Emerging Trends in Smart Self-Healing Coatings: A Focus on Micro/Nanocontainer Technologies for Enhanced Corrosion Protection
Abstract
1. Introduction
1.1. Advantages of Smart Self-Healing Coatings
1.2. Research Gaps and Disadvantages and Their Challenges
2. Exploring the World of Micro/Nanocontainers: Key to Advanced Extrinsic Self-Healing Coatings
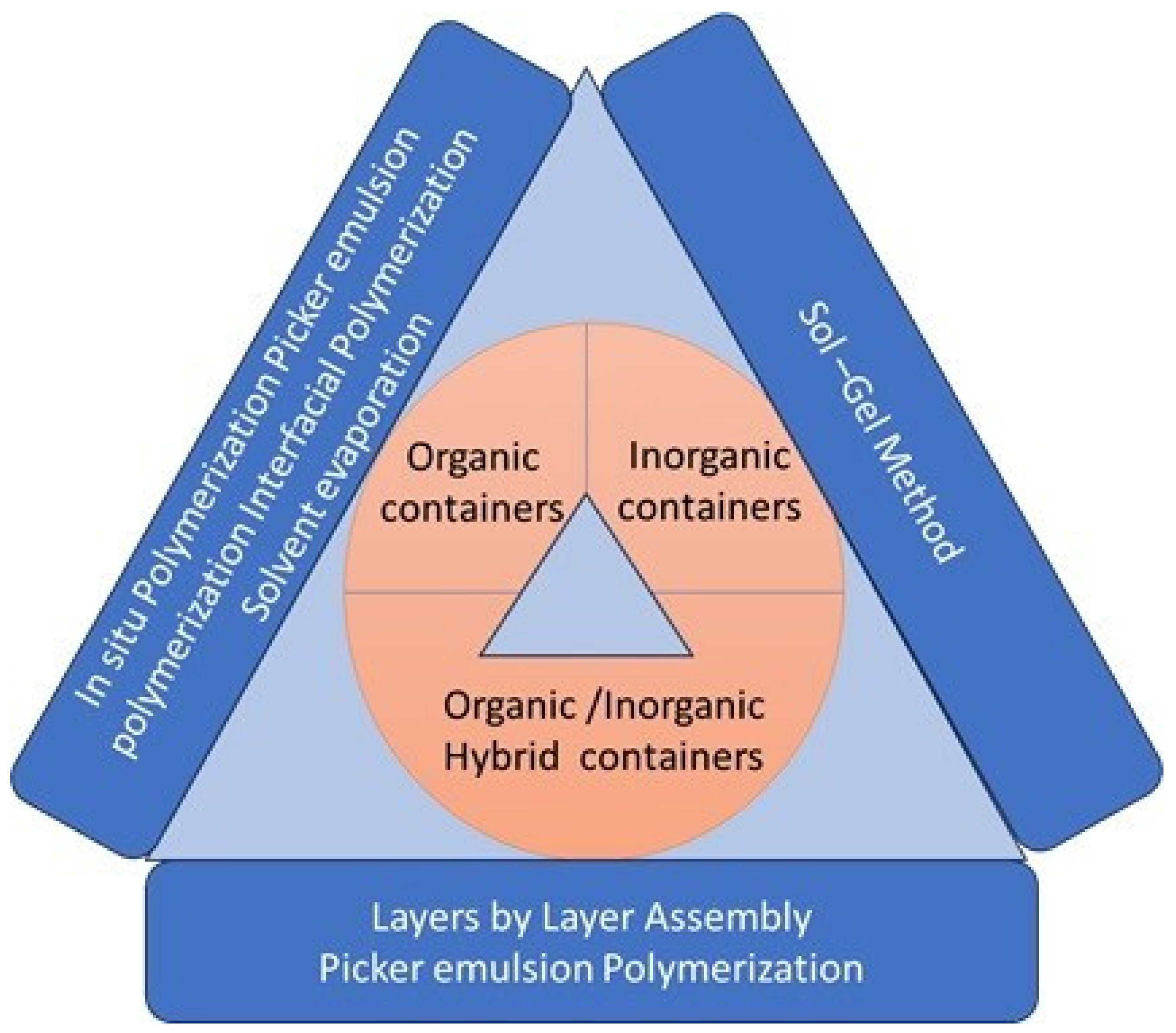
2.1. Organic Micro/Nanocontainers: A Vital Component in Self-Healing Materials
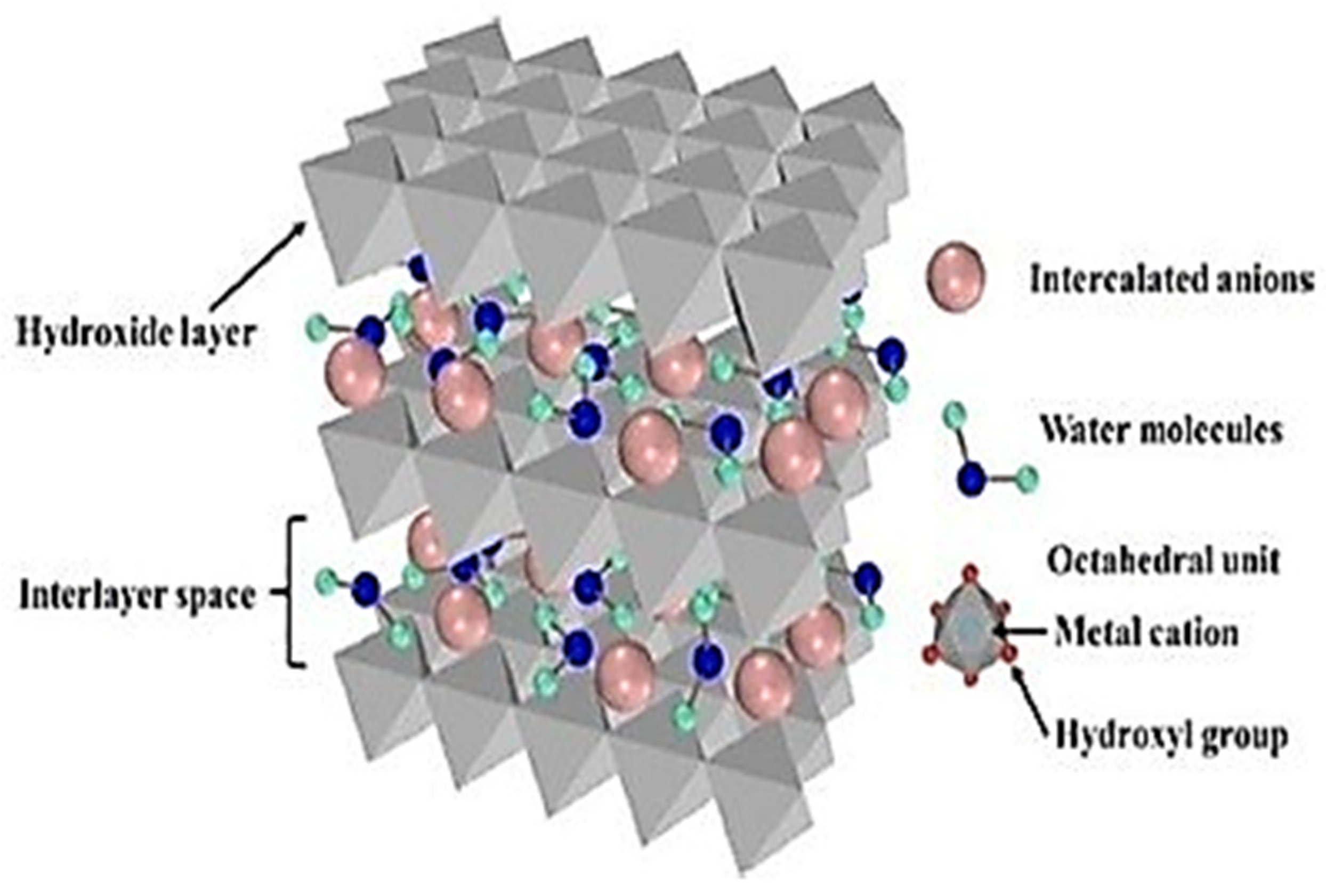
2.2. Inorganic Micro/Nanocontainers: Focusing on Their Structure, Applications, and Limitations
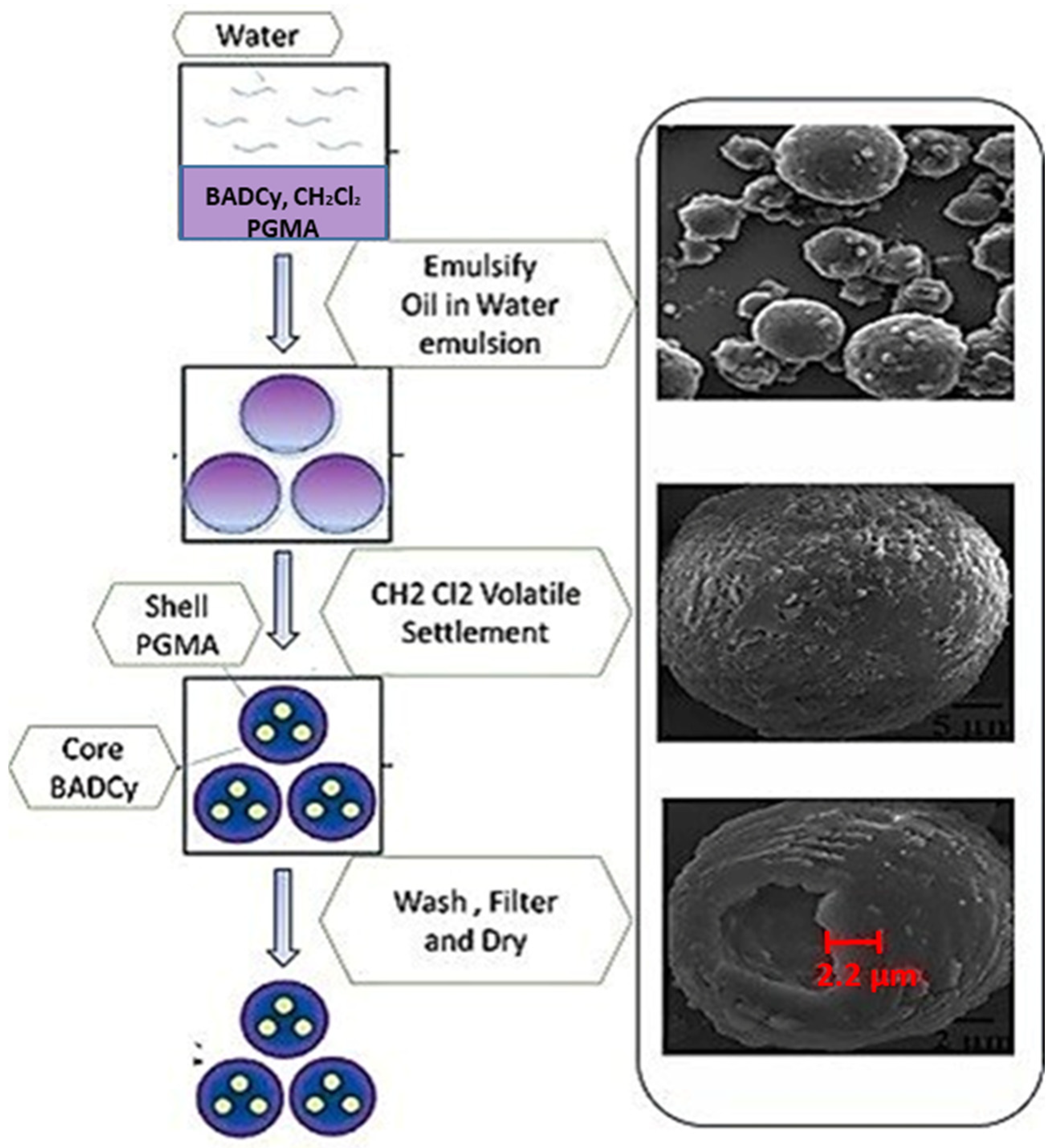
2.3. Micro/Nanocontainers: Their Composition, Manufacturing Methods, and Applications
3. Exploring the Dynamic Release Patterns of Encapsulated Agents from Micro and Nanocontainers
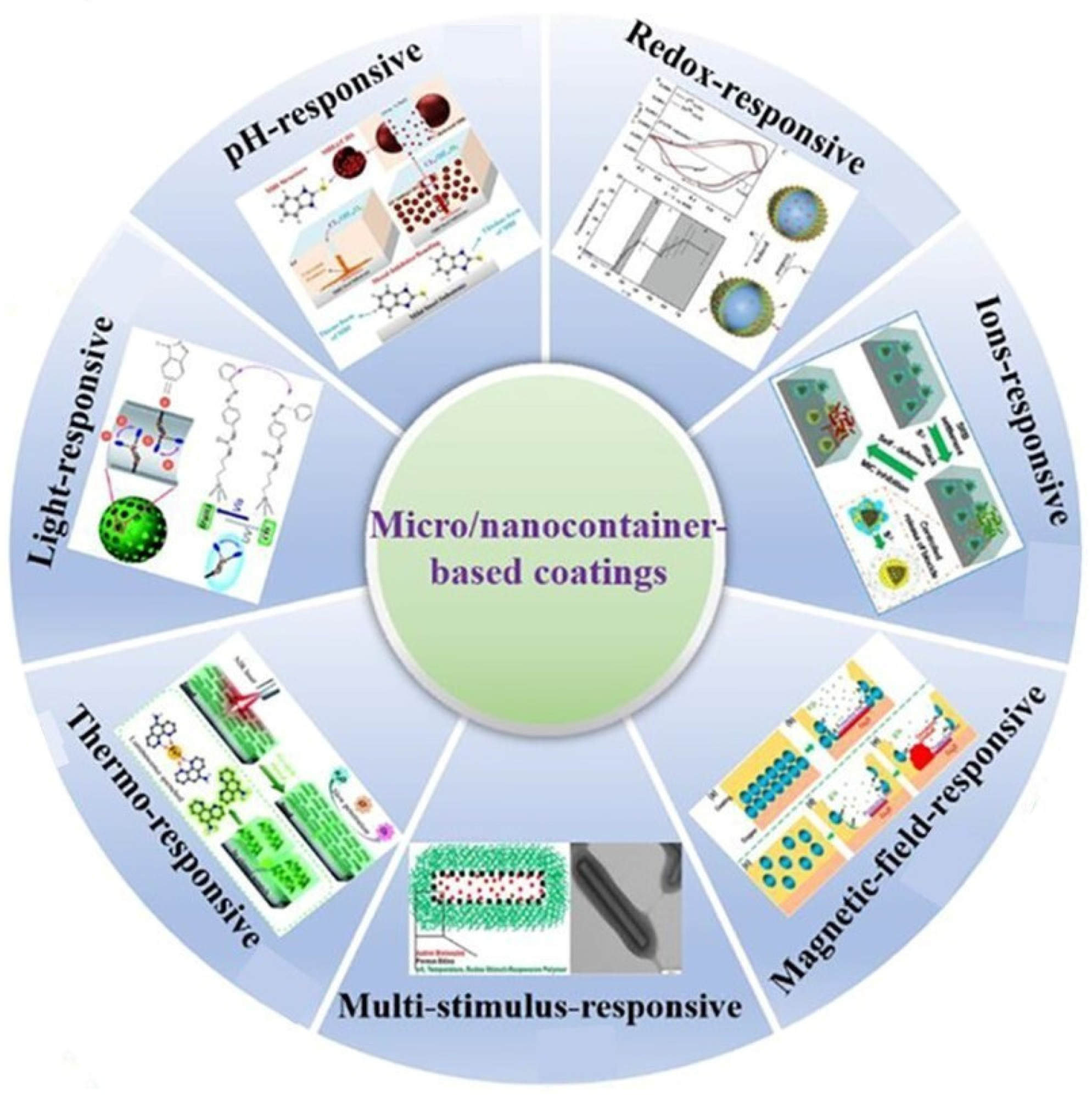
4. Stimulus-Responsive Coatings Represent an Advancement in Material Science
4.1. pH-Responsive Coatings
4.2. Redox-Responsive Coatings Represent a Sophisticated Approach in the Field of Smart Materials, Particularly in the Context of Corrosion Protection
4.3. Ions-Responsive Coatings Represent a Novel Class of Smart Materials That Are Tailored to Respond to Specific Aggressive Ions Present in Corrosive Environments
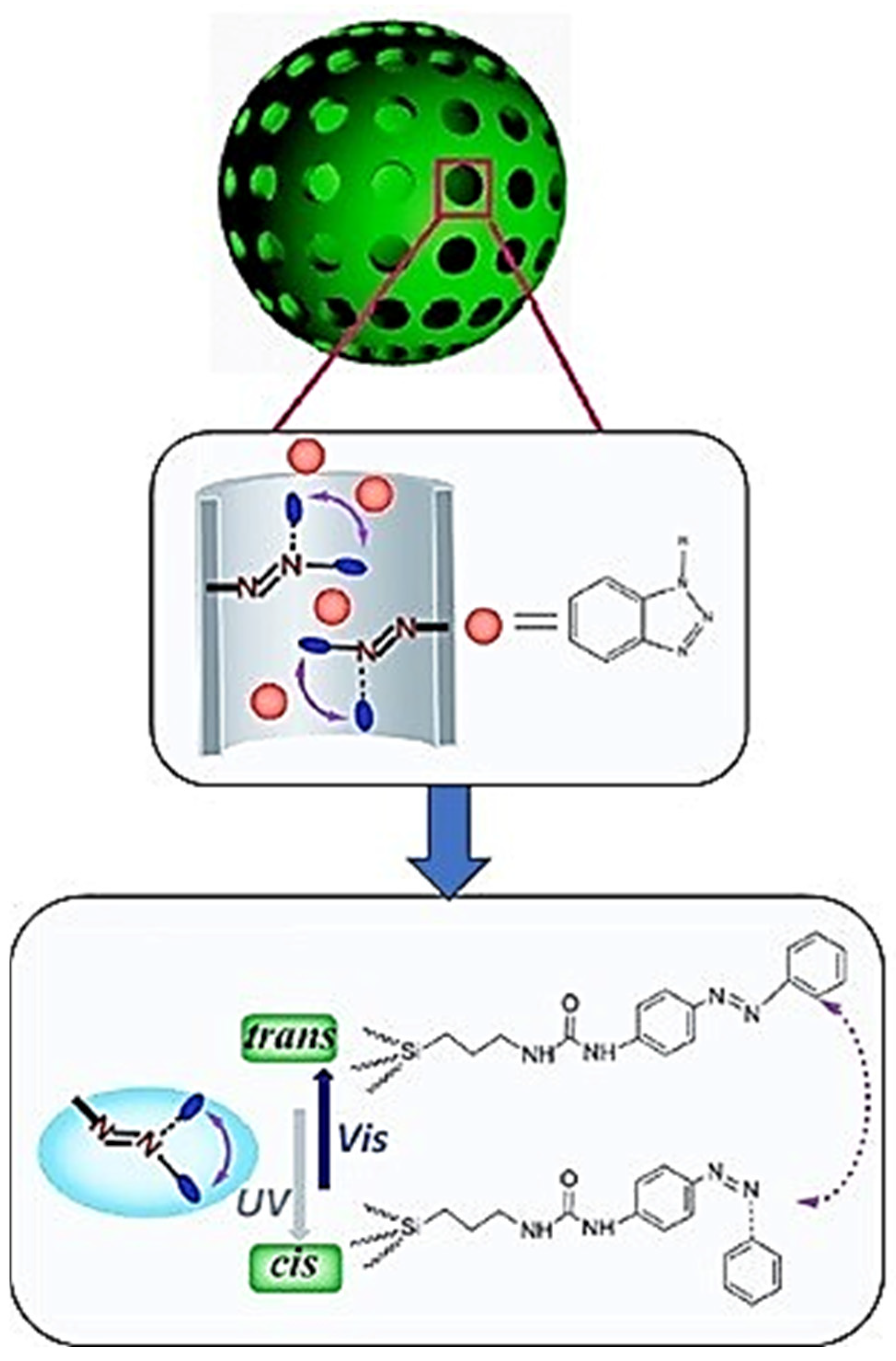
4.4. Light-Responsive Coatings
4.5. Thermo-Responsive Coatings
4.6. Magnetic-Field Responsive Coatings
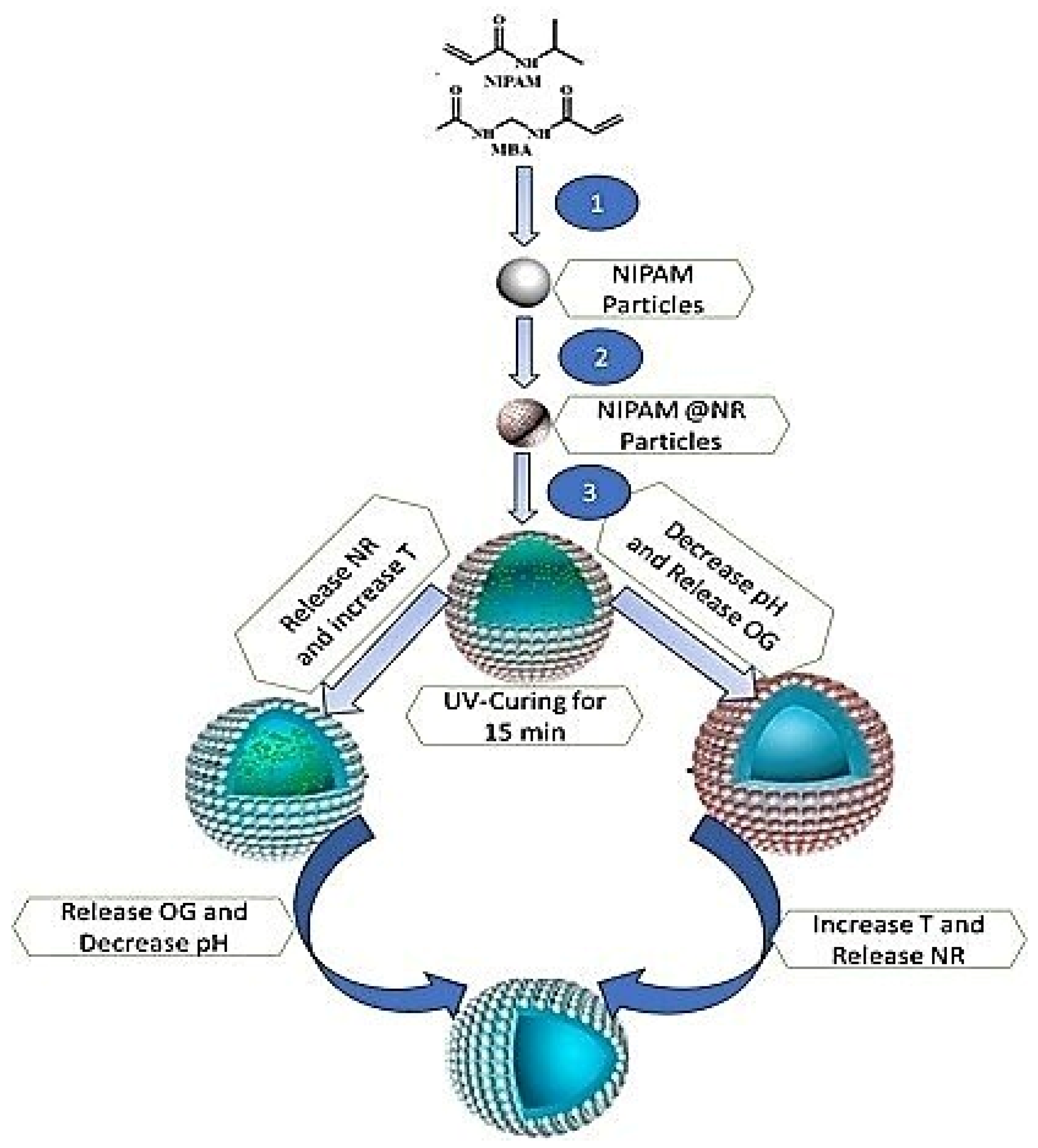
4.7. Multistimulus-Responsive Coatings
5. Application of the Micro/Nanocontainers in Functional Coatings
5.1. Self-Reporting and Self-Healing Coatings
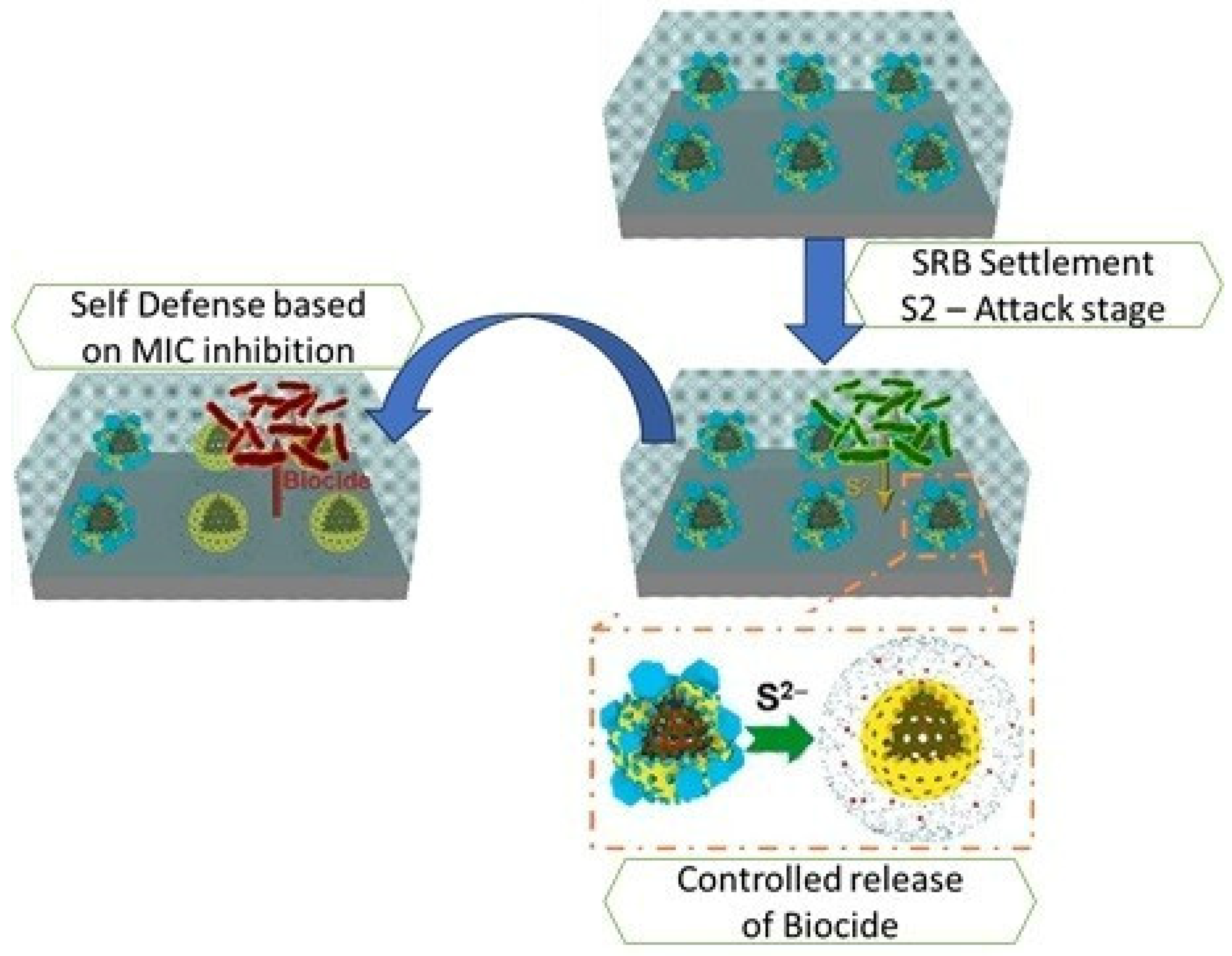
5.2. Anti-Microbial and Anti-Fouling Coatings
5.3. Self-Lubrication Coatings
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Chaouiki, A.; Chafiq, M.; Ko, Y.G. Engineering organic-inorganic frameworks as functionalized coating with remarkable active sites for boosted durability of anti-corrosion protection. J. Environ. Chem. Eng. 2023, 11, 110935. [Google Scholar] [CrossRef]
- Manivasagam, G.; Dhinasekaran, D.; Rajamanickam, A. Biomedical implants: Corrosion and its prevention—A review. Recent Patents Corros. Sci. 2010, 2, 40–54. [Google Scholar] [CrossRef]
- Singh, E. The Wetting of Two Dimensional Nanomaterials and Their Industrial Applications. Ph.D. Thesis, Rensselaer Polytechnic Institute, Troy, NY, USA, 2014. [Google Scholar]
- Khairi, M.T.M.; Ibrahim, S.; Yunus, M.A.M.; Faramarzi, M.; Sean, G.P.; Pusppanathan, J.; Abid, A. Ultrasound computed tomography for material inspection: Principles, design and applications. Measurement 2019, 146, 490–523. [Google Scholar] [CrossRef]
- Chawla, S.L. Materials Selection for Corrosion Control; ASM International: Detroit, MI, USA, 1993. [Google Scholar]
- Aljibori, H.S.; Alamiery, A.; Kadhum, A.A.H. Advances in corrosion protection coatings: A comprehensive review. Int. J. Corros. Scale. Inhib. 2023, 12, 1476–1520. [Google Scholar]
- Ahmad, S.; Habib, S.; Nawaz, M.; Shakoor, R.; Kahraman, R.; Al Tahtamouni, T.M. The role of polymeric matrices on the performance of smart self-healing coatings: A review. J. Ind. Eng. Chem. 2023, 124, 40–67. [Google Scholar] [CrossRef]
- Purkait, M.K.; Mondal, P.; Changmai, M.; Volli, V.; Shu, C.M. Hazards and Safety in Process Industries: Case Studies; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar]
- Zhang, R.; Yu, X.; Yang, Q.; Cui, G.; Li, Z. The role of graphene in anti-corrosion coatings: A review. Constr. Build. Mater. 2021, 294, 123613. [Google Scholar] [CrossRef]
- Cao, Z.; Qin, Z.; Duns, G.J.; Huang, Z.; Chen, Y.; Wang, S.; Deng, R.; Nie, L.; Luo, X. Repair of Infected Bone Defects with Hydrogel Materials. Polymers 2024, 16, 281. [Google Scholar] [CrossRef]
- Chen, Z.; Scharnagl, N.; Zheludkevich, M.L.; Ying, H.; Yang, W. Micro/nanocontainer-based intelligent coatings: Synthesis, performance and applications—A review. Chem. Eng. J. 2023, 451, 138582. [Google Scholar] [CrossRef]
- Thakur, A.; Kaya, S.; Kumar, A. Recent innovations in nano container-based self-healing coatings in the construction industry. Curr. Nanosci. 2022, 18, 203–216. [Google Scholar] [CrossRef]
- Cui, G.; Bi, Z.; Wang, S.; Liu, J.; Xing, X.; Li, Z.; Wang, B. A comprehensive review on smart anti-corrosive coatings. Prog. Org. Coatings 2020, 148, 105821. [Google Scholar] [CrossRef]
- Zehra, S.; Mobin, M.; Aslam, R.; Bhat, S.U.I. Nanocontainers: A comprehensive review on their application in the stimuli-responsive smart functional coatings. Prog. Org. Coatings 2023, 176, 107389. [Google Scholar] [CrossRef]
- Gong, H.; Yu, C.; Zhang, L.; Xie, G.; Guo, D.; Luo, J. Intelligent lubricating materials: A review. Compos. Part B Eng. 2020, 202, 108450. [Google Scholar] [CrossRef]
- Yao, W.; Wu, L.; Pan, F. Self-Healing Coatings. In Advances in Corrosion Control of Magnesium and Its Alloys; CRC Press: Boca Raton, FL, USA, 2024; pp. 375–398. [Google Scholar]
- Ahmadi, N.; Ramazani, A. Hybrid Magnetic Nanocatalysts for Organic Synthesis. In Synthetic Applications; Walter de Gruyter GmbH & Co KG.: Berlin, Germany, 2022; Volume 71. [Google Scholar]
- Chen, S.; Huang, Z.; Yuan, M.; Huang, G.; Guo, H.; Meng, G.; Feng, Z.; Zhang, P. Trigger and response mechanisms for controlled release of corrosion inhibitors from micro/nanocontainers interpreted using endogenous and exogenous stimuli: A review. J. Mater. Sci. Technol. 2022, 125, 67–80. [Google Scholar] [CrossRef]
- Yun, X.-W.; Tang, B.; Xiong, Z.-Y.; Wang, X.-G. Understanding self-assembly, colloidal behavior and rheological properties of graphene derivatives for high-performance supercapacitor fabrication. Chin. J. Polym. Sci. 2020, 38, 423–434. [Google Scholar] [CrossRef]
- Aouadi, S.M.; Gu, J.; Berman, D. Self-healing ceramic coatings that operate in extreme environments: A review. J. Vac. Sci. Technol. A 2020, 38, 050802. [Google Scholar] [CrossRef]
- Zhang, F.; Ju, P.; Pan, M.; Zhang, D.; Huang, Y.; Li, G.; Li, X. Self-healing mechanisms in smart protective coatings: A review. Corros. Sci. 2018, 144, 74–88. [Google Scholar] [CrossRef]
- Fenker, M.; Balzer, M.; Kappl, H. Corrosion protection with hard coatings on steel: Past approaches and current research efforts. Surf. Coat. Technol. 2014, 257, 182–205. [Google Scholar] [CrossRef]
- Tripathi, D.; Sharma, R.K.; Oztop, H.F.; Natarajan, R. Nanomaterials and Nanoliquids: Applications in Energy and Environment; Springer Nature: Berlin, Germany, 2023. [Google Scholar]
- Liu, T.; Ma, L.; Wang, X.; Wang, J.; Qian, H.; Zhang, D.; Li, X. Self-healing corrosion protective coatings based on micro/nanocarriers: A review. Corros. Commun. 2021, 1, 18–25. [Google Scholar] [CrossRef]
- Wu, Z. Design of Functional Materials for Encapsulation and Controllable Re-Lease. Ph.D. Thesis, University of Massachusetts Lowell, Lowell, MA, USA, 2021. [Google Scholar]
- Leclercq, L.; Nardello-Rataj, V. 16 Pickering Emulsions and Biomass. In Biorefinery: From Biomass to Chemicals and Fuels; De Gruyter: Villeneuve d’Ascq, France, 2021; Volume 537. [Google Scholar]
- Erol, M.; Mustafov, S.D.; Akalın, S.A.; Uzunbayır, B.; Özdemir, E.T.; Coşkun, B.; Ertekin, Z. Carbon Nanostructures for Automotive and Aerospace Applications. In Handbook of Functionalized Carbon Nanostructures: From Synthesis Methods to Applications; Springer International Publishing: Cham, Switzerland, 2023; pp. 1–29. [Google Scholar]
- Huang, Z.; Shao, G.; Li, L. Micro/nano functional devices fabricated by additive manufacturing. Prog. Mater. Sci. 2023, 131, 101020. [Google Scholar] [CrossRef]
- Christian, P. Nanomaterials: Properties, Preparation and Applications. In Environmental and Human Health Impacts of Nanotechnology; Wiley-Blackwell: Hoboken, NJ, USA, 2009; pp. 31–77. [Google Scholar]
- Ouarga, A.; Lebaz, N.; Tarhini, M.; Noukrati, H.; Barroug, A.; Elaissari, A.; Ben Youcef, H. Towards smart self-healing coatings: Advances in micro/nano-encapsulation processes as carriers for anti-corrosion coatings development. J. Mol. Liq. 2022, 354, 118862. [Google Scholar] [CrossRef]
- Chen, Y.; Wei, W.; Zhu, Y.; Luo, J.; Liu, R.; Liu, X. Synthesis of temperature/pH dualstimuli- response multicompartmental microcapsules via pickering emulsion for preprogrammable payload release. ACS Appl. Mater. Interfaces 2020, 12, 4821–4832. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Sun, P.; Liu, H.; Zhao, Q.; Niu, Y.; Zhao, D. Stimulus responsive microcapsules and their aromatic applications. J. Control. Release 2022, 351, 198–214. [Google Scholar] [CrossRef]
- Chafiq, M.; Chaouiki, A.; Ko, Y.G. Advances in COFs for energy storage devices: Harnessing the potential of covalent organic framework materials. Energy Storage Mater. 2023, 63, 103014. [Google Scholar] [CrossRef]
- Liang, R.-R.; Jiang, S.-Y.; Ru-Han, A.; Zhao, X. Two-dimensional covalent organic frameworks with hierarchical porosity. Chem. Soc. Rev. 2020, 49, 3920–3951. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Jin, K.; Wang, W.; Li, W.; Wang, T.; Yang, T.; Cheng, J.; Zhao, Z.; Chen, S. A long-term anti-corrosion and cathodic delamination resistant epoxy coating based on COF grafted GO nanofillers. J. Ind. Eng. Chem. 2023, 128, 222–234. [Google Scholar] [CrossRef]
- Zhang, C.; Li, W.; Liu, C.; Zhang, C.; Cao, L.; Kong, D.; Wang, W.; Chen, S. Effect of covalent organic framework modified graphene oxide on anticorrosion and self-healing properties of epoxy resin coatings. J. Colloid Interface Sci. 2022, 608, 1025–1039. [Google Scholar] [CrossRef] [PubMed]
- Qian, B.; Zheng, Z.; Liu, C.; Li, M.; D’sa, R.A.; Li, H.; Graham, M.; Michailidis, M.; Kantserev, P.; Vinokurov, V.; et al. Microcapsules prepared via pickering emulsion polymerization for multifunctional coatings. Prog. Org. Coatings 2020, 147, 105785. [Google Scholar] [CrossRef]
- Karakuş, S.; Özeroğlu, C.; Kahyaoğlu, M.İ.; Tan, E.; İlgar, M.; Orhan, Z.; Albayrak, N. One-Dimensional Polymeric Nanocomposites-Based Microcontainers for Biomedical Applications. In One-Dimensional Polymeric Nanocomposites; CRC Press: Boca Raton, FL, USA, 2023; pp. 417–432. [Google Scholar]
- Ghimire, P.P.; Jaroniec, M. Renaissance of Stöber method for synthesis of colloidal particles: New developments and opportunities. J. Colloid Interface Sci. 2021, 584, 838–865. [Google Scholar] [CrossRef]
- Karaman, D.; Pamukçu, A.; Karakaplan, M.B.; Kocaoglu, O.; Rosenholm, J.M. Recent advances in the use of mesoporous silica nanoparticles for the diagnosis of bacterial infections. Int. J. Nanomed. 2021, 16, 6575–6591. [Google Scholar] [CrossRef]
- Chen, Z.; Li, X.; Gong, B.; Scharnagl, N.; Zheludkevich, M.L.; Ying, H.; Yang, W. Double Stimuli-Responsive Conducting Polypyrrole Nanocapsules for Corrosion-Resistant Epoxy Coatings. ACS Appl. Mater. Interfaces 2022, 15, 2067–2076. [Google Scholar] [CrossRef]
- Dubey, M.; Wadhwa, S.; Mathur, A.; Kumar, R. Progress in mesoporous ceria: A review on synthesis strategies and catalytic applications. Appl. Surf. Sci. Adv. 2022, 12, 100340. [Google Scholar] [CrossRef]
- Liu, C.; Jin, Z.; Zhao, H.; Wang, L. Triple-action smart nanocontainers enhanced protective coatings with superior active and passive properties. Prog. Org. Coatings 2020, 148, 105887. [Google Scholar] [CrossRef]
- Ma, L.; Wang, J.; Wang, Y.; Guo, X.; Wu, S.; Fu, D.; Zhang, D. Enhanced active corrosion protection coatings for aluminum alloys with two corrosion inhibitors co-incorporated in nanocontainers. Corros. Sci. 2022, 208, 110663. [Google Scholar] [CrossRef]
- Zaitoon, A.; Luo, X.; Lim, L. Triggered and controlled release of active gaseous/volatile compounds for active packaging applications of agri-food products: A review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 541–579. [Google Scholar] [CrossRef] [PubMed]
- Kordas, G. Nanocontainers Against Biofouling and Corrosion Degradation of Materials: A Short Review With Prospects. Front. Nanotechnol. 2022, 4, 813908. [Google Scholar] [CrossRef]
- Palo, E.; Zhang, H.; Lastusaari, M.; Salomäki, M.O. Nanometer-thick ion-selective polyelectrolyte multilayer coatings to inhibit the disintegration of inorganic upconvert-ing nanoparticles. ACS Appl. Nano Mater. 2020, 3, 6892–6898. [Google Scholar] [CrossRef]
- Olivieri, F.; Castaldo, R.; Cocca, M.; Gentile, G.; Lavorgna, M. Mesoporous silica nanoparticles as carriers of active agents for smart anticorrosive organic coatings: A critical review. Nanoscale 2021, 13, 9091–9111. [Google Scholar] [CrossRef] [PubMed]
- Leyva-Jiménez, F.J.; Oliver-Simancas, R.; Castangia, I.; Rodríguez-García, A.M.; Alañón, M.E. Comprehensive review of natural based hydrogels as an upcoming trend for food packing. Food Hydrocoll. 2023, 135, 108124. [Google Scholar] [CrossRef]
- Chuhadiya, S.; Himanshu; Suthar, D.; Patel, S.; Dhaka, M. Metal organic frameworks as hybrid porous materials for energy storage and conversion devices: A review. Coord. Chem. Rev. 2021, 446, 214115. [Google Scholar] [CrossRef]
- Shahini, M.; Mohammadloo, H.E.; Ramezanzadeh, M.; Ramezanzadeh, B. Recent innovations in synthesis/characterization of advanced nano-porous metal-organic frameworks (MOFs); current/future trends with a focus on the smart anti-corrosion features. Mater. Chem. Phys. 2022, 276, 125420. [Google Scholar] [CrossRef]
- Wang, D.-G.; Liang, Z.; Gao, S.; Qu, C.; Zou, R. Metal-organic framework-based materials for hybrid supercapacitor application. Co-ord. Chem. Rev. 2020, 404, 213093. [Google Scholar] [CrossRef]
- Bayer, I.S. Controlled Drug Release from Nanoengineered Polysaccharides. Pharmaceutics 2023, 15, 1364. [Google Scholar] [CrossRef] [PubMed]
- Malekjani, N.; Jafari, S.M. Modeling the release of food bioactive ingredients from carriers/nanocarriers by the empirical, semiempirical, and mechanistic models. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3–47. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Qian, B.; Hou, P.; Song, Z. Stimulus responsive zeolitic imidazolate framework to achieve corrosion sensing and active protecting in polymeric coatings. ACS Appl. Mater. Interfaces 2021, 13, 4429–4441. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Qiang, Y.; Zhao, W.; Zhang, S. 2-Mercaptobenzimidazole-inbuilt metalorganic-frameworks modified graphene oxide towards intelligent and excellent anti-corrosion coating. Corros. Sci. 2021, 191, 109715. [Google Scholar] [CrossRef]
- Su, Y.; Ojo, O.F.; Tsengam, I.K.M.; He, J.; McPherson, G.L.; John, V.T.; Valla, J.A. Thermoresponsive coatings on hollow particles with mesoporous shells serve as stimuli-responsive gates to species encapsulation and release. Langmuir 2018, 34, 14608–14616. [Google Scholar] [CrossRef] [PubMed]
- Imanieh, A. Afshar, Corrosion protection of aluminum by smart coatings containing layered double hydroxide (LDH) nanocontainers. J. Mater. Res. Technol. 2019, 8, 3004–3023. [Google Scholar] [CrossRef]
- Zheludkevich, M.L.; Tedim, J.; Ferreira, M.G.S. “Smart” coatings for active corrosion protection based on multi-functional micro and nanocontainers. Electrochim. Acta 2012, 82, 314–323. [Google Scholar] [CrossRef]
- Ding, C.; Xu, J.; Tong, L.; Gong, G.; Jiang, W.; Fu, J. Design and fabrication of a novel stimulus-feedback anticorrosion coating featured by rapid self-healing functionality for the protection of magnesium alloy. ACS Appl. Mater. Interfaces 2017, 9, 21034–21047. [Google Scholar] [CrossRef]
- Li, C.; Zhao, X.; Meng, C.; Zhang, T.; Sun, S.; Hu, S. Application of hollow mesoporous organosilica nanoparticles as pH and redox double stimuli-responsive nanocontainer in the controlled release of corrosion inhibitor molecules. Prog. Org. Coatings 2021, 159, 106437. [Google Scholar] [CrossRef]
- Ma, L.; Wang, J.; Zhang, D.; Huang, Y.; Huang, L.; Wang, P.; Qian, H.; Li, X.; Terryn, H.A.; Mol, J.M.C. Dual-action self-healing protective coatings with photothermal responsive corrosion inhibitor nanocontainers. Chem. Eng. J. 2021, 404, 127118. [Google Scholar] [CrossRef]
- Leal, D.A.; Riegel-Vidotti, I.C.; Ferreira, M.G.S.; Marino, C.E.B. Smart coating based on double stimuli-responsive microcapsules containing linseed oil and benzotriazole for active corrosion protection. Corros. Sci. 2018, 130, 56–63. [Google Scholar] [CrossRef]
- Paret, N.; Trachsel, A.; Berthier, D.L.; Herrmann, A. Developing multi stimuli-responsive core/shell microcapsules to control the release of volatile compounds. Macromol. Mater. Eng. 2018, 304, 1800599. [Google Scholar] [CrossRef]
- Xiong, W.; Tang, J.; Zhu, G.; Han, N.; Schlangen, E.; Dong, B.; Wang, X.; Xing, F. A novel capsule-based self-recovery system with a chloride ion trigger. Sci. Rep. 2015, 5, 10866. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, C.; Xie, H.; Sun, W.; Chen, X.; Wang, X.; Yang, Z.; Liu, G. Calcium alginate gel capsules loaded with inhibitor for corrosion protection of downhole tube in oilfields. Corros. Sci. 2015, 90, 296–304. [Google Scholar] [CrossRef]
- Lv, L.-P.; Jiang, S.; Inan, A.; Landfester, K.; Crespy, D. Redox-responsive release of active payloads from depolymerized nanoparticles. RSC Adv. 2017, 7, 8272–8279. [Google Scholar] [CrossRef]
- Boostani, S.; Jafari, S.M. A comprehensive review on the controlled release of encapsulated food ingredients; fundamental concepts to design and applications. Trends Food Sci. Technol. 2021, 109, 303–321. [Google Scholar] [CrossRef]
- Fahmi, M.Z.; Prasetya, R.A.; Dzikri, M.F.; Sakti, S.C.W.; Yuliarto, B. MnFe2O4 nanoparticles/cellulose acetate composite nanofiber for controllable release of naproxen. Mater. Chem. Phys. 2020, 250, 123055. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Singh, S.; Bahmid, N.A.; Mehany, T.; Shyu, D.J.H.; Assadpour, E.; Malekjani, N.; Castro-Muñoz, R.; Jafari, S.M. Release of Encapsulated Bioactive Compounds from Active Packaging/Coating Materials and Its Modeling: A Systematic Review. Colloids Interfaces 2023, 7, 25. [Google Scholar] [CrossRef]
- Adsul, S.H.; Bagale, U.D.; Sonawane, S.H.; Subasri, R. Release rate kinetics of corrosion inhibitor loaded halloysite nanotube-based anticorrosion coatings on magnesium alloy AZ91D. J. Magnes. Alloy. 2021, 9, 202–215. [Google Scholar] [CrossRef]
- Ghodke, S.A.; Sonawane, S.H.; Bhanvase, B.A.; Mishra, S.; Joshi, K.S. Studies on fragrance delivery from inorganic nanocontainers: Encapsulation, release and modeling studies. J. Inst. Eng. (India) Ser. E 2015, 96, 45–53. [Google Scholar] [CrossRef]
- Ulaeto, S.B.; Rajan, R.; Pancrecious, J.K.; Rajan, T.; Pai, B. Developments in smart anticorrosive coatings with multifunctional characteristics. Prog. Org. Coat. 2017, 111, 294–314. [Google Scholar] [CrossRef]
- Grumezescu, V.; Grumezescu, A. (Eds.) Materials for Biomedical Engineering: Thermoset and Thermoplastic Polymers; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Li, Y.; Jiang, J.; Han, S. Preparation of intelligent corrosion resistant coatings based on pH-responsive silica nanocontainers. Microporous Mesoporous Mater. 2023, 349, 112418. [Google Scholar] [CrossRef]
- Wei, H.; Wang, Y.; Guo, J.; Shen, N.Z.; Jiang, D.; Zhang, X.; Yan, X.; Zhu, J.; Wang, Q.; Shao, L.; et al. Advanced micro/nanocapsules for self-healing smart anticorrosion coatings. J. Mater. Chem. A 2015, 3, 469–480. [Google Scholar] [CrossRef]
- Cong, Y.; Chen, K.; Zhou, S.; Wu, L. Synthesis of pH and UV dual-responsive microcapsules with high loading capacity and their application in self-healing hydrophobic coatings. J. Mater. Chem. A 2015, 3, 19093–19099. [Google Scholar] [CrossRef]
- Lombardo, D.; Kiselev, M.A.; Caccamo, M.T. Smart nanoparticles for drug delivery application: Development of versatile nanocarrier platforms in biotechnology and nanomedicine. J. Nanomater. 2019, 2019, e3702518. [Google Scholar] [CrossRef]
- Altinbasak, I.; Arslan, M.; Sanyal, R.; Sanyal, A. Pyridyl disulfide-based thiol–disulfide exchange reaction: Shaping the design of redox-responsive polymeric materials. Polym. Chem. 2020, 11, 7603–7624. [Google Scholar] [CrossRef]
- Kankala, R.K.; Zhang, H.; Liu, C.; Kanubaddi, K.R.; Lee, C.; Wang, S.; Cui, W.; Santos, H.A.; Lin, K.; Chen, A. Metal species–encapsulated mesoporous silica nanoparticles: Current advancements and latest breakthroughs. Adv. Funct. Mater. 2019, 29, 1902652. [Google Scholar] [CrossRef]
- Ding, C.; Fu, J. Smart Anticorrosion Coatings Based on Nanocontainers. In Smart Nanocontainers; Elsevier: Amsterdam, The Netherlands, 2020; pp. 413–429. [Google Scholar]
- Sun, S.; Zhao, X.; Cheng, M.; Wang, Y.; Li, C.; Hu, S. Facile preparation of redox-responsive hollow mesoporous silica spheres for the encapsulation and controlled release of corrosion inhibitors. Prog. Org. Coat. 2019, 136, 105302. [Google Scholar] [CrossRef]
- Tang, R.; Wang, X.; Chen, Z.; Liu, Y.; Yang, W. An S2-responsive nanocontainer for inhibiting microbial corrosion caused by sulfate-reducing bacteria. Colloids Surf. A Physicochem. Eng. Asp. 2023, 663, 131110. [Google Scholar] [CrossRef]
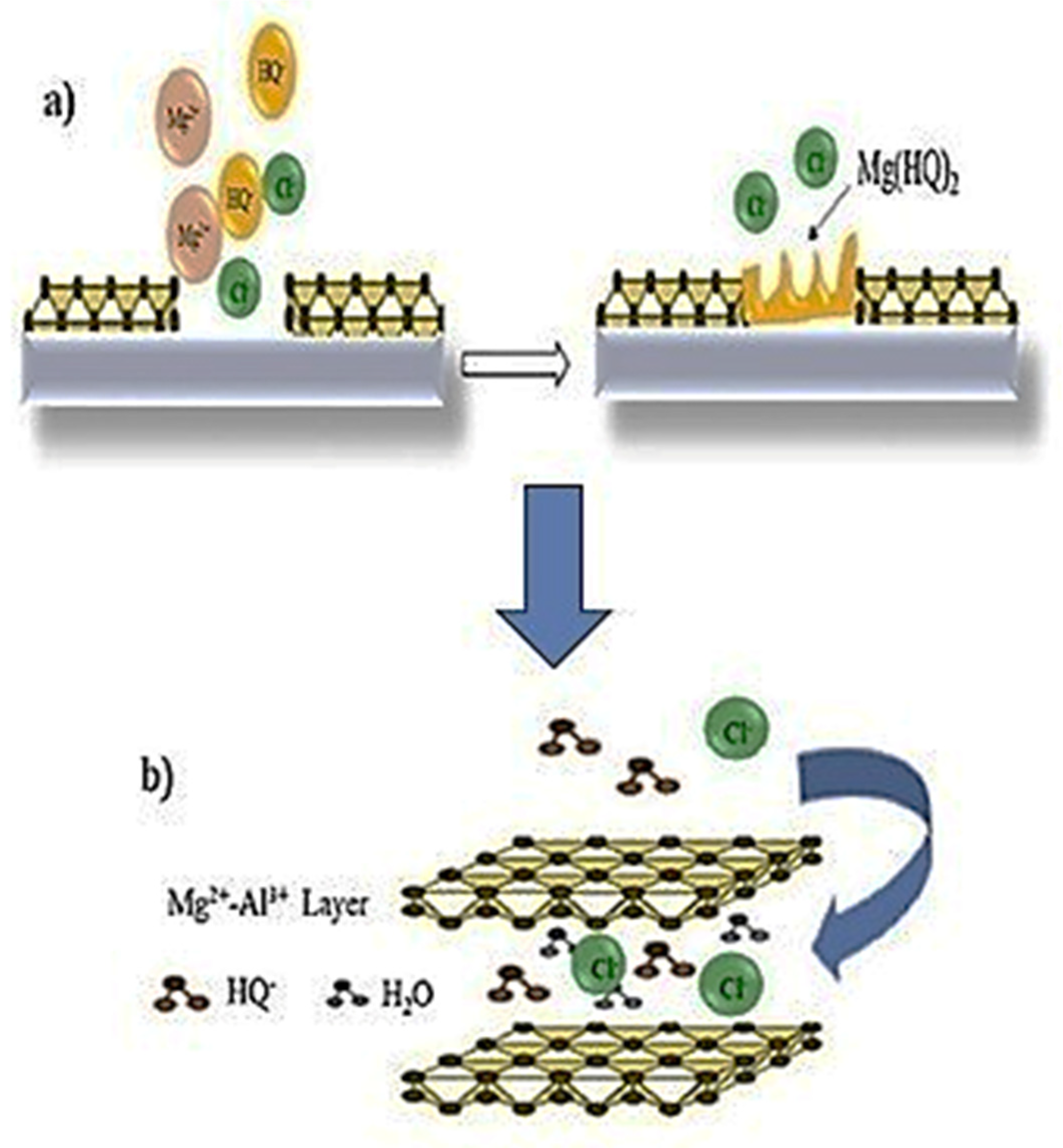
- Cao, Y.; Zheng, D.; Zhang, F.; Pan, J.; Lin, C. Layered double hydroxide (LDH) for multi-functionalized corrosion protection of metals: A review. J. Mater. Sci. Technol. 2022, 102, 232–263. [Google Scholar] [CrossRef]
- Jagtap, A.; Wagle, P.G.; Jagtiani, E.; More, A.P. Layered double hydroxides (LDHs) for coating applications. J. Coat. Technol. Res. 2022, 19, 1009–1032. [Google Scholar] [CrossRef]
- He, X.; Chiu, C.; Esmacher, M.J.; Liang, H. Nanostructured photocatalytic coatings for corrosion protection and surface repair. Surf. Coatings Technol. 2013, 237, 320–327. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, P.; Tan, W.; Hao, W.; Ma, L.; Wang, J.; Liu, T.; Zhang, F.; Ren, C.; Liu, W.; et al. Photothermal and pH dual-responsive self-healing coating for smart corrosion protection. J. Mater. Sci. Technol. 2022, 107, 34–42. [Google Scholar] [CrossRef]
- Li, W.; Ni, X.; Zhang, X.; Lei, Y.; Guo, J.; Jin, J.; You, B. UV-NIR dual-responsive nanocomposite coatings with healable, superhydrophobic, and contaminantresistant properties. ACS Appl. Mater. Interfaces 2020, 12, 48101–48108. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Chen, R.; Jin, Z.; Liu, J. Engineering hollow mesoporous silica nanocontainers with molecular switches for continuous self-healing anticorrosion coating. J. Mater. Chem. A 2015, 3, 9510–9516. [Google Scholar] [CrossRef]
- Cheng, L.; Liu, C.; Zhao, H.; Wang, L. Photothermal-triggered shape memory coatings with active repairing and corrosion sensing properties. J. Mater. Chem. A 2021, 9, 22509–22521. [Google Scholar] [CrossRef]
- Zadehnajar, P.; Akbari, B.; Amini, A.; Tayebi, L. Shape-Memory Polymers in Cartilage Tissue Engineering. In Cartilage: From Biology to Biofabrication; Springer Nature Singapore: Singapore, 2023; pp. 307–331. [Google Scholar]
- Menon, A.V.; Madras, G.; Bose, S. The journey of self-healing and shape memory polyurethanes from bench to translational research. Polym. Chem. 2019, 10, 4370–4388. [Google Scholar] [CrossRef]
- Li, G.L.; Möhwald, H.; Shchukin, D.G. Silica/polymer double-walled hybrid nanotubes: Synthesis and application as stimuli-responsive nanocontainers in selfhealing coatings. ACS Nano 2013, 7, 2470–2478. [Google Scholar] [CrossRef]
- Liu, C.; Wu, H.; Qiang, Y.; Zhao, H.; Wang, L. Design of smart protective coatings with autonomous self-healing and early corrosion reporting properties. Corros. Sci. 2021, 184, 109355. [Google Scholar] [CrossRef]
- Wang, T.; Du, J.; Ye, S.; Tan, L.; Fu, J. Triple-stimuli-responsive smart nanocontainers enhanced self-healing anticorrosion coatings for protection of aluminum alloy. ACS Appl. Mater. Interfaces 2019, 11, 4425–4438. [Google Scholar] [CrossRef] [PubMed]
- Abu-Thabit, N.Y.; Makhlouf, A.S.H. Fundamental of Smart Coatings and Thin Films: Synthesis, Deposition Methods, and Industrial Applications. In Advances in Smart Coatings and Thin Films for Future Industrial and Biomedical Engineering Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 3–35. [Google Scholar]
- Yang, C.; Xu, W.; Meng, X.; Shi, X.; Shao, L.; Zeng, X.; Yang, Z.; Li, S.; Liu, Y.; Xia, X. A pH-responsive hydrophilic controlled release system based on ZIF-8 for selfhealing anticorrosion application. Chem. Eng. J. 2021, 415, 128985. [Google Scholar] [CrossRef]
- Zhao, Y.; Jiang, F.; Chen, Y.-Q.; Hu, J.-M. Coatings embedded with GO/MOFs nanocontainers having both active and passive protecting properties. Corros. Sci. 2020, 168, 108563. [Google Scholar] [CrossRef]
- Barman, M.; Mahmood, S.; Augustine, R.; Hasan, A.; Thomas, S.; Ghosal, K. Natural halloysite nanotubes /chitosan based bio-nanocomposite for delivering norfloxacin, an anti-microbial agent in sustained release manner. Int. J. Biol. Macromol. 2020, 162, 1849–1861. [Google Scholar] [CrossRef] [PubMed]
- Ulaeto, S.B.; Nair, A.V.; Pancrecious, J.K.; Karun, A.S.; Mathew, G.M.; Rajan, T.; Pai, B. Smart nanocontainer-based anticorrosive bio-coatings: Evaluation of quercetin for corrosion protection of aluminium alloys. Prog. Org. Coat. 2019, 136, 105276. [Google Scholar] [CrossRef]
- Guo, Q.B.; Lau, K.T.; Zheng, B.F.; Rong, M.Z.; Zhang, M.Q. Imparting ultra-low friction and wear rate to epoxy by the incorporation of microencapsulated lubricant? Macromol. Mater. Eng. 2009, 294, 20–24. [Google Scholar] [CrossRef]
- Yan, H.; Fan, X.; Cai, M.; Song, S.; Zhu, M. Amino-functionalized Ti3C2Tx loading ZIF-8 nanocontainer@benzotriazole as multifunctional composite filler towards self-healing epoxy coating. J. Colloid Interface Sci. 2021, 602, 131–145. [Google Scholar] [CrossRef]




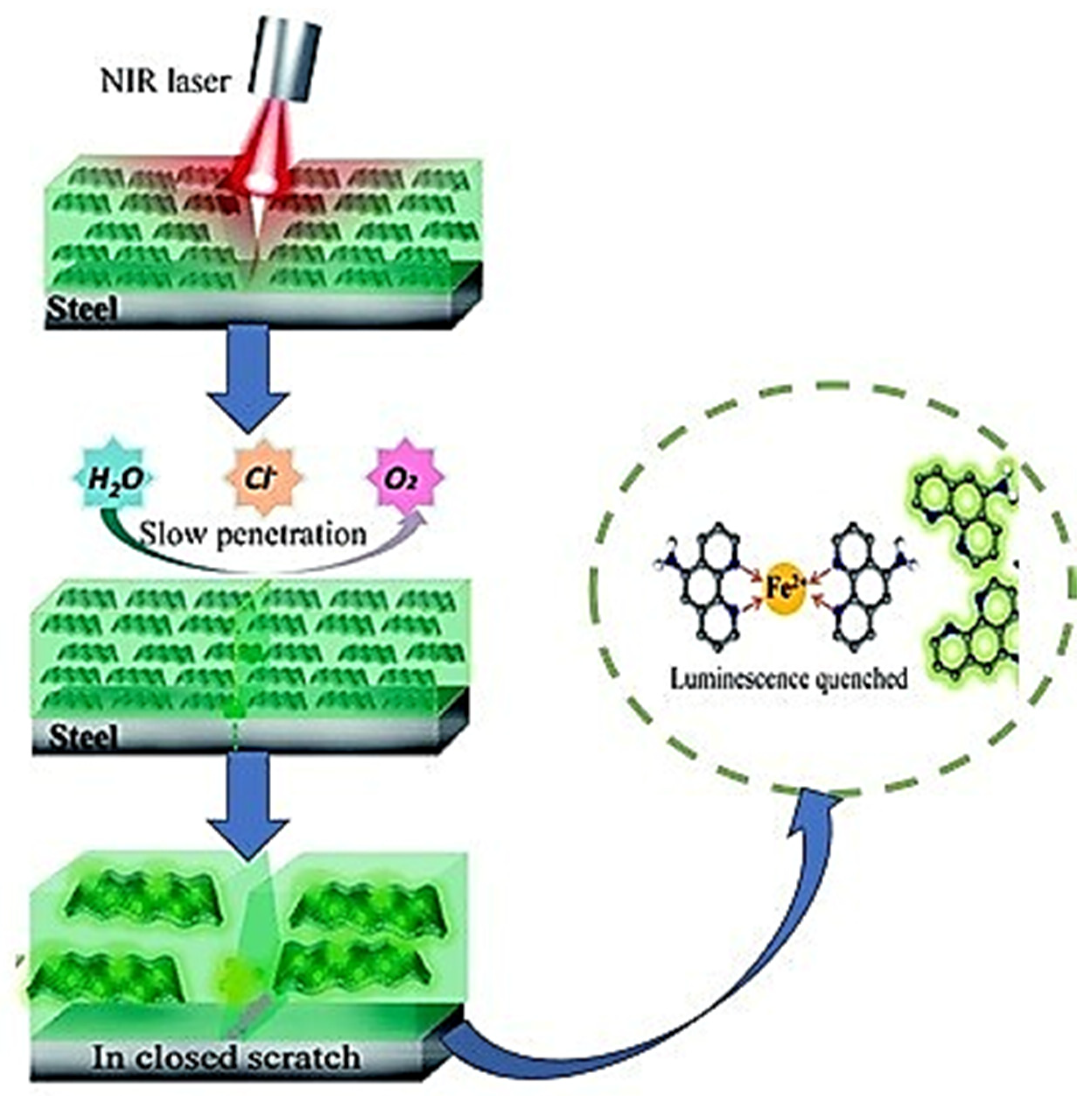

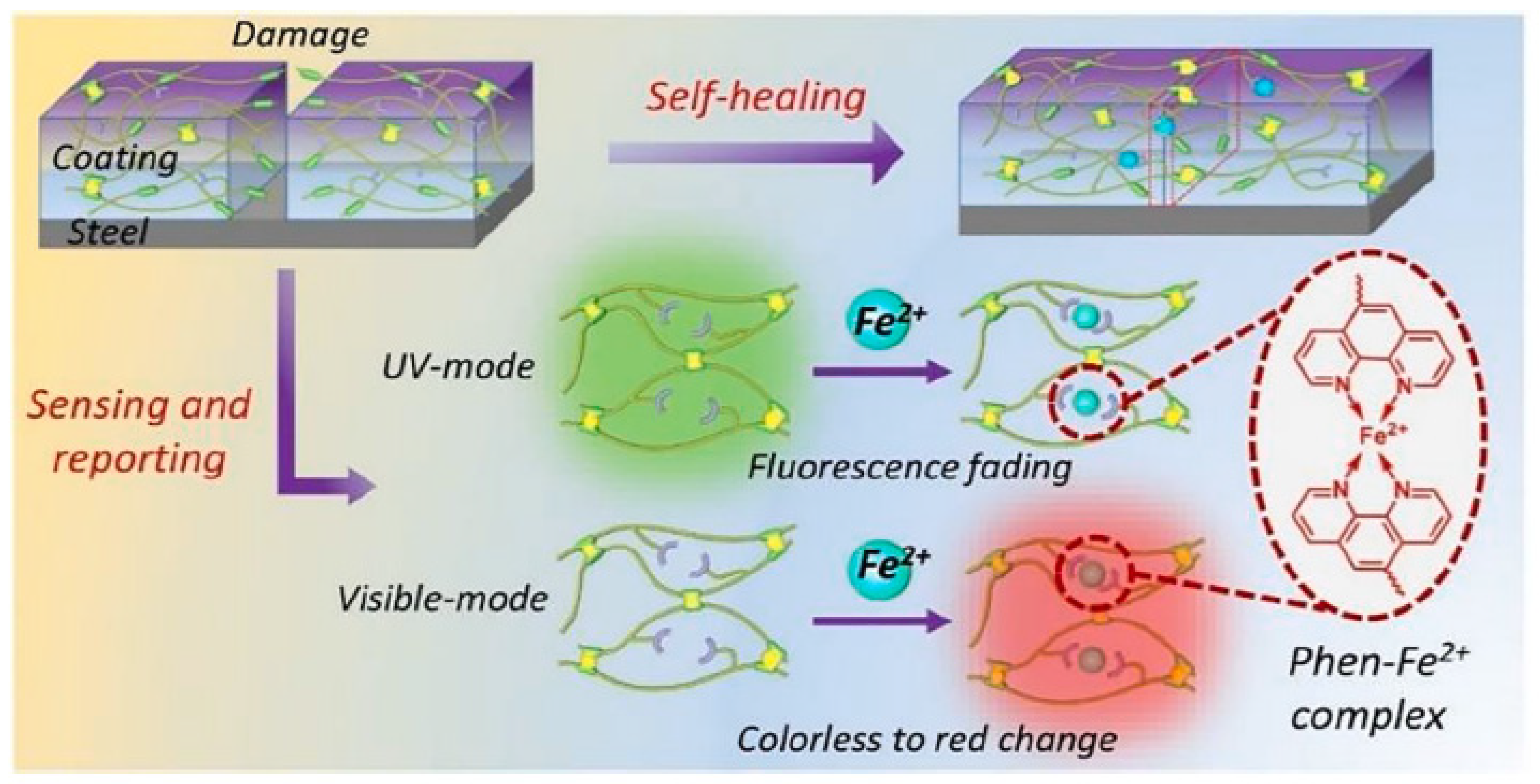
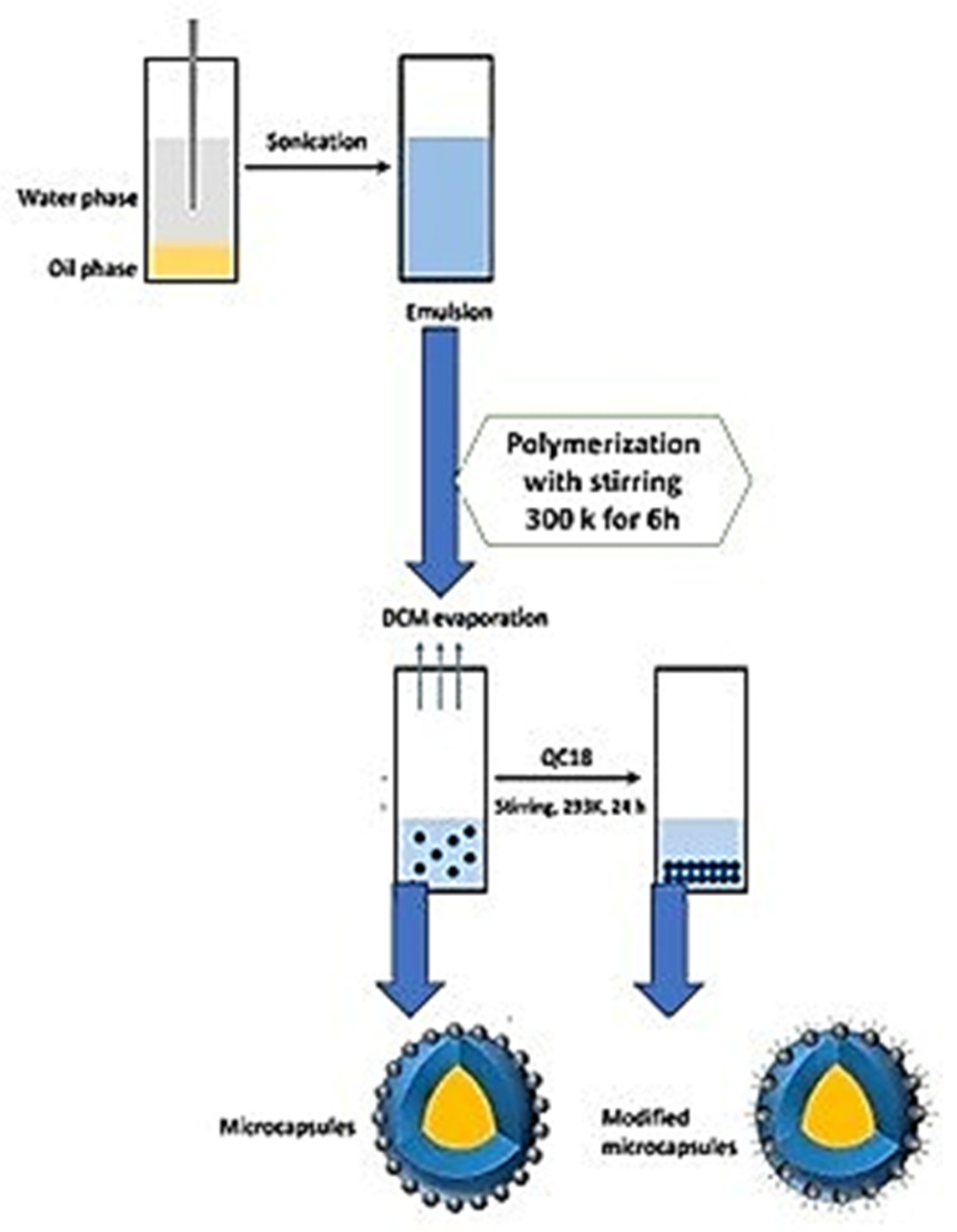
| SNo | Trigger Factor | Type | Micro/Nanocontainers | Inhibitors/Healing Agent | Reference |
|---|---|---|---|---|---|
| 1 | pH | Organic/inorganic hybrid micro/nanocontainers | ZIF | Benzimidazole | [55] |
| pH | MOF/graphene oxide | 2-Mercaptobenzimidazole (MBI) | [56] | ||
| pH | SiO2/polyelectrolyte layers | BTA | [56] | ||
| Thermo | Silica-poly(N-isopropylacrylamide) | rhodamine B | [57] | ||
| 2 | pH | Inorganic micro/nanocontainers | LDH | sodium molybdate | [58] |
| pH | Graphene | BTA | [59] | ||
| Redox | Fe3O4@mSiO2 | 8-HQ | [60] | ||
| pH/redox | Mesoporous SiO2 | 2-mercaptobenzothiazole (MBT) | [61] | ||
| NIR | TiN@mesoporous SiO2 | BTA | [62] | ||
| NIR/UV | TiO2/carbon black nanoparticles | fluorine silane | [62] | ||
| 3 | Mechanical/pH | Poly(urea-ormaldehyde) | BTA | [63] | |
| UV | Poly(urea-urethane) | 2-oxoacetates | [64] | ||
| Chloride ions | Alginate hydrogel capsules | silver | [65] | ||
| Thermo | Calcium alginate gel capsules | Imidazoline quaternary ammonium salt | [66] | ||
| Redox | Polyaniline/poly(2,5-dimercapto-1,3,4-thiadiazole) | 2,5-dimercapto-1,3,4-thiadiazole | [67] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanyal, S.; Park, S.; Chelliah, R.; Yeon, S.-J.; Barathikannan, K.; Vijayalakshmi, S.; Jeong, Y.-J.; Rubab, M.; Oh, D.H. Emerging Trends in Smart Self-Healing Coatings: A Focus on Micro/Nanocontainer Technologies for Enhanced Corrosion Protection. Coatings 2024, 14, 324. https://doi.org/10.3390/coatings14030324
Sanyal S, Park S, Chelliah R, Yeon S-J, Barathikannan K, Vijayalakshmi S, Jeong Y-J, Rubab M, Oh DH. Emerging Trends in Smart Self-Healing Coatings: A Focus on Micro/Nanocontainer Technologies for Enhanced Corrosion Protection. Coatings. 2024; 14(3):324. https://doi.org/10.3390/coatings14030324
Chicago/Turabian StyleSanyal, Simpy, SeonJu Park, Ramachandran Chelliah, Su-Jung Yeon, Kaliyan Barathikannan, Selvakumar Vijayalakshmi, Ye-Jin Jeong, Momna Rubab, and Deog Hawn Oh. 2024. "Emerging Trends in Smart Self-Healing Coatings: A Focus on Micro/Nanocontainer Technologies for Enhanced Corrosion Protection" Coatings 14, no. 3: 324. https://doi.org/10.3390/coatings14030324
APA StyleSanyal, S., Park, S., Chelliah, R., Yeon, S.-J., Barathikannan, K., Vijayalakshmi, S., Jeong, Y.-J., Rubab, M., & Oh, D. H. (2024). Emerging Trends in Smart Self-Healing Coatings: A Focus on Micro/Nanocontainer Technologies for Enhanced Corrosion Protection. Coatings, 14(3), 324. https://doi.org/10.3390/coatings14030324








