS functional groups [50,51,52], to model the functionalized graphene with separated functional groups.
In terms of the preparation of nanodroplets, Hou et al. [53] investigated the effects of NaCl nanodroplets with different concentrations on the wettability of the tobermorite surface. The results indicated no significant contact angle variation at mass fractions below 12%, whereas a pronounced contact angle shift occurred at a 16% mass fraction. Consequently, to amplify the responses of the original tobermorite surface and different FGS functional groups to ions, a water nanodroplet with a density of 1 g/cm3 and NaCl nanodroplet with a mass fraction of 16% were selected. The initial shape of the nanodroplets is cubic, and their sizes are a = b = c = 35.5 Å and α = β = γ = 90°, respectively. Finally, the droplets were placed at the position of 3.5 Å above the interfaces, and the vacuum layer (>100 Å) was set to cut off the influence of the tobermorite bottom on the droplets.
2.2. Force Fields and Simulation Environments
A ClayFF force field [54] was employed to simulate the interactions between the tobermorite substrate and droplets. It has been validated as effective for cement-based systems [55,56]. The simple point charge (SPC) water model [57], commonly paired with the ClayFF force field, was utilized to describe water molecules and hydroxyl groups, enabling accurate prediction of the structural and dynamic characteristics of aqueous and NaCl solution systems. The consistent valence force field (CVFF) [58] was utilized to simulate the interatomic potential of the graphene-based coatings, which is suitable for graphene and functionalized graphene structures [23,59,60]. In addition, the force field combination of (ClayFF + CVFF) is extensively adopted to investigate the interactions among the tobermorite substrate, graphene-based materials and solutions [23,50,61]. The force field parameters used in this paper can be found in Refs. [54,58].
All simulations in this study were performed in the Lammps software package (version 3 Mar 20) [62]. Initially, droplets were positionally constrained to facilitate 500 ps of NVT ensemble relaxation for the tobermorite substrate and graphene-based materials, achieving stable initial interfacial configurations. Subsequently, the droplets were released and the whole system continued to relax for 3 ns under the NVT ensemble to reach equilibrium. The temperature was set to 300 K and the timestep was set to 1 fs. Periodic boundary conditions were applied in the dimensions of X, Y and Z, and the long-range Coulomb interaction was calculated by the pppm method. Finally, the atomic configurations were recorded every 1 ps for analyzing the structural and dynamic properties of the composite models.
3. Results and Discussion
3.1. Quantitative Characterization of Droplet Wettability
In this section, different wetting behaviors of water and NaCl droplets on the interfaces of tobermorite and tobermorite coated with FGSs are described through the changes in centroid height, contact angles and interaction energy. Figure 2 shows the representative snapshots of the wetting process of NaCl nanodroplets at different composite interfaces. Firstly, the initial cubic droplets collapse under the influence of interface attraction and take on a shape resembling a segment of a sphere due to surface tension, showing rapid diffusion within 500 ps. Subsequently, droplet shapes stabilize, indicating that the droplets have reached the equilibrium configurations at composite interfaces. Furthermore, Figure 3a,b show the changes in the centroid heights of water and NaCl droplets over time during the wetting processes. The centroid heights of the droplets decline rapidly within 500 ps, followed by a gradual decrease. Finally, the centroid heights exhibit stable fluctuations at the interfaces of the tobermorite composited with three graphene-based coatings, while a slight decreasing trend persists on the original tobermorite surface. This demonstrates that the strong hydrophilicity of the tobermorite surface causes water and ions to exhibit continuous permeation properties.
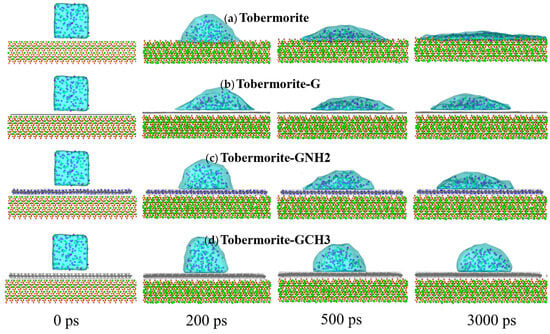
Figure 2.
Wetting processes of NaCl droplets on the surfaces of (a) tobermorite, (b) tobermorite-G, (c) tobermorite-GNH2 and (d) tobermorite-GCH3, respectively.
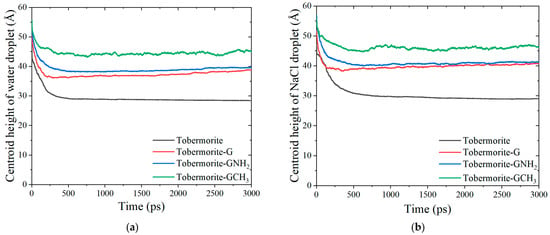
Figure 3.
The changes in centroid heights with time of (a) water and (b) NaCl droplets on tobermorite and tobermorite coated with three graphene-based material interfaces.
To quantitatively compare the wettabilities of water and NaCl droplets at various interfaces, the contact angle was calculated as an important index. Higher contact angles signify interfaces resistant to solution penetration, indicating enhanced durability. Figure 4 depicts a geometric diagram for the calculation of contact angle. The calculation formulae are included at the lower left corner, in which R represents the radius of the sphere, r represents the contact radius of the droplet along the horizontal direction and h is the height of the droplet along the vertical direction. Table 1 shows the contact angle of water and NaCl nanodroplets at tobermorite and three composite interfaces. The water droplet on the tobermorite surface exhibits a contact angle of 13.6°, which is close to the reported values of 7.98–13.84° by Hou et al. [63] and 12.8° by Wang et al. [64]. Compared with the tobermorite surface, the contact angles of water droplets at tobermorite/G, tobermorite/G-NH2 and tobermorite/G-CH3 composite interfaces exhibit increases of 15%, 55% and 448%, while those of NaCl droplets at the above three composite interfaces show rises of 136%, 220% and 524%, respectively. The statistical results confirm that the contact angles of the graphene-based coatings all increase compared with those on the tobermorite surface, and the contact angle increments under NaCl droplet wetting are obviously larger than those under water droplet wetting. In addition, FGS coatings are more effective than graphene coating in reducing wettability. Notably, the G-CH3 surface displays hydrophobicity under the NaCl droplet wetting. On the one hand, the thicknesses of FGSs could weaken the wettability transparency phenomenon of tobermorite [65]. On the other hand, the functional groups of FGSs may exhibit higher repulsion to water molecules and ions compared with the hydroxyl and Ca2+ on the tobermorite surface.
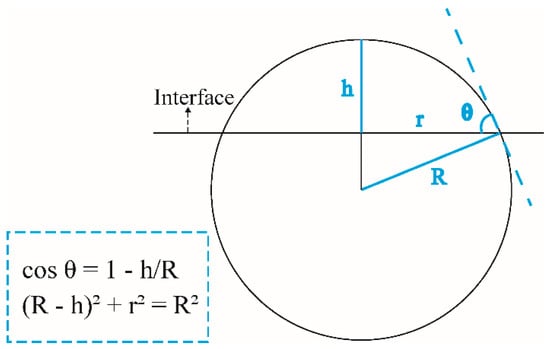
Figure 4.
Schematic diagram for the calculation of droplet contact angle.

Table 1.
Contact angles of water and NaCl droplets at different composite interfaces.
Further, the interaction energy between water/NaCl droplets and the composite interfaces was calculated. As shown in Figure 5, the tobermorite surface exhibits strong interaction energy with droplets attributable to its strong hydrophilicity. With the addition of graphene-based materials, the interaction energy decreases significantly, which can be ranked as tobermorite > tobermorite-G > tobermorite-GNH2 > tobermorite-GCH3. It is indicated that the increasing interaction energy results in the stronger interface hydrophilicity and the smaller contact angle. Notably, NaCl displays higher energy than water on tobermorite whereas this relationship reverses on coated surfaces. This demonstrates distinct ion adsorption properties on tobermorite relative to coated interfaces, further modulating water molecule distribution around surfaces and consequently influencing water–interface interactions. To elucidate the influences of distributions and local structures of water molecules and ions at different interfaces on wettability, the atomic intensity and the radial distribution function (RDF) are further analyzed as important indicators.
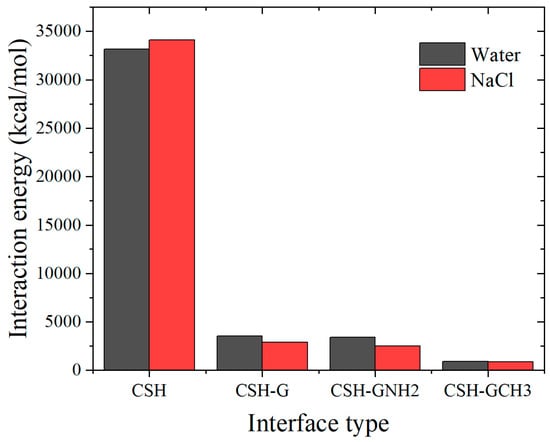
Figure 5.
Interaction energy of water and NaCl droplets with different interfaces.
3.2. Atomic Intensity Distribution Properties
Atomic intensity profiles characterize both atomic spatial positions/concentrations and distribution ranges. Herein, atomic intensity is defined as the number density of atoms within thin layers along a specified axis. Thus, the change in wetting property can be reflected from the perspective of spatial distribution. Figure 6a–d depict the distributions of water and ion intensities on different composite interfaces, with ionic distribution details provided in upper-right insets. The surface line positions of the composite interface are defined as the center lines of -OH, CG, -NH2 and -CH3, respectively, which are the interfaces in direct contact with water molecules and ions. Figure 6a shows pronounced OW (water oxygen) and Na+ peaks within the surface line of tobermorite, confirming that molecular/ion penetration promotes wetting. However, with the addition of the graphene-based coatings, the peak locations of both water molecules and ions are outside their surface lines. As shown in Figure 6b, although the peak intensity of Ow on the tobermorite/graphene interface still exhibits a high value under water droplet wetting, that under NaCl droplet wetting significantly decreases and the peak range increases compared with the tobermorite surface. This proves that the distribution of water molecules in the droplet is significantly affected by the ion distribution. Additionally, as shown in Figure 6c,d, the intensities of water molecules and ions on the interfaces of FGSs are further reduced, primarily governed by the functional groups. Based on the intensity curves, Figure 6e further illustrates the atomic distribution ranges in each system, specifically defined as the horizontal axis span covered by the intensity curve. The results show that the distribution ranges of water molecules and ions increase simultaneously with the addition of graphene-based coatings. According to their distribution ranges, it can be ranked as tobermorite/G-CH3 > tobermorite/G-NH2 > tobermorite/G > tobermorite. Combined with the results of Table 1, the wider the distribution range of water and ions, the greater the contact angle of droplets at the interfaces.
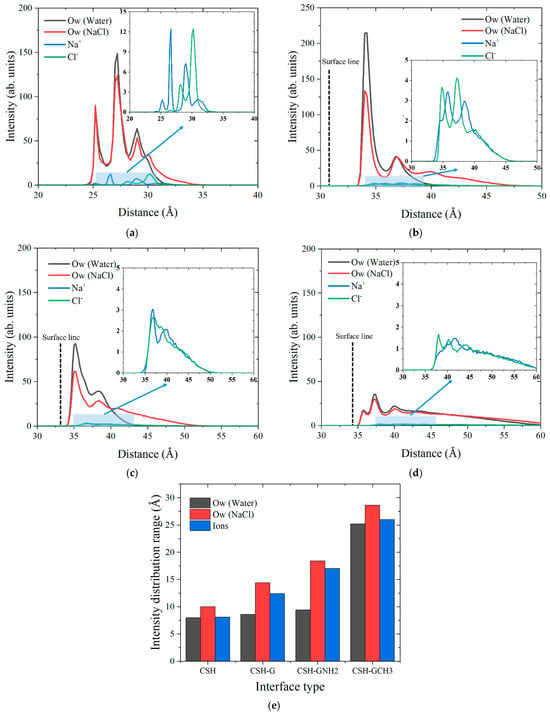
Figure 6.
The intensity distributions of Ow, Na+ and Cl− ions perpendicular to the surfaces of (a) tobermorite, (b) tobermorite-G, (c) tobermorite-GNH2 and (d) tobermorite-GCH3. (e) Intensity distribution ranges of Ow and ions with the change in interface types.
Furthermore, Figure 7a–d show the structural snapshots of water molecules and ions at the pristine tobermorite interface and various coating interfaces, and the relative distances between the average lowest position of water molecules and ions along the vertical surface direction after equilibrium are marked. The penetration depths of water molecules are higher than those of ions, and only the water molecules and ions of the tobermorite system have penetrated the surface line, which corresponds to the intensity curves described in Figure 6. The relative distances between water molecules and ions on the coating surfaces can be ranked as tobermorite/G-CH3 > tobermorite/G-NH2 > tobermorite/G, showing a positive correlation with the distribution range of ions (Figure 6e). This illustrates that expanded ionic distributions reduce surface ion deposition, governed by functional-group/water, functional-group/ion, and ion/water interactions.
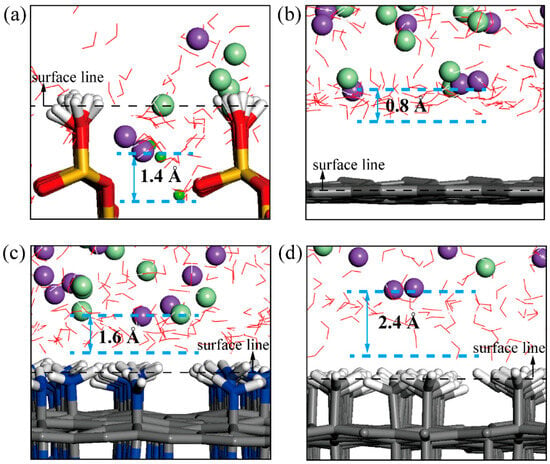
Figure 7.
Position distribution of water molecules and ions relative to the surfaces of (a) tobermorite, (b) tobermorite-G, (c) tobermorite-GNH2 and (d) tobermorite-GCH3.
3.3. Local Structures Among Nanodroplets, Tobermorite and FGSs
Tobermorite and its graphene-based coatings have different interactions with water and ions, which can be reflected in the bonding strength structurally, leading to different wetting behaviors. As a powerful tool, RDF was used to analyze the radius and strength of bonding between different atoms [66], and the formula is as follows:
where N represents the total atom numbers of the system,
is the average density and
represents the probability that the particle exists at the distance
from the center particle.
In addition, the coordination number (CN) is calculated by taking the peak valley position of the first peak of the corresponding RDF curve as the truncation radius. As shown in Figure 8a,b, there are large and wide RDF peaks between the OOH of tobermorite, Ow and Na+, indicating the strong bonding interactions between water molecules, ions and the tobermorite surface. In addition, OOH-Na exhibits two characteristic peaks, indicating that the tobermorite substrate has multiple bonding effects on Na+ ions (including Na+ ions captured and adsorbed by the tobermorite surface). On the contrary, the RDFs between graphene-based surfaces and water molecules only show weak peaks, whereas no distinct RDF peaks are observed between graphene-based surfaces and Na+. Among them, CG-CH3-Ow and CG-CH3-Na show the minimum interaction, demonstrating that the G-CH3 surface exerts maximal repulsion toward both water and ions. Different surface interactions further influence the self-interaction between water molecules (Ow-Ow) in nanodroplets and the ionic hydration structures. As shown in Figure 8c, as the interface–water interaction increases, the Ow-Ow interaction decreases. It can be ranked as tobermorite-GCH3 > tobermorite-GNH2 > tobermorite-G > tobermorite. Furthermore, the calculation results of the CN indicate that the CN between functional group atoms in the graphene-based coating and water molecules is significantly lower than that between -OH groups of tobermorite and water molecules. This is attributable to the high electronegativity of the tobermorite surface, which provides more binding sites for water adsorption. Additionally, the CN among water molecules within the droplet serves as an indicator of molecular cohesiveness, exhibiting a negative correlation with the CN between interfaces and water molecules.
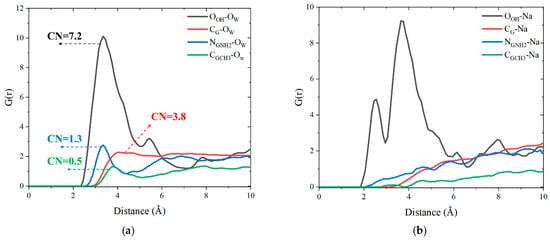
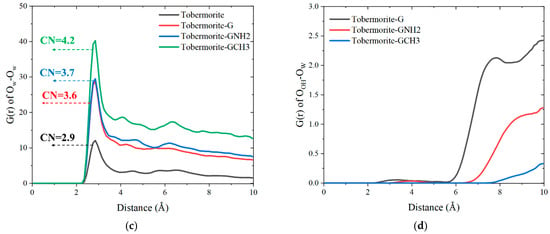
Figure 8.
RDF curves of (a) the atoms of functional groups and Ow, (b) the atoms of functional groups and Na+, (c) Ow-Ow and (d) OOH-Ow of graphene-based coating systems.
In addition, Figure 8d evaluates the influences of the tobermorite surface on water molecules with the addition of graphene-based coatings. After the block of pure graphene coating, the RDF curve of OOH-Ow still exhibits a gentle peak at 8.45 Å, while it has no obvious peaks after the block of GNH2 and G-CH3 coatings. This implies that, compared with the graphene coating, the presence of FGS functional groups can better weaken the wettability transparency phenomenon of the tobermorite surface on nanodroplets. Collectively, the above analyses elucidate the differences in the local structures among different substrate interfaces, water molecules and ions within droplets. Furthermore, the bonding structures between interfaces and ions will affect those between ions and water molecules, leading to changes in the cohesion of nanodroplets dominated by ionic hydration shells.
According to the results mentioned above, the addition of ions elevates the interface contact angle, attributable to the formation of Na-Ow and Cl-Hw hydration shells. As shown in Figure 9a,b, the Na-Ow RDF displays distinct peaks at 2.35 Å and 4.45 Å, and that of Cl-Hw shows obvious peaks at 2.25 Å and 3.65 Å. This confirms that both Na+ and Cl− can form two layers of hydration shells with water molecules, thereby constraining the movement of surrounding water molecules to a certain extent, which is defined as the “ionic cage restriction”. The schematic diagrams of the ionic hydration shell structure are shown in Figure 9c,d.
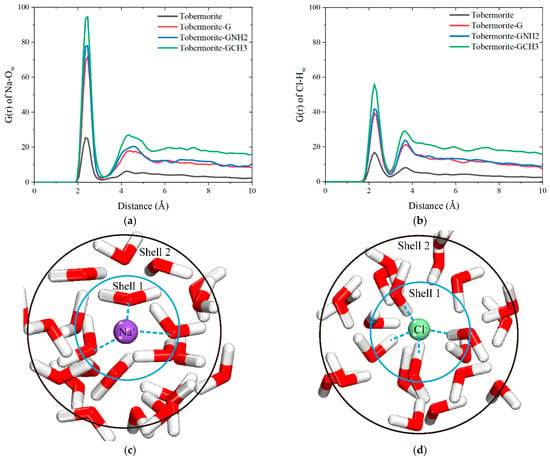
Figure 9.
RDF curves of (a) Na-Ow and (b) Cl-Hw in tobermorite and tobermorite–graphene-based coating systems. Schematic diagrams of ionic hydration shell structures of (c) Na-Ow and (d) Cl-Hw.
Overall, the effectiveness of “ionic cage restriction” is significantly influenced by the properties of the wetting surface. As the interaction between the surface and ions increases, the ionic solidification effect is enhanced, leading to a reduced interaction between the ions and water molecules (Figure 8b and Figure 9a). As a result, ions have a reduced ability to coordinate effectively with the water molecules within the droplet, thereby inhibiting the “ionic cage restriction” effect. For instance, the strong interaction between ions and the tobermorite surface results in weaker interactions between the ions and water molecules, which leads to the deposition of ions on the tobermorite surface, reducing the droplet cohesion induced by ions (the aggregating behavior caused by the attraction of ions to surrounding water molecules). It also explains the decrease in the interfacial interaction energy of graphene-based systems under NaCl droplet wetting (Figure 5). In contrast, the graphene-based coating repels ions away from the substrate surface, thereby preserving their hydration shells as intact and amplifying the ionic cage restriction. The amplification immobilizes more water molecules within the droplet and lowers the liquid–solid affinity, thereby finally manifesting as a notable reduction in water uptake of the bulk cement paste, conferring enhanced durability under saline exposure. In conclusion, the strong ionic deposition effect on the tobermorite surface is a disadvantage to the restriction of the ionic cage on the wettability of water molecules, while the addition of graphene-based coatings amplifies the influence of the ionic cage restriction.
The RDF is further used to analyze the interfacial bonding characteristics between graphene-based coatings and the tobermorite substrate. Pronounced interfacial bonding behavior indicates superior adhesion of graphene-based coatings onto tobermorite surfaces, which is an important factor for evaluating the properties of graphene-based coating materials. As shown in Figure 10a,c, the RDFs of NG-NH2-HOH, HG-NH2-OOH, CG-CH3-HOH and HG-CH3-OOH show obvious peaks at 1.95 Å, 2.75 Å, 2.85 Å and 2.85 Å, respectively, and have the characteristics of multiple-peak distribution. These distinct short-range peaks indicate that atoms within the functional groups of FGSs form bonds with surface oxygen and hydrogen atoms of the -OH groups in tobermorite. This configuration establishes a mechanical anchoring mechanism, thereby enhancing interfacial interlocking performance. The schematic diagrams of interface connection structures are displayed in Figure 10b,d. For the graphene sheet lacking functional groups, Figure 10e exhibits only a single and broad RDF peak, indicating weak adhesion to the tobermorite surface. After clarifying the interfacial structure between graphene-based coatings and the tobermorite surface, dynamic bonding stability emerges as another critical performance metric for interfacial bonding evaluation. Quantitative analysis of this property will be addressed subsequently.
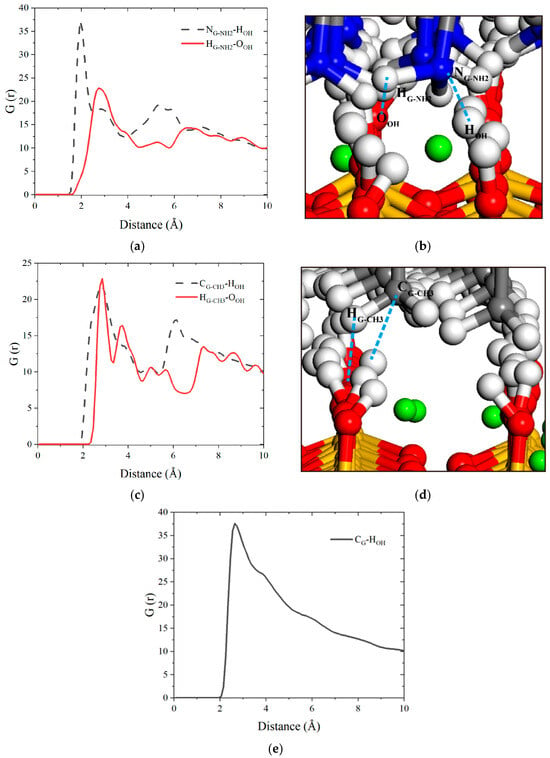
Figure 10.
RDF curves of (a) -NH2 and -OH, (c) -CH3 and -OH and (e) graphene and -OH, respectively. Local structure diagrams between (b) G-NH2 surface, (d) G-CH3 surface and tobermorite surface.
3.4. Dipole Moment Magnitude Distribution of Water Molecules
The dipole moment magnitude of water molecules can be employed to analyze the hydrophilic and hydrophobic properties of different interfaces, which enables a deeper understanding of water wetting behaviors under interfacial influences [67]. The magnitude of the dipole moment is equal to the product of the distance between the centers of positive and negative charges and the amount of charge carried by the charge center, with its direction pointing from the positive charge center to the negative charge center. The downshift in dipole moment distribution indicates that the surface hydrophilicity is weakened, while the opposite indicates the increase in surface hydrophilicity. The water dipole moment distribution within 2 Å of the surface line of different interfaces was calculated, as shown in Figure 11. In the tobermorite system, the mean magnitude of the dipole moment is approximately 2.5 D, which is consistent with previous research [36,64]. The value is weaker than the 2.51 D reported by Youssef et al. [67], as the water molecules that they studied were constrained by the silica chains. Moreover, this value exceeds that of bulk water molecules (2.44 D), which can be attributed to the strong electric field of the hydrophilic interface that polarizes water molecules beyond the self-polarization level of ordinary liquid water [68]. With the addition of graphene-based coatings, the dipole moment curve shifts to the left. After GNH2 modification, the dipole moment of water molecules decreases to 2.46 D, indicating a reduction in water molecule polarity, although it remains higher than the mean dipole moment of bulk water. This could be attributed to the weak hydrogen bonding interaction between GNH2 and water molecules. After GCH3 functionalization, the mean dipole moment of water molecules approximates 2.43 D, approaching that of bulk water and demonstrating increased disorder among water molecules. Consequently, the surface modification significantly modulates the polarity of water molecules, thereby governing interfacial wetting behavior.
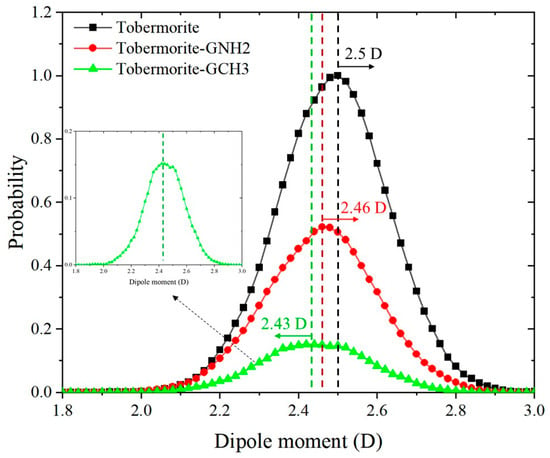
Figure 11.
The water dipole moment magnitude distributions of different interfaces.
3.5. Bond Stability Analysis Between Interfaces and Droplets
The time correlation function (TCF), as an important indicator for describing the dynamic properties of atomic pairs [66], is widely used to describe the bonding stabilities among interfaces, water molecules and ions [69], which can be expressed by the following formula:
where
is a binary value. If two particles are kept a bonding state with elapsed time t, the value is 1; otherwise, it is 0. In addition, the first peak valley position of the RDF curve for bonded atomic pairs is used as the cutoff radius to assess whether a chemical bond is effectively formed. Therefore, the bonding lifetime between different particle pairs can be expressed by the TCF. The higher the TCF values, the more stable the bonds.
As described in Section 3.3, G-NH2 and G-CH3 can form good interface connections structurally. Further, Figure 12a illustrates the TCFs between the atoms of various graphene-based coating surfaces and the HOH on the tobermorite surface. The TCFs of CG-NH2-HOH and CG-CH3-HOH maintain values above 0.8 throughout the 100 ps simulation, whereas CG-HOH exhibits values below 0.6, indicating that, compared to the two types of FGS nanosheets, graphene nanosheets lack functional groups to interact with the hydroxyl groups on the tobermorite surface, resulting in enhanced atomic vibrations and diminished interfacial stability. As shown in Figure 12b,c, the TCFs of OOH-Ow and OOH-Na at 100 ps are 0.79 and 0.83, respectively. In contrast, the TCFs for NG-NH2-Ow and CG-CH3-Ow decreased to 0.18 and 0.17, while those for NG-NH2-Na and CG-CH3-Na diminished to 0.33 and 0.25. The magnitude of TCFs for the interface–water molecules and interface–ions can be ranked as follows: tobermorite > G-NH2 > G-CH3. Combined with the RDFs of OOH-Ow and OOH-Na in Section 3.3, it can be deduced that the tobermorite surface exhibits significant capability to form strong and stable hydrogen bonds and ionic bonds with water molecules and ions. Consequently, this leads to a prolonged residence time of water and ions on the tobermorite surface, thereby establishing a stable wetting environment at the interface. Such a phenomenon facilitates the sustained wetting of droplets on the tobermorite surface. Conversely, the hydrogen bonds and ionic bonds formed between droplets and the FGS surface exhibit elevated instability, which accelerates the turnover rate of interfacial chemical bonds, thereby reducing droplet wetting at FGS interfaces. Furthermore, the dynamic properties of ion hydration structures within droplets are modulated by varying surface characteristics. As illustrated in Figure 12d, the bonding stability relationship of Na-Ow is delineated as tobermorite > G-NH2 > G-CH3. This is consistent with the previously discussed bonding stability of interface–Na, suggesting that lower solidification of ions by the surface leads to the formation of unstable ion hydration shells to inhibit wetting behavior. From the point of view of dynamics, this section demonstrates that unstable interfacial chemical bonds suppress wetting and amplify this inhibitory effect in ionic environments.
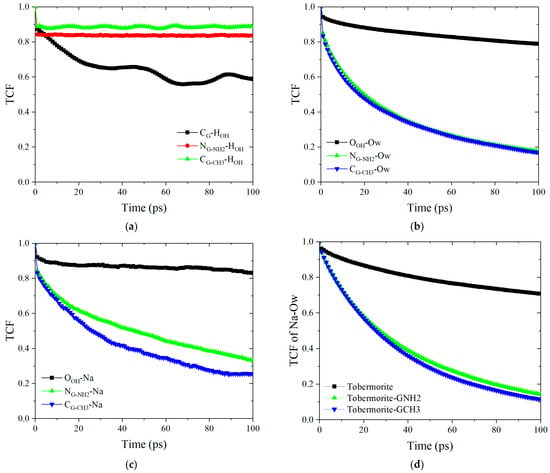
Figure 12.
TCFs: (a) tobermorite and graphene-based coatings, (b) different interfaces and water molecules, (c) different interfaces and Na+ ions and (d) Na+ ions and water molecules.
4. Conclusions
In this study, the wetting behaviors of water and NaCl droplets on the surfaces of tobermorite and its crosslinked graphene-based coatings were investigated by MD simulations. To illustrate the mechanisms of wetting and hydrophobic protection at different interfaces, contact angle, energy, local structure and dynamic properties are analyzed as important indicators. The following conclusions can be summarized:
- (1)
- The combination of FGSs with a tobermorite substrate can significantly reduce the wettability of water and NaCl droplets. The wettability of droplets on different interfaces can be ranked as tobermorite > graphene > G-NH2 > G-CH3. Compared with the tobermorite surface, the increase in contact angle at the interface of graphene-based coatings under NaCl droplet wetting is significantly greater than that under water droplet wetting, which largely depends on the functional group properties of graphene-based coatings.
- (2)
- Structurally, the strong OOH-Ow and OOH-Na bonding structures on the tobermorite surface promote the wettability, whereas weak interactions between Ow, Na and graphene functional groups suppress it. The surface–ion configuration further influences the ionic hydration shell, and graphene-based coatings intensify ionic cage confinement. Moreover, compared with pristine graphene, functionalized graphene sheets exhibit stronger interfacial interlocking, thereby improving coating adhesion.
- (3)
- Dynamically, wettability correlates with interfacial bonding stability of water and ions. Hydrophilic sites on tobermorite prolong binding of water and ions, facilitating sustained droplet spreading. Conversely, hydrophobic functional groups in graphene coatings reduce bond lifetimes, inhibiting wetting. Critically, interfacial bonding stability modulates ionic hydration shell integrity: weak immobilization at FGS interfaces generates more free ionic hydration shells, thereby enhancing ionic suppression of wetting.
Atomic-level simulations demonstrate that hydrophobic and salt-blocking effects of FGS functional groups, ionic cage restriction and stable interfacial interlocking collectively suppress wetting in FGS–tobermorite systems. After addressing key implementation challenges, including large-scale production, dispersion issues and the long-term stability of FGSs, the research findings could provide potential application support for concrete interface protection methods and increasing corrosion durability, such as incorporating FGSs within concrete to enhance its overall anti-corrosion performance, or applying FGSs as a surface coating to create a hydrophobic barrier, reducing moisture and ion ingress.
Author Contributions
Conceptualization, T.L.; Methodology, T.L. and D.H.; Software, T.L. and D.H.; Validation, Q.L. and W.Y.; Investigation, F.H., X.Z. and W.Y.; Writing—original draft, T.L.; Writing—review & editing, F.H., X.Z. and K.Z.; Visualization, Q.L.; Funding acquisition, T.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Natural Science Foundation of China (Grant No. 42401168), the Natural Science Foundation of Chongqing (Grant No. CSTB2025NSCQ-GPX0864), the Science and Technology Research Program of Chongqing Municipal Education Commission (Grant No. KJQN202500736).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
Author Te Liang was employed by the company Xizang Communications Development Group Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Mehta, P.K.; Monteiro, P.J. Concrete: Microstructure, Properties, and Materials; McGraw-Hill Education: Columbus, OH, USA, 2014. [Google Scholar]
- Landsman, M.R.; Sujanani, R.; Brodfuehrer, S.H.; Cooper, C.M.; Darr, A.G.; Davis, R.J.; Kim, K.; Kum, S.; Nalley, L.K.; Nomaan, S.M. Water treatment: Are membranes the panacea? Annu. Rev. Chem. Biomol. Eng. 2020, 11, 559–585. [Google Scholar] [CrossRef]
- Law, K.-Y. Definitions for Hydrophilicity, Hydrophobicity, and Superhydrophobicity: Getting the Basics Right; ACS Publications: Washington, DC, USA, 2014; pp. 686–688. [Google Scholar]
- Graham, J.R.; Creegan, P.J.; Hamilton, W.S.; Hendrickson, J.; Kaden, R.A.; McDonald, J.E.; Noble, G.E.; Schrader, E.K. Erosion of concrete in hydraulic structures. ACI Mater. J. 1987, 84, 136–156. [Google Scholar] [CrossRef]
- Hilo, A.N.; Ghasham, T.S.; Hamedi, M.H.; Ayoob, N.S.; Abd, A.H. Numerical and experimental study of abrasion erosion in hydraulic structures of high-velocity water flow. In Proceedings of the 2019 12th International Conference on Developments in eSystems Engineering (DeSE), Kazan, Russia, 7–10 October 2019; pp. 415–420. [Google Scholar]
- Guo, J.J.; Wang, K.; Guo, T.; Yang, Z.Y.; Zhang, P. Effect of Dry-Wet Ratio on Properties of Concrete Under Sulfate Attack. Materials 2019, 12, 2755. [Google Scholar] [CrossRef]
- Powers, T.C. A working hypothesis for further studies of frost resistance of concrete. J. Proc. ACI 1945, 16, 245–272. [Google Scholar]
- Di Mundo, R.; Labianca, C.; Carbone, G.; Notarnicola, M. Recent advances in hydrophobic and icephobic surface treatments of concrete. Coatings 2020, 10, 449. [Google Scholar] [CrossRef]
- Al-Kheetan, M.J.; Rahman, M.M.; Chamberlain, D.A. Fundamental interaction of hydrophobic materials in concrete with different moisture contents in saline environment. Constr. Build. Mater. 2019, 207, 122–135. [Google Scholar] [CrossRef]
- Pan, X.; Shi, Z.; Shi, C.; Ling, T.-C.; Li, N. A review on concrete surface treatment Part I: Types and mechanisms. Constr. Build. Mater. 2017, 132, 578–590. [Google Scholar] [CrossRef]
- Wu, Y.; Dong, L.; Shu, X.; Yang, Y.; She, W.; Ran, Q. A review on recent advances in the fabrication and evaluation of superhydrophobic concrete. Compos. Part B Eng. 2022, 237, 109867. [Google Scholar] [CrossRef]
- Di Mundo, R.; Petrella, A.; Notarnicola, M. Surface and bulk hydrophobic cement composites by tyre rubber addition. Constr. Build. Mater. 2018, 172, 176–184. [Google Scholar] [CrossRef]
- Qu, L.; Song, W.; Wang, Q.; Xu, S.; Hou, C. Effects of hydrophobic modified fly ash on resistance of chloride corrosion and water penetration of cement mortar in the early hydration stage. J. Build. Eng. 2023, 64, 105573. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, H.; Zeze, A.L.P.; Liu, X.; Tao, M. Coating performance, durability and anti-corrosion mechanism of organic modified geopolymer composite for marine concrete protection. Cem. Concr. Compos. 2022, 129, 104495. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, H.; Yuan, L.; Wang, Z.; Guo, H.; Zhang, S.; Li, G. Multi-component synergistically modified hydrophobic coatings used in concrete surface: High bonding properties and corrosion resistance. J. Build. Eng. 2025, 112, 113718. [Google Scholar] [CrossRef]
- Chuah, S.; Pan, Z.; Sanjayan, J.G.; Wang, C.M.; Duan, W.H. Nano reinforced cement and concrete composites and new perspective from graphene oxide. Constr. Build. Mater. 2014, 73, 113–124. [Google Scholar] [CrossRef]
- Norhasri, M.M.; Hamidah, M.; Fadzil, A.M. Applications of using nano material in concrete: A review. Constr. Build. Mater. 2017, 133, 91–97. [Google Scholar] [CrossRef]
- Bautista-Gutierrez, K.P.; Herrera-May, A.L.; Santamaría-López, J.M.; Honorato-Moreno, A.; Zamora-Castro, S.A. Recent progress in nanomaterials for modern concrete infrastructure: Advantages and challenges. Materials 2019, 12, 3548. [Google Scholar] [CrossRef]
- Liu, C.; Huang, X.; Wu, Y.-Y.; Deng, X.; Liu, J.; Zheng, Z.; Hui, D. Review on the research progress of cement-based and geopolymer materials modified by graphene and graphene oxide. Nanotechnol. Rev. 2020, 9, 155–169. [Google Scholar] [CrossRef]
- Zhi, D.; Li, T.; Li, J.; Ren, H.; Meng, F. A review of three-dimensional graphene-based aerogels: Synthesis, structure and application for microwave absorption. Compos. Part B Eng. 2021, 211, 108642. [Google Scholar]
- Krystek, M.; Pakulski, D.; Górski, M.; Szojda, L.; Ciesielski, A.; Samorì, P. Electrochemically Exfoliated Graphene for High-Durability Cement Composites. ACS Appl. Mater. Interfaces 2021, 13, 23000–23010. [Google Scholar] [CrossRef]
- Du, H.; Gao, H.J.; Dai Pang, S. Improvement in concrete resistance against water and chloride ingress by adding graphene nanoplatelet. Cem. Concr. Res. 2016, 83, 114–123. [Google Scholar] [CrossRef]
- Hou, D.S.; Zhang, Q.E.; Zhang, J.H.; Wang, P. Molecular Modeling of Capillary Transport in the Nanometer Pore of Nanocomposite of Cement Hydrate and Graphene/GO. J. Phys. Chem. C 2019, 123, 15557–15568. [Google Scholar] [CrossRef]
- Zeng, H.; Zhou, R.; Yu, J.; Hu, Y.; Qu, S.; Chen, J.; Hou, S. Experiment and Modelling of Degradation Mechanism of Cement Mortar with Graphene Oxide Nanosheets under Sulfate Attack. Cem. Concr. Compos. 2024, 155, 105833. [Google Scholar] [CrossRef]
- Indukuri, C.S.R.; Nerella, R. Enhanced transport properties of graphene oxide based cement composite material. J. Build. Eng. 2021, 37, 102174. [Google Scholar] [CrossRef]
- Joshi, R.; Carbone, P.; Wang, F.-C.; Kravets, V.G.; Su, Y.; Grigorieva, I.V.; Wu, H.; Geim, A.K.; Nair, R.R. Precise and ultrafast molecular sieving through graphene oxide membranes. Science 2014, 343, 752–754. [Google Scholar] [CrossRef]
- Zheng, W.; Chen, W.; Feng, T.; Li, W.; Liu, X.; Dong, L.; Fu, Y. Enhancing chloride ion penetration resistance into concrete by using graphene oxide reinforced waterborne epoxy coating. Prog. Org. Coat. 2020, 138, 105389. [Google Scholar] [CrossRef]
- Korayem, A.H.; Ghoddousi, P.; Javid, A.S.; Oraie, M.; Ashegh, H. Graphene oxide for surface treatment of concrete: A novel method to protect concrete. Constr. Build. Mater. 2020, 243, 118229. [Google Scholar] [CrossRef]
- Mohammed, A.; Sanjayan, J.G.; Duan, W.; Nazari, A. Incorporating graphene oxide in cement composites: A study of transport properties. Constr. Build. Mater. 2015, 84, 341–347. [Google Scholar] [CrossRef]
- Liang, C.; Zhao, P.; Xie, N.; Wang, S.; Huang, Y.; Lu, L.; Cheng, X. Enhanced comprehensive performance of polymer-CSA cement coating with graphene oxide. Constr. Build. Mater. 2023, 363, 129885. [Google Scholar] [CrossRef]
- Antolín-Rodríguez, A.; Merino-Maldonado, D.; Rodríguez-González, Á.; Fernández-Raga, M.; González-Domínguez, J.M.; Juan-Valdés, A.; García-González, J. Statistical study of the effectiveness of surface application of graphene oxide as a coating for concrete protection. Coatings 2023, 13, 213. [Google Scholar] [CrossRef]
- Barbhuiya, S.; Das, B.B. Molecular dynamics simulation in concrete research: A systematic review of techniques, models and future directions. J. Build. Eng. 2023, 76, 107267. [Google Scholar] [CrossRef]
- Pellenq, R.J.M.; Kushima, A.; Shahsavari, R.; Van Vliet, K.J.; Buehler, M.J.; Yip, S.; Ulm, F.J. A realistic molecular model of cement hydrates. Proc. Natl. Acad. Sci. USA 2009, 106, 16102–16107. [Google Scholar]
- Tao, L.; Shahsavari, R. Diffusive, Displacive Deformations and Local Phase Transformation Govern the Mechanics of Layered Crystals: The Case Study of Tobermorite. Sci. Rep. 2017, 7, 5907. [Google Scholar] [CrossRef]
- Zhu, X.; Zaoui, A.; Sekkal, W. Wettability and work of adhesion of water nanodroplet on (001) surface of cement paste. Cem. Concr. Res. 2022, 159, 106896. [Google Scholar] [CrossRef]
- Zheng, H.; Duan, Y.; Liu, L.; Wang, P.; Pang, B.; Duan, H.; Hou, D. Molecular structure and dynamics of water on the surfaces of cement hydration products and associated Minerals: Nanoscale wettability behavior. Appl. Surf. Sci. 2025, 687, 162274. [Google Scholar] [CrossRef]
- Li, G.; Akbar, A.; Zhang, L.-W.; Rosei, F.; Liew, K. Surface modification strategy for controlling wettability and ionic diffusion behaviors of calcium silicate hydrate. Appl. Surf. Sci. 2023, 622, 156993. [Google Scholar] [CrossRef]
- Gong, P.; Ren, Q.; Peng, S.; Mei, K.; Zhang, C.; Wu, Y.; Cheng, X. Influence of graphene oxide on the self-healing of cement paste fractures in CCUS cementing: A combined analysis of experiments and molecular dynamics simulations. Constr. Build. Mater. 2023, 404, 133067. [Google Scholar] [CrossRef]
- Miao, J.; Hu, J.; Lu, S.; Wang, S.; Lin, J.; Gao, Y. Molecular dynamics investigation on the crack-bridging role of coated graphene oxide on steel fiber reinforced concrete. Case Stud. Constr. Mater. 2025, 22, e04689. [Google Scholar] [CrossRef]
- Min, B.; Chen, G.; Sun, Y.; Li, K.; Chen, X.; Wang, Z. Enhancing the fracture properties of carbon fiber-calcium silicate hydrate interface through graphene oxide. Mater. Des. 2024, 241, 112916. [Google Scholar] [CrossRef]
- Wei, L.; Liu, G.; Wang, J. The influence of temperature and functional groups of graphene-reinforced CSH: Molecular dynamics study. Mater. Sci. Eng. B 2024, 303, 117336. [Google Scholar] [CrossRef]
- He, J.; Zhang, C. Effect of temperature on NaCl migration properties within nano-channels of graphene, graphene oxide, and nano-silica modified cementitious composites: A molecular dynamics study. Diam. Relat. Mater. 2025, 157, 112456. [Google Scholar] [CrossRef]
- Lai, L.; Chen, L.; Zhan, D.; Sun, L.; Liu, J.; Lim, S.H.; Poh, C.K.; Shen, Z.; Lin, J. One-step synthesis of NH2-graphene from in situ graphene-oxide reduction and its improved electrochemical properties. Carbon 2011, 49, 3250–3257. [Google Scholar] [CrossRef]
- Zhang, C.; Hao, R.; Liao, H.; Hou, Y. Synthesis of amino-functionalized graphene as metal-free catalyst and exploration of the roles of various nitrogen states in oxygen reduction reaction. Nano Energy 2013, 2, 88–97. [Google Scholar] [CrossRef]
- Gan, L.; Zhou, J.; Ke, F.; Gu, H.; Li, D.; Hu, Z.; Sun, Q.; Guo, X. Tuning the properties of graphene using a reversible gas-phase reaction. NPG Asia Mater. 2012, 4, e31. [Google Scholar] [CrossRef]
- Liao, L.; Song, Z.; Zhou, Y.; Wang, H.; Xie, Q.; Peng, H.; Liu, Z. Photoinduced methylation of graphene. Small 2013, 9, 1348–1352. [Google Scholar] [CrossRef] [PubMed]
- Qomi, M.A.; Krakowiak, K.; Bauchy, M.; Stewart, K.; Shahsavari, R.; Jagannathan, D.; Brommer, D.B.; Baronnet, A.; Buehler, M.J.; Yip, S. Combinatorial molecular optimization of cement hydrates. Nat. Commun. 2014, 5, 4960. [Google Scholar] [CrossRef]
- de Souza, F.B.; Shamsaei, E.; Chen, S.; Sagoe-Crentsil, K.; Duan, W. Controlled growth and ordering of poorly-crystalline calcium-silicate-hydrate nanosheets. Commun. Mater. 2021, 2, 84. [Google Scholar] [CrossRef]
- Hamid, S. The crystal structure of the 11Å natural tobermorite Ca2.25[Si3O7.5(OH)1.5]·1H2O. Z. Für Krist.-Cryst. Mater. 1981, 154, 189–198. [Google Scholar] [CrossRef]
- Yu, J.; Zheng, Q.; Hou, D.; Zhang, J.; Li, S.; Jin, Z.; Wang, P.; Yin, B.; Wang, X. Insights on the capillary transport mechanism in the sustainable cement hydrate impregnated with graphene oxide and epoxy composite. Compos. Part B Eng. 2019, 173, 106907. [Google Scholar] [CrossRef]
- Jiao, S.; Xu, Z. Selective gas diffusion in graphene oxides membranes: A molecular dynamics simulations study. ACS Appl. Mater. Interfaces 2015, 7, 9052–9059. [Google Scholar] [CrossRef]
- Min, B.; Wang, P.; Li, S.; Wang, Z. Mechanical influence of graphene oxide in the interface between calcium silicate hydrate and quartz: A molecular dynamics study. Constr. Build. Mater. 2022, 325, 126597. [Google Scholar] [CrossRef]
- Hou, D.; Yang, Q.; Wang, P.; Wang, M.; Zhang, Y.; Wang, X.; Zhang, J. Concentration-induced wettability alteration of nanoscale NaCl solution droplets on the CSH surface. Phys. Chem. Chem. Phys. 2021, 23, 7449–7461. [Google Scholar] [CrossRef]
- Cygan, R.T.; Liang, J.-J.; Kalinichev, A.G. Molecular models of hydroxide, oxyhydroxide, and clay phases and the development of a general force field. J. Phys. Chem. B 2004, 108, 1255–1266. [Google Scholar] [CrossRef]
- Kalinichev, A.G.; Wang, J.; Kirkpatrick, R.J. Molecular dynamics modeling of the structure, dynamics and energetics of mineral–water interfaces: Application to cement materials. Cem. Concr. Res. 2007, 37, 337–347. [Google Scholar] [CrossRef]
- Tang, S.; Hubao, A.; Chen, J.; Yu, W.; Yu, P.; Chen, E.; Deng, H.; He, Z. The interactions between water molecules and C-S-H surfaces in loads-induced nanopores: A molecular dynamics study. Appl. Surf. Sci. 2019, 496, 143744. [Google Scholar] [CrossRef]
- Berendsen, H.J.; Postma, J.P.; van Gunsteren, W.F.; Hermans, J. Interaction models for water in relation to protein hydration. In Intermolecular Forces; Springer: Berlin/Heidelberg, Germany, 1981; pp. 331–342. [Google Scholar]
- Dauber-Osguthorpe, V.A.R.P.; Osguthorpe, D.J.; Wolff, J.; Genest, M.; Hagler, A.T. Structure and energetics of ligand binding to proteins: Escherichia coli dihydrofolate reductase-trimethoprim, a drug-receptor system. Proteins Struct. Funct. Bioinform. 1988, 4, 31–47. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Qiao, G.; Hou, D.; Jin, Z.; Wang, M.; Zhang, J.; Sun, G. Functionalization enhancement interfacial bonding strength between graphene sheets and calcium silicate hydrate: Insights from molecular dynamics simulation. Constr. Build. Mater. 2020, 261, 120500. [Google Scholar] [CrossRef]
- Mabudi, A.; Noaparast, M.; Gharabaghi, M.; Vasquez, V.R. A molecular dynamics study on the wettability of graphene-based silicon dioxide (glass) surface. Colloids Surf. A Physicochem. Eng. Asp. 2019, 569, 43–51. [Google Scholar]
- Lu, Z.; Yu, J.; Yao, J.; Hou, D. Experimental and molecular modeling of polyethylene fiber/cement interface strengthened by graphene oxide. Cem. Concr. Compos. 2020, 112, 103676. [Google Scholar] [CrossRef]
- Plimpton, S.; Crozier, P.; Thompson, A. LAMMPS-large-scale atomic/molecular massively parallel simulator. Sandia Natl. Lab. 2007, 18, 43. [Google Scholar]
- Hou, D.; Zheng, H.; Wang, P.; Wan, X.; Wang, M.; Wang, H. Molecular insight in the wetting behavior of nanoscale water droplet on CSH surface: Effects of Ca/Si ratio. Appl. Surf. Sci. 2022, 587, 152811. [Google Scholar] [CrossRef]
- Wang, P.; Duan, Y.; Zheng, H.; Chen, Z.; Wang, M.; Wang, X.; Li, H.; Hou, D. Molecular structure and dynamics of water on the surface of cement hydration products: Wetting behavior at nanoscale. Appl. Surf. Sci. 2023, 611, 155713. [Google Scholar] [CrossRef]
- Rafiee, J.; Mi, X.; Gullapalli, H.; Thomas, A.V.; Yavari, F.; Shi, Y.; Ajayan, P.M.; Koratkar, N.A. Wetting transparency of graphene. Nat. Mater. 2012, 11, 217–222. [Google Scholar] [CrossRef]
- Chen, Z.; Xu, W.; Tang, L. Theory and Practice of Molecular Simulation; Chemical Industry Press: Beijing, China, 2007. [Google Scholar]
- Youssef, M.; Pellenq, R.J.; Yildiz, B. Glassy nature of water in an ultraconfining disordered material: The case of calcium-silicate-hydrate. J. Am. Chem. Soc. 2011, 133, 2499–2510. [Google Scholar] [CrossRef]
- Coudert, F.-X.; Vuilleumier, R.; Boutin, A. Dipole moment, hydrogen bonding and IR spectrum of confined water. ChemPhysChem 2006, 7, 2464. [Google Scholar] [CrossRef]
- Huang, J.; Chen, R.; Zhou, Y.; Ming, J.; Liu, J. Molecular design and experiment of ion transport inhibitors towards concrete sustainability. Cem. Concr. Compos. 2022, 133, 104710. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).