1. Introduction
Non-ferrous metal alloys are generally isotropic materials, which are much easier to repair than composite materials. However, polymer composites containing carbon or glass fibres have improved characteristics such as excellent fatigue resistance, high stiffness, and durability. When considering metal materials themselves, problems related to fatigue properties and corrosion may be encountered. Despite the improved characteristics of polymeric composites, their poor ability to be repaired, for example, in aircraft skins, resulted in the development of fibre metal laminates (FML) in the 1980s [
1]. These laminates contain alternating metal layers such as aluminium, titanium, or magnesium alloys combined with a polymer composite, such as one containing carbon or glass fibres. In this way, the features of two different structures are combined, which provide new properties, such as improved repairability or increased impact resistance. Moreover, FMLs ensure low moisture absorption, are lightweight, and have high resistance to the propagation of fatigue cracks perpendicular to the fibre direction, due to the fibre bridging mechanism [
2,
3,
4,
5,
6].
Botelho et al. [
4] and Papanicolaou et al. [
7] claim that the creation of a permanently bonded contact surface between the metal alloys and the polymer was particularly important in the case of FMLs combined with fibre-reinforced composites. The authors have also reported a significant difference in the mechanical performance between fibre-reinforced composites and FMLs, with the latter showing improved characteristics.
When considering the defects that may occur in FMLs, attention should be paid to the formation of cracks at the interface between the metallic and polymer layers, the occurrence of damage between the polymer layers, and poor adhesion related to the improper preparation of the metal surface. The degradation of laminates, especially those made of aluminium or magnesium combined with layers of polymeric composites based on carbon fibres, may result in the occurrence of galvanic corrosion, particularly in a NaCl electrolyte medium. Ostapiuk et al. [
8] proved that FMLs with a surface prepared by anodising aluminium in sulfuric and chromic acid containing carbon fibres are prone to greater and faster corrosion than those containing glass fibres. The formation of a galvanic cell at the junction of two different layers, with distinct corrosion parameters, is responsible for the increased corrosion rate. The surface treatment of the metal layer before bonding is the most important factor in achieving high adhesion. Chemical conversion [
9,
10], micro-arc oxidation [
11,
12,
13], electrochemical deposition [
8,
14], and the sol–gel process [
15] were applied to improve the corrosion resistance of magnesium alloys.
To address the issue of accelerated corrosion in FMLs and protect the polymer/laminate interface, there are three distinct measures that can be considered: the surface treatment of the metal layer, the introduction of nano/micro fillers (e.g., carbon nanotubes or nanoclay) at the interface of the layers, and the incorporation of a repair agent between the layers (such as micro-/nanocapsules or microparticles). Self-healing materials have been the subject of study for several years and are currently employed in various industries. Among these materials, microcapsules are particularly interesting due to their versatility. The use of self-healing microcapsules allows a material to repair itself in response to external forces or changes in corrosion conditions.
Ostapiuk et al. [
14] used microcapsules in research on the interlaminar shear strength (ILSS) of FMLs. Their study focused on observing the effect of structure healing on the interface between the layers of magnesium and a polymer composite containing carbon fibres. Several self-healing agents have been widely proposed. Self-healing microcapsules are advantageous because of their versatility, simplicity, and ease of preparation and use. The use of microcapsules (MCs) for this application was first reported by White et al. [
16], who described the storage of a self-healing agent in capsules dispersed in resin. The growth of an interlayer crack ruptures the microcapsules, releasing their core content, a self-healing substance, into the fracture zone. This promotes the self-healing and repair of the layers. Kessler et al. [
11] reported on the incorporation of 20 wt% of microcapsules into the contact surface of a carbon fibre–epoxy laminate. They observed an improvement in resistance to interfacial cracking and a 37% self-healing efficiency. However, the original strength was reduced by almost 20%. Ostapiuk et al. [
14] reported that the presence of microcapsules does not impair the interlayer failure properties of the FML, especially when adding 1.5 wt% of microcapsules containing isophorone diisocyanate (IPDI). The microcapsules in the FML can also act as reinforcement in the layer. Jin et al. [
9] reported that the primary fracture toughness of the interlaminar epoxy–steel joint was improved in the presence of dicyclopentadiene (DCPD)-filled urea-formaldehyde microcapsules, using a double cantilever beam (DCB) fracture specimen. The research by Jin et al. [
10] revealed that incorporating polymer hollow and self-healing microcapsules at the interface reduced the fracture toughness of epoxy–steel substrates during the DCB fracture test.
The electrochemical changes that occur during the corrosion process and the mechanisms associated with the corrosion phenomenon are still being investigated, as well as the changes in pH. Electrochemically or pH-responsive materials can then be used to detect and control corrosion.
Microcapsules capable of controlling the release of their internal substance can be designed for use in self-healing applications that involve corrosion. Typically, microcapsules release their contents when they are mechanically broken. However, pH-sensitive microcapsules release their contents in response to corrosion. It is widely understood that mechanical damage to coatings is one of the primary causes of metal corrosion. Additionally, other types of coating defects, such as air bubbles, uneven thickness, penetration, porosity, or edge effects, should also be considered as they can result in the inadequate corrosion protection of the coating.
The microcapsule wall is designed to decompose and release the encapsulated contents in response to changes in pH at the cathodic site of localised corrosion. Various compounds, such as corrosion indicators, inhibitors, self-healing agents, and dyes, can be encapsulated for corrosion applications.
The importance of this work lies on the use of microcapsules in FML systems capable of offering double protection, both through the creation of a barrier and the anti-corrosion effect due to the encapsulation of the corrosion inhibitor. Such a system can provide active protection against both mechanical damage and corrosive factors. In this work, a simple procedure for the synthesis of polyurethane/polyurea (PU/PUa) microcapsules was used. In this article, the self-healing behaviour of FMLs and their coatings were assessed using open circuit potential (OCP), potentiodynamic polarisation, electrochemical impedance spectroscopy (EIS), and a corrosion chamber. The main objective of this work was to assess the self-healing capability of a magnesium/PEO layer utilising microcapsules in a corrosive environment. Furthermore, the study observed the corrosion resistance and self-healing phenomena of fibre metal laminates.
2. Materials and Methods
Isophorone diisocyanate (IPDI), with a purity of 98%, is the isocyanate to be encapsulated. It is commercially known as Desmodur® I and was supplied by Covestro AG (Leverkusen, Germany). The shell precursor, a polyisocyanate called Desmodur® RC, was kindly supplied by CIPADE S.A, Braga, Portugal. The emulsion stabiliser, gum arabic (GA), was purchased from Fisher Chemical (Porto, Salvo, Portugal). The active sources of H—poly(ethyleneimine) (PEI) aqueous solution (Mw 60,000, 50 wt% in H2O) and triethoxy(octyl)silane (n-OTES)—were obtained from Sigma-Aldrich (St. Louis, MO, USA).
2.1. Microcapsule Manufacturing
An oil-in-water (O/W) microemulsion system combined with interfacial polymerization processing was used to manufacture the MCs. The emulsion was produced by vigorously stirring the O and W phases. The O phase consisted of 4.85 wt% of the emulsion and was stirred at a speed of 1200 rpm for 10 min at room temperature (RT) using an Ultra-Turrax (IKA T25 digital ULTRA TURRAX, IKA, Frankfurt, Germany). The O phase of the emulsion was composed of two isocyanates: Desmodur® I, intended to be encapsulated, and Desmodur® RC, a solution of polyisocyanurate of toluene diisocyanate (TDI) in ethyl acetate at 25 wt%. The first isocyanate, the one intended to be encapsulated, comprised 51.2 wt% of the O phase, and Desmodur® RC comprised the remaining 48.8 wt%. The W phase was composed of water and gum arabic (GA), an emulsion stabiliser, present at 4.7 wt%. The emulsion system was subjected to a mild agitation of 500 rpm at 50 °C, and the active H sources, n-OTES (triethoxy(octyl)silane) and an aqueous solution of PEI (polyethylenimine), were added to the synthesis to contribute to the MC shell formation. The synthesis was carried out under the abovementioned conditions for 3 h and 30 min. The maturity of the MCs was evaluated during their fabrication using optical microscopy. Once the necessary maturity was achieved, the MCs were washed with water and centrifuged. The final MCs were dried at atmospheric pressure and room temperature for 96 h and could then be directly added to the sol–gel on magnesium alloy AZ31 and FMLs.
2.2. FML Manufacturing
The FMLs were configured as 2 plies of magnesium and 1 ply of polymer composite, forming a structure that is based on carbon/epoxy resin. The magnesium AZ31 alloy (0.5 mm, Luoyang Shengte Corp., Luoyang, China) served as the metallic layer in the FMLs. The polymer composite was made of unidirectional carbon fibre/epoxy prepreg tape (0.131 mm for one layer, AS7J Hexcel, Stamford, CT, USA).
The FMLs were manufactured in a high-quality autoclave chamber system. During the cure cycle, the pressure used in the autoclave was −0.08 MPa, and a vacuum of −0.02 MPa was applied. The curing temperature was 135 °C, the curing time was 2 h, and the heating/cooling rate was 0.033 K s−1.
2.3. FML Surface Preparation
The plasma electrolytic oxidation (PEO) process was the first stage in preparing the surface of the magnesium AZ31 alloy, which had a thickness of 0.5 mm. The process involved several steps: (i) cleaning/degreasing the surface in an alkaline Na2SiO3 solution; (ii) activating it in 10% HF; (iii) rinsing it in distilled water; and (iv) cleaning the coated samples in distilled water and drying them in controlled ambient-like conditions (Micro Arc, Gliwice, Poland). The aqueous electrolyte used for the PEO process contained 50 g L−1 of sodium metasilicate Na2SiO3 and 40 g L−1 of sodium hydroxide. A constant current of 5 A dm−2 was employed, with a high voltage of up to 400 V and a DC power source used to supply a unipolar pulse of 0.5 ms “on” time and 4.5 ms “off” time. This corresponded to a work cycle frequency of 200 Hz. The PEO treatment process was conducted for 10 min, and the pH was 13.1.
2.4. FML with Microcapsules
The magnesium AZ31 alloy with the PEO layer was coated with a sol–gel. The commercial EC 2333 (3 M Scotch WeldTM, New York, NY, USA) primer, based on resin and inorganic–organic silanes (sol–gel based on toluene, 3-(trimethoxysilyl)propyl glycidyl ether), was used. The primer was applied to the Mg/PEO surface using an atomiser after being mixed with selected amounts of MCs. Afterwards, the samples were dried for 72 h at room temperature in a dryer. A total of 5 wt% of microcapsules was added to the surface of the magnesium alloy AZ31 and FML.
2.5. OCP, Potentiodynamic Polarisation, and EIS Tests
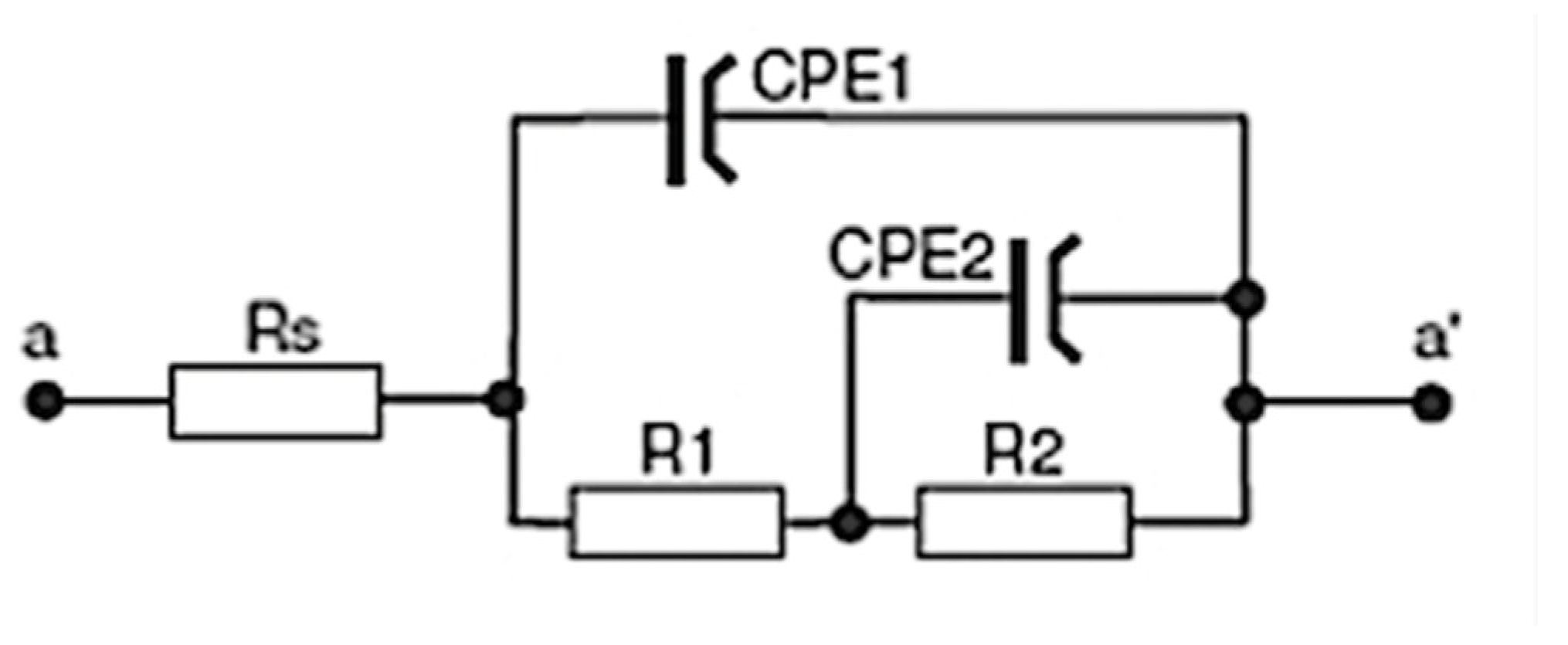
The corrosion resistance of the AZ31 Mg substrate with the sol–gel coating containing the MCs was evaluated using open circuit potential (OCP), polarisation curve, and impedance (EIS) tests. The tests were conducted in a 5 wt% NaCl (aq) solution at room temperature. An Atlas 0531 potentiostat (Atlas-Sollich, Rębiechowo, Poland) with a Frequency Response Analyser (FRA) was used to measure the tests. The samples were directly analysed in the measurement program. The area of the coatings being investigated was limited to 5 cm2, using a flat cell. Three samples were used per test, and the average results are shown on the graphs. The solution was exposed to air and left unstirred.
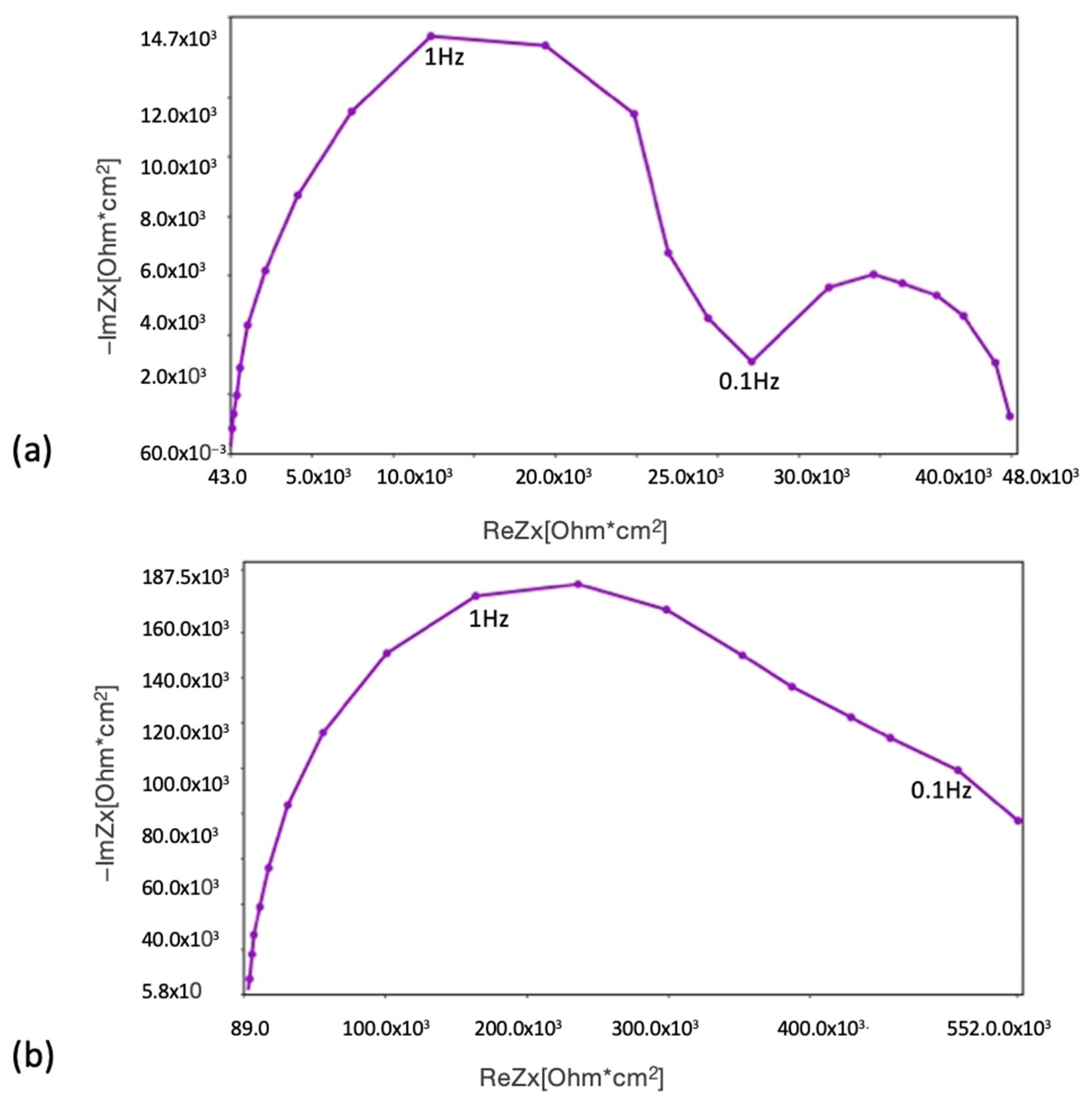
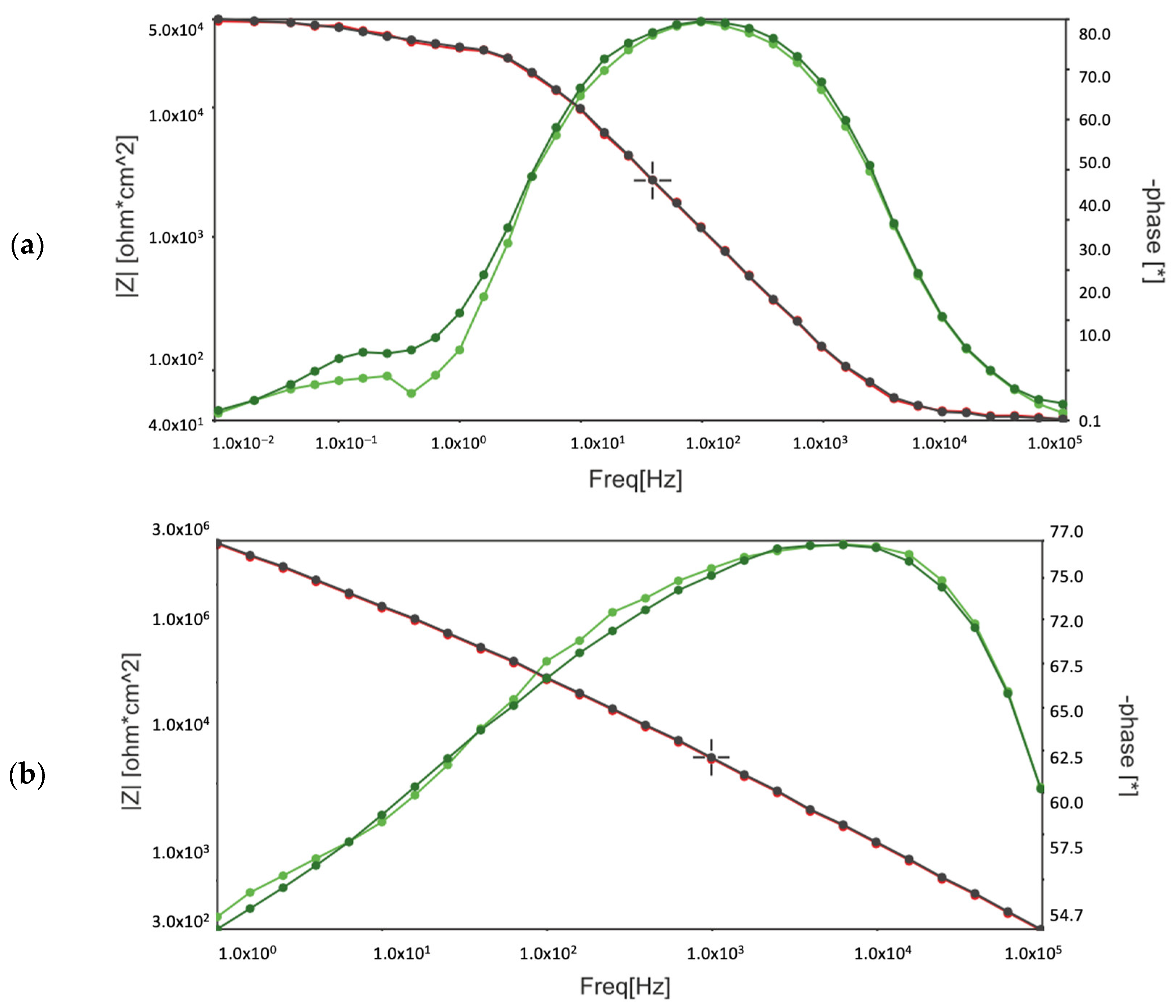
The three-electrode cell consisted of a saturated calomel reference electrode (SCE), a platinum foil electrode, and the test sample (as the working electrode in a horizontal position). A neutral 5% NaCl solution was used for the OCP and potentiodynamic tests at ambient temperature. The NaCl solution used in the corrosion chamber was the same, in order to show the main comparative results. The potentiodynamic current–potential curves were recorded by polarising the specimen from 100 kHz to 0.01 Hz relative to the OCP at a scan rate of 1 mV/s. Next, the EIS tests were carried out after 1 h and 150 h of immersion. The amplitude of the sinusoidal signal was 10 mV versus the open circuit potential, and the frequency span was 100 kHz to 0.01 Hz. The impedance spectra are presented in the form of Bode and Nyquist plots.
2.6. Experiment in Corrosion Chamber
Tests were conducted in a corrosion salt chamber (ASCOTT CC450 xp, Ascott Analytical Equipment Limited, Staffordshire, UK) on magnesium AZ31 and FMLs with and without MCs. A 5 wt% NaCl (aq) electrolyte was used at 37 °C and 95% relative humidity (RH) for six weeks, following EN ISO 9227 [
6]. After each week, three samples were collected. An X-shaped graze was made on the coating of the magnesium AZ31 alloy to expose the substrate and assess the healing process of the MCs. The scratch test involved manually creating 2 cm crossed-scratched lines (X-cut) on samples of magnesium alloy AZ31 using a razor blade, with appropriate surface preparation. The scratches had a depth of approximately 50 μm. The scratched samples were then observed using stereoscopic microscopy.
The tests were performed in a 5 wt% NaCl (aq) solution after three different periods of two, four, and six weeks for both the magnesium AZ31 alloy and the FML. The dimensions of the FML samples were 50 mm × 50 mm, and the edges were coated with epoxy to protect against moisture penetration into the composite and prevent undesired premature corrosion at the edges.
2.7. Macro- and Micro-Structure Analysis
The macro- and micro-structures of the samples before and after the corrosion tests were examined using a stereoscopic microscope (NIKON SMZ 1500, Nikon, Tokyo, Japan) and a scanning electron microscope (SEM, NovaNanoSEM 450, FEI Company Japan Ltd., Tokyo, Japan). The SEM was operated in a low vacuum mode, with a pressure of 100 Pa and voltages of 10 and 15 keV applied.
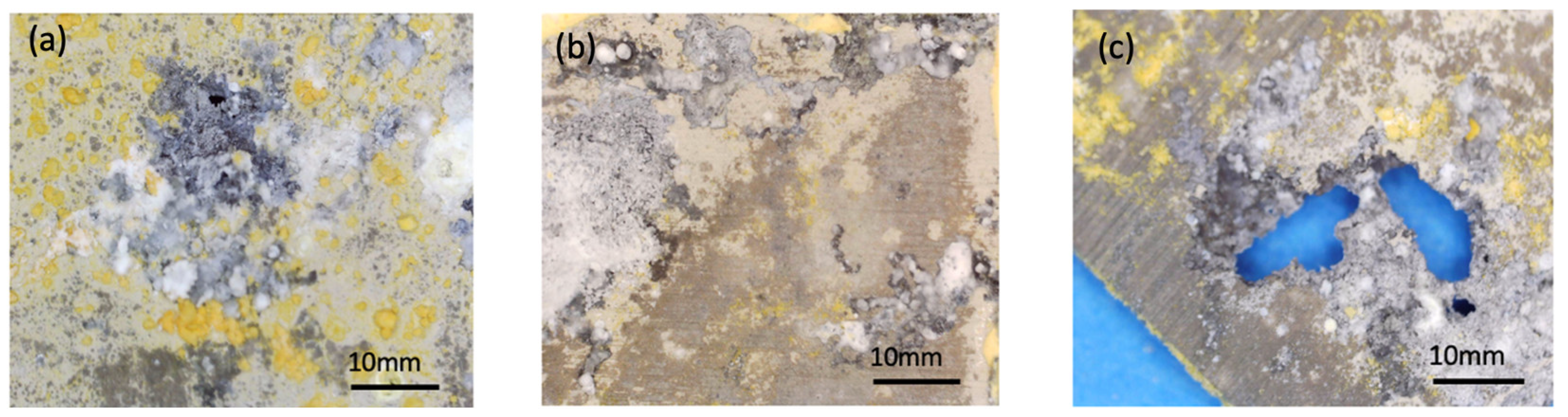
4. Discussion
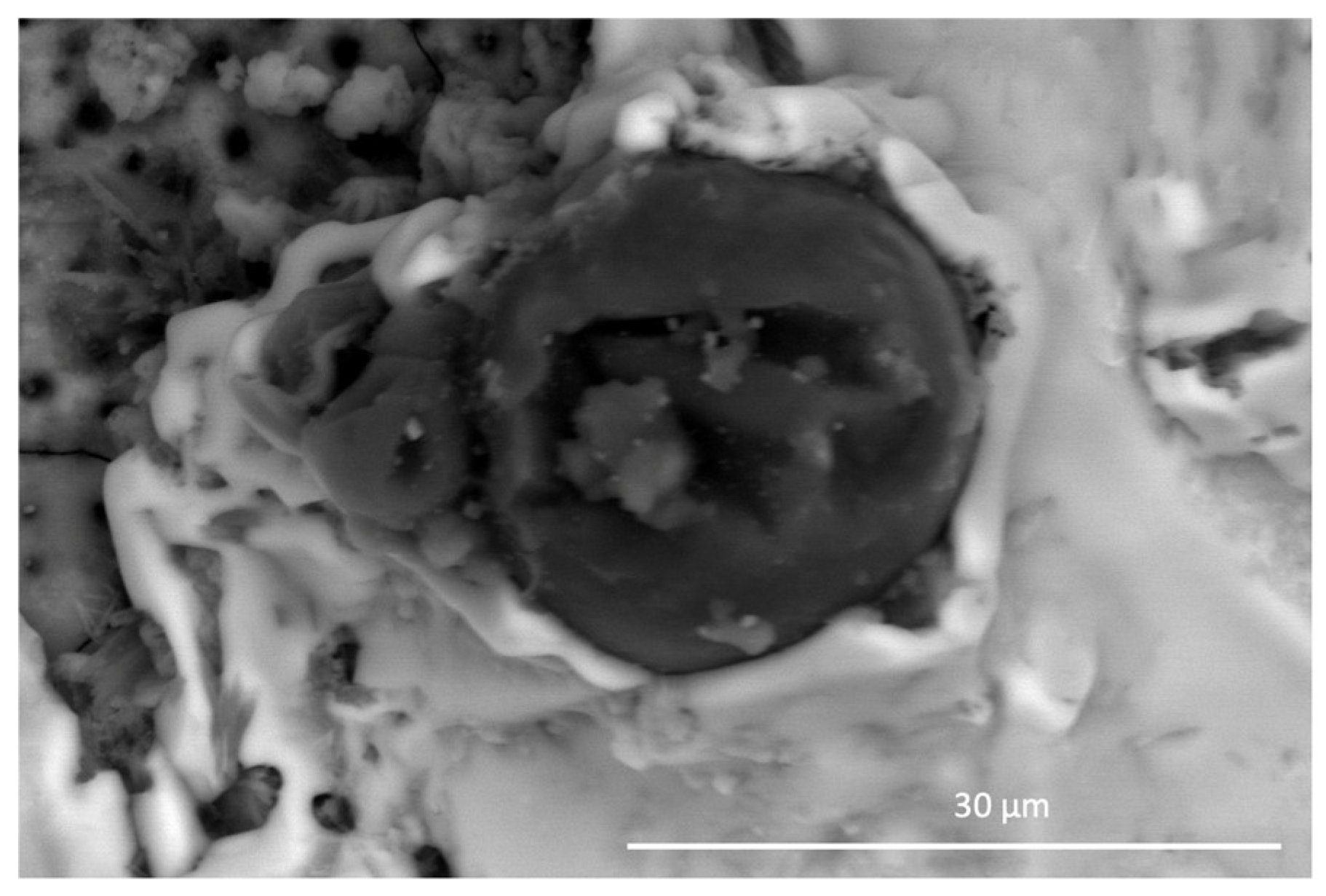
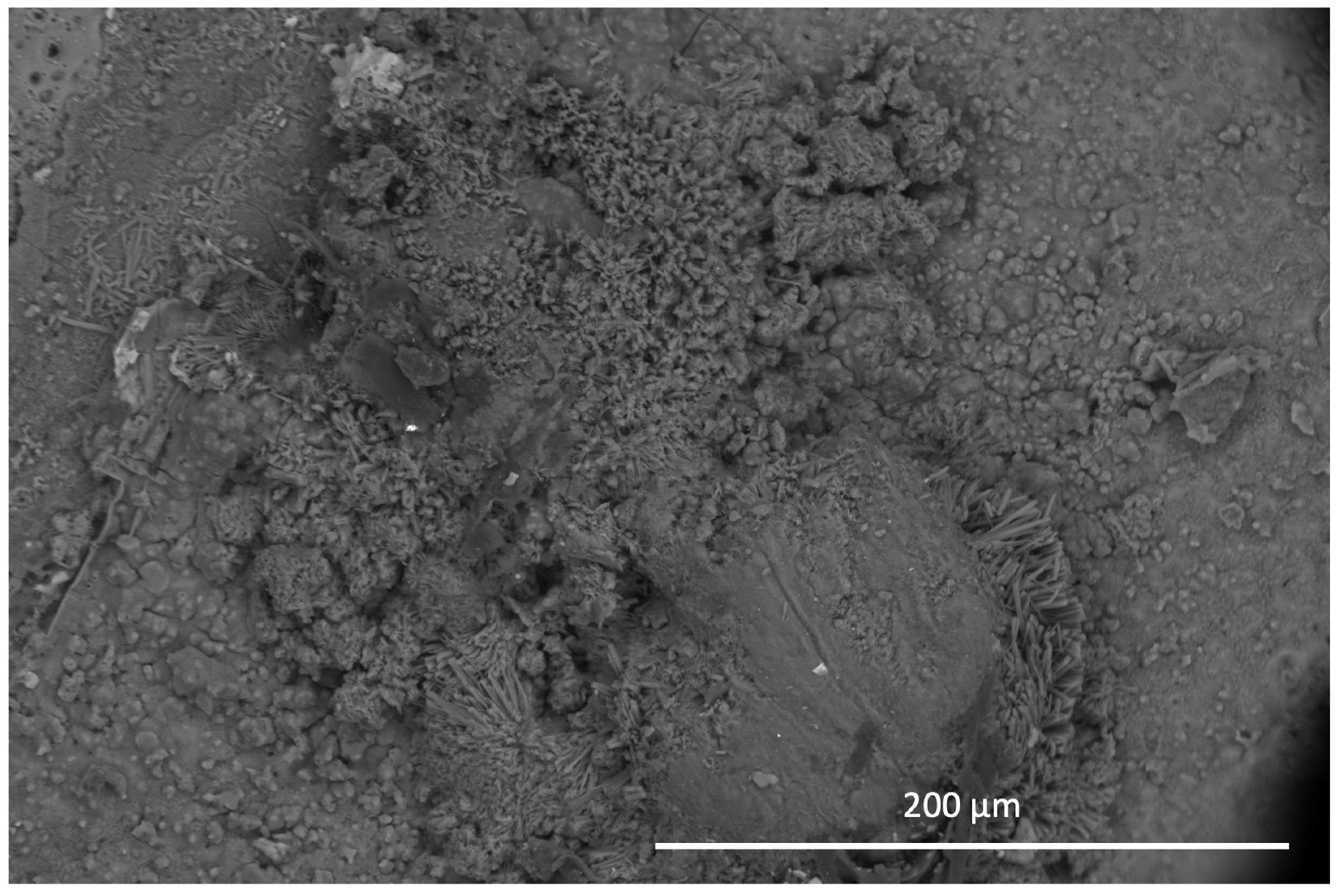
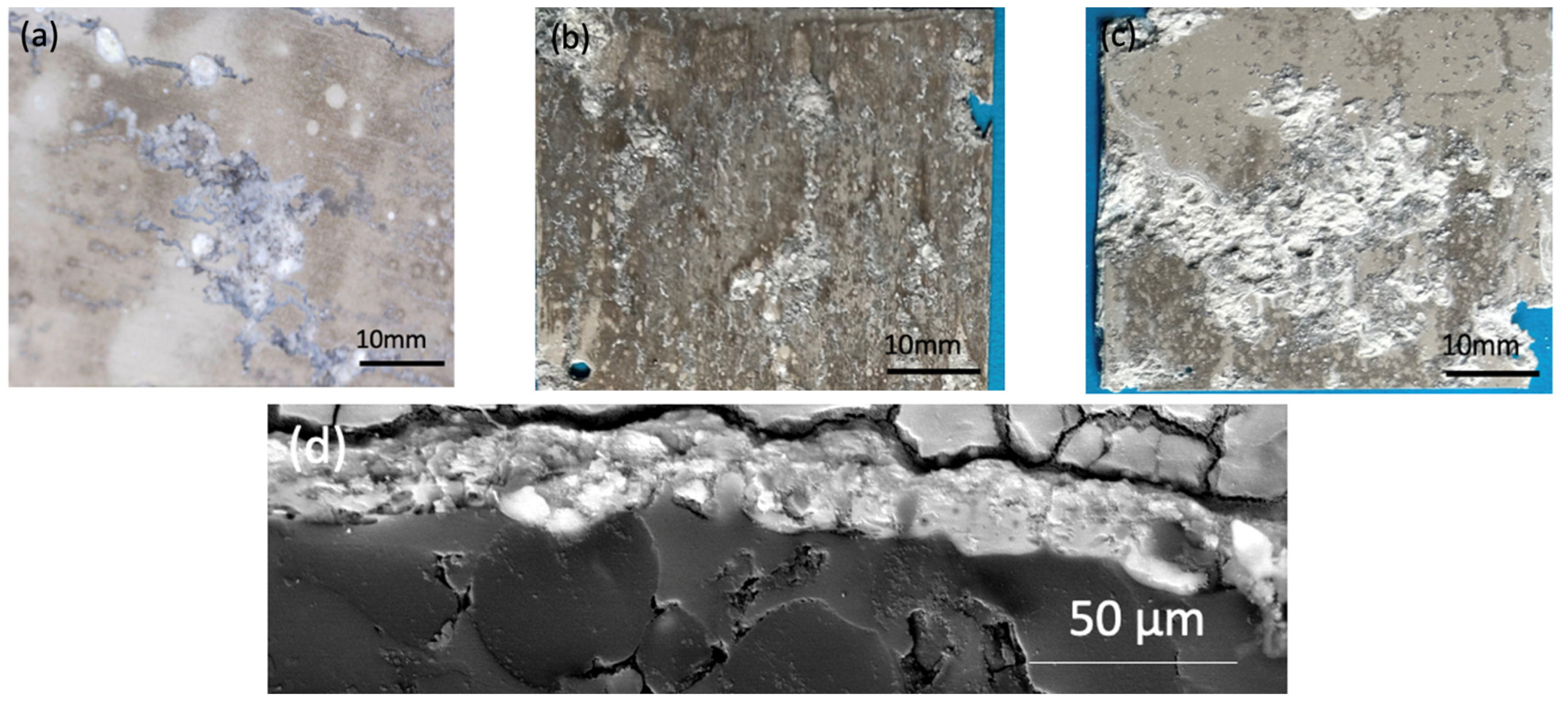
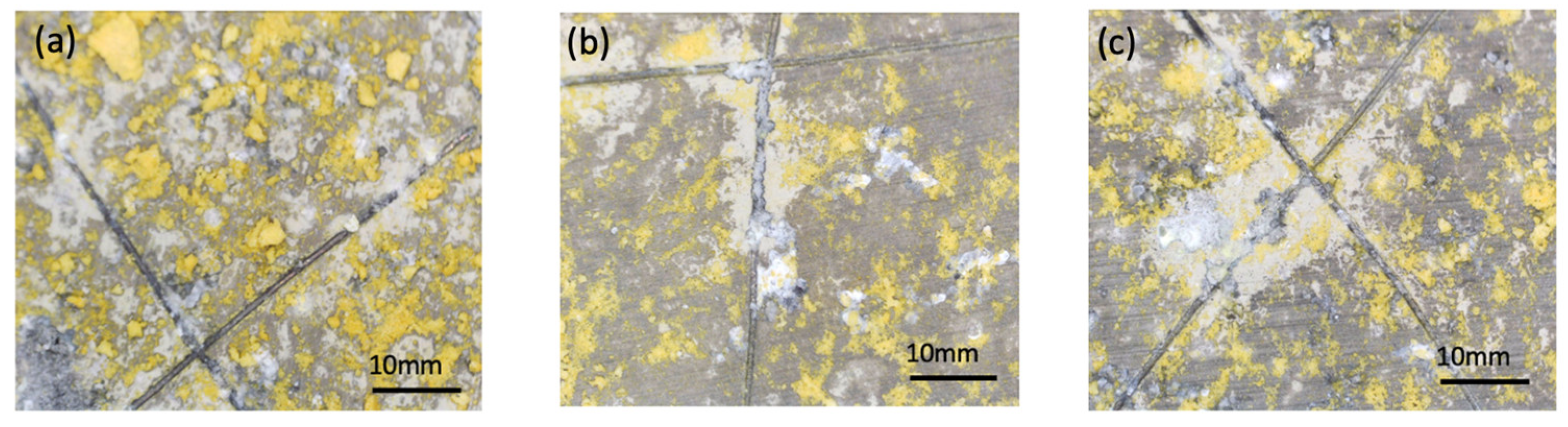
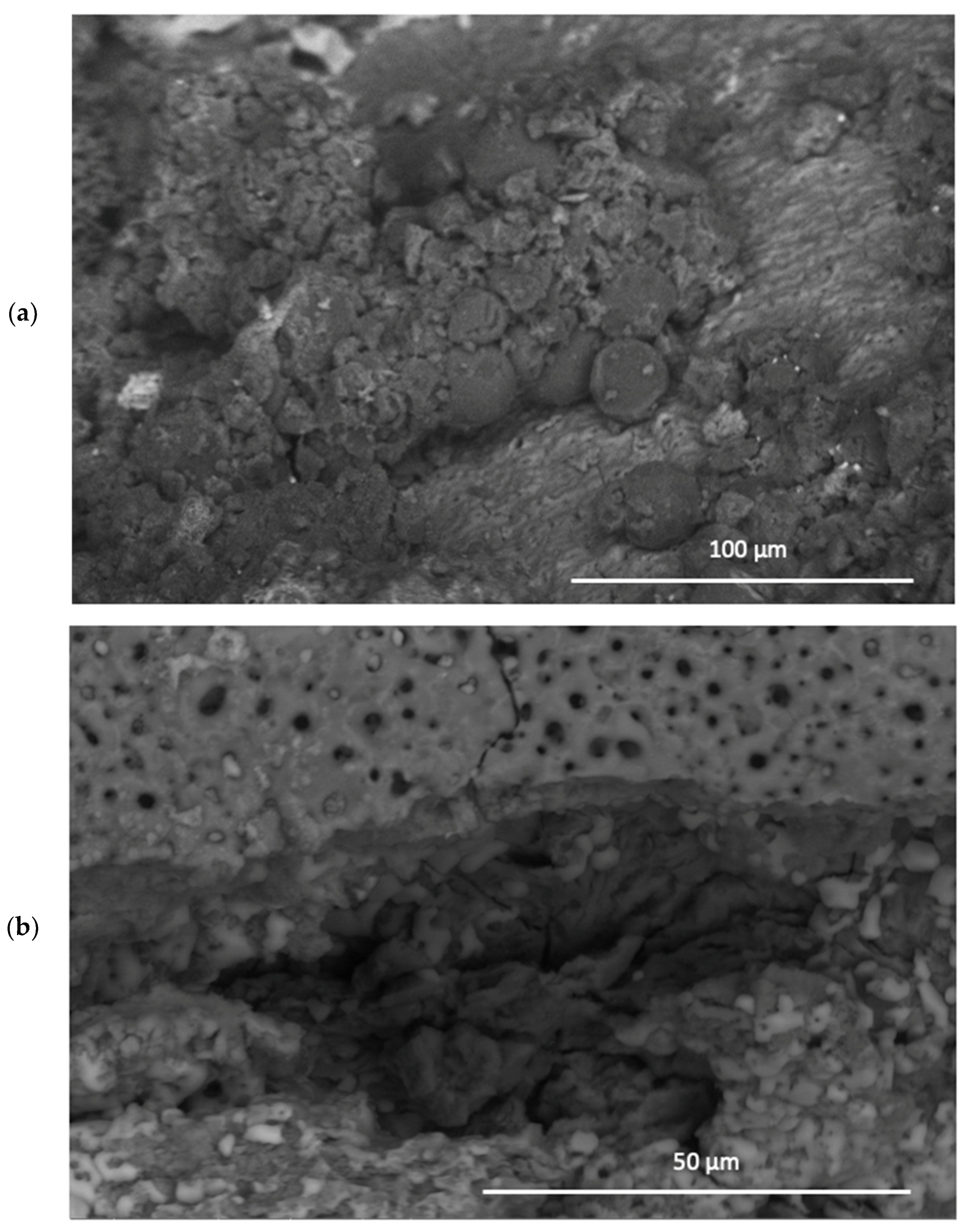
All samples were exposed to a 5 wt% NaCl environment in a corrosion chamber for 6 weeks. The MCs show a uniform distribution throughout the coating in the samples to which they were added. On the other hand, the samples without MCs reveal the phenomenon of intergranular corrosion. The SEM micrographs of the scratched coating and the self-healing coating are displayed in
Figure 10. The IPDI polymerised and formed a solid layer. Indeed, the IPDI was released from the MCs and automatically covered the scratch on the coating surface. The MCs are shown to provide excellent corrosion protection under the abovementiones conditions. After 48 h of exposure to the NaCl solution, no signs of corrosion were observed.
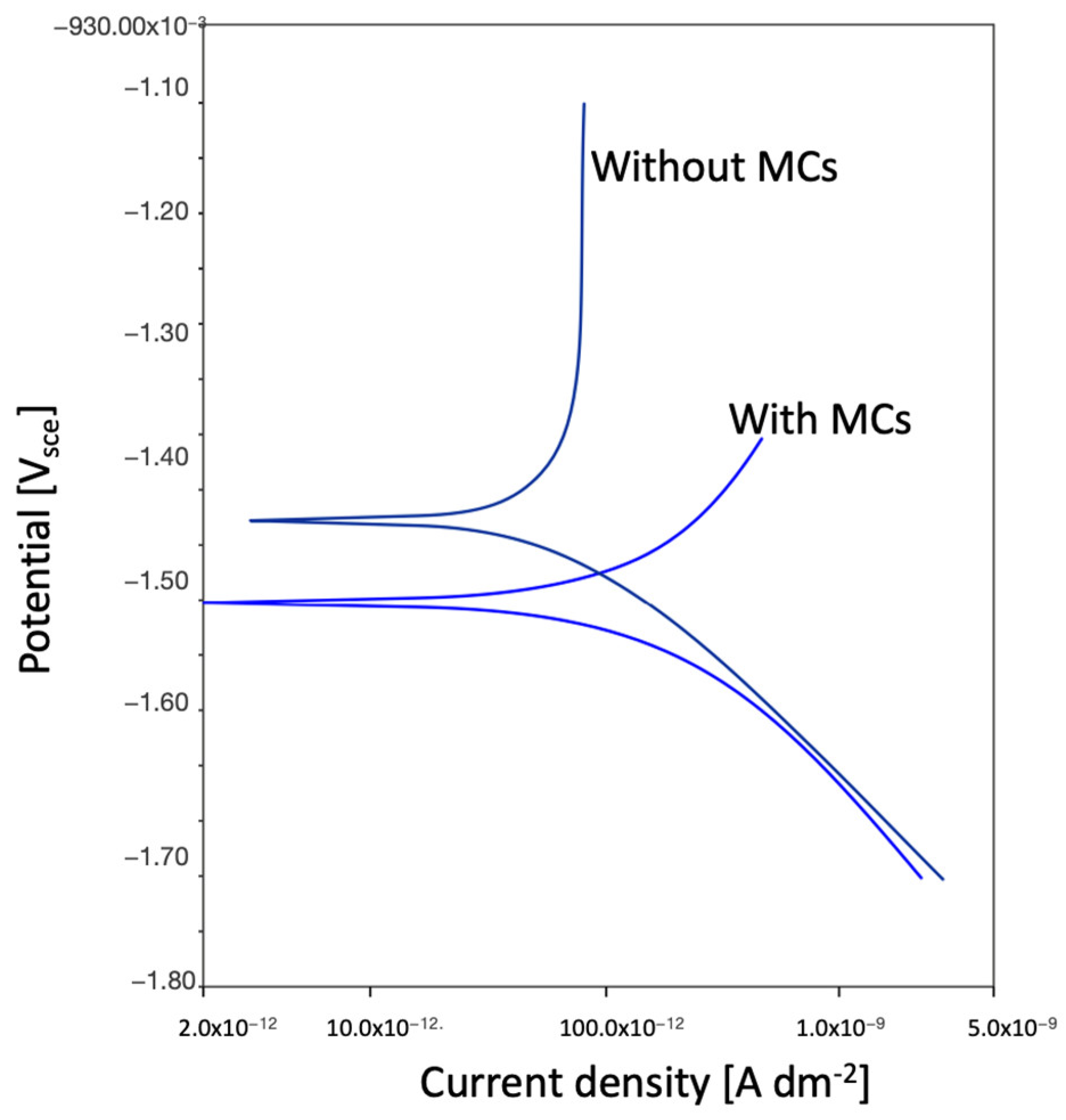
Comparing the test results obtained from EIS, potentiodynamic tests, and the corrosion chamber, it can be seen that the MCs layer obtained on AZ31 fulfils its role. It is noticeable that without MCs, corrosion clearly progresses after 1 h. However, self-healing mechanisms occur in the polymer layer with MCs. In these tests, the same pattern of corrosion phenomena, especially pitting corrosion, was observed. However, the polymer layer with MCs clearly affects the protection of the magnesium layer against subsequent corrosion phenomena. When pitting corrosion occurs, MCs release a self-healing agent, thereby reducing the effects of corrosion.
An issue that needs to be considered is the solubility of the corrosion inhibitors. When formulating the coating, it is important to select an inhibitor that can offer effective corrosion protection. The “solubility window” should be taken into account, as low solubility can lead to a reduction or absence of ions that inhibit corrosion. On the other hand, using too many inhibitors can cause them to wash out and diffuse onto the metal surface. If solubility is higher, the inhibitors may leach out of the coating and be lost, resulting in limited protection for the substrate over a certain period of time.
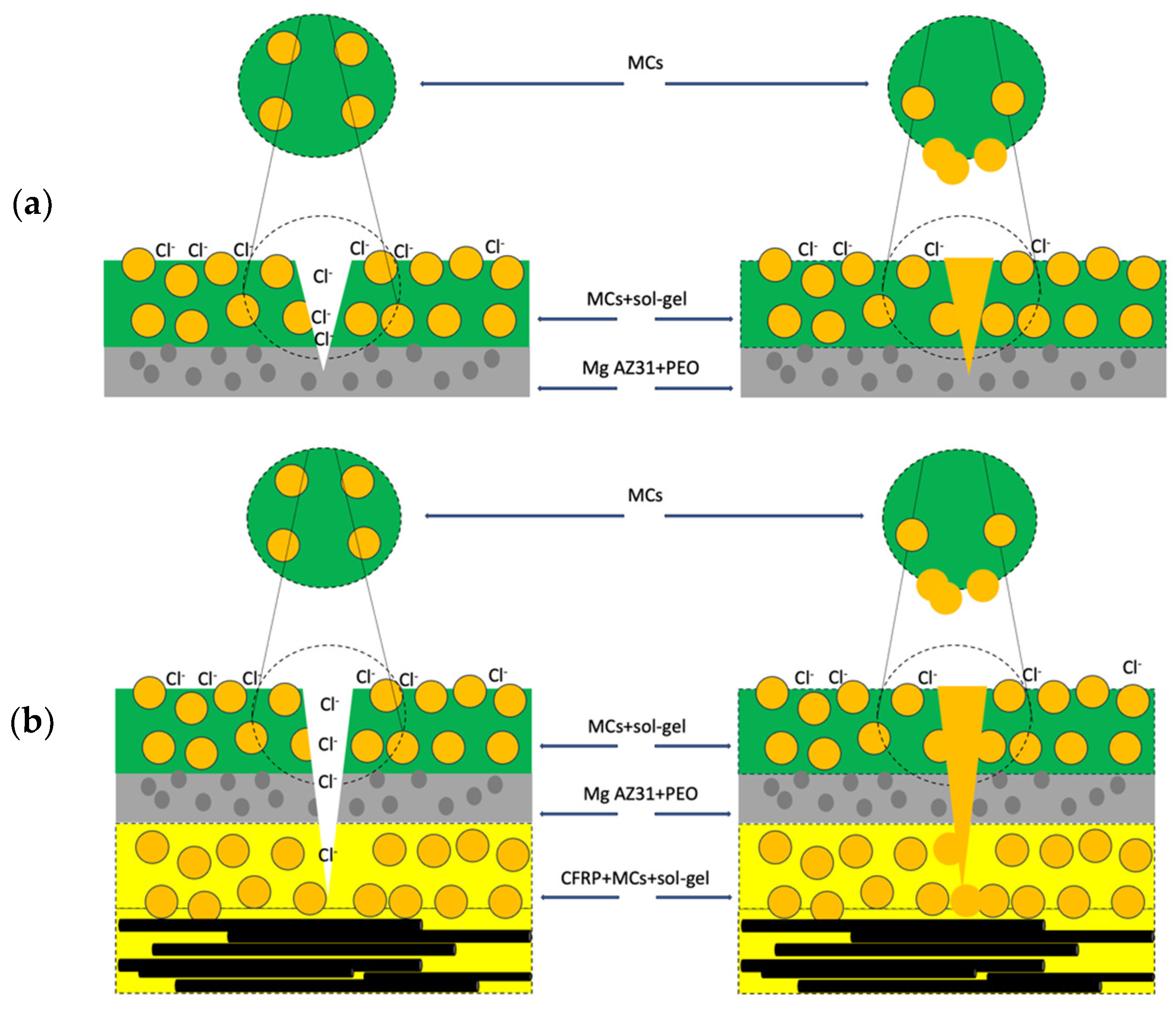
A diagram illustrating the inhibition mechanism provided by the MCs loaded with IPDI on the Mg/PEO and in the FML is presented in
Figure 11. This diagram reveals the hydrophobic-based corrosion mechanism during the adsorption process.