Abstract
The two-dimensional electrochemical Y2C’s low work function and strong charge transfer qualities limit its applicability in catalysis due to its poor catalytic activity. In this paper, based on density functional theory calculations, we use two techniques to increase the HER catalytic activity of the Y2C monolayer: substitution doping (XC) and adsorption doping (XT) of non-metal (X = N, P, O, S, and F). The results showed that the absolute values of hydrogen free energies (ΔGH*) of the substitutional dopants of PC, SC and adsorptive dopants of NT, OT, ST, and PT had increased catalytic activity compared with those of the pristine Y2C monolayer (−0.673 eV). It was highlighted that the adsorption doping of PT can further reduce the adsorption free energy of the pristine Y2C monolayer to −0.19 eV, which is close to the optimal zero value, and the binding energy of the hydrogen atoms on the Y2C surface significantly increased from −0.913 to −0.438 eV, which is more favorable for the desorption of hydrogen atoms. These results demonstrate that the doping of non-metals activates the adsorption of hydrogen atoms on monolayer Y2C and provides a feasible method for hydrogen generation.
1. Introduction
Global demand for all types of energy is rising in tandem with the growing population. Expert predictions state that over the course of the 28-year period from 2012 to 2040, there will be a significant increase in the total global demand for energy in its most basic form, or primary energy, which includes both renewable and conventional fossil fuels like coal and non-renewable energy sources like solar and wind power, oil, and natural gas [1,2]. Approximately 80% of this demand for energy will still come from traditional fossil fuels, and the extensive use of these fuels can result in serious environmental issues [3]. It is critical to locate clean, plentiful, and renewable energy sources in order to reduce environmental pollution and solve the world’s energy dilemma [4,5,6,7,8,9]. Hydrogen’s zero-pollution characteristic has made it a potential substitute for fossil fuels [9,10,11,12]. Electrochemical water decomposition (EWD) is a green alternative for the efficient synthesis of high-quality hydrogen [13,14]. Many investigations and theories have revealed that the Gibbs free energy of hydrogen adsorbed on the electrode surface is the primary component in influencing the hydrogen precipitation reaction activity [15]. Platinum is currently the best HER catalyst material [16,17]. However, its high cost and unavailability have substantially limited its wide application in reality [18,19,20,21,22]. In order to achieve the large-scale and sustainable production of H2, researchers need to identify new materials or adapt existing materials to increase HER performance given their limited economic efficiency and storage capacity. In the past few years, 2D materials have been proposed as effective HER catalysts due to their large specific surface area, abundant active sites, tunable electronic properties, and good electrocatalytic performance, such as transition metal carbon/oxygen/phosphorus/sulfides [23,24,25,26,27], graphene-like carbon nitrides (g-C3N4) [28,29], layered double hydroxides (LDHs) [30], monoatomic catalysts (SACs) [31], and two-dimensional electronic compounds (2D-electrides) [32].
In recent years, two-dimensional electrides (2D-electrides) as new two-dimensional (2D) materials have been exploited in catalysis because to their high electron transport capacity, low work function, and excellent catalytic activity [33,34,35]. For example, the electride [Ca24Al28O64]4+·4e− (commonly abbreviated as C12A7: e−) has a low figure of merit and good electron transfer ability and is employed in the synthesis of ammonia-catalyzed organics, light-emitting diodes, and other materials [36]. Ru-loaded Y5Si3 (Ru/Y5Si3) is a very efficient catalyst for ammonia synthesis because of its excellent electron transfer capacity, which can minimize the activation energy of the ammonia synthesis reaction [37]. The Cu-based intermetallic electride LaCu0.67Si1.33 has high carrier densities and low work functions, which can effectively reduce the activation energy of chemical reactions and exhibit excellent catalytic activity in various hydrogenation reactions [38]. Recently, a layered electride material, yttriumized, with dicarbon-containing 2D anionic electrons has been disclosed. This multilayer electride (Y2C) has been proposed as a candidate for prospective HER catalysts due to its low work function (2.7 eV) and efficient charge-transfer characteristics, broad pH application, and good stability [39,40,41,42]. However, its weak catalytic activity (ΔGH* = −0.673 eV) limits its application in catalysis. Catalyst modification via elemental doping is widely used to enhance HER performance. According to Shi et al., P doping at a concentration of 2.85 weight percent significantly increased the catalytic activity of Mo2C because of the increased electron density close to the Fermi level of the compound. This led to a decrease in Mo-H bonding and a faster rate of HER kinetics [43]. Wu et al., doped non-metal oxygen (O) elements into Co2P to increase the HER activity of Co2P in alkaline media [44]. Sun et al., inserted N dopants into WC with a considerable rise in the (H-atom) hydrogen-atom binding energy from −1.05 eV to −0.72 eV on the surface of WC (001), which is more favorable for hydrogen atoms desorption [45]. Therefore, elemental doping is a powerful technique for altering the electronic structure and optimizing the physical properties of a compound, therefore dramatically enhancing the HER performance of the compound.
In this work, we examine the hydrogen (H) adsorption on the structure of the two-dimensional electrochemical Y2C monolayer using first-principles density-functional theory (DFT) computations. A non-metal doping technique is suggested to raise Y2C monolayers’ HER catalytic activity. The outcomes demonstrate that this technique can raise the HER catalytic activity of Y2C monolayers to a better level with Gibbs free energy |ΔGH*| < 0.2 eV.
2. Computational Methods
In this investigation, density functional theory (DFT) calculations were conducted with the MedeA platform and based on the Vienna ab initio simulation package (MedeA-VASP) [46,47,48]. The projected augmented wave (PAW) [49] generalized gradient approximation of Perdew–Burke–Ernzerhof (GGA-PBE) [50,51] pseudopotential was applied to represent the electron exchange correlation energy. The cutoff energy was set to 500 eV. 4 × 4 × 1 supercell of the Y2C monolayer and 15 Å vacuum perpendicular to the 2D plane were employed to prevent the interactions from the periodic boundary condition. The electronic characteristics and structural optimizations were estimated, with the force converged to 0.01 eV/Å and the energy converged to 1.0 × 10−5 eV/atom. Furthermore, a denser 5 × 5 × 1 k-point grid was used for density of states (DOS) calculations, a 3 × 3 × 1 k-point grid for static self-consistent calculations, and a 1 × 1 × 1 k-point grid for structural optimizations were used to sample the first Brillouin zones. Ab initio molecular dynamics (AIMD) [52] simulations utilizing the Nose–Hoover thermostat (NVT) ensemble were run for 10 ps at 300 K with a time step of 2.0 fs in order to verify the thermal stability. The formation energy of non-metal (XC = N, P, O, S, F) dopants embedded in the C vacancy was calculated according to:
The formation energy of non-metal (XT = N, P, O, S, F) dopants adsorbed above the three Y vacancies was calculated according to:
where Ef (XC@Y2C) and Ef (XT@Y2C) signify the total Y2C energies for the substitution of C atoms by non-metallic X atoms and for the adsorption of non-metallic X atoms onto the hollow sites of the three Y, respectively. E(Y2C) and E(C) signify the energies of the monolayer of Y2C and the energies of the graphitic carbon, respectively. For the non-metallic elements, a portion of the chemical potential originates from their solid state; the P and S atoms’ chemical potentials are calculated from their orthorhombic phase, while the C atoms’ chemical potential is derived from graphene. The energies of an atom in F2, O2, and N2 are the chemical potentials of the F, O, and N atoms, respectively. The second component originates from the gas phase. The hydrogen evolution reaction (HER) mechanism under standard conditions is:
The Gibbs free energy is formulated as:
where and are the variations in the zero-point energy and entropy between the adsorbed and gas phase hydrogen and is the hydrogen adsorption energy. In this case, the acidic HER process is considered at standard conditions (p = 1 bar, pH = 0, and T = 298.15 K), and based on the calculations of Nørskov et al. [53,54]. We rewrote the equation as .
3. Results and Discussion
3.1. Catalytic Properties of Pristine Y2C
The Y2C monolayer with space group R-3m has a two-dimensional crystal structure made up of two Y layers sandwiched between a C layer. Each surface Y atom is coordinated to three C atoms, while each C atom is covalently bound to six Y atoms. The Y-C bond length is 2.482 Å, and the optimized Y2C monolayer lattice parameter is a = b = 3.62 Å. This result is consistent with the literature [39,40,41,42]. Long-term operation of the two-dimensional structural framework requires stability. Consequently, by using ab initio arithmetic molecular dynamics (AIMD) simulations with the Nose–Hoover thermal bath technique, the thermal stability of the Y2C monolayer structure was assessed. After 10 ps simulation, the Y2C monolayer structure remained intact at 300 K. Figure 1 shows the final structure and energy distribution during the AIMD simulation, and the Y2C monolayer structure did not undergo deformation, indicating its good thermal stability at room temperature.
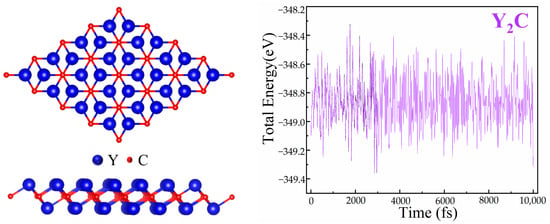
Figure 1.
Final structures after molecular dynamics simulation for 10 ps (left panel) and the corresponding energy profiles (right panel).
The catalytic activity of HER is frequently characterized by the Gibbs free energy of hydrogen adsorption (ΔGH*). To guarantee the highest catalytic activity, the value of ΔGH* on the catalyst should be very near zero. The adsorption behavior of hydrogen atoms on the surface of the Y2C monolayer was first computed to identify the most advantageous adsorption sites in order to investigate the HER catalytic activity of Y2C. On top of three Y atoms on the surface lies the best active site for H adsorption, as shown in Figure 2. There are three basic reactions for HER, which are the Vollmer reaction, the Tafel reaction, and the Helovsky reaction. The Volmer reaction, or electrochemical adsorption (H+ + e− → H*), is the initial stage. Hydrogen atoms are adsorbed onto the catalyst’s active sites during this process. In the subsequent phase, known as desorption, the adsorbed H* atoms undergo reduction to generate H2 molecules through either the Tafel reaction (H+ + e− + H* → H2) or the Heyrovsky reaction (H* + H* → H2). However, the values of Y2C adsorption and desorption processes are close to 1 eV, which suggests that hydrogen atoms are difficult to desorb from the catalyst surface. Our DFT calculations confirm the low HER catalytic activity of the pristine Y2C monolayer, which is reflected by the corresponding ΔGH* value (−0.673 eV).
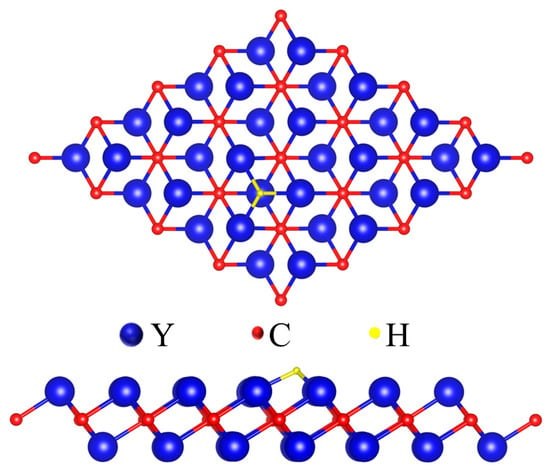
Figure 2.
Top and side views of the considered H adsorption sites for Y2C.
3.2. Catalytic Properties of Y2C Doped with Non-Metallic Atoms
Therefore, to further tune the ΔGH* value to zero, we explored the doping of Y2C monolayers with simple atoms N, P, O, S, and F. The doping of Y2C monolayers with simple atoms N, P, O, S, and F was also explored. In two-dimensional transition metal carbides, the true active site is generally dominated by cations, and the surrounding anionic environment generally modulates the cations and enhances activity. Due to the strong or weak binding to the reaction intermediates, the doped metal elements lead to the complexity of the active metal sites; therefore, we influenced the electronic structure of the metal ions by substituting the original anions of the compounds with non-metal atoms (X = N, P, O, S, and F) and influence the interactions of the active sites with the reactants, intermediary adsorption, and the final products during the reaction process, which modulates the intrinsic activity, to further improve catalytic performance. For the doping of non-metallic atom X, two doping configurations were considered: (a) one non-metal X atom replacing one C atom, XC, and (b) non-metal X adsorbing on the top of the hollow site of three Y center, XT, as shown in Figure 3.
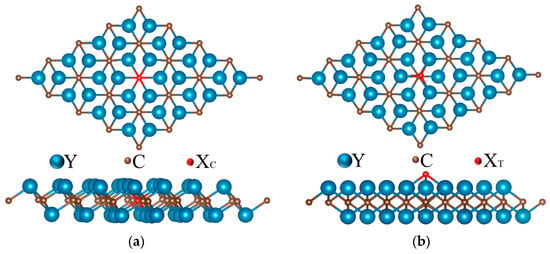
Figure 3.
Top view and side view of (a) XC @Y2C and (b) XT @Y2C after doping.
The formation energies (Ef) of XC@Y2C and XT@Y2C doping can be used to estimate the likelihood of a doping reaction occurring based on the Ef values: a negative value indicates that the doping reaction proceeds spontaneously, and a positive value indicates that additional energy is required to overcome the energy barrier when dopant atoms are introduced into the host structure, which is calculated as shown in Table 1. For non-metallic substitutional doping, the formation energies of NC, OC, SC, and FC are all negative, except for PC, which has a positive formation energy of 1.215 eV, indicating that the formation process of NC, OC, SC, and FC is exothermic and the formation reaction of PC is heat-absorbing. Interestingly, the formation energies of all XT are negative, indicating that non-metallic elements are readily adsorbed on Y2C. In addition, the formation energy of the F atom is the largest, confirming the strong adsorption of non-metallic elements on Y2C.

Table 1.
The formation energy Ef (eV) for N, P, O, S, and F dopants.
In order to evaluate the catalytic activity of the Y2C monolayer surface after doping with non-metal atoms, the optimal adsorption sites on the 4 × 4 × 1 supercell model were first investigated, and since hydrogen adsorption can take place at all possible positions on all surfaces, it was necessary to find the most energetically favorable configuration with different adsorption sites of H, as shown in Figure 4. A suitable value of ΔEH* is necessary in the HER reaction to enhance desorption, as illustrated in Table 2. In this regard, NT@Y2C (0.1 eV), PT@Y2C (−0.438), and OT@Y2C (−0.492 eV) exhibit ΔEH* close to half of that of Y2C (−0.913 eV), showing equilibrium ΔEH* values relative to Y2C throughout adsorption and desorption.
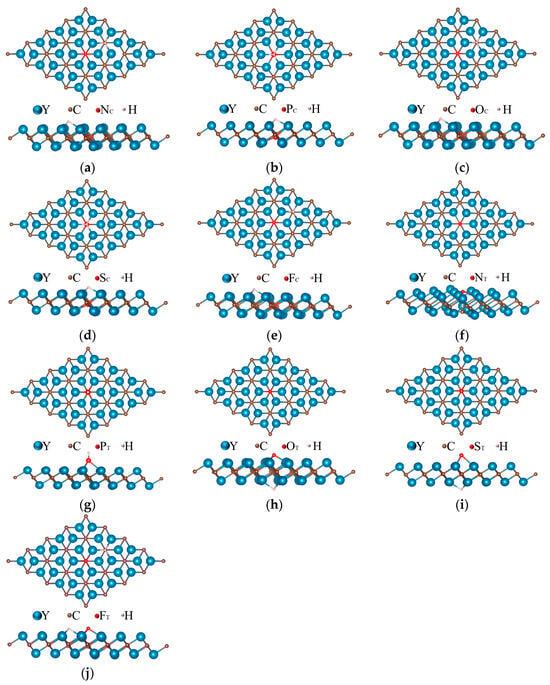
Figure 4.
Top and side views of the considered H adsorption sites for (a) NC@Y2C, (b) PC@Y2C, (c) OC@Y2C, (d) SC@Y2C, (e) FC@Y2C, (f) NT@Y2C, (g) PT@Y2C, (h) OT@Y2C, (i) ST@Y2C, and (j) FT@Y2C.

Table 2.
Calculated adsorption energies ΔEH* (eV) for N, P, O, S, and F dopants.
Table 3 and Figure 5 show the ΔGH* of N, P, O, S, and F doping on the monolayer Y2C surface. On the fully mono-doped surface, the catalytic hydrogenolysis ability of monolayer Y2C doped with non-metallic NC, OC, FC, and FT is comparable to that of the pristine Y2C monolayer, which is unsuitable for HER. The ΔGH* values of PC, SC, NT, OT and ST doped on the monolayer Y2C surface are in the range of −0.25~−0.5 eV, and compared to pure transition metallizers (Table 4), the introduction of surface heteroatom (non-metallic atom) dopants in MXenes can activate their surfaces and further increase the number of active sites, thus improving catalytic activity, which are significantly lower in number compared to those of pristine Y2C, indicating an increase in its HER activity. Notably, compared to the Gibbs free energy of H adsorption (ΔGH*) of the above five doped configurations, only PT doped on the monolayer Y2C surface has |ΔGH*| less than 0.2 eV, indicating the highest catalytic activity. This is consistent with the results analyzed later that P atoms have the least electronegativity and that doping increases the charge density of the space charge layer in this region, increasing the catalytic activity of its surface. In addition, charge transfer is another indicator used to characterize the adsorption strength of the Y2C substrate with H atoms. The less charge transfer, the weaker the interaction between the substrate and H atoms. As shown in Figure 6, the Bader charge analysis reveals that the ΔGH* of non-metal substitution and adsorption doping in monolayer Y2C is negatively and linearly correlated with the transferred charge.

Table 3.
Hydrogen adsorption free energies ΔGH* (eV) for N, P, O, S, and F dopants.
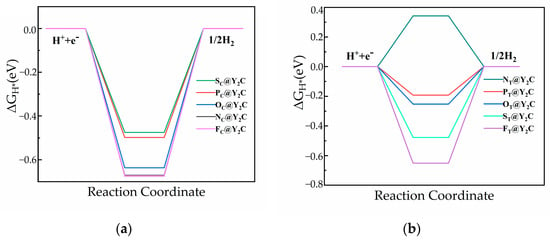
Figure 5.
Gibbs free energy diagram at 1/16 hydrogen coverage: (a) ΔGH* (XC@Y2C) and (b) ΔGH* (XT@Y2C).

Table 4.
Hydrogen adsorption free energies ΔGH* (eV) for transition metal compounds.
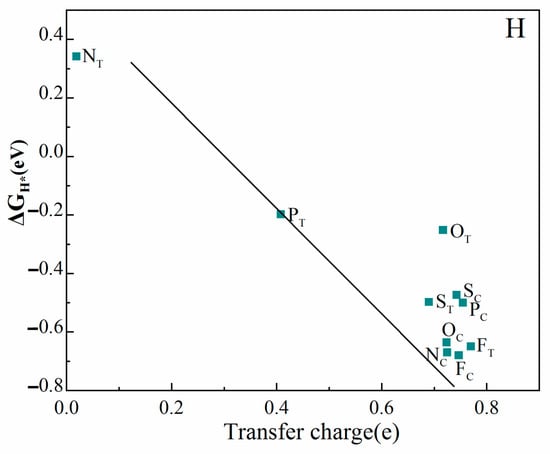
Figure 6.
ΔGH* as function of the Bader transfer charge of H atoms.
To determine the explanation for the increased HER performance, we studied the electronic structure of Y2C. As shown in Figure 7, we calculated the total and projected DOS of the Y2C structure without H. By examining the density of states, we showed that the electronic density of states at the Fermi energy level is not zero after doping with N, P, O, S, and F. This implies that the two-dimensional electrode monolayer Y2C is still a conductor after doping with non-metallic materials. This implies that the two-dimensional electrode monolayer Y2C remains a conductor despite doping with non-metallic elements, indicating metallic conductivity. To further illustrate the charge transfer capabilities of monolayer Y2C, Figure 8 illustrates the varied charge densities of monolayers XC@Y2C and XT@Y2C without H adsorption. As can be observed from the figure, the electronic state of Y2C can be regulated due to the varied electronegativity of the non-metal, in which the Y-X bonding interaction reduces the electron density surrounding the C atom, thus weakening the Y-C bond. Compared with pure Y2C, the inclusion of non-metallic atoms generates more electrons on the X-Y2C surface, lowers the number of C-H interactions, accelerates the detachment of H* from the surface, and favors the production of H2. Therefore, it is conceivable to participate and contribute to the HER process by introducing new non-metallic elements to control the transition metal ions or activate their own anions to attain the perfect H adsorption energy (near to zero) and thereby improve HER efficiency.
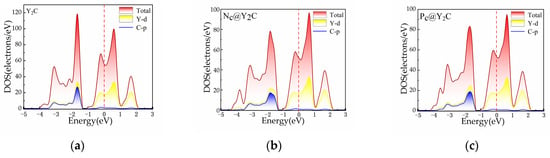
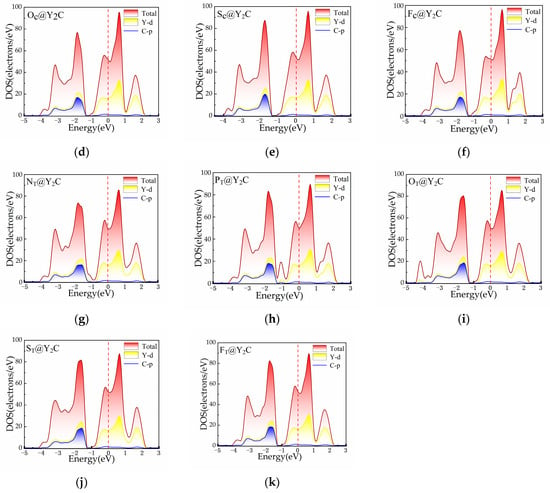
Figure 7.
The total and projected density of states (PDOS): (a) Y2C, (b) NC@Y2C, (c) PC@Y2C, (d) OC@Y2C, (e) SC@Y2C, (f) FC@Y2C, (g) NT@Y2C, (h) PT@Y2C, (i) OT@Y2C, (j) ST@Y2C, and (k) FT@Y2C.
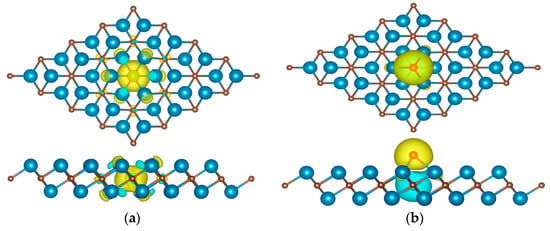
Figure 8.
The charge density difference of (a) XC@Y2C and (b) XT@Y2C, where the yellow and blue regions indicate the charge density accumulation and depletion, respectively. The isosurface is set to 0.005 e/bohr3.
4. Conclusions
In this paper, the effect of surface doping on the catalytic performance of two-dimensional material Y2C monolayers in a hydrogen precipitation reaction (HER) is systematically investigated by using density functional theory (DFT). Substitution and adsorption doping effects including nitrogen (N), phosphorus (P), oxygen (O), sulfur (S), and fluorine (F) elements were considered. The thermodynamic stability of each doped surface was confirmed by calculating the formation energy. Hydrogen adsorption free energy calculations were used to assess the HER catalytic activity of the doped Y2C monolayers. The results show that compared to pristine Y2C, PC@Y2C (ΔGH* = −0.50 eV), SC@Y2C (ΔGH* = −0.474 eV), NT@Y2C (ΔGH* = 0.341 eV), OT@Y2C (ΔGH* = −0.252 eV), and ST@Y2C (ΔGH* = −0.478 eV) all enhance the catalytic activity of Y2C. In particular, PT@Y2C, (ΔGH* = −0.192 eV) showed the most excellent HER catalytic activity and its active site was easy to form. P doping leads to a new density of states peak in Y2C around the Fermi energy level, which can further improve the electrical conductivity of the material. In addition, P doping made the P site a new active site and reduced the free energy of hydrogen adsorption at the C site in Y2C. This study not only gives an in-depth explanation and prediction of the hydrogen uptake catalytic activity of non-metal doped Y2C monolayers but also provides essential theoretical advice for the design of efficient and stable two-dimensional material-based catalysts.
Author Contributions
Conceptualization, C.L. and N.S.; methodology, C.L.; software, N.S.; validation, C.L. and N.S.; formal analysis, C.L.; investigation, C.L.; resources, N.S.; data curation, C.L.; writing—original draft preparation, C.L.; writing—review and editing, N.S.; supervision, Y.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
Special thanks to my tutor Su Ningning for her guidance and help and my family for their support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ahmad, T.; Zhang, D. A critical review of comparative global historical energy consumption and future demand: The story told so far. Energy Rep. 2020, 6, 1973–1991. [Google Scholar] [CrossRef]
- Ahmad, T.; Chen, H. A review on machine learning forecasting growth trends and their real-time applications in different energy systems. SCS 2020, 54, 102010. [Google Scholar] [CrossRef]
- Güney, T. Solar energy, governance and CO2 emissions. RENEY 2022, 184, 791–798. [Google Scholar] [CrossRef]
- Salah, B.; Abdelgawad, A.; El-Demellawi, J.K.; Lu, Q.; Xia, Z.; Abdullah, A.M.; Eid, K. Scalable One-Pot Fabrication of Carbon-Nanofiber-Supported Noble-Metal-Free Nanocrystals for Synergetic-Dependent Green Hydrogen Production: Unraveling Electrolyte and Support Effects. ACS Appl. Mater. Interfaces 2024, 16, 18768–18781. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Zhu, M.; Li, Y. The Adsorption Behavior of Hydrogen on the PuO2(111) Surface: A DFT+U Study. Coatings 2024, 14, 195. [Google Scholar] [CrossRef]
- Wawrzyńczak, A.; Feliczak-Guzik, A. Hydrogen Production Using Modern Photocatalysts. Coatings 2024, 14, 366. [Google Scholar] [CrossRef]
- Jiao, S.; Yao, Z.; Xue, F.; Lu, Y.; Liu, M.; Deng, H.; Ma, X.; Liu, Z.; Ma, C.; Huang, H.; et al. Defect-rich one-dimensional MoS2 hierarchical architecture for efficient hydrogen evolution: Coupling of multiple advantages into one catalyst. Appl. Catal. B Environ. 2019, 258, 117964. [Google Scholar] [CrossRef]
- Song, D.; Wang, Y.; Lu, X.; Gao, Y.; Li, Y.; Gao, F. Ag nanoparticles-decorated nitrogen-fluorine co-doped monolayer MoS2 nanosheet for highly sensitive electrochemical sensing of organophosphorus pesticides. Sens. Actuators B Chem. 2018, 267, 5–13. [Google Scholar] [CrossRef]
- Li, C.; Zhu, D.; Cheng, S.; Zuo, Y.; Wang, Y.; Ma, C.; Dong, H. Recent research progress of bimetallic phosphides-based nanomaterials as cocatalyst for photocatalytic hydrogen evolution. Chin. Chem. Lett. 2022, 33, 1141–1153. [Google Scholar] [CrossRef]
- Turner, J.A. Sustainable Hydrogen Production. Science 2004, 305, 972–974. [Google Scholar] [CrossRef]
- Kolar, J. Alternative energy technologies. J. Environ. Qual. 2001, 10, 45–54. [Google Scholar] [CrossRef]
- Crabtree, G.W.; Dresselhaus, M.S.; Buchanan, M.V. The Hydrogen Economy. Phys. Today 2004, 57, 39–44. [Google Scholar] [CrossRef]
- Huang, T.; Shen, T.; Gong, M.; Deng, S.; Lai, C.; Liu, X.; Zhao, T.; Teng, L.; Wang, D. Ultrafine Ni-B nanoparticles for efficient hydrogen evolution reaction. Chin. J. Catal. 2019, 40, 1867–1873. [Google Scholar] [CrossRef]
- Benck, J.D.; Hellstern, T.R.; Kibsgaard, J.; Chakthranont, P.; Jaramillo, T.F. Catalyzing the Hydrogen Evolution Reaction (HER) with Molybdenum Sulfide Nanomaterials. ACS Catal. 2014, 4, 3957–3971. [Google Scholar] [CrossRef]
- Jiao, Y.; Zheng, Y.; Jaroniec, M.; Qiao, S.Z. Design of electrocatalysts for oxygen- and hydrogen-involving energy conversion reactions. Chem. Soc. Rev. 2015, 44, 2060–2086. [Google Scholar] [CrossRef]
- Amiinu, I.S.; Pu, Z.; Liu, X.; Owusu, K.A.; Monestel, H.G.R.; Boakye, F.O.; Zhang, H.; Mu, S. Multifunctional Mo–N/C@MoS2 Electrocatalysts for HER, OER, ORR, and Zn–Air Batteries. Adv. Funct. Mater. 2017, 27, 1702300. [Google Scholar] [CrossRef]
- Kang, M.; Lin, C.; Yang, H.; Guo, Y.; Liu, L.; Xue, T.; Liu, Y.; Gong, Y.; Zhao, Z.; Zhai, T.; et al. Proximity Enhanced Hydrogen Evolution Reactivity of Substitutional Doped Monolayer WS2. ACS Appl. Mater. Interfaces 2021, 13, 19406–19413. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Luo, W.; Ma, L.; Yu, M.; Ren, X.; He, M.; Polen, S.; Click, K.; Garrett, B.; Lu, J.; et al. Dimeric [Mo2S12]2− Cluster: A Molecular Analogue of MoS2 Edges for Superior Hydrogen-Evolution Electrocatalysis. Angew. Chem. Int. Ed. 2015, 54, 15181–15185. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, C.; Huang, Y.; Fu, J. Recent advances in electrocatalysts for neutral and large-current-density water electrolysis. Nano Energy 2021, 80, 105545. [Google Scholar] [CrossRef]
- Shi, W.; Yin, G.; Yu, S.; Hu, T.; Wang, X.; Wang, Z. Atomic precision tailoring of two-dimensional MoSi2N4 as electrocatalyst for hydrogen evolution reaction. J. Mater. Sci. 2022, 57, 18535–18548. [Google Scholar] [CrossRef]
- Liu, S.; Ban, J.; Shi, H.; Wu, Z.; Shao, G.; Cao, G.; Hu, J. Near solution-level conductivity of polyvinyl alcohol based electrolyte and the application for fully compliant Al-air battery. Chem. Eng. J. 2022, 431, 134283. [Google Scholar] [CrossRef]
- Kataoka, H.; Saito, Y.; Sakai, T.; Quartarone, E.; Mustarelli, P. Conduction Mechanisms of PVDF-Type Gel Polymer Electrolytes of Lithium Prepared by a Phase Inversion Process. J. Phys. Chem. B 2000, 104, 11460–11464. [Google Scholar] [CrossRef]
- Bai, S.; Wang, C.; Deng, M.; Gong, M.; Bai, Y.; Jiang, J.; Xiong, Y. Surface Polarization Matters: Enhancing the Hydrogen-Evolution Reaction by Shrinking Pt Shells in Pt–Pd–Graphene Stack Structures. Angew. Chem. Int. Ed. 2014, 53, 12120–12124. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Chen, P.; Zhou, T. A Bifunctional Hybrid Electrocatalyst for Oxygen Reduction and Evolution: Cobalt Oxide Nanoparticles Strongly Coupled to B, N-Decorated Graphene. Angew. Chem. Int. Ed. 2017, 56, 7121–7125. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhou, L.; Zhang, W. Stabilizing sulfur vacancy defects by performing “click” chemistry of ultrafine palladium to trigger a high-efficiency hydrogen evolution of MoS2. Nanoscale 2020, 12, 9943–9949. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Liu, H.; Qu, J. Earth-Rich Transition Metal Phosphide for Energy Conversion and Storage. Adv. Energy Mater. 2016, 6, 1600087. [Google Scholar] [CrossRef]
- Vrubel, H.; Hu, X. Molybdenum Boride and Carbide Catalyze Hydrogen Evolution in both Acidic and Basic Solutions. Angew. Chem. Int. Ed. 2012, 51, 12703–12706. [Google Scholar] [CrossRef]
- Abdelgawad, A.; Salah, B.; Lu, Q.; Abdullah, A.M.; Chitt, M.; Ghanem, A.; Al-Hajri, R.S.; Eid, K. Template-free synthesis of M/g-C3N4 (M = Cu, Mn, and Fe) porous one-dimensional nanostructures for green hydrogen production. J. Electroanal. Chem. 2023, 938, 117426. [Google Scholar] [CrossRef]
- Salah, B.; Abdelgawad, A.; Lu, Q.; Ipadeola, A.K.; Luque, R.; Eid, K. Synergistically interactive MnFeM (M = Cu, Ti, and Co) sites doped porous g-C3N4 fiber-like nanostructures for an enhanced green hydrogen production. Green Chem. 2023, 25, 6032–6040. [Google Scholar] [CrossRef]
- Song, B.; Jin, S. Two Are Better than One: Heterostructures Improve Hydrogen Evolution Catalysis. Joule 2017, 1, 220–221. [Google Scholar] [CrossRef]
- Su, J.; Zhuang, L.; Zhang, S.; Liu, Q.; Zhang, L.; Hu, G. Single atom catalyst for electrocatalysis. Chin. Chem. Lett. 2021, 32, 2947–2962. [Google Scholar] [CrossRef]
- Liu, L.; Wang, C.; Zhang, L.; Liu, C.; Niu, C.; Zeng, Z.; Ma, D.; Jia, Y. Surface Van Hove Singularity Enabled Efficient Catalysis in Low-Dimensional Systems: CO Oxidation and Hydrogen Evolution Reactions. J. Phys. Chem. Lett. 2022, 13, 740–746. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, G. Recent Advances and Applications of Inorganic Electrides. J. Phys. Chem. Lett. 2020, 11, 3841–3852. [Google Scholar] [CrossRef] [PubMed]
- Hosono, H.; Kitano, M. Advances in Materials and Applications of Inorganic Electrides. Chem. Rev. 2021, 121, 3121–3185. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Bao, A.; Mo, G. Effect of multi-walled carbon nanotubes on the electrochemical performance of LiVPO4F cathode material for rechargeable lithium-ion batteries. Solid State Ion. 2014, 264, 45–48. [Google Scholar] [CrossRef]
- Khan, K.; Tareen, A.K.; Aslam, M.; Thebo, K.H.; Khan, U.; Wang, R.; Shams, S.S.; Han, Z.; Ouyang, Z. A comprehensive review on synthesis of pristine and doped inorganic room temperature stable mayenite electride, [Ca24Al28O64]4+(e−)4 and its applications as a catalyst. Prog. Solid State Chem. 2019, 54, 1–19. [Google Scholar] [CrossRef]
- Lu, Y.; Li, J.; Tada, T.; Toda, Y.; Ueda, S.; Yokoyama, T.; Kitano, M.; Hosono, H. Water Durable Electride Y5Si3: Electronic Structure and Catalytic Activity for Ammonia Synthesis. J. Am. Chem. Soc. 2016, 138, 3970–3973. [Google Scholar] [CrossRef] [PubMed]
- Ye, T.-N.; Lu, Y.; Li, J.; Nakao, T.; Yang, H.; Tada, T.; Kitano, M.; Hosono, H. Copper-Based Intermetallic Electride Catalyst for Chemoselective Hydrogenation Reactions. J. Am. Chem. Soc. 2017, 139, 17089–17097. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, Z.; Lei, H.; Toda, Y.; Matsuishi, S.; Kamiya, T.; Ueda, S.; Hosono, H. Two-Dimensional Transition-Metal Electride Y2C. Chem. Mater. 2014, 26, 6638–6643. [Google Scholar] [CrossRef]
- Aliakbari, A.; Amiri, P.; Salehi, H. First-principles investigation of the structural and dynamical stability, electronic and thermal properties of two-dimensional Yn+1Cn (n = 1, 2, and 3) MXenes. FlatChem 2022, 31, 100328. [Google Scholar] [CrossRef]
- Wang, H.; Choi, J.-H. Hydrogen Adsorption on the Vertical Heterostructures of Graphene and Two-Dimensional Electrides: A First-Principles Study. ACS Omega 2022, 7, 16063–16069. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Jin, K.-H.; Zhang, S.; Liu, F. Topological Electride Y2C. Nano Lett. 2018, 18, 1972–1977. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Nie, K.; Shao, Z.-J.; Gao, B.; Lin, H.; Zhang, H.; Liu, B.; Wang, Y.; Zhang, Y.; Sun, X.; et al. Phosphorus-Mo2C@carbon nanowires toward efficient electrochemical hydrogen evolution: Composition, structural and electronic regulation. Energy Environ. Sci. 2017, 10, 1262–1271. [Google Scholar] [CrossRef]
- Xu, K.; Ding, H.; Zhang, M.; Chen, M.; Hao, Z.; Zhang, L.; Wu, C.; Xie, Y.J.A.M. Regulating Water-Reduction Kinetics in Cobalt Phosphide for Enhancing HER Catalytic Activity in Alkaline Solution. Adv. Mater. 2017, 29, 1606980. [Google Scholar] [CrossRef] [PubMed]
- Han, N.; Yang, K.R.; Lu, Z.; Li, Y.; Xu, W.; Gao, T.; Cai, Z.; Zhang, Y.; Batista, V.S.; Liu, W.; et al. Nitrogen-doped tungsten carbide nanoarray as an efficient bifunctional electrocatalyst for water splitting in acid. Nat. Commun. 2018, 9, 924. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Wang, Y. Generalized gradient approximation for the exchange-correlation hole of a many-electron system. Phys. Rev. B 1996, 54, 16533. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [PubMed]
- Sa, B.; Li, Y.-L.; Qi, J.; Ahuja, R.; Sun, Z.J.J.O.P.C.C. Strain Engineering for Phosphorene: The Potential Application as a Photocatalyst. J. Phys. Chem. C 2014, 118, 26560–26568. [Google Scholar] [CrossRef]
- Nørskov, J.K.; Bligaard, T.; Logadottir, A.; Kitchin, J.R.; Chen, J.G.; Pandelov, S.; Stimming, U. Trends in the Exchange Current for Hydrogen Evolution. J. Electrochem. Soc. 2005, 152, J23. [Google Scholar] [CrossRef]
- Mir, S.H.; Chakraborty, S.; Jha, P.C.; Wärnå, J.; Soni, H.; Jha, P.K.; Ahuja, R. Two-dimensional boron: Lightest catalyst for hydrogen and oxygen evolution reaction. Appl. Phys. Lett. 2016, 109, 053903. [Google Scholar] [CrossRef]
- Liu, B.; Chen, Z.; Xiong, R.; Yang, X.; Zhang, Y.; Xie, T.; Wen, C.; Sa, B. Enhancing hydrogen evolution reaction performance of transition metal doped two-dimensional electride Ca2N. Chin. Chem. Lett. 2023, 34, 107643. [Google Scholar] [CrossRef]
- Cao, X.; Bai, H.; Wu, W.; Bao, H.; Li, Y. Improving the catalytic activity of two-dimensional Mo2C for hydrogen evolution reaction by doping and vacancy defects. Int. J. Hydrogen Energy 2022, 47, 38517–38523. [Google Scholar] [CrossRef]
- Gong, Q.; Wang, Y.; Hu, Q.; Zhou, J.; Feng, R.; Duchesne, P.N.; Zhang, P.; Chen, F.; Han, N.; Li, Y.; et al. Ultrasmall and phase-pure W2C nanoparticles for efficient electrocatalytic and photoelectrochemical hydrogen evolution. Nat. Commun. 2016, 7, 13216. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).