Efficient Photocatalytic Reduction of Hexavalent Chromium by NiCo2S4/BiOBr Heterogeneous Photocatalysts
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation
2.2. Characterizations
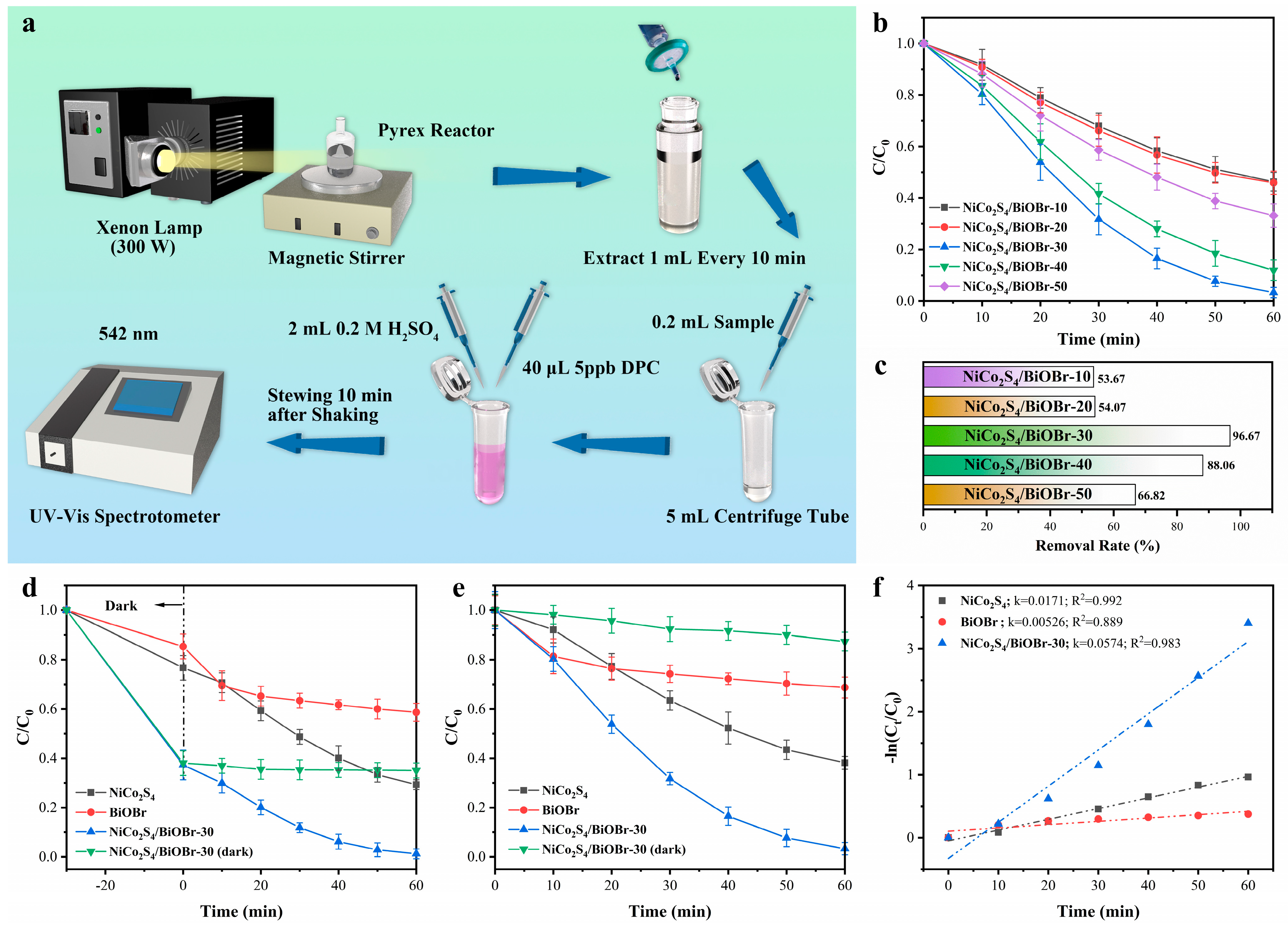
2.3. Photocatalytic Performances
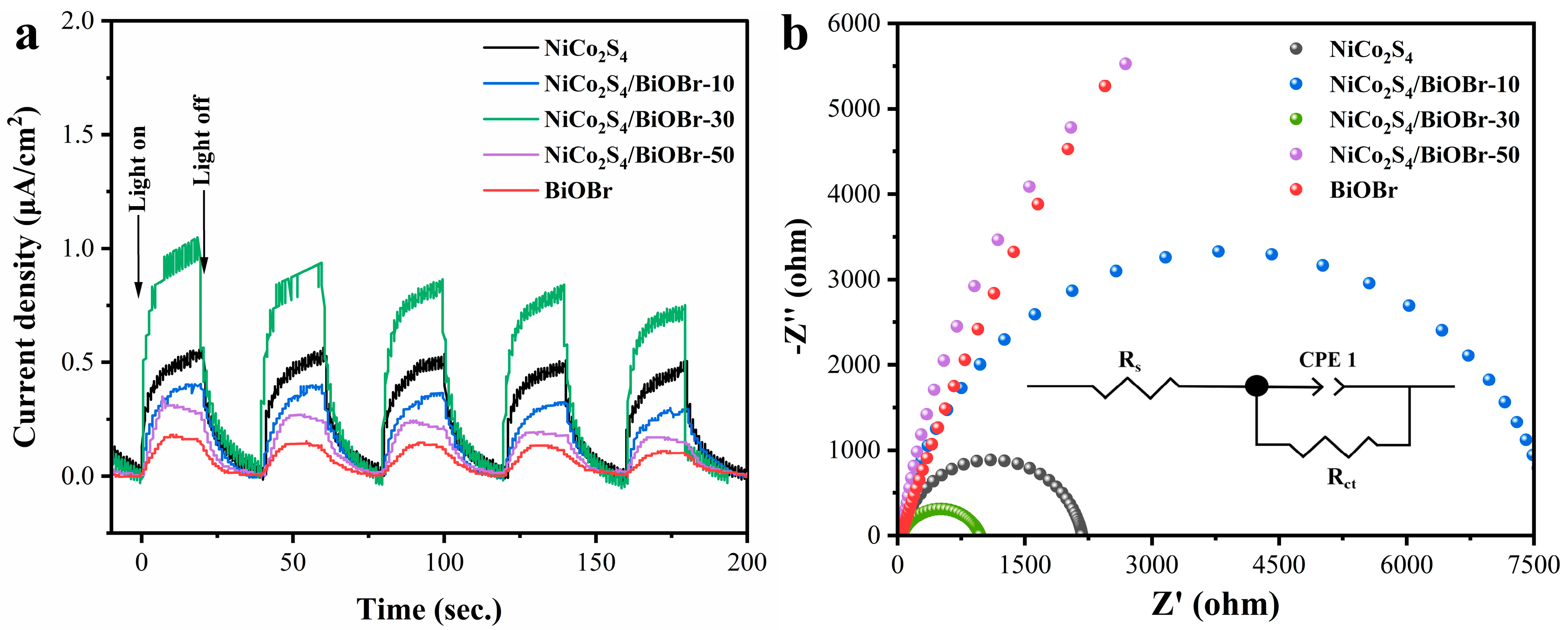
2.4. Electrochemical Measurements
2.5. Simulated Calculation Details
3. Results and Discussion
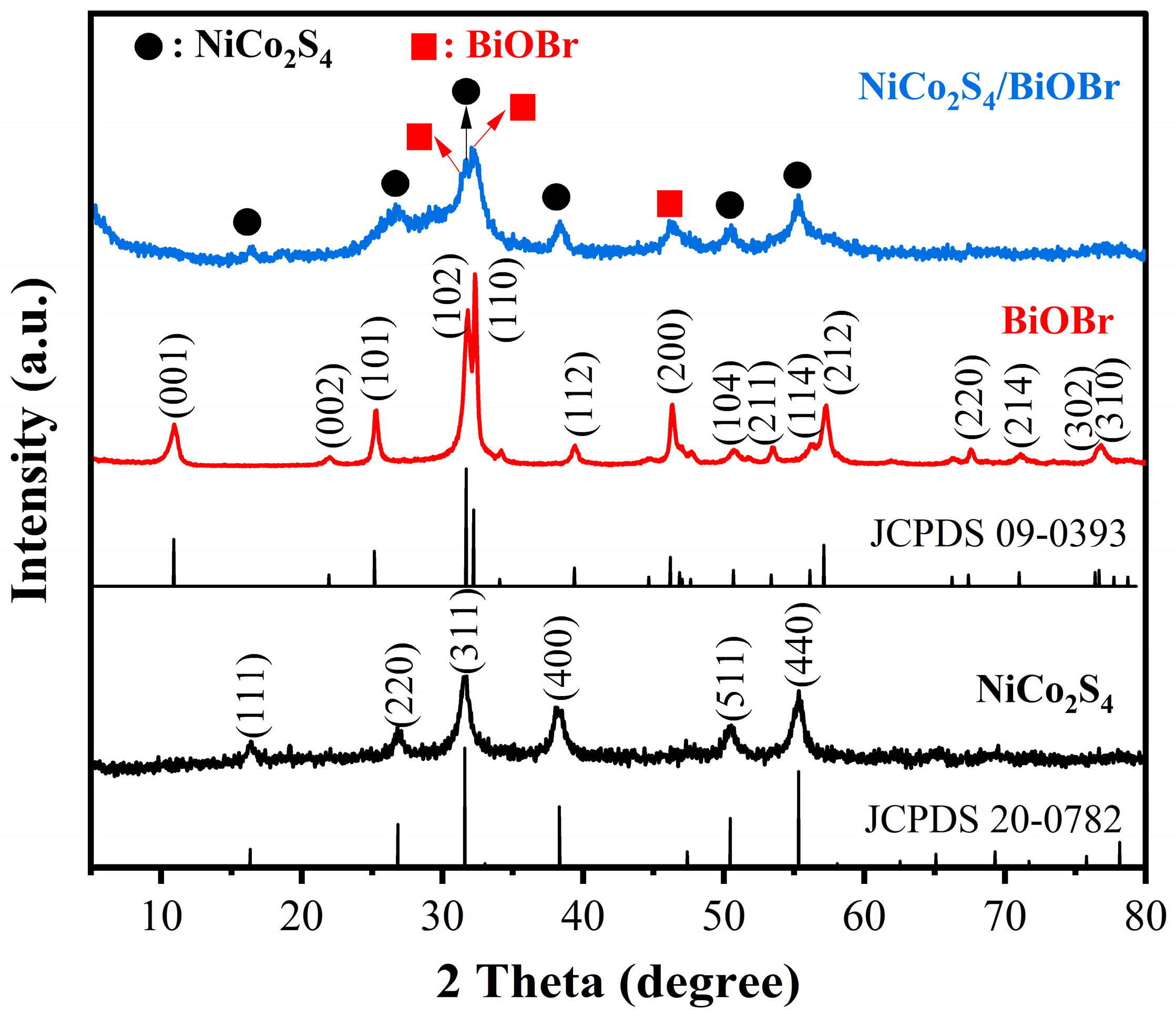
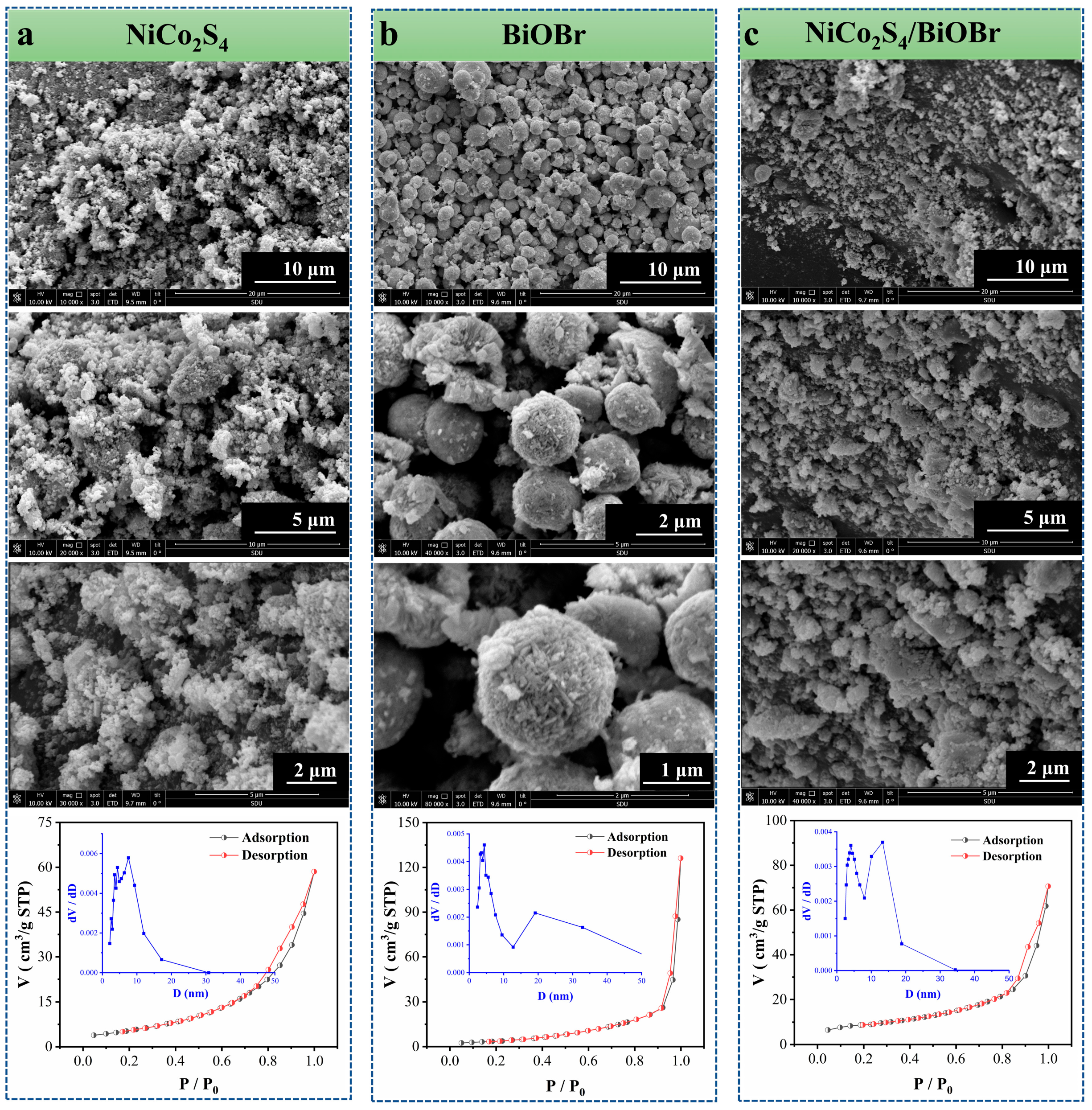
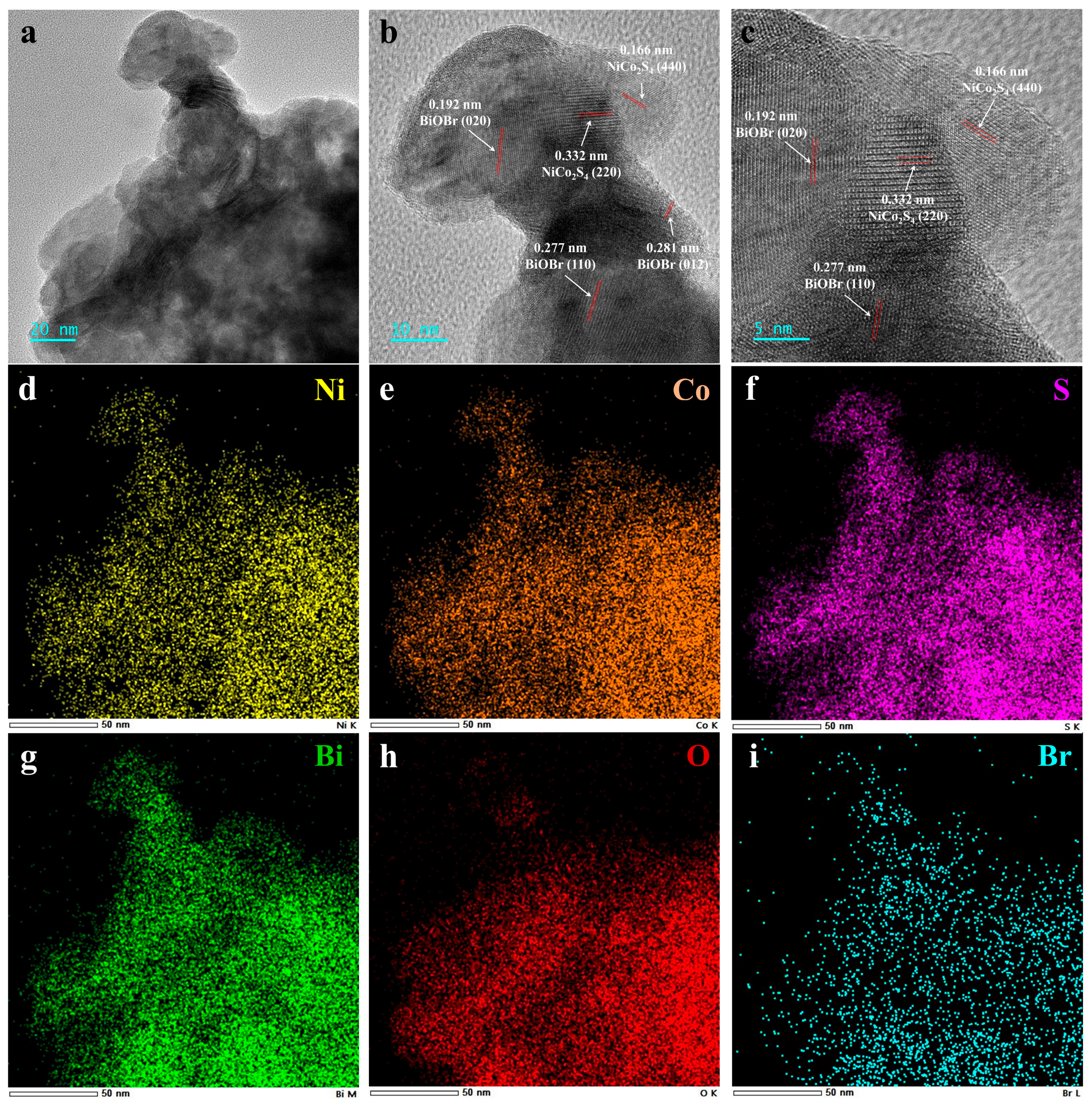
3.1. Structural Characterizations
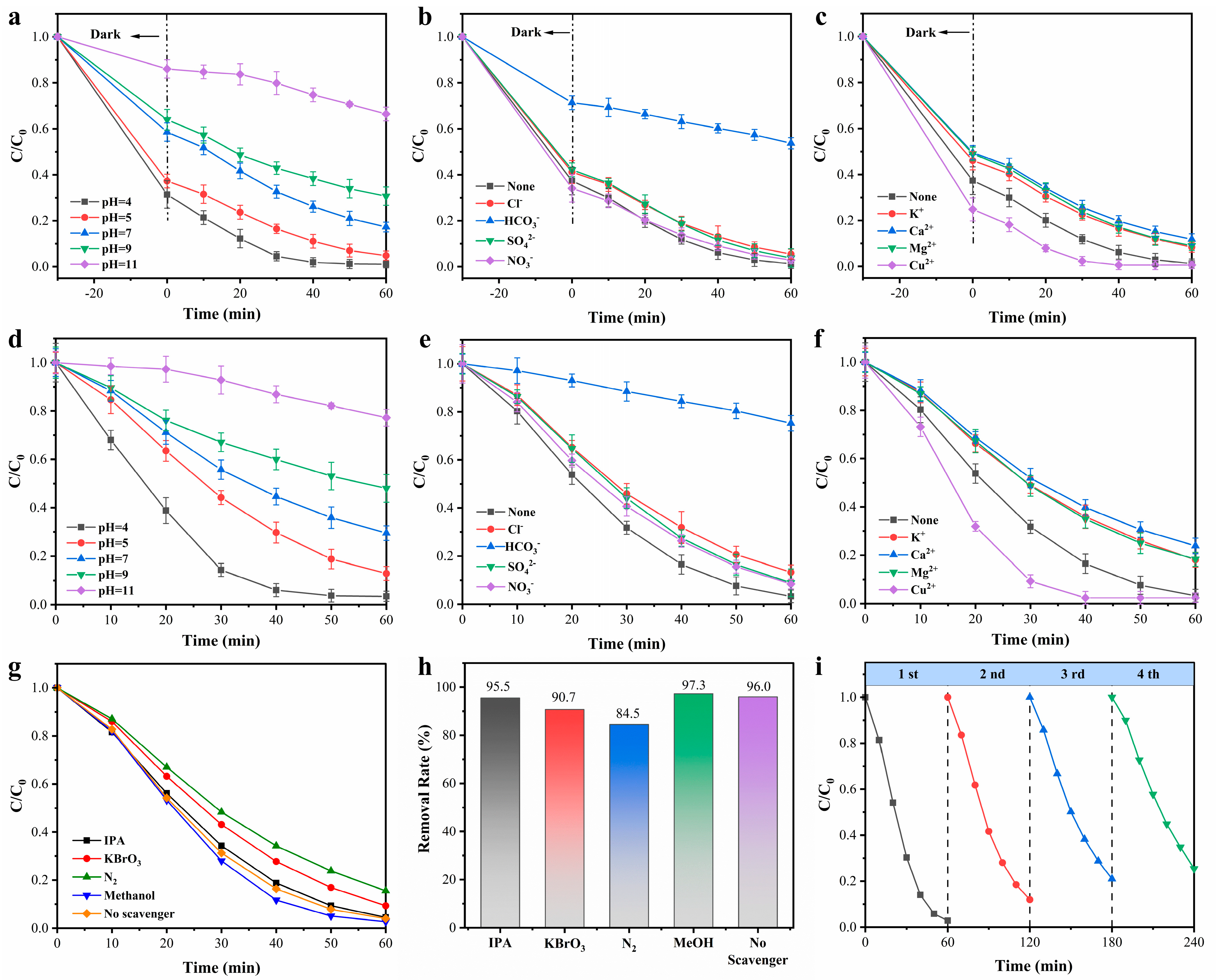
3.2. Photocatalytic Performances
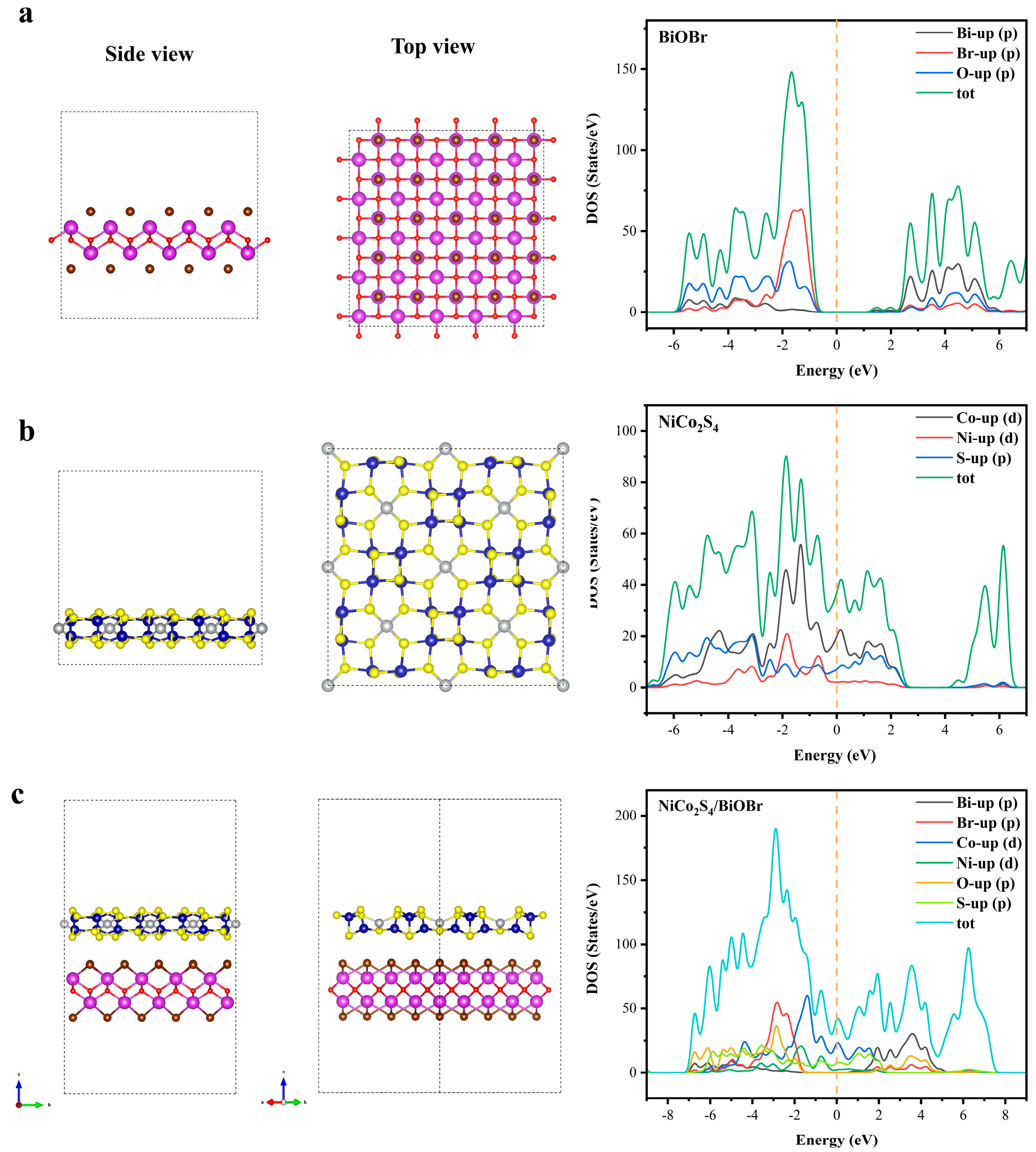
3.3. Enhanced Photocatalytic Cr(VI) Reduction Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Su, H.; Chen, H.; Wen, B.; Lu, L.; Zhang, D.; Wang, H. Chitosan-based fluorescent inverse opal particles for Cr(VI) sensing. NPJ Clean Water 2023, 6, 70. [Google Scholar] [CrossRef]
- Jin, Q.; Dai, M.; Zhan, X.; Wang, S.; He, Z. Carbon nanotubes and graphene composites used in Cr(VI) detection techniques: A review. J. Alloys Compd. 2022, 922, 166268. [Google Scholar] [CrossRef]
- Kapoor, R.T.; Bani Mfarrej, M.F.; Alam, P.; Rinklebe, J.; Ahmad, P. Accumulation of chromium in plants and its repercussion in animals and humans. Environ. Pollut. 2022, 301, 119044. [Google Scholar] [CrossRef] [PubMed]
- Vielee, S.T.; Buchanan, W.J.; Roof, S.H.; Kahloon, R.; Evans, E.; Isibor, J.; Patel, M.; Meaza, I.; Lu, H.; Williams, A.R.; et al. Chromium selectively accumulates in the rat hippocampus after 90 days of exposure to Cr(VI) in drinking water and induces age- and sex-dependent metal dyshomeostasis. Toxics 2024, 12, 722. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Xu, Z.; Xue, Y.; Li, F.; Bi, J.; Liu, J.; Wang, S.; Guo, X.; Zhang, P.; Yuan, J. Application prospect of Ion-imprinted polymers in harmless treatment of heavy metal wastewater. Molecules 2024, 29, 3160. [Google Scholar] [CrossRef]
- Minisy, I.M.; Taboubi, O.; Hromádková, J.; Bober, P. Aerogels of polypyrrole/tannic acid with nanofibrillated cellulose for the removal of hexavalent chromium ions. Gels 2024, 10, 415. [Google Scholar] [CrossRef]
- El Gaayda, J.; Rachid, Y.; Titchou, F.E.; Barra, I.; Hsini, A.; Yap, P.; Oh, W.; Swanson, C.; Hamdani, M.; Akbour, R.A. Optimizing removal of chromium (VI) ions from water by coagulation process using central composite design: Effectiveness of grape seed as a green coagulant. Sep. Purif. Technol. 2023, 307, 128805. [Google Scholar] [CrossRef]
- Zhang, X.; Zou, W.; Ding, L.; Chen, J. Polypyrrole confined in network of composite hydrogel as adsorbent for enhanced adsorption of Cr(VI) in aqueous solutions. Polym. Bull. 2023, 81, 6325–6348. [Google Scholar] [CrossRef]
- Xu, P.; Zhang, R.; Gong, J.; Luo, Y.; Zhuang, Y.; Zhang, P. S-scheme WO3/SnIn4S8 heterojunction for water purification: Enhanced photocatalytic performance and mechanism. Catalysts 2023, 13, 1450. [Google Scholar] [CrossRef]
- Jawed, A.; Golder, A.K.; Pandey, L.M. Bio-based iron oxide nanoparticles forming bi-functional chitosan composite adsorbent for Cr(VI) decontamination. Chem. Eng. J. 2024, 481, 148411. [Google Scholar] [CrossRef]
- Yu, Z.; Yang, Y.; Zhuang, H.; Shan, S.; Beldean-Galea, M.; Xue, Q.; Shen, X.; Li, S. In-situ growth of MIL-53 (Fe) on charcoal sponge as a highly efficient and recyclable photocatalyst for removal of Cr(VI). Rare Met. 2024, 43, 4344–4355. [Google Scholar] [CrossRef]
- Wang, C.; You, C.; Rong, K.; Shen, C.; Yang, F.; Li, S. An S-scheme MIL-101(Fe)-on-BiOCl heterostructure with oxygen vacancies for boosting photocatalytic removal of Cr(VI). Acta Phys.-Chim. Sin. 2024, 40, 2307045. [Google Scholar] [CrossRef]
- Tang, J.; Zeng, Q.; Chen, Z.; Huang, X. Electrochemical indirect measurement for the study of glutathione adsorption on multiwalled carbon nanotubes and activated carbon. Acta Phys.-Chim. Sin. 2012, 28, 1269–1274. [Google Scholar] [CrossRef]
- Qiu, K.; Li, W. Adsorption of hexavalent chromium ions on multi-wall carbon nanotubes in aqueous solution. Acta Phys.-Chim. Sin. 2006, 22, 1542–1546. [Google Scholar] [CrossRef]
- Zheng, Z.Y.; Tian, S.; Feng, Y.X.; Zhao, S.; Li, X.; Wang, S.G.; He, Z.L. Recent advances of photocatalytic coupling technologies for wastewater treatment. Chin. J. Catal. 2023, 54, 88–136. [Google Scholar] [CrossRef]
- Feng, H.; Yu, Y.; Jiang, S.; Shang, J.; Cheng, Y.; Wang, L.; Hao, W.; Wang, T. Synthesis of magnetic core-shell iron nanochains for potential applications in Cr(VI) ion pollution treatment. Rare Met. 2021, 40, 176–179. [Google Scholar] [CrossRef]
- Zhang, Q.; Cheng, Y.; Liu, W.; Che, H.; Ao, Y. Efficient piezo-assisted near-infrared-light-driven Cr(VI) reduction over Bi2S3 nanowires transformed from ultrathin Bi2WO6 nanosheets. Rare Met. 2024, 43, 4333–4343. [Google Scholar] [CrossRef]
- Xu, J.; Gu, H.; Chen, M.; Li, X.; Zhao, H.; Yang, H. Dual Z-scheme Bi2TaO7/Bi2S3/SnS2 photocatalyst with high performance for Cr(VI) reduction and TC degradation under visible light irradiation. Rare Met. 2022, 41, 2417–2428. [Google Scholar] [CrossRef]
- Hou, X.; Wang, Z.; Fang, C.; Cheng, Y.; Li, T. A facile synthesis of supported amorphous iron oxide with high performance for Cr(VI) removal from aqueous solution under visible light irradiation. J. Mater. Sci. Technol. 2019, 35, 323–329. [Google Scholar] [CrossRef]
- Pandey, K.; Saharan, B.S.; Kumar, R.; Jabborova, D.; Duhan, J.S. Modern-day green strategies for the removal of chromium from wastewater. J. Xenobiot. 2024, 14, 1670–1696. [Google Scholar] [CrossRef]
- Gao, Y.; Yang, X.; Lu, X.; Li, M.; Wang, L.; Wang, Y. Kinetics and mechanisms of Cr(VI) removal by nZVI: Influencing parameters and nodification. Catalysts 2022, 12, 999. [Google Scholar] [CrossRef]
- Tang, H.; Deng, S.; Chu, Z.; Shangguan, Y.; Liang, J.; Zheng, Q.; Wang, R.; Chen, H. Three-in-One: Coupling chemical reduction, photoreduction, and ion-exchange mechanisms in greigite/red soil-based analcime zeolite composites for Cr(VI) remediation in groundwater. ACS ES&T Eng. 2023, 4, 466–477. [Google Scholar] [CrossRef]
- Li, Z.; Dong, J.; Azi, F.; Feng, X.; Ge, Z.; Yang, S.; Sun, Y.; Guan, X.; Dong, M. Mechanism of Cr(VI) removal by polyphenols-rich bacterial cellulose gel produced from fermented wine pomace. NPJ Clean Water 2024, 7, 21. [Google Scholar] [CrossRef]
- Yuan, X.; Deng, S.; Fang, D.; Li, Y.; Yu, X.; Huang, L. Layer-by-layer adsorption behavior of Cr(VI) on MoS2@Mt induced by sulfamethoxazole. Sep. Purif. Technol. 2025, 354, 129261. [Google Scholar] [CrossRef]
- Zuo, J.; Li, W.; Xia, Z.; Zhao, T.; Tan, C.; Wang, Y.; Li, J. Preparation of modified biochar and its adsorption of Cr(VI) in aqueous solution. Coatings 2023, 13, 1884. [Google Scholar] [CrossRef]
- Sun, X.; Zeng, Q.; Feng, C. Adsorption kinetics of Chromium(VI) onto an anion exchange fiber containing polyamine. Acta Phys.-Chim. Sin. 2009, 25, 1951–1957. [Google Scholar] [CrossRef]
- Mi, B.; Wang, Y. Performance and mechanism of porous carbons derived from niomass as adsorbent for removal of Cr(VI). Processes 2024, 12, 2229. [Google Scholar] [CrossRef]
- Cao, D.; Zhou, Y.; Jiang, H.; Feng, X.; Liu, X.; Li, W.; Liu, J.; Tang, A.; Kong, D. Always positive covalent organic nanosheet enabling pH-independent adsorption and removal of Cr(VI). J. Hazard. Mater. 2024, 465, 133420. [Google Scholar] [CrossRef]
- Dong, L.; Liang, J.; Li, Y.; Hunang, S.; Wei, Y.; Bai, X.; Jin, Z.; Zhang, M.; Qu, J. Effect of coexisting ions on Cr(VI) adsorption onto surfactant modified Auricularia auricula spent substrate in aqueous solution. Ecotoxicol. Environ. Saf. 2018, 166, 390–400. [Google Scholar] [CrossRef]
- Zhou, H.; Chen, Y. Effect of acidic surface functional groups on Cr(VI) removal by activated carbon from aqueous solution. Rare Metals 2010, 29, 333–338. [Google Scholar] [CrossRef]
- Li, S.; Cai, M.; Wang, C.; Liu, Y.; Li, N.; Zhang, P.; Li, X. Rationally designed Ta3N5/BiOCl S-scheme heterojunction with oxygen vacancies for elimination of tetracycline antibiotic and Cr(VI): Performance, toxicity evaluation and mechanism insight. J. Mater. Sci. Technol. 2022, 123, 177–190. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Y.; Yuan, D.; Huang, W.; Ding, J.; Wan, H.; Dai, W.-L.; Guan, G. One step method of structure engineering porous graphitic carbon nitride for efficient visible-light photocatalytic reduction of Cr(VI). J. Mater. Sci. Technol. 2021, 71, 211–220. [Google Scholar] [CrossRef]
- Liu, J.; Li, Y.; Ke, J.; Wang, Z.; Xiao, H. Synergically improving light harvesting and charge transportation of TiO2 nanobelts by deposition of MoS2 for enhanced photocatalytic removal of Cr(VI). Catalysts 2017, 7, 7010030. [Google Scholar] [CrossRef]
- Xiao, L.; Zhang, J.; Lu, T.; Zhou, G.; Ren, Y.; Zheng, Z.; Yuan, X.; Wang, S.; He, Z. High-strength TiO2/TPU composite fiber based textiles for organic pollutant removal. NPJ Clean Water 2024, 7, 98. [Google Scholar] [CrossRef]
- Bi, H.; Yin, X.; He, J.; Song, H.; Lu, S.; Ma, Y.; Han, Z. Conjugated organic component-functionalized hourglass-type phosphomolybdates for visible-light photocatalytic Cr(VI) reduction in wide pH range. Rare Met. 2023, 42, 3638–3650. [Google Scholar] [CrossRef]
- Guo, M.; Wu, Z.; Zhang, M.; Huang, Z.; Zhang, K.; Wang, B.; Tu, J. Coupling interface constructions of FeOOH/NiCo2S4 by microwave-assisted method for efficient oxygen evolution reaction. Rare Met. 2023, 42, 1847–1857. [Google Scholar] [CrossRef]
- Ran, M.; Wang, M.; Hu, Z.; Huang, Y.; Wang, L.; Wu, L.; Yuan, M.; Zhang, J.; Li, B.; Van Tendeloo, G.; et al. A hollow core-shell TiO2/NiCo2S4 Z-Scheme heterojunction photocatalyst for efficient hydrogen evolution. J. Mater. Sci. Technol. 2025, 212, 182–191. [Google Scholar] [CrossRef]
- Xiong, Z.; Hou, Y.; Yuan, R.; Ding, Z.; Ong, W.; Wang, S. Hollow NiCo2S4 nanospheres as a cocatalyst to support ZnIn2S4 nanosheets for visible-light-driven hydrogen production. Acta Phys.-Chim. Sin. 2022, 38, 2111021. [Google Scholar] [CrossRef]
- Mushtaq, M.A.; Kumar, A.; Yasin, G.; Tabish, M.; Arif, M.; Ajmal, S.; Raza, W.; Naseem, S.; Zhao, J.; Li, P.; et al. Multivalent sulfur vacancy-rich NiCo2S4@MnO2 urchin-like heterostructures for ambient electrochemical N2 reduction to NH3. Small 2024, 20, e2310431. [Google Scholar] [CrossRef]
- Ni, C.; Wang, X.; Cai, X.; Yu, C.; Wu, Q.; Shen, Y.; Hao, C. Construction of core-shell heterostructures Co3S4@NiCo2S4 as cathode and covalent organic framework derived carbon as anode for hybrid supercapacitors. J. Mater. Sci. Technol. 2025, 210, 233–245. [Google Scholar] [CrossRef]
- Li, J.; Zhou, J.; Zhou, Q.; Wang, X.; Guo, C.; Li, M. Promoting the Na+-storage of NiCo2S4 hollow nanospheres by surfacing Ni–B nanoflakes. J. Mater. Sci. Technol. 2021, 82, 114–121. [Google Scholar] [CrossRef]
- Zhao, M.; Zhu, L.; Fu, B.; Jiang, S.; Zhou, Y.; Song, Y. Sodium ion storage performance of NiCo2S4 hexagonal nanosheets. Acta Phys.-Chim. Sin. 2019, 35, 193–199. [Google Scholar] [CrossRef]
- Jin, Q.; Zheng, Z.; Feng, Y.; Tian, S.; He, Z. Multi-walled carbon nanotubes modified NiCo2S4 for the efficient photocatalytic reduction of hexavalent chromium. C 2023, 9, 99. [Google Scholar] [CrossRef]
- Lu, J.; Gu, S.; Li, H.; Wang, Y.; Guo, M.; Zhou, G. Review on multi-dimensional assembled S-scheme heterojunction photocatalysts. J. Mater. Sci. Technol. 2023, 160, 214–239. [Google Scholar] [CrossRef]
- Wu, K.; Jiang, R.; Zhao, Y.; Mao, L.; Gu, X.; Cai, X.; Zhu, M. Hierarchical NiCo2S4/ZnIn2S4 heterostructured prisms: High-efficient photocatalysts for hydrogen production under visible-light. J. Colloid Interf. Sci. 2022, 619, 339–347. [Google Scholar] [CrossRef]
- Feng, Y.B.; Du, Y.; Du, M.X.; Li, Z.F.; He, Z.L.; Yang, K.; Lv, X.J.; Jiang, N.; Liu, Y. Facile constructing novel 3D porous g-C3N4/BiOBr0.2I0.8 hybrids: Efficient charge separation for visible-light photocatalysis. J. Alloys Compd. 2018, 767, 241–252. [Google Scholar] [CrossRef]
- López-Cuéllar, E.; Martínez-de la Cruz, A.; Morales-Ibarra, R.; Garza-Navarro, M.; Olivares-Cortez, J. Thin films of bismuth oxyhalides (BiOX, X = Cl, Br, I) deposited by thermal evaporation for the decontamination of water and air by photocatalysis. Catalysts 2024, 14, 716. [Google Scholar] [CrossRef]
- Xiong, J.; Zeng, H.; Xu, S.; Peng, J.; Liu, F.; Wang, L. Enhancing the intrinsic properties of flower-like BiOI by S-doping toward excellent photocatalytic performances. J. Mater. Sci. Technol. 2022, 118, 181–189. [Google Scholar] [CrossRef]
- Wang, C.; Liu, N.; Zhao, X.; Tian, Y.; Chen, X.; Zhang, Y.; Fan, L.; Hou, B. C-doped BiOCl/Bi2S3 heterojunction for highly efficient photoelectrochemical detection and photocatalytic reduction of Cr(VI). J. Mater. Sci. Technol. 2023, 164, 188–197. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Yuan, Y. S-scheme BiOBr/Bi2WO6 with oxygen vacancies for synergistic photodegradation and hydrogen generation: Mechanisms insight and DFT calculations. J. Alloys Compd. 2024, 995, 174755. [Google Scholar] [CrossRef]
- Hu, J.; Hong, C.; Zhao, C.; Si, Y.; Xing, Y.; Ling, W.; Zhang, B.; Li, Z.; Wang, Y.; Feng, L.; et al. Nitrogen self-doped hierarchical porous carbon via penicillin fermentation residue (PR) hydrothermal carbonization (HTC) and activation for supercapacitance. J. Alloys Compd. 2022, 918, 165452. [Google Scholar] [CrossRef]
- Li, M.; Ramachandran, R.; Sakthivel, T.; Wang, F.; Xu, Z. Siloxene: An advanced metal-free catalyst for efficient photocatalytic reduction of aqueous Cr(VI) under visible light. Chem. Eng. J. 2021, 421, 129728. [Google Scholar] [CrossRef]
- Wan, Z.; Mao, Q.; Xiang, J.; Ma, D.; Tang, H. Greatly increased visible-light photocatalytic activity of SnS2/carbon nanotube composite for Cr(VI) reduction: Insights into effects of solid acid structure. J. Mater. Sci. Technol. 2023, 161, 233–244. [Google Scholar] [CrossRef]
- Liu, C.; Xiao, W.; Liu, X.; Wang, Q.; Hu, J.; Zhang, S.; Xu, J.; Zhang, Q.; Zou, Z. Rationally designed Ti3C2 MXene/CaIn2S4 Schottky heterojunction for enhanced photocatalytic Cr(VI) reduction: Performance, influence factors and mechanism. J. Mater. Sci. Technol. 2023, 161, 123–135. [Google Scholar] [CrossRef]
- Ai, L.; Zha, M.; Cai, W.; Tan, C.; Guo, N.; Xu, M.; Leng, C.; Ma, Q.; Feng, L.; Zhou, B.; et al. Construction of freestanding BiOBr/CCFs composites: Enhanced carrier separation achieving efficient photocatalytic performance. Chem. Eng. Sci. 2024, 295, 120136. [Google Scholar] [CrossRef]
- Li, D.; Gong, Y.; Pan, C. Facile synthesis of hybrid CNTs/NiCo2S4 composite for high performance supercapacitors. Sci. Rep. 2016, 6, 29788. [Google Scholar] [CrossRef]
- Shuai, C.; Mo, Z.; Niu, X.; Yang, X.; Liu, G.; Wang, J.; Liu, N.; Guo, R. Hierarchical NiCo2S4 nanosheets grown on graphene to catalyze the oxygen evolution reaction. J. Mater. Sci. 2019, 55, 1627–1636. [Google Scholar] [CrossRef]
- Guan, H.; Dong, Y.; Kang, X.; Han, Y.; Cheng, Z.; Han, L.; Xie, L.; Chen, W.; Zhang, J. Extraordinary electrochemical performance of lithium-sulfur battery with 2D ultrathin BiOBr/rGO sheet as an efficient sulfur host. J. Colloid Interf. Sci. 2022, 626, 374–383. [Google Scholar] [CrossRef]
- Sun, W.; Liu, J.; Ran, F.; Li, N.; Li, Z.; Li, Y.; Wang, K. Step-scheme CsPbBr3/BiOBr photocatalyst with oxygen vacancies for efficient CO2 photoreduction. Dalton Trans. 2024, 53, 14018–14027. [Google Scholar] [CrossRef]
- Qin, W.; Yang, Q.; Ye, H.; Xie, Y.; Shen, Z.; Guo, Y.; Deng, Y.; Ling, Y.; Yu, J.; Luo, G.; et al. Novel 2D/2D BiOBr/Zn(OH)2 photocatalysts for efficient photoreduction CO2. Sep. Purif. Technol. 2023, 306, 122721. [Google Scholar] [CrossRef]
- Zhang, G.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. Fabrication of Bi2MoO6/ZnO hierarchical heterostructures with enhanced visible-light photocatalytic activity. Appl. Catal. B-Environ. 2019, 250, 313–324. [Google Scholar] [CrossRef]
- Wu, M.; Xu, N.; Chen, B.; Yang, J.; Shen, M.; Li, Q.; Li, M.; Liu, W.; Lian, J.; Wang, R. Photocatalytic reduction of Cr(VI) by AgIn5S8/ZnIn2S4 heterojunction under visible light: Experimental and density functional theory study. J. Environ. Chem. Eng. 2024, 12, 112880. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.; Xu, Z.; Su, H.; Bian, X.; Zhang, S.; Dong, X.; Zeng, L.; Zeng, T.; Feng, M.; et al. Carbon quantum dots implanted CdS nanosheets: Efficient visible-light-driven photocatalytic reduction of Cr(VI) under saline conditions. Appl. Catal. B-Environ. 2020, 262, 118306. [Google Scholar] [CrossRef]
- Szabó, M.; Kalmár, J.; Ditrói, T.; Bellér, G.; Lente, G.; Simic, N.; Fábián, I. Equilibria and kinetics of chromium(VI) speciation in aqueous solution—A comprehensive study from pH 2 to 11. Inorg. Chim. Acta 2018, 472, 295–301. [Google Scholar] [CrossRef]
- Li, W.; Chai, L.; Du, B.; Chen, X.; Sun, R. Full-Lignin-Based adsorbent for removal of Cr(VI) from waste water. Sep. Purif. Technol. 2023, 306, 122644. [Google Scholar] [CrossRef]
- Islam, J.B.; Furukawa, M.; Tateishi, I.; Kawakami, S.; Katsumata, H.; Kaneco, S. Enhanced photocatalytic reduction of toxic Cr(VI) with Cu modified ZnO nanoparticles in presence of EDTA under UV illumination. SN Appl. Sci. 2019, 1, 1282. [Google Scholar] [CrossRef]
- Dai, M.; Yu, H.; Chen, W.; Qu, K.; Zhai, D.; Liu, C.; Zhao, S.; Wang, S.; He, Z. Boosting photocatalytic activity of CdLa2S4/ZnIn2S4 S-scheme heterojunctions with spatial separation of photoexcited carries. Chem. Eng. J. 2023, 470, 144240. [Google Scholar] [CrossRef]
- Dai, M.; He, Z.; Cao, W.; Zhang, J.; Chen, W.; Jin, Q.; Que, W.; Wang, S. Rational construction of S-scheme BN/MXene/ZnIn2S4 heterojunction with interface engineering for efficient photocatalytic hydrogen production and chlorophenols degradation. Sep. Purif. Technol. 2023, 309, 123004. [Google Scholar] [CrossRef]
- Xiao, L.; Zhang, S.; Cui, H.; Chang, J.; Feng, Y.; Wang, S.; He, Z. Promoted photocatalytic performances over Ti3+-B co-doped TiO2/BN with high carrier transfer and absorption capabilities driven by SWCNT addition. Mater. Sci. Semicond. Process. 2024, 177, 108364. [Google Scholar] [CrossRef]
- Chen, W.; Dai, M.; Xiang, L.; Zhao, S.; Wang, S.; He, Z. Assembling S-scheme heterojunction between basic bismuth nitrate and bismuth tungstate with promoting charges’ separation for accelerated photocatalytic sulfamethazine degradation. J. Mater. Sci. Technol. 2024, 171, 185–197. [Google Scholar] [CrossRef]
- Jia, H.; He, W.; Zhang, B.; Yao, L.; Yang, X.; Zheng, Z. Facile synthesis of bismuth oxyhalide nanosheet films with distinct conduction type and photo-induced charge carrier behavior. Appl. Surf. Sci. 2018, 441, 832–840. [Google Scholar] [CrossRef]
- Zheng, Z.; Du, T.; Chen, P.; Yue, Q.; Wang, H.; Zhou, L.; Wang, Y. 2D/1D nested hollow porous ZnIn2S4/g-C3N4 heterojunction based on morphology modulation for photocatalytic CO2 reduction. J. Environ. Chem. Eng. 2024, 12, 112971. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, S.; Xu, R.; Jin, Q.; Wang, S.; Ren, Y.; Huang, Y.; Zheng, Z.; Xiao, L.; Zhai, D.; Wang, S.; et al. Efficient Photocatalytic Reduction of Hexavalent Chromium by NiCo2S4/BiOBr Heterogeneous Photocatalysts. Coatings 2024, 14, 1492. https://doi.org/10.3390/coatings14121492
Qin S, Xu R, Jin Q, Wang S, Ren Y, Huang Y, Zheng Z, Xiao L, Zhai D, Wang S, et al. Efficient Photocatalytic Reduction of Hexavalent Chromium by NiCo2S4/BiOBr Heterogeneous Photocatalysts. Coatings. 2024; 14(12):1492. https://doi.org/10.3390/coatings14121492
Chicago/Turabian StyleQin, Shumeng, Ruofan Xu, Qiu Jin, Sen Wang, Yi Ren, Yulin Huang, Ziye Zheng, Lihui Xiao, Dong Zhai, Shuguang Wang, and et al. 2024. "Efficient Photocatalytic Reduction of Hexavalent Chromium by NiCo2S4/BiOBr Heterogeneous Photocatalysts" Coatings 14, no. 12: 1492. https://doi.org/10.3390/coatings14121492
APA StyleQin, S., Xu, R., Jin, Q., Wang, S., Ren, Y., Huang, Y., Zheng, Z., Xiao, L., Zhai, D., Wang, S., & He, Z. (2024). Efficient Photocatalytic Reduction of Hexavalent Chromium by NiCo2S4/BiOBr Heterogeneous Photocatalysts. Coatings, 14(12), 1492. https://doi.org/10.3390/coatings14121492







