A Strategy of Candle Soot-Based Photothermal Icephobic Superhydrophobic Surface
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of PDMS/Al Surface
2.3. Fabrication of PDMS/Candle Soot@PDMS/Al (PDMS/CS@PDMS/Al) Surface
2.4. Characterization of the PDMS/CS@PDMS/Al Surface
2.5. Visualization of Droplet De-Icing and Anti-Icing Processes on the PDMS/CS60@PDMS/Al Surface
3. Results
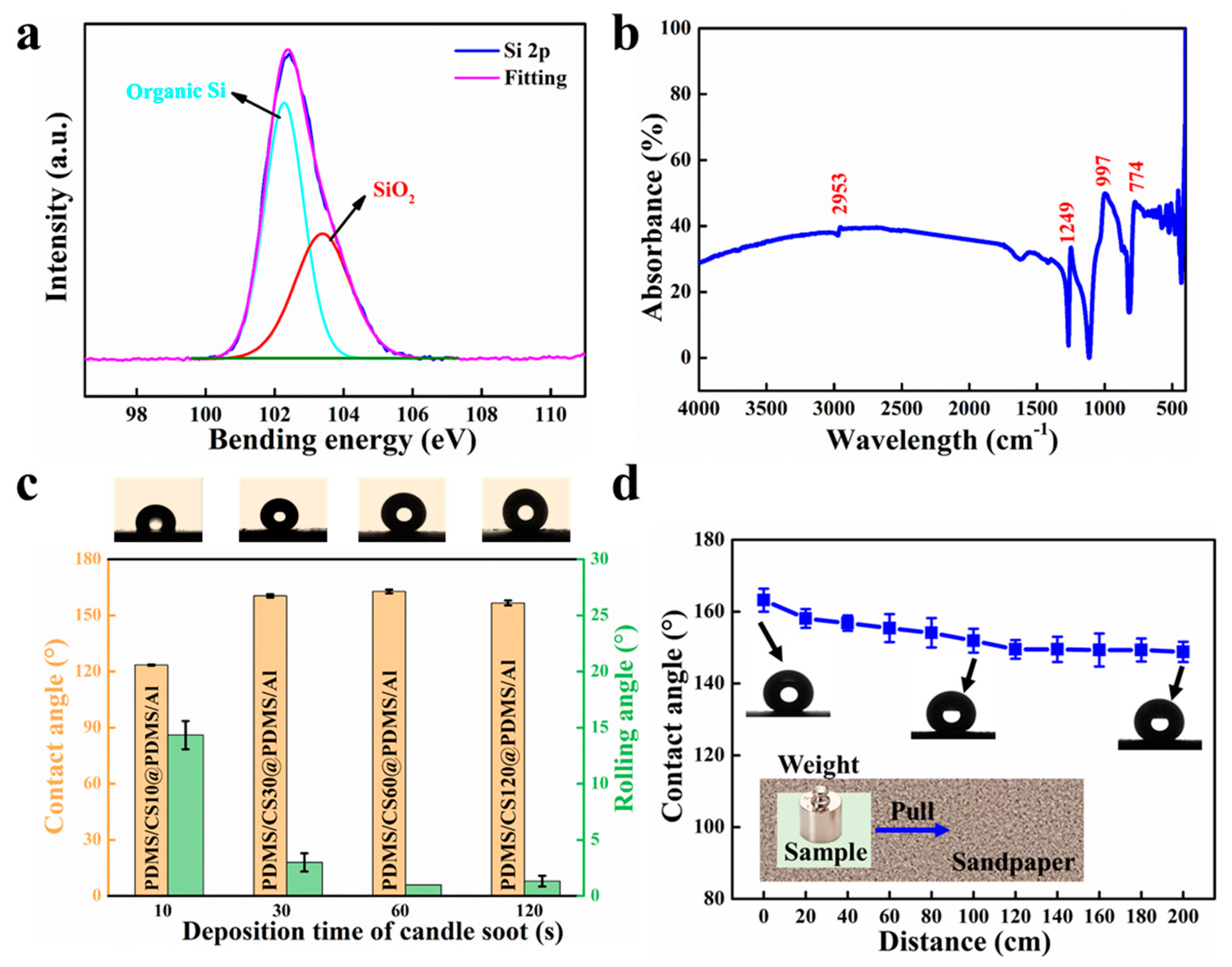
3.1. Fabrication and Characterization of PDMS/CS60@PDMS/Al
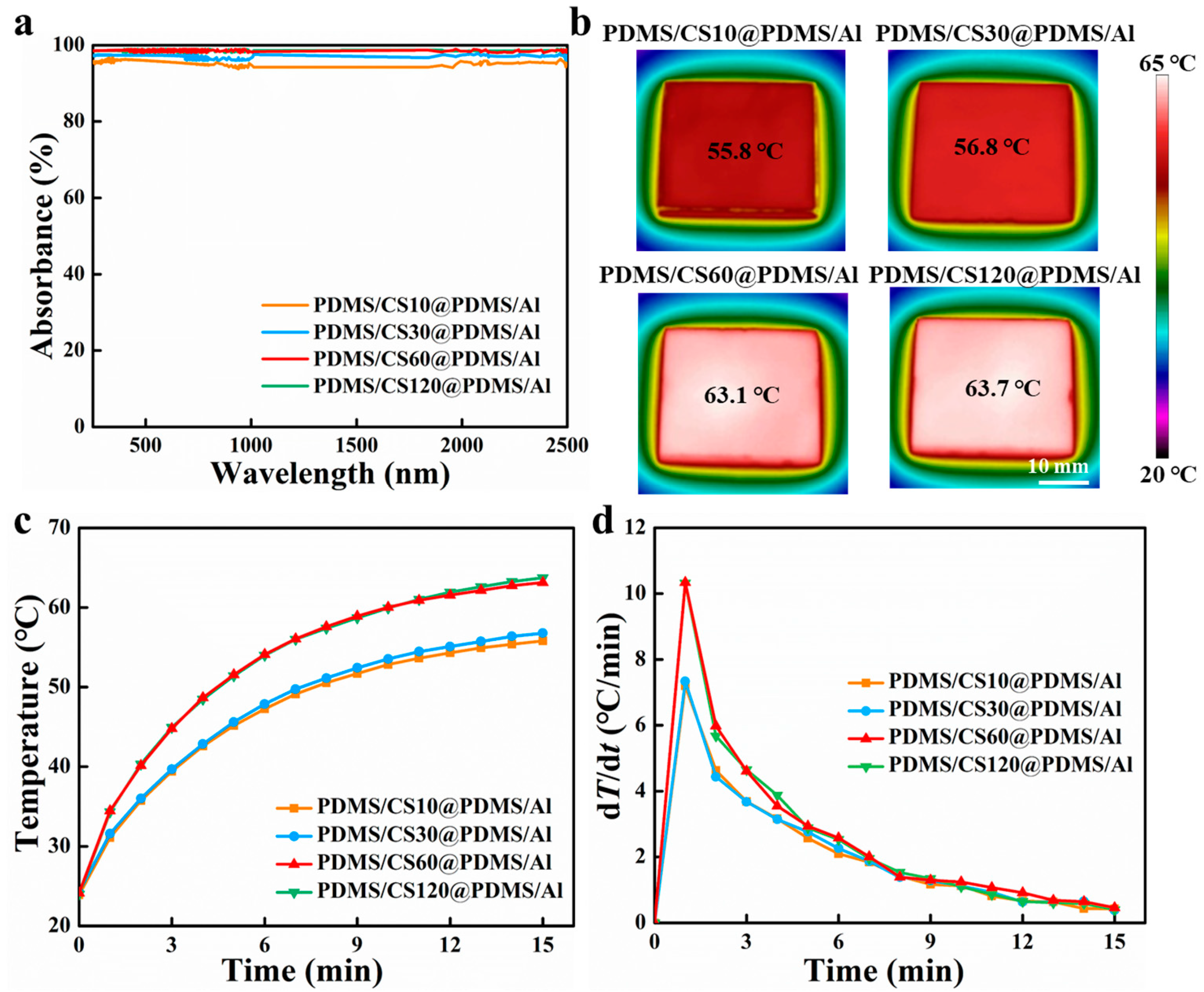
3.2. Photothermal Performance of PDMS/CS60@PDMS/Al
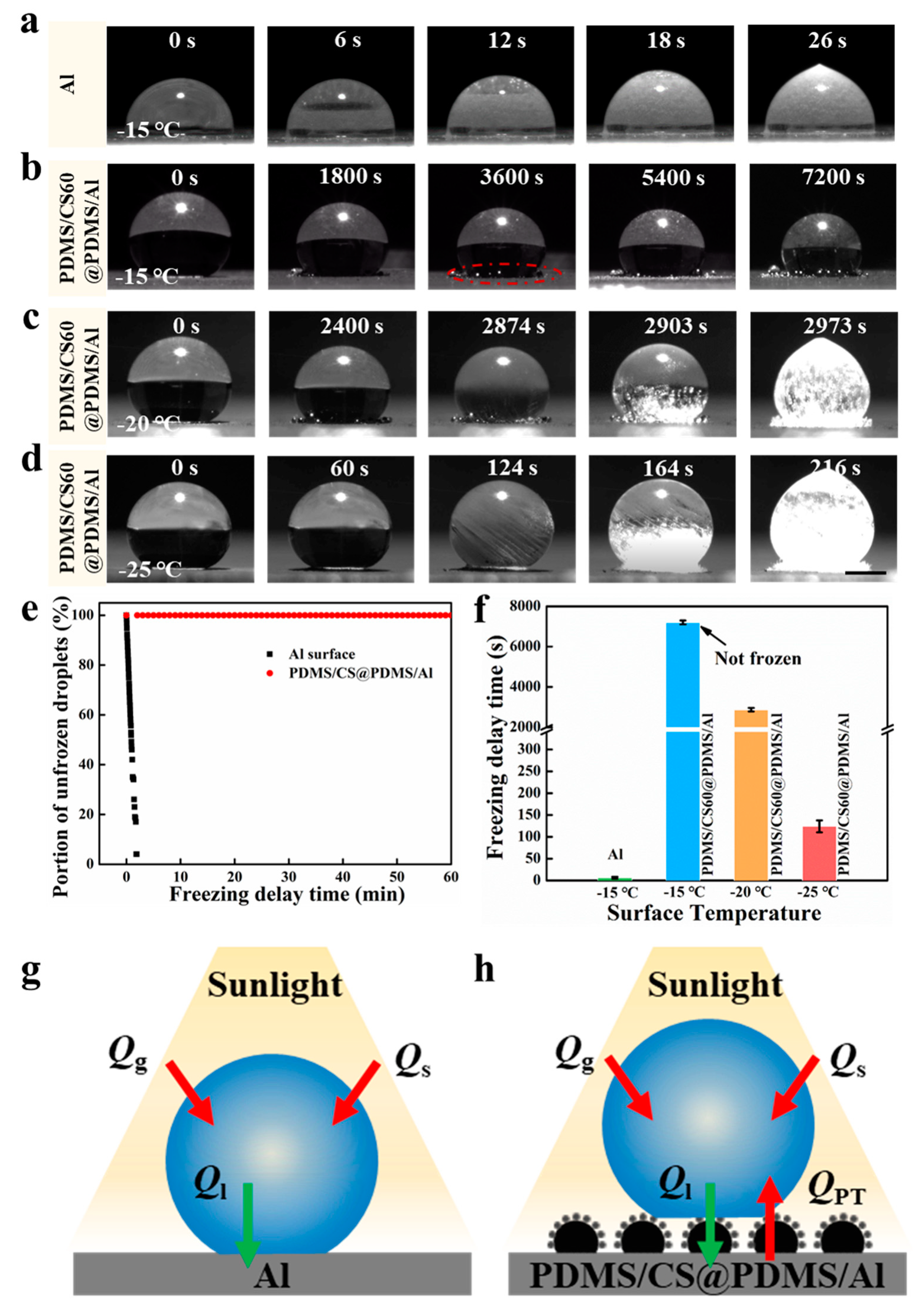
3.3. Anti-Icing Performance of PDMS/CS60@PDMS/Al
3.4. Mechanism of Anti-Icing Permance of PDMS/CS60@PDMS/Al
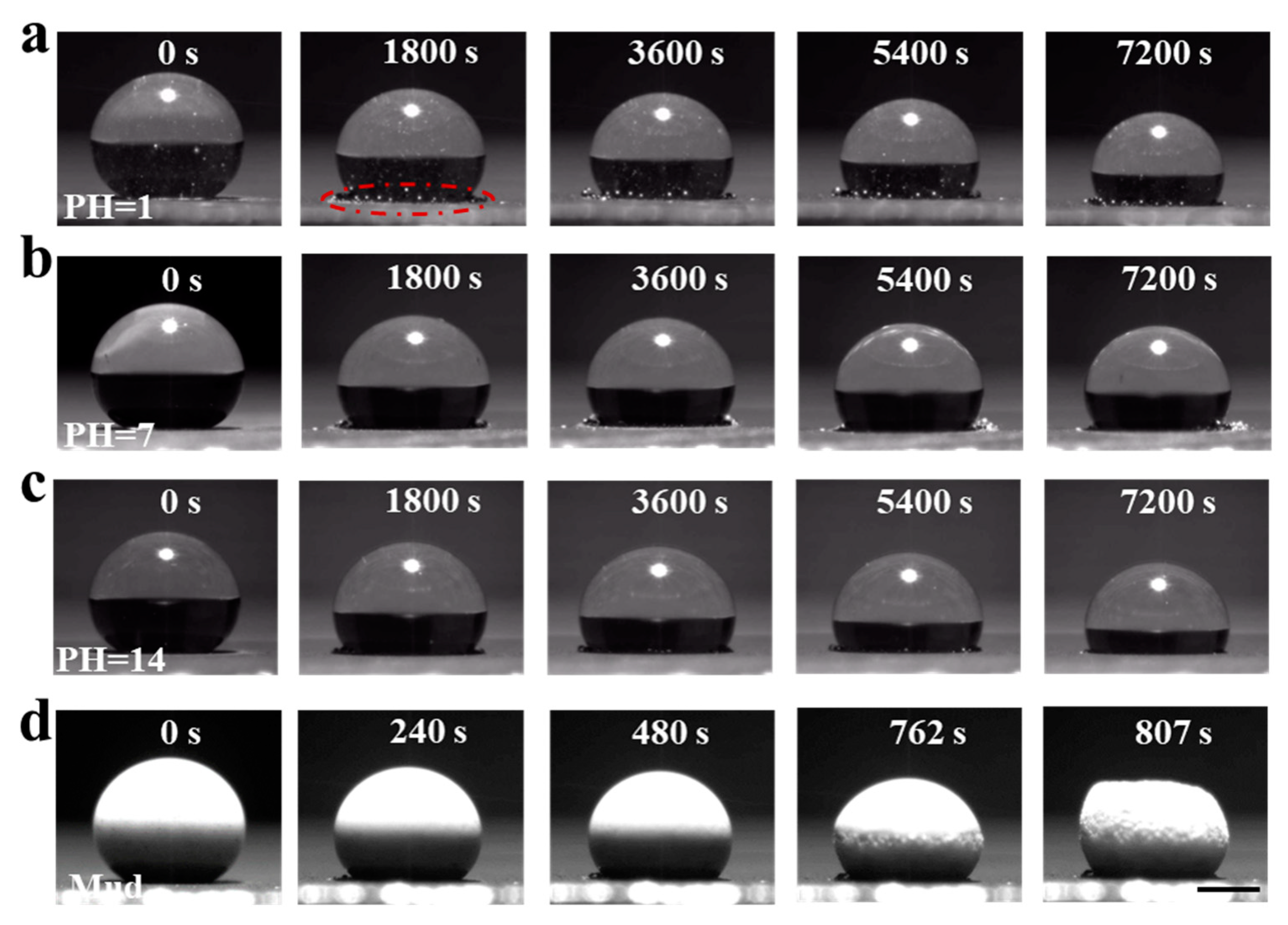
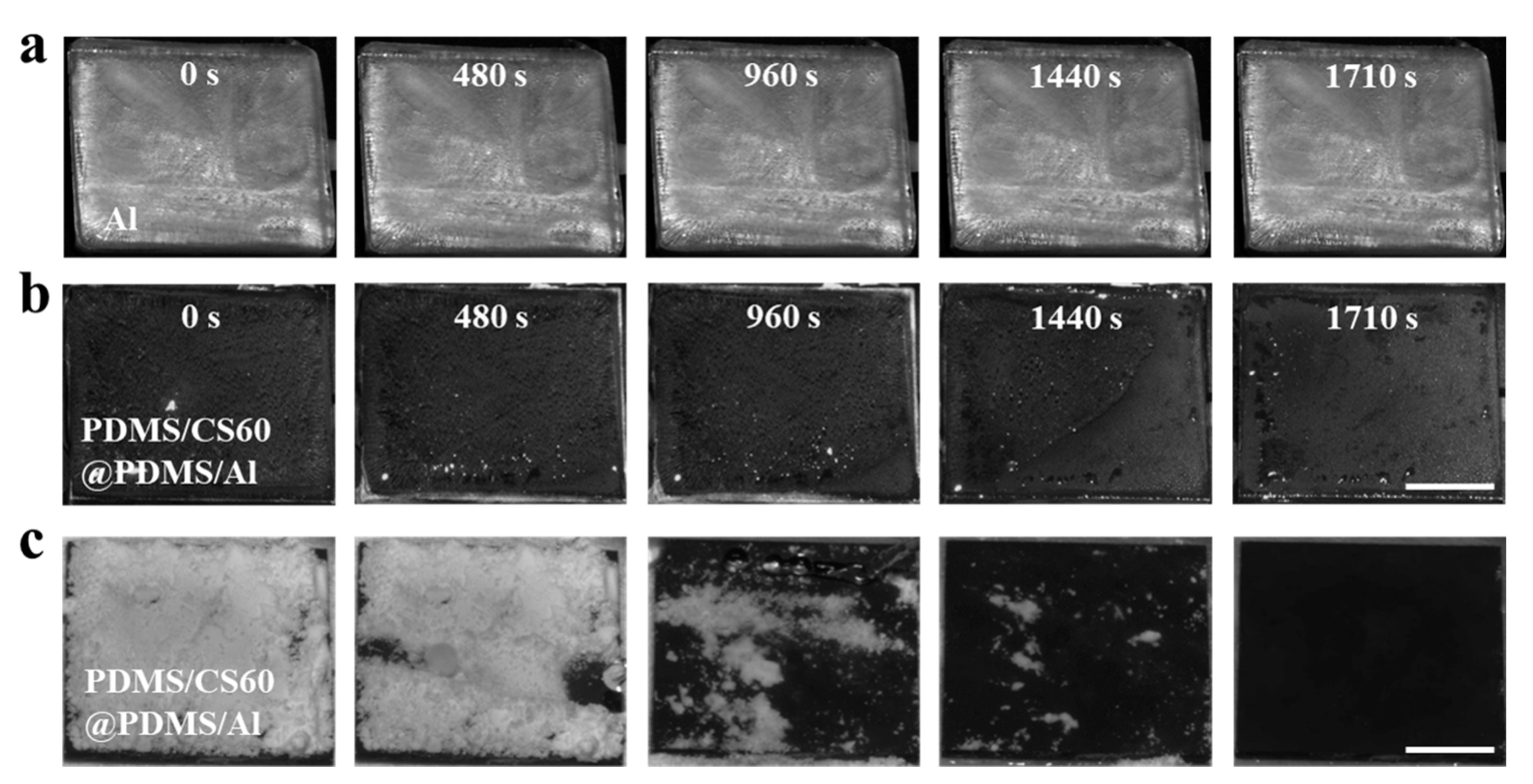
3.5. De-Icing Performance and Self-Cleaning Performance of PDMS/CS60@PDMS/Al
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.; Li, X.; Jin, J.; Liu, J.; Yan, Y.; Han, Z.; Ren, L. Anti-Icing Property of Bio-Inspired Micro-Structure Superhydrophobic Surfaces and Heat Transfer Model. Appl. Surf. Sci. 2017, 400, 498–505. [Google Scholar] [CrossRef]
- Zhang, H.; Jin, Z.; Jiao, M.; Yang, Z. Experimental Investigation of the Impact and Freezing Processes of a Water Droplet on Different Cold Concave Surfaces. Int. J. Therm. Sci. 2018, 132, 498–508. [Google Scholar] [CrossRef]
- Gao, L.; Liu, Y.; Hu, H. An Experimental Investigation of Dynamic Ice Accretion Process on a Wind Turbine Airfoil Model Considering Various Icing Conditions. Int. J. Heat Mass Transf. 2019, 133, 930–939. [Google Scholar] [CrossRef]
- Yirtici, O.; Ozgen, S.; Tuncer, I.H. Predictions of Ice Formations on Wind Turbine Blades and Power Production Losses Due to Icing. Wind Energy 2019, 22, 945–958. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, H.; Zhu, Y.; Liu, X.; Wang, Z.; Chen, J. A Robust Superhydrophobic Anti-Icing/de-Icing Composite Coating with Electrothermal and Auxiliary Photothermal Performances. Compos. Sci. Technol. 2022, 227, 109578. [Google Scholar] [CrossRef]
- Rashid, T.; Khawaja, H.A.; Edvardsen, K. Review of Marine Icing and Anti-/de-Icing Systems. J. Mar. Eng. Technol. 2016, 15, 79–87. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Y.; Huang, Q. Progress on Ultrasonic Guided Waves De-Icing Techniques in Improving Aviation Energy Efficiency. Renew. Sustain. Energy Rev. 2017, 79, 638–645. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Y.; Su, F. Damage Accumulation Model of Ice Detach Behavior in Ultrasonic De-Icing Technology. Renew. Energy 2020, 153, 1396–1405. [Google Scholar] [CrossRef]
- Ding, L.; Chang, S.; Yi, X.; Song, M. Coupled Thermo-Mechanical Analysis of Stresses Generated in Impact Ice during in-Flight de-Icing. Appl. Therm. Eng. 2020, 181, 115681. [Google Scholar] [CrossRef]
- Shome, A.; Das, A.; Borbora, A.; Dhar, M.; Manna, U. Role of Chemistry in Bio-Inspired Liquid Wettability. Chem. Soc. Rev. 2022, 51, 5452–5497. [Google Scholar] [CrossRef]
- Rykaczewski, K.; Anand, S.; Subramanyam, S.B.; Varanasi, K.K. Mechanism of Frost Formation on Lubricant-Impregnated Surfaces. Langmuir 2013, 29, 5230–5238. [Google Scholar] [CrossRef] [PubMed]
- Stamatopoulos, C.; Hemrle, J.; Wang, D.; Poulikakos, D. Exceptional Anti-Icing Performance of Self-Impregnating Slippery Surfaces. ACS Appl. Mater. Interfaces 2017, 9, 10233–10242. [Google Scholar] [CrossRef] [PubMed]
- Kreder, M.J.; Alvarenga, J.; Kim, P.; Aizenberg, J. Design of Anti-Icing Surfaces: Smooth, Textured or Slippery? Nat. Rev. Mater. 2016, 1, 15003. [Google Scholar] [CrossRef]
- Jamil, M.I.; Wang, Q.; Ali, A.; Hussain, M.; Aziz, T.; Zhan, X.; Zhang, Q. Slippery Photothermal Trap for Outstanding Deicing Surfaces. J Bionic Eng 2021, 18, 548–558. [Google Scholar] [CrossRef]
- Guo, P.; Zheng, Y.; Wen, M.; Song, C.; Lin, Y.; Jiang, L. Icephobic/Anti-Icing Properties of Micro/Nanostructured Surfaces. Adv. Mater. 2012, 24, 2642–2648. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Gong, Q.; Zhan, S.; Jiang, L.; Zheng, Y. Robust Anti-Icing Performance of a Flexible Superhydrophobic Surface. Adv. Mater. 2016, 28, 7729–7735. [Google Scholar] [CrossRef]
- Shen, Y.; Wang, G.; Tao, J.; Zhu, C.; Liu, S.; Jin, M.; Xie, Y.; Chen, Z. Anti-Icing Performance of Superhydrophobic Texture Surfaces Depending on Reference Environments. Adv. Mater. Interfaces 2017, 4, 1700836. [Google Scholar] [CrossRef]
- Jung, S.; Dorrestijn, M.; Raps, D.; Das, A.; Megaridis, C.M.; Poulikakos, D. Are Superhydrophobic Surfaces Best for Icephobicity? Langmuir 2011, 27, 3059–3066. [Google Scholar] [CrossRef]
- Chen, C.; Tian, Z.; Luo, X.; Jiang, G.; Hu, X.; Wang, L.; Peng, R.; Zhang, H.; Zhong, M. Cauliflower-like Micro-Nano Structured Superhydrophobic Surfaces for Durable Anti-Icing and Photothermal de-Icing. Chem. Eng. J. 2022, 450, 137936. [Google Scholar] [CrossRef]
- Xie, Z.; Wang, H.; Geng, Y.; Li, M.; Deng, Q.; Tian, Y.; Chen, R.; Zhu, X.; Liao, Q. Carbon-Based Photothermal Superhydrophobic Materials with Hierarchical Structure Enhances the Anti-Icing and Photothermal Deicing Properties. ACS Appl. Mater. Interfaces 2021, 13, 48308–48321. [Google Scholar] [CrossRef]
- Zhao, Y.; Yan, C.; Hou, T.; Dou, H.; Shen, H. Multifunctional Ti3C2Tx MXene-Based Composite Coatings with Superhydrophobic Anti-Icing and Photothermal Deicing Properties. ACS Appl. Mater. Interfaces 2022, 14, 26077–26087. [Google Scholar] [CrossRef]
- Hou, M.; Jiang, Z.; Sun, W.; Chen, Z.; Chu, F.; Lai, N.-C. Efficient Photothermal Anti-/Deicing Enabled by 3D Cu2-xS Encapsulated Phase Change Materials Mixed Superhydrophobic Coatings. Adv. Mater. 2024, 36, 2310312. [Google Scholar] [CrossRef]
- Sun, W.; Wei, Y.; Feng, Y.; Chu, F. Anti-Icing and Deicing Characteristics of Photothermal Superhydrophobic Surfaces Based on Metal Nanoparticles and Carbon Nanotube Materials. Energy 2024, 286, 129656. [Google Scholar] [CrossRef]
- Jiang, G.; Chen, L.; Zhang, S.; Huang, H. Superhydrophobic SiC/CNTs Coatings with Photothermal Deicing and Passive Anti-Icing Properties. ACS Appl. Mater. Interfaces 2018, 10, 36505–36511. [Google Scholar] [CrossRef]
- Xie, Z.; Wang, H.; Li, M.; Tian, Y.; Deng, Q.; Chen, R.; Zhu, X.; Liao, Q. Photothermal Trap with Multi-Scale Micro-Nano Hierarchical Structure Enhances Light Absorption and Promote Photothermal Anti-Icing/Deicing. Chem. Eng. J. 2022, 435, 135025. [Google Scholar] [CrossRef]
- Xie, H.; Xu, W.-H.; Du, Y.; Gong, J.; Niu, R.; Wu, T.; Qu, J.-P. Cost-Effective Fabrication of Micro-Nanostructured Superhydrophobic Polyethylene/Graphene Foam with Self-Floating, Optical Trapping, Acid-/Alkali Resistance for Efficient Photothermal Deicing and Interfacial Evaporation. Small 2022, 18, 2200175. [Google Scholar] [CrossRef]
- Xie, H.; Wei, J.; Duan, S.; Zhu, Q.; Yang, Y.; Chen, K.; Zhang, J.; Li, L.; Zhang, J. Non-Fluorinated and Durable Photothermal Superhydrophobic Coatings Based on Attapulgite Nanorods for Efficient Anti-Icing and Deicing. Chem. Eng. J. 2022, 428, 132585. [Google Scholar] [CrossRef]
- Miao, S.; Liu, X.; Chen, Y. Freezing as a Path to Build Micro-Nanostructured Icephobic Coatings. Adv. Funct. Mater. 2023, 33, 2212245. [Google Scholar] [CrossRef]
- Jamil, M.I.; Zhan, X.; Chen, F.; Cheng, D.; Zhang, Q. Durable and Scalable Candle Soot Icephobic Coating with Nucleation and Fracture Mechanism. ACS Appl. Mater. Interfaces 2019, 11, 31532–31542. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, G.; Li, L.; Xu, C.; Lv, X.; Zhang, H.; Yao, W. Icephobic Behaviors of Superhydrophobic Amorphous Carbon Nano-Films Synthesized from a Flame Process. J. Colloid Interface Sci. 2019, 552, 613–621. [Google Scholar] [CrossRef]
- Wu, S.; Du, Y.; Alsaid, Y.; Wu, D.; Hua, M.; Yan, Y.; Yao, B.; Ma, Y.; Zhu, X.; He, X. Superhydrophobic Photothermal Icephobic Surfaces Based on Candle Soot. Proc. Natl. Acad. Sci. USA 2020, 117, 11240–11246. [Google Scholar] [CrossRef]
- MacNeil, J.; Volaric, L. Incomplete Combustion with Candle Flames: A Guided-Inquiry Experiment in the First-Year Chemistry Lab. J. Chem. Educ. 2003, 80, 302. [Google Scholar] [CrossRef]
- Liang, C.-J.; Liao, J.-D.; Li, A.-J.; Chen, C.; Lin, H.-Y.; Wang, X.-J.; Xu, Y.-H. Relationship between Wettabilities and Chemical Compositions of Candle Soots. Fuel 2014, 128, 422–427. [Google Scholar] [CrossRef]
- Qahtan, T.F.; Gondal, M.A.; Alade, I.O.; Dastageer, M.A. Fabrication of Water Jet Resistant and Thermally Stable Superhydrophobic Surfaces by Spray Coating of Candle Soot Dispersion. Sci. Rep. 2017, 7, 7531. [Google Scholar] [CrossRef]
- Garbassi, F.; Balducci, L.; Chiurlo, P.; Deiana, L. A Study of Surface Modification of Silica Using XPS, DRIFT and NMR. Appl. Surf. Sci. 1995, 84, 145–151. [Google Scholar] [CrossRef]
- Yang, C.; Tartaglino, U.; Persson, B.N.J. Influence of Surface Roughness on Superhydrophobicity. Phys. Rev. Lett. 2006, 97, 116103. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, J.; Zhang, D.; Li, L.; Zhu, Y. Preparation and Characterization of Superhydrophobic Surface Based on Polydimethylsiloxane (PDMS). J. Adhes. Sci. Technol. 2019, 33, 1870–1881. [Google Scholar] [CrossRef]
- Zheng, Q.; Lü, C. Size Effects of Surface Roughness to Superhydrophobicity. Procedia IUtam 2014, 10, 462–475. [Google Scholar] [CrossRef]
- Das, A.; Shome, A.; Manna, U. Porous and Reactive Polymeric Interfaces: An Emerging Avenue for Achieving Durable and Functional Bio-Inspired Wettability. J. Mater. Chem. A 2021, 9, 824–856. [Google Scholar] [CrossRef]
- Dash, S.; de Ruiter, J.; Varanasi, K.K. Photothermal Trap Utilizing Solar Illumination for Ice Mitigation. Sci. Adv. 2018, 4, eaat0127. [Google Scholar] [CrossRef]
- Marín, A.G.; Enríquez, O.R.; Brunet, P.; Colinet, P.; Snoeijer, J.H. Universality of Tip Singularity Formation in Freezing Water Drops. Phys. Rev. Lett. 2014, 113, 054301. [Google Scholar] [CrossRef] [PubMed]
- Graeber, G.; Schutzius, T.M.; Eghlidi, H.; Poulikakos, D. Spontaneous Self-Dislodging of Freezing Water Droplets and the Role of Wettability. Proc. Natl. Acad. Sci. USA 2017, 114, 11040–11045. [Google Scholar] [CrossRef] [PubMed]
- Snoeijer, J.H.; Brunet, P. Pointy Ice-Drops: How Water Freezes into a Singular Shape. Am. J. Phys. 2012, 80, 764–771. [Google Scholar] [CrossRef]
- Jung, S.; Tiwari, M.K.; Poulikakos, D. Frost Halos from Supercooled Water Droplets. Proc. Natl. Acad. Sci. USA 2012, 109, 16073–16078. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, Y.; Liu, Y.; Xu, R.; Liu, S.; Zhou, F. Robust Photothermal Coating Strategy for Efficient Ice Removal. ACS Appl. Mater. Interfaces 2020, 12, 46981–46990. [Google Scholar] [CrossRef]
- Xue, C.-H.; Li, H.-G.; Guo, X.-J.; Ding, Y.-R.; Liu, B.-Y.; An, Q.-F.; Zhou, Y. Superhydrophobic Anti-Icing Coatings with Self-Deicing Property Using Melanin Nanoparticles from Cuttlefish Juice. Chem. Eng. J. 2021, 424, 130553. [Google Scholar] [CrossRef]
- Boinovich, L.B.; Emelyanenko, A.M. Recent Progress in Understanding the Anti-Icing Behavior of Materials. Adv. Colloid Interface Sci. 2024, 323, 103057. [Google Scholar] [CrossRef]
- Hu, H.; Larson, R.G. Marangoni Effect Reverses Coffee-Ring Depositions. J. Phys. Chem. B 2006, 110, 7090–7094. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Han, B.; Zhang, Y.; Li, Q.; Zhang, Y.-L.; Han, D.-D.; Sun, H.-B. Laser-Induced Graphene Tapes as Origami and Stick-On Labels for Photothermal Manipulation via Marangoni Effect. Adv. Funct. Mater. 2021, 31, 2006179. [Google Scholar] [CrossRef]
- Wang, X.; Lu, Y.; Zhang, Q.; Wang, K.; Carmalt, C.J.; Parkin, I.P.; Zhang, Z.; Zhang, X. Durable Fire Retardant, Superhydrophobic, Abrasive Resistant and Air/UV Stable Coatings. J. Colloid Interface Sci. 2021, 582, 301–311. [Google Scholar] [CrossRef]

| Surfaces | Freezing Delay Time (s) | Reference |
|---|---|---|
| PDMS/CS60@PDMS/Al in this work | 7200 | This work |
| Bio-inspired micro-structure surface | 1938 | [1] |
| Nanostructured surface | 1740 | [15] |
| MWCNT based photothermal surface | 1800 | [45] |
| Cuttlefish juice based photothermal surface | 144 | [46] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qian, C.; Wang, L.; Li, Q.; Chen, X. A Strategy of Candle Soot-Based Photothermal Icephobic Superhydrophobic Surface. Coatings 2024, 14, 612. https://doi.org/10.3390/coatings14050612
Qian C, Wang L, Li Q, Chen X. A Strategy of Candle Soot-Based Photothermal Icephobic Superhydrophobic Surface. Coatings. 2024; 14(5):612. https://doi.org/10.3390/coatings14050612
Chicago/Turabian StyleQian, Chenlu, Lu Wang, Qiang Li, and Xuemei Chen. 2024. "A Strategy of Candle Soot-Based Photothermal Icephobic Superhydrophobic Surface" Coatings 14, no. 5: 612. https://doi.org/10.3390/coatings14050612
APA StyleQian, C., Wang, L., Li, Q., & Chen, X. (2024). A Strategy of Candle Soot-Based Photothermal Icephobic Superhydrophobic Surface. Coatings, 14(5), 612. https://doi.org/10.3390/coatings14050612





