Thermoelastic Vibration of Nickel Film Irradiated by Femtosecond Laser: Molecular Dynamics Study
Abstract
:1. Introduction
2. Computational Models
3. Results and Discussion
3.1. Temperature Evolution and Laser-Induced Stress
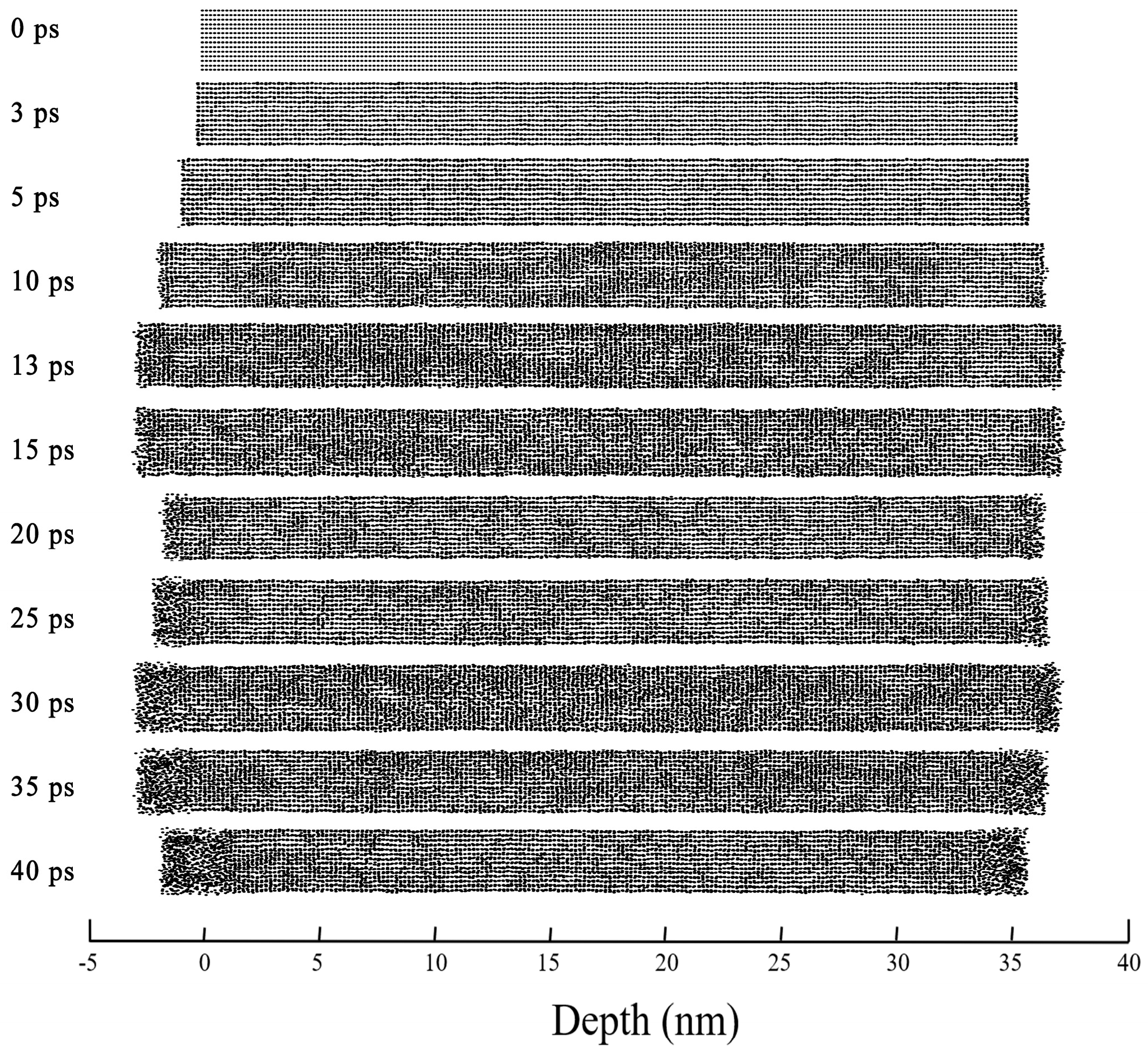
3.2. Thermoelastic Vibration and Atomic Structure Transformation
3.3. The Mechanism of Thermoelastic Vibration
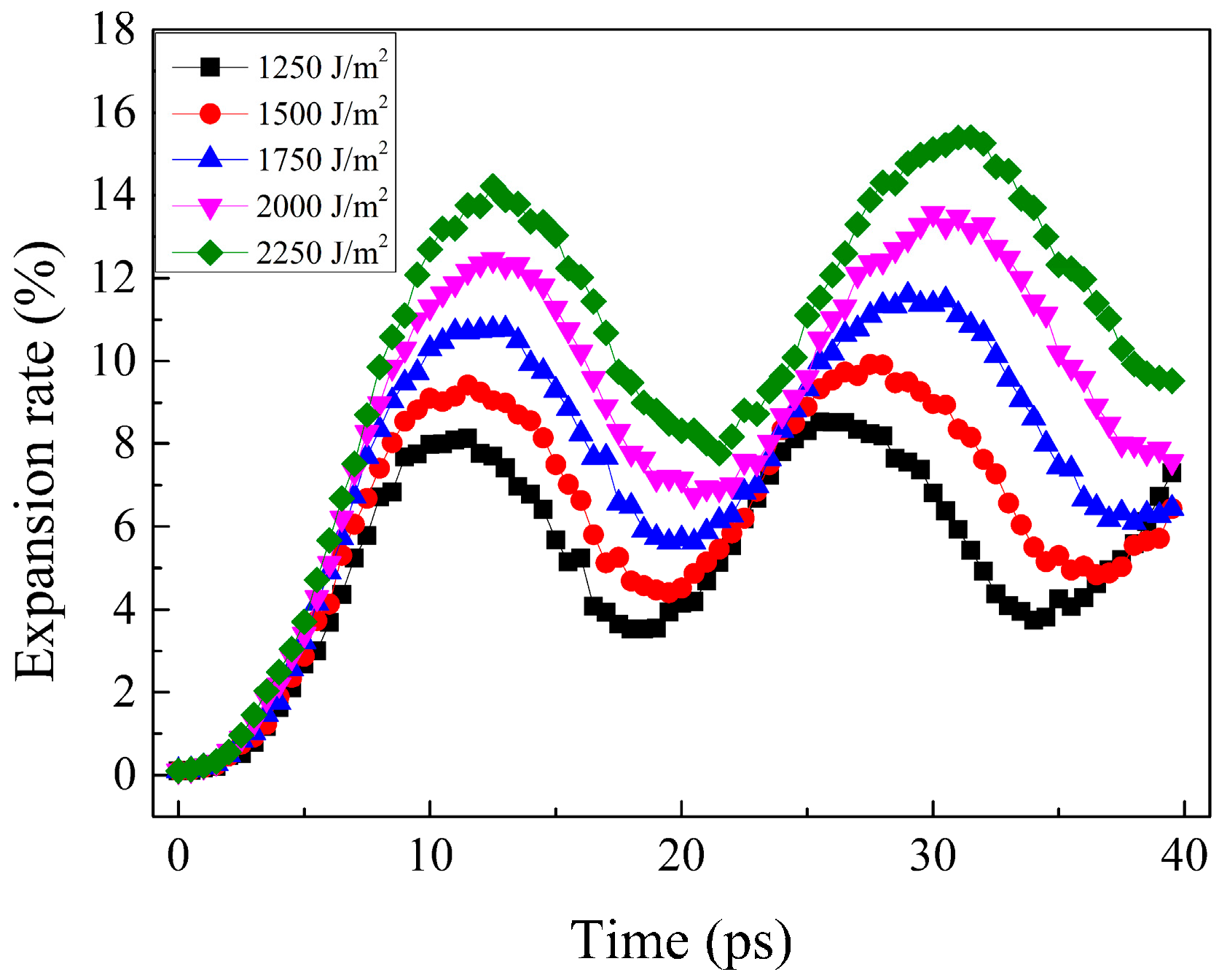
3.4. Effect of Fluence on Thermoelastic Vibration
4. Conclusions
- (1)
- Stress waves are generated in nickel films under femtosecond laser irradiation. The laser-induced stress wave propagates and reflects continuously in the film, which leads to thermoelastic vibration in the nonthermal ablation region.
- (2)
- Thermoelastic vibration can be divided into two stages, which are bounded by thermal equilibrium. Before the thermal balance, the deposition of laser energy in the nickel film increases the energy of the collective atomic motion, which leads to continuous expansion behavior. After reaching the thermal balance, the relaxation of laser-induced stress leads to the periodic expansion and contraction of the nickel film, accompanied by changes in structure and temperature.
- (3)
- The amplitude of thermoelastic vibration increases with increasing fluence, which is the result of the increase in the energy of collective atomic motion. At high fluence, the propagation time of the stress wave is prolonged by a large expansion rate and more intense melting.
- (4)
- Reducing laser energy deposition in the nonthermal ablation region is an effective way to reduce thermoelastic vibration. This can be achieved by reducing the size of the femtosecond laser spot. When a femtosecond laser is applied to a metal, it may be feasible to transform a single pulse into multiple pulses with a small spot size.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Putzer, M.; da Silva, G.R.; Michael, K.; Schröder, N.; Schudeleit, T.; Bambach, M.; Wegener, K. Geometrical modeling of ultrashort pulse laser ablation with redeposition and analysis of the influence of spot size and shape. Mater. Des. 2024, 246, 113357. [Google Scholar] [CrossRef]
- Ullah, N.; Cui, J.; Wei, F.; Yin, H.; Mei, X. Ablation threshold, structural modification, and sensing of graphene oxide thin film induced by UV nanosecond laser. Surf. Interfaces 2024, 52, 104991. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, Z.; Zhao, Z.; Liu, Y.; Wang, C.; Xu, W. Multimodal Deep-Learning Framework for Accurate Prediction of Wettability Evolution of Laser-Textured Surfaces. ACS Appl. Mater. Interfaces 2023, 15, 10261–10272. [Google Scholar] [CrossRef]
- Ha, J. Superhydrophilic Surface Creation and Its Temporal Transition to Hydrophobicity on Copper via Femtosecond Laser Texturing. Coatings 2024, 14, 1107. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, Z.; Yang, Z.; Wang, C. Femtosecond Laser-Induced Evolution of Surface Micro-Structure in Depth Direction of Nickel-Based Alloy. Appl. Sci. 2022, 12, 8464. [Google Scholar] [CrossRef]
- Ma, S.; McDonald, J.; Tryon, B.; Yalisove, S.; Pollock, T. Femtosecond laser ablation regimes in a single-crystal superalloy. Metall. Mater. Trans. A 2007, 38, 2349–2357. [Google Scholar] [CrossRef]
- Ivanov, D.S.; Zhigilei, L.V. Combined atomistic-continuum modeling of short-pulse laser melting and disintegration of metal films. Phys. Rev. B 2003, 68, 064114. [Google Scholar] [CrossRef]
- Shugaev, M.V.; Zhigilei, L.V. Thermodynamic analysis and atomistic modeling of subsurface cavitation in photomechanical spallation. Comput. Mater. Sci. 2019, 166, 311–317. [Google Scholar] [CrossRef]
- Richardson, C.; Ehrlich, M.; Wagner, J. Interferometric detection of ultrafast thermoelastic transients in thin films: Theory with supporting experiment. JOSA B 1999, 16, 1007–1015. [Google Scholar] [CrossRef]
- Sun, Y.; Saka, M.; Li, J.; Yang, J. Ultrafast laser-induced thermoelastic behavior in metal films. Int. J. Mech. Sci. 2010, 52, 1202–1207. [Google Scholar] [CrossRef]
- Wang, X.; Xu, X. Thermoelastic wave induced by pulsed laser heating. Appl. Phys. A 2001, 73, 107–114. [Google Scholar] [CrossRef]
- Maznev, A.; Hohlfeld, J.; Güdde, J. Surface thermal expansion of metal under femtosecond laser irradiation. J. Appl. Phys. 1997, 82, 5082–5085. [Google Scholar] [CrossRef]
- Gusev, V.; Desmet, C.; Lauriks, W.; Glorieux, C.; Thoen, J. Theory of Scholte, leaky Rayleigh, and lateral wave excitation via the laser-induced thermoelastic effect. J. Acoust. Soc. Am. 1996, 100, 1514–1528. [Google Scholar] [CrossRef]
- Alexopoulou, V.E.; Markopoulos, A.P. A critical assessment regarding two-temperature models: An investigation of the different forms of two-temperature models, the various ultrashort pulsed laser models and computational methods. Arch. Comput. Methods. Eng. 2024, 31, 93–123. [Google Scholar] [CrossRef]
- Gusev, V.; Kolomenskii, A.A.; Hess, P. Effect of melting on the excitation of surface acoustic wave pulses by UV nanosecond laser pulses in silicon. Appl. Phys. A 1995, 61, 285–298. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, Z.; Wang, C.; Zhang, Q.; Zheng, S.; Xu, W. Mechanisms of femtosecond laser ablation of Ni3Al: Molecular dynamics study. Opt. Laser Technol. 2021, 133, 106505. [Google Scholar] [CrossRef]
- Etcheverry, J.; Mesaros, M. Molecular dynamics simulation of the production of acoustic waves by pulsed laser irradiation. Phys. Rev. B 1999, 60, 9430. [Google Scholar] [CrossRef]
- Yao, J.; Qi, D.; Liang, H.; He, Y.; Yao, Y.; Jia, T.; Yang, Y.; Sun, Z.; Zhang, S. Exploring femtosecond laser ablation by snapshot ultrafast imaging and molecular dynamics simulation. Ultrafast Sci. 2022, 2022, 9754131. [Google Scholar] [CrossRef]
- Lin, Z.; Zhigilei, L.V. Time-resolved diffraction profiles and atomic dynamics in short-pulse laser-induced structural transformations: Molecular dynamics study. Phys. Rev. B Condens. Matter. Mater. Phys. 2006, 73, 184113. [Google Scholar] [CrossRef]
- Schrider, K.J.; Torralva, B.; Yalisove, S.M. The dynamics of femtosecond pulsed laser removal of 20 nm Ni films from an interface. Appl. Phys. Lett. 2015, 107, 124101. [Google Scholar] [CrossRef]
- Leveugle, E.; Ivanov, D.S.; Zhigilei, L.V. Photomechanical spallation of molecular and metal targets: Molecular dynamics study. Appl. Phys. A 2004, 79, 1643–1655. [Google Scholar] [CrossRef]
- Lin, G.; Jiang, L.; Ji, P.; Sun, J.; Hu, J.; Lian, Y. Ultrafast melting, spallation, and phase explosion in femtosecond laser processing on nickel film surface investigated by atomistic simulation and transient reflectivity microscopy. Opt. Laser Technol. 2025, 180, 111404. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, Y.; Hu, M.; Chen, X. An improved three-dimensional two-temperature model for multi-pulse femtosecond laser ablation of aluminum. J. Appl. Phys. 2015, 117, 063104. [Google Scholar] [CrossRef]
- Zhigilei, L.V.; Lin, Z.; Ivanov, D.S. Atomistic modeling of short pulse laser ablation of metals: Connections between melting, spallation, and phase explosion. J. Phys. Chem. C 2009, 113, 11892–11906. [Google Scholar] [CrossRef]
- Kudryashov, S.I.; Danilov, P.A.; Bezhanov, S.G.; Rudenko, A.A.; Ionin, A.A.; Uryupin, S.A.; Umanskaya, S.; Smirnov, N.A. Plasmon-enhanced two-photon absorption of infrared femtosecond laser pulses in thin gold films. JETP Lett. 2019, 109, 382–386. [Google Scholar] [CrossRef]
- Tsibidis, G.D. The influence of dynamical change of optical properties on the thermomechanical response and damage threshold of noble metals under femtosecond laser irradiation. J. Appl. Phys. 2018, 123, 085903. [Google Scholar] [CrossRef]
- Kudryashov, S.I.; Ionin, A.A. Multi-scale fluence-dependent dynamics of front-side femtosecond laser heating, melting and ablation of thin supported aluminum film. Int. J. Heat Mass Transfer. 2016, 99, 383–390. [Google Scholar] [CrossRef]
- Cheng, C.; Xu, X. Mechanisms of decomposition of metal during femtosecond laser ablation. Phys. Rev. B Condens. Matter. Mater. Phys. 2005, 72, 165415. [Google Scholar] [CrossRef]
- Nedialkov, N.; Atanasov, P.; Amoruso, S.; Bruzzese, R.; Wang, X. Laser ablation of metals by femtosecond pulses: Theoretical and experimental study. Appl. Surf. Sci. 2007, 253, 7761–7766. [Google Scholar] [CrossRef]
- Zhigilei, L.V.; Lin, Z.; Ivanov, D.S.; Leveugle, E.; Duff, W.H.; Thomas, D.; Sevilla, C.; Guy, S.J. Atomic/molecular-level simulations of laser–materials interactions. In Laser-Surface Interactions for New Materials Production: Tailoring Structure and Properties; Springer: Berlin/Heidelberg, Germany, 2010; pp. 43–79. [Google Scholar]
- Wang, X.; Xu, X. Molecular dynamics simulation of thermal and thermomechanical phenomena in picosecond laser material interaction. Int. J. Heat. Mass. Transf. 2003, 46, 45–53. [Google Scholar] [CrossRef]
- Murphy, R.D.; Torralva, B.; Yalisove, S.M. The role of an interface on Ni film removal and surface roughness after irradiation by femtosecond laser pulses. Appl. Phys. Lett. 2013, 102, 181602. [Google Scholar] [CrossRef]
- Das, D.; McDonald, J.; Yalisove, S.; Pollock, T. Depth-profiling study of a thermal barrier coated superalloy using femtosecond laser-induced breakdown spectroscopy. Spectrochim. Acta Part B At. Spectrosc. 2008, 63, 27–36. [Google Scholar] [CrossRef]
- Bonse, J.; Baudach, S.; Krüger, J.; Kautek, W.; Lenzner, M. Femtosecond laser ablation of silicon–modification thresholds and morphology. Appl. Phys. A 2002, 74, 19–25. [Google Scholar] [CrossRef]








| Parameter | Value | References |
|---|---|---|
| γ constant | 1065 J/m3K2 | [7,21,27] |
| k0 constant | 91 W/mK | [7,21,27] |
| g electron-phonon coupling | 3.6 × 1017 W/m3K | [7,21,27] |
| coefficient | ||
| R reflectivity | 0.62 | [7] |
| Lp optical absorption length | 13.5 nm | [7] |
| τL FWHM pulse width | 500 fs | |
| D dissociation energy | 0.4205 ev | [27] |
| rε equilibrium distance | 0.278 nm | [27] |
| b constant | 14.199 nm−1 | [27] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, W.; Gu, Y.; Yang, Z.; Zhang, Z. Thermoelastic Vibration of Nickel Film Irradiated by Femtosecond Laser: Molecular Dynamics Study. Coatings 2025, 15, 1. https://doi.org/10.3390/coatings15010001
Zhao W, Gu Y, Yang Z, Zhang Z. Thermoelastic Vibration of Nickel Film Irradiated by Femtosecond Laser: Molecular Dynamics Study. Coatings. 2025; 15(1):1. https://doi.org/10.3390/coatings15010001
Chicago/Turabian StyleZhao, Wanrong, Yucheng Gu, Zenan Yang, and Zhen Zhang. 2025. "Thermoelastic Vibration of Nickel Film Irradiated by Femtosecond Laser: Molecular Dynamics Study" Coatings 15, no. 1: 1. https://doi.org/10.3390/coatings15010001
APA StyleZhao, W., Gu, Y., Yang, Z., & Zhang, Z. (2025). Thermoelastic Vibration of Nickel Film Irradiated by Femtosecond Laser: Molecular Dynamics Study. Coatings, 15(1), 1. https://doi.org/10.3390/coatings15010001






