The Influence of the Photoinitiating System on Residual Monomer Contents and Photopolymerization Rate of a Model Pigmented UV/LED Nail Gel Formulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zareanshahraki, F.; Mannari, V. “Green” UV-LED Gel Nail Polishes from Bio-based Materials. Intern. J. Cosmet. Sci. 2018, 40, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, A.; Jin, J. Photopolymerization in 3D Printing. ACS Appl. Polym. Mater. 2019, 1, 593–611. [Google Scholar] [CrossRef]
- Graça, A.; Bom, S.; Martins, A.M.; Ribeiro, H.M.; Marto, J. Vat-Based Photopolymerization 3D Printing: From Materials to Topical and Transdermal Applications. Asian J. Pharm. Sci. 2024, 19, 100940. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Monem, M.M. Naturally Derived Photoinitiators for Dental and Biomaterials Applications. Eur. Dent. Res. Biomater. J. 2020, 1, 72–78. [Google Scholar] [CrossRef]
- Mieriņa, I.; Grigale-Sorocina, Z.; Birks, I. The Chemistry of Behind the UV-Curable Nail Polishes. Polymers 2025, 17, 1166. [Google Scholar] [CrossRef]
- Klang, J.; Lu, J.; Vappala, I. Nal Polish Composition Based on Solvent-Free Aqueous Polyurethane Dispersions. WO2015165897 A1, 28 April 2015. [Google Scholar]
- Goudjil, K. Metamorphic Nail Polish. US5730961A, 24 March 1998. [Google Scholar]
- Vu, T.; Conger, C.; Larsen, D.M.; Valia, D.; Schoon, D.D. Compositions and Methods for UV-Curable Cosmetic Nail Coatings. US8901199B2, 23 October 2014. [Google Scholar]
- Chemtob, A.; De Paz-Simon, H.; Croutxé-Barghorn, C.; Rigolet, S. UV-activated Silicone Oligomer Cross-linking through Photoacid and Photobase Organocatalysts. J. Appl. Polym. Sci. 2014, 131, 39875. [Google Scholar] [CrossRef]
- Zareanshahraki, F.; Mannari, V. Optimizing the Performance of Sustainable Nail Gel Compositions Using a Mixture Experimental Design Methodology. Prog. Org. Coat. 2021, 153, 106168. [Google Scholar] [CrossRef]
- Dietliker, K.; Li, Z. Photoinitiators for UV Inkjet Applications. In Inkjet Printing in Industry; Zapka, W., Ed.; Wiley: Hoboken, NJ, USA, 2022; pp. 199–278. ISBN 978-3-527-34780-3. [Google Scholar]
- Pojnar, K.; Pilch-Pitera, B.; Kisiel, M.; Zioło, A.; Kędzierski, M. UV-Curable Powder Transparent Coatings Based on Oligo (Meth) Acrylic Resins. Polimery 2024, 69, 11–24. [Google Scholar] [CrossRef]
- Hayeri, T.N.; Mannari, V. Non-acrylate UV-LED Nail Gel with High Bio-renewable Content Based on Hydrophobic Organic-inorganic Hybrid System. Intern. J. Cosmet. Sci. 2025, 47, 398–410. [Google Scholar] [CrossRef]
- Du, X.-N.; He, Y.; Chen, Y.-W.; Liu, Q.; Sun, L.; Sun, H.-M.; Wu, X.-F.; Lu, Y. Decoding Cosmetic Complexities: A Comprehensive Guide to Matrix Composition and Pretreatment Technology. Molecules 2024, 29, 411. [Google Scholar] [CrossRef]
- Lang, M.; Hirner, S.; Wiesbrock, F.; Fuchs, P. A Review on Modeling Cure Kinetics and Mechanisms of Photopolymerization. Polymers 2022, 14, 2074. [Google Scholar] [CrossRef]
- Elkhoury, K.; Zuazola, J.; Vijayavenkataraman, S. Bioprinting the Future Using Light: A Review on Photocrosslinking Reactions, Photoreactive Groups, and Photoinitiators. SLAS Technol. 2023, 28, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Müller, S.M.; Schlögl, S.; Wiesner, T.; Haas, M.; Griesser, T. Recent Advances in Type I Photoinitiators for Visible Light Induced Photopolymerization. ChemPhotoChem 2022, 6, e202200091. [Google Scholar] [CrossRef]
- Gupta, V.; Mohapatra, S.; Mishra, H.; Farooq, U.; Kumar, K.; Ansari, M.; Aldawsari, M.; Alalaiwe, A.; Mirza, M.; Iqbal, Z. Nanotechnology in Cosmetics and Cosmeceuticals—A Review of Latest Advancements. Gels 2022, 8, 173. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, P.; Das, K.; Goldust, M.; Wambier, C.G. Emerging Technologies in Hair and Nail Diagnosis and Treatment. Dermatol. Rev. 2024, 5, e251. [Google Scholar] [CrossRef]
- Yang, X.; Liu, X.; Chau, Y.Y.; Qin, X.; Zhu, H.; Peng, L.; Chan, K.W.Y.; Wang, Z. Role of Chemistry in Nature-Inspired Skin Adhesives. Chem. Sci. 2025, 16, 10665–10690. [Google Scholar] [CrossRef]
- Condini, A. Uv-Curable Protective Coating for the Inner Surface of Steel Pipes. Ph.D. Thesis, University of Trento, Trento, Italy, 2024. [Google Scholar]
- Kowalska, A.; Sokolowski, J.; Bociong, K. The Photoinitiators Used in Resin Based Dental Composite—A Review and Future Perspectives. Polymers 2021, 13, 470. [Google Scholar] [CrossRef]
- Li, C.; Liu, S.; Liu, Z.; Hu, X. Study on the Interaction Between Verapamil Hydrochloride and Eosin Y by Absorption, Fluorescence and Resonance Rayleigh Scattering Spectra and Their Analytical Applications. J. Fluoresc. 2011, 21, 723–732. [Google Scholar] [CrossRef]
- Bonardi, A.H.; Dumur, F.; Grant, T.M.; Noirbent, G.; Gigmes, D.; Lessard, B.H.; Fouassier, J.-P.; Lalevée, J. High Performance Near-Infrared (NIR) Photoinitiating Systems Operating under Low Light Intensity and in the Presence of Oxygen. Macromolecules 2018, 51, 1314–1324. [Google Scholar] [CrossRef]
- Dressano, D.; Palialol, A.R.; Xavier, T.A.; Braga, R.R.; Oxman, J.D.; Watts, D.C.; Marchi, G.M.; Lima, A.F. Effect of Diphenyliodonium Hexafluorophosphate on the Physical and Chemical Properties of Ethanolic Solvated Resins Containing Camphorquinone and 1-Phenyl-1,2-Propanedione Sensitizers as Initiators. Dent. Mater. 2016, 32, 756–764. [Google Scholar] [CrossRef]
- Gómez, M.L.; Montejano, H.A.; Previtali, C.M. Excited States Interaction of Polycyclic Aromatic Hydrocarbons with Diphenyliodonium Chloride. J. Photochem. Photobiol. A Chem. 2008, 197, 18–24. [Google Scholar] [CrossRef]
- Tomal, W.; Ortyl, J. Water-Soluble Photoinitiators in Biomedical Applications. Polymers 2020, 12, 1073. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Xu, C.; Wu, X.; Liao, Q.; Xiong, Y.; Li, Z.; Tang, H. Photobleachable Cinnamoyl Dyes for Radical Visible Photoinitiators. Dye. Pigment. 2020, 178, 108350. [Google Scholar] [CrossRef]
- Bednarczyk, P.; Pawlikowska, M.; Czech, Z. Primers Used in UV-Curable Nail Varnishes. Int. J. Adhes. Adhes. 2017, 74, 177–180. [Google Scholar] [CrossRef]
- Lee, B.C.; Pangeni, R.; Na, J.; Koo, K.-T.; Park, J.W. Preparation and in Vivo Evaluation of a Highly Skin- and Nail-Permeable Efinaconazole Topical Formulation for Enhanced Treatment of Onychomycosis. Drug Deliv. 2019, 26, 1167–1177. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.T.; West, J.L. Photopolymerizable Hydrogels for Tissue Engineering Applications. Biomaterials 2002, 23, 4307–4314. [Google Scholar] [CrossRef]
- Allushi, A.; Kutahya, C.; Aydogan, C.; Kreutzer, J.; Yilmaz, G.; Yagci, Y. Conventional Type II Photoinitiators as Activators for Photoinduced Metal-Free Atom Transfer Radical Polymerization. Polym. Chem. 2017, 8, 1972–1977. [Google Scholar] [CrossRef]
- Chen, M.; Zhong, M.; Johnson, J.A. Light-Controlled Radical Polymerization: Mechanisms, Methods, and Applications. Chem. Rev. 2016, 116, 10167–10211. [Google Scholar] [CrossRef]
- Bednarczyk, P.; Balcerak-Woźniak, A.; Kabatc-Borcz, J.; Czech, Z. High Potential of New Dyeing Photoinitiators for Fast Curing of (Meth)Acrylate Compositions under Low Intensity UV–Vis Light. Polym. Chem. 2023, 14, 3931–3949. [Google Scholar] [CrossRef]
- Asmusen, S.; Arenas, G.; Cook, W.D.; Vallo, C. Photobleaching of Camphorquinone during Polymerization of Dimethacrylate-Based Resins. Dent. Mater. 2009, 25, 1603–1611. [Google Scholar] [CrossRef]
- Cook, W.D. Photopolymerization Kinetics of Dimethacrylates Using the Camphorquinone/Amine Initiator System. Polymer 1992, 33, 600–609. [Google Scholar] [CrossRef]
- Ogunyinka, A.; Palin, W.M.; Shortall, A.C.; Marquis, P.M. Photoinitiation Chemistry Affects Light Transmission and Degree of Conversion of Curing Experimental Dental Resin Composites. Dent. Mater. 2007, 23, 807–813. [Google Scholar] [CrossRef]
- Schneider, L.F.J.; Cavalcante, L.M.; Prahl, S.A.; Pfeifer, C.S.; Ferracane, J.L. Curing Efficiency of Dental Resin Composites Formulated with Camphorquinone or Trimethylbenzoyl-Diphenyl-Phosphine Oxide. Dent. Mater. 2012, 28, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Jakubiak, J.; Allonas, X.; Fouassier, J.P.; Sionkowska, A.; Andrzejewska, E.; Linden, L.Å.; Rabek, J.F. Camphorquinone–Amines Photoinitating Systems for the Initiation of Free Radical Polymerization. Polymer 2003, 44, 5219–5226. [Google Scholar] [CrossRef]
- Burdick, J.A.; Mason, M.N.; Anseth, K.S. In Situ Forming Lactic Acid Based Orthopaedic Biomaterials: Influence of Oligomer Chemistry on Osteoblast Attachment and Function. J. Biomater. Sci. Polym. Ed. 2001, 12, 1253–1265. [Google Scholar] [CrossRef] [PubMed]
- Declercq, H.A.; Gorski, T.L.; Tielens, S.P.; Schacht, E.H.; Cornelissen, M.J. Encapsulation of Osteoblast Seeded Microcarriers into Injectable, Photopolymerizable Three-Dimensional Scaffolds Based on d,l -Lactide and ε-Caprolactone. Biomacromolecules 2005, 6, 1608–1614. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Spencer, P.; Yao, X.; Ye, Q. Effect of Coinitiator and Water on the Photoreactivity and Photopolymerization of HEMA/Camphoquinone-based Reactant Mixtures. J. Biomed. Mater. Res. 2006, 78A, 721–728. [Google Scholar] [CrossRef]
- Kamoun, E.A.; Winkel, A.; Eisenburger, M.; Menzel, H. Carboxylated Camphorquinone as Visible-Light Photoinitiator for Biomedical Application: Synthesis, Characterization, and Application. Arab. J. Chem. 2016, 9, 745–754. [Google Scholar] [CrossRef]
- Andrzejewska, E. Photopolymerization Kinetics of Multifunctional Monomers. Prog. Polym. Sci. 2001, 26, 605–665. [Google Scholar] [CrossRef]
- Fouassier, J.P.; Allonas, X.; Burget, D. Photopolymerization Reactions under Visible Lights: Principle, Mechanisms and Examples of Applications. Prog. Org. Coat. 2003, 47, 16–36. [Google Scholar] [CrossRef]
- Carioscia, J.A.; Lu, H.; Stanbury, J.W.; Bowman, C.N. Thiol-Ene Oligomers as Dental Restorative Materials. Dent. Mater. 2005, 21, 1137–1143. [Google Scholar] [CrossRef]
- Hoyle, C.E.; Bowman, C.N. Thiol–Ene Click Chemistry. Angew. Chem. Int. Ed. 2010, 49, 1540–1573. [Google Scholar] [CrossRef]
- Schmoldt, A.; Benthe, H.F.; Haberland, G. Digitoxin Metabolism by Rat Liver Microsomes. Biochem. Pharmacol. 1975, 24, 1639–1641. [Google Scholar] [CrossRef]
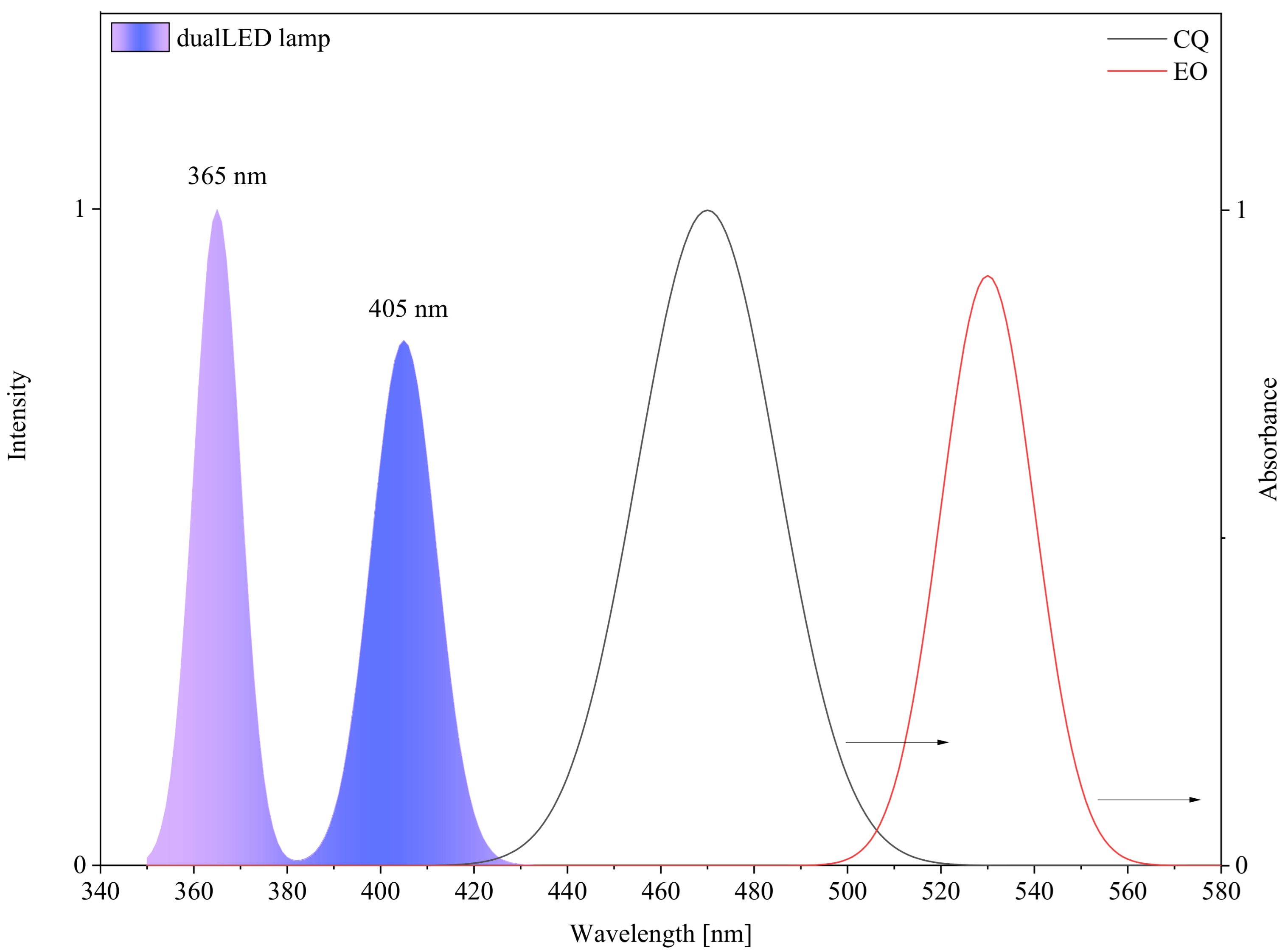
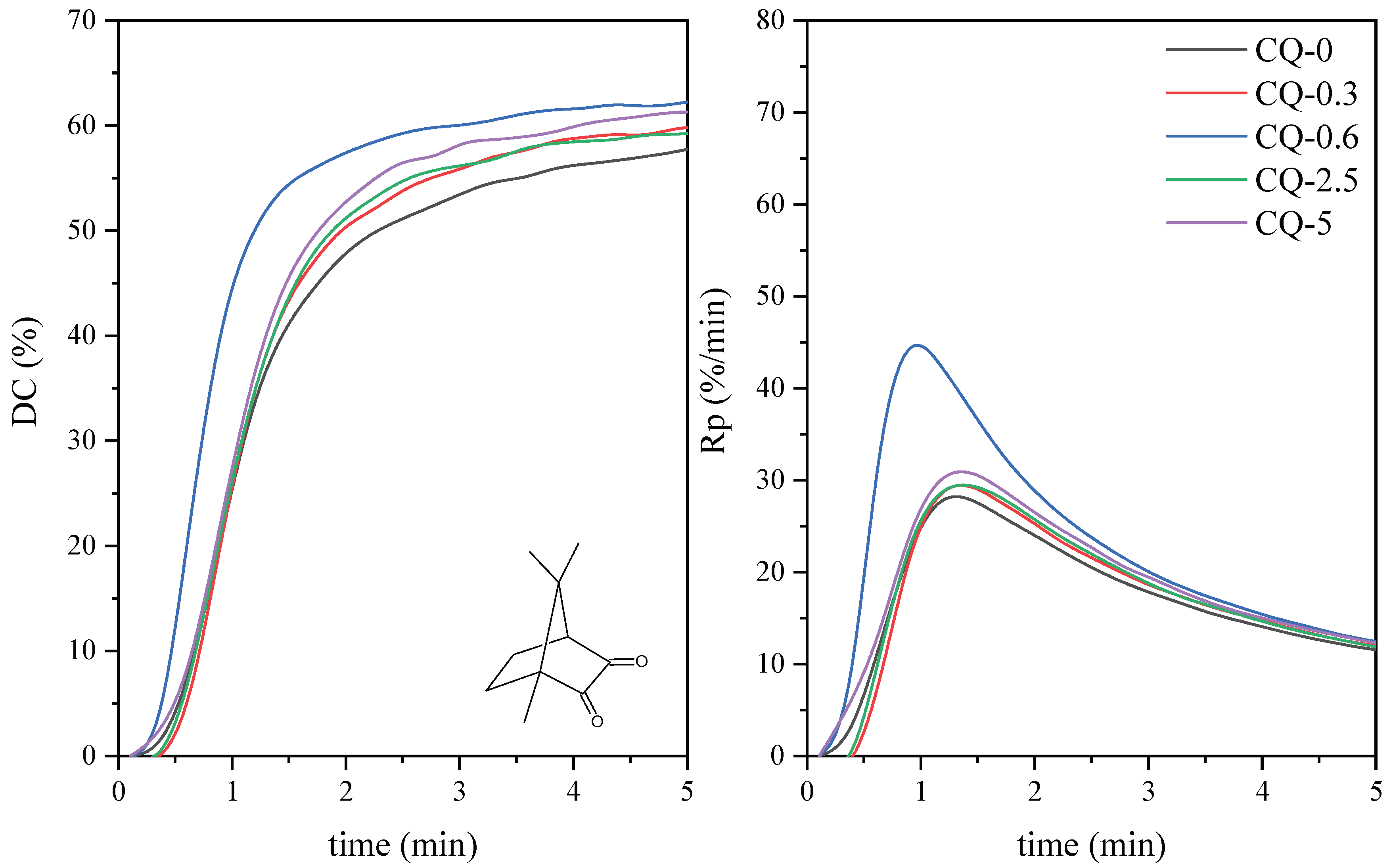
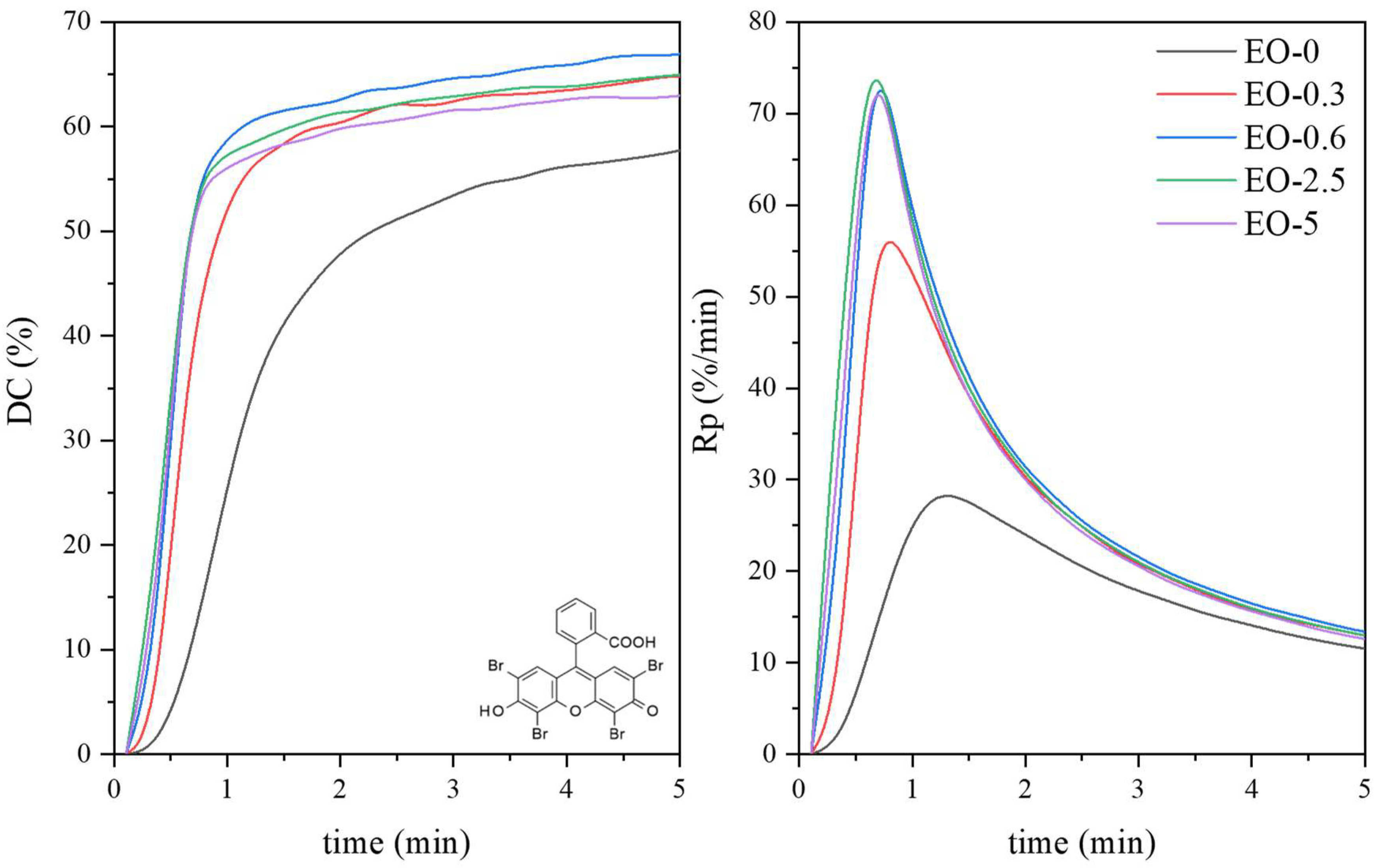


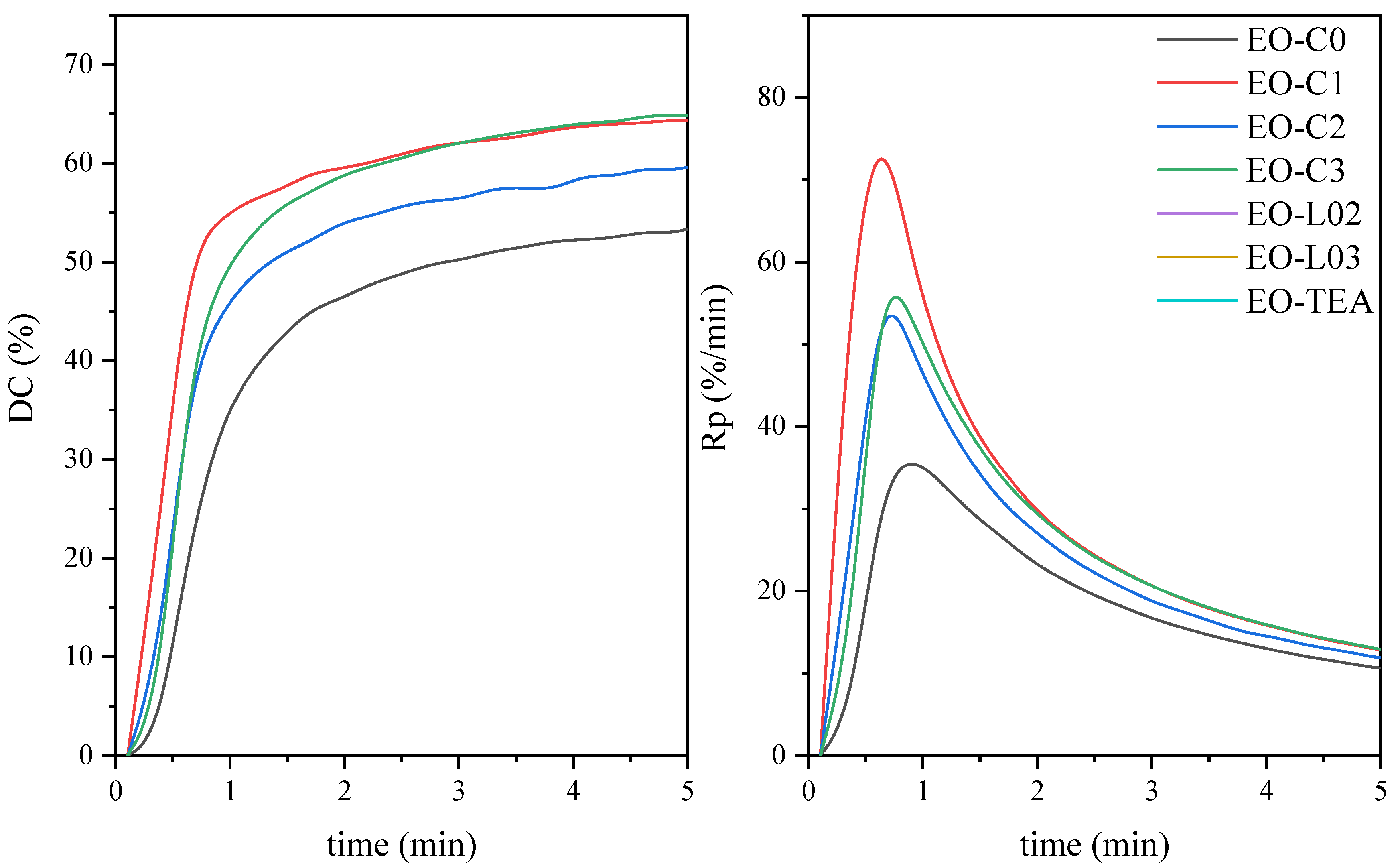
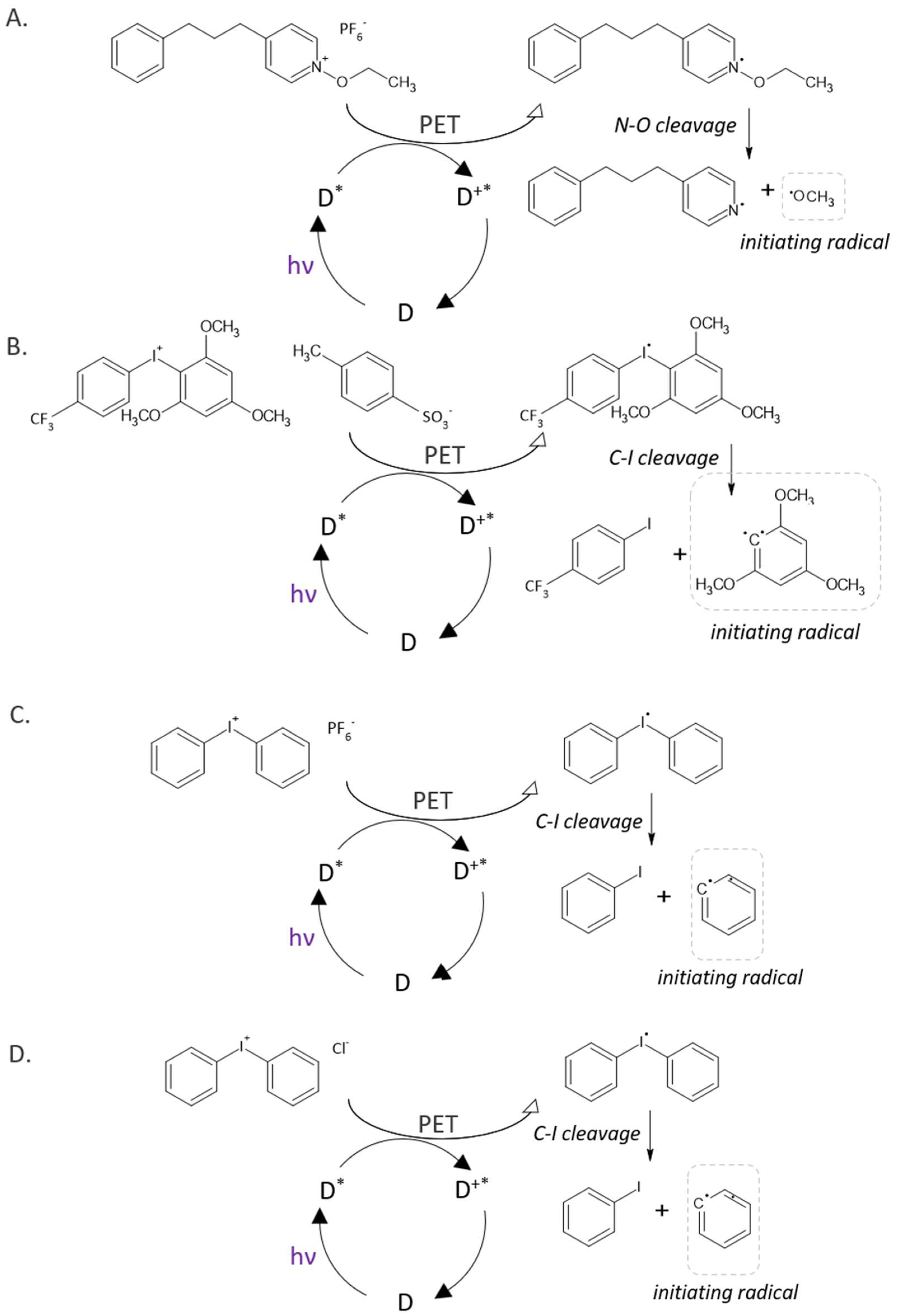
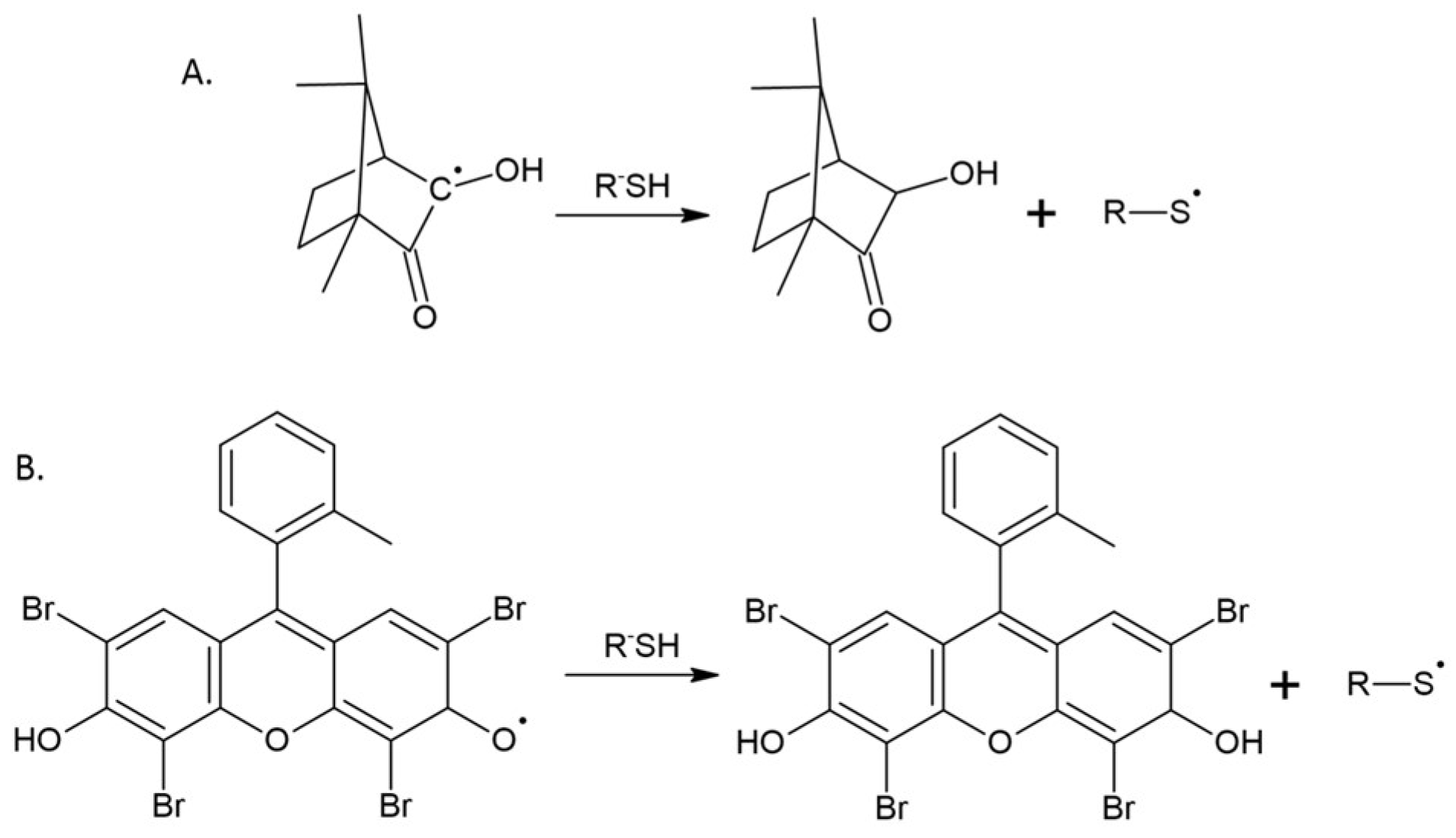

| Symbol | Chemical Name | Structure | Absorption Range [nm] | Absorbance Maximum [nm] |
|---|---|---|---|---|
| CQ | Camphorquinone | 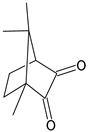 | 360–510 [22] | 468 [22] |
| EO | Eosin-Y |  | 300−700 [23] | 515 [23] |
| Symbol | Chemical Name | Structure | Absorbance Maximum [nm] |
|---|---|---|---|
| C0 | N-ethoxy-4-(3-phenylpropyl)pyridinium hexafluorophosphate | 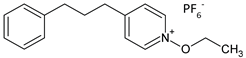 | 256 |
| C1 | [3-(trifluoromethyl)phenyl](2,4,6-trimethoxyphenyl)iodonium p-toluenesulfonate | 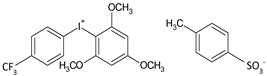 | 202 [24] |
| C2 | diphenyliodonium hexafluorophosphate |  | 400 [25] |
| C3 | diphenyliodonium chloride |  | 214, 226 [26] |
| Sample Name | NG [wt.%] | CQ [wt.%] | EO [wt.%] | Coinitiator | |
|---|---|---|---|---|---|
| Name | [wt.%] | ||||
| CQ-0 | 100 | - | - | - | - |
| CQ-0.3 | 100 | 0.3 | - | - | - |
| CQ-0.6 | 100 | 0.6 | - | - | - |
| CQ-2.5 | 100 | 2.5 | - | - | - |
| CQ-5 | 100 | 5 | - | - | - |
| EO-0 | 100 | - | - | - | - |
| EO-0.3 | 100 | - | 0.3 | - | - |
| EO-0.6 | 100 | - | 0.6 | - | - |
| EO-2.5 | 100 | - | 2.5 | - | - |
| EO-5 | 100 | - | 5 | - | - |
| CQ-C0 | 100 | 5 | - | C0 | 3 |
| CQ-C1 | 100 | 5 | - | C1 | 3 |
| CQ-C2 | 100 | 5 | - | C2 | 3 |
| CQ-C3 | 100 | 5 | - | C3 | 3 |
| CQ-L02 | 100 | 5 | - | L02 | 3 |
| CQ-L03 | 100 | 5 | - | L03 | 3 |
| CQ-TEA | 100 | 5 | - | TEA | 3 |
| EO-C0 | 100 | 5 | - | C0 | 3 |
| EO-C1 | 100 | 5 | - | C1 | 3 |
| EO-C2 | 100 | 5 | - | C2 | 3 |
| EO-C3 | 100 | 5 | - | C3 | 3 |
| EO-L02 | 100 | 5 | - | L02 | 3 |
| EO-L03 | 100 | 5 | - | L03 | 3 |
| EO-TEA | 100 | 5 | - | TEA | 3 |
| Property | Camphorquinone | Eosin Y | ||
|---|---|---|---|---|
| +Thiol | +Amine | +Thiol | +Amine | |
| Radical Generation Efficiency | High | Moderate | High | Moderate |
| Polymerization Rate (Rp) | High (up to 73 %/min) | Moderate | High (up to 70 %/min) | Moderate |
| Degree of Conversion (DC) | 67% | 50%–60% | 65% | 45%–55% |
| Light Penetration support | High | Moderate | High | Moderate |
| Residual monomer content | Low | Moderate | Medium | Low |
| Compatibility with dual-LED (365/405 nm) | High | High | Moderate | Moderate |
| Stability in formulation | Moderate | High | Moderate | High |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bednarczyk, P.; Rożniakowski, K. The Influence of the Photoinitiating System on Residual Monomer Contents and Photopolymerization Rate of a Model Pigmented UV/LED Nail Gel Formulation. Coatings 2025, 15, 1125. https://doi.org/10.3390/coatings15101125
Bednarczyk P, Rożniakowski K. The Influence of the Photoinitiating System on Residual Monomer Contents and Photopolymerization Rate of a Model Pigmented UV/LED Nail Gel Formulation. Coatings. 2025; 15(10):1125. https://doi.org/10.3390/coatings15101125
Chicago/Turabian StyleBednarczyk, Paulina, and Kamil Rożniakowski. 2025. "The Influence of the Photoinitiating System on Residual Monomer Contents and Photopolymerization Rate of a Model Pigmented UV/LED Nail Gel Formulation" Coatings 15, no. 10: 1125. https://doi.org/10.3390/coatings15101125
APA StyleBednarczyk, P., & Rożniakowski, K. (2025). The Influence of the Photoinitiating System on Residual Monomer Contents and Photopolymerization Rate of a Model Pigmented UV/LED Nail Gel Formulation. Coatings, 15(10), 1125. https://doi.org/10.3390/coatings15101125







