Preparation Process Optimization for Melamine Resin-Covered Pomelo Peel Flavonoid Antibacterial Microcapsules and Their Effect on Waterborne Paint Film Performance
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Preparation of Microcapsules
2.3. Paint Film Preparation Method
2.4. Testing and Characterization
2.4.1. Microencapsulation Yield and Coverage Rate
2.4.2. Characterization of Morphology and Chemical Composition
2.4.3. Paint Film Antibacterial Experiment
2.4.4. Paint Film Optical Property Test
2.4.5. Mechanical Properties of Paint Films
3. Results and Discussion
3.1. Analysis of the Yield and Coverage Rate of the Microcapsules
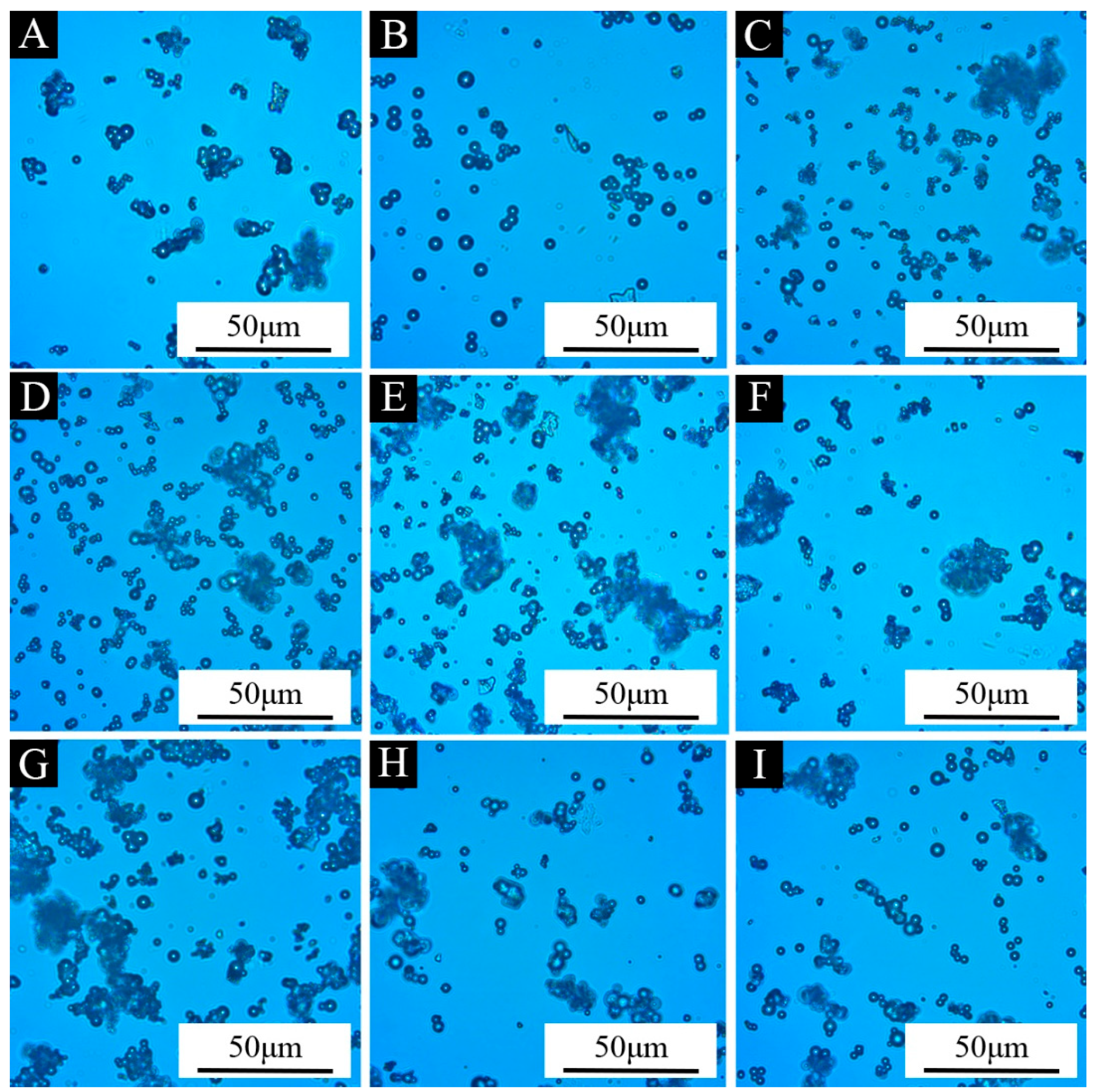
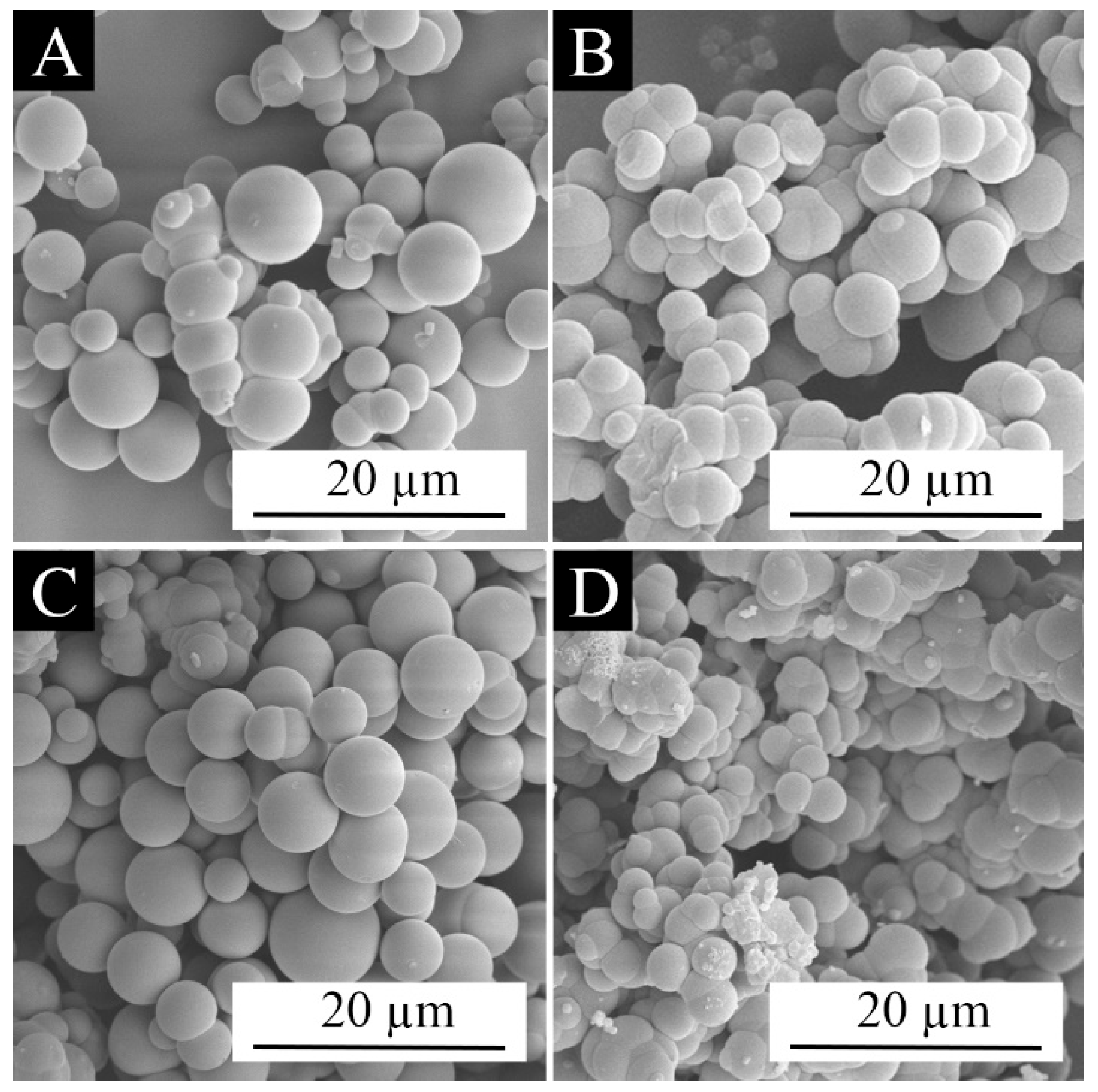
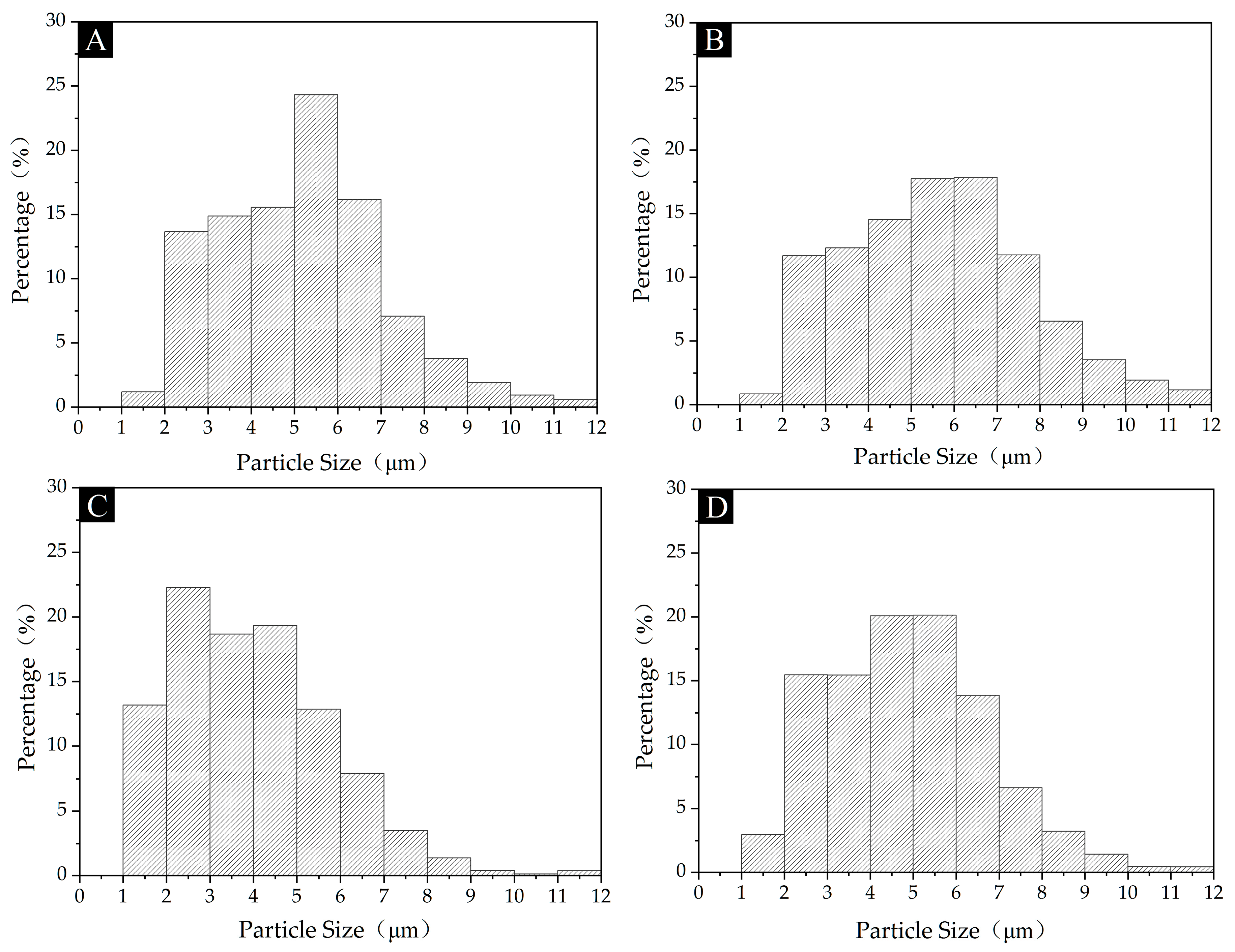
3.2. Morphological Analysis of Microcapsules
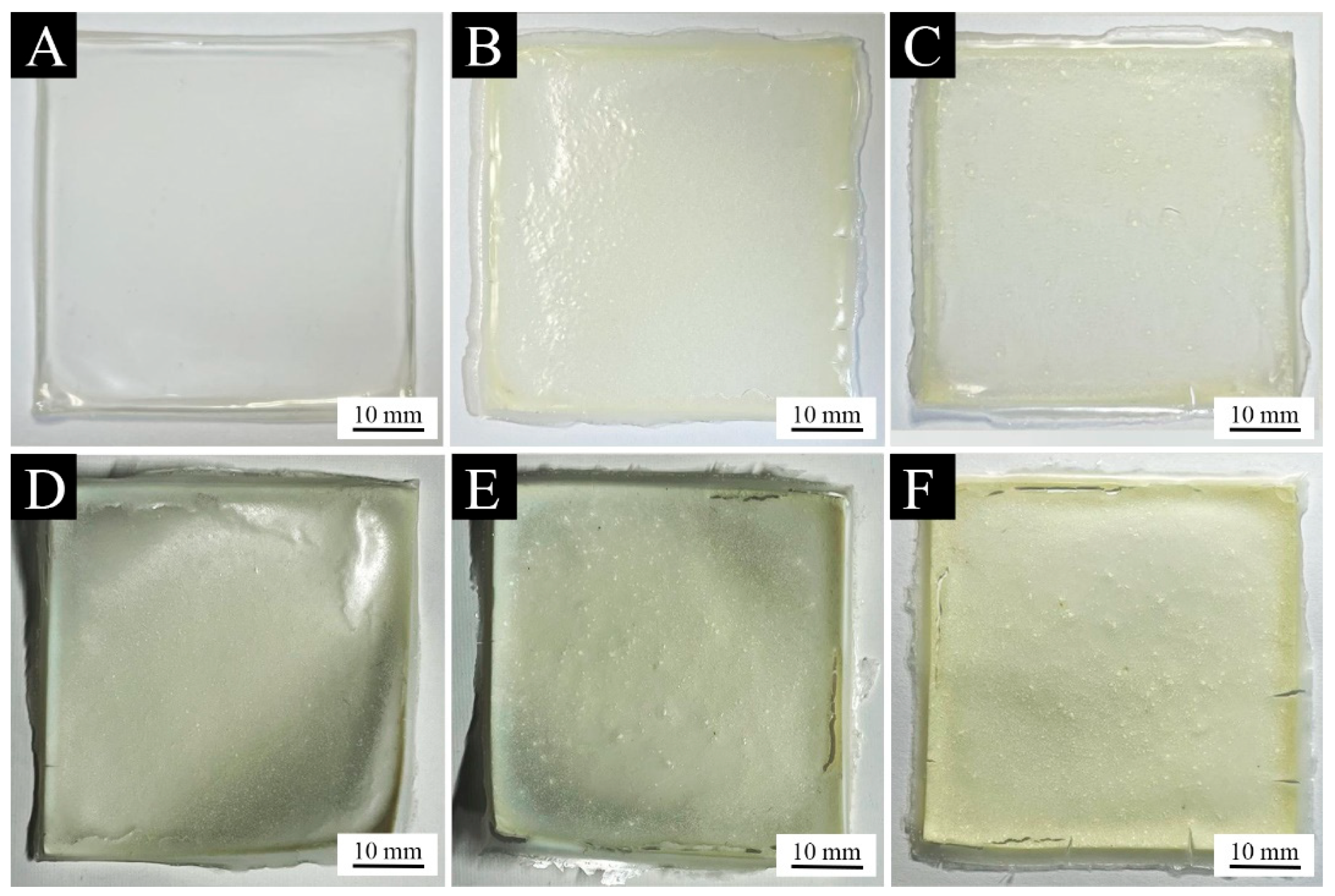
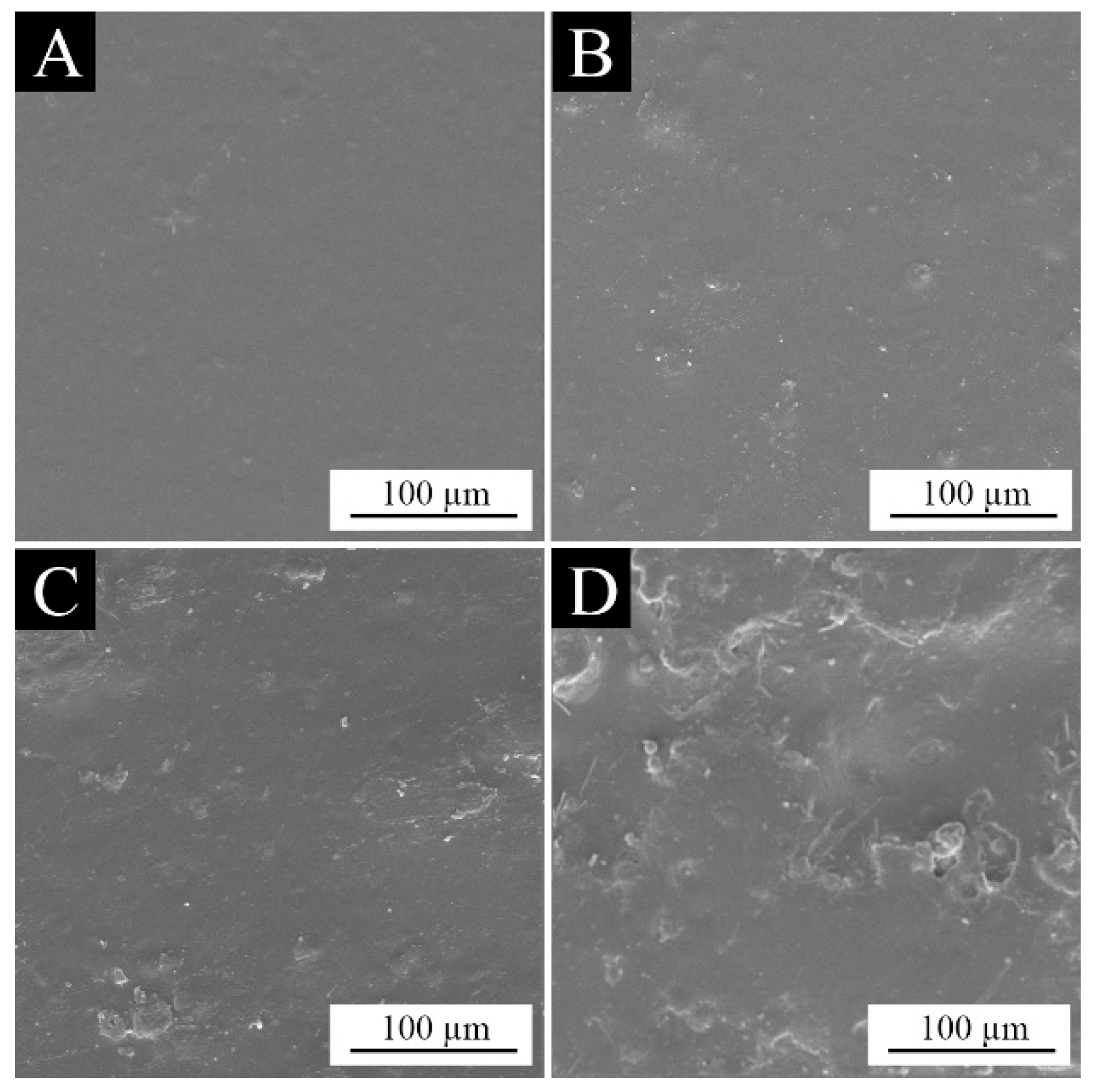
3.3. Analysis of the Morphology of the Paint Films
3.4. Chemical Composition Analysis of Paint Films
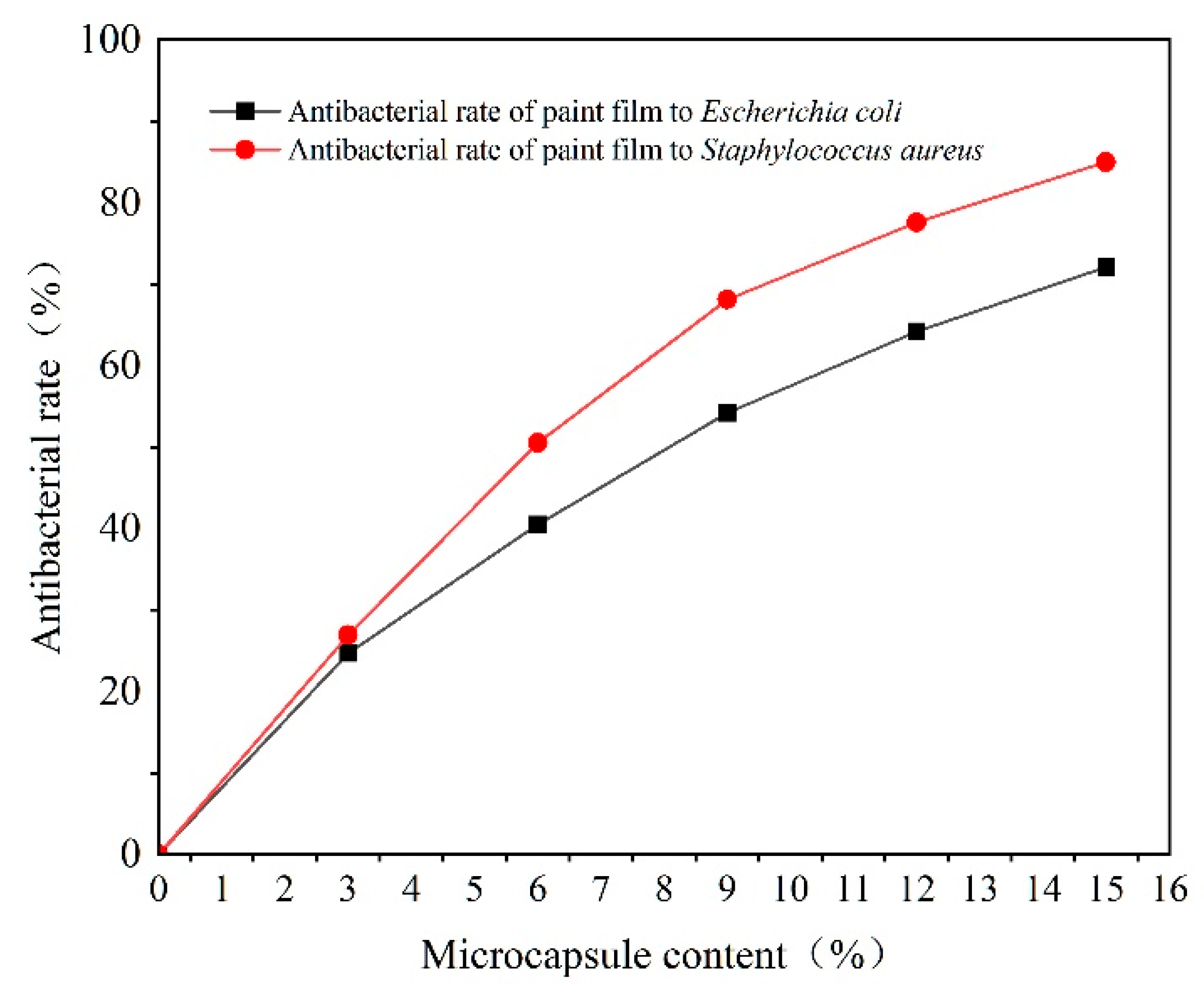
3.5. Analysis of the Effect of Microcapsules with Different Content Levels on the Antibacterial Properties of Paint Films
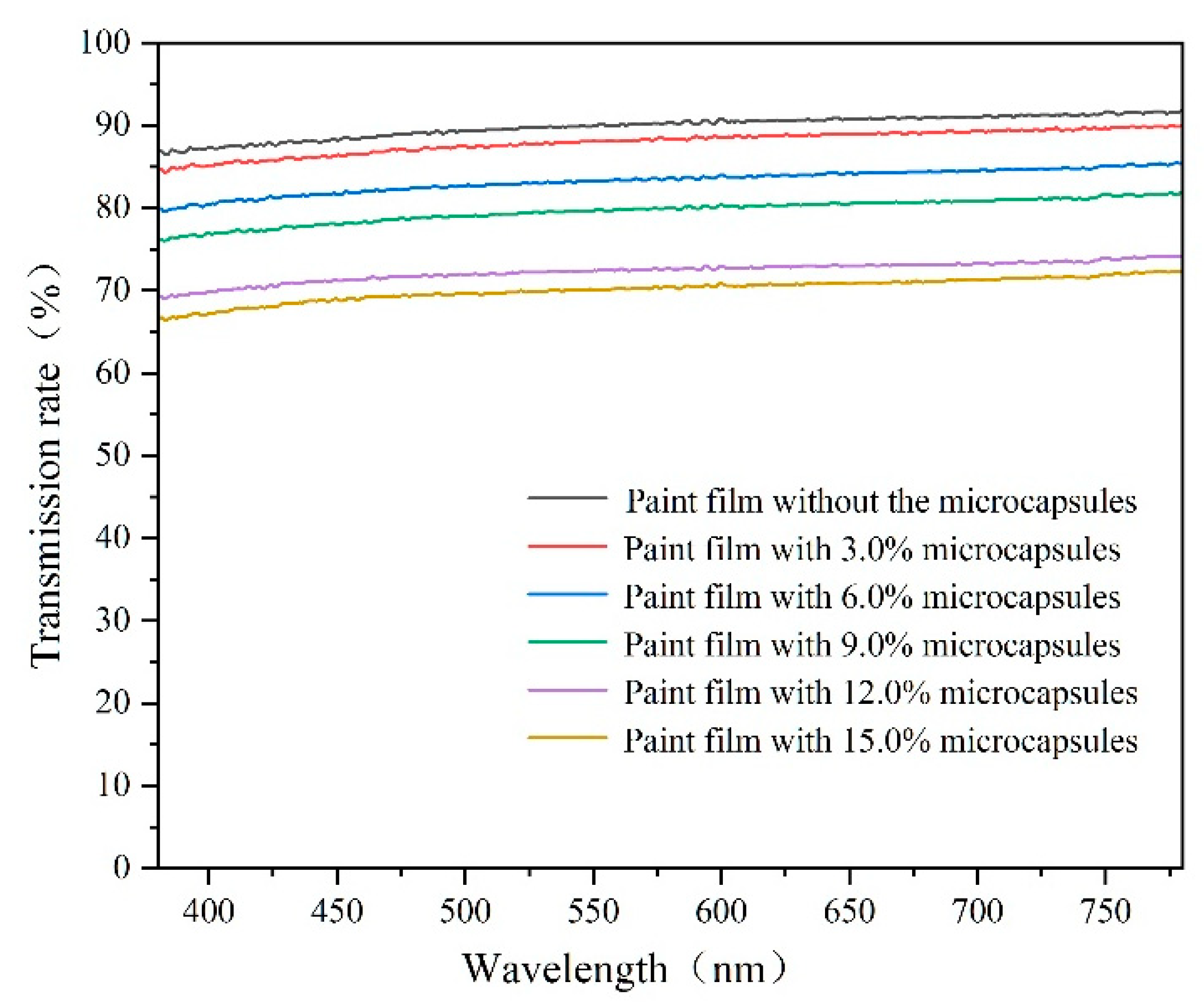
3.6. Analysis of the Effect of Microcapsules with Different Content Levels on the Optical Properties of Paint Films
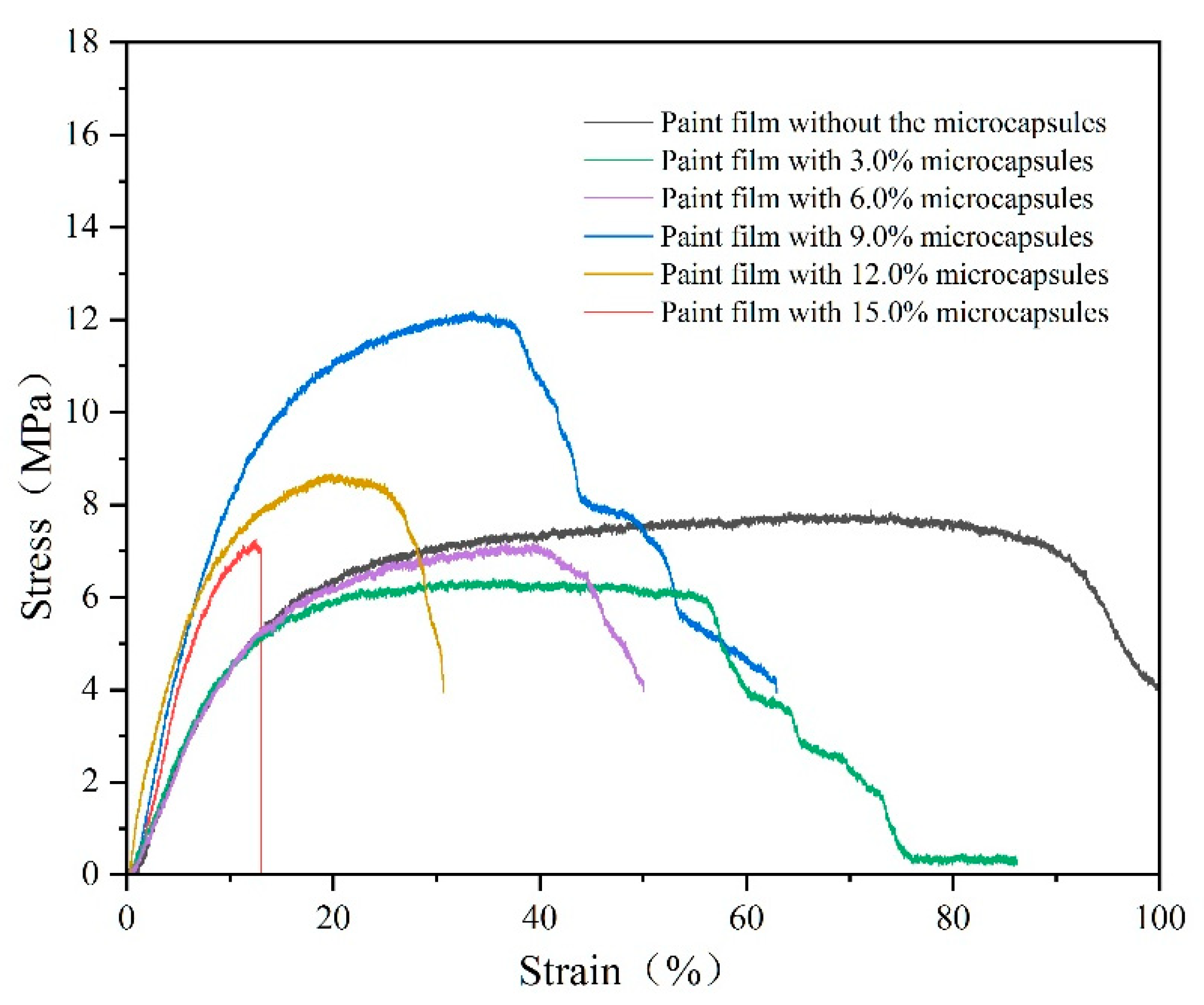
3.7. Effect of Microcapsules with Different Content Levels on the Mechanical Properties of Paint Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, Y.J.; Xie, C.Y. City garden environment research: Optimal design can improve the therapeutic benefit of therapy garden. Fresen. Environ. Bull. 2020, 29, 11615–11623. [Google Scholar]
- Han, M.J.N.; Kim, M.J. Green environments and happiness level in housing areas toward a sustainable life. Sustainability 2019, 11, 4768. [Google Scholar] [CrossRef]
- Tellnes, L.G.F.; Alfredsen, G.; Flaete, P.O.; Gobakken, L.R. Effect of service life aspects on carbon footprint: A comparison of wood decking products. Holzforschung 2020, 74, 426–433. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, W.; Kasal, A.; Erdil, Y.Z. The State of the Art of Biomechanics Applied in Ergonomic Furniture Design. Appl. Sci. 2023, 13, 12120. [Google Scholar] [CrossRef]
- Hu, W.G.; Liu, Y.; Konukcu, A.C. Study on withdrawal load resistance of screw in wood-based materials: Experimental and numerical. Wood Mater. Sci. Eng. 2023, 18, 334–343. [Google Scholar] [CrossRef]
- Hu, W.; Fu, W.; Zhao, Y. Optimal design of the traditional Chinese wood furniture joint based on experimental and numerical method. Wood. Res. 2024, 69, 50–59. [Google Scholar] [CrossRef]
- Jiao, C.Y.; Shao, Q.; Wu, M.Y.; Zheng, B.; Guo, Z.; Yi, J.M.; Zhang, J.X.; Lin, J.; Wu, S.D.; Dong, M.Y.; et al. 2-(3,4-Epoxy) ethyltriethoxysilane-modified waterborne acrylic resin: Preparation and property analysis. Polymer 2020, 190, 122196. [Google Scholar] [CrossRef]
- Ge, H.W.; Zhang, J.F.; Yuan, Y.; Liu, J.C.; Liu, R.; Liu, X.Y. Preparation of organicinorganic hybrid silica nanoparticles with contact antibacterial properties and their application in UV-curable coatings. Prog. Org. Coat. 2017, 106, 20–26. [Google Scholar] [CrossRef]
- Hu, W.; Zhang, J. Effect of growth rings on acoustic emission characteristic signals of southern yellow pine wood cracked in mode I. Constr. Build. Mater. 2022, 329, 127092. [Google Scholar] [CrossRef]
- Chen, B.; Yu, X.; Hu, W. Experimental and numerical studies on the cantilevered leg joint and its reinforced version commonly used in modern wood furniture. Bioresources 2022, 17, 3952–3964. [Google Scholar] [CrossRef]
- Revuelta, M.V.; Bogdan, S.; Gamez-Espinosa, E.; Deya, M.C.; Romagnoli, R. Green antifungal waterborne coating based on essential oil microcapsules. Prog. Org. Coat. 2020, 151, 106101. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, J.; Wu, Z. Fabrication of coatings with structural color on a wood surface. Coatings 2020, 10, 32. [Google Scholar] [CrossRef]
- Chen, B.; Xia, H.; Hu, W. The design and evaluation of the three-dimensional corner joints used in wooden furniture frames: Experimental and numerical. Bioresources 2022, 17, 2143–2156. [Google Scholar] [CrossRef]
- Chen, C.J.; Kuang, Y.D.; Zhu, S.Z.; Burgert, I.; Keplinger, T.; Gong, A.; Li, T.; Berglund, L.; Eichhorn, S.J.; Hu, L.B. Structureproperty-function relationships of natural and engineered wood. Nat. Rev. Mater. 2020, 5, 642–666. [Google Scholar] [CrossRef]
- Yan, X.X.; Han, Y.; Yin, T.Y. Synthesis of urea-formaldehyde microcapsule containing fluororesin and its effect on performances of waterborne coatings on wood surface. Polymers 2021, 13, 1674. [Google Scholar] [CrossRef]
- Julaeha, E.; Eddy, D.R.; Wahyudi, T.; Ningsih, B.A.; Nurzaman, M.; Permadi, N.; Herlina, T.; Al Anshori, J. Coacervate Microcapsules of Citrus aurantifolia Essential Oil (LOs): Optimization and Their Antibacterial Activity Study. ChemistrySelect 2022, 7, e202200187. [Google Scholar] [CrossRef]
- Hu, W.; Wan, H. Comparative study on weathering durability properties of phenol formaldehyde resin modified sweetgum and southern pine specimens. Maderas-Cienc. Tecnol. 2022, 24, 17. [Google Scholar] [CrossRef]
- Hu, W.; Liu, Y.; Li, S. Characterizing mode I fracture behaviors of wood using compact tension in selected system crack propagation. Forests 2021, 12, 1369. [Google Scholar] [CrossRef]
- Yan, X.X.; Wang, L. Preparation of shellac resin microcapsules coated with urea formaldehyde resin and properties of waterborne paint films for Tilia amurensis Rupr. Membranes 2020, 10, 278. [Google Scholar] [CrossRef]
- Hu, W.; Li, S.; Liu, Y. Vibrational characteristics of four wood species commonly used in wood products. Bioresources 2021, 16, 7101–7111. [Google Scholar] [CrossRef]
- Hu, W.; Chen, B. A methodology for optimizing tenon geometry dimensions of mortise-and-tenon joint wood products. Forests 2021, 12, 478. [Google Scholar] [CrossRef]
- Zhi, L.; Zhang, C.Q.; Liu, Z.Z.; Liu, T.; Dou, X.Y.; Chen, Y.Q.; Ou, R.X.; Wang, Q.W. Flexible decorative wood veneer with high strength, wearability and moisture penetrability enabled by infiltrating castor oil-based waterborne polyurethanes. Compos. Part B-Eng. 2022, 230, 109502. [Google Scholar] [CrossRef]
- Yan, X.X.; Han, Y.; Yin, T.Y. Coating process optimization and self-healing performance evaluation of shellac microcapsules coated with melamine/rice husk powder. Appl. Sci. 2021, 11, 8373. [Google Scholar] [CrossRef]
- Wang, J.J.; Lu, Y.Z.; Chu, Q.D.; Ma, C.L.; Cai, L.R.; Shen, Z.H.; Chen, H. Facile construction of superhydrophobic surfaces by coating fluoroalkylsilane/silica composite on a modified hierarchical structure of wood. Polymers 2022, 12, 813. [Google Scholar] [CrossRef] [PubMed]
- Rosu, L.; Varganici, C.D.; Mustata, F.; Rosu, D.; Rosca, I.; Rusu, T. Epoxy coatings based on modified vegetable oils for wood surface protection against fungal degradation. ACS Appl. Mater. Inter. 2020, 12, 14443–14458. [Google Scholar] [CrossRef] [PubMed]
- Han, S.J.; Chen, Y.P.; Lyu, S.Y.; Chen, Z.L.; Wang, S.Q.; Fu, F. Effects of processing conditions on the properties of paraffin/melamine-urea-formaldehyde microcapsules prepared by in situ polymerization. Colloids Surf. B 2019, 585, 124046. [Google Scholar] [CrossRef]
- Huang, N.; Yan, X.X. Preparation of Aloe-Emodin Microcapsules and Its Effect on Antibacterial and Optical Properties of Water-Based Coating. Polymers 2023, 15, 1728. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Bawazir, M.; Dhall, A.; Kim, H.E.; He, L.; Heo, J.; Hwang, G. Implication of Surface Properties, Bacterial Motility, and Hydrodynamic Conditions on Bacterial Surface Sensing and Their Initial Adhesion. Front. Bioeng. Biotechnol. 2021, 9, 643722. [Google Scholar] [CrossRef]
- Weng, M.; Zhu, Y.; Mao, W.; Zhou, J.; Xu, W. Nano-Silica/Urea-Formaldehyde Resin-Modified Fast-Growing Lumber Performance Study. Forests 2023, 14, 1440. [Google Scholar] [CrossRef]
- Hu, W.; Zhang, J. Bolt-Bearing Yield Strength of Three-Layered Cross-Laminated Timber Treated with Phenol Formaldehyde Resin. Forests 2020, 11, 551. [Google Scholar] [CrossRef]
- Barbaz-Isfahani, R.; Saber-Samandari, S.; Salehi, M. Novel electrosprayed enhanced microcapsules with different nanoparticles containing healing agents in a single multicore microcapsule. Int. J. Biol. Macromol. 2022, 200, 532–542. [Google Scholar] [CrossRef]
- Hu, W.; Liu, N. Comparisons of finite element models used to predict bending strength of mortise-and-tenon joints. Bioresources 2020, 15, 5801–5811. [Google Scholar] [CrossRef]
- Yuan, H.Z.; Li, G.X.; Yang, L.J.; Yan, X.J.; Yang, D.B. Development of melamine-formaldehyde resin microcapsules with low formaldehyde emission suited for seed treatment. Colloids. Surf. B. 2015, 128, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yu, J.; Jiang, M.; Li, J. Effect of selective enhancement on the bending performance of fused deposition methods 3D-printed PLA models. Bioresources 2024, 19, 2660–2669. [Google Scholar] [CrossRef]
- Tao, Y.; Yan, X.X. Influence of HLB Value of Emulsifier on the Properties of Microcapsules and Self-Healing Properties of Waterborne Coatings. Polymers 2022, 14, 1304. [Google Scholar] [CrossRef]
- Wang, C.; Zhou, Z. Optical properties and lampshade design applications of PLA 3D printing materials. Bioresources 2023, 18, 1545–1553. [Google Scholar] [CrossRef]
- Das, S.; Das, A.; Bhavya, T.; Nivashini, S.R. Molecular characterisation and antibacterial activity of Aloe barbadensis miller on textiles. J. Text. Inst. 2020, 111, 1116–1122. [Google Scholar] [CrossRef]
- Xiong, X.Y.; Li, X.B.; Zhu, Z.F.; Zhang, E.D.; Shi, J.; Lu, M.G. Antibacterial and alkali-responsive cationic waterborne polyurethane based on modification of aloe emodin. Chem. Res. Chin. Univ. 2023, 39, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.J.; Wang, H.; Liu, D.; Zheng, Z.N. Surface attachment of natural antimicrobial coatings onto conventional polypropylene nonwoven fabric and its antimicrobial performance assessment. J. Food. Protect. 2018, 81, 172–177. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, C.; Ding, K.; Jiang, M. Immersion polishing post-treatment of PLA 3D printed formed parts on its surface and mechanical performance. Bioresources 2023, 18, 7995–8006. [Google Scholar] [CrossRef]
- Luo, Z.; Xu, W.; Wu, S. Performances of Green Velvet Material (PLON) Used in Upholstered Furniture. Bioresources 2023, 18, 5108–5119. [Google Scholar] [CrossRef]
- Luo, Y.; Xu, W. Optimization of Panel Furniture Plates Rework Based on Intelligent Manufacturing. Bioresources 2023, 18, 5198–5208. [Google Scholar] [CrossRef]
- Wang, C.; Yu, J.; Jiang, M.H.; Li, J.Y. Effect of slicing parameters on the light transmittance of 3D-printed polyethylene terephthalate glycol products. Bioresources 2024, 19, 500–509. [Google Scholar] [CrossRef]
- Aichayawanich, S.; Ngaouwthong, C. Antimicrobial Activity of Crude Extract from Pomelo Peel against Staphylococcus aureus. Adv. Mat. Res. 2012, 550–553, 1871–1874. [Google Scholar] [CrossRef]
- Thavanapong, N.; Wetwitayaklung, P. Comparison of Essential Oils Compositions of Citrus maxima Merr. Peel Obtained by Cold Press and Vacuum Stream Distillation Methods and of Its Peel and Flower Extract Obtained by Supercritical Carbon Dioxide Extraction Method and Their Antimicrobial Activity. J. Essent. Oil Res. 2020, 22, 71–77. [Google Scholar]
- GB/T 21866-2008; Test Method and Effect for Antibacterial Capability of Paints Film. Standardization Administration of the People’s Republic of China: Beijing, China, 2008.
- GB/T 4789.2-2016; National Food Safety Standard Food Microbiological Examination: Aerobic Plate Count. Standardization Administration of the People’s Republic of China: Beijing, China, 2016.
- GB/T 11186.3-1989; Methods for Measuring the Colour of Paint Films. Part Ⅲ: Calculation of Colour Differences. Standardization Administration of the People’s Republic of China: Beijing, China, 1990.
- GB/T 4893.4-2013; Test of Surface Coatings of Furniture. Standardization Administration of the People’s Republic of China: Beijing, China, 2013.
- Zotiadis, C.; Patrikalos, I.; Loukaidou, V.; Korres, D.M.; Karantonis, A.; Vouyiouka, S. Self-healing coatings based on poly(urea-formaldehyde) microcapsules: In situ polymerization, capsule properties and application. Prog. Org. Coat. 2021, 161, 106475. [Google Scholar] [CrossRef]
- An, S.; Lee, M.W.; Yarin, A.L.; Yoon, S.S. A review on corrosion-protective extrinsic self-healing: Comparison of microcapsulebased systems and those based on core-shell vascular networks. Chem. Eng. J. 2018, 344, 206–220. [Google Scholar] [CrossRef]
- Scheiner, M.; Dickens, T.J.; Okoli, O. Progress towards self-healing polymers for composite structural applications. Polymer 2016, 83, 260–282. [Google Scholar] [CrossRef]




| Material | Molecular Formula | MW (g/mol) | CAS No. | Concentration (%) |
|---|---|---|---|---|
| Melamine | CH4N2O | 60.06 | 57-13-6 | 99.0 |
| Formaldehyde solution | - | - | - | 37.0 |
| Triethanolamine | C6H15NO3 | 149.19 | 102-71-6 | 99.9 |
| Citric acid monohydrate | C6H10O8 | 210.14 | 5949-29-1 | 99.9 |
| Sodium dodecyl benzene sulfonate | C18H29NaO3S | 348.48 | 25155-30-0 | 99.9 |
| Anhydrous ethanol | C2H6O | 46.07 | 64-17-5 | 99.9 |
| Level | Factor A m(core material): m(wall material) | Factor B Temperature (°C) | Factor C Stirring Speed (rpm) | Factor D Emulsifier Concentration (%) |
|---|---|---|---|---|
| 1 | 0.08:1 | 50 | 600 | 1 |
| 2 | 0.10:1 | 60 | 800 | 2 |
| 3 | 0.12:1 | 70 | 1000 | 3 |
| Sample | m(core material): m(wall material) | Temperature (°C) | Stirring Speed (rpm) | Emulsifier Concentration (%) |
|---|---|---|---|---|
| 1 | 0.08:1 | 50 | 600 | 1 |
| 2 | 0.08:1 | 60 | 800 | 2 |
| 3 | 0.08:1 | 70 | 1000 | 3 |
| 4 | 0.10:1 | 50 | 800 | 3 |
| 5 | 0.10:1 | 60 | 1000 | 1 |
| 6 | 0.10:1 | 70 | 600 | 2 |
| 7 | 0.12:1 | 50 | 1000 | 2 |
| 8 | 0.12:1 | 60 | 600 | 3 |
| 9 | 0.12:1 | 70 | 800 | 1 |
| Test Type | Sample | Melamine (g) | Formaldehyde Solution (g) | Wall Material (g) | Core Material (g) | Ethanol (mL) | Emulsifier (g) | Deionized Water (g) |
|---|---|---|---|---|---|---|---|---|
| Orthogonal test | 1 | 10.00 | 19.30 | 17.13 | 1.37 | 12.33 | 2.74 | 271.26 |
| 2 | 10.00 | 19.30 | 17.13 | 1.37 | 12.33 | 5.48 | 268.52 | |
| 3 | 10.00 | 19.30 | 17.13 | 1.37 | 12.33 | 8.22 | 265.78 | |
| 4 | 10.00 | 19.30 | 17.13 | 1.71 | 15.39 | 10.26 | 331.74 | |
| 5 | 10.00 | 19.30 | 17.13 | 1.71 | 15.39 | 3.42 | 320.58 | |
| 6 | 10.00 | 19.30 | 17.13 | 1.71 | 15.39 | 6.84 | 335.16 | |
| 7 | 10.00 | 19.30 | 17.13 | 2.06 | 18.54 | 8.24 | 403.76 | |
| 8 | 10.00 | 19.30 | 17.13 | 2.06 | 18.54 | 12.36 | 399.64 | |
| 9 | 10.00 | 19.30 | 17.13 | 2.06 | 18.54 | 4.12 | 407.88 | |
| Single-factor test | 10 | 10.00 | 19.30 | 17.13 | 2.06 | 18.54 | 8.24 | 403.76 |
| 11 | 10.00 | 19.30 | 17.13 | 2.06 | 18.54 | 8.24 | 403.76 | |
| 12 | 10.00 | 19.30 | 17.13 | 2.06 | 18.54 | 8.24 | 403.76 | |
| 13 | 10.00 | 19.30 | 17.13 | 2.06 | 18.54 | 8.24 | 403.76 | |
| 14 | 10.00 | 19.30 | 17.13 | 2.06 | 18.54 | 8.24 | 403.76 |
| Content of the Microcapsules (%) | Microcapsule Weight (g) | Waterborne Coating Weight (g) |
|---|---|---|
| 0 | 0 | 1.00 |
| 3.0 | 0.03 | 0.97 |
| 6.0 | 0.06 | 0.94 |
| 9.0 | 0.09 | 0.91 |
| 12.0 | 0.12 | 0.88 |
| 15.0 | 0.15 | 0.85 |
| Item | Sample | Factor A m(core material): m(wall material) | Factor B Temperature (°C) | Factor C Stirring Speed (rpm) | Factor D Emulsifier Concentration (%) | Yield Rate (%) |
|---|---|---|---|---|---|---|
| Analysis of range | 1 | 0.08:1 | 50 | 600 | 1 | 48 |
| 2 | 0.08:1 | 60 | 800 | 2 | 58 | |
| 3 | 0.08:1 | 70 | 1000 | 3 | 55 | |
| 4 | 0.10:1 | 50 | 800 | 3 | 54 | |
| 5 | 0.10:1 | 60 | 1000 | 1 | 61 | |
| 6 | 0.10:1 | 70 | 600 | 2 | 53 | |
| 7 | 0.12:1 | 50 | 1000 | 2 | 57 | |
| 8 | 0.12:1 | 60 | 600 | 3 | 66 | |
| 9 | 0.12:1 | 70 | 800 | 1 | 55 | |
| Mean value 1 | 53.667 | 53.333 | 55.667 | 54.667 | ||
| Mean value 2 | 56.000 | 61.667 | 55.667 | 56.000 | ||
| Mean value 3 | 59.333 | 54.333 | 57.667 | 58.333 | ||
| Range | 5.666 | 8.667 | 2.000 | 3.666 | ||
| Primary and secondary order | B > A > D > C | |||||
| Optimal level | A3 | B2 | C3 | D3 | ||
| Optimal scheme | A3 B2 C3 D3 | |||||
| Analysis of variance | Quadratic sum | 48.667 | 130.667 | 8 | 20.667 | |
| Free degree | 2 | 2 | 2 | 2 | ||
| F-ratio | 0.936 | 2.513 | 0.154 | 0.397 | ||
| F-critical value | 4.640 | 4.640 | 4.640 | 4.640 | ||
| Significance | ||||||
| Item | Sample | Factor A m(core material): m(wall material) | Factor B Temperature (°C) | Factor C Stirring Speed (rpm) | Factor D Emulsifier Concentration (%) | Coverage Rate (%) |
|---|---|---|---|---|---|---|
| Analysis of range | 1 | 0.08:1 | 50 | 600 | 1 | 10 |
| 2 | 0.08:1 | 60 | 800 | 2 | 15 | |
| 3 | 0.08:1 | 70 | 1000 | 3 | 14 | |
| 4 | 0.10:1 | 50 | 800 | 3 | 13 | |
| 5 | 0.10:1 | 60 | 1000 | 1 | 12 | |
| 6 | 0.10:1 | 70 | 600 | 2 | 20 | |
| 7 | 0. 12:1 | 50 | 1000 | 2 | 11 | |
| 8 | 0. 12:1 | 60 | 600 | 3 | 15 | |
| 9 | 0.12:1 | 70 | 800 | 1 | 21 | |
| Mean value 1 | 13.000 | 11.333 | 15.000 | 14.333 | ||
| Mean value 2 | 15.000 | 14.000 | 16.333 | 15.333 | ||
| Mean value 3 | 15.667 | 18.333 | 12.333 | 14.000 | ||
| Range | 2.667 | 7.000 | 4.000 | 1.333 | ||
| Primary and secondary order | B > C > A > D | |||||
| Optimal level | A3 | B3 | C2 | D2 | ||
| Optimal scheme | A3 B3 C2 D2 | |||||
| Analysis of variance | Quadratic sum | 11.556 | 74.889 | 24.889 | 2.889 | |
| Free degree | 2 | 2 | 2 | 2 | ||
| F-ratio | 0.405 | 2.623 | 0.872 | 0.104 | ||
| F-critical value | 4.460 | 4.460 | 4.460 | 4.460 | ||
| Significance | ||||||
| Sample | Temperature (°C) | Yield Rate (%) | Coverage Rate (%) |
|---|---|---|---|
| 10 | 40 | 45 | 16 |
| 11 | 50 | 47 | 17 |
| 12 | 60 | 51 | 20 |
| 13 | 70 | 46 | 20 |
| 14 | 80 | 42 | 22 |
| Microcapsule Content (%) | Average Number of Recovered Escherichia coli (CFU·Piece−1) | Antibacterial Rate against Escherichia coli (%) | Average Number of Recovered Staphylococcus aureus (CFU·Piece−1) | Antibacterial Rate against Staphylococcus aureus (%) |
|---|---|---|---|---|
| 0 | 190 | - | 432 | - |
| 3.0 | 143 | 24.7 | 316 | 26.9 |
| 6.0 | 113 | 40.5 | 214 | 50.5 |
| 9.0 | 87 | 54.2 | 138 | 68.1 |
| 12.0 | 68 | 64.2 | 97 | 77.6 |
| 15.0 | 53 | 72.1 | 65 | 85.0 |
| Microcapsule Content (%) | L | a | b | ΔE |
|---|---|---|---|---|
| 0 | 81.91 | 1.73 | −2.27 | - |
| 3.0 | 79.47 | 1.10 | −1.43 | 2.66 |
| 6.0 | 78.94 | 1.10 | −1.03 | 3.28 |
| 9.0 | 78.43 | 0.93 | 0.67 | 4.63 |
| 12.0 | 76.10 | 0.63 | 0.97 | 6.74 |
| 15.0 | 73.23 | 0.54 | 1.36 | 9.48 |
| Microcapsule Content (%) | 20° (%) | 60° (%) | 85° (%) | Gloss Loss Rate (%) |
|---|---|---|---|---|
| 0 | 6.10 | 17.45 | 31.17 | - |
| 3.0 | 2.80 | 9.13 | 3.77 | 47.7 |
| 6.0 | 2.57 | 8.67 | 2.63 | 50.3 |
| 9.0 | 2.17 | 7.07 | 1.43 | 59.5 |
| 12.0 | 1.67 | 5.43 | 0.83 | 68.9 |
| 15.0 | 1.53 | 5.00 | 0.77 | 71.3 |
| Microcapsule Content (%) | Elongation at Break (%) |
|---|---|
| 0 | 18.9 |
| 3.0 | 12.7 |
| 6.0 | 10.8 |
| 9.0 | 10.2 |
| 12.0 | 7.9 |
| 15.0 | 3.8 |
| Microcapsule Content (%) | Roughness (μm) |
|---|---|
| 0 | 0.27 |
| 3.0 | 0.84 |
| 6.0 | 1.75 |
| 9.0 | 2.97 |
| 12.0 | 3.90 |
| 15.0 | 3.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, T.; Yan, X. Preparation Process Optimization for Melamine Resin-Covered Pomelo Peel Flavonoid Antibacterial Microcapsules and Their Effect on Waterborne Paint Film Performance. Coatings 2024, 14, 654. https://doi.org/10.3390/coatings14060654
Ding T, Yan X. Preparation Process Optimization for Melamine Resin-Covered Pomelo Peel Flavonoid Antibacterial Microcapsules and Their Effect on Waterborne Paint Film Performance. Coatings. 2024; 14(6):654. https://doi.org/10.3390/coatings14060654
Chicago/Turabian StyleDing, Tingting, and Xiaoxing Yan. 2024. "Preparation Process Optimization for Melamine Resin-Covered Pomelo Peel Flavonoid Antibacterial Microcapsules and Their Effect on Waterborne Paint Film Performance" Coatings 14, no. 6: 654. https://doi.org/10.3390/coatings14060654
APA StyleDing, T., & Yan, X. (2024). Preparation Process Optimization for Melamine Resin-Covered Pomelo Peel Flavonoid Antibacterial Microcapsules and Their Effect on Waterborne Paint Film Performance. Coatings, 14(6), 654. https://doi.org/10.3390/coatings14060654





