Engineering g-C3N4/Bi2WO6 Composite Photocatalyst for Enhanced Photocatalytic CO2 Reduction
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
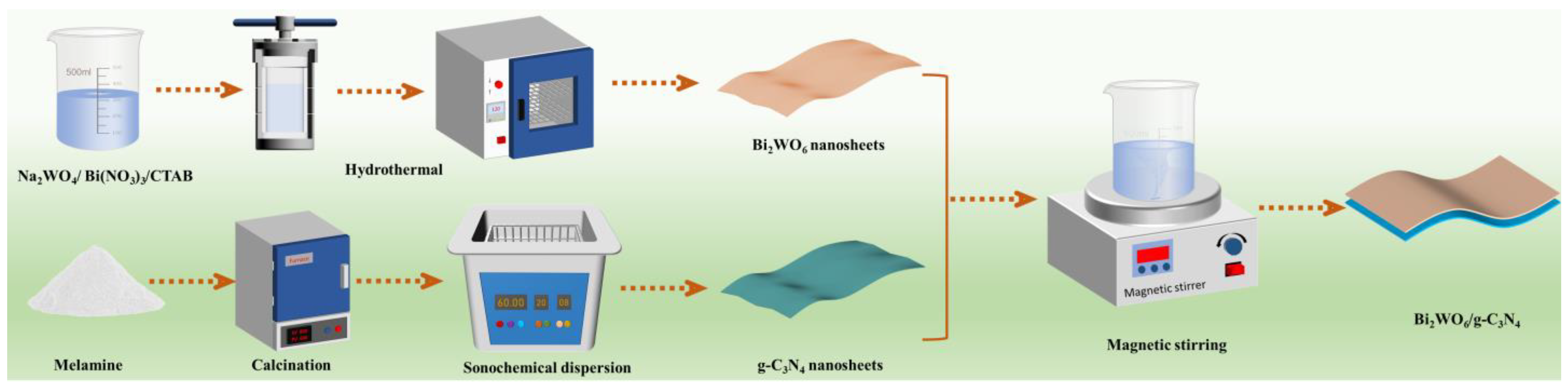
2.2. Synthesis of Bi2WO6
2.3. Synthesis of g-C3N4/Bi2WO6 Composite Photocatalyst
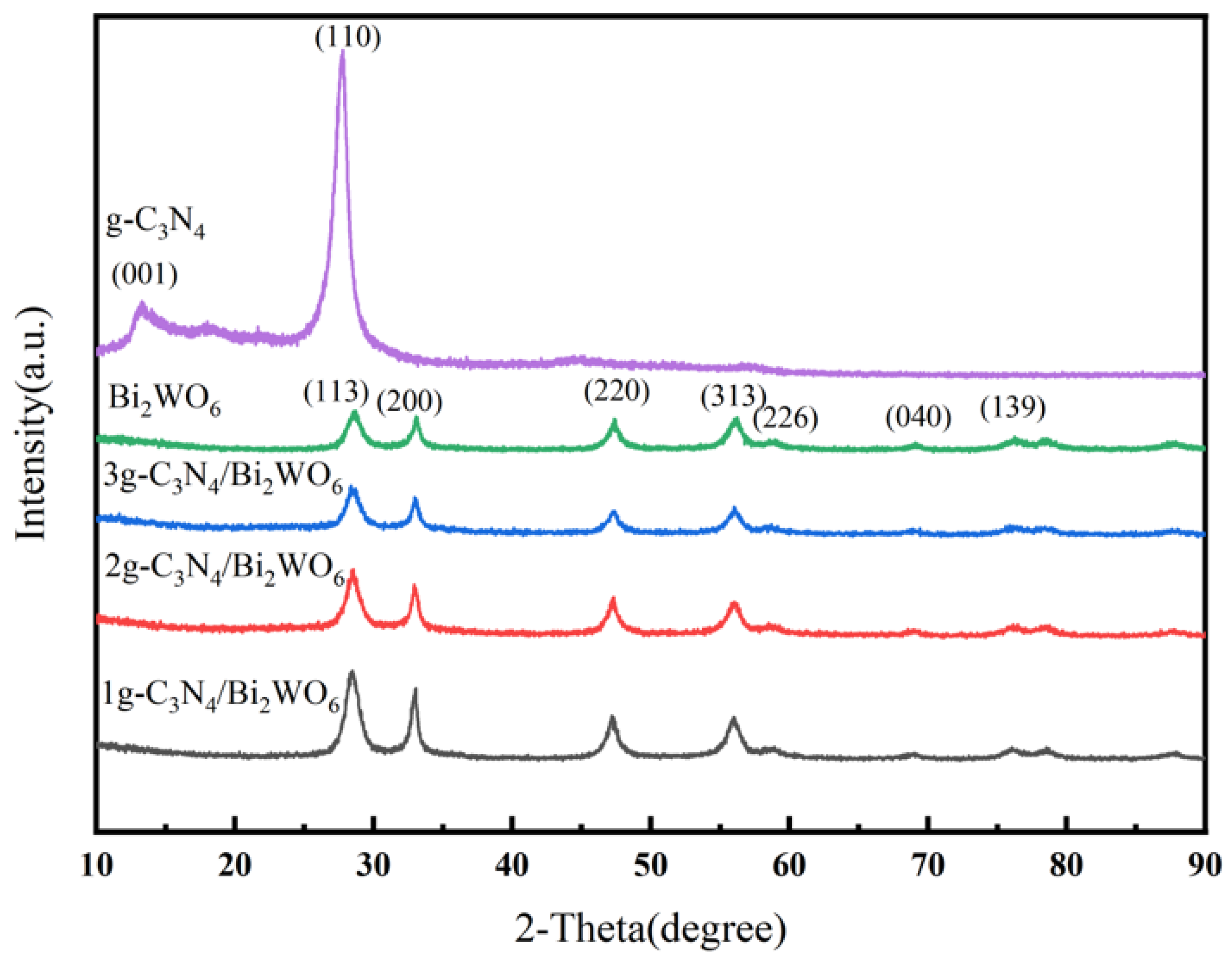
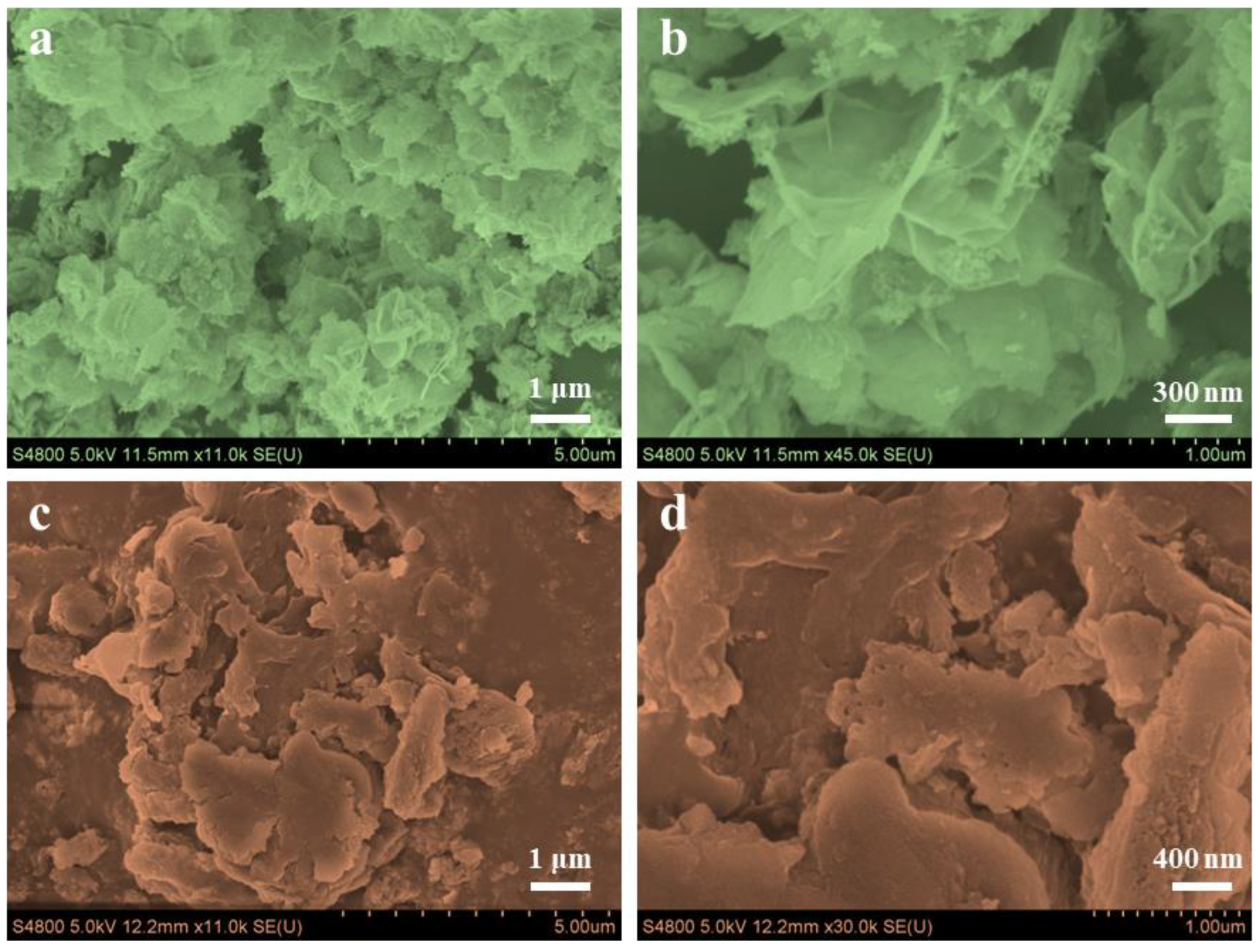
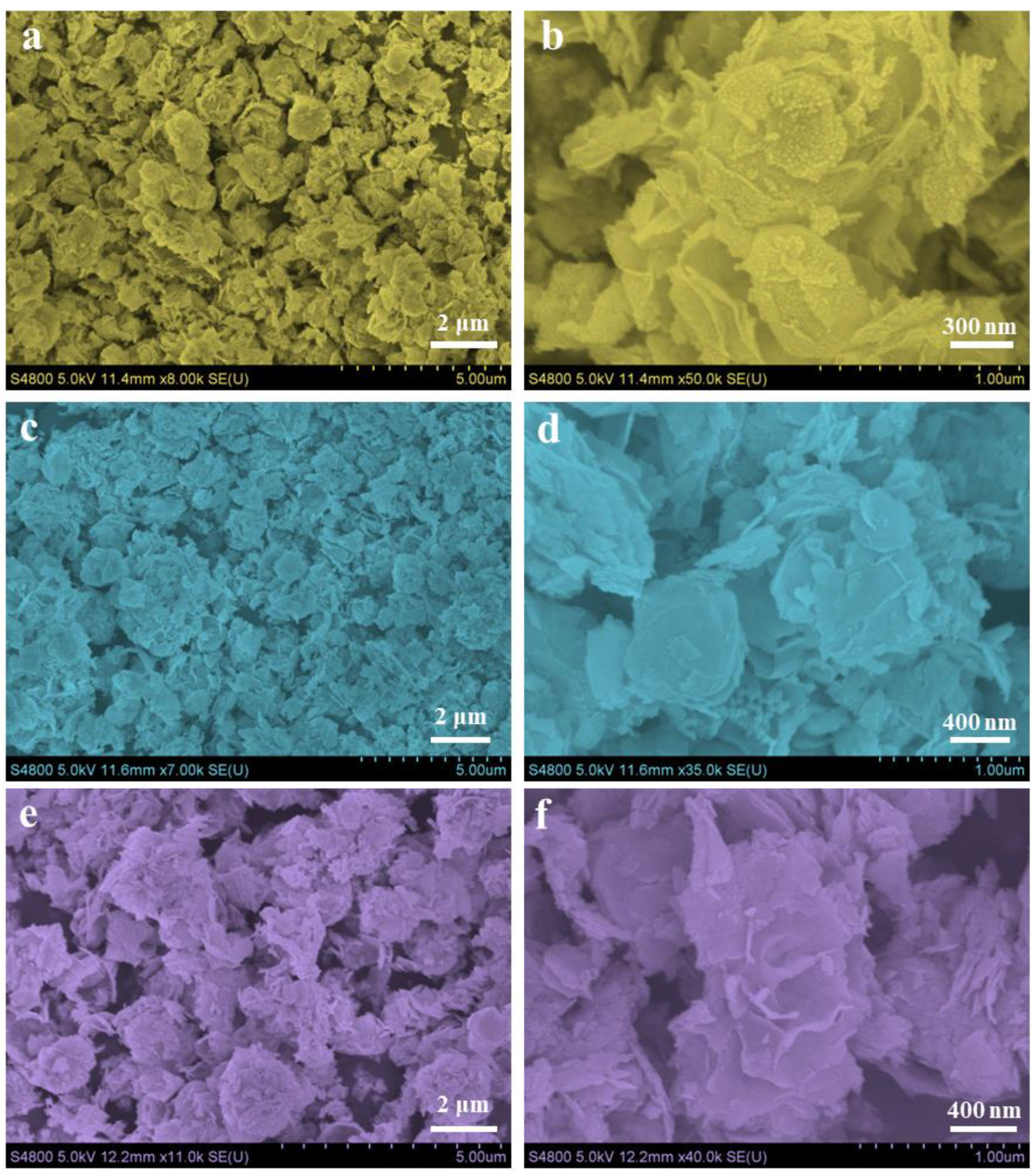
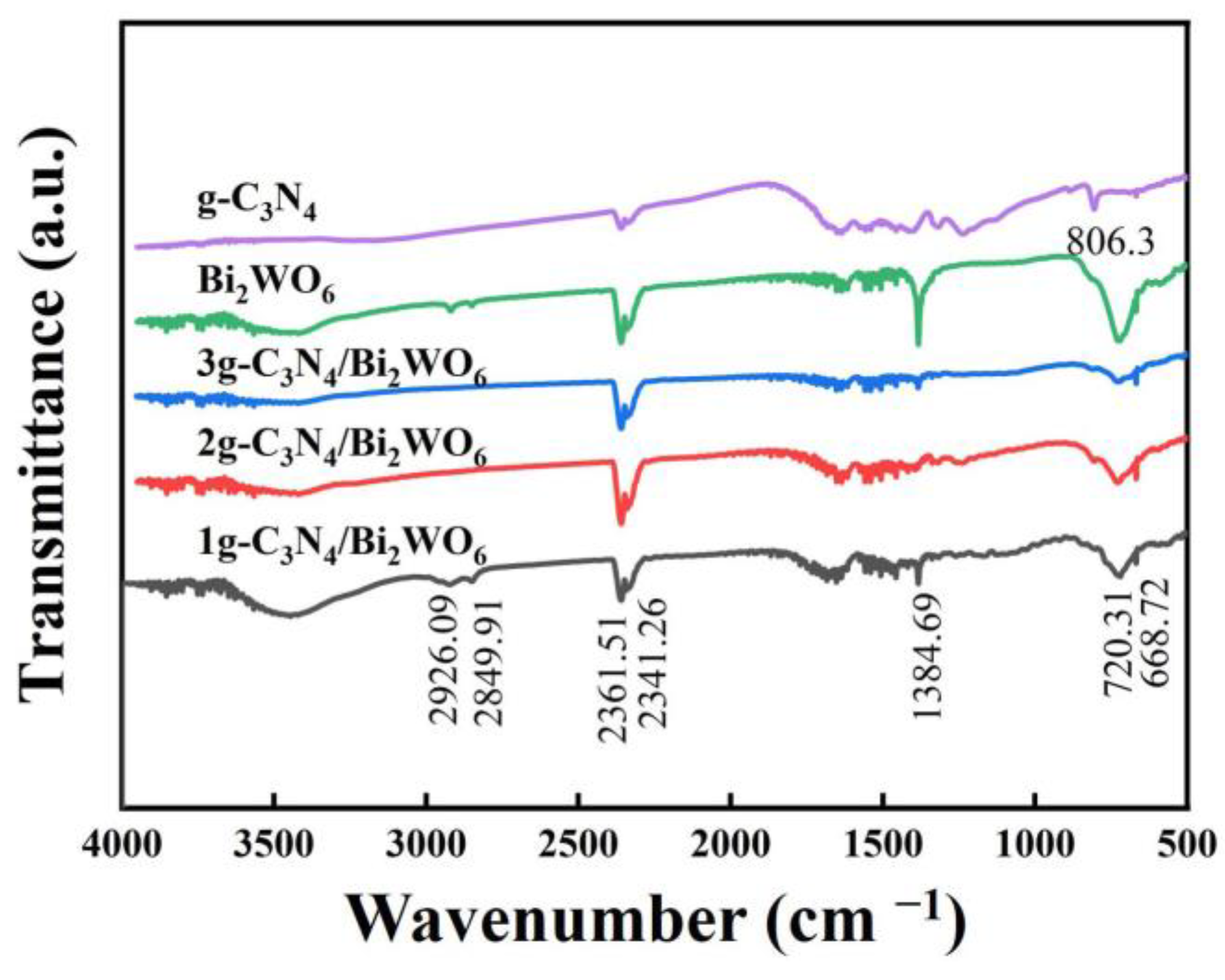
2.4. Characterization
2.5. Photocatalytic Experiments
2.6. Photoelectrochemical Testing
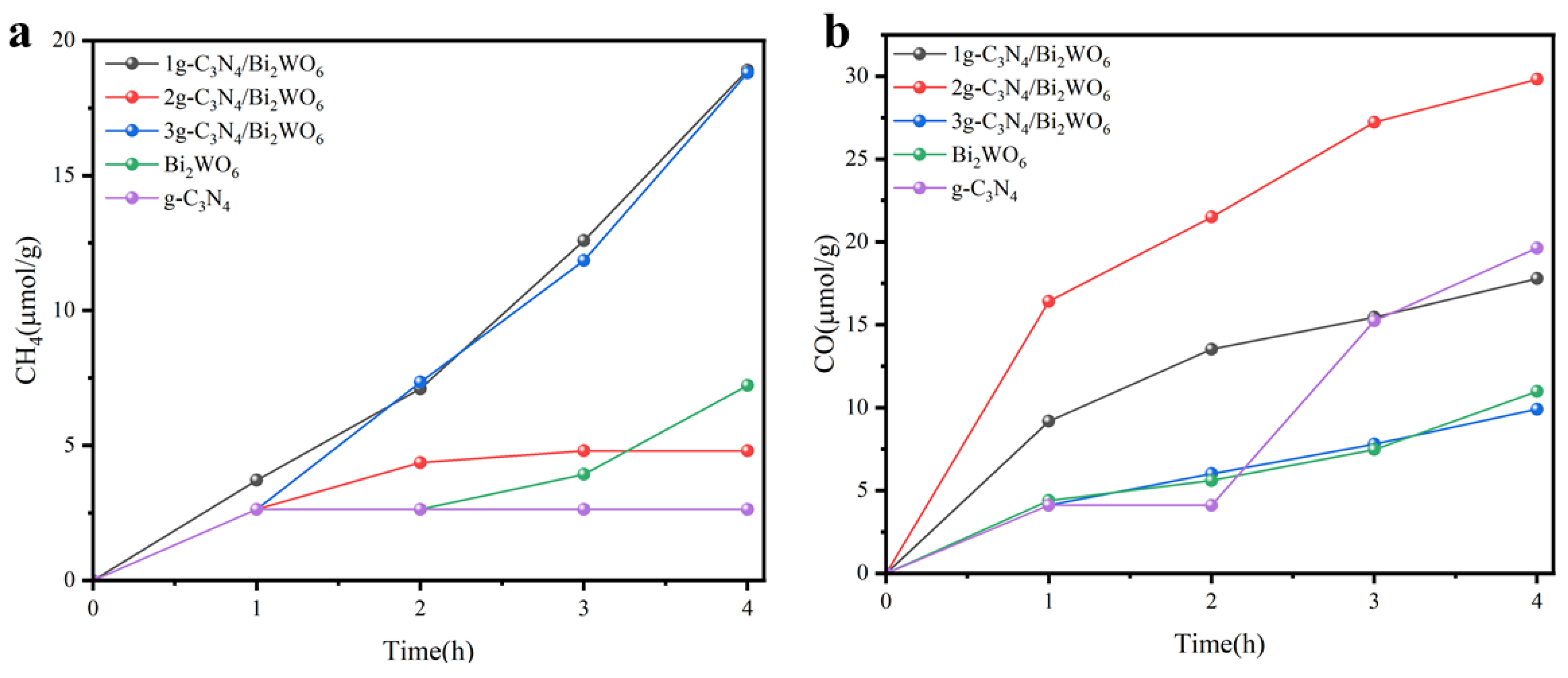
3. Results
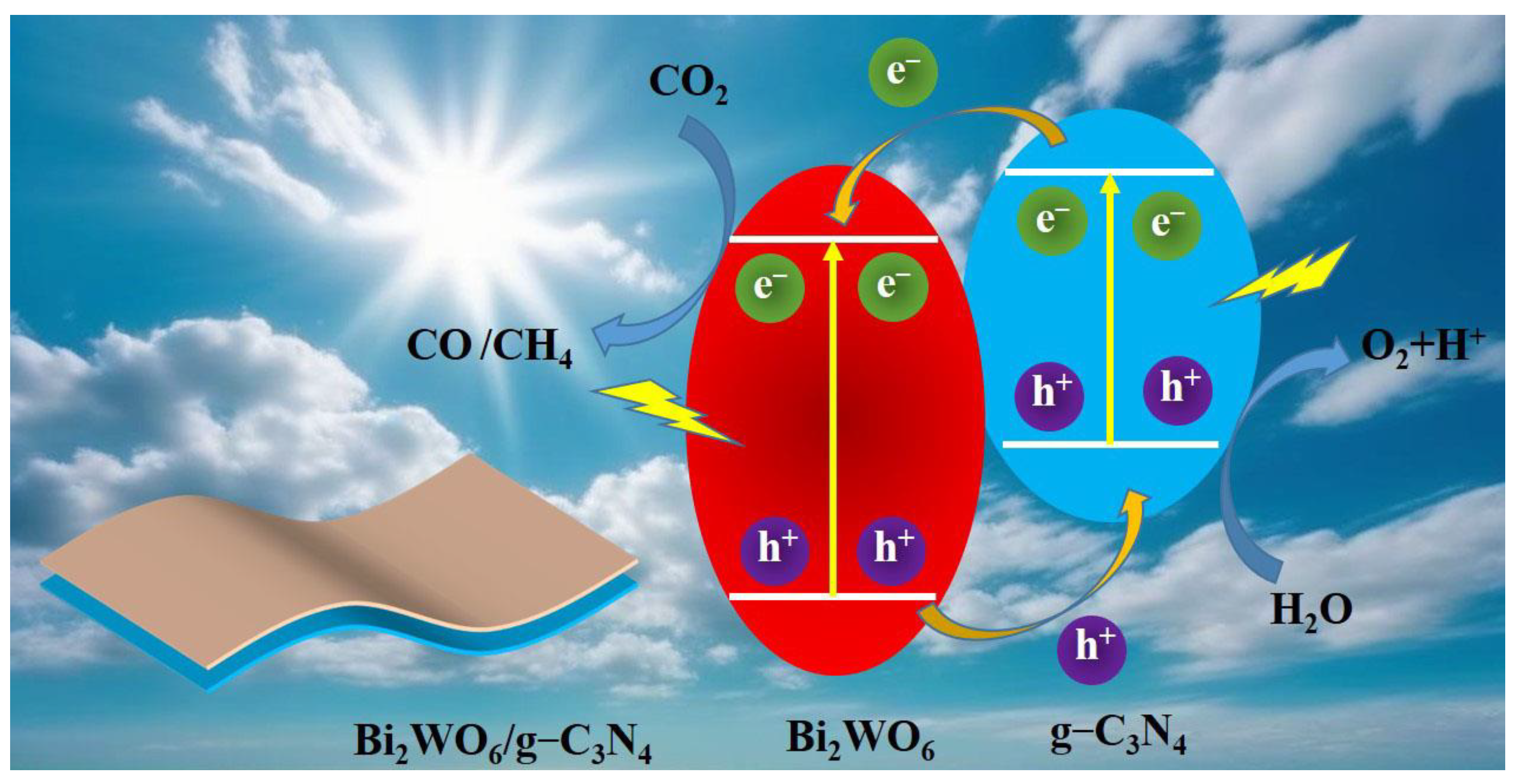
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Solomon, S.; Plattner, G.-K.; Knutti, R.; Friedlingstein, P. Irreversible climate change due to carbon dioxide emissions. Proc. Natl. Acad. Sci. USA 2009, 106, 1704–1709. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, T.; Kayani, U.N.; Gul, A.; Haider, S.A.; Ahmad, S. Carbon emissions, environmental distortions, and impact on growth. Energy Econ. 2023, 126, 107040. [Google Scholar] [CrossRef]
- Centi, G.; Quadrelli, E.A.; Perathoner, S. Catalysis for CO2 conversion: A key technology for rapid introduction of renewable energy in the value chain of chemical industries. Energy Environ. Sci. 2013, 6, 1711–1731. [Google Scholar] [CrossRef]
- Mustafa, A.; Lougou, B.G.; Shuai, Y.; Wang, Z.; Tan, H. Current technology development for CO2 utilization into solar fuels and chemicals: A review. J. Energy Chem. 2020, 49, 96–123. [Google Scholar] [CrossRef]
- Zhao, L.; Hou, H.; Wang, L.; Bowen, C.R.; Wang, J.; Yan, R.; Zhan, X.; Yang, H.; Yang, M.; Yang, W. Atomic-level surface modification of ultrathin Bi2WO6 nanosheets for boosting photocatalytic CO2 reduction. Chem. Eng. J. 2024, 480, 148033. [Google Scholar] [CrossRef]
- Hou, H.; Bowen, C.R.; Yang, D.; Yang, W. Cation-exchange-upgraded nanostructures for photocatalysts. Chem 2024, 10, 800–831. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, X.; Lee, A.F. Synthetic strategies to nanostructured photocatalysts for CO2 reduction to solar fuels and chemicals. J. Mater. Chem. A 2015, 3, 14487–14516. [Google Scholar] [CrossRef]
- Kumar, A.; Hasija, V.; Sudhaik, A.; Raizada, P.; Van Le, Q.; Singh, P.; Pham, T.-H.; Kim, T.; Ghotekar, S.; Nguyen, V.-H. Artificial leaf for light-driven CO2 reduction: Basic concepts, advanced structures and selective solar-to-chemical products. Chem. Eng. J. 2022, 430, 133031. [Google Scholar] [CrossRef]
- Huan, L.; Huitong, D.; Huanhuan, L.; Hua, W.; Wenlei, Z.; Yang, Z. Recent developments in metal nanocluster-based catalysts for improving photocatalytic CO2 reduction performance. Microstructures 2023, 3, 2023024. [Google Scholar]
- Schultz, D.M.; Yoon, T.P. Solar synthesis: Prospects in visible light photocatalysis. Science 2014, 343, 1239176. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Li, Q.; Xu, D. Recent progress in semiconductor-based nanocomposite photocatalysts for solar-to-chemical energy conversion. Adv. Energy Mater. 2017, 7, 1700529. [Google Scholar] [CrossRef]
- Jiao, X.; Zheng, K.; Liang, L.; Li, X.; Sun, Y.; Xie, Y. Fundamentals and challenges of ultrathin 2D photocatalysts in boosting CO2 photoreduction. Chem. Soc. Rev. 2020, 49, 6592–6604. [Google Scholar] [CrossRef]
- Liu, G.; Liu, S.; Lai, C.; Qin, L.; Zhang, M.; Li, Y.; Xu, M.; Ma, D.; Xu, F.; Liu, S. Strategies for enhancing the photocatalytic and electrocatalytic efficiency of covalent triazine frameworks for CO2 reduction. Small 2024, 20, 2307853. [Google Scholar] [CrossRef] [PubMed]
- Kaiyue, H.; Jiayu, T.; Zhifu, Z.; Daming, Z.; Xiangjiu, G. Direct Z-scheme photocatalytic systems based on vdW heterostructures for water splitting and CO2 reduction: Fundamentals and recent advances. Microstructures 2024, 4, 2024021. [Google Scholar]
- Ong, W.-J.; Tan, L.-L.; Ng, Y.H.; Yong, S.-T.; Chai, S.-P. Graphitic carbon nitride (g-C3N4)-based photocatalysts for artificial photosynthesis and environmental remediation: Are we a step closer to achieving sustainability? Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef] [PubMed]
- Muhmood, T.; Ahmad, I.; Haider, Z.; Haider, S.K.; Shahzadi, N.; Aftab, A.; Ahmed, S.; Ahmad, F. Graphene-like graphitic carbon nitride (g-C3N4) as a semiconductor photocatalyst: Properties, classification, and defects engineering approaches. Mater. Today Sustain. 2023, 25, 100633. [Google Scholar] [CrossRef]
- Li, Y.; Wang, L.; Gao, X.; Xue, Y.; Li, B.; Zhu, X. Construction of a graphitic carbon nitride-based photocatalyst with a strong built-in electric field via π–π stacking interactions boosting photocatalytic CO2 reduction. J. Mater. Chem. A 2024, 12, 7807–7816. [Google Scholar] [CrossRef]
- Bhanderi, D.; Lakhani, P.; Modi, C.K. Graphitic carbon nitride (g-C3N4) as an emerging photocatalyst for sustainable environmental applications: A comprehensive review. RSC Sustain. 2024, 2, 265–287. [Google Scholar] [CrossRef]
- Stefa, S.; Skliri, E.; Gagaoudakis, E.; Kiriakidis, G.; Kotzias, D.; Papagiannakopoulos, P.; Konsolakis, M.; Mao, S.; Binas, V. Visible light photocatalytic oxidation of NO using g-C3N4 nanosheets: Stability, kinetics, and effect of humidity. Appl. Phys. A 2024, 130, 451. [Google Scholar] [CrossRef]
- Xu, Q.; Xia, Z.; Zhang, J.; Wei, Z.; Guo, Q.; Jin, H.; Tang, H.; Li, S.; Pan, X.; Su, Z. Recent advances in solar-driven CO2 reduction over g-C3N4-based photocatalysts. Carbon Energy 2023, 5, e205. [Google Scholar] [CrossRef]
- He, B.; Cui, Y.; Lei, Y.; Li, W.; Sun, J. Design and application of g-C3N4-based materials for fuels photosynthesis from CO2 or H2O based on reaction pathway insights. J. Colloid Interface Sci. 2023, 629, 825–846. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Shao, G.; Yang, W. Recent advances in g-C3N4-based photocatalysts incorporated by MXenes and their derivatives. J. Mater. Chem. A 2021, 9, 13722–13745. [Google Scholar] [CrossRef]
- Song, X.-L.; Chen, L.; Gao, L.-J.; Ren, J.-T.; Yuan, Z.-Y. Engineering g-C3N4 based materials for advanced photocatalysis: Recent advances. Green Energy Environ. 2022, 42, 166–167. [Google Scholar] [CrossRef]
- Chen, T.; Liu, L.; Hu, C.; Huang, H. Recent advances on Bi2WO6-based photocatalysts for environmental and energy applications. Chinese J. Catal. 2021, 42, 1413–1438. [Google Scholar] [CrossRef]
- Li, B.; Liu, X.J.; Zhu, H.W.; Guan, H.P.; Guo, R.T. A Review on Bi2WO6-Based Materials for Photocatalytic CO2 Reduction. Small 2024, 20, 2406074. [Google Scholar] [CrossRef]
- Supriya, S. Tailoring layered structure of bismuth-based aurivillius perovskites: Recent advances and future aspects. Coordin. Chem. Rev. 2023, 479, 215010. [Google Scholar] [CrossRef]
- Belousov, A.; Parkhacheva, A.; Suleimanov, E.; Shafiq, I. Potential of Bi2WO6-based heterojunction photocatalysts for environmental remediation. Materials Today Chem. 2023, 32, 101633. [Google Scholar] [CrossRef]
- Mandyal, P.; Guleria, A.; Sharma, R.; Sambyal, S.; Priye, A.; Fang, B.; Shandilya, P. Insight into the properties, morphologies and photocatalytic applications of S-scheme Bi2WO6. J. Environ. Chem. Eng. 2022, 10, 108918. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, B.; He, H.; Yang, S.; Duan, X.; Wang, S. Bismuth-based complex oxides for photocatalytic applications in environmental remediation and water splitting: A review. Sci. Total Environ. 2022, 804, 150215. [Google Scholar] [CrossRef]
- Ling, G.Z.S.; Ng, S.F.; Ong, W.J. Tailor-engineered 2D cocatalysts: Harnessing electron–hole redox center of 2D g-C3N4 photocatalysts toward solar-to-chemical conversion and environmental purification. Adv. Funct. Mater. 2022, 32, 2111875. [Google Scholar] [CrossRef]
- Li, Y.; He, Z.; Liu, L.; Jiang, Y.; Ong, W.-J.; Duan, Y.; Ho, W.; Dong, F. Inside-and-out modification of graphitic carbon nitride (g-C3N4) photocatalysts via defect engineering for energy and environmental science. Nano Energy 2023, 105, 108032. [Google Scholar] [CrossRef]
- Ge, L.; Han, C.; Liu, J. Novel visible light-induced g-C3N4/Bi2WO6 composite photocatalysts for efficient degradation of methyl orange. Appl. Catal. B-Environ. 2011, 108, 100–107. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, Y.; Wang, D.; He, W.; Fang, X.; Zhao, C.; Pan, J.; Liu, D.; Liu, S.; Chen, T. Nanoengineering construction o g-C3N4/Bi2WO6 S-scheme heterojunctions for cooperative enhanced photocatalytic CO2 reduction and pollutant degradation. Sep. Purif. Technol. 2025, 354, 128893. [Google Scholar] [CrossRef]
- Yang, Z.; Zeng, H.; Xiong, J.; Peng, D.; Liu, S. F-doped Bi2WO6 with rich oxygen vacancies for boosting photo-oxidation/reduction activity. Colloid. Surface A 2023, 675, 132035. [Google Scholar] [CrossRef]
- Wang, J.; Tang, L.; Zeng, G.; Liu, Y.; Zhou, Y.; Deng, Y.; Wang, J.; Peng, B. Plasmonic Bi metal deposition and g-C3N4 coating on Bi2WO6 microspheres for efficient visible-light photocatalysis. ACS Sustain. Chem. Eng. 2017, 5, 1062–1072. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, W.; Luo, W.; Chen, X.; Zhu, Y. Ultrathin nanosheets g-C3N4@ Bi2WO6 core-shell structure via low temperature reassembled strategy to promote photocatalytic activity. Appl. Catal. B Environ. 2018, 237, 633–640. [Google Scholar] [CrossRef]
- Li, M.; Zhang, L.; Fan, X.; Zhou, Y.; Wu, M.; Shi, J. Highly selective CO2 photoreduction to CO over g-C3N4/Bi2WO6 composites under visible light. J. Mater. Chem. A 2015, 3, 5189–5196. [Google Scholar] [CrossRef]
- Shi, L.; Wang, T.; Zhang, H.; Chang, K.; Ye, J. Electrostatic self-assembly of nanosized carbon nitride nanosheet onto a zirconium metal–organic framework for enhanced photocatalytic CO2 reduction. Adv. Funct. Mater. 2015, 25, 5360–5367. [Google Scholar] [CrossRef]
- Huang, Q.; Yu, J.; Cao, S.; Cui, C.; Cheng, B. Efficient photocatalytic reduction of CO2 by amine-functionalized g-C3N4. Appl. Surf. Sci. 2015, 358, 350–355. [Google Scholar] [CrossRef]
- Wang, J.C.; Yao, H.C.; Fan, Z.Y.; Zhang, L.; Wang, J.S.; Zang, S.Q.; Li, Z.J. Indirect Z-scheme BiOI/g-C3N4 photocatalysts with enhanced photoreduction CO2 activity under visible light irradiation. ACS Appl. Mater. Interfaces. 2016, 8, 3765–3775. [Google Scholar] [CrossRef]
- Bai, Y.; Ye, L.; Wang, L.; Shi, X.; Wang, P.; Bai, W.; Wong, P.K. g-C3N4/Bi4O5I2 heterojunction with I3−/I− redox mediator for enhanced photocatalytic CO2 conversion. Appl. Catal. B 2016, 194, 98–104. [Google Scholar] [CrossRef]
- Jo, W.; Kumar, S.; Eslava, S.; Tonda, S. Construction of Bi2WO6/RGO/g-C3N4 2D/2D/2D hybrid Z-scheme heterojunctions with large interfacial contact area for efficient charge separation and high-performance photoreduction of CO2 and H2O into solar fuels. Appl. Catal. B. 2018, 239, 586–598. [Google Scholar] [CrossRef]
- Murugesan, P.; Narayanan, S.; Manickam, M. Experimental studies on photocatalytic reduction of CO2 using AgBr decorated g-C3N4 composite in TEA mediated system. J. CO2 Util. 2017, 22, 250–261. [Google Scholar] [CrossRef]
- Li, Z.; Kim, R.; Vollmer, S.; Subramanian, S.V. Direct Z-scheme WO3/graphitic carbon nitride nanocomposites for the photoreduction of CO2. ACS Appl. Nano. Mater. 2020, 3, 1298–1306. [Google Scholar] [CrossRef]
- Yang, M.; Zhan, X.-Q.; Ou, D.-L.; Wang, L.; Zhao, L.-L.; Yang, H.-L.; Liao, Z.-Y.; Yang, W.-Y.; Ma, G.-Z.; Hou, H.-L. Efficient visible-light-driven hydrogen production with Ag-doped flower-like ZnIn2S4 microspheres. Rare Met. 2024. [Google Scholar] [CrossRef]
- Yuan, X.; Shen, D.; Zhang, Q.; Zou, H.; Liu, Z.; Peng, F. Z-scheme Bi2WO6/CuBi2O4 heterojunction mediated by interfacial electric field for efficient visible-light photocatalytic degradation of tetracycline. Chem. Eng. J. 2019, 369, 292–301. [Google Scholar] [CrossRef]
- Zhan, X.; Ou, D.; Zheng, Y.; Li, B.; Xu, L.; Yang, H.; Yang, W.; Zhang, H.; Hou, H.; Yang, W. Boosted photocatalytic hydrogen production over two-dimensional/two-dimensional Ta3N5/ReS2 van der Waals heterojunctions. J. Colloid Interface Sci. 2023, 629, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Zhu, H.; Deng, L.; Yang, L.; Shen, Y.; Fan, Q.; Le, Z.; Xie, Z. Heterostructured g-C3N4/Bi2WO6 Composites as Highly Efficient Photocatalysts for Selective Atmospheric Oxidation of Thiols to Disulfides. ACS Appl. Mater. Interfaces 2024, 16, 56073–56081. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zou, R.; Chen, Z.; Tu, W.; Xia, R.; Iwuoha, E.I.; Peng, X. Engineering a Hydrophobic–Hydrophilic Diphase in a Bi2WO6–C3N4 Heterojunction for Solar-Powered CO2 Reduction. ACS Catal. 2024, 14, 138–147. [Google Scholar] [CrossRef]


| Title 1 | Light Source | Production | Ref. |
|---|---|---|---|
| g-C3N4/Bi2WO6 | 300 W Xe lamp with λ > 420 nm filter | CO: 5.19 μmol/g/h | [37] |
| g-C3N4@UIO66 | 400–800 nm | CO: 9.9 μmol/g/h | [38] |
| Amine-g-C3N4 | 300 W Xe lamp | CH4: 0.34 μmol/g/h | [39] |
| BiOI/g-C3N4 | 300 W Xe lamp | CO: 3.44 μmol/g/h | [40] |
| g-C3N4/Bi4O5I2 | 300 W Xe lamp with λ > 400 nm filter | CO: 45.6 μmol/g/h | [41] |
| Bi2WO6/RGO/ g-C3N4 | 300 W Xe lamp with λ > 420 nm filter | CH4: 2.51 μmol/g/h CO: 15.93 μmol/g/h | [42] |
| AgBr/g-C3N4 | UV light, 365 nm | CH4: 30.76 μmol/g/h | [43] |
| WO3/g-C3N4 | 8 W Hg Lamp | CO: 14.6 | [44] |
| g-C3N4/Bi2WO6 | 300 W Xe lamp | CH4: 18.90 μmol/g/h CO: 29.82 μmol/g/h | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.; Ni, L.; Ogino, K.; Sun, H.; Bi, J.; Hou, H. Engineering g-C3N4/Bi2WO6 Composite Photocatalyst for Enhanced Photocatalytic CO2 Reduction. Coatings 2025, 15, 32. https://doi.org/10.3390/coatings15010032
Chen W, Ni L, Ogino K, Sun H, Bi J, Hou H. Engineering g-C3N4/Bi2WO6 Composite Photocatalyst for Enhanced Photocatalytic CO2 Reduction. Coatings. 2025; 15(1):32. https://doi.org/10.3390/coatings15010032
Chicago/Turabian StyleChen, Wenxing, Lingzhe Ni, Kenji Ogino, Hong Sun, Jinghui Bi, and Huilin Hou. 2025. "Engineering g-C3N4/Bi2WO6 Composite Photocatalyst for Enhanced Photocatalytic CO2 Reduction" Coatings 15, no. 1: 32. https://doi.org/10.3390/coatings15010032
APA StyleChen, W., Ni, L., Ogino, K., Sun, H., Bi, J., & Hou, H. (2025). Engineering g-C3N4/Bi2WO6 Composite Photocatalyst for Enhanced Photocatalytic CO2 Reduction. Coatings, 15(1), 32. https://doi.org/10.3390/coatings15010032






