High-Temperature Effects on TGO Growth and Al Depletion in TBCs of Ni-Based Superalloy GTD111
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
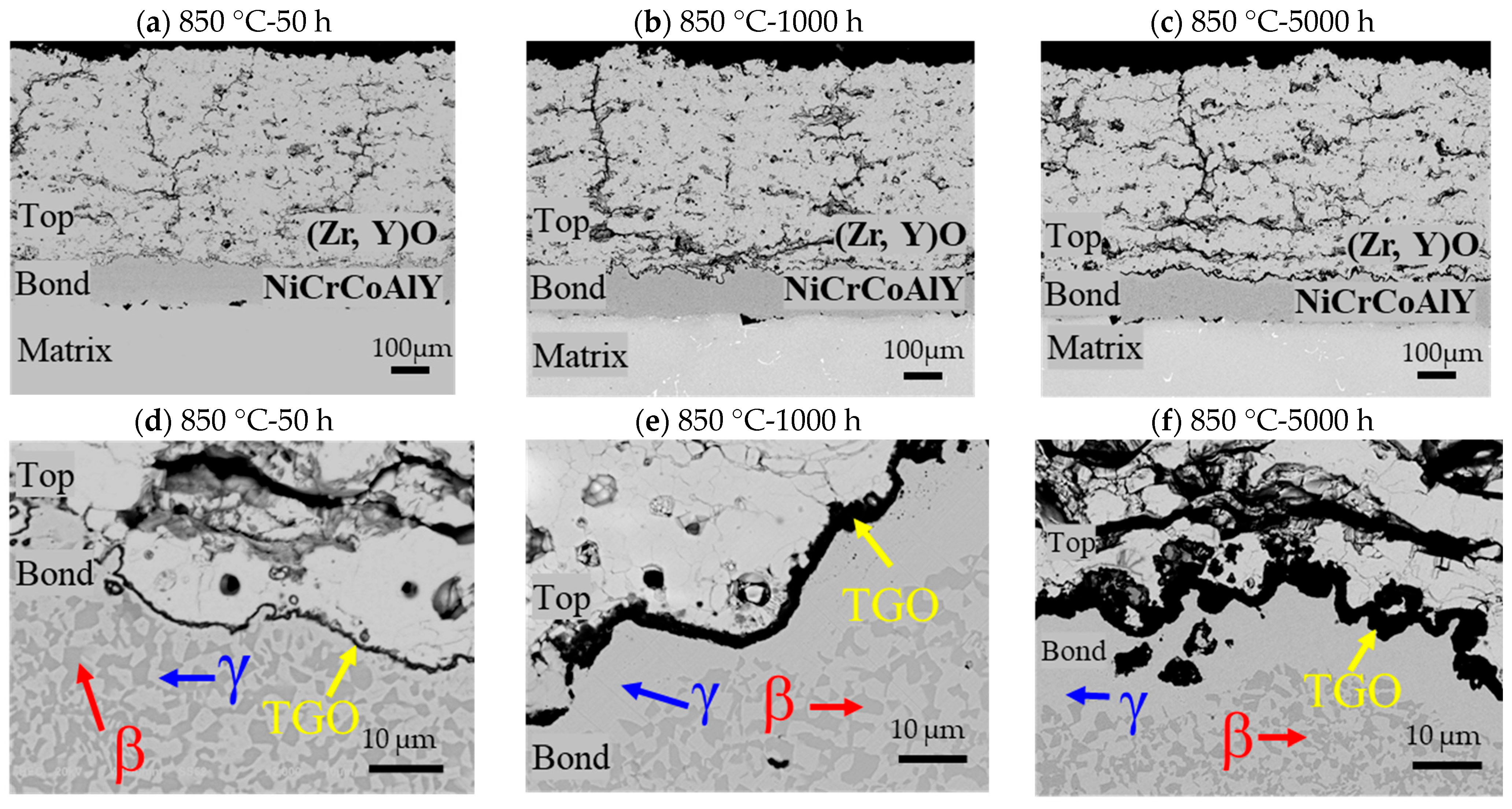
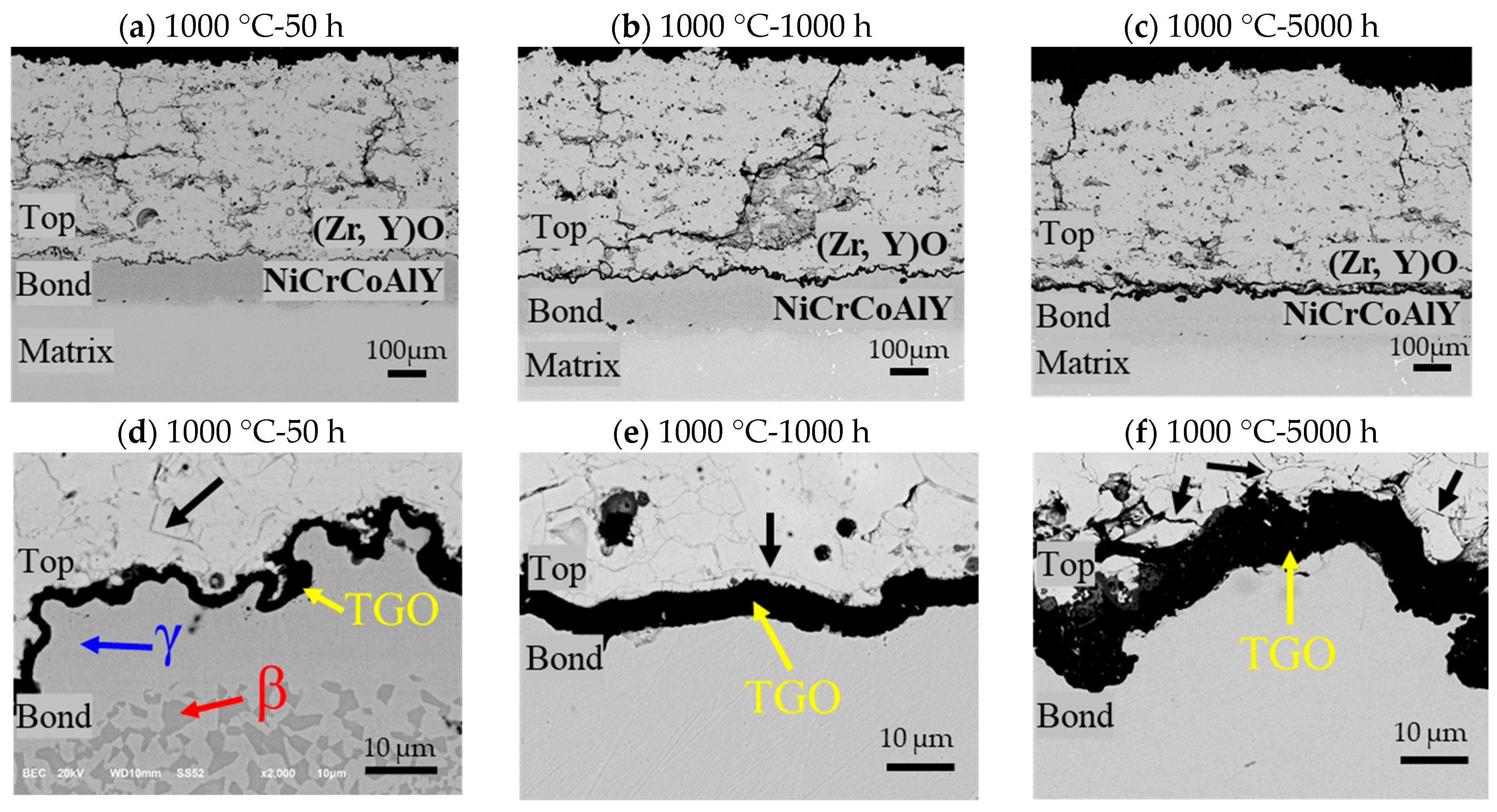
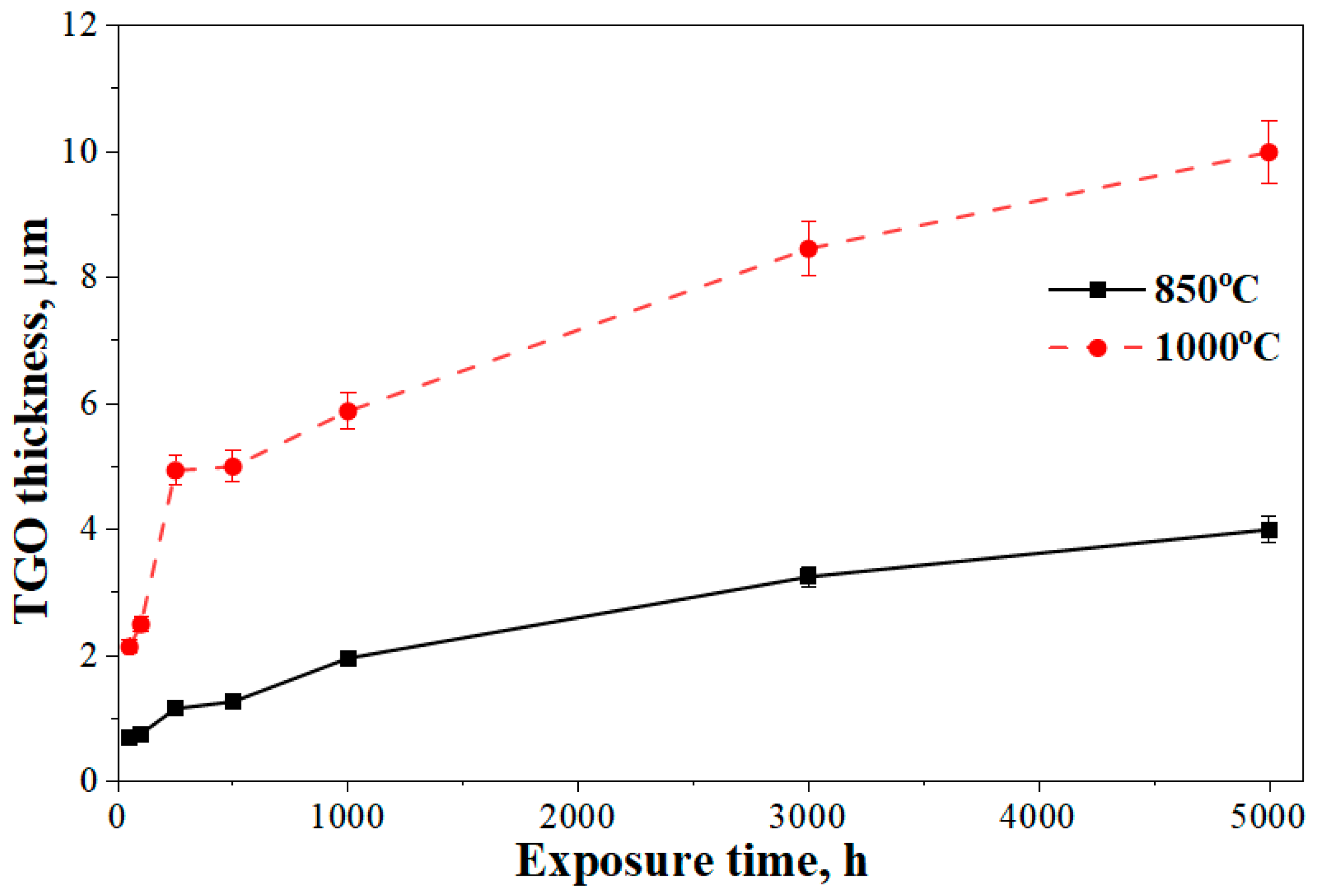
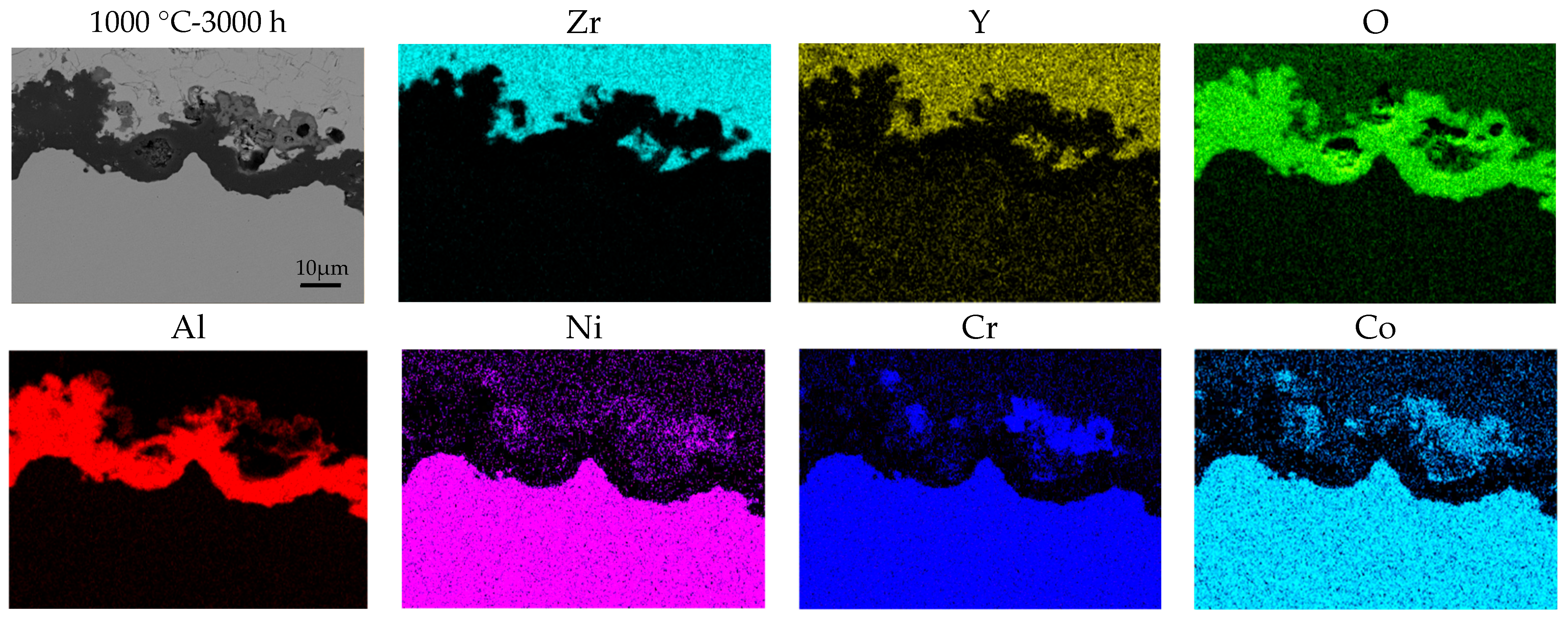
- The top coat, bond coat, and thermally grown oxide (TGO) are comprised of (Zr, Y)O, Ni-Cr-Co-Al, and Al2O3, respectively. TGO thickness increases from 0.7 µm at 850 °C/50 h to 9.9 µm at 1000–5000 h, showing accelerated growth at higher temperature and longer exposure.
- From 850 °C to 250 h onward, the Al in the β-NiAl phase of the bond coat depletes, leading to the thickening of the Al2O3 layer and the formation of a less protective γ-phase, which weakens the overall bond coat.
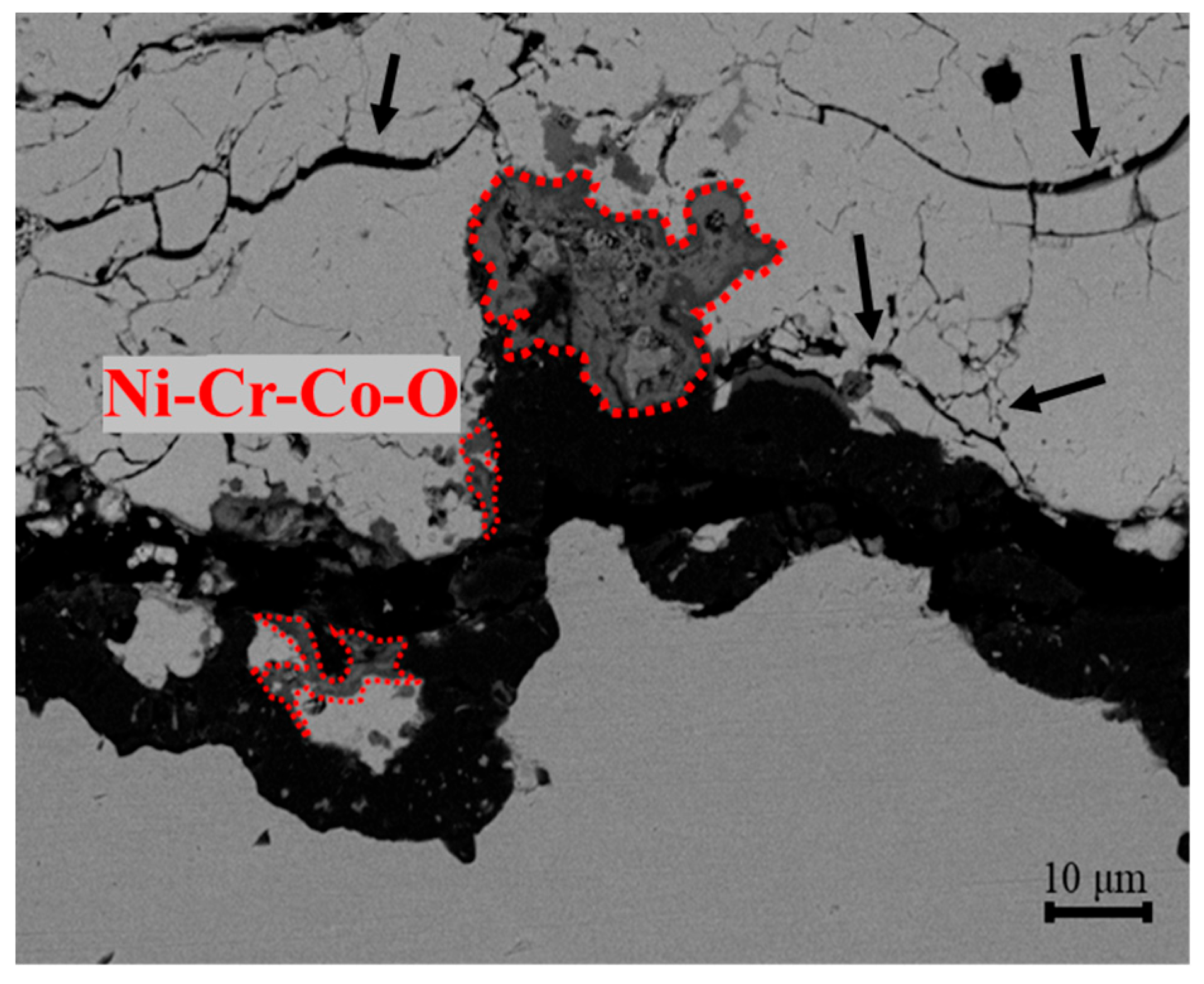
- In all the specimens exposed from 850 °C for 500 h to 1000 °C for 5000 h, spinel mixed metal oxides, such as Ni-Cr-Co-O, formed in the TGO layer due to the diffusion of elements like Ni, Cr, and Co, deteriorating the bond coat’s condition, as evidenced by the increased cracks and defects in the TBCs due to the high stress at the interface between the TGO layer and the top coat. While no gross spallation occurs during isothermal exposure up to 5000 h, the observed TGO thickening and interfacial microcracking indicate heightened spallation risk under cyclic thermal loading.
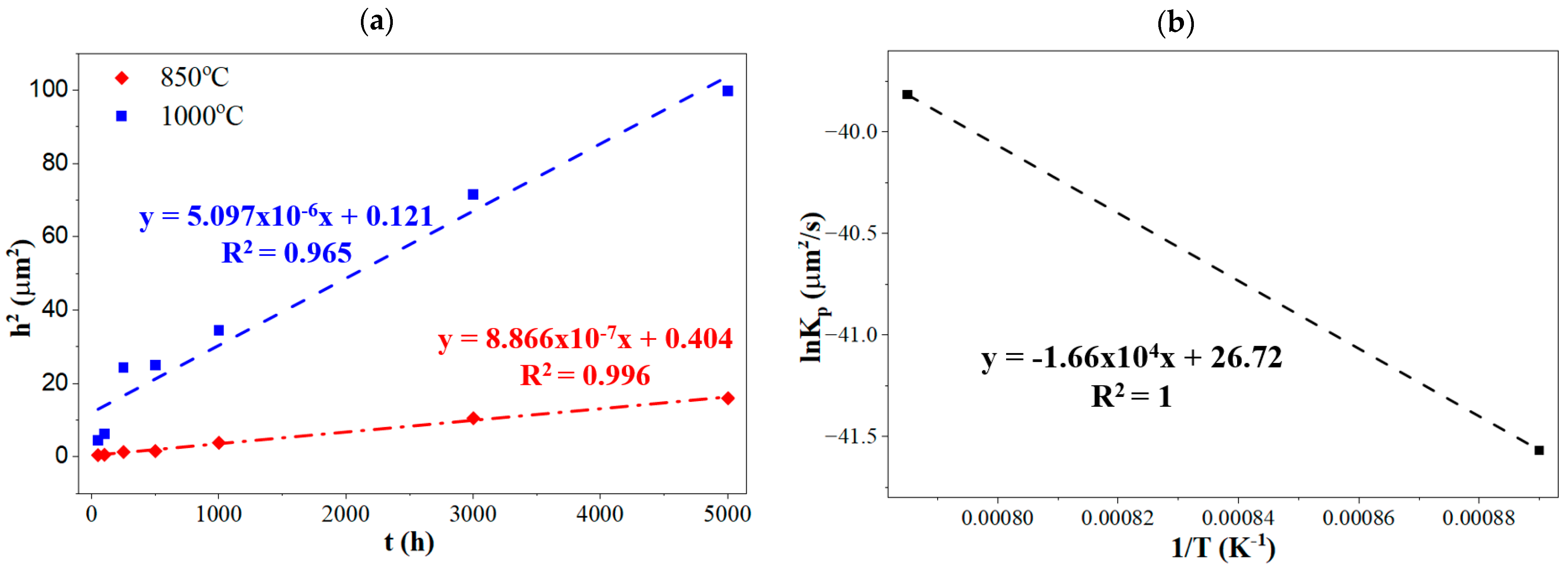
- The TGO layer also thickened steadily with higher temperatures and longer exposure, reaching about ~9.9 μm. This thickening, along with the cracks, increases the risk of TBC system failure. Increased time and temperature lead to a higher growth rate in the TGO layer. Quantitatively, increases linearly with time (quadratic law), giving and . The latter (or the at 1000 °C) is ~5.8 times higher at 1000 °C. The progressive TGO thickening therefore raises growth and thermal mismatch stresses at the TGO/YSZ interface, accelerating crack initiation.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Padture, N.P.; Gell, M.; Jordan, E.H. Thermal barrier coatings for gas-turbine engine applications. Science 2002, 296, 280–284. [Google Scholar] [CrossRef]
- Pint, B.A.; Haynes, J.A.; Zhang, Y. Substrate; bond coat compositions: Factors affecting alumina scale growth; spallation. Surf. Coat. Technol. 2015, 282, 82–91. [Google Scholar] [CrossRef]
- Zhu, D.; Miller, R.A. Thermal barrier coatings for advanced gas turbine engines. Mater. Sci. Eng. A 1997, 245, 150–153. [Google Scholar] [CrossRef]
- Peng, X. Metallic coatings for high-temperature oxidation resistance. In Thermal Barrier Coatings; Woodhead Publishing: Cambridge, UK, 2011; pp. 53–74. [Google Scholar] [CrossRef]
- Evans, A.G.; Mumm, D.R.; Hutchinson, J.W.; Meier, G.H.; Pettit, F.S. Mechanisms controlling the durability of thermal barrier coatings. Prog. Mater. Sci. 2001, 46, 505–553. [Google Scholar] [CrossRef]
- Chen, X.; He, M.Y.; Spitsberg, I.; Fleck, N.A.; Hutchinson, J.W.; Evans, A.G. Mechanisms governing the high temperature erosion of thermal barrier coatings. Wear 2004, 256, 735–746. [Google Scholar] [CrossRef]
- Ma, K.; Xie, C.; Li, Y.; Yang, B.; Jin, Y.; Wang, H.; Zeng, Z.; Li, Y.; Ye, X. Oxidation Behavior of Aluminide Coatings on Cobalt-Based Superalloys by a Vapor Phase Aluminizing Process. Materials 2024, 17, 5897. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Huang, B.; He, L.; Mu, R.; Tian, H.; Xu, Z. Thermal cycling behavior and failure mechanism of Yb2O3-doped yttria-stabilized zirconia thermal barrier coatings. Mater. Today Commun. 2023, 34, 105409. [Google Scholar] [CrossRef]
- Liu, D.; Mu, R.; He, L.; Li, S.; Yang, W. Failure behaviour of EB-PVD YSZ thermal barrier coatings under simulated aero-engine operating conditions. Surf. Coat. Technol. 2023, 474, 130027. [Google Scholar] [CrossRef]
- Pour-Ali, S.; Tavangar, R.; Akhtari, F.; Hejazi, S. High-Temperature Oxidation Behavior of GTD-111 Ni-Based Superalloy with an Ultrafine-Grained Surface at 900 °C. Corros. Sci. 2023, 212, 110935. [Google Scholar] [CrossRef]
- Freborg, A.M.; Ferguson, B.L.; Brindley, W.J.; Petrus, G.J. Modeling Oxidation Induced Stresses in Thermal Barrier Coatings. Mater. Sci. Eng. A 1998, 245, 182–190. [Google Scholar] [CrossRef]
- Abdelgawad, A.; Al-Athel, K. Effect of TGO Thickness, Pores and Creep on the Developed Residual Stresses in Thermal Barrier Coatings under Cyclic Loading Using SEM Image-Based Finite Element Model. Ceram. Int. 2021, 47, 20064–20076. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Ma, Z.; Dong, S.Z.; Xu, M.M.; Li, S.; Zhang, C.; Jiang, C.Y.; Bao, Z.B.; Zhu, S.L.; Wang, F.H. High-Temperature Oxidation Behaviour of Refurbished (Ni,Pt)Al Coating on Ni-Based Superalloy at 1100 °C. Corros. Sci. 2021, 187, 109521. [Google Scholar] [CrossRef]
- Zhang, P.; Yuan, K.; Peng Ru Li, X.; Johansson, S. Long-term oxidation of MCrAlY coatings at 1000 °C and an Al-activity based coating life criterion. Surf. Coat. Technol. 2017, 332, 12–21. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Zhang, C.; Xu, N.; Bao, Z. Oxidation Behavior and Oxide Transformation of a Pt-Modified Aluminide Coating at Moderate High Temperature. Crystals 2021, 11, 972. [Google Scholar] [CrossRef]
- Haynes, J.A.; Pint, B.A.; Zhang, Y.; Wright, I.G. Comparison of the Oxidation Behavior of β; γ–γ′ NiPtAl Coatings. Surf. Coat. Technol. 2009, 204, 816–819. [Google Scholar] [CrossRef]
- Liu, D.; Jing, Y.; Cui, X.; Jin, G.; Chen, Z.; Wang, X.; Liu, A.; Li, Q.; Tian, H.; Fang, Y. Study of toughening behavior of SiC whiskers on 8YSZ thermal barrier coatings. Surf. Coat. Technol. 2023, 455, 129232. [Google Scholar] [CrossRef]
- Leng, K.; Rincon Romero, A.; Hussain, T. Multilayer GZ/YSZ thermal barrier coating from suspension and solution precursor thermal spray. J. Eur. Ceram. Soc. 2023, 43, 4991–5003. [Google Scholar] [CrossRef]
- Kumar, M.; Majumdar, J.D.; Manna, I. Development of Gd2O3-doped yttria-stabilized zirconia-based thermal barrier coating for improved high-temperature oxidation and erosion resistance. Ceram. Int. 2023, 49, 38081–38093. [Google Scholar] [CrossRef]
- Emine, B.; Daniel, E.M.; Georg, M.; Robert, V. Gadolinium zirconate/YSZ thermal barrier coatings: Processing and thermal cycling behavior. J. Am. Ceram. Soc. 2014, 97, 4045–4051. [Google Scholar] [CrossRef]
- Strangman, T. Damage mechanisms, life prediction, and development of life models for thermal barrier coatings. Surf. Coat. Technol. 2007, 201, 2087–2103. [Google Scholar] [CrossRef]
- Chen, Y.; Yao, Z.; Wang, J.; Dong, J.; Ren, M.; Peng, J.; Yang, H.; Leng, L. Microstructural Evolution and Diffusion Mechanism of MCrAlY Coated Nickel-Based Superalloy Turbine Blades after Serviced for 47,000 h. Surf. Coat. Technol. 2024, 493, 131288. [Google Scholar] [CrossRef]
- Brenneman, J.; Wei, J.; Sun, Z.; Liu, L.; Zou, G.; Zhou, Y. Oxidation Behavior of GTD111 Ni-Based Superalloy at 900 °C in Air. Corros. Sci. 2015, 100, 267–274. [Google Scholar] [CrossRef]
- Dong, H.; Yang, G.-J.; Li, C.-X.; Luo, X.-T.; Li, C.-J. Effect of TGO Thickness on Thermal Cyclic Lifetime; Failure Mode of Plasma-Sprayed TBCs. J. Am. Ceram. Soc. 2014, 97, 1226–1232. [Google Scholar] [CrossRef]
- Liu, H.; Huang, J.; Wang, Z.; Qiu, Z.; Zheng, Z.; Wu, Y.; Yuan, S.; Zeng, D. Composite structure of YSZ embedded in NiCoCrAlTaY bond coat induces thin and multilayered Al2O3 film to extend the thermal cycle life of thermal barrier coatings. Surf. Coat. Technol. 2023, 475, 130104. [Google Scholar] [CrossRef]
- Ge, M.T.; Li, Y.M.; Tan, Z.H.; Tao, X.P.; Yang, Y.H.; Liu, J.D.; Liu, J.L.; Zou, M.K.; Zhang, C.H.; Zhang, S.; et al. Influence of Ta + Al on the microstructure evolution of two Ru-containing Ni-based single crystal superalloys deposited with γ′ + β NiAl coating at extremely high temperature. Surf. Coat. Technol. 2023, 472, 129893. [Google Scholar] [CrossRef]
- Jiang, X.; Song, W.; Liang, J.; Zhang, D.; Zhou, Y.; Sun, X.; Li, J. Effect of coatings on microstructure and oxidation behavior of the Ni-based single crystal superalloys containing different Ru contents. Surf. Coat. Technol. 2023, 474, 130092. [Google Scholar] [CrossRef]
- Fan, J.; Wang, Q.; Ning, X.; Li, L.; Sun, Z. Failure mechanisms for Gd2O3–Yb2O3 co-doped YSZ thermal barrier coatings under high-temperature gradient. Ceram. Int. 2024, 50, 28563–28572. [Google Scholar] [CrossRef]
- Chen, W.R.; Wu, X.; Marple, B.R.; Patnaik, P.C. Oxidation and Crack Nucleation/Growth in an Air-Plasma-Sprayed Thermal Barrier Coating with NiCrAlY Bond Coat. Surf. Coat. Technol. 2005, 197, 109–115. [Google Scholar] [CrossRef]
- Jackson, R.D.; Taylor, M.P.; Evans, H.E.; Li, X.-H. Oxidation Study of an EB-PVD MCrAlY Thermal Barrier Coating System. Oxid. Met. 2011, 76, 259–271. [Google Scholar] [CrossRef]
- Taylor, C.D.; Tossey, B.M. High temperature oxidation of corrosion resistant alloys from machine learning. Npj Mater Degrad. 2021, 5, 38. [Google Scholar] [CrossRef]
- Birks, N.; Meier, G.H.; Pettit, F.S. Introduction to the High-Temperature Oxidation of Metals, 2nd ed.; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Grabke, H.J. Oxidation of NiAl and FeAl. Intermetallics 1999, 7, 1153–1158. [Google Scholar] [CrossRef]
- Chen, H.; Barman, T. Thermo-Calc; DICTRA Modelling of the β-Phase Depletion Behaviour in CoNiCrAlY Coating Alloys at Different Al Contents. Comput. Mater. Sci. 2018, 147, 103–114. [Google Scholar] [CrossRef]
- Gheno, T.; Liu, X.L.; Lindwall, G.; Liu, Z.-K.; Gleeson, B. Experimental Study and Thermodynamic Modeling of the Al–Co–Cr–Ni System. Sci. Technol. Adv. Mater. 2015, 16, 055001. [Google Scholar] [CrossRef] [PubMed]
- Thermo-Calc Software AB. Diffusion Module (DICTRA). Available online: https://thermocalc.com/products/add-on-modules/diffusion-module-dictra (accessed on 7 September 2025).
- Teixeira, V.; Andritschky, M.; Fischer, W.; Buchkremer, H.P.; Stöver, D. Effects of deposition temperature and thermal cycling on residual stress state in zirconia-based thermal barrier coatings. Surf. Coat. Technol. 1999, 103–111. [Google Scholar] [CrossRef]
- Cui, J.; Saito, H.; Sato, K.; Ichikawa, Y.; Ogawa, K.; Nakashima, M.; Suzuki, A.; Sato, F. Degradation behavior of yttria-stabilized zirconia in thermal barrier coatings under reducing environments after short-term heat treatment. Ceram. Int. 2024, 50, 23. [Google Scholar] [CrossRef]
- He, J.; Sharobem, T. Influence of heat treatment on thermal cyclic fatigue of TBC systems. Surf. Coat. Technol. 2019, 379, 125050. [Google Scholar] [CrossRef]
- Yan, G.; Sun, Y.; Zhao, X.; Liu, W.; Wang, Q.; Li, C.; Yang, L.; Zhou, Y. The enhanced thermal shock resistance performance induced by interface effect in blade-level La2Ce2O7/YSZ thermal barrier coating. Appl. Surf. Sci. 2023, 619, 156723. [Google Scholar] [CrossRef]
| Elements | Cr | Co | Mo | W | Ta | Ti | Al | C | Zr | Ni |
|---|---|---|---|---|---|---|---|---|---|---|
| GTD111 | 14.0 | 10.0 | 1.5 | 4.3 | 4.7 | 2.7 | 4.0 | 0.08 | 0.03 | Bal. |
| Temperature (°C) | Duration (h) | ||||||
|---|---|---|---|---|---|---|---|
| 850 | 50 | 100 | 250 | 500 | 1000 | 3000 | 5000 |
| 1000 | 50 | 100 | 250 | 500 | 1000 | 3000 | 5000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Battulga, N.-E.; He, Y.; Kim, Y.; Kang, Y.; Jung, J.; Shin, K.; Lee, J.-H. High-Temperature Effects on TGO Growth and Al Depletion in TBCs of Ni-Based Superalloy GTD111. Coatings 2025, 15, 1145. https://doi.org/10.3390/coatings15101145
Battulga N-E, He Y, Kim Y, Kang Y, Jung J, Shin K, Lee J-H. High-Temperature Effects on TGO Growth and Al Depletion in TBCs of Ni-Based Superalloy GTD111. Coatings. 2025; 15(10):1145. https://doi.org/10.3390/coatings15101145
Chicago/Turabian StyleBattulga, Nomin-Erdene, Yinsheng He, Youngdae Kim, Yeonkwan Kang, Jinesung Jung, Keesam Shin, and Je-Hyun Lee. 2025. "High-Temperature Effects on TGO Growth and Al Depletion in TBCs of Ni-Based Superalloy GTD111" Coatings 15, no. 10: 1145. https://doi.org/10.3390/coatings15101145
APA StyleBattulga, N.-E., He, Y., Kim, Y., Kang, Y., Jung, J., Shin, K., & Lee, J.-H. (2025). High-Temperature Effects on TGO Growth and Al Depletion in TBCs of Ni-Based Superalloy GTD111. Coatings, 15(10), 1145. https://doi.org/10.3390/coatings15101145






