In Situ Observation of the Austenite Grains Growth Behavior in the Austenitizing Process of Nb–Ti Micro-Alloyed Medium Manganese Steel
Abstract
1. Introduction
2. Experimental Materials and Methods
3. Results
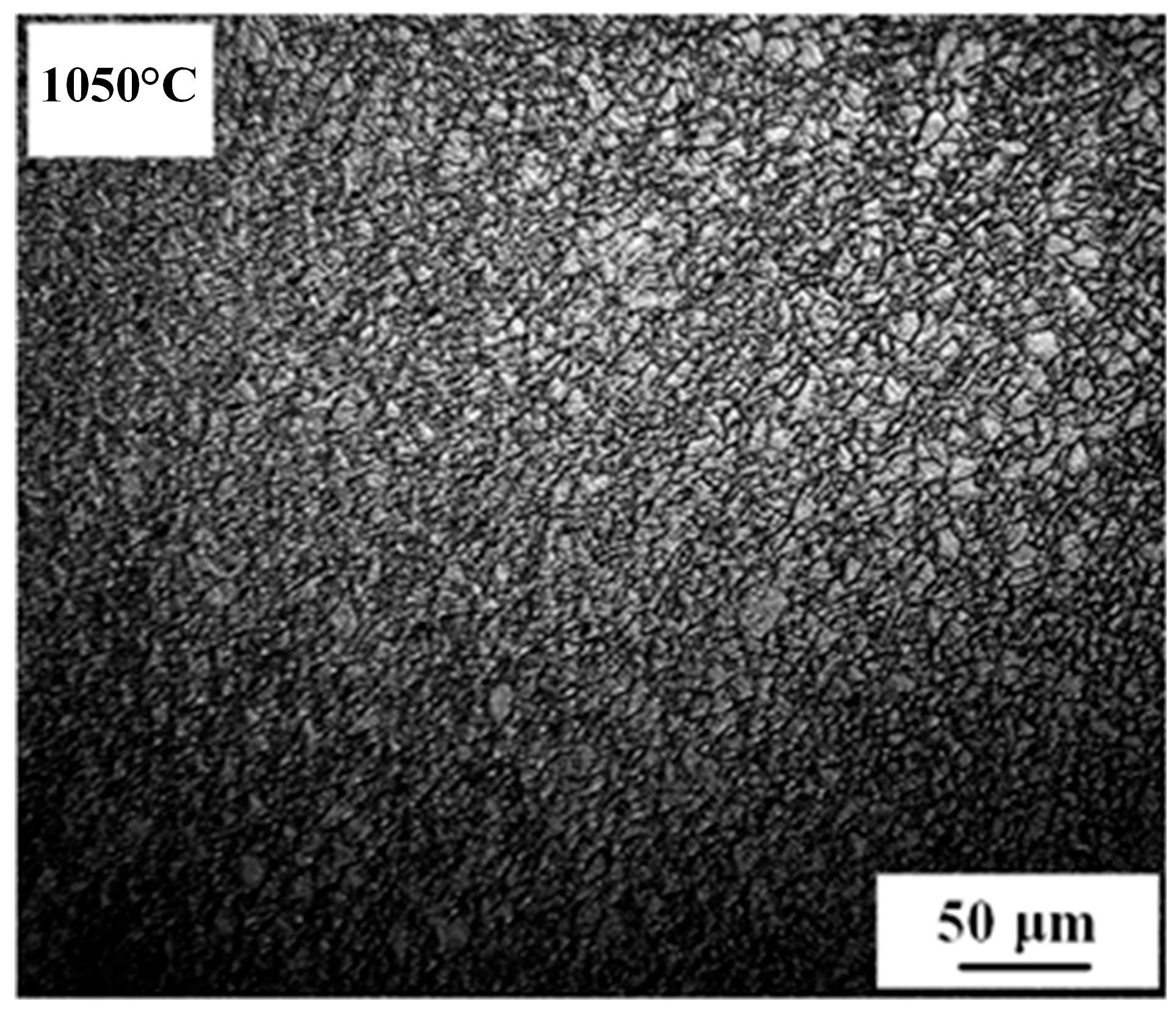
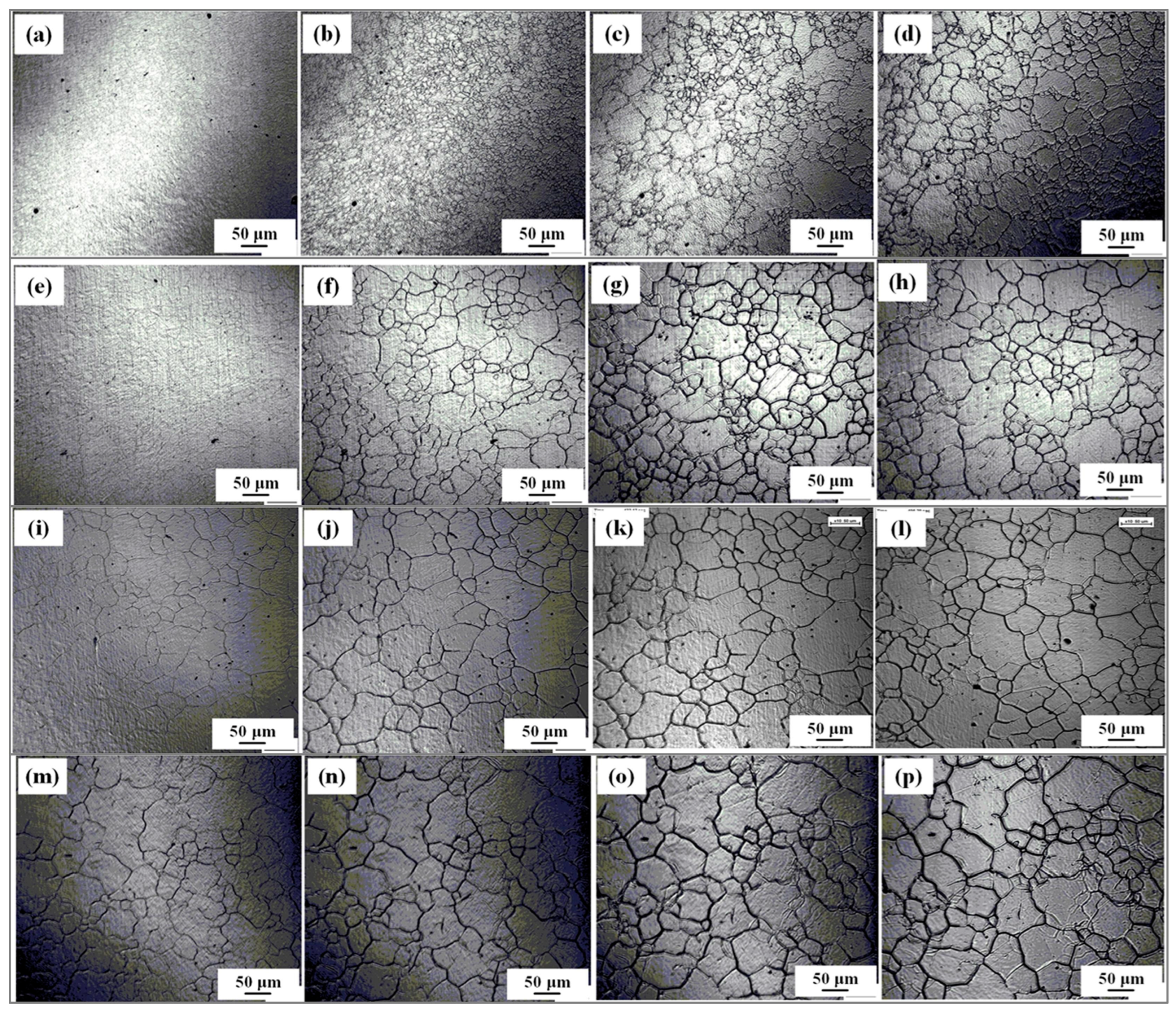
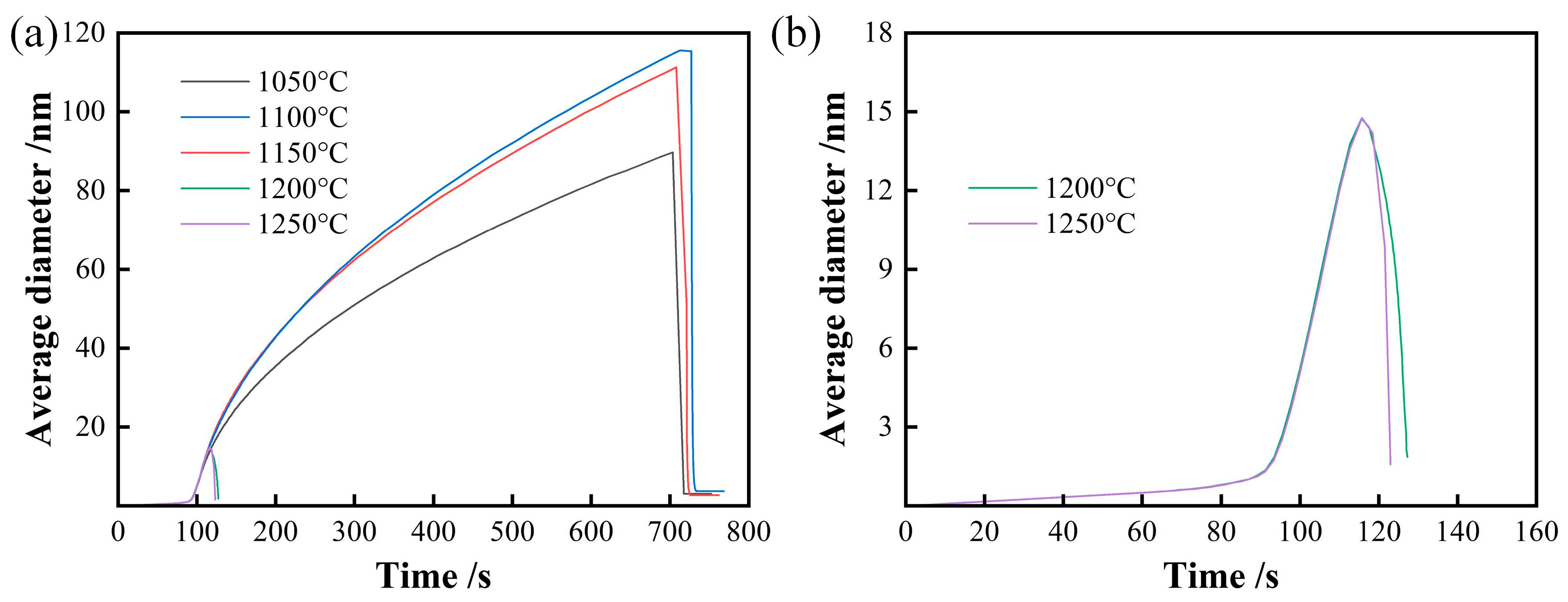
3.1. Growth Behavior of Austenite Grains During Heating Process
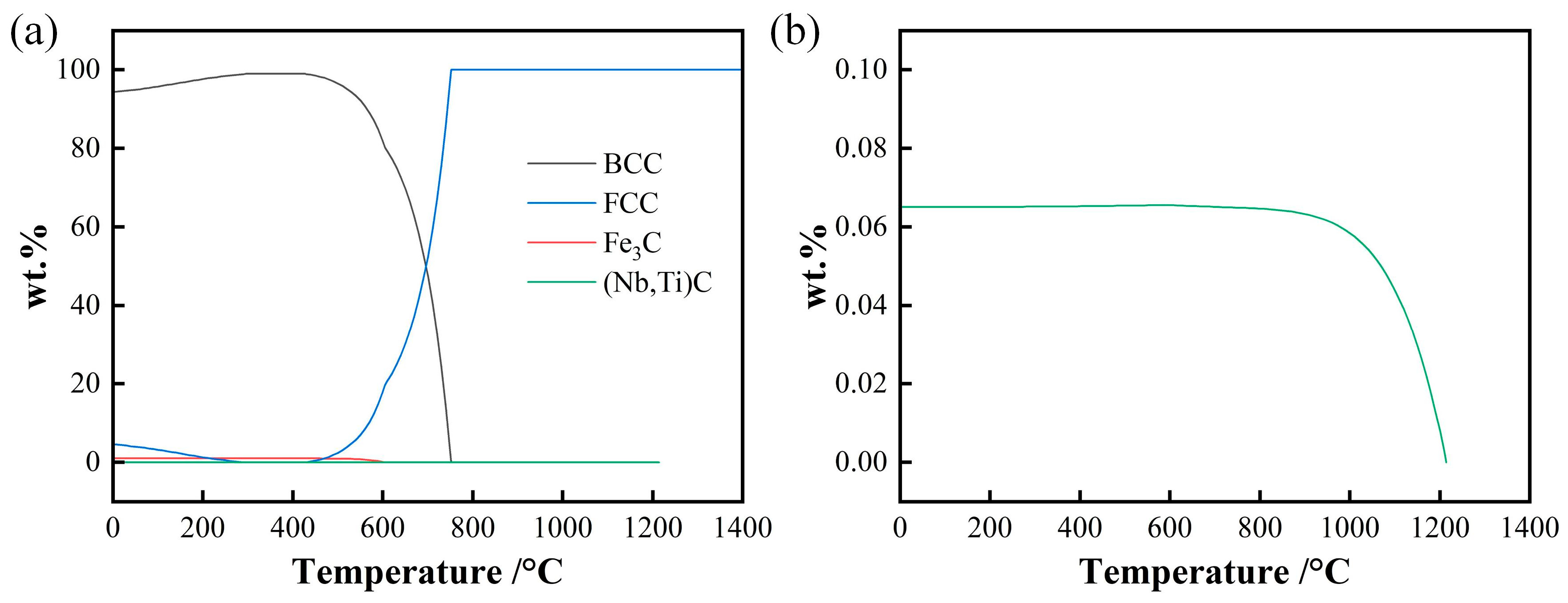
3.2. Thermodynamics Calculation of Phase Zones and Carbides Average Diameter
4. Discussion
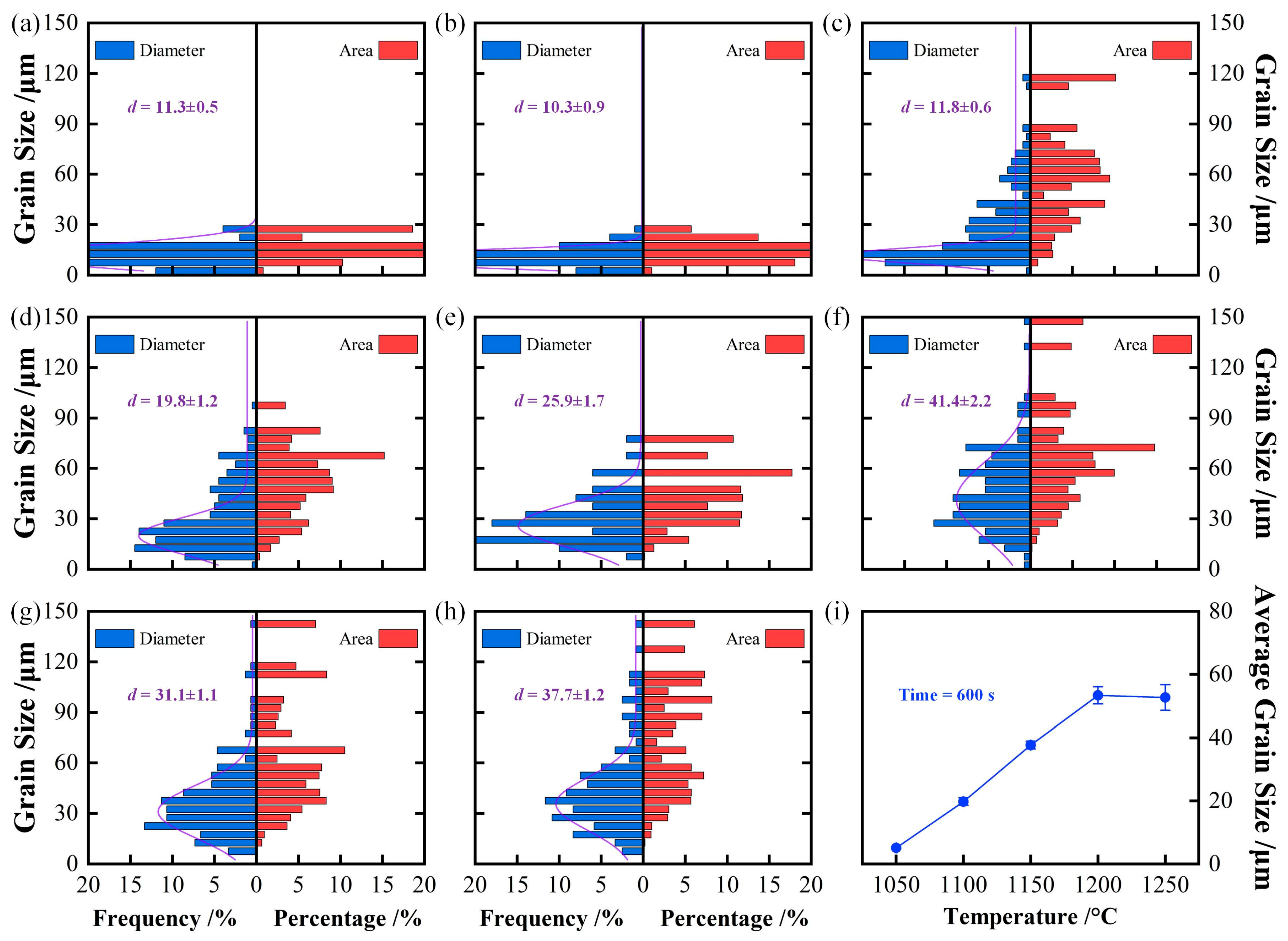
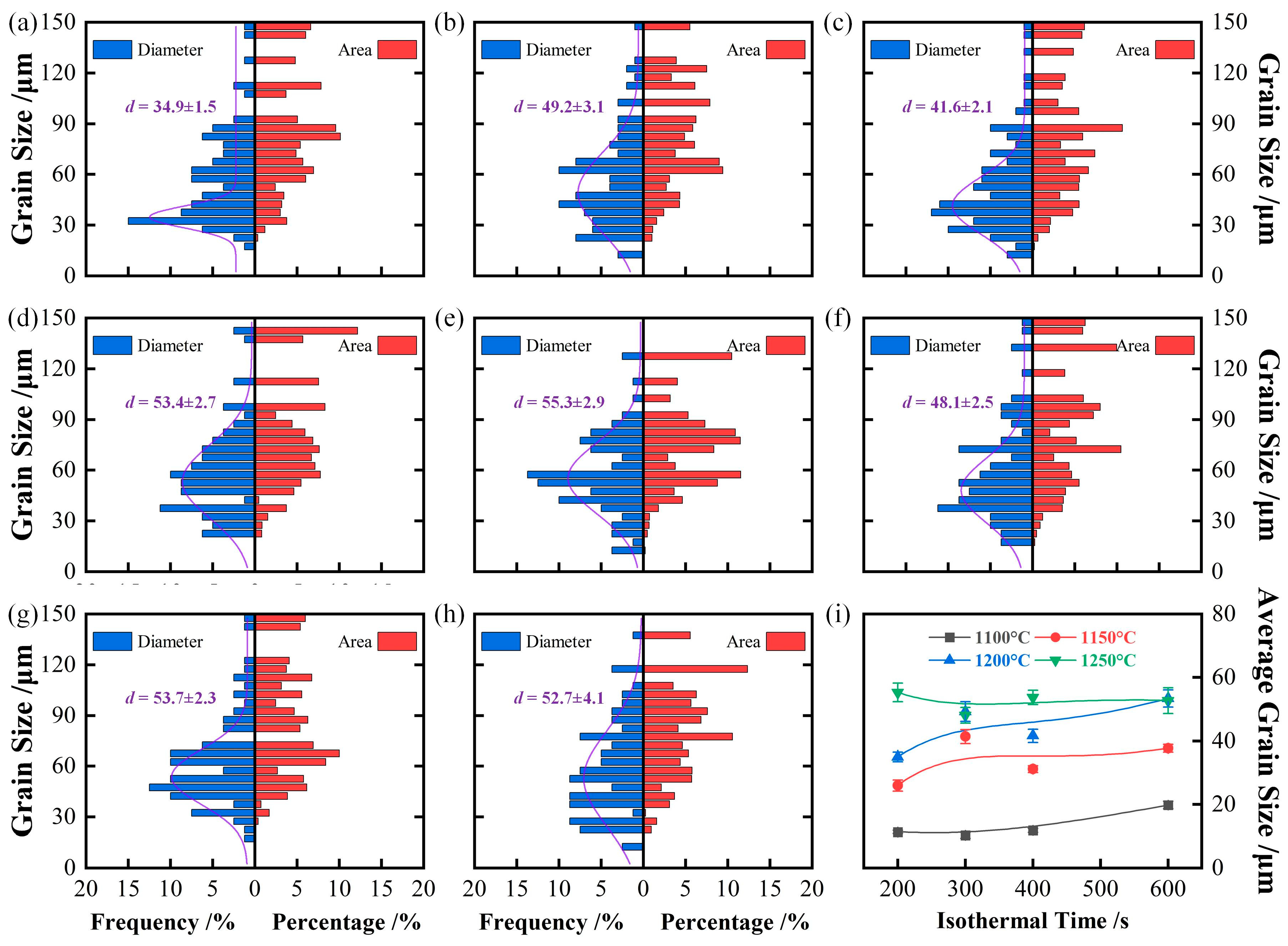
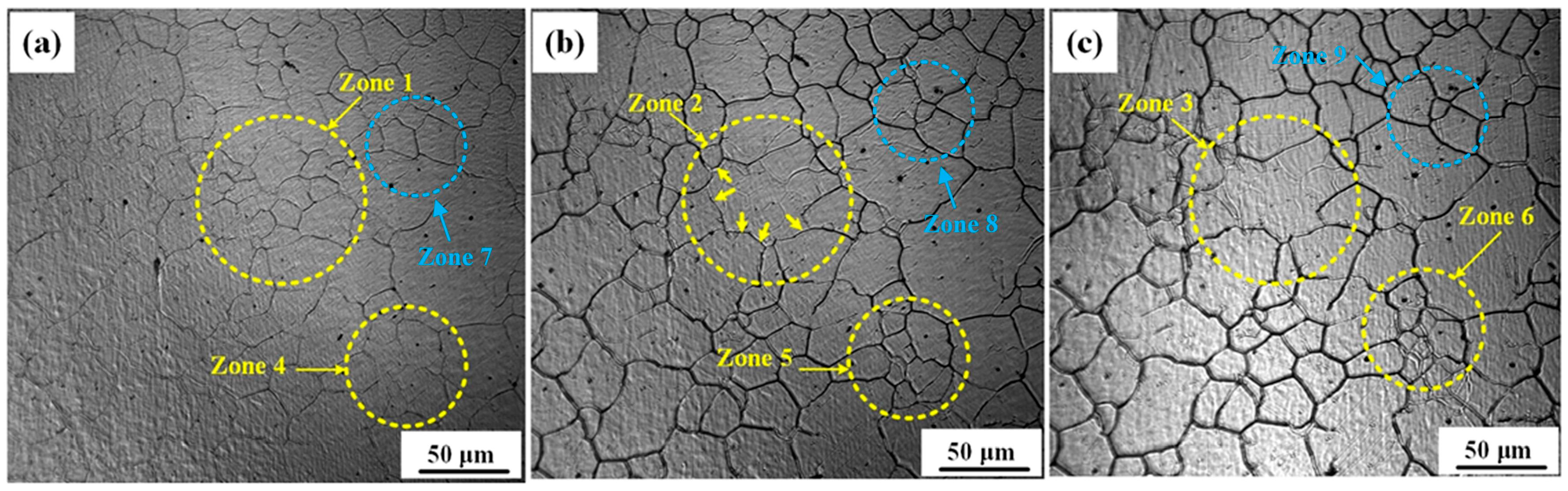
4.1. Effect of Isothermal Temperature on Austenite Grain Growth Behavior
4.2. Austenite Grain Growth Mechanism During Isothermal
4.3. Consideration of Heating Process for Medium Manganese Steel Continuous Casting Billet
5. Conclusions
- (1).
- The average austenite grain sizes of the specimen isothermally held at 1050 °C, 1100 °C, 1150 °C, 1200 °C, and 1250 °C for 600 s are 3.2, 19.8, 37.7, 53.4, and 52.7 μm, respectively. The average austenite grain sizes change from about 3 μm at 1050 °C to over 50 μm at 1250°C.
- (2).
- When the grain boundary is a small-angle grain boundary, one grain boundary will split into several dislocations. With the extension of heating time, the lattice orientation difference further decreases, and the remaining dislocations may merge into new grain boundaries. When the grain boundary is a large-angle grain boundary, only grain boundary movement can occur.
- (3).
- The weight fraction of precipitates below 1000 °C is about 0.065%, and precipitates gradually dissolve from about 1000 °C to 1200 °C. A portion of the precipitates nucleate and grow during the heating and holding stage at a holding temperature of 1050~1150 °C, and the precipitates dissolve completely during heating when the holding temperature exceeds 1200 °C.
- (4).
- The most suitable heating temperature for the medium manganese steel in this paper is from 1100 °C to 1150 °C, taking into account influences such as grain size, grain boundary damage, and deformation resistance.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, J.P.; Zhao, S.; Ma, S.N.; Chen, C.; Ding, H.H.; Ren, R.M. Research on the dynamic recrystallization mechanism of high manganese steel under severe wear conditions. Wear 2025, 15, 206226. [Google Scholar] [CrossRef]
- Cai, Z.H.; Ding, H.; Misra, R.D.K.; Ying, Z.Y. Austenite stability and deformation behavior in a cold-rolled transformation-induced plasticity steel with medium manganese content. Acta Mater. 2015, 84, 229–236. [Google Scholar] [CrossRef]
- Hu, J.; Li, X.Y.; Meng, Q.W.; Wang, L.Y.; Li, Y.Z.; Xu, W. Tailoring retained austenite and mechanical property improvement in Al-Si-V containing medium Mn steel via direct intercritical rolling. Mater. Sci. Eng. A 2022, 855, 143904. [Google Scholar] [CrossRef]
- Wolke, S.; Smaga, M.; Beck, T. Influence of surface morphologies and defects on the high cycle fatigue life of high manganese TWIP steel. Int. J. Fatigue 2025, 200, 109089. [Google Scholar] [CrossRef]
- Chu, J.H.; Cheng, Z.Y.; Dou, P.J.; Pan, X.; Wang, Z.M.; Li, Y.Y.; Tao, N.; Yue, Q.; Chang, L.Z. Corrosion behaviour of magnesia-carbon refractories by high manganese steel melts. Corros. Sci. 2025, 246, 112753. [Google Scholar] [CrossRef]
- Soleimani, M.; Kalhor, A.; Mirzadeh, H. Transformation-induced plasticity (TRIP) in advanced steels: A review. Mater. Sci. Eng. A 2020, 795, 140023. [Google Scholar] [CrossRef]
- Cai, F.J.; Wang, H.S.; Wang, Y.; Kong, Y.; Fan, R.Q.; Yan, F.K. Enhanced stability of retained austenite to facilitate deformation compatibility of surrounding ferrite in a medium manganese steel. J. Mater. Res. Technol. 2025, 36, 3425–3435. [Google Scholar] [CrossRef]
- Dong, Y.; Zhang, B.; Zhao, M.M.; Du, Y.; Misra, R.D.K.; Du, L.X. Investigation of austenite decomposition behavior and relationship to mechanical properties in continuously cooled medium-Mn steel. Mater. Sci. Eng. A 2022, 831, 142208. [Google Scholar] [CrossRef]
- Dai, Z.B.; Chen, H.; Ding, R.; Lu, Q.; Zhang, C.; Yang, Z.G.; van der Zwaag, S. Fundamentals and application of solid-state phase transformations for advanced high strength steels containing metastable retained austenite. Mater. Sci. Eng. R Rep. 2021, 143, 100590. [Google Scholar] [CrossRef]
- Luo, H.W.; Shi, J.; Wang, C.; Cao, W.Q.; Sun, X.J.; Dong, H. Experimental and numerical analysis on formation of stable austenite during the intercritical annealing of 5Mn steel. Acta Mater. 2011, 59, 4002–4014. [Google Scholar] [CrossRef]
- Han, J. A critical review on medium-Mn steels: Mechanical properties governed by microstructural morphology. Steel Res. Int. 2023, 94, 2200238. [Google Scholar] [CrossRef]
- Zhang, H.L.; Sun, M.Y.; Liu, Y.X.; Ma, D.P.; Xu, B.; Huang, M.X.; Li, D.Z.; Li, Y.Y. Ultrafine-grained dual-phase maraging steel with high strength and excellent cryogenic toughness. Acta Mater. 2021, 211, 116878. [Google Scholar] [CrossRef]
- Kuzmina, M.; Ponge, D.; Raabe, D. Grain boundary segregation engineering and austenite reversion turn embrittlement into toughness: Example of a 9 wt.% medium Mn steel. Acta Mater. 2015, 86, 182–192. [Google Scholar] [CrossRef]
- Liao, Z.H.; Dong, Y.; Du, Y.; Wang, X.N.; Qi, M.; Wu, H.Y.; Gao, X.H.; Gao, L.X. Effects of different intercritical annealing processes on microstructure and cryogenic toughness of newly designed medium-Mn and low-Ni steel. J. Mater. Res. Technol. 2023, 23, 1471–1486. [Google Scholar] [CrossRef]
- Sun, B.H.; Palanisamy, D.; Ponge, D.; Gault, B.; Fazeli, F.; Scott, C.; Yue, S.; Raabe, D. Revealing fracture mechanisms of medium manganese steels with and without delta-ferrite. Acta Mater. 2019, 164, 683–696. [Google Scholar] [CrossRef]
- Yu, S.; Du, L.; Hu, J.; Misra, R.D.K. Effect of hot rolling temperature on the microstructure and mechanical properties of ultra-low carbon medium manganese steel. Mater. Sci. Eng. A 2018, 731, 149–155. [Google Scholar] [CrossRef]
- van Tol, R.T.; Zhao, L.; Sietsma, J. Kinetics of austenite decomposition in manganese-based steel. Acta Mater. 2014, 64, 33–40. [Google Scholar] [CrossRef]
- Hu, H.L.; Zhao, M.J.; Rong, L.J. Retarding the precipitation of η phase in Fe-Ni based alloy through grain boundary engineering. J. Mater. Sci. Technol. 2020, 47, 152–161. [Google Scholar] [CrossRef]
- Baram, M.; Chatain, D.; Kaplan, W.D. Nanometer-thick equilibrium films: The interface between thermodynamics and atomistics. Science 2011, 332, 206–209. [Google Scholar] [CrossRef]
- Kokkula, P.C.; Samanta, S.; Mandal, S.; Singh, S.B. Kinetics of pearlite transformation: The effect of grain boundary engineering. Acta Mater. 2025, 284, 120641. [Google Scholar] [CrossRef]
- Han, R.Y.; Yang, G.W.; Xu, D.M.; Xu, Y.W.; Fu, Z.X. New approach to modeling of ultra-refined austenite grain size in medium manganese martensitic steel. J. Mater. Sci. 2025, 60, 2640–2657. [Google Scholar] [CrossRef]
- Zhang, X.G.; Ren, Y.J.; Zhang, J.; Yang, X.D.; Xie, X.; Liu, H.; Yang, W.C. Effects of prior austenite grain size on reversion kinetics of different crystallographic austenite in a low carbon steel. Mater. Charact. 2022, 190, 112025. [Google Scholar] [CrossRef]
- Avila, D.D.S.; Offerman, S.E.; Santofimia, M.J. Modeling the effect of prior austenite grain size on bainite formation kinetics. Acta Mater. 2024, 266, 119656. [Google Scholar] [CrossRef]
- Hanamura, T.; Torizuka, S.; Tamura, S.; Enokida, S.; Takechi, H. Effect of austenite grain size on transformation behavior, microstructure and mechanical properties of 0.1C–5Mn martensitic steel. ISIJ Inter. 2013, 53, 2218–2225. [Google Scholar] [CrossRef]
- Lejcek, P.; Sob, M.; Paidar, V. Interfacial segregation and grain boundary embrittlement: An overview and critical assessment of experimental data and calculated results. Prog. Mater. Sci. 2017, 87, 83–139. [Google Scholar] [CrossRef]
- Wang, J.; Janisch, R.; Madsen, G.K.H.; Drauz, R. First-principles study of carbon segregation in bcc iron symmetrical tilt grain boundaries. Acta Mater. 2016, 115, 259–268. [Google Scholar] [CrossRef]
- Huber, L.; Grabowski, B.; Militzer, M.; Neugebauer, J.; Rottler, J. Ab-initio modelling of solute segregation energies to a general grain boundary. Acta Mater. 2017, 132, 138–148. [Google Scholar] [CrossRef]
- Rohrer, G.S. The role of grain boundary energy in grain boundary complexion transitions. Curr. Opin. Solid State Mater. Sci. 2016, 20, 231–239. [Google Scholar] [CrossRef]
- Lejček, P.; Hofmann, S. Modeling grain boundary segregation by prediction of all the necessary parameters. Acta Mater. 2019, 170, 253–267. [Google Scholar] [CrossRef]
- Burke, J.E.; Turnbull, D. Recrystallization and grain growth. Prog. Met. Phys. 1952, 3, 220–224. [Google Scholar] [CrossRef]
- Aust, K.T.; Rutter, J.W. Grain boundary migration in high purity lead and lead–tin alloys. Trans. Metall. Soc. AIME 1959, 215, 119–127. [Google Scholar]
- Lücke, K.; Stüwe, H.P. On the theory of impurity controlled grain boundary motion. Acta Mater. 1971, 19, 1087–1099. [Google Scholar] [CrossRef]
- McLean, D. Grain Boundaries in Metals; Oxford University Press: Oxford, UK, 1957; pp. 116–149. [Google Scholar]
- Nishizawa, T. Thermodynamics of Microstructures; ASM International: Almere, The Netherlands, 2008; pp. 157–159. [Google Scholar]
- Zener, C.; Smith, C. Grains, Phases and Interfaces: Interpretation of Microstructures. Trans. Metall. Soc. AIME 1948, 175, 15–51. [Google Scholar]
- Bignon, M.; Bernacki, M. Particle pinning during grain growth—A new analytical model for predicting the mean limiting grain size but also grain size heterogeneity in a 2D polycrystalline context. Acta Mater. 2024, 277, 120174. [Google Scholar] [CrossRef]
- Dong, Y.; Xiang, L.Y.; Zhu, C.J.; Du, Y.; Xiong, Y.; Zhang, X.Y.; Du, L.X. Analysis of phase transformation thermodynamics and kinetics and its relationship to structure-mechanical properties in a medium-Mn high strength steel. J. Mater. Res. Technol. 2023, 27, 5411–5423. [Google Scholar] [CrossRef]
- Dong, Y.; Qi, M.; Du, Y.; Wu, H.Y.; Gao, X.H.; Du, L.X. Significance of retained austenite stability on yield point elongation phenomenon in a hot-rolled and intercritically annealed medium-Mn steel. Steel Res. Int. 2022, 93, 2200400. [Google Scholar] [CrossRef]
- Yang, D.P.; Wu, D.; Yi, H.L. Reverse transformation from martensite into austenite in a medium-Mn steel. Scr. Mater. 2019, 161, 1–5. [Google Scholar] [CrossRef]
- Moszner, F.; Povoden-Karadeniz, E.; Pogatscher, S.; Uggowitzer, P.J.; Estrin, Y.; Gerstl, S.S.A.; Kozeschnik, E.; Lo, J.F. Reverse α′→γ transformation mechanisms of martensitic Fe–Mn and age-hardenable Fe–Mn–Pd alloys upon fast and slow continuous heating. Acta Mater. 2014, 72, 99–109. [Google Scholar] [CrossRef]

| C | Si | Mn | P | S | Nb | Ti |
|---|---|---|---|---|---|---|
| 0.08 | 0.25 | 4.52 | 0.011 | 0.0019 | 0.018 | 0.036 |
| 200 s | 300 s | 400 s | 600 s | |
|---|---|---|---|---|
| 1100 °C | 11.3 ± 0.5 | 10.3 ± 0.9 | 11.8 ± 0.6 | 19.8 ± 1.2 |
| 1150 °C | 25.9 ± 1.7 | 41.4 ± 2.2 | 31.1 ± 1.1 | 37.7 ± 1.2 |
| 1200 °C | 34.9 ± 1.5 | 49.2 ± 3.1 | 41.6 ± 2.1 | 53.4 ± 2.7 |
| 1250 °C | 55.3 ± 2.9 | 48.1 ± 2.5 | 53.7 ± 2.3 | 52.7 ± 4.1 |
| 200 s | 300 s | 400 s | 600 s | |||||
|---|---|---|---|---|---|---|---|---|
| Diameter Frequency | Area Percentage | Diameter Frequency | Area Percentage | Diameter Frequency | Area Percentage | Diameter Frequency | Area Percentage | |
| 1100 °C | 0.0 | 0.0 | 0.0 | 0.0 | 2.7 | 22.6 | 2.0 | 11.0 |
| 1150 °C | 0.0 | 0.0 | 6.9 | 28.0 | 5.3 | 31.1 | 13.3 | 49.7 |
| 1200 °C | 21.3 | 53.7 | 19.0 | 51.1 | 16.0 | 49.3 | 17.5 | 46.5 |
| 1250 °C | 17.5 | 41.1 | 20.0 | 53.5 | 22.5 | 53.3 | 21.3 | 51.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, G.; Du, Y.; Sun, C.; Gao, X.; Wu, H.; Du, L. In Situ Observation of the Austenite Grains Growth Behavior in the Austenitizing Process of Nb–Ti Micro-Alloyed Medium Manganese Steel. Coatings 2025, 15, 1144. https://doi.org/10.3390/coatings15101144
Yuan G, Du Y, Sun C, Gao X, Wu H, Du L. In Situ Observation of the Austenite Grains Growth Behavior in the Austenitizing Process of Nb–Ti Micro-Alloyed Medium Manganese Steel. Coatings. 2025; 15(10):1144. https://doi.org/10.3390/coatings15101144
Chicago/Turabian StyleYuan, Guangpeng, Yu Du, Chao Sun, Xiuhua Gao, Hongyan Wu, and Linxiu Du. 2025. "In Situ Observation of the Austenite Grains Growth Behavior in the Austenitizing Process of Nb–Ti Micro-Alloyed Medium Manganese Steel" Coatings 15, no. 10: 1144. https://doi.org/10.3390/coatings15101144
APA StyleYuan, G., Du, Y., Sun, C., Gao, X., Wu, H., & Du, L. (2025). In Situ Observation of the Austenite Grains Growth Behavior in the Austenitizing Process of Nb–Ti Micro-Alloyed Medium Manganese Steel. Coatings, 15(10), 1144. https://doi.org/10.3390/coatings15101144








