High-Temperature Sulfate Corrosion Resistance and Wear Performance of NiCr-Cr3C2 Coatings for the Water Wall of Power Plant Boilers
Abstract
1. Introduction
2. Experiment
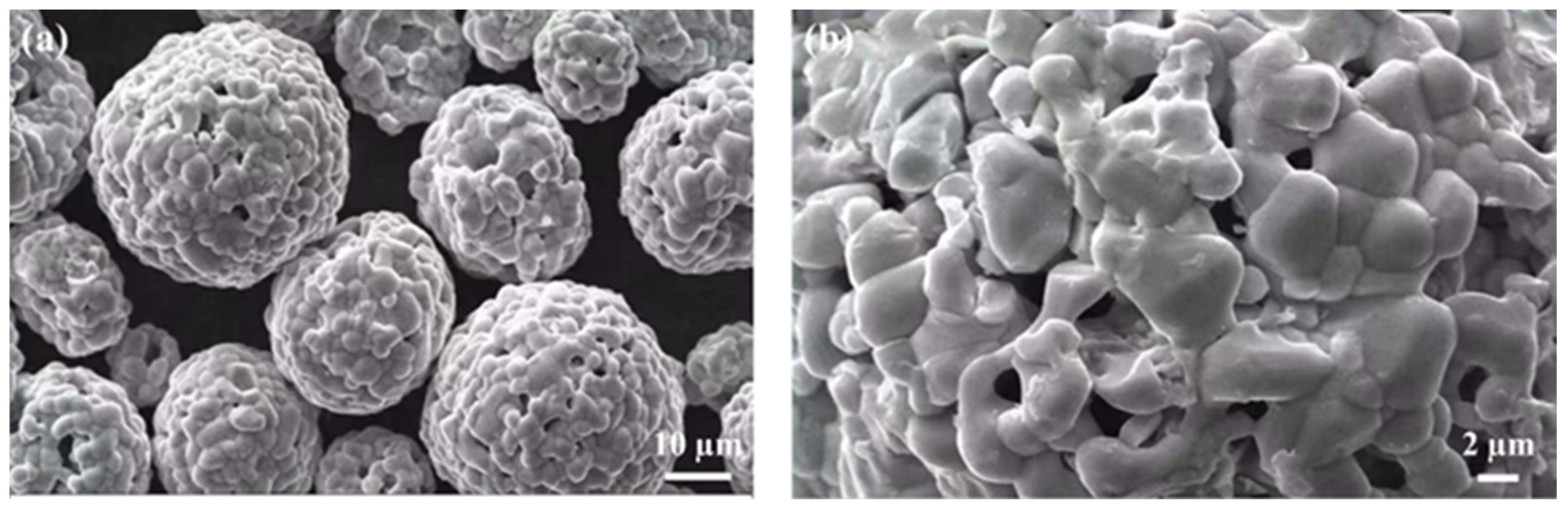
2.1. Materials
2.2. Thermal Spraying Process
2.3. Microstructural Characterization of the Coatings
2.4. Microhardness and Bonding Strength of the Coatings
2.5. High-Temperature Sulfate Corrosion Test
2.6. High-Temperature Tribological Performance Test of Coatings
3. Results and Discussion
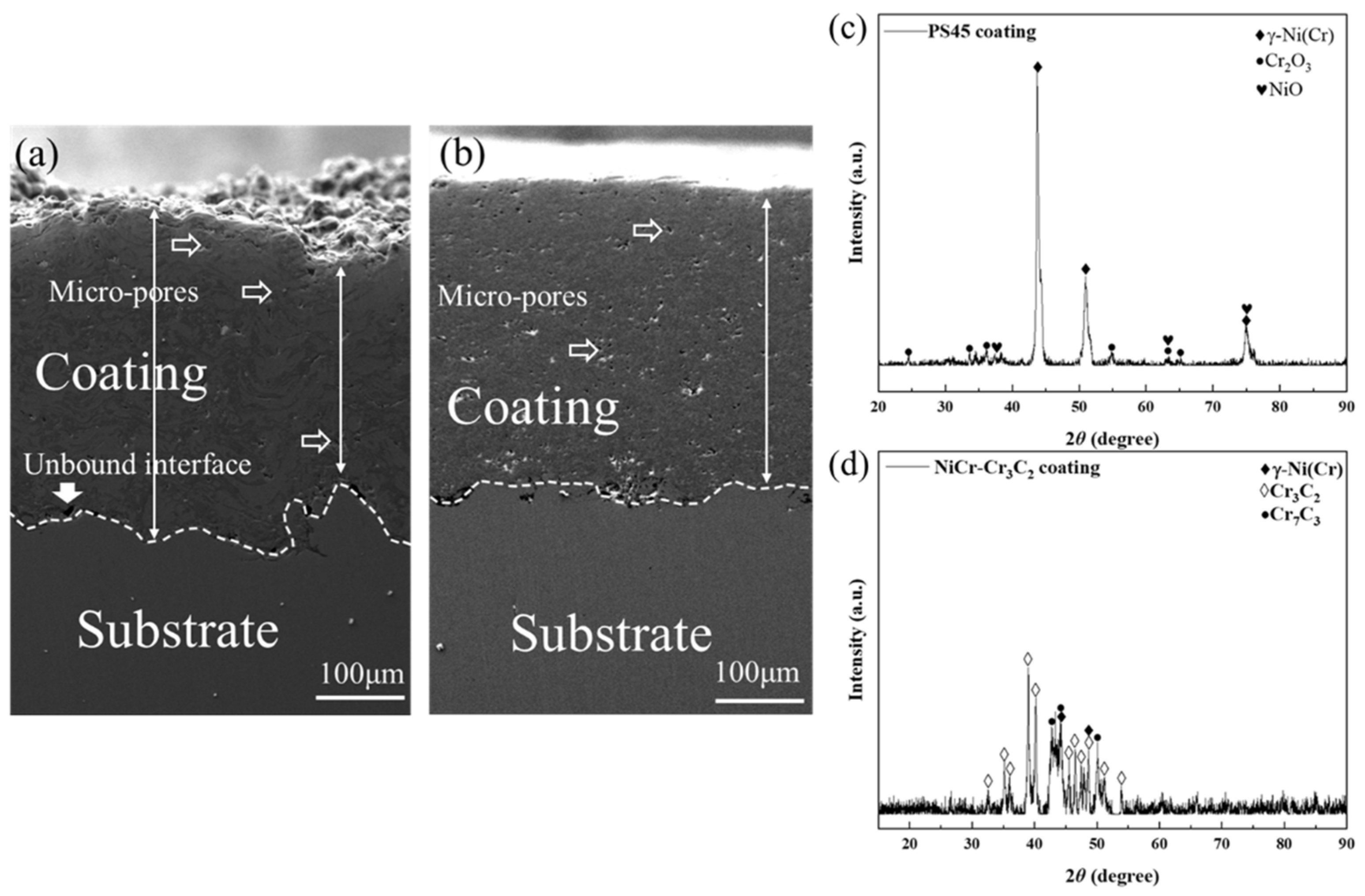
3.1. Microstructural Characterization of Coatings
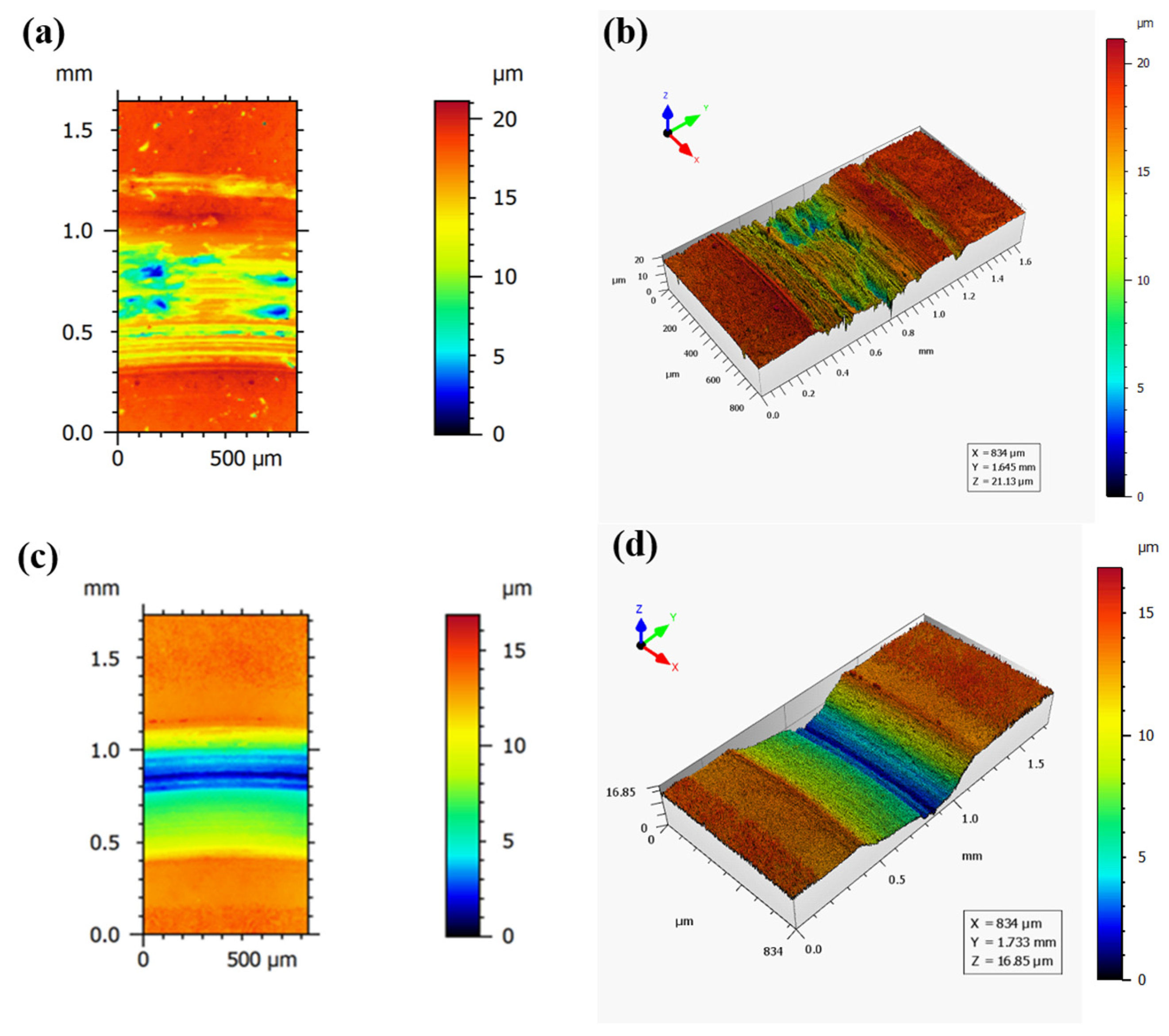
3.2. Mechanical Properties of the Coatings
3.3. Evaluation of High-Temperature Sulfate Corrosion Resistance of Coatings
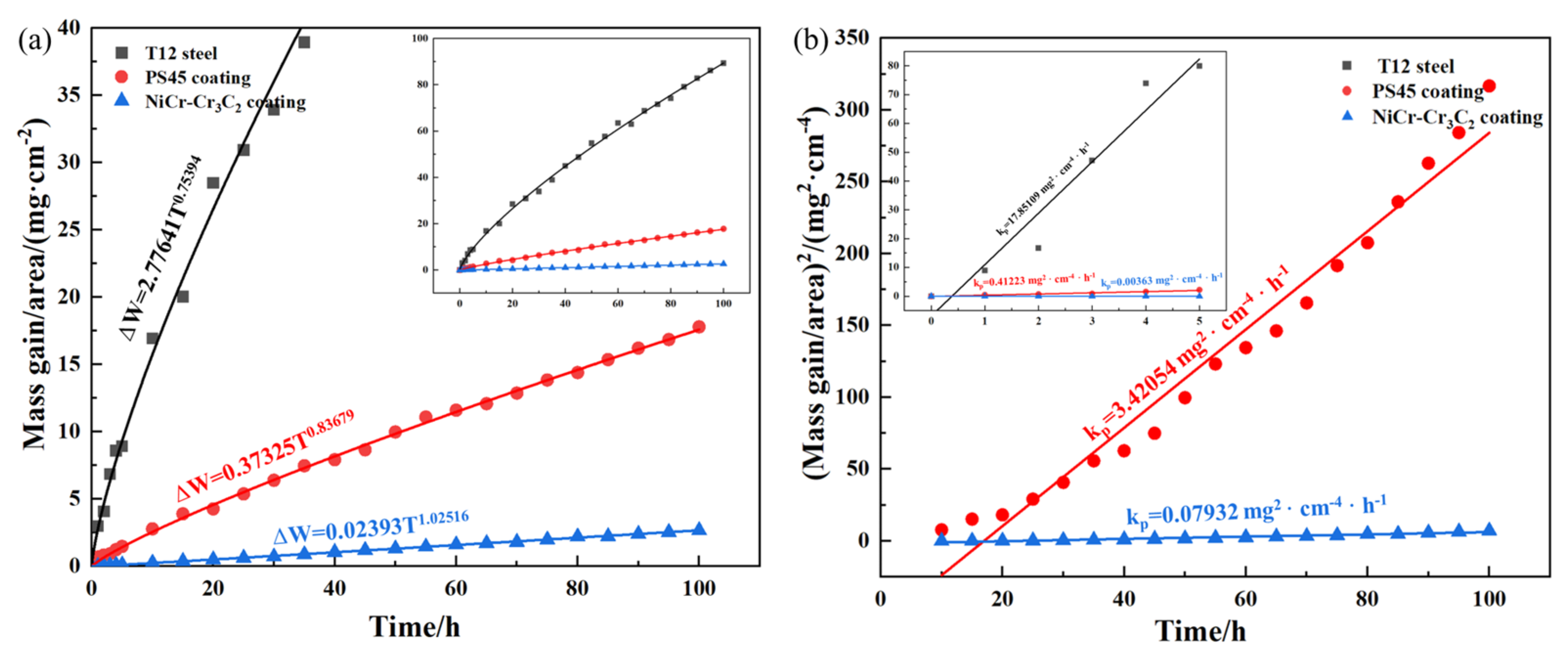
3.3.1. High-Temperature Sulfate Corrosion Kinetics of the Coatings
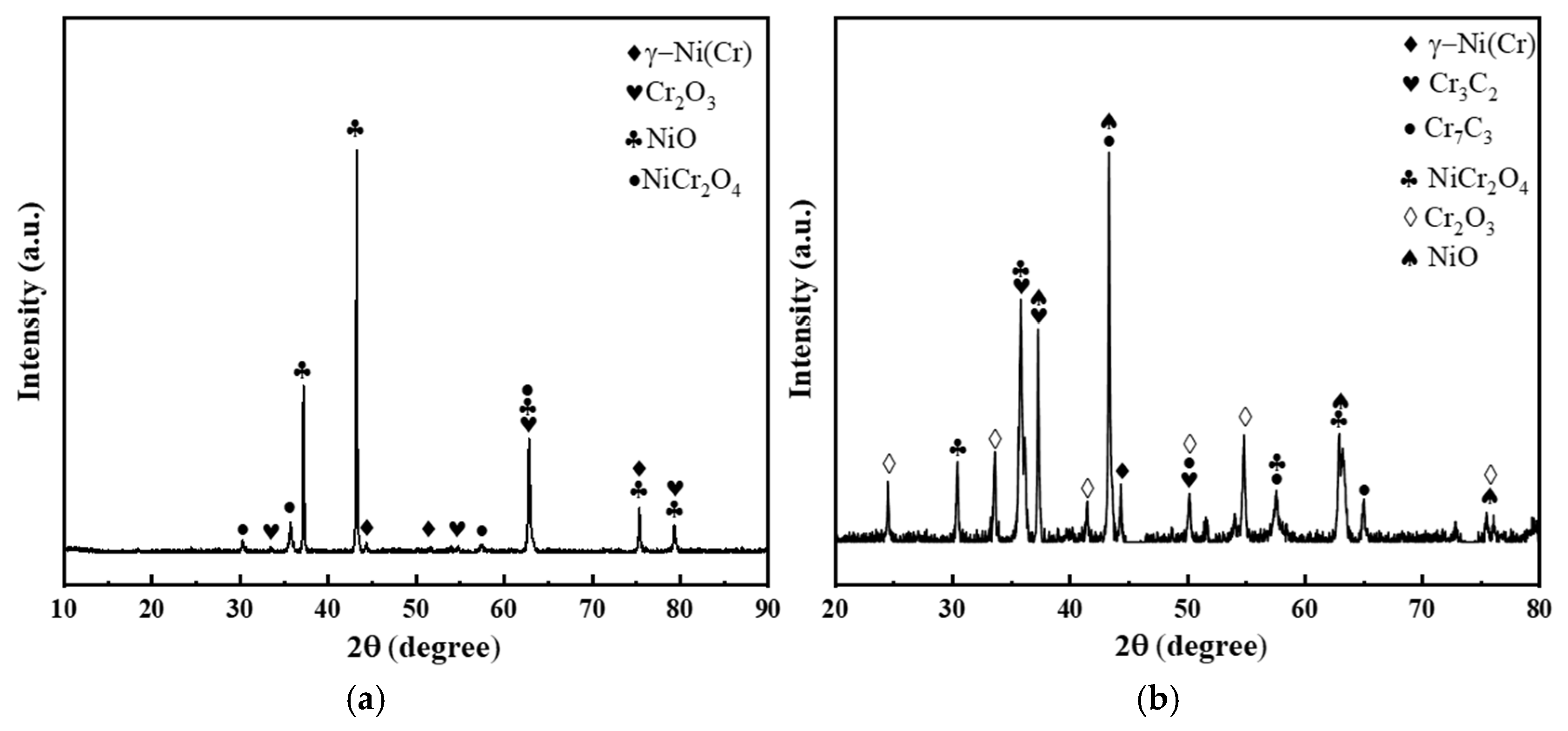
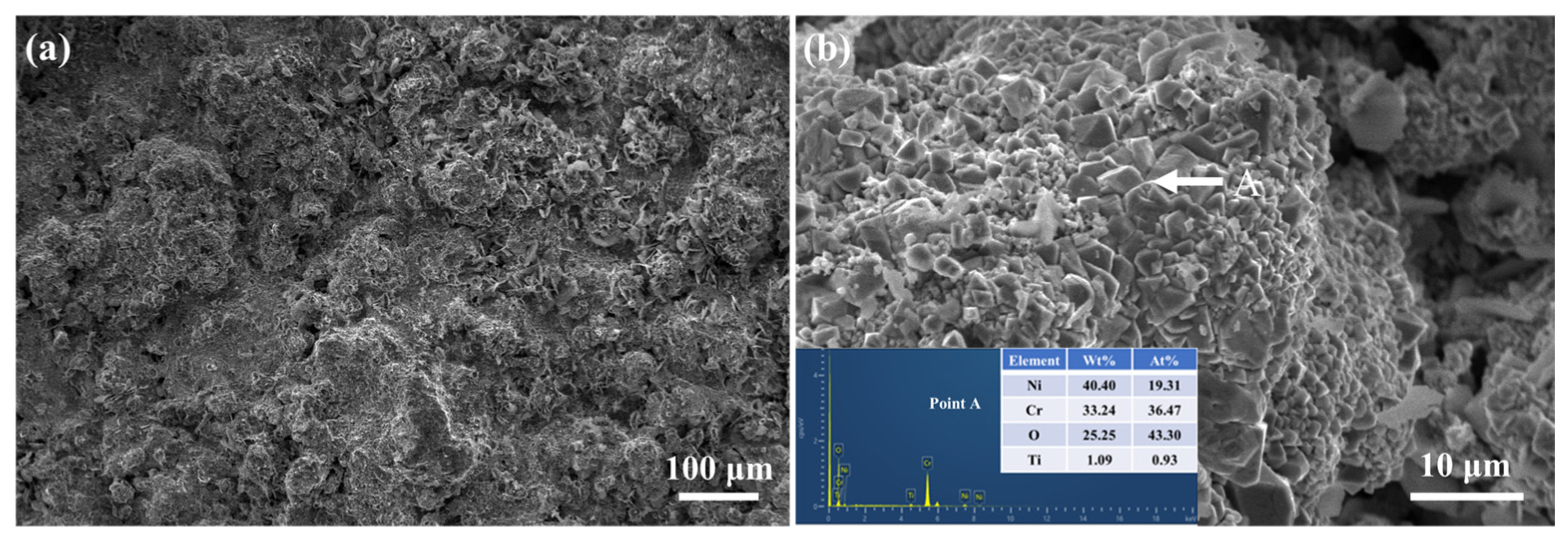
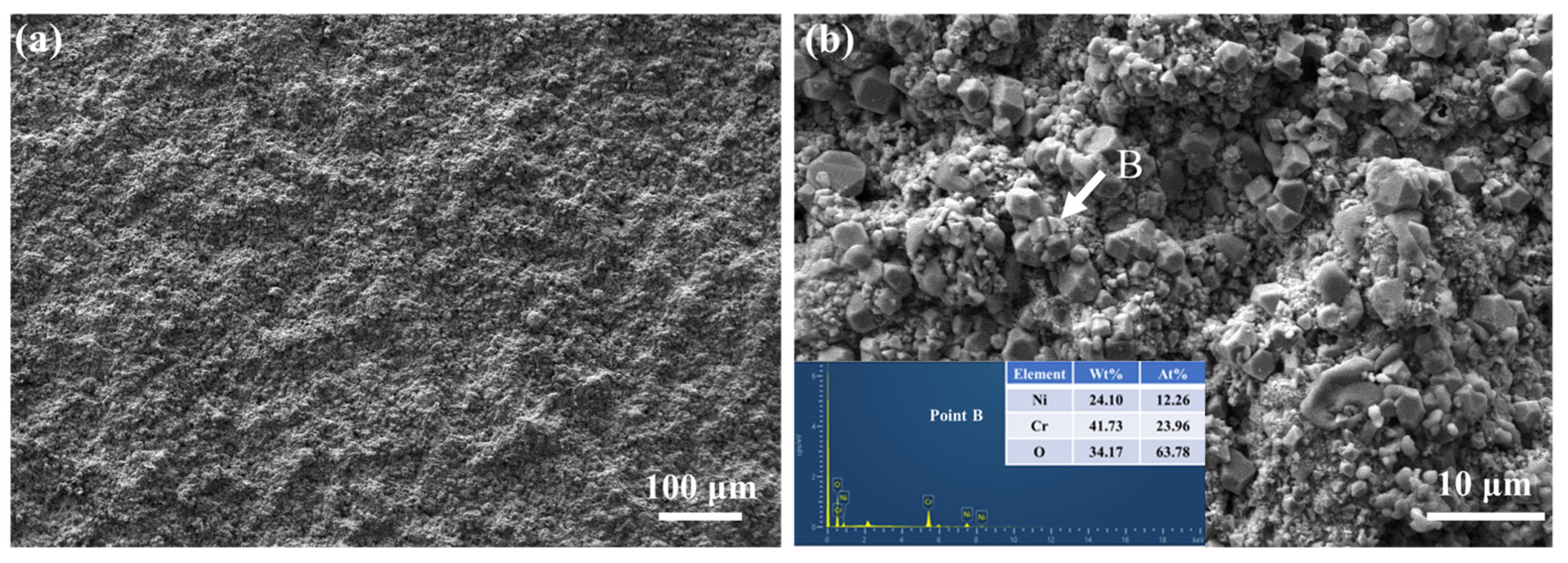
3.3.2. Phase Composition and Surface Morphology Evolution of Coatings After High-Temperature Sulfate Corrosion
3.3.3. Mechanism of High-Temperature Sulfate Corrosion Resistance in NiCr–Cr3C2 Coatings
3.4. High-Temperature Tribological Properties of Coatings
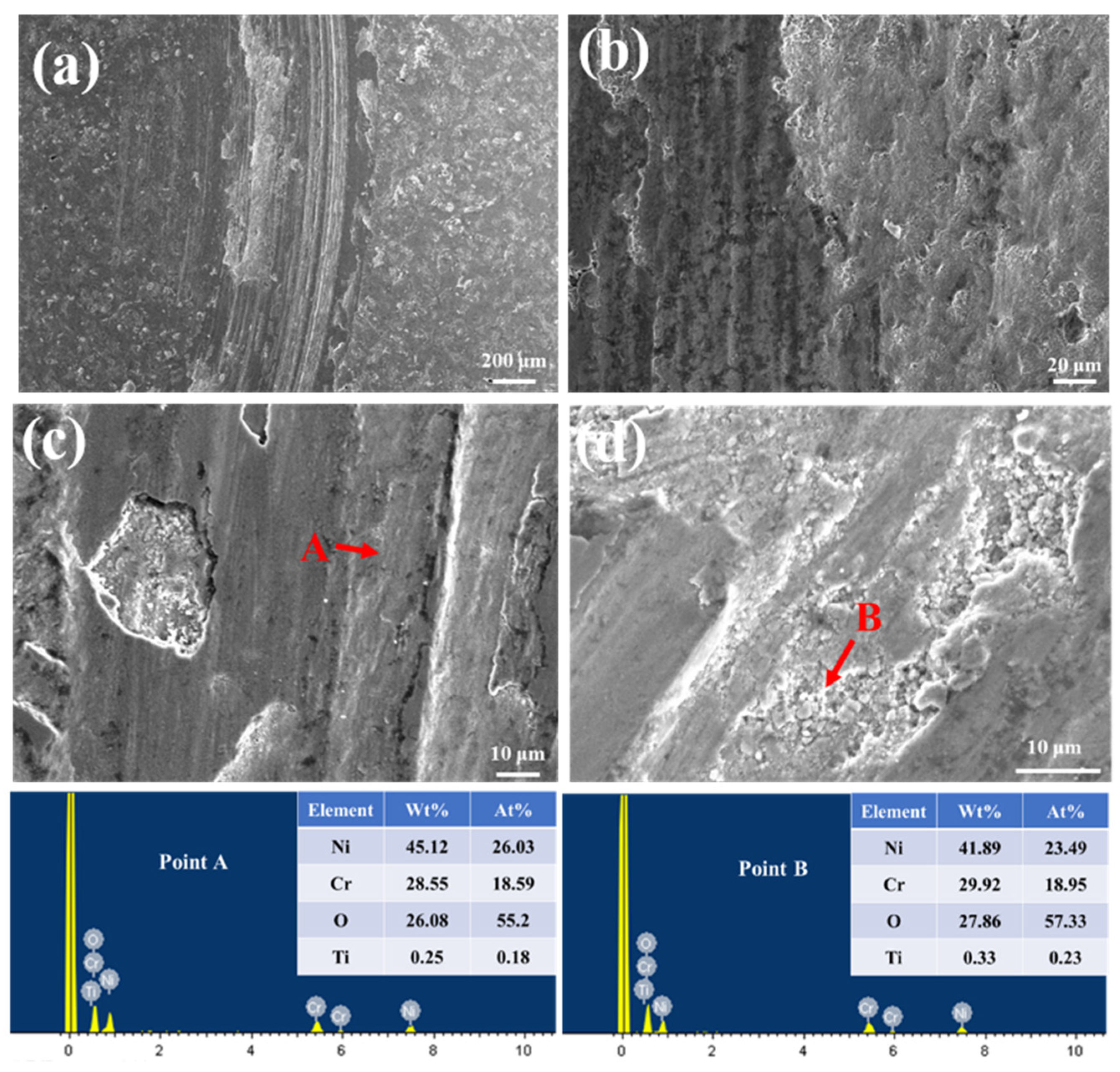
3.4.1. High-Temperature Friction and Wear Behavior
3.4.2. High-Temperature Wear Resistance Mechanisms
4. Conclusions
- 1.
- The HVOF-sprayed NiCr–Cr3C2 coating exhibited a dense microstructure with low porosity (1.54%), high microhardness (965 HV0.3), and superior bonding strength (61.20 MPa), significantly outperforming the arc-sprayed PS45 coating.
- 2.
- After 100 h of corrosion at 750 °C, the NiCr–Cr3C2 coating showed the lowest mass gain (2.7 mg/cm2): only 3.02% of the substrate and 15.17% of the PS45 coating. Its excellent corrosion resistance originated from the formation of a protective Cr2O3/NiCr2O4 layer via the basic fluxing mechanism, further enhanced by the high Cr content through selective oxidation, which effectively inhibited corrosive penetration.
- 3.
- Both coatings experienced oxidative, abrasive, and adhesive wear at 750 °C. The NiCr–Cr3C2 coating demonstrated a lower stable friction coefficient (~0.4). However, the NiCr–Cr3C2 coating showed comparable wear volume to the PS45 coating due to the binder phase softening and subsequent hard phase detachment, which highlights the need for improved high-temperature binder stability.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, Z.; Li, Z.; Zhu, Q.; Jing, J. Gas/particle flow and combustion characteristics and NOx emissions of a new swirl coal burner. Energy 2011, 36, 709–723. [Google Scholar] [CrossRef]
- Liu, G.; Zheng, L.; Gao, L.; Zhang, H.; Peng, Z. The characterization of coal quality from the Jining coalfield. Energy 2005, 30, 1903–1914. [Google Scholar] [CrossRef]
- You, C.; Xu, X. Coal combustion and its pollution control in China. Energy 2010, 35, 4467–4472. [Google Scholar] [CrossRef]
- Le Bris, T.; Cadavid, F.; Caillat, S.; Pietrzyk, S.; Blondin, J.; Baudoin, B. Coal combustion modelling of large power plant, for NOx abatement. Fuel 2007, 86, 2213–2220. [Google Scholar] [CrossRef]
- Dong, J.; Fan, H.; Wu, X.; Zhou, T.; Zhang, J.; Zhang, Z. Study on the effect of flame offset on water wall tube temperature in 600 °C and 700 °C ultra-supercritical boiler. Combust. Sci. Technol. 2018, 191, 472–490. [Google Scholar] [CrossRef]
- Song, T.; Wang, Z. Research Progress on High-temperature Oxidation of Austenitic Stainless Steel for Ultra-superCritical Boilers. IOP Conf. Ser. Mater. Sci. Eng. 2020, 735, 012008. [Google Scholar] [CrossRef]
- Phongphiphat, A.; Ryu, C.; Yang, Y.B.; Finney, K.N.; Leyland, A.; Sharifi, V.N.; Swithenbank, J. Investigation into high-temperature corrosion in a large-scale municipal waste-to-energy plant. Corros. Sci. 2010, 52, 3861–3874. [Google Scholar] [CrossRef]
- Kaur, M.; Singh, H.; Prakash, S. Surface engineering analysis of detonation-gun sprayed Cr3C2–NiCr coating under high-temperature oxidation and oxidation–erosion environments. Surf. Coat. Technol. 2011, 206, 530–541. [Google Scholar] [CrossRef]
- Kung, S.C. Further Understanding of Furnace Wall Corrosion in Coal-Fired Boilers. Corrosion 2013, 70, 749–763. [Google Scholar] [CrossRef]
- Reiche, M.; Grahl, S.; Beckmann, M. Advanced monitoring of the fouling process on water walls. Fuel 2018, 216, 436–444. [Google Scholar] [CrossRef]
- Wang, S.; Xin, Y. Anaysis on High-temperature Corrosion and Erosion of Water-Cooler Tube. Corros. Sci. Prot. Technol. 2010, 22, 243–246. [Google Scholar]
- Skrifvars, B.-J.; Backman, R.; Hupa, M.; Salmenoja, K.; Vakkilainen, E. Corrosion of superheater steel materials under alkali salt deposits Part 1: The effect of salt deposit composition and temperature. Corros. Sci. 2008, 50, 1274–1282. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, X.; Cheng, J.; Lin, J.; Zhou, Z. Cavitation Erosion Resistance of Fe-Based Amorphous/Nanocrystal Coatings Prepared by High-Velocity Arc Spraying. J. Therm. Spray Technol. 2014, 23, 742–749. [Google Scholar] [CrossRef]
- Cheng, J.; Liu, D.; Liang, X.; Chen, Y. Wear Behaviors of Arc-Sprayed FeBSiNb Amorphous Coatings. Tribol. Lett. 2015, 60, 22. [Google Scholar] [CrossRef]
- Chen, P.H.; Liu, Z.L.; Li, R.Q.; Qiu, C.J.; Li, X.Q. Thermal-sprayed coating of optimally mixed ceramic powders on stainless steel with enhanced corrosion resistance. J. Iron. Steel Res. Int. 2018, 25, 207–212. [Google Scholar] [CrossRef]
- Yuan, Z.F.; Xie, S.S.; Yu, X.T.; Liu, J.; Du, P.; Li, Z.H. Wear-resistant coating spraying technique for wear-proofing cover of coke dry quenching furnace. J. Iron Steel Res. Int. 2018, 26, 123–129. [Google Scholar] [CrossRef]
- He, D.; Dong, N.; Jiang, J. Corrosion Behavior of Arc Sprayed Nickel-Base Coatings. J. Therm. Spray Technol. 2007, 16, 850–856. [Google Scholar] [CrossRef]
- Ji, G.C.; Li, C.J.; Wang, Y.Y.; Li, W.Y. Erosion performance of HVOF-sprayed Cr3C2-NiCr coatings. J. Therm. Spray Technol. 2007, 16, 557–565. [Google Scholar] [CrossRef]
- Matthews, S.; James, B.; Hyland, M. The Effect of Heat Treatment on the Oxidation Mechanism of Blended Powder Cr3C2-NiCr Coatings. J. Therm. Spray Technol. 2009, 19, 119–127. [Google Scholar] [CrossRef]
- Ghosh, D.; Mitra, S.K. Plasma sprayed Cr3C2–Ni–Cr coating for oxidation protection of 2·25Cr–1Mo steel. Surf. Eng. 2014, 31, 342–348. [Google Scholar] [CrossRef]
- Hussain, T.; Dudziak, T.; Simms, N.J.; Nicholls, J.R. Fireside corrosion behavior of HVOF and plasma-sprayed coatings in advanced coal/biomass co-fired power plants. J. Therm. Spray Technol. 2013, 22, 797–807. [Google Scholar] [CrossRef]
- Yadav, A.S.; Mishra, S.B. Comparative erosion performance of HVOF and LVOF sprayed NiCrSiBCFe-WC-Co coating. Surf. Eng. 2024, 40, 575–590. [Google Scholar] [CrossRef]
- Sudhakara, D.; Jeyasimmana, D.; Duraiselvamb, M. Dry Sliding Wear Behavior of Cr3C2-NiCr Coating on Austenitic Stainless Steel. Int. J. Core Eng. Manag. 2015, 1, 215–225. [Google Scholar]
- Chatha, S.S.; Sidhu, H.S.; Sidhu, B.S. Characterisation and corrosion-erosion behaviour of carbide based thermal spray coatings. J. Miner. Mater. Charact. Eng. 2012, 11, 569–586. [Google Scholar] [CrossRef]
- Mehta, J.; Mittal, V.K.; Gupta, P. Role of thermal spray coatings on wear, erosion and corrosion behavior: A review. J. Appl. Sci. Eng. 2017, 20, 445–452. [Google Scholar]
- Schubert, J.; Česánek, Z.; Bláhová, O. Mechanical Properties of HVOF Sprayed CrC-NiCr Coating Exposed to Hot Corrosion Environment. Key Eng. Mater. 2018, 784, 141–146. [Google Scholar] [CrossRef]
- Chatha, S.S.; Sidhu, H.S.; Sidhu, B.S. High temperature hot corrosion behaviour of NiCr and Cr3C2–NiCr coatings on T91 boiler steel in an aggressive environment at 750 °C. Surf. Coat. Technol. 2012, 206, 3839–3850. [Google Scholar] [CrossRef]
- Oksa, M.; Auerkari, P.; Salonen, J.; Varis, T. Nickel-based HVOF coatings promoting high temperature corrosion resistance of biomass-fired power plant boilers. Fuel Process. Technol. 2014, 125, 236–245. [Google Scholar] [CrossRef]
- Somasundaram, B.; Kadoli, R.; Ramesh, M. Evaluation of Thermocyclic Oxidation Behavior of HVOF Sprayed (Cr3C2-35% NiCr) + 5% Si Coatings on Boiler Tube Steels. Procedia Mater. Sci. 2014, 5, 398–407. [Google Scholar] [CrossRef]
- Manjunatha, K.; Giridhara, G.; Shivalingappa, D. High temperature erosion behaviour of high-velocity oxy-fuel sprayed CNT/NiCr-Cr3C2 composite coatings. Surf. Coat. Technol. 2022, 448, 128900. [Google Scholar]
- Gok, K.; Ada, H.D.; Kilicaslan, N.; Gok, A. A Review of CFD Modeling of Erosion-induced Corrosion Formation in Water Jets Using FEA. J. Mech. Mater. Mech. Res. 2023, 6, 14–22. [Google Scholar] [CrossRef]
- ISO 14916:2017; Thermal Spraying—Determination of Tensile Adhesive Strength. ISO: Geneva, Switzerland, 2017.
- Simons, E.L.; Browning, G.V.; Liebhafsky, H.A. Sodium Sulfate in Gas Turbines. Corrosion 1955, 11, 17–26. [Google Scholar] [CrossRef]
- Bornstein, N.S.; DeCrescente, M.A. The role of sodium in the accelerated oxidation phenomenon termed sulfidation. Met. Trans. 1971, 2, 2875–2883. [Google Scholar] [CrossRef]
- Shores, D.A.; Fang, W. Transport of Oxidant in Molten Na2SO4 in O2-SO2-SO3 Environments. J. Electrochem. Soc. 1981, 128, 346–348. [Google Scholar] [CrossRef]
- Otsuka, N.; Rapp, R.A. Hot Corrosion of Preoxidized Ni by a Thin Fused Na2SO4 Film at 900 °C. J. Electrochem. Soc. 1990, 137, 46. [Google Scholar] [CrossRef]
- Ding, Y.; Hussain, T.; Mccartney, D.G. High-temperature oxidation of HVOF thermally sprayed NiCr–Cr3C2 coatings: Micro-structure and kinetics. J. Mater. Sci. 2015, 50, 6808–6821. [Google Scholar] [CrossRef]
- Bala, N.; Singh, H.; Prakash, S. Accelerated hot corrosion studies of cold spray Ni–50Cr coating on boiler steels. Mater. Des. 2010, 31, 244–253. [Google Scholar] [CrossRef]
- Berger, L.M.; Woydt, M.; Zimmermann, S.; Keller, H.; Schwier, G.; Enžl, R.; Thiele, S. Tribological behavior of HVOF-sprayed Cr3C2-NiCr and TiC-based coatings under high-temperature dry sliding conditions. In Proceedings of the International Thermal Spray Conference, Osaka, Japan, 10–12 May 2004; pp. 468–477. [Google Scholar]





| Element | Ni | Cr | C |
|---|---|---|---|
| Content | 16.32 | 72.28 | 11.39 |
| Element | Ni | Cr | Ti | Al | Fe |
|---|---|---|---|---|---|
| Content | 57.28 | 40.75 | 1.00 | 0.16 | 0.10 |
| Spray Parameters | PS45 Coating |
|---|---|
| Arc voltage (V) | 24 |
| Current (A) | 150 |
| Air pressure (kPa) | 600 |
| Spray distance (mm) | 150 |
| Spray Parameters | NiCr-Cr3C2 Coating |
|---|---|
| Carrier gas flow rate(gph) | 26 |
| Kerosene flow rate(gph) | 3 |
| Oxygen flow rate (scfh) | 2000 |
| Powder feed rate (rpm) | 3 |
| Spray distance (mm) | 350 |
| Gun traverse speed (mm·s−1) | 200 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Zhang, Z.; Zhou, C.; Jin, F.; Cai, Y.; Ni, Y.; Ma, X.; Fan, C.; Xiang, S.; Song, D. High-Temperature Sulfate Corrosion Resistance and Wear Performance of NiCr-Cr3C2 Coatings for the Water Wall of Power Plant Boilers. Coatings 2025, 15, 1152. https://doi.org/10.3390/coatings15101152
Zhang H, Zhang Z, Zhou C, Jin F, Cai Y, Ni Y, Ma X, Fan C, Xiang S, Song D. High-Temperature Sulfate Corrosion Resistance and Wear Performance of NiCr-Cr3C2 Coatings for the Water Wall of Power Plant Boilers. Coatings. 2025; 15(10):1152. https://doi.org/10.3390/coatings15101152
Chicago/Turabian StyleZhang, Hang, Zhao Zhang, Cheng Zhou, Fangzhou Jin, Yongfeng Cai, Yifan Ni, Xinmin Ma, Chenghao Fan, Shulin Xiang, and Dan Song. 2025. "High-Temperature Sulfate Corrosion Resistance and Wear Performance of NiCr-Cr3C2 Coatings for the Water Wall of Power Plant Boilers" Coatings 15, no. 10: 1152. https://doi.org/10.3390/coatings15101152
APA StyleZhang, H., Zhang, Z., Zhou, C., Jin, F., Cai, Y., Ni, Y., Ma, X., Fan, C., Xiang, S., & Song, D. (2025). High-Temperature Sulfate Corrosion Resistance and Wear Performance of NiCr-Cr3C2 Coatings for the Water Wall of Power Plant Boilers. Coatings, 15(10), 1152. https://doi.org/10.3390/coatings15101152






