The Influence of an Alternating Current Field on Pack Boriding for Medium Carbon Steel at Moderate Temperature
Abstract
1. Introduction
2. Experimental Procedure
3. Results
3.1. The Current Intensity
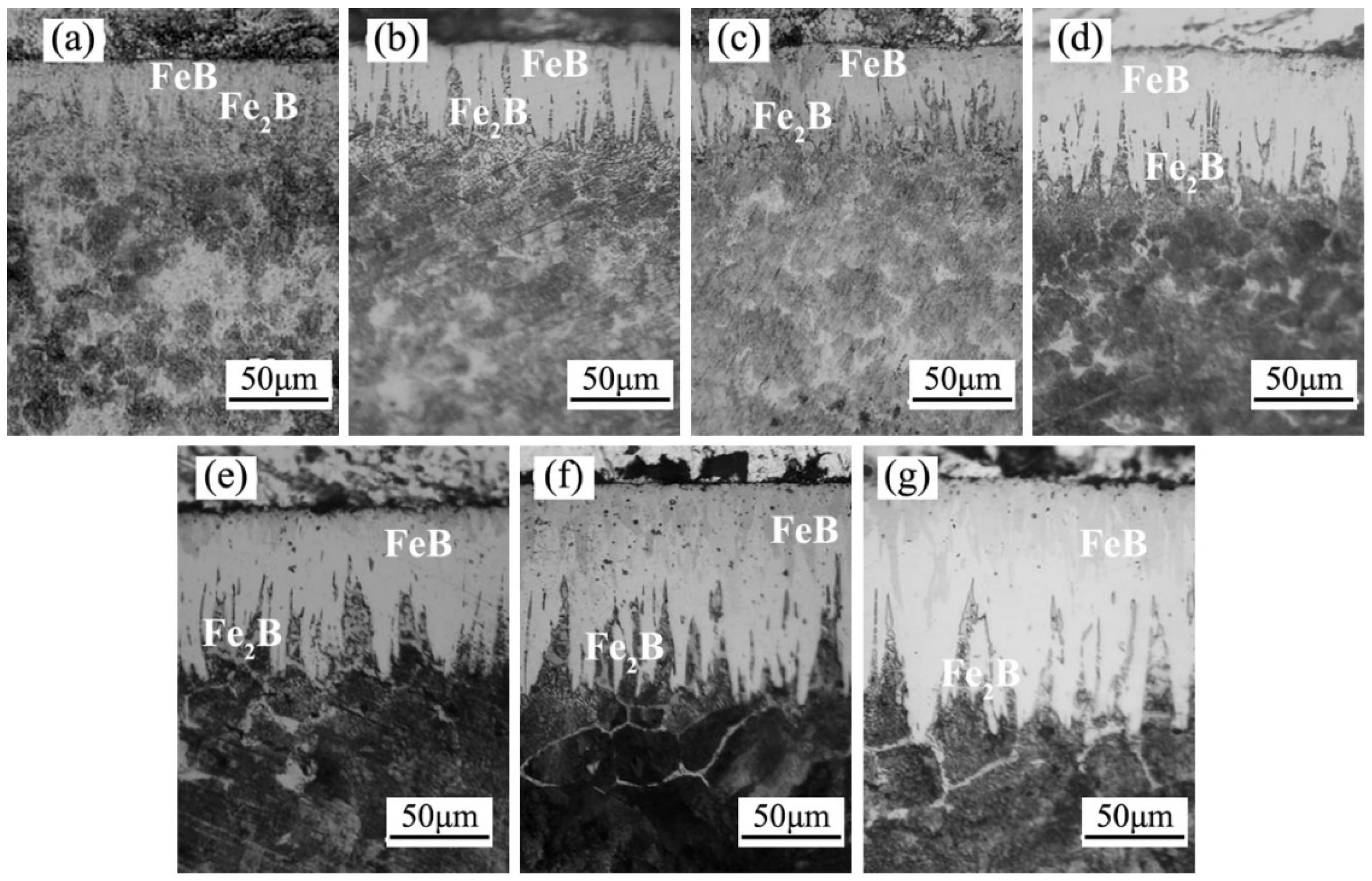
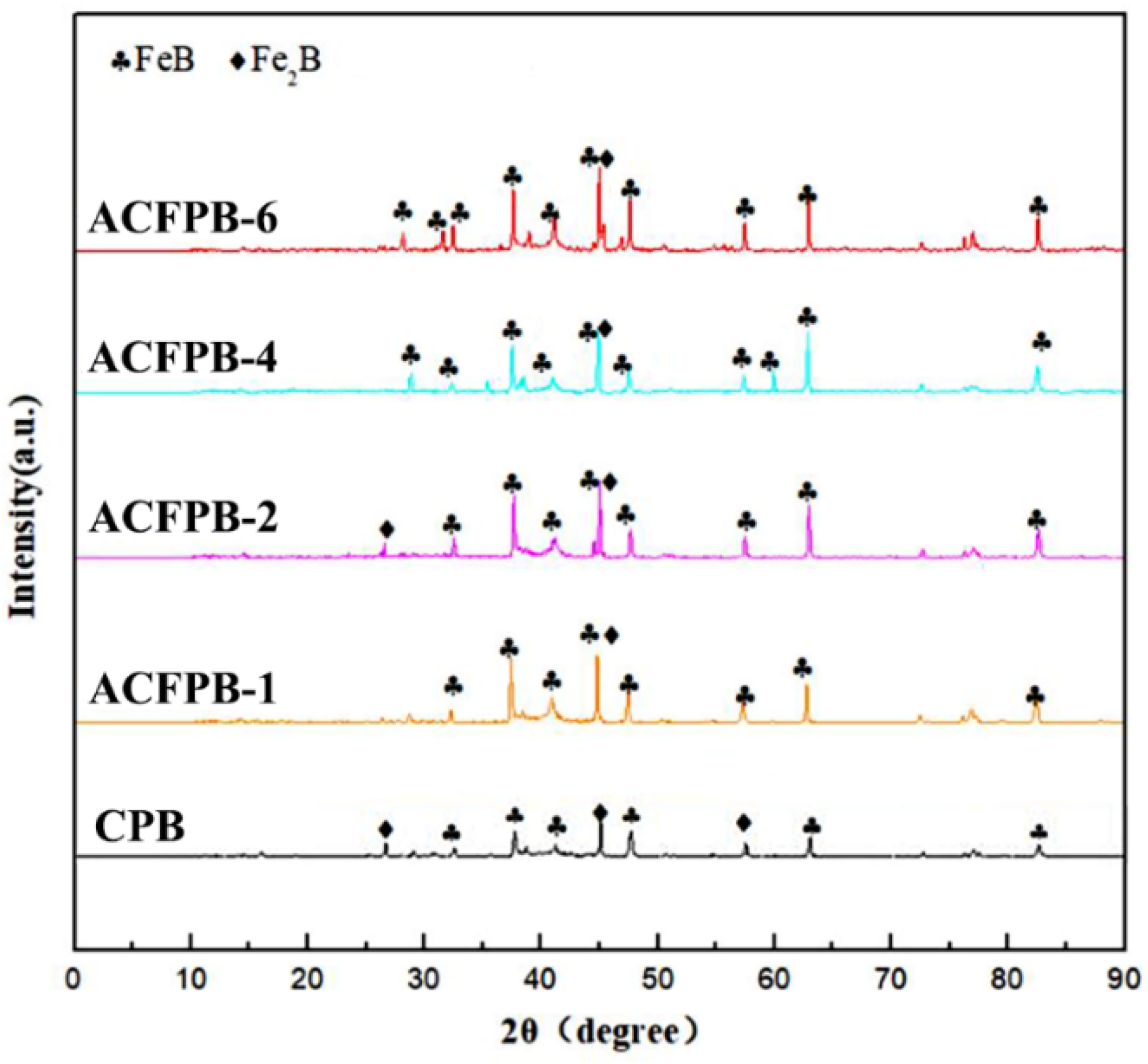
3.1.1. Microstructure of Boride Layer
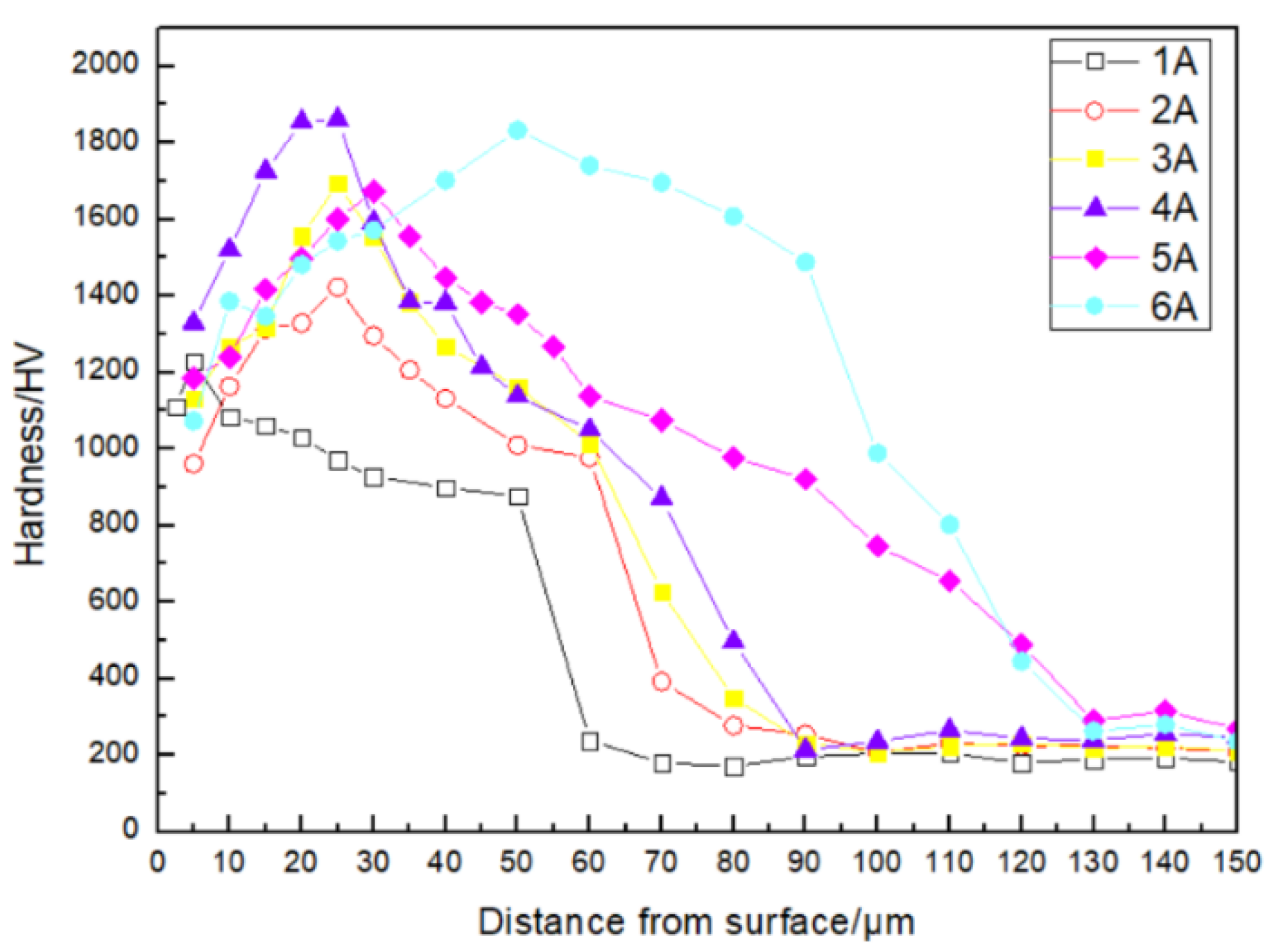
3.1.2. Microhardness of Boride Layer
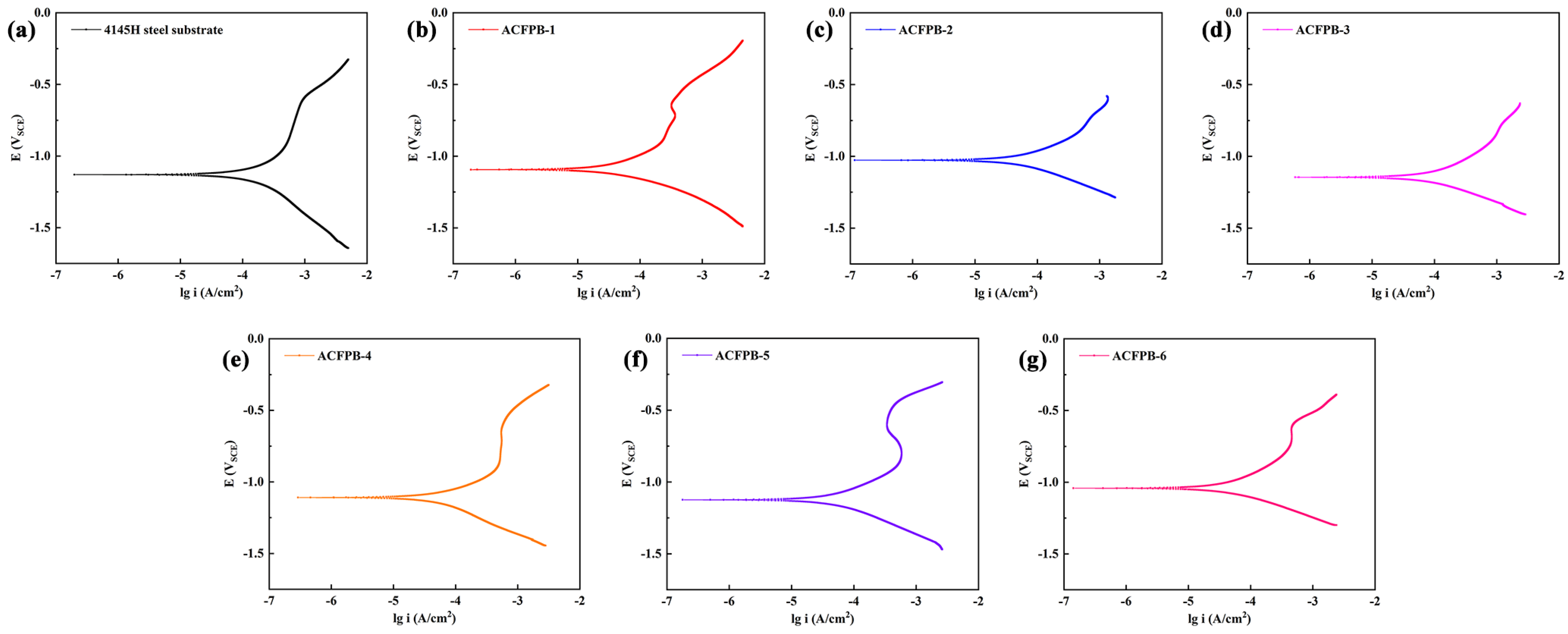
3.1.3. Corrosion Resistance of Boride Layer
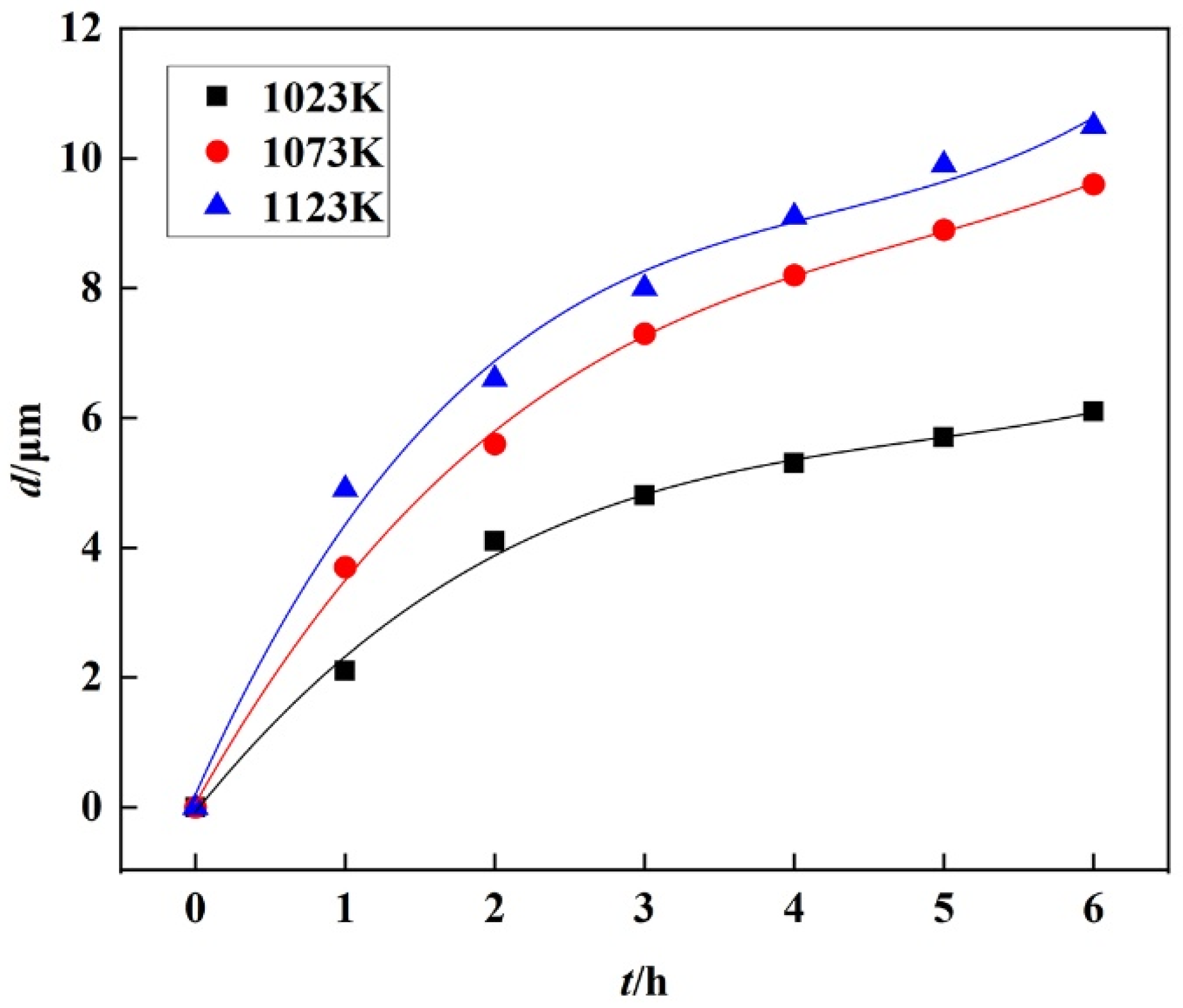
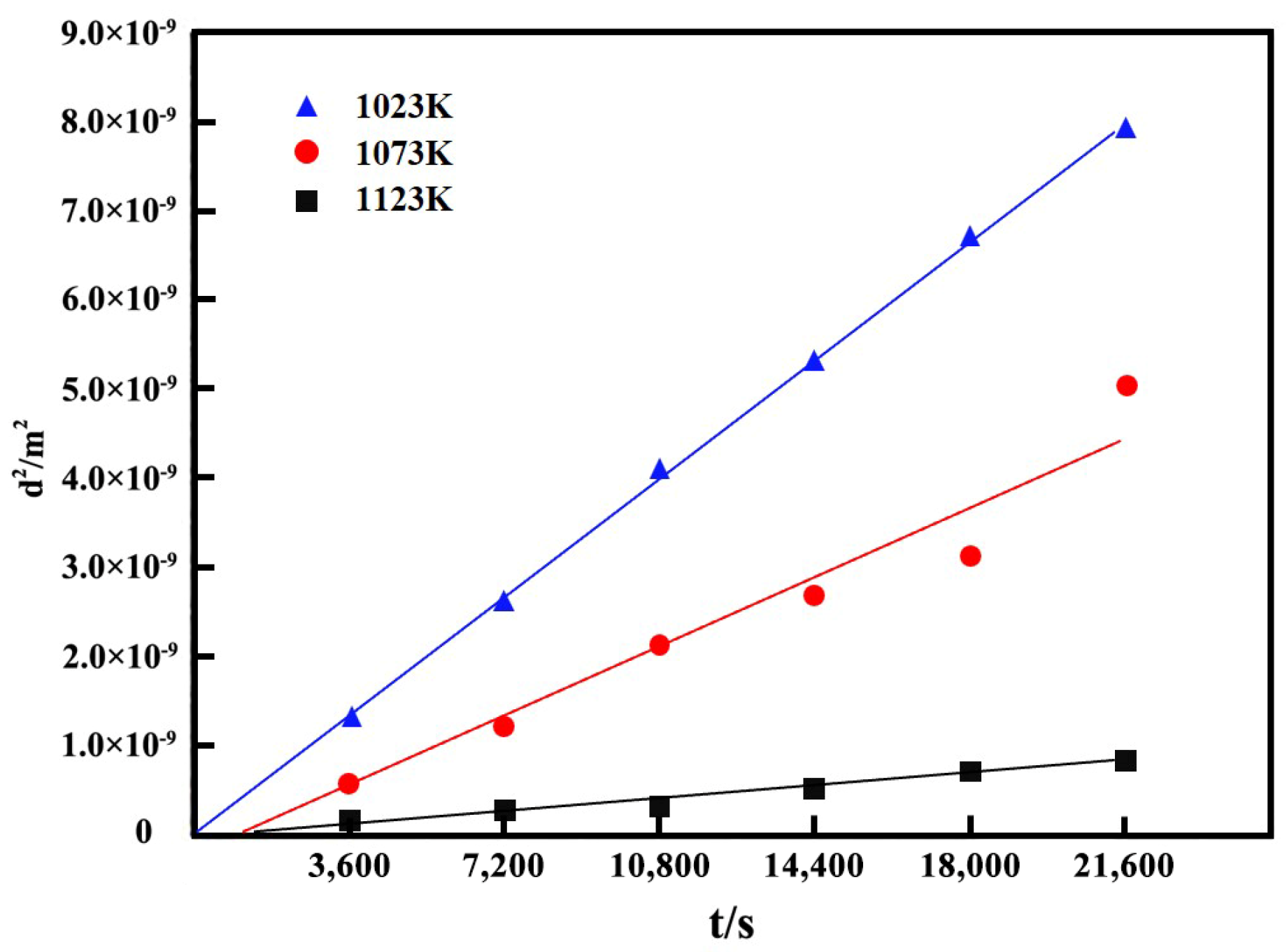
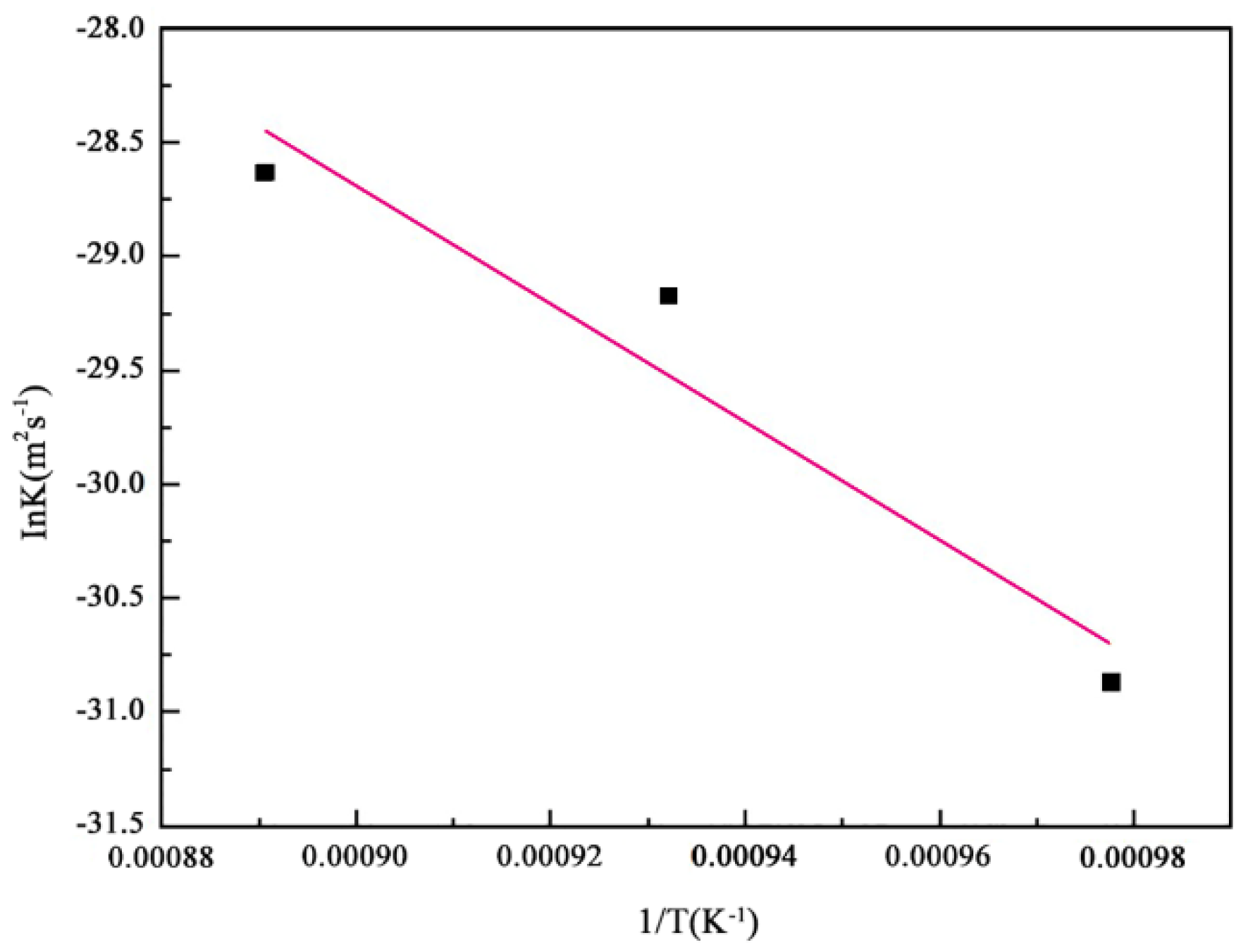
3.2. Growth Kinetics of Boride Layer
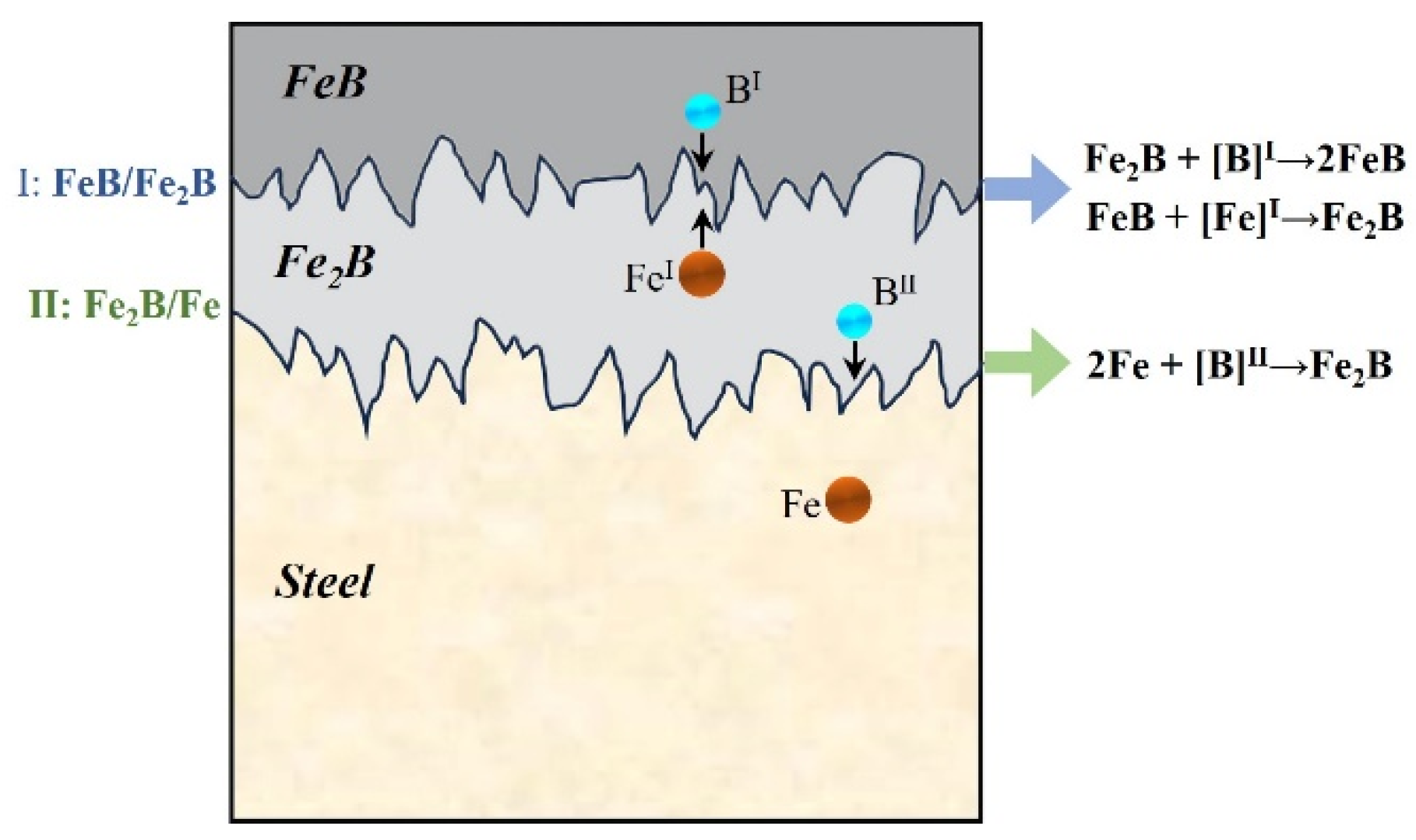
3.3. Discussion
4. Conclusions
- The microstructure of the boride layer obtained through AC field boriding resembled that of conventional boriding, comprised of an outer FeB and an inner Fe2B on the 4145H steel. In addition, as the current density increased, the thickness of the boride layer gradually increased, and the thickness obtained at a current density of 6A was five times that of conventional boriding.
- The AC field boriding process was primarily controlled by diffusion. Based on the parabolic growth law of the boride layer, this study established a growth kinetic model within the temperature range of 1023–1123 K.
- The AC field boriding samples exhibit a relatively flat microhardness curve and exhibit significantly reduced corrosion current density and corrosion rate in a 3.5 wt.% NaCl solution. The application of AC field boriding has enhanced the microhardness and corrosion resistance of 4145H steel. This is primarily attributed to the AC field’s ability to increase the number of active boron atoms and accelerate the diffusion of iron and boron atoms, thereby effectively increasing the thickness of the boride layer and the proportion of Fe2B phase within it.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, X.Y.; Paul, K.C.; Ding, C.X. Surface modification of titanium, titanium alloys, and related materials for biomedical applications. Mater. Sci. Eng. R Rep. 2004, 47, 49–121. [Google Scholar] [CrossRef]
- Aich, S.; Chandran, K.S.R. TiB Whisker Coating on Titanium Surfaces by Solid-State Diffusion: Synthesis, Microstructure, and Mechanical Properties. Metall. Mater. Trans. A-Phys. Metall. Mater. Sci. 2002, 33, 3489–3498. [Google Scholar] [CrossRef]
- Oliveira, C.K.N.; Casteletti, L.C.; Cuma, B.; Lombardi Neto, A.; Totten, G.E.; Heck, S.C. Production and characterization of boride layers on AISI D2 tool steel. Vacuum 2010, 84, 792–796. [Google Scholar] [CrossRef]
- Li, Y.H.; Luo, D.F.; Zhang, S.P. QPQ technology and resistance to hydrogen sulfide corrosion. Heat Treat. Met. 2010, 08, 97–100. [Google Scholar]
- Makuch, N.; Kulka, M.; Keddam, M.; Taktak, S.; Ataibis, V.; Dziarski, P. Growth kinetics and some mechanical properties of two-phase boride layers produced on commercially pure titanium during plasma paste boriding. Thin Solid Film. 2017, 626, 25–37. [Google Scholar] [CrossRef]
- Sarma, B.; Tikekar, N.M.; Chandran, R.S.K. Kinetics of growth of superhard boride layers during solid state diffusion of boron into titanium. Ceram. Int. 2012, 38, 6795–6805. [Google Scholar] [CrossRef]
- Morsi, K.; Patel, V.V.; Naraghi, S.; Garay, J. Processing of titanium-titanium boride dual matrix composites. J. Mater. Process. Technol. 2007, 196, 236–242. [Google Scholar] [CrossRef]
- Usta, M.; Ozbek, I.; Bindal, C.; Ucisik, A.H.; Ingole, S.; Liang, H. A comparative study of borided pure niobium, tungsten and chromium. Vacuum 2006, 80, 1321–1325. [Google Scholar] [CrossRef]
- Chail, G.; Kangas, P. Super and hyper duplex stainless steels: Structures, properties and application. Procedia Struct. Integr. 2016, 2, 1755–1762. [Google Scholar] [CrossRef]
- Sahin, S.; Meric, C. Investigation of the effect of boronizing on cast irons. Mater. Res. Bull. 2002, 37, 971–979. [Google Scholar] [CrossRef]
- Sen, U.; Sen, S.; Koksal, S.; Yilmaz, F. Fracture toughness of borides formed on boronized ductile iron. Mater. Des. 2004, 26, 175–179. [Google Scholar] [CrossRef]
- Sen, U.; Sen, S. The fracture toughness of borides formed on boronized cold work tool steels. Mater. Charact. 2003, 50, 261–267. [Google Scholar] [CrossRef]
- Tang, L.N.; Yan, M.F. Effects of rare earths addition on the microstructure, wear and corrosion resistances of plasma nitrided 30CrMnSiA steel. Surf. Coat. Technol. 2011, 206, 2363–2370. [Google Scholar] [CrossRef]
- Allaoui, O.; Bouaouadja, N.; Saindernan, G. Characterization of boronized layers on a XC38 steel. Surf. Coat. Technol. 2006, 201, 3475–3482. [Google Scholar] [CrossRef]
- Yu, L.G.; Chen, X.J.; Khor, K.A.; Sundararajan, G. FeB/Fe2B phase transformation during SPS pack-boriding: Boride layer growth kinetics. Acta Mater. 2005, 53, 2361–2368. [Google Scholar] [CrossRef]
- Ipek, M.; Efe, G.C.; Ozbek, I.; Zeytin, S.; Bindal, C. Investigation of Boronizing Kinetics of AISI 51100 Steel. J. Mater. Eng. Performance 2012, 21, 733–738. [Google Scholar] [CrossRef]
- Xu, B.; Li, M.S. Effect on decreasing the eigen-brittleness of boride layer on its friction and wear behavior. Mech. Eng. 2002, 38, 131–134. [Google Scholar] [CrossRef]
- Peng, Z.H.; Yang, Z.B.; Wang, S.W.; Guo, Y.W. Effect of rare earth on structure and properties of boronzing layer. Heat Treat. Met. 2004, 29, 31–34. [Google Scholar]
- Huang, Z.R.; Li, P.N.; Xie, F.; Pan, J.W. Effect of rare elements on powder boro-carbo-nitnding at low temperature. J. Rare Earths 2000, 18, 205–209. [Google Scholar]
- Anthymidis, K.G.; Stergioudis, G.; Tsipas, D.N. Boride coatings on non-ferrous materials in a fluidized bed reactor and their properties. Sci. Technol. Adv. Mater. 2002, 3, 303–311. [Google Scholar] [CrossRef]
- Zhang, Q.X.; Ye, W.P.; Zang, J.H.; Zhang, L. Study temperature property of bonding medium under microwave field. Hot Work. Technol. 2007, 36, 53–55. [Google Scholar]
- Yang, H.P.; Wu, X.C.; Yang, Z.; Pu, S.; Wang, H. Enhanced boromzing kinetics of alloy steel assisted by surface mechanical attrition treatments. J. Alloys Compd. 2014, 590, 388–395. [Google Scholar] [CrossRef]
- Jauhari, I.; Yusof, H.A.M.; Saidan, R. Superplastic boronizing of duplex stainless steel under dual compression method. Mater. Sci. Eng. A 2011, 528, 8106–8110. [Google Scholar] [CrossRef]
- Xie, F.; Cheng, J.; Pan, J.W. Influences of current field frequency on alternating current field enhanced pack boriding on 45 steel. China Surf. Eng. 2018, 31, 39–44. [Google Scholar]
- Cheng, J.; Xie, F.; Sun, L.; Xie, F. A kinetic investigation on alternating current field enhanced powder-pack boriding of Q215 steel. Sci. Sin. Technol. 2014, 44, 315–322. [Google Scholar]
- Xie, F.; Sun, L.; Cheng, J. Alternating current field assisted pack boriding to Fe2B coating. Surf. Eng. 2013, 29, 240–243. [Google Scholar] [CrossRef]
- Campos, I.; Hernandez, E.J.; Contreras, A.; Rosales-Lopez, J.L.; Valdez-Zayas, E.; Mejía-Caballero, I.; Martínez-Trinidad, J. Pulsed-DC powder-pack boriding: Growth kinetics of boride layers on an AISI 316L stainless steel and Inconel 718 superalloy. Surf. Coat. Technol. 2021, 421, 127404. [Google Scholar] [CrossRef]
- Alcantar-Martínez, L.M.; Ruiz-Trabolsi, P.A.; Tadeo-Rosas, R.; Miranda-Hernández, J.G.; Cabrera-Sierra, R.; Velázquez, J.C.; Hernández-Sánchez, E. Improving the Surface Properties of an API 5L Grade B Pipeline Steel by Applying the Boriding Process-Part II: On the Changes in the Mechanical Properties. Coating 2023, 13, 470. [Google Scholar] [CrossRef]
- Ding, H.Z.; Li, H.; Qiu, W.Q. Single-phase Fe2B layer prepared by variable electric field assisted pack-boriding at low temperature. Heat Treat. Met. 2024, 49, 195–199. [Google Scholar]
- Ocak-Araz, S.; Birden, A.; Bayca, U.B.O. Effect of Powder-Pack Boronizing on the Microhardness, Wear, and Corrosion Behaviors of AISI 304L Steel. J. Mater. Eng. Perform. 2024, 33, 166–172. [Google Scholar] [CrossRef]
- Cheng, J.; Xie, F.; Sun, L.; Zhu, L.; Pan, J. Characterization of alternating current field enhanced pack boriding for 45 carbon steel at low and medium temperatures. Acta Metall. Sin. 2014, 50, 1311–1318. [Google Scholar]
- Wang, X.J.; Xie, F.; Cheng, J.; Pan, J.W. Phase structure and property of boriding case of 45 steel by direct current field enhanced pack boronizing. Trans. Mater. Heat Treat. 2015, 36, 197–202. [Google Scholar]
- Li, C.M.; Shen, B.L.; Li, G.J.; Yang, C. Effect of boronizing temperature and time on microstructure and abrasion wear resistance of Cr12Mn2V2 high chromium cast iron. Surf. Coat. Technol. 2008, 202, 5882–5886. [Google Scholar] [CrossRef]
- Chen, F.S.; Wang, K.L. The kinetics and mechanism of multi-component diffusion on AISI 1045 steel. Surf. Coat. Technol. 1999, 115, 239–248. [Google Scholar] [CrossRef]
- Campos, I.; Bautista, O.; Ramírez, G.; Islas, M.; De La Parra, J.; Zúñiga, L. Effect of boron paste thickness on the growth kinetics of Fe2B boride layers during the boriding process. Appl. Surf. Sci. 2005, 243, 429–436. [Google Scholar] [CrossRef]
- Jiang, B.L.; Lei, T.Q. On the controlling factors in growth of boronizing layer and its kinetic analysis. Acta Metall. Sin. 1990, 27, A11–A15. [Google Scholar]
- Xie, F.; Cheng, J.; Zou, W.D.; Pan, J.W. Effects of Alternating Current Field on Growth of Boride Layer on Q215 Steel by Pack-boriding at 800 °C. Hot Work. Technol. 2019, 48, 88–90. [Google Scholar]
- Kondo, T.; Yasuhara, M.; Kuramoto, T.; Kodera, Y.; Ohyanagi, M.; Munir, Z.A. Effect of pulsed DC current on atomic diffusion of Nb-C diffusion couple. J. Mater. Sci. 2008, 43, 6400–6405. [Google Scholar] [CrossRef]
- Yang, H.; Wu, X.; Cao, G.; Yang, Z. Enhanced boronizing kinetics and high temperature wear resistance of H13 steel with boriding treatment assisted by air blast shot peening. Surf. Coat. Technol. 2016, 307, 506–516. [Google Scholar] [CrossRef]
- Genel, K. Boriding kinetics of H13 steel. Vacuum 2006, 80, 451–457. [Google Scholar] [CrossRef]
- Martini, C.; Palombarini, G.; Carbucicchio, M. Mechanism of thermochemical growth of iron borides on iron. J. Mater. Sci. 2004, 39, 933–937. [Google Scholar] [CrossRef]
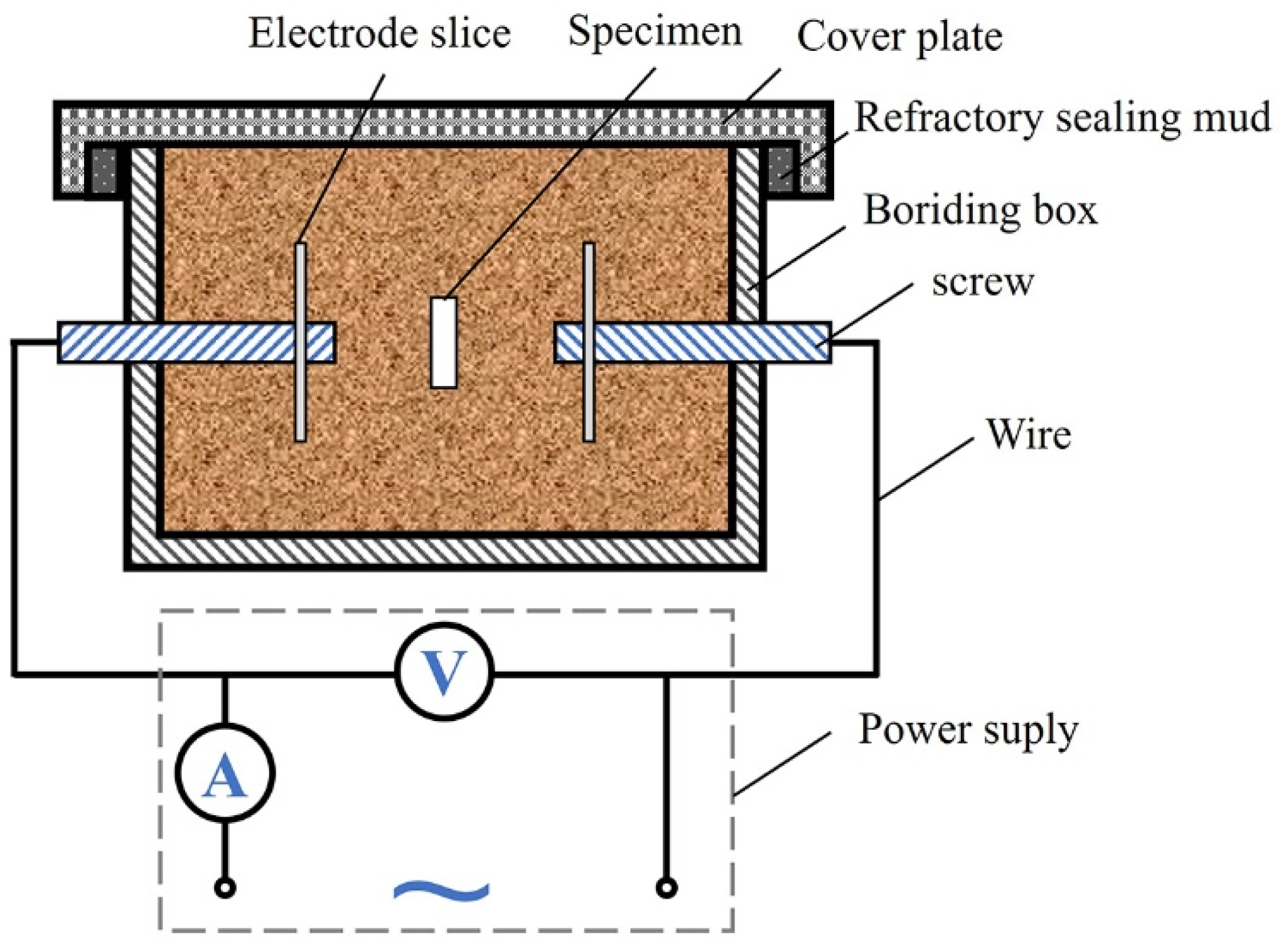

| C | Si | Mn | P | S | Cr | Mo | Fe |
|---|---|---|---|---|---|---|---|
| 0.42~0.49 | 0.15~0.35 | 0.65~1.1 | ≤0.035 | 0 ≤ 0.04 | 0.75~1.2 | 0.15~0.25 | Bal |
| Components | Percentage | Manufacturers |
|---|---|---|
| B4C | 5% | Hebei Tengshuang Metal Materials Co., Ltd. (Xingtai, China) |
| C | 2% | Guangdong Fangxin Biotechnology Co., Ltd. (Shaoguan, China) |
| KBF4 | 5% | Suichang Shenlonggu Charcoal Industry Co., Ltd. (Lishui, China) |
| SiC | 88% | Henan Kesheng Abrasive Materials Co., Ltd. (Zhengzhou, China) |
| Boriding Medium | Boriding Temperature /K | Boriding Time /h | The Current Intensity /A | |
|---|---|---|---|---|
| CPB | 5%B4C + 2%C + 5%KBF4 + 88%SiC | 1073 | 4 | / |
| ACFPB-1 | 1 | |||
| ACFPB-2 | 2 | |||
| ACFPB-3 | 3 | |||
| ACFPB-4 | 4 | |||
| ACFPB-5 | 5 | |||
| ACFPB-6 | 6 |
| The Current Intensity/A | Ecorr/V | icorr/A·cm−2 | Vcorr/mm·a−1 | |
|---|---|---|---|---|
| Bare 4145H | / | −1.13 | 1.21 × 10−3 | 14.20 |
| ACFPB-1 | 1 | −1.09 | 4.16 × 10−5 | 0.49 |
| ACFPB-2 | 2 | −1.02 | 6.02 × 10−5 | 0.71 |
| ACFPB-3 | 3 | −1.14 | 1.09 × 10−4 | 1.28 |
| ACFPB-4 | 4 | −1.11 | 8.10 × 10−5 | 0.95 |
| ACFPB-5 | 5 | −1.12 | 5.63 × 10−5 | 0.66 |
| ACFPB-6 | 6 | −1.04 | 4.84 × 10−5 | 0.57 |
| Boriding Medium | The Current Intensity/A | Boriding Temperature /K | Boriding Time /h |
|---|---|---|---|
| 5%B4C + 2%C + 5%KBF4 + 88%SiC | 4 | 1023, 1073, 1123 | 0 |
| 1 | |||
| 2 | |||
| 3 | |||
| 4 | |||
| 5 | |||
| 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Liu, W.; Yuan, J.; Yuan, J.; Zhou, X.; Pan, T.; Ren, Y. The Influence of an Alternating Current Field on Pack Boriding for Medium Carbon Steel at Moderate Temperature. Coatings 2025, 15, 39. https://doi.org/10.3390/coatings15010039
Li X, Liu W, Yuan J, Yuan J, Zhou X, Pan T, Ren Y. The Influence of an Alternating Current Field on Pack Boriding for Medium Carbon Steel at Moderate Temperature. Coatings. 2025; 15(1):39. https://doi.org/10.3390/coatings15010039
Chicago/Turabian StyleLi, Xiaoxiao, Wei Liu, Jianguang Yuan, Jiaye Yuan, Xiaobao Zhou, Taijun Pan, and Yanjie Ren. 2025. "The Influence of an Alternating Current Field on Pack Boriding for Medium Carbon Steel at Moderate Temperature" Coatings 15, no. 1: 39. https://doi.org/10.3390/coatings15010039
APA StyleLi, X., Liu, W., Yuan, J., Yuan, J., Zhou, X., Pan, T., & Ren, Y. (2025). The Influence of an Alternating Current Field on Pack Boriding for Medium Carbon Steel at Moderate Temperature. Coatings, 15(1), 39. https://doi.org/10.3390/coatings15010039






