Silver Nanoparticles–Chitosan Nanocomposites as Protective Coatings for Dental Remineralization Treatment: An In Vitro Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Preparation and Characterization of Chitosan Gel with AgNPs
2.3. Sample Preparation
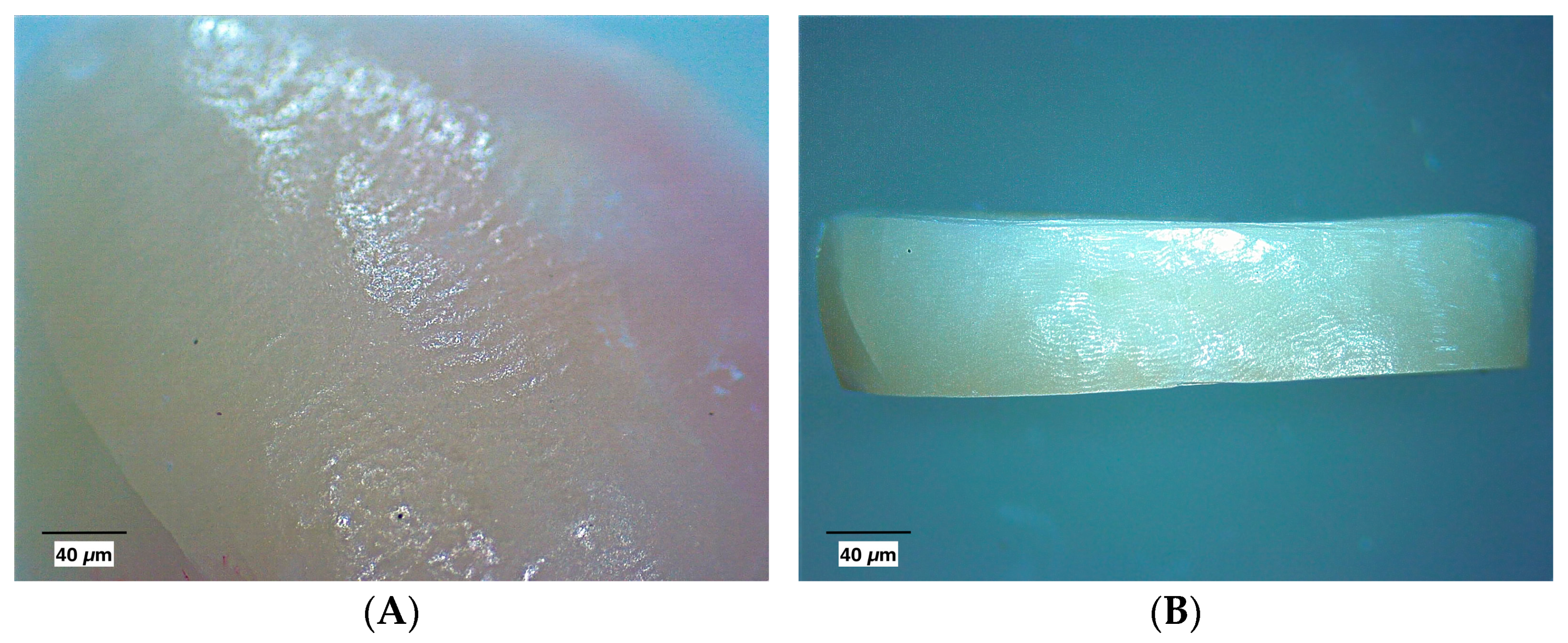
2.4. Macroscopic Evaluation
2.5. Laser Fluorescence Measurement
2.6. Artificial Demineralization of the Sample Surfaces
2.7. Application of Remineralizing Agents
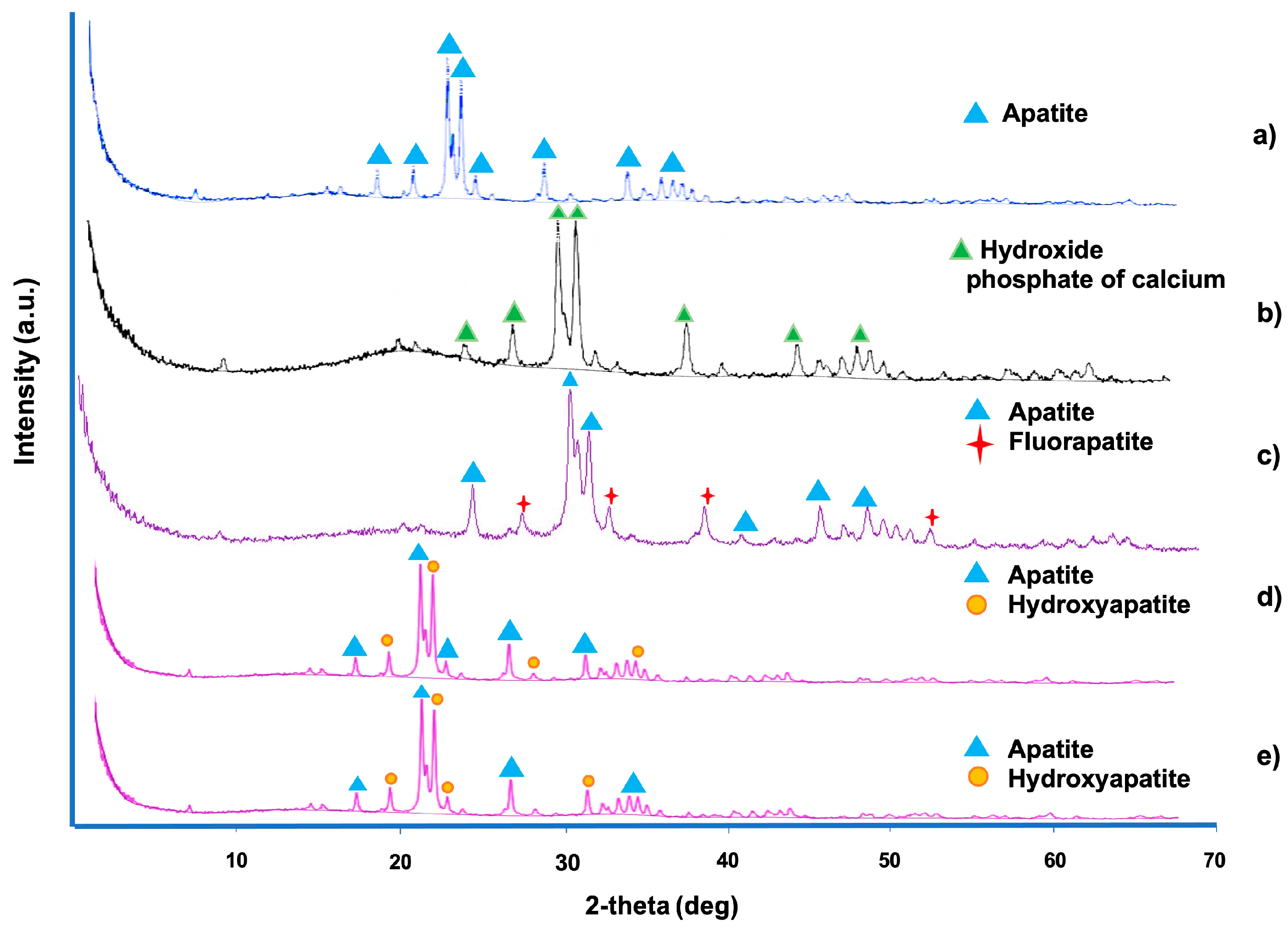
2.8. X-Ray Diffraction
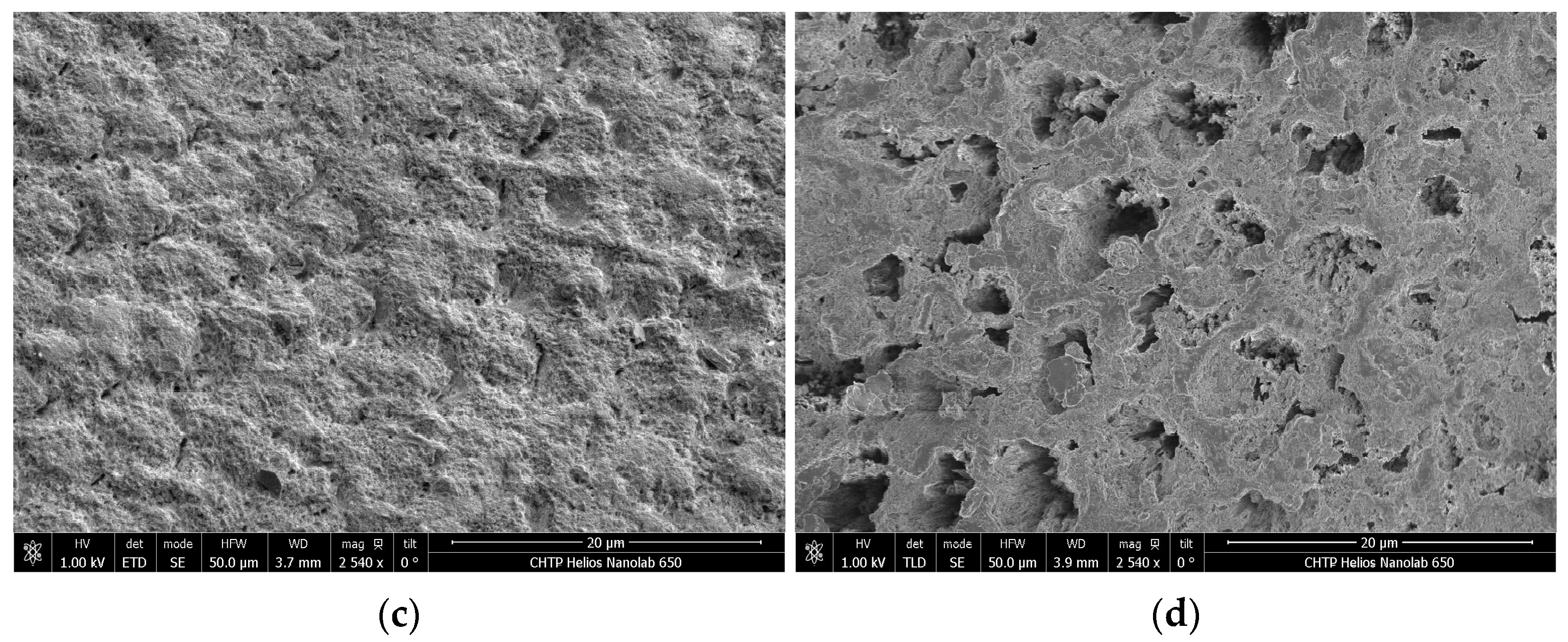
2.9. Scanning Electron Microscopy and Energy-Dispersive X-Ray Analyses
2.10. Statistical Analysis
3. Results
3.1. Macroscopic Evaluation
3.2. Laser Fluorescence
3.3. X-Ray Diffraction
3.4. Scanning Electron Microscopy and EDX Analysis
4. Discussion
5. Conclusions
- The changes evaluated on the enamel surface after applying FV + CG + AgNPs are related to the remineralization process, extending its effect up to 168 h compared to FV.
- The absence of pigmentation on the enamel surface is also highlighted.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- John, H.; Franklin, G.-G.; Catherine, F. Biological factors in dental caries: Role of saliva and dental plaque in the dynamic process of demineralization and remineralization (part 1). J. Clin. Pediatr. Dent. 2003, 28, 47–52. [Google Scholar]
- Chen, H.; Gu, L.; Liao, B.; Zhou, X.; Cheng, L.; Ren, B. Advances of Anti-Caries Nanomaterials. Molecules 2020, 25, 5047. [Google Scholar] [CrossRef]
- Kim, M.-J.; Li, M.-J.; Kim, K.-M.; Yang, S.-Y.; Seo, Y.-J.; Chul, C.S.; Ren, B. Enamel Demineralization Resistance and Remineralization by Various Fluoride-Releasing Dental Restorative Materials. Materials 2021, 14, 4554. [Google Scholar] [CrossRef]
- Chhatwani, S.; Hoppe, J.; Naumova, E.A.; Arnold, W.H.; Möhlhenrich, S.C.; Bizhang, M.; Danesh, G. Fluoride ion release characteristics of fluoride-containing varnishes-an in vitro study. Appl. Sci. 2021, 11, 1452. [Google Scholar] [CrossRef]
- Parisay, I.; Boskabady, M.; Bagheri, H.; Babazadeh, S.; Hoseinzadeh, M.; Esmaeilzadeh, F. Investigating the efficacy of a varnish containing gallic acid on remineralization of enamel lesions: An in vitro study. BMC Oral Health 2024, 24, 175. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, B.H.; Rajendra, A.; Veitz-Keenan, A.; Niederman, R. The effect of silver diamine fluoride in preventing caries in the primary dentition: A systematic review and meta-analysis. Caries Res. 2019, 53, 24–32. [Google Scholar] [CrossRef]
- Tejaswi, B.; Gopal Sree, V.; Sivapriya, E.; Archana, D.; PradeepKumar, A.R. Nanoparticles in caries prevention: A review. J. Glob. Oral Health 2021, 4, 56–66. [Google Scholar] [CrossRef]
- Salas-López, E.K.; Pierdant-Pérez, M.; Hernández-Sierra, J.F.; Ruíz, F.; Mandeville, P.; Pozos-Guillén, A.J. Effect of silver nanoparticle-added pit and fissure sealant in the prevention of dental caries in children. J. Clin. Pediatr. Dent. 2017, 41, 48–52. [Google Scholar] [CrossRef]
- Pichaiaukrit, W.; Thamrongananskul, N.; Siralertmukul, K.; Swasdison, S. Fluoride varnish containing chitosan demonstrated sustained fluoride release. Dent. Mater. J. 2019, 38, 1036–1042. [Google Scholar] [CrossRef]
- Ghafar, H.; Khan, M.I.; Sarwar, H.S.; Yaqoob, S.; Hussain, S.Z.; Tariq, I.; Madni, A.U.; Shahnaz, G.; Sohail, M.F. Development and Characterization of Bioadhesive Film Embedded with Lignocaine and Calcium Fluoride Nanoparticles. AAPS PharmSciTech 2020, 21, 60. [Google Scholar] [CrossRef]
- Memarpour, M.; Shafiei, F.; Rafiee, A.; Soltani, M.; Dashti, M.H. Effect of hydroxyapatite nanoparticles on enamel remineralization and estimation of fissure sealant bond strength to remineralized tooth surfaces: An in vitro study. BMC Oral. Health 2019, 19, 92. [Google Scholar] [CrossRef] [PubMed]
- Abedi, M.; Ghasemi, Y.; Nemati, M.M. Nanotechnology in toothpaste: Fundamentals, trends, and safety. Heliyon 2024, 10, e24949. [Google Scholar] [CrossRef] [PubMed]
- Surija, I.; Gunawan, H.A.; Amir, L.R. Effect of chitosan on the enamel demineralization process in vitro: An enamel solubility test. J. Phys. Conf. Ser. 2018, 1073, 052005. [Google Scholar] [CrossRef]
- Sámano-Valencia, C.; Martinez-Castanon, G.A.; Martínez-Gutiérrez, F.; Ruiz, F.; Toro-Vázquez, J.F.; Morales-Rueda, J.A.; Espinosa-Cristóbal, L.F.; Alonso, N.V.Z.; Martínez, N.N. Characterization and biocompatibility of chitosan gels with silver and gold nanoparticles. J. Nanomater. 2014, 2014, 543419. [Google Scholar] [CrossRef]
- Rajan, R.; Krishnan, R.; Bhaskaran, B.; Kumar, S.V. A Polarized Light Microscopic Study to Comparatively evaluate Four Remineralizing Agents on Enamel viz CPP-ACPF, ReminPro, SHY-NM and Colgate Strong Teeth. Int. J. Clin. Pediatr. Dent. 2015, 8, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Yamakami, S.A.; Faraoni, J.J.; Lia, N.S.N.D.; Regula, F.B.; Ohyama, H.; Palma-Dibb, R.G. Effect of an experimental chitosan/casein gel on demineralized enamel under a cariogenic challenge. Dent. Med. Probl. 2022, 59, 531–538. [Google Scholar] [CrossRef]
- Seredin, P.; Goloshchapov, D.; Kashkarov, V.; Emelyanova, A.; Buylov, N.; Barkov, K.; Ippolitov, Y.; Khmelevskaia, T.; Mahdy, I.A.; Mahdy, M.A.; et al. Biomimetic Mineralization of Tooth Enamel Using Nanocrystalline Hydroxyapatite under Various Dental Surface Pretreatment Conditions. Biomimetics 2022, 7, 111. [Google Scholar] [CrossRef]
- Shaalan, O.O. DIAGNOdent versus International Caries Detection and Assessment System in detection of incipient carious lesions: A diagnostic accuracy study. J. Conserv. Dent. 2023, 26, 199–206. [Google Scholar] [CrossRef] [PubMed]
- El Moshy, S.; Abbass, M.M.S.; El-Motayam, A.M. Biomimetic remineralization of acid etched enamel using agarose hydrogel model. F1000Res 2018, 7, 1476. [Google Scholar] [CrossRef]
- Sámano-Valencia, C.; Martinez-Castanon, G.A.; Martínez-Martínez, R.E.; Loyola-Rodríguez, J.P.; Reyes-Macías, J.F.; Ortega-Zarzosa, G.; Niño-Martínez, N. Bactericide efficiency of a combination of chitosan gel with silver nanoparticles. Mater. Lett. 2013, 106, 413–416. [Google Scholar] [CrossRef]
- Noronha, V.T.; Paula, A.J.; Durán, G.; Galembeck, A.; Cogo-Müller, K.; Franz-Montan, M.; Durán, N. Silver nanoparticles in dentistry. Dent. Mater. 2017, 33, 1110–1126. [Google Scholar] [CrossRef]
- Ruan, Q.; Zhang, Y.; Yang, X.; Nutt, S.; Moradian-Oldak, J. An amelogenin-chitosan matrix promotes assembly of an enamel-like layer with a dense interface. Acta Biomater. 2013, 9, 7289–7297. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Q.; Liberman, D.; Bapat, R.; Chandrababu, K.B.; Phark, J.H.; Moradian-Oldak, J. Efficacy of amelogenin-chitosan hydrogel in biomimetic repair of human enamel in pH-cycling systems. J. Biomed. Eng. Inform. 2015, 2, 119. [Google Scholar] [CrossRef] [PubMed]
- Nimbeni, S.B.; Nimbeni, B.S.; Divakar, D.D. Role of chitosan in remineralization of enamel and dentin: A systematic review. Int. J. Clin. Pediatr. Dent. 2021, 14, 562–568. [Google Scholar] [CrossRef] [PubMed]
- NouhzadehMalekshah, S.; Fekrazad, R.; Bargrizan, M.; Kalhori, K.A. Evaluation of laser fluorescence in combination with photosensitizers for detection of demineralized lesions. Photodiag. Photodyn. Ther. 2019, 26, 300–305. [Google Scholar] [CrossRef]
- Kolumban, A.; Moldovan, M.; Țig, I.A.; Chifor, I.; Cuc, S.; Bud, M.; Badea, M.E. An evaluation of the demineralizing effects of various acidic solutions. Appl. Sci. 2021, 11, 8270. [Google Scholar] [CrossRef]
- Enax, J.; Fandrich, P.; Schulze zur Wiesche, E.; Epple, M. The Remineralization of Enamel from Saliva: A Chemical Perspective. Dent. J. 2024, 12, 339. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lynch, R.J.M.; Watson, T.F.; Banerjee, A. Remineralisation of enamel white spot lesions pre-treated with chitosan in the presence of salivary pellicle. J. Dent. 2018, 72, 21–28. [Google Scholar] [CrossRef]
- Üstün, N.; Aktören, O. Analysis of efficacy of the self-assembling peptide-based remineralization agent on artificial enamel lesions. Microsc. Res. Tech. 2019, 82, 1065–1072. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Sun, X.; Kishen, A.; Deng, X.; Yang, X.; Wang, H.; Cong, C.; Wang, Y.; Wu, M. Biomimetic remineralization of demineralized enamel with nano-complexes of phosphorylated chitosan and amorphous calcium phosphate. J. Mater. Sci. Mater. Med. 2014, 25, 2619–2628. [Google Scholar] [CrossRef]
- Bijle, M.N.; Abdalla, M.M.; Ashraf, U.; Siu, K.W.; Tsoi, J.; Yiu, C.K.Y. The acid-resistance potential of arginine-fluoride varnish treated enamel. J. Mech. Behav. Biomed. Mater. 2022, 125, 104763. [Google Scholar] [CrossRef]
- Zhao, I.S.; Yin, I.X.; Mei, M.L.; Lo, E.C.M.; Tang, J.; Li, Q.; So, L.Y.; Chu, C.H. Remineralising dentine caries using sodium fluoride with silver nanoparticles: An in vitro study. Int. J. Nanomed. 2020, 15, 2829–2839. [Google Scholar] [CrossRef]
- Dai, L.L.; Mei, M.L.; Chu, C.H.; Lo, E.C.M. Remineralizing effect of a new strontium-doped bioactive glass and fluoride on demineralized enamel and dentine. J. Dent. 2021, 108, 103633. [Google Scholar] [CrossRef]
- Ren, J.; Rao, J.; Wang, H.; He, W.; Feng, J.; Wei, D.; Zhao, B.; Wang, X.; Bian, W. Synergistic remineralization of enamel white spot lesions using mesoporous bioactive glasses loaded with amorphous calcium phosphate. Front. Bioeng. Biotechnol. 2023, 11, 1109195. [Google Scholar] [CrossRef] [PubMed]
- Bijle, M.N.; Abdalla, M.M.; Ashraf, U.; Ekambaram, M.; Yiu, C.K.Y. Enamel remineralization potential of arginine-fluoride varnish in a multi-species bacterial pH-cycling model. J. Dent. 2021, 104, 103528. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Y.; Wong, H.M.; McGrath, C.P.J.; Li, Q.L. In vitro and in vivo evaluation of electrophoresis-aided casein phosphopeptide-amorphous calcium phosphate remineralisation system on pH-cycling and acid-etching demineralised enamel. Sci. Rep. 2018, 8, 8904. [Google Scholar] [CrossRef] [PubMed]
- Wassel, M.O.; Sherief, D.I. Ion release and enamel remineralizing potential of miswak, propolis and chitosan nano-particles based dental varnishes. Pediatr. Dent. J. 2019, 29, 1–10. [Google Scholar] [CrossRef]
- Vitiello, F.; Tosco, V.; Monterubbianesi, R.; Orilisi, G.; Gatto, M.L.; Sparabombe, S.; Memé, L.; Mengucci, P.; Putignano, A.; Orsini, G. Remineralization Efficacy of Four Remineralizing Agents on Artificial Enamel Lesions: SEM-EDS Investigation. Materials 2022, 15, 4398. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Boyes, V.; Festy, F.; Lynch, R.J.M.; Watson, T.F.; Banerjee, A. In-vitro subsurface remineralisation of artificial enamel white spot lesions pre-treated with chitosan. Dent. Mater. 2018, 34, 1154–1167. [Google Scholar] [CrossRef]
- Muşat, V.; Anghel, E.M.; Zaharia, A.; Atkinson, I.; Mocioiu, O.C.; Buşilă, M.; Alexandru, P. A chitosan–agarose polysaccharide-based hydrogel for biomimetic remineralization of dental enamel. Biomolecules 2021, 11, 1137. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.; Wang, J.; Gao, F. The application of chitosan nanostructures in stomatology. Molecules 2021, 26, 6315. [Google Scholar] [CrossRef] [PubMed]
- Deokar, K.K.; Shashikiran, N.; Maurya, A.; Gaonkar, N.; Gugwad, S.; Hadakar, S.; Taur, S. Comparative Evaluation of Chitosan Nanoparticles, Silver Diamine Fluoride and Acidulated Phosphate Fluoride Gel on Microhardness of Artificial Carious Lesions Created on Extracted Teeth. J. Clin. Diagn. Res. 2020, 14, 20. [Google Scholar] [CrossRef]
- Hanafy, R.A.; El-Fattah, A.A.; Mostafa, D.; Kandil, S. Biomimetic chitosan against bioinspired nanohydroxyapatite for repairing enamel surfaces. Bioinspired Biomim. Nanobiomater. 2020, 9, 85–94. [Google Scholar] [CrossRef]
- Magalhães, T.C.; Teixeira, N.M.; França, R.S.; Denadai, Â.M.L.; Dos Santos, R.L.; Carlo, H.L.; Munchow, E.A.; de Carvalho, F.G. Synthesis of a Chitosan nanoparticle suspension and its protective effects against enamel demineralization after an in vitro Cariogenic challenge. J. Appl. Oral Sci. 2021, 29, e20210120. [Google Scholar] [CrossRef]
- Xu, V.W.; Nizami, M.Z.I.; Yin, I.X.; Lung, C.Y.K.; Yu, O.Y.; Chu, C.H. Caries Management with Non-Metallic Nanomaterials: A Systematic Review. Int. J. Nanomed. 2022, 17, 5809–5824. [Google Scholar] [CrossRef] [PubMed]
- Wahied, D.M.; Ezzeldin, N.; Abdelnabi, A.; Othman, M.S.; El Rahman, M.H.A. Evaluation of Surface Properties of Two Remineralizing Agents after Modification by Chitosan Nano Particles: An In vitro Study. Contemp. Clin. Dent. 2023, 14, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Zhang, O.L.; Niu, J.Y.; Yin, I.X.; Yu, O.Y.; Mei, M.L.; Chu, C.H. Bioactive Materials for Caries Management: A Literature Review. Dent. J. 2023, 11, 59. [Google Scholar] [CrossRef]
- Tristán-López, J.-D.; Niño-Martínez, N.; Kolosovas-Machuca, E.-S.; Patiño-Marín, N.; De Alba-Montero, I.; Bach, H.; Martínez-Castañón, G.-A. Application of Silver Nanoparticles to Improve the Antibacterial Activity of Orthodontic Adhesives: An In Vitro Study. Int. J. Mol. Sci. 2023, 24, 1401. [Google Scholar] [CrossRef] [PubMed]




| Initial a | After b | Exposition Time | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | 24 h | 48 h | 120 h | 168 h | |||||||||||
| n | Mean | SD | Mean | SD | p * | Mean | SD | Mean | SD | Mean | SD | Mean | SD | p ** | |
| 1 | 16 | 6.44 | 1.79 | 66.00 | 29.49 | <0.05 | 61.75 ** | 16.03 ** | 81.5 ** | 21.76 ** | 61.25 ** | 32.36 ** | 36.50 ** | 11.21 ** | <0.05 |
| 2 | 16 | 6.94 | 2.41 | 64.56 | 32.77 | 46.75 ** | 12.04 ** | 48.25 | 35.38 | 80.5 ** | 37.00 ** | 34.75 ** | 11.35 ** | ||
| 3 | 16 | 7.25 | 2.44 | 65.06 | 30.55 | 47.00 ** | 6.22 ** | 77.50 ** | 28.44 ** | 12.5 | 4.43 | 37.00 ** | 22.14 ** | ||
| 4 | 16 | 8.31 | 2.55 | 76.75 | 21.92 | 81.75 ** | 8.06 ** | 36.25 | 11.12 | 30.5 | 13.77 | 33.50 ** | 1.91 ** | ||
| 5 | 16 | 7.06 | 2.29 | 70.50 | 29.35 | 14.25 | 6.95 | 10.50 | 3.70 | 27.75 | 12.04 | 12.25 | 5.68 | ||
| 6 | 16 | 6.00 | 1.41 | 61.44 | 29.83 | 28.25 | 12.04 | 13.50 | 1.73 | 7.5 | 1.73 | 4.75 | 1.50 | ||
| Exposition Time | Treatment | Phase | a/Å * | b/Å * | c/Å * |
|---|---|---|---|---|---|
| Healthy enamel | Apatite | 9.43 | 9.43 | 6.87 | |
| Demineralized enamel | Calcium phosphate hydroxide | 9.45 | 9.45 | 6.89 | |
| 24 h | Fluoride varnish | Carbonate Hidroxiapatite | 9.44 | 9.44 | 6.88 |
| Fluoride varnish + chitosan gel | Apatite | 9.43 | 9.43 | 6.88 | |
| Fluoride varnish + NpAg + chitosan gel | Apatite Fluorapatite | 9.45 9.45 | 9.45 9.45 | 6.89 6.88 | |
| NpAg + chitosan gel | Apatite | 9.43 | 9.43 | 6.87 | |
| 48 h | Fluoride varnish | Apatite | 9.45 | 9.45 | 6.88 |
| Fluoride varnish + chitosan gel | Apatite | 9.45 | 9.45 | 6.88 | |
| Fluoride varnish + NpAg + chitosan gel | Hidroxiapatite | 9.43 | 9.45 | 6.87 | |
| NpAg + chitosan gel | Apatite | 9.43 | 9.43 | 6.87 | |
| 120 h | Fluoride varnish | Apatite | 9.42 | 9.42 | 6.86 |
| Fluoride varnish + chitosan gel | Apatite | 9.42 | 9.42 | 6.87 | |
| Fluoride varnish + NpAg + chitosan gel | Apatite Hidroxiapatite | 9.42 9.42 | 9.42 9.42 | 6.86 6.85 | |
| NpAg + chitosan gel | Apatite Potasium, calcium | 9.47 3.88 | 9.47 3.88 | 6.89 16.63 | |
| 168 h | Fluoride varnish | Carbonate Hidroxiapatite | 9.44 | 9.44 | 6.87 |
| Fluoride varnish + chitosan gel | Apatite | 9.43 | 9.43 | 6.86 | |
| Fluoride varnish + NpAg + chitosan gel | Apatite | 9.42 | 9.42 | 6.86 | |
| NpAg + chitosan gel | Apatite | 9.45 | 9.45 | 6.88 |
| Author(s) | Aim | Bioactive Materials | Main Results | Ref. |
|---|---|---|---|---|
| Deokar et al. | To evaluate and compare the efficacy of the remineralizing potential of acidulated phosphate fluoride gel, chitosan nanoparticles, and silver diamine fluoride on the microhardness of artificial carious lesions created on extracted teeth. | Chitosan nanoparticles, silver diamine fluoride, and acidulated phosphate fluoride gel |
| [42] |
| Hanafy et al. | Chitosan was employed as a novel biomimetic mineralization model to repair damaged enamel to compare its performance with that of bioinspired zinc-doped nanohydroxyapatite. | Chitosan–hydrogel and a zinc-doped nanohydroxyapatite |
| [43] |
| Muşat et al. | To induce the biomimetic remineralization of acid-etched human enamel in artificial saliva containing fluoride under agarose and novel chitosan–agarose hydrogels action without a toxic crosslinking agent for chitosan. | Chitosan (CS) and agarose (A) in a biopolymer-based hydrogel |
| [40] |
| Magalhães et al. | Synthesize, characterize, and determine the effects of a ChNPs suspension on human enamel after cariogenic challenge via pH cycling. | Chitosan nanoparticles |
| [44] |
| Yamakami et al. | Evaluate the effects of an experimental chitosan/casein gel on enamel demineralization/remineralization in an environment with a high cariogenic challenge. | Chitosan/casein gel |
| [16] |
| Xu et al. | Review the types, properties, and potential uses of non-metallic nanomaterials systematically for managing dental caries. | Biological organic nanomaterials, synthetic organic nanomaterials, carbon-based nanomaterials, and selenium nanomaterials |
| [45] |
| Wahied et al. | Evaluate the effect of chitosan nanoparticles on the remineralization of the demineralized enamel surface after being added to nano-hydroxyapatite and nano-calcium phosphate materials. | Nano-hydroxyapatite and nano-calcium phosphate |
| [46] |
| Zhang et al. | The purpose of this literature review is to provide an overview of current bioactive materials for caries management. | Bioactive materials for caries management |
| [47] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Rodríguez, K.A.A.; de González, W.Y.E.; Castañeda Monroy, V.; Murphy, S.; Martínez-Castañón, G.-A.; Bach, H.; Niño-Martínez, N. Silver Nanoparticles–Chitosan Nanocomposites as Protective Coatings for Dental Remineralization Treatment: An In Vitro Study. Coatings 2025, 15, 40. https://doi.org/10.3390/coatings15010040
de Rodríguez KAA, de González WYE, Castañeda Monroy V, Murphy S, Martínez-Castañón G-A, Bach H, Niño-Martínez N. Silver Nanoparticles–Chitosan Nanocomposites as Protective Coatings for Dental Remineralization Treatment: An In Vitro Study. Coatings. 2025; 15(1):40. https://doi.org/10.3390/coatings15010040
Chicago/Turabian Stylede Rodríguez, Katleen A. Aguirre, Wendy Y. Escobar de González, Vianney Castañeda Monroy, Sean Murphy, Gabriel-Alejandro Martínez-Castañón, Horacio Bach, and Nereyda Niño-Martínez. 2025. "Silver Nanoparticles–Chitosan Nanocomposites as Protective Coatings for Dental Remineralization Treatment: An In Vitro Study" Coatings 15, no. 1: 40. https://doi.org/10.3390/coatings15010040
APA Stylede Rodríguez, K. A. A., de González, W. Y. E., Castañeda Monroy, V., Murphy, S., Martínez-Castañón, G.-A., Bach, H., & Niño-Martínez, N. (2025). Silver Nanoparticles–Chitosan Nanocomposites as Protective Coatings for Dental Remineralization Treatment: An In Vitro Study. Coatings, 15(1), 40. https://doi.org/10.3390/coatings15010040









