Influence of HNT-ZnO Nanofillers on the Performance of Epoxy Resin Composites for Marine Applications
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation
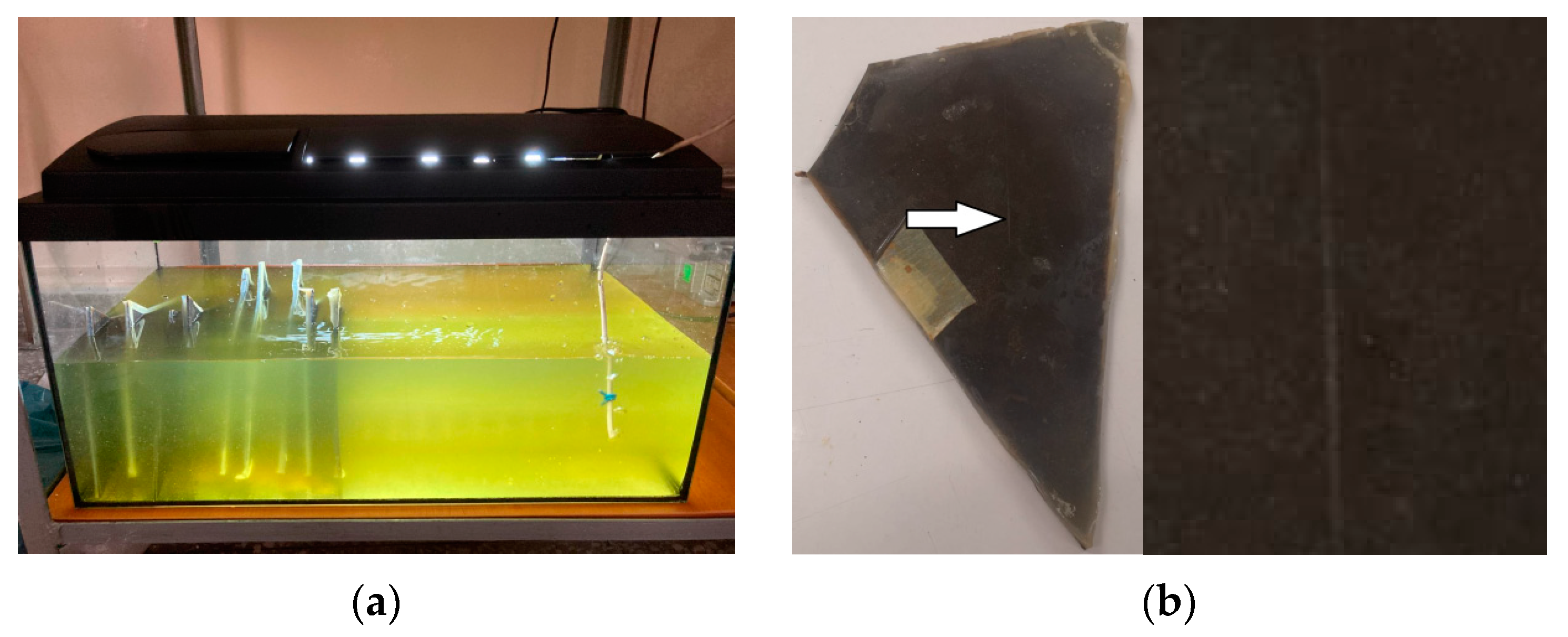
2.3. Characterization
3. Results and Discussion
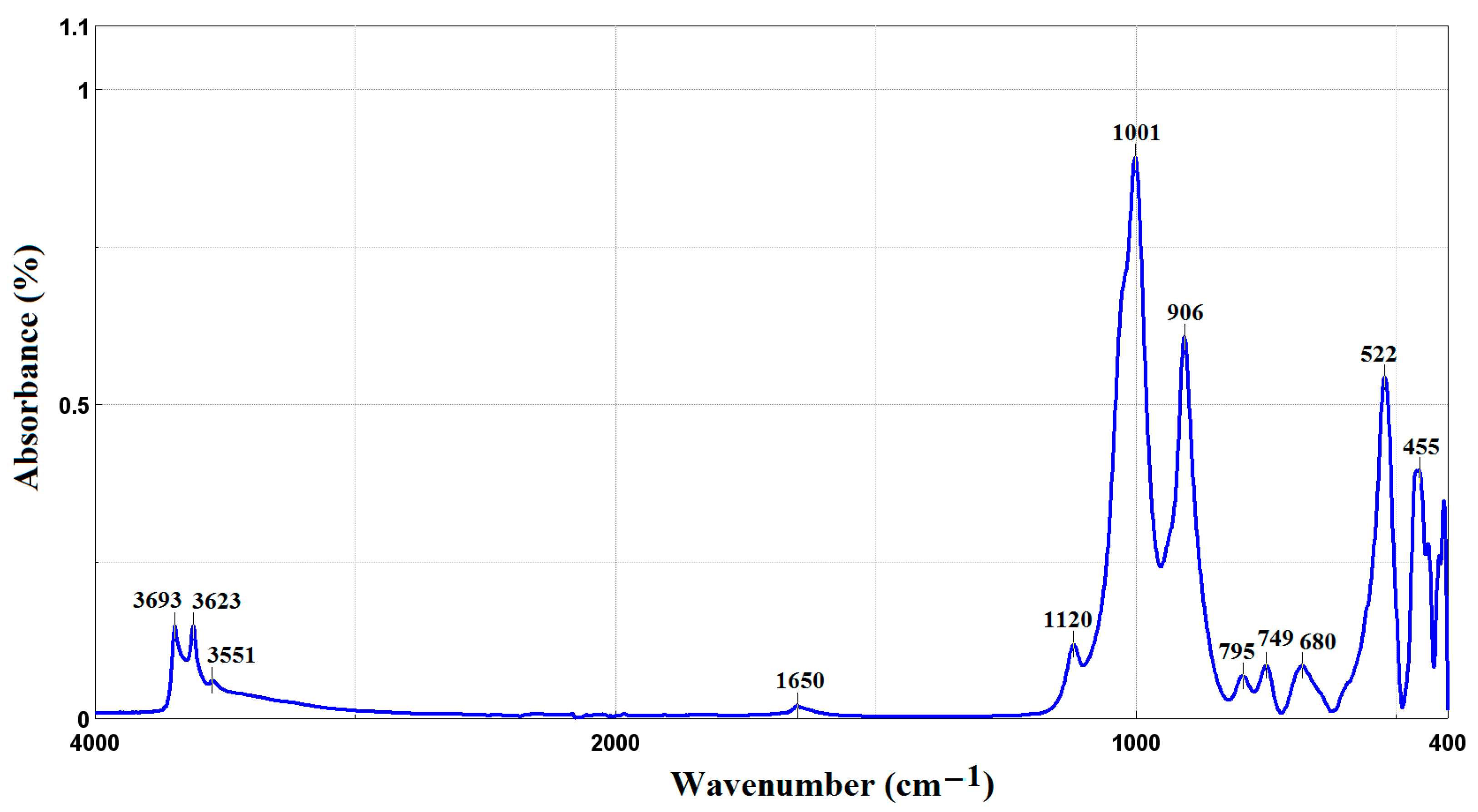
3.1. FTIR-ATR Spectroscopy
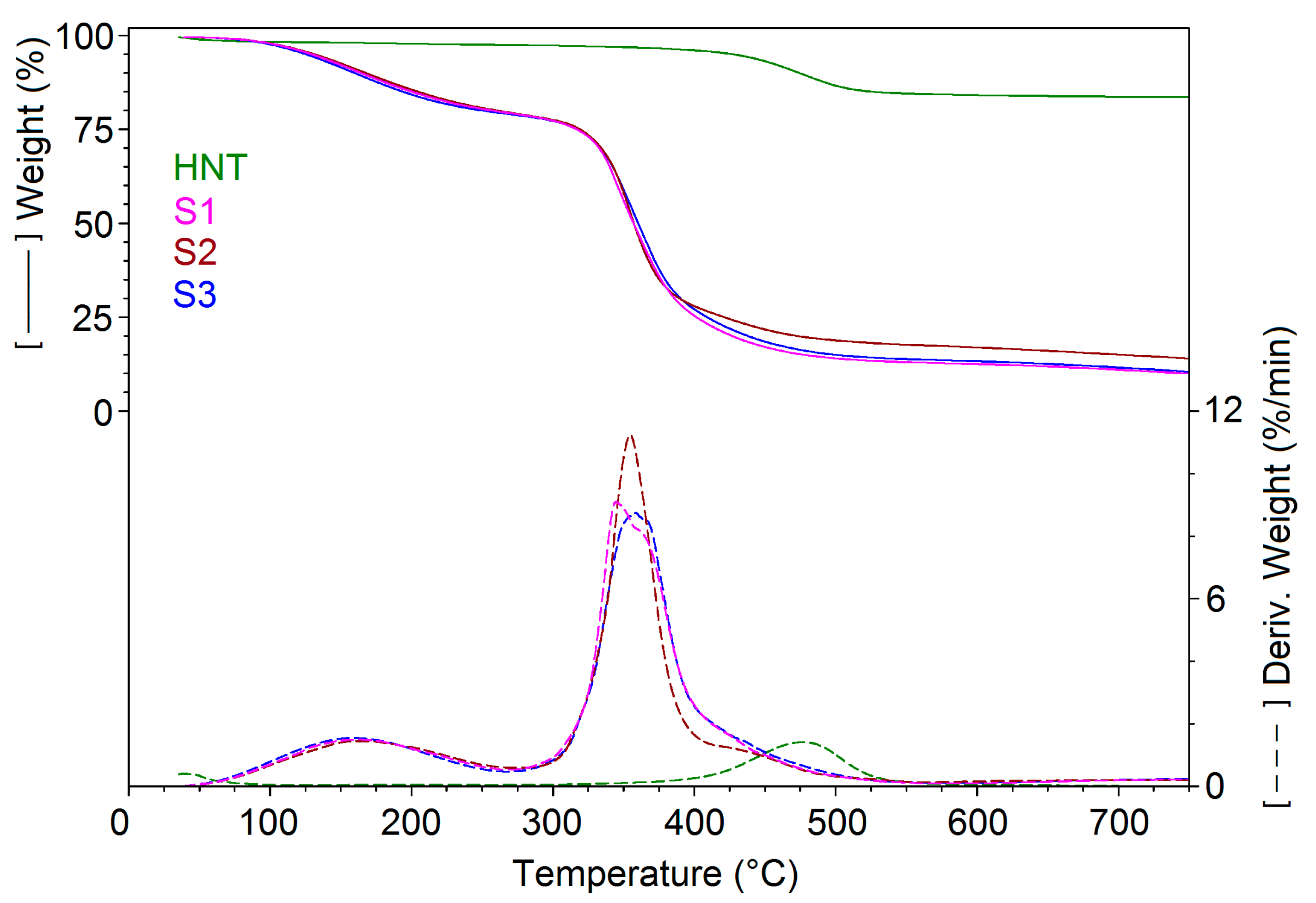
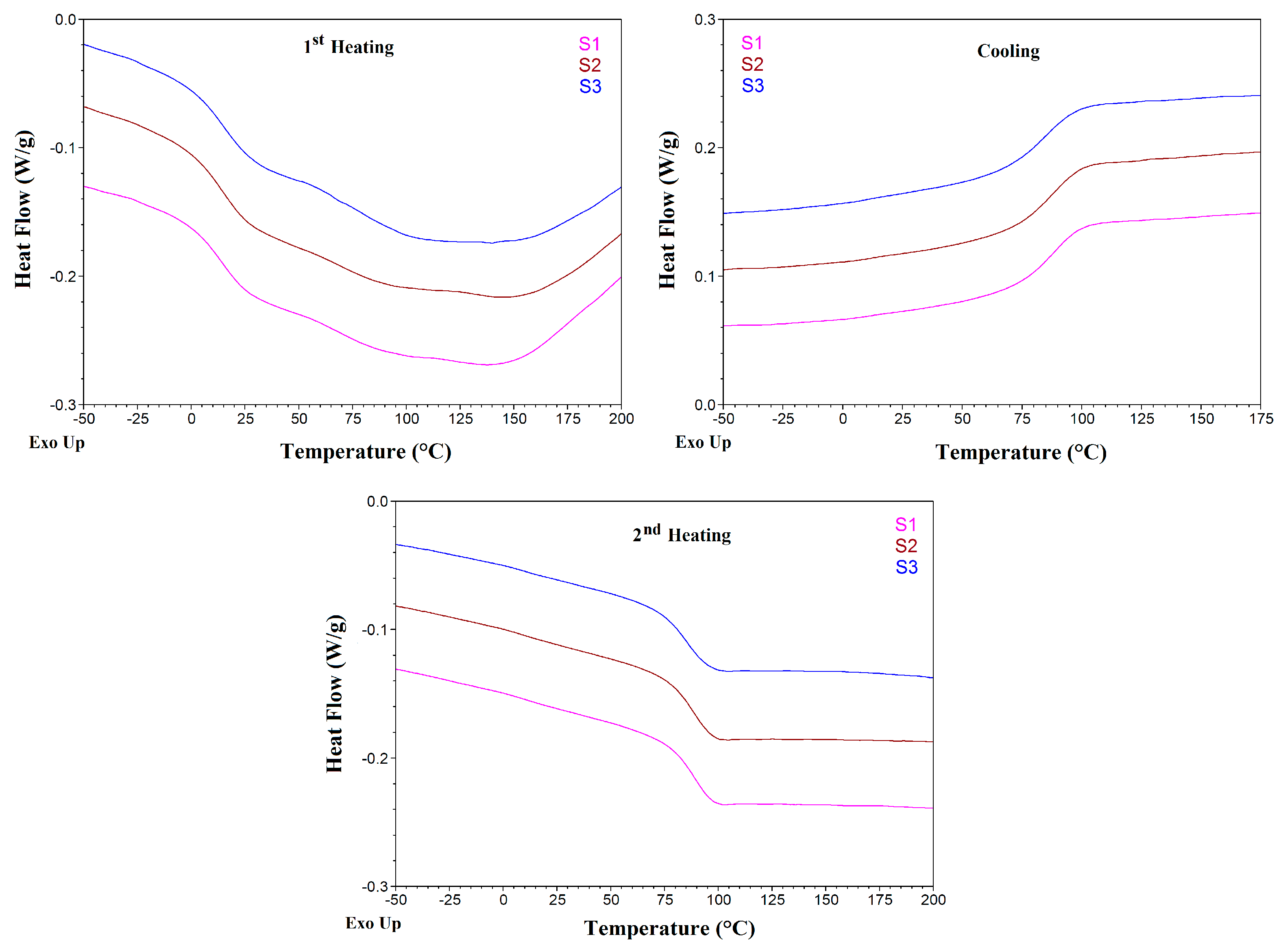
3.2. TGA and DSC Analysis
3.3. TEM Analysis
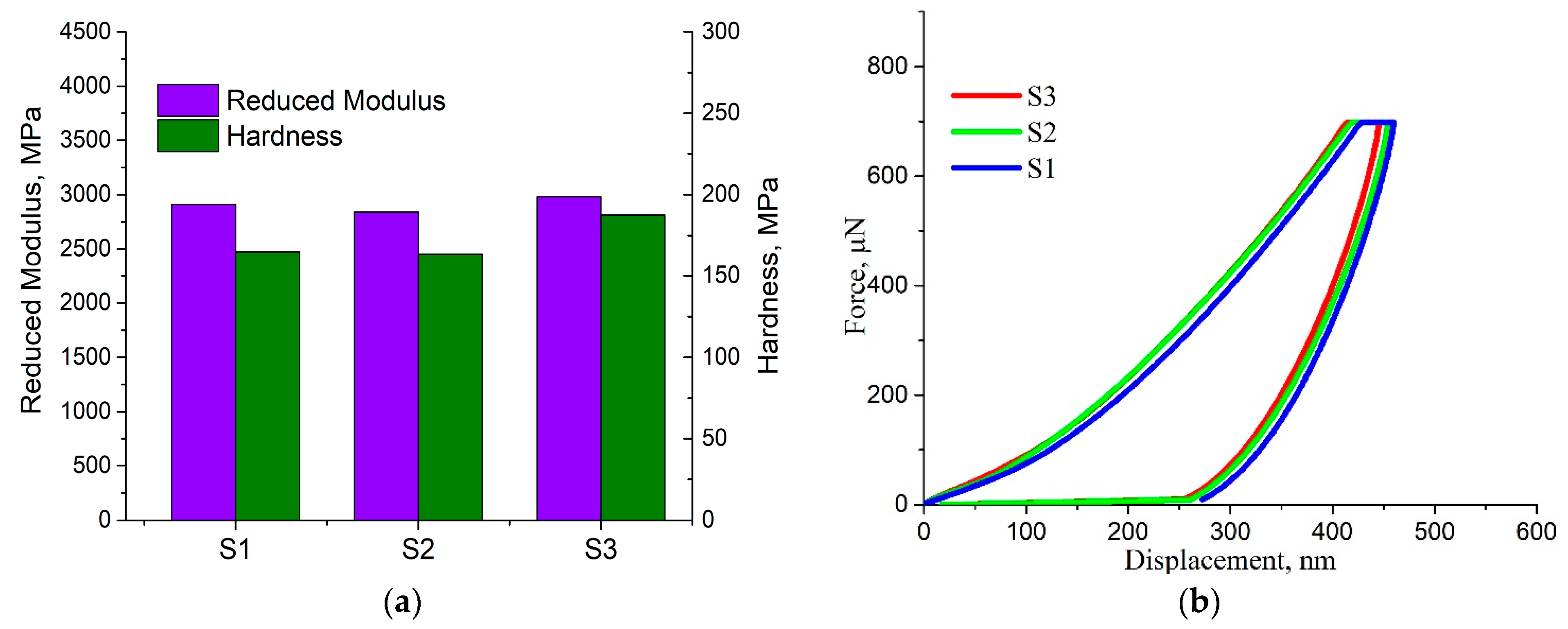
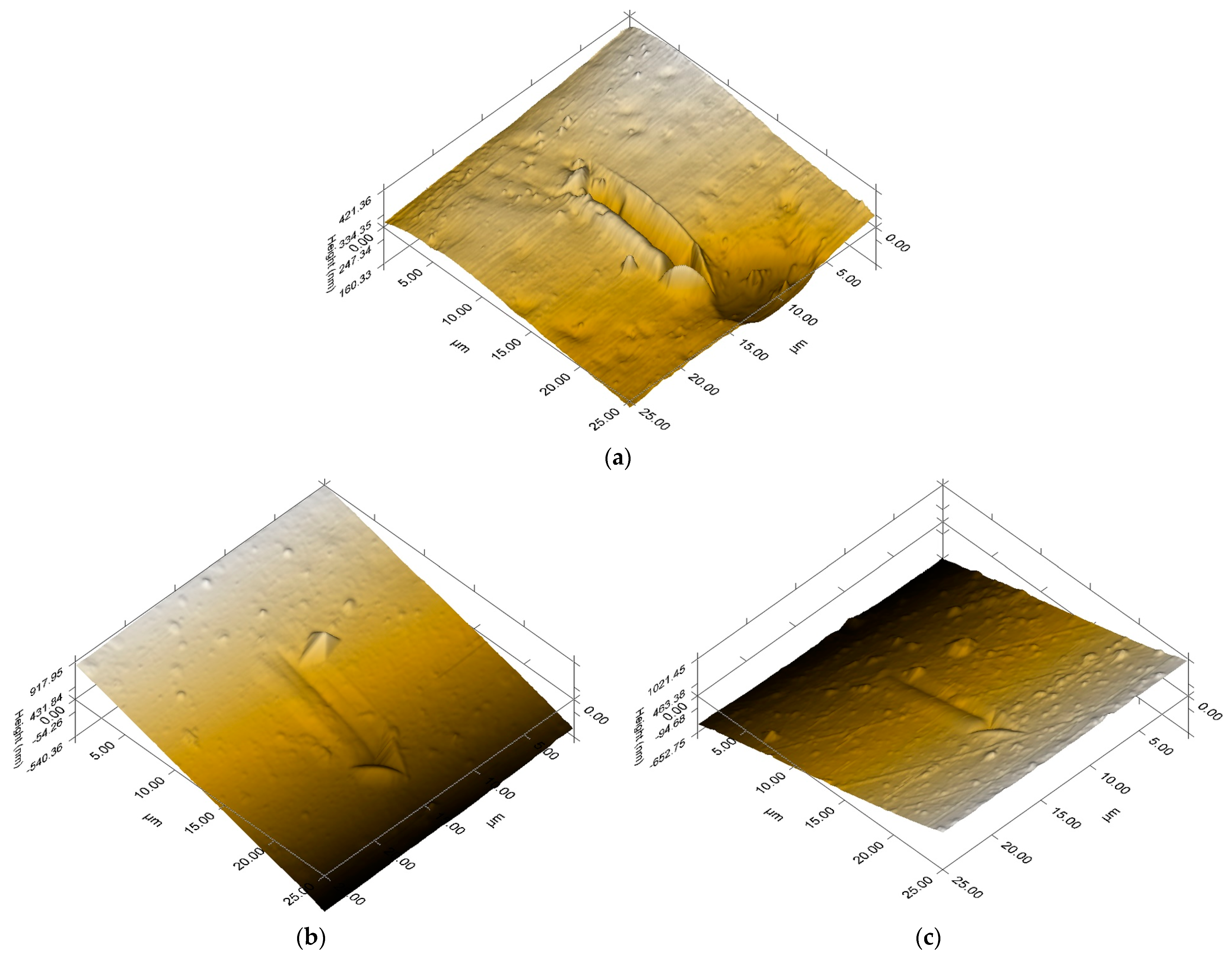
3.4. Nanoindentation Analysis Method
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alam, M.A.; Samad, U.A.; Anis, A.; Alam, M.; Ubaidullah, M.; Al-Zahrani, S.M. Effects of SiO2 and zno nanoparticles on epoxy coatings and its performance investigation using thermal and nanoindentation technique. Polymers 2021, 13, 1490. [Google Scholar] [CrossRef] [PubMed]
- Samad, U.A.; Alam, M.A.; Anis, A.; Sherif, E.-S.M.; Al-Mayman, S.I.; Al-Zahrani, S.M. Effect of incorporated ZnO nanoparticles on the corrosion performance of SiO2 nanoparticle-based mechanically robust epoxy coatings. Materials 2020, 13, 3767. [Google Scholar] [CrossRef] [PubMed]
- Abdellaoui, H.; Raji, M.; Bouhfid, R.; Qaiss, A.e.k. Investigation of the deformation behavior of epoxy-based composite materials. In Failure Analysis in Biocomposites, Fibre-Reinforced Composites and Hybrid Composites, 2nd ed.; Jawaid, M., Thariq, M., Saba, N., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 29–49. [Google Scholar]
- Junid, R.; Siregar, J.P.; Endot, N.A.; Razak, J.A.; Wilkinson, A.N. Optimization of Glass Transition Temperature and Pot Life of Epoxy Blends Using Response Surface Methodology (RSM). Polymers 2021, 13, 3304. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, X.; Ren, J.; Chang, M.; He, B.; Zhang, C. Effects of nano-ZnO and nano-SiO2 particles on properties of PVA/xylan composite films. Int. J. Biol. Macromol. 2019, 132, 978–986. [Google Scholar] [CrossRef] [PubMed]
- Becker, O.; Varley, R.; Simon, G. Morphology, thermal relaxations and mechanical properties of layered silicate nanocomposites based upon high-functionality epoxy resins. Polymer 2002, 43, 4365. [Google Scholar] [CrossRef]
- Rashvand, M.; Ranjbar, Z.; Rastegar, S. Preserving Anti-Corrosion Properties of Epoxy Based Coatings Simultaneously Exposed to Humidity and UV-Radiation Using Nano Zinc Oxide. J. Electrochem. Soc. 2012, 159, C129. [Google Scholar] [CrossRef]
- Sunny, A.T.; Vijayan, P.P.; Adhikari, R.; Mathew, S.; Thomas, S. Copper oxide nanoparticle in an epoxy network: Microstructure, chain confinement and mechanical behavior. Phys. Chem. Chem. Phys. 2016, 18, 19655–19667. [Google Scholar] [CrossRef] [PubMed]
- Suntako, R. Cure Characteristics and Mechanical Properties of ZnO Nanoparticles as Activator in Unfilled Natural Rubbe. Adv. Mater. Res. 2014, 1044–1045, 23–26. [Google Scholar] [CrossRef]
- Ding, K.H.; Wang, G.L.; Zhang, M. Characterization of mechanical properties of epoxy resin reinforced with submicron-sized ZnO prepared via in situ synthesis method. Mater. Des. 2011, 32, 3986–3991. [Google Scholar] [CrossRef]
- Thipperudrappa, S.; Ullal Kini, A.; Hiremath, A. Influence of zinc oxide nanoparticles on the mechanical and thermal responses of glass fiber-reinforced epoxy nanocomposites. Polym. Compos. 2020, 41, 174e81. [Google Scholar] [CrossRef]
- Liu, C.; Daneshvar, F.; Hawkins, S.; Kotaki, M.; Sue, H.J. High dielectric constant epoxy nano-composites containing ZnO quantum dots decorated carbon nanotube. J. Appl. Polym. Sci. 2020, 138, e49778. [Google Scholar] [CrossRef]
- Samad, U.A.; Alam, M.A.; Chafidz, A.; Al-Zahrani, S.M.; Alharthi, N.H. Enhancing mechanical properties of epoxy/polyaniline coating with addition of ZnO nanoparticles: Nanoindentation characterization. Prog. Org. Coat. 2018, 119, 109–115. [Google Scholar] [CrossRef]
- Srikanth, C.; Madhu, G.M. Effect of nano CdO-ZnO content on structural, thermal, optical, mechanical and electrical properties of epoxy composites. J. Met. Mater. Miner. 2023, 33, 38–52. [Google Scholar] [CrossRef]
- Hardoň, Š.; Kúdelčík, J.; Trnka, P.; Totzauer, P.; Hornak, J.; Michal, O. The influence of ZnO nanoparticles on the dielectric properties of epoxy resin. In Proceedings of the Applied Physics of Condensed Matter, Strba, Slovakia, 19–21 June 2019. [Google Scholar]
- Al-Lhaibi, S.A.; Al-Shabander, B.M. Study the effect of ZnO nanoparticles reinforced sawdust /epoxy composites on mechanical properties. Dig. J. Nanomater. Biostruct. 2022, 17, 851–860. [Google Scholar] [CrossRef]
- Lorero, I.; Campo, M.; Del Rosario, G.; López, F.A.; Prolongo, S.G. New Manufacturing Process of Composites Reinforced with ZnO Nanoparticles Recycled from Alkaline Batteries. Polymers 2020, 12, 1619. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Zhang, Z.; Kang, R.; Chen, Y.; Hou, X.; Wu, Y.; Wang, M.; Wang, B.; Cui, J.; Jiang, N.; et al. Enhanced thermal conductivity of epoxy composites filled with tetrapod-shaped ZnO. RSC Adv. 2018, 8, 12337–12343. [Google Scholar] [CrossRef]
- Hawkins, S.A.; Yao, H.; Wang, H.; Sue, H.J. Tensile properties and electrical conductivity of epoxy composite thin films containing zinc oxide quantum dots and multi-walled carbon nanotubes. Carbon 2017, 115, 18–27. [Google Scholar] [CrossRef]
- Halder, S.; Prasad, T.; Khan, N.I.; Goyat, M.S.; Chauhan, S.R. Superior mechanical properties of poly vinyl alcohol-assisted ZnO nanoparticle reinforced epoxy composites. Mater. Chem. Phys. 2017, 192, 198–209. [Google Scholar] [CrossRef]
- Sari, M.G.; Vahabi, H.; Gabrion, X.; Laheurte, P.; Zarrintaj, P.; Formela, K.; Saeb, M.R. An attempt to mechanistically explain the viscoelastic behavior of transparent epoxy/starch-modified ZnO nanocomposite coatings. Prog. Org. Coat. 2018, 119, 171–182. [Google Scholar] [CrossRef]
- Shi, X.; Nguyen, T.A.; Suo, Z.; Liu, Y.; Avci, R. Effect of nanoparticles on the anticorrosion and mechanical properties of epoxy coating. Surf. Coat. Technol. 2009, 204, 237–245. [Google Scholar] [CrossRef]
- Tonelli, M.; Perini, I.; Ridi, F.; Baglioni, P. Improving the properties of antifouling hybrid composites: The use of Halloysites as nano-containers in epoxy coatings. Colloids Surf. A Physicochem. Eng. Asp. 2021, 623, 126779. [Google Scholar] [CrossRef]
- Kaybal, H.B.; Ulus, H.; Avcı, A. Seawater aged basalt/epoxy composites: Improved bearing performance with halloysite nanotube reinforcement. Fibers Polym. 2021, 22, 1643–1652. [Google Scholar] [CrossRef]
- Pourhashem, S.; Saba, F.; Duan, J.; Rashidie, A.; Guan, F.; Nezhad, E.G.; Hou, B. Polymer/Inorganic nanocomposite coatings with superior corrosion protection perfor-mance: A review. J. Ind. Eng. Chem. 2020, 88, 29–57. [Google Scholar] [CrossRef]
- Purcar, V.; Şomoghi, R.; Niţu, S.G.; Nicolae, C.-A.; Alexandrescu, E.; Gîfu, I.C.; Gabor, A.R.; Stroescu, H.; Ianchiş, R.; Căprărescu, S.; et al. The Effect of Different Coupling Agents on Nano-ZnO Materials Obtained via the Sol–Gel Process. Nanomaterials 2017, 7, 439. [Google Scholar] [CrossRef] [PubMed]
- Somoghi, R.; Purcar, V.; Alexandrescu, E.; Gifu, I.C.; Ninciuleanu, C.M.; Cotrut, C.M.; Oancea, F.; Stroescu, H. Synthesis of Zinc Oxide Nanomaterials via Sol-Gel Process with Anti-Corrosive Effect for Cu, Al and Zn Metallic Substrates. Coatings 2021, 11, 444. [Google Scholar] [CrossRef]
- ASTM D 3363; Standard Test Method for Film Hardness by Pencil Test. ASTM International: West Conshohocken, PA, USA, 2022.
- Vuluga, Z.; Corobea, M.; Elizetxea, C.; Ordonez, M.; Ghiurea, M.; Raditoiu, V.; Nicolae, C.; Florea, D.; Iorga, M.; Somoghi, R.; et al. Morphological and Tribological Properties of PMMA/Halloysite Nanocomposites. Polymers 2018, 10, 816. [Google Scholar] [CrossRef] [PubMed]
- Arat, R.; Uyanık, N. Surface Modification of Nanoclays with Styrene-Maleic Anhydride Copolymers. Nat. Resour. 2017, 8, 159–171. [Google Scholar] [CrossRef]
- Thakura, S.; Bhattacharyab, R.; Neogia, S.; Neogi, S. Enhancement of Microwave Absorption Properties of Epoxy by Sol–Gel-Synthesised ZnO Nanoparticles. Indian Chem. Eng. 2016, 58, 310–324. [Google Scholar] [CrossRef]
- Dhanapal, D.; Ranjitha, J.; Vijayalakshmi, S.; Sagadevan, S. Fabrication of tetraglycidyl epoxy nano-composites functionalized with amine-terminated zinc oxide with improved mechanical and thermal properties. J. Mater. Res. Technol. 2022, 21, 3947–3960. [Google Scholar] [CrossRef]
- Ramírez-Herrera, C.A.; Cruz-Cruz, I.; Jiménez-Cedeño, I.H.; Martínez-Romero, O.; Elías-Zúñiga, A. Influence of the Epoxy Resin Process Parameters on the Mechanical Properties of Produced Bidirectional [±45°] Carbon/Epoxy Woven Composites. Polymers 2021, 13, 1273. [Google Scholar] [CrossRef] [PubMed]
- Moussa, S.; Namouchi, F.; Guermazi, H. Elaboration, structural and optical investigations of ZnO/epoxy nanocomposites. Eur. Phys. J. Plus 2015, 130, 152. [Google Scholar] [CrossRef]
- Thakor, S.G.; Rana, V.A.; Vankar, H.P.; Pandit, T.R. Dielectric spectroscopy and structural characterization of nano-filler-loaded epoxy resin. J. Adv. Dielectr. 2021, 11, 2150011. [Google Scholar] [CrossRef]
- Ramezanzadeh, B.; Attar, M.M.; Farzam, M. Effect of ZnO nanoparticles on the thermal and mechanical properties of epoxy-based nanocomposite. J. Therm. Anal. Calorim. 2011, 103, 731–739. [Google Scholar] [CrossRef]
- Vuluga, Z.; Sanporean, C.-G.; Panaitescu, D.M.; Teodorescu, G.M.; Corobea, M.C.; Nicolae, C.A.; Gabor, A.R.; Raditoiu, V. The Effect of SEBS/Halloysite Masterbatch Obtained in Different Extrusion Conditions on the Properties of Hybrid Polypropylene/Glass Fiber Composites for Auto Parts. Polymers 2021, 13, 3560. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.N.; Pham, T.V.A.; Le, M.L.P.; Nguyen, T.P.T.; Tran, V.M. Synthesis of amorphous silica and sulfonic acid functionalized silica used as reinforced phase for polymer electrolyte membrane. Adv. Nat. Sci. Nanosci. 2013, 4, 045007. [Google Scholar] [CrossRef]
- Ravindra, R.T.; Kaneko, S.; Endo, T.; Lakshmi, R.S. Spectroscopic Characterization of Bentonite. J. Laser Opt. Photonics 2017, 4, 171. [Google Scholar]
- Wu, Z.; Li, S.; Liu, M.; Wang, Z.; Liu, X. Liquid oxygen compatible epoxy resin: Modification and characterization. RSC Adv. 2015, 5, 11325–11333. [Google Scholar] [CrossRef]
- Du, M.; Guo, B.; Lei, Y.; Liu, M.; Jia, D. Carboxylated butadiene–styrene rubber/halloysite nanotube nanocomposites: Interfacial interaction and performance. Polymer 2008, 49, 4871–4876. [Google Scholar] [CrossRef]
- Lim, K.; Chow, W.S.; Pung, S.Y. Accelerated Weathering and UV Protection-Ability of Poly(Lactic Acid) Nanocomposites Containing Zinc Oxide Treated Halloysite Nanotube. J. Polym. Environ. 2019, 27, 1746–1759. [Google Scholar] [CrossRef]
- Muhammad, A.N.S.; Farooq, A.; Lodhi, M.; Deen, K. Performance evaluation of epoxy coatings containing different fillers in natural and simulated environmental conditions for corrosion resistance. J. Biomed. Eng. Biosci. 2019, 3, 1. [Google Scholar]
- Tomaszewska, J.; Sterzyński, T.; Woźniak-Braszak, A.; Banaszak, M. Review of Recent Developments of Glass Transition in PVC Nanocomposites. Polymers 2021, 13, 4336. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.C.; Whang, W.T.; Hung, C.H.; Chiang, P.C.; Hsiao, K.N. Effect of the polyimide structure and ZnO concentration on the morphology and characteristics of polyimide/ZnO nanohybrid films. Macromol. Chem. Phys. 2005, 206, 291–298. [Google Scholar] [CrossRef]
- Baghdadi, Y.N.; Youssef, L.; Bouhadir, K.; Harb, M.; Mustapha, S.; Patra, D.; Tehrani-Bagha, A.R. The effects of modified zinc oxide nanoparticles on the mechanical/thermal properties of epoxy resin. J. Appl. Polym. Sci. 2020, 137, 49330. [Google Scholar] [CrossRef]
- Ammar, S.; Ramesh, K.; Vengadaesvaran, B.; Ramesh, S.; Arof, A.K. Amelioration of anticorrosion and hydrophobic properties of epoxy/PDMS composite coatings containing nano ZnO particles. Prog. Org. Coat. 2016, 92, 54–65. [Google Scholar] [CrossRef]
- Adroja, P.P.; Ghumara, R.Y.; Parsania, P.H. Dynamic DSC curing kinetic study of epoxy resin of 1,3-bis(4-hydroxyphenyl) prop-2-en-1-one using aromatic diamines and phthalic anhydride. J. Appl. Polym. Sci. 2013, 130, 572–578. [Google Scholar] [CrossRef]
- Lizundia, E.; Serna, I.; Axpe, E.; Vilas, J.L. Free-volume effects on the thermomechanical performance of epoxy-SiO2 nanocomposites. J. Appl. Polym. Sci. 2017, 134, 45216. [Google Scholar] [CrossRef]
- Panahandeh, N.; Torabzadeh, H.; Aghaee, M.; Hasani, E.; Safa, S. Effect of incorporation of zinc oxide nanoparticles on mechanical properties of conventional glass ionomer cements. J. Conserv. Dent. Endod. 2018, 21, 130–135. [Google Scholar]
- Li, J.H.; Hong, R.Y.; Li, M.Y.; Li, H.Z.; Zheng, Y.; Ding, J. Effects of ZnO nanoparticles on the mechanical and antibacterial properties of polyurethane coatings. Prog. Org. Coat. 2009, 64, 504–509. [Google Scholar] [CrossRef]
- Karasinski, E.N.; Da Luz, M.G.; Lepienski, C.M.; Coelho, L.A.F. Nanostructured coating based on epoxy/metal oxides: Kinetic curing and mechanical properties. Thermochim. Acta 2013, 569, 167–176. [Google Scholar] [CrossRef]
- Vlad-Cristea, M.; Riedl, B.; Blanchet, P.; Jimenez-Pique, E. Nanocharacterization techniques for investigating the durability of wood coatings. Eur. Polym. J. 2012, 48, 441–453. [Google Scholar] [CrossRef]
- Skaja, A.; Fernando, D.; Croll, S. Mechanical property changes and degradation during accelerated weathering of polyesterurethane coatings. J. Coat. Technol. Res. 2006, 3, 41–51. [Google Scholar] [CrossRef]
- Parimalam, M.; Islam, M.R.; Yunus, R.M. Effects of nanosilica, zinc oxide, titatinum oxide on the performance of epoxy hybrid nanocoating in presence of rubber latex. Polym. Test. 2018, 70, 197–207. [Google Scholar] [CrossRef]
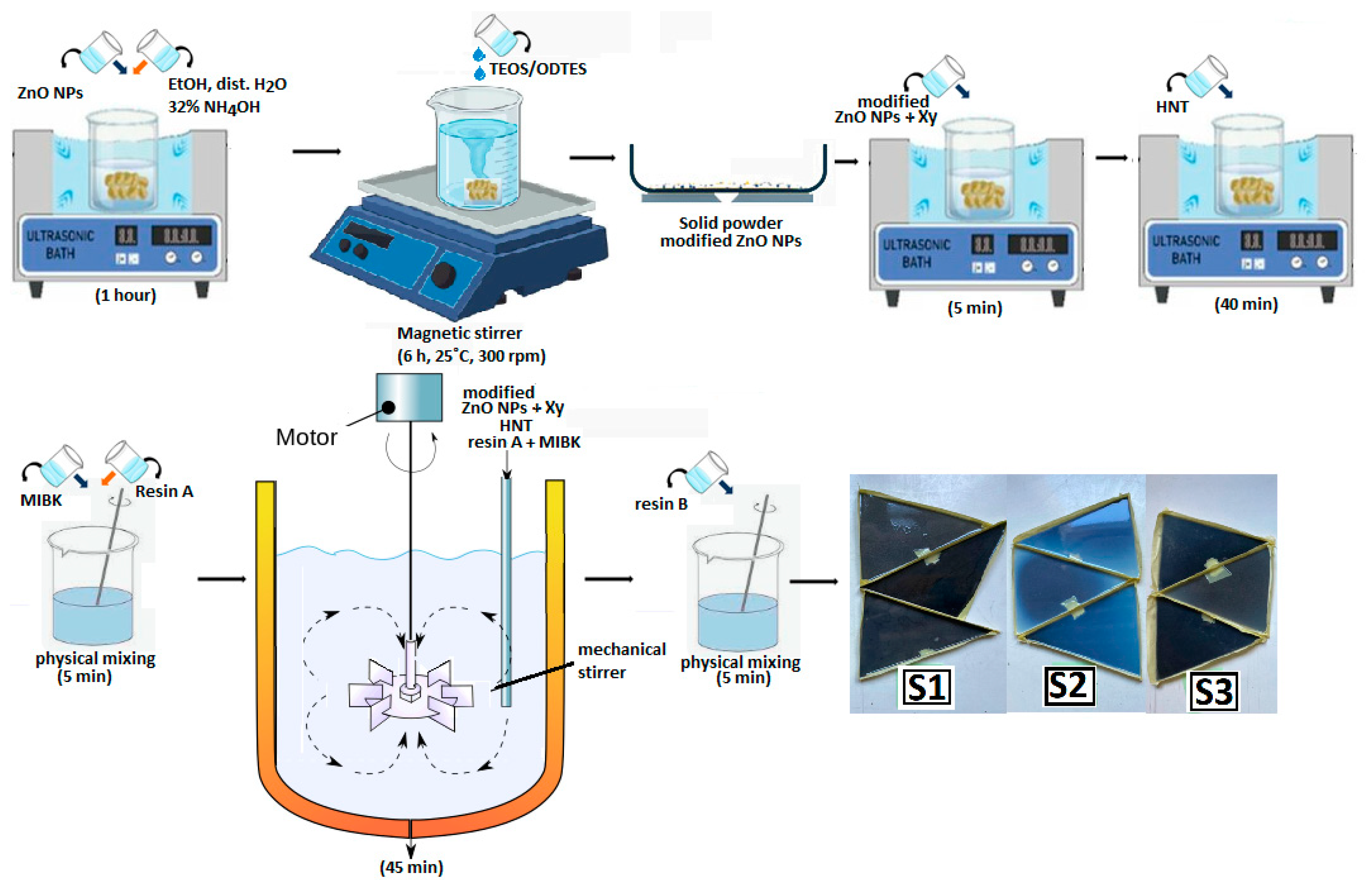

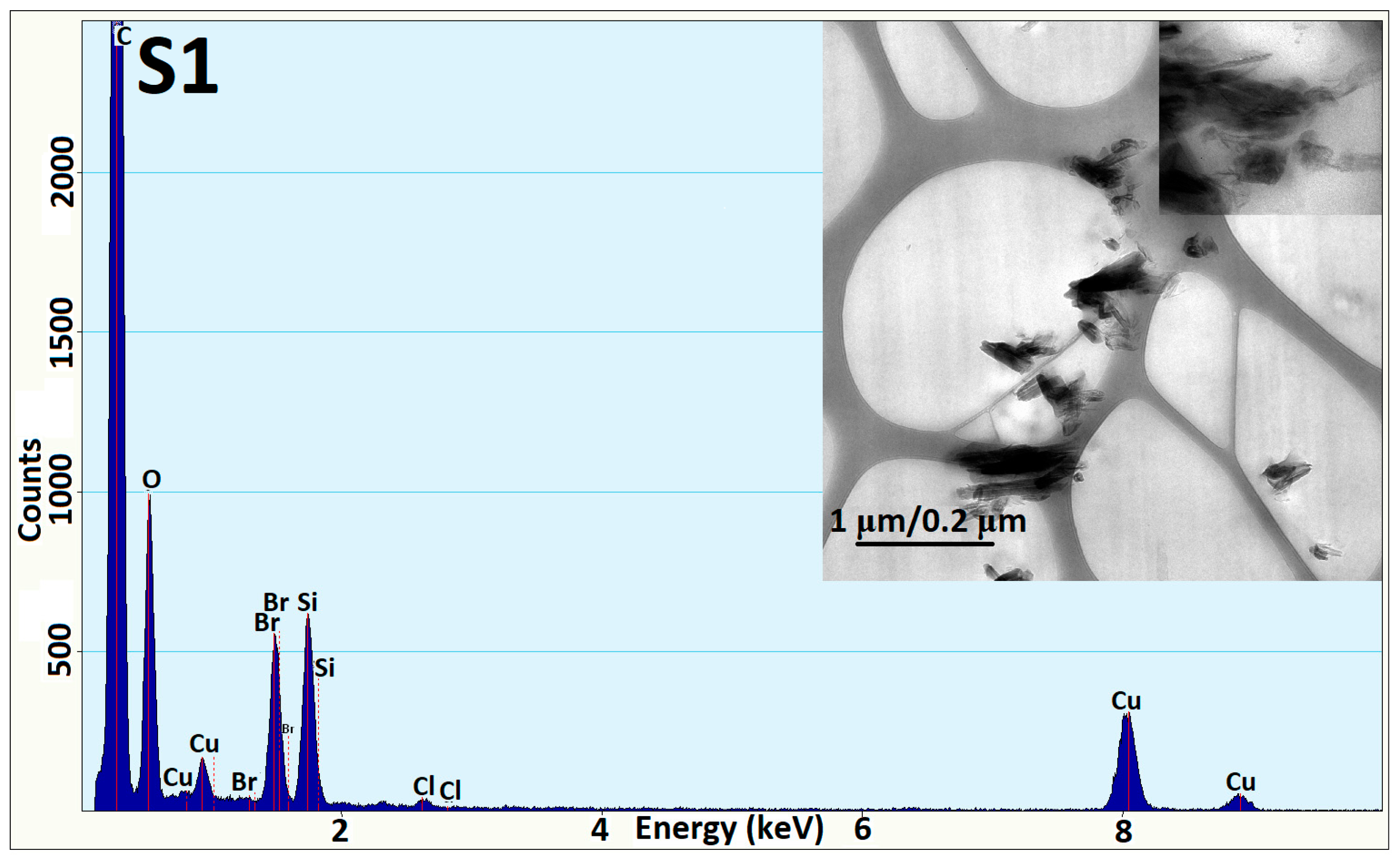
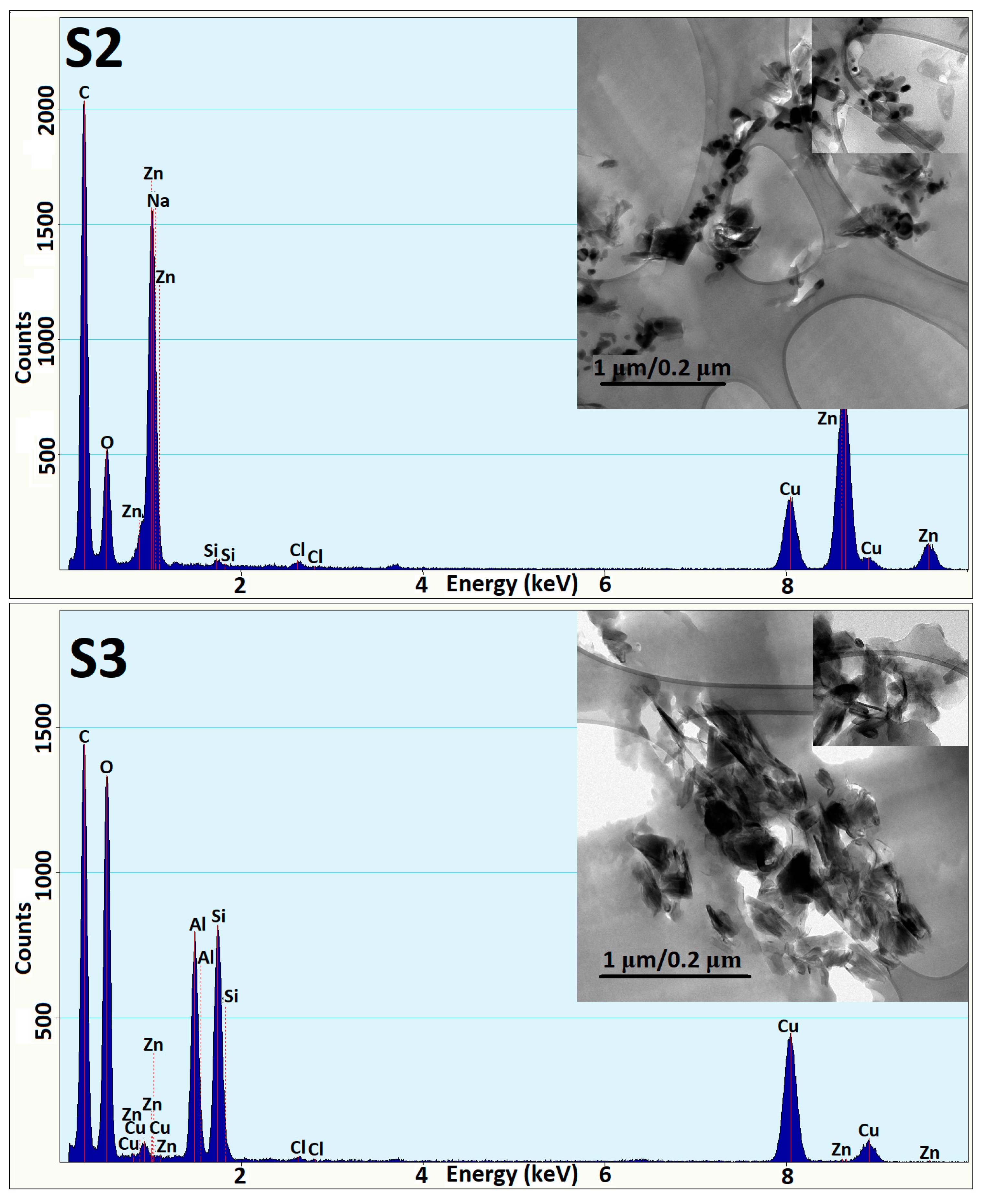
| Sample | Resin A (g) | Hardener B (g) | MIBK (g) | Xy (g) | HNT (g) | ZnO-ODTES (g) | Commercial ZnO (g) |
|---|---|---|---|---|---|---|---|
| S1 | 66.6 | 33.3 | 8 | 8 | 5 | - | - |
| S2 | - | 1 | |||||
| S3 | 1 | - |
| Sample | 30–265 °C | 265–575 °C | 575–750 °C | Residue at 750 °C | ||
|---|---|---|---|---|---|---|
| Wt. loss % | Tmax 1 °C | Wt. loss % | Tmax °C | Wt. loss % | N2 % | |
| HNT | 2.04 | 201.6 | 13.22 | 476.7 | 0.64 | 84.10 |
| S1 | 20.20 | 162.0 | 66.85 | 344.6 | 2.77 | 10.19 |
| S2 | 20.44 | 165.1 | 61.78 | 354.6 | 3.49 | 14.28 |
| S3 | 20.46 | 158.1 | 65.76 | 358.4 | 3.06 | 10.72 |
| Sample | Glass Transition | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1st Heating | Cooling | 2nd Heating | ||||||||||
| Onset (°C) | Tg (I) (°C) | End (°C) | ΔCp J/(g °C) | Onset (°C) | Tg (I) (°C) | End (°C) | ΔCp J/(g °C) | Onset (°C) | Tg (I) (°C) | End (°C) | ΔCp J/(g °C) | |
| S1 | 1.5 | 14.4 | 25.7 | 0.33 | 99.7 | 89.7 | 72.0 | 0.33 | 77.1 | 88.9 | 96.6 | 0.30 |
| S2 | 0.6 | 14.1 | 26.0 | 0.35 | 99.5 | 87.1 | 70.8 | 0.34 | 76.7 | 88.1 | 96.9 | 0.30 |
| S3 | 2.3 | 15.0 | 27.8 | 0.35 | 97.7 | 85.8 | 68.2 | 0.32 | 73.4 | 85.6 | 95.2 | 0.30 |
| Sample | Roughness (Rms, nm) | Coefficient of Friction (μ) | |
|---|---|---|---|
| Before Scratch | After Scratch | ||
| S1 | 65.007 | 40.170 | 0.40 ± 0.04 |
| S2 | 423.812 | 401.013 | 0.40 ± 0.04 |
| S3 | 418.214 | 430.878 | 0.40 ± 0.06 |
| Sample | 2H | H | B |
|---|---|---|---|
| S1 | Visible scratch | Visible scratch | No visible scratch |
| S2 | Visible scratch | No visible scratch | No visible scratch |
| S3 | Visible scratch | No visible scratch | No visible scratch |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Şomoghi, R.; Mihai, S.; Teodorescu, G.-M.; Vuluga, Z.; Gabor, A.R.; Nicolae, C.-A.; Trică, B.; Vătău, D.M.S.; Oancea, F.; Stănciulescu, C.M. Influence of HNT-ZnO Nanofillers on the Performance of Epoxy Resin Composites for Marine Applications. Coatings 2024, 14, 532. https://doi.org/10.3390/coatings14050532
Şomoghi R, Mihai S, Teodorescu G-M, Vuluga Z, Gabor AR, Nicolae C-A, Trică B, Vătău DMS, Oancea F, Stănciulescu CM. Influence of HNT-ZnO Nanofillers on the Performance of Epoxy Resin Composites for Marine Applications. Coatings. 2024; 14(5):532. https://doi.org/10.3390/coatings14050532
Chicago/Turabian StyleŞomoghi, Raluca, Sonia Mihai, George-Mihail Teodorescu, Zina Vuluga, Augusta Raluca Gabor, Cristian-Andi Nicolae, Bogdan Trică, Daniel Mihai Stănescu Vătău, Florin Oancea, and Cătălin Marian Stănciulescu. 2024. "Influence of HNT-ZnO Nanofillers on the Performance of Epoxy Resin Composites for Marine Applications" Coatings 14, no. 5: 532. https://doi.org/10.3390/coatings14050532
APA StyleŞomoghi, R., Mihai, S., Teodorescu, G.-M., Vuluga, Z., Gabor, A. R., Nicolae, C.-A., Trică, B., Vătău, D. M. S., Oancea, F., & Stănciulescu, C. M. (2024). Influence of HNT-ZnO Nanofillers on the Performance of Epoxy Resin Composites for Marine Applications. Coatings, 14(5), 532. https://doi.org/10.3390/coatings14050532









