Optimized Functionalization of Graphene Oxide for Enhanced Mechanical Properties in Epoxy Resin Composites
Abstract
1. Introduction
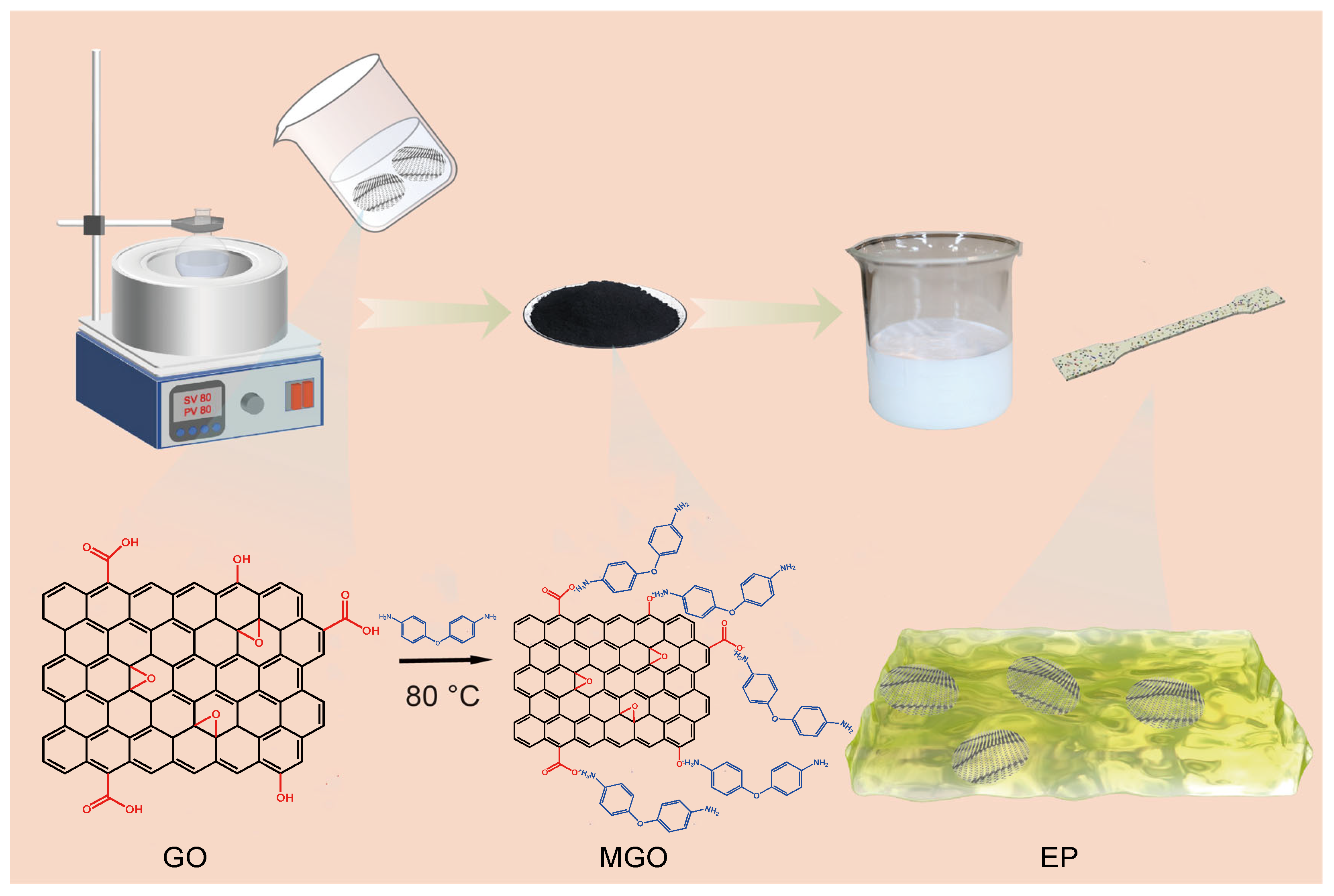
2. Experiments
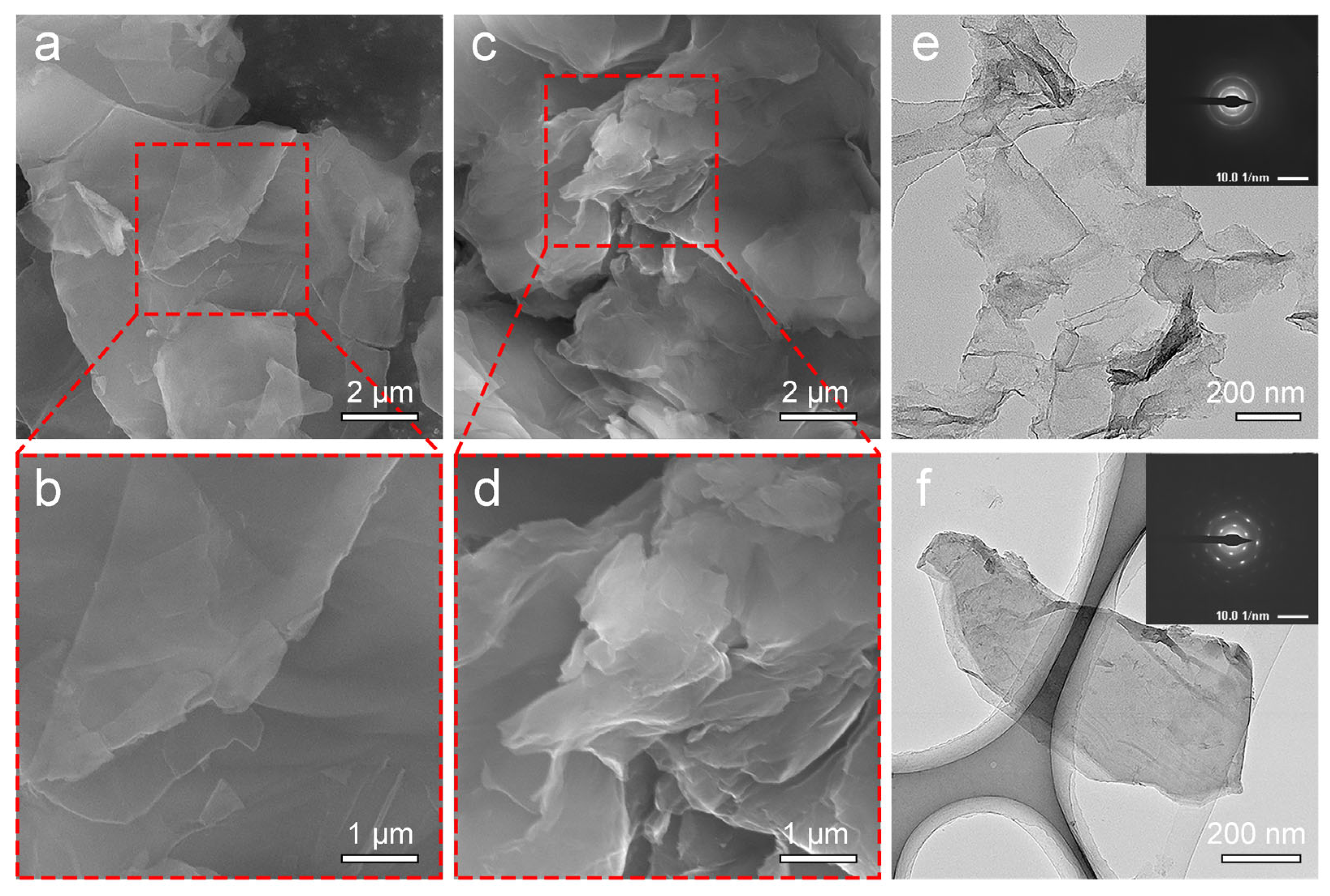
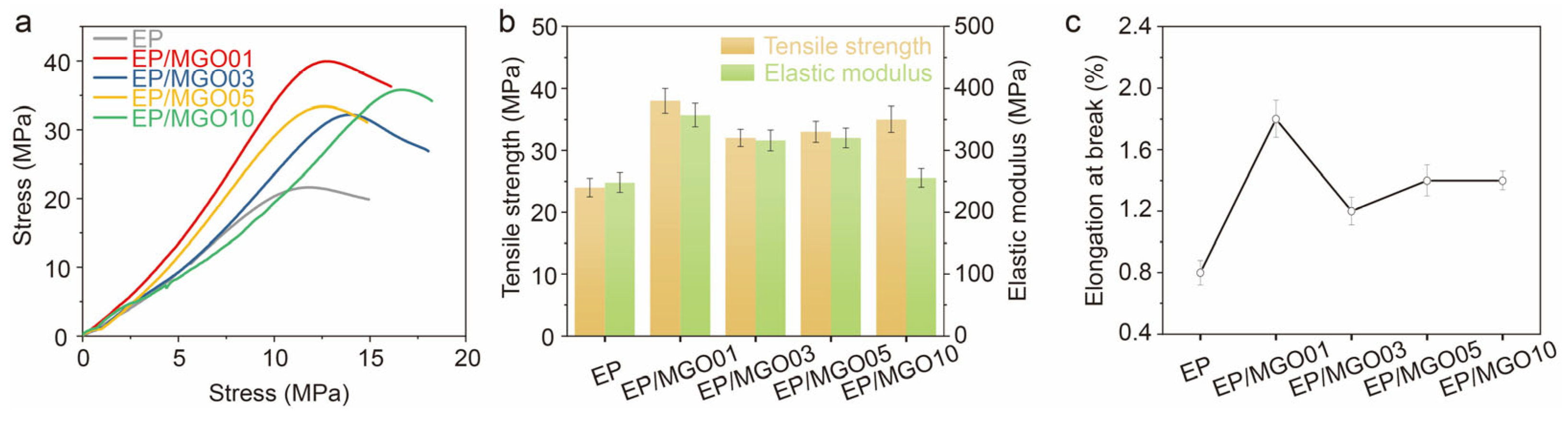
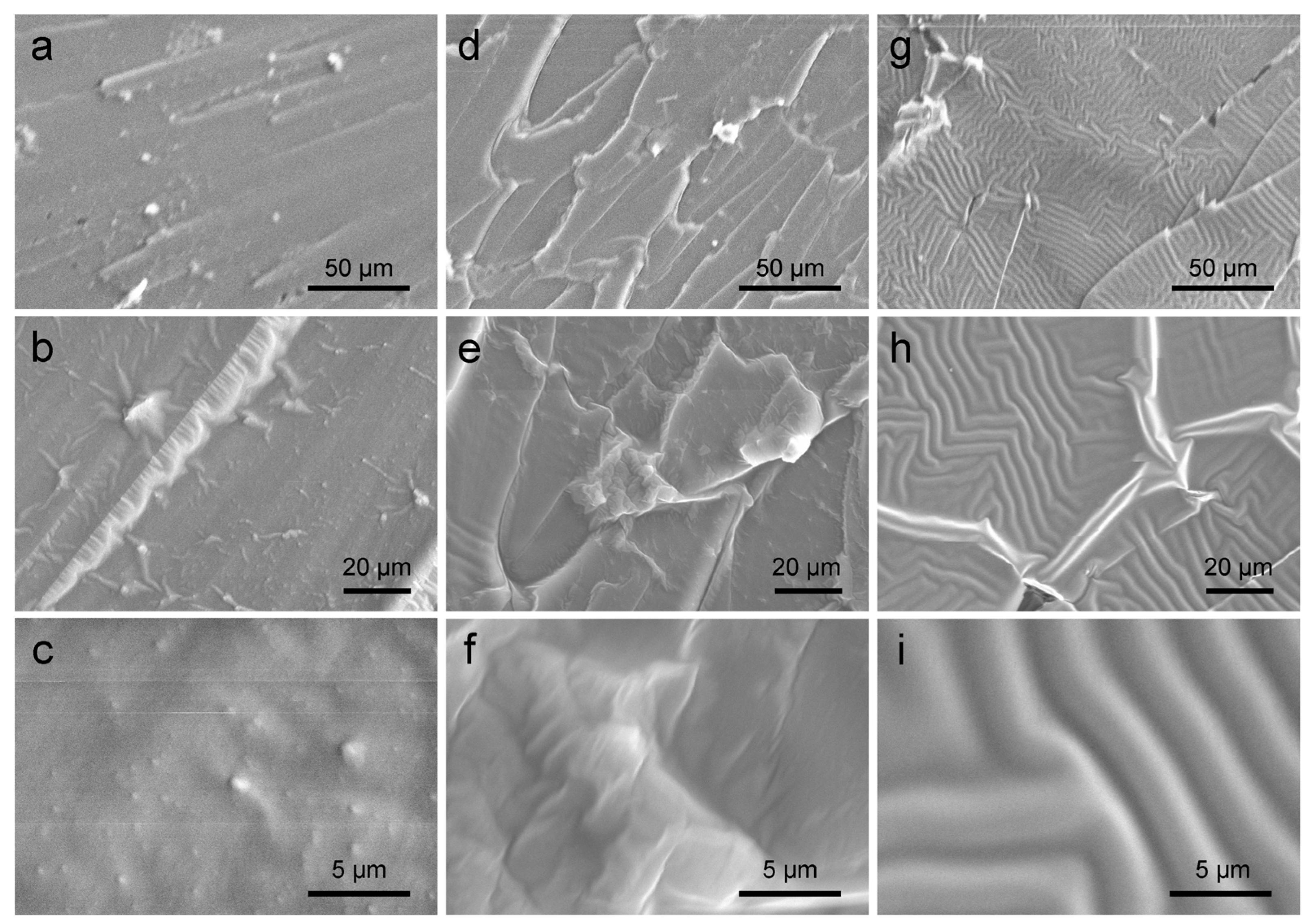
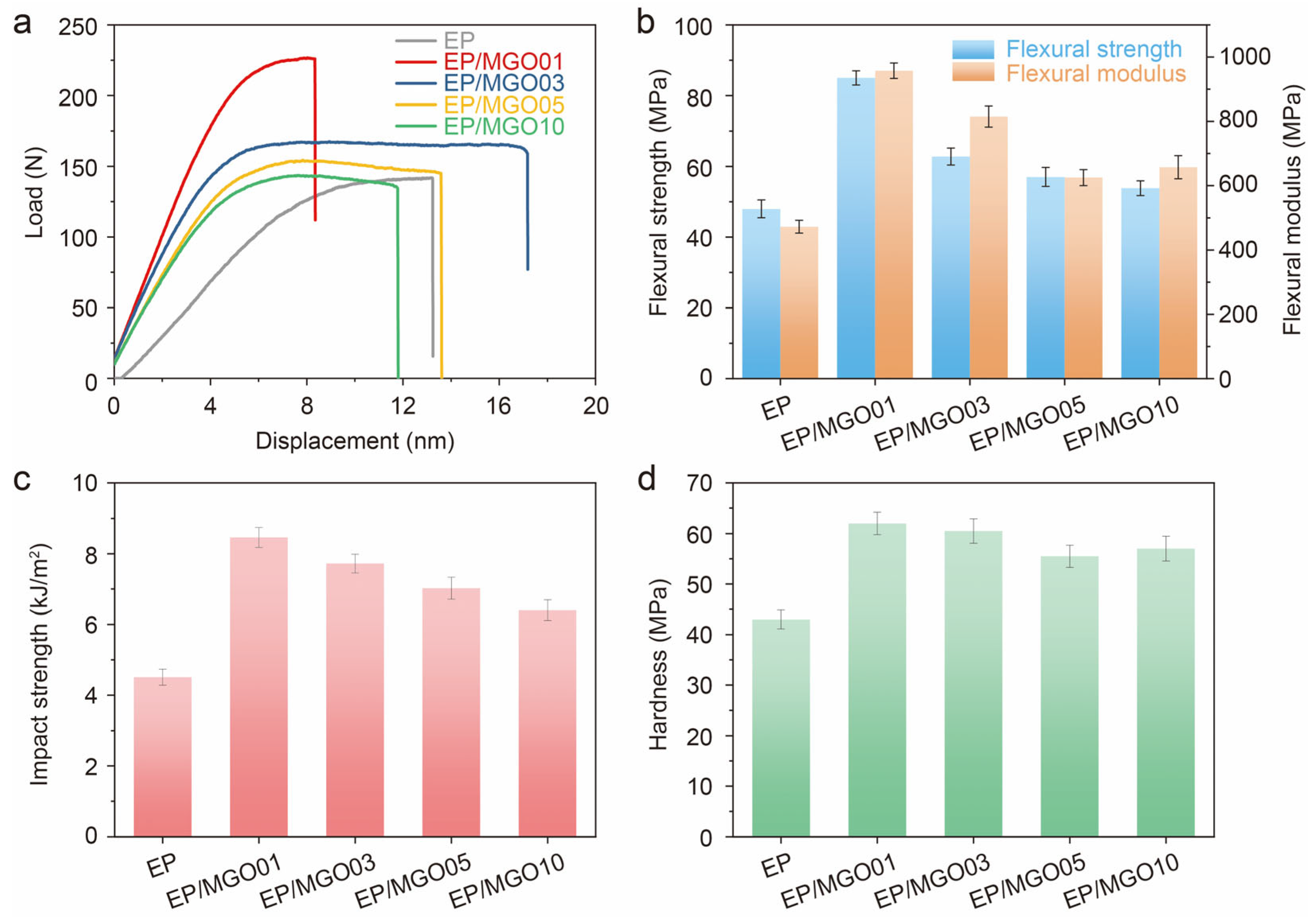
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Paolillo, S.; Bose, R.K.; Santana, M.H.; Grande, A.M. Intrinsic Self-Healing Epoxies in Polymer Matrix Composites (PMCs) for Aerospace Applications. Polymers 2021, 13, 201. [Google Scholar] [CrossRef] [PubMed]
- Salunke, D.R.; Gopalan, V. Thermal and Electrical behaviors of Boron Nitride/Epoxy reinforced polymer matrix composite—A review. Polym. Compos. 2021, 42, 1659–1669. [Google Scholar] [CrossRef]
- Wang, Z.; Jia, Z.; Feng, X.; Zou, Y. Graphene nanoplatelets/epoxy composites with excellent shear properties for construction adhesives. Compos. Part B Eng. 2018, 152, 311–315. [Google Scholar] [CrossRef]
- Jeong, S.-G.; Cha, J.; Kim, S.; Seo, J.; Lee, J.-H.; Kim, S. Preparation of thermal-enhanced epoxy resin adhesive with organic PCM for applying wood flooring. J. Therm. Anal. Calorim. 2014, 117, 1027–1034. [Google Scholar] [CrossRef]
- Baig, M.M.A.; Samad, M.A. Epoxy\Epoxy Composite\Epoxy Hybrid Composite Coatings for Tribological Applications—A Review. Polymers 2021, 13, 179. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Ma, S.; Yue, H.; Wang, S.; Zhu, J. Comparison of Hydrogenated Bisphenol A and Bisphenol A Epoxies: Curing Behavior, Thermal and Mechanical Properties, Shape Memory Properties. Macromol. Res. 2018, 26, 529–538. [Google Scholar] [CrossRef]
- Khan, M.; Khurram, A.A.; Li, T.; Zhao, T.; Subhani, T.; Gul, I.; Ali, Z.; Patel, V. Synergistic effect of organic and inorganic nano fillers on the dielectric and mechanical properties of epoxy composites. J. Mater. Sci. Technol. 2018, 34, 2424–2430. [Google Scholar] [CrossRef]
- Sun, J.; Qian, L.; Li, J. Toughening and strengthening epoxy resin with flame retardant molecular structure based on tyrosine. Polymer 2021, 230, 124045. [Google Scholar] [CrossRef]
- Orlov, A.; Konstantinova, A.; Korotkov, R.; Yudaev, P.; Mezhuev, Y.; Terekhov, I.; Gurevich, L.; Chistyakov, E. Epoxy Compositions with Reduced Flammability Based on DER-354 Resin and a Curing Agent Containing Aminophosphazenes Synthesized in Bulk Isophoronediamine. Polymers 2022, 14, 3592. [Google Scholar] [CrossRef]
- Wan, Y.-J.; Gong, L.-X.; Tang, L.-C.; Wu, L.-B.; Jiang, J.-X. Mechanical properties of epoxy composites filled with silane-functionalized graphene oxide. Compos. Part A Appl. Sci. Manuf. 2014, 64, 79–89. [Google Scholar] [CrossRef]
- Naebe, M.; Wang, J.; Amini, A.; Khayyam, H.; Hameed, N.; Li, L.H.; Chen, Y.; Fox, B. Mechanical Property and Structure of Covalent Functionalised Graphene/Epoxy Nanocomposites. Sci. Rep. 2014, 4, 4375. [Google Scholar] [CrossRef]
- Sun, Y.W.; Papageorgiou, D.G.; Humphreys, C.J.; Dunstan, D.J.; Puech, P.; Proctor, J.E.; Bousige, C.; Machon, D.; San-Miguel, A. Mechanical properties of graphene. Appl. Phys. Rev. 2021, 8, 021310. [Google Scholar] [CrossRef]
- Kovalev, S.; Hafez, H.A.; Tielrooij, K.-J.; Deinert, J.-C.; Ilyakov, I.; Awari, N.; Alcaraz, D.; Soundarapandian, K.; Saleta, D.; Germanskiy, S.; et al. Electrical tunability of terahertz nonlinearity in graphene. Sci. Adv. 2021, 7, eabf9809. [Google Scholar] [CrossRef] [PubMed]
- Orek, C.; Bartolomei, M.; Coletti, C.; Bulut, N. Graphene as Nanocarrier for Gold(I)-Monocarbene Complexes: Strength and Nature of Physisorption. Molecules 2023, 28, 3941. [Google Scholar] [CrossRef] [PubMed]
- Konstantinova, A.; Yudaev, P.; Orlov, A.; Loban, O.; Lukashov, N.; Chistyakov, E. Aryloxyphosphazene-Modified and Graphite-Filled Epoxy Compositions with Reduced Flammability and Electrically Conductive Properties. J. Compos. Sci. 2023, 7, 417. [Google Scholar] [CrossRef]
- Papageorgiou, D.G.; Li, Z.; Liu, M.; Kinloch, I.A.; Young, R.J. Mechanisms of mechanical reinforcement by graphene and carbon nanotubes in polymer nanocomposites. Nanoscale 2020, 12, 2228–2267. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Yang, J.; Curtis, T.E.; Luo, S.; Huang, D.; Feng, Z.; Morales-Ferreiro, J.O.; Sapkota, P.; Lei, F.; Zhang, J.; et al. Exfoliated Graphene Leads to Exceptional Mechanical Properties of Polymer Composite Films. ACS Nano 2019, 13, 1097–1106. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Song, F.; Ding, Y.; Shao, M. The incorporation of graphene to enhance mechanical properties of polypropylene self-reinforced polymer composites. Mater. Des. 2020, 195, 109073. [Google Scholar] [CrossRef]
- Belyaeva, L.A.; van Deursen, P.M.G.; Barbetsea, K.I.; Schneider, G.F. Hydrophilicity of Graphene in Water through Transparency to Polar and Dispersive Interactions. Adv. Mater. 2018, 30, 1703274. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, H.; Wang, Z.; Sui, W.; Gong, Y.; Cui, J.; Ao, Y.; Shang, L. Functional boron nitride/graphene oxide three-dimensional skeleton co-heat transfer epoxy resin composite. J. Alloys Compd. 2024, 985, 173935. [Google Scholar] [CrossRef]
- González, M.C.R.; Leonhardt, A.; Stadler, H.; Eyley, S.; Thielemans, W.; De Gendt, S.; Mali, K.S.; De Feyter, S. Multicomponent Covalent Chemical Patterning of Graphene. ACS Nano 2021, 15, 10618–10627. [Google Scholar] [CrossRef] [PubMed]
- Assies, L.; Fu, C.; Kovaříček, P.; Bastl, Z.; Drogowska, K.A.; Lang, J.; Guerra, V.L.P.; Samorì, P.; Orgiu, E.; Perepichka, D.F.; et al. Dynamic covalent conjugated polymer epitaxy on graphene. J. Mater. Chem. C 2019, 7, 12240–12247. [Google Scholar] [CrossRef]
- Valenta, L.; Kovaříček, P.; Valeš, V.; Bastl, Z.; Drogowska, K.A.; Verhagen, T.A.; Cibulka, R.; Kalbáč, M. Spatially Resolved Covalent Functionalization Patterns on Graphene. Angew. Chem. Int. Ed. 2018, 58, 1324–1328. [Google Scholar] [CrossRef] [PubMed]
- Nan, D.; Li, X.; Liu, Q.; Wang, B.; Gao, X.; He, N.; Xu, Y.; Liu, J. Epoxy primer topcoat syncretic coating prepared by using modified graphene oxide incorporated with TiO2 nanoparticles. Mater. Today Sustain. 2023, 21, 100282. [Google Scholar] [CrossRef]
- Nan, D.; Li, X.; Li, D.; Liu, Q.; Wang, B.; Gao, X.; Ma, T.; He, N.; Xu, Y.; Dong, J. Preparation and Anticorrosive Performance of Waterborne Epoxy Resin Composite Coating with Amino-Modified Graphene Oxide. Polymers 2022, 15, 27. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, D.; Chen, J.; Huo, D.; Gao, X.; Dong, J.; Yin, Y.; Liu, J.; Nan, D. Melamine-Modified Graphene Oxide as a Corrosion Resistance Enhancing Additive for Waterborne Epoxy Resin Coatings. Coatings 2024, 14, 488. [Google Scholar] [CrossRef]
- GB/T 13525-1992; Test Method for Tensile-Impact Property of Plastics. State Technical Supervision Bureau: Beijing, China, 1992.
- GB/T 6569-2006; Fine Ceramics (Advanced Ceramics, Advanced Technical Ceramics)—Test Method for Flexural Strength of Monolithic Ceramics at Room Temperature. Standardization Administration of China: Beijing, China, 2006.
- Pourhashem, S.; Vaezi, M.R.; Rashidi, A.; Bagherzadeh, M.R. Exploring corrosion protection properties of solvent based epoxy-graphene oxide nanocomposite coatings on mild steel. Corros. Sci. 2017, 115, 78–92. [Google Scholar] [CrossRef]
- Wan, Y.-J.; Tang, L.-C.; Gong, L.-X.; Yan, D.; Li, Y.-B.; Wu, L.-B.; Jiang, J.-X.; Lai, G.-Q. Grafting of epoxy chains onto graphene oxide for epoxy composites with improved mechanical and thermal properties. Carbon 2014, 69, 467–480. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Yu, B.; Chen, J.; Huo, D.; Liu, J.; Nan, D. Optimized Functionalization of Graphene Oxide for Enhanced Mechanical Properties in Epoxy Resin Composites. Coatings 2024, 14, 609. https://doi.org/10.3390/coatings14050609
Li X, Yu B, Chen J, Huo D, Liu J, Nan D. Optimized Functionalization of Graphene Oxide for Enhanced Mechanical Properties in Epoxy Resin Composites. Coatings. 2024; 14(5):609. https://doi.org/10.3390/coatings14050609
Chicago/Turabian StyleLi, Xin, Bing Yu, Jie Chen, Dongxia Huo, Jun Liu, and Ding Nan. 2024. "Optimized Functionalization of Graphene Oxide for Enhanced Mechanical Properties in Epoxy Resin Composites" Coatings 14, no. 5: 609. https://doi.org/10.3390/coatings14050609
APA StyleLi, X., Yu, B., Chen, J., Huo, D., Liu, J., & Nan, D. (2024). Optimized Functionalization of Graphene Oxide for Enhanced Mechanical Properties in Epoxy Resin Composites. Coatings, 14(5), 609. https://doi.org/10.3390/coatings14050609





