Abstract
There is a scarcity of research on the characterization of the behaviour of titanium and its alloys in highly corrosive environments. These materials are highly recommended for use in various industries such as aviation, maritime, medical, and chemical, due to their perceived superior corrosion resistance. This research examines the mechanical and corrosion characteristics of Ti6Al4V material when exposed to solutions containing 9% NaCl, 25% HCl, and a mixture of 9% NaCl and 25% HCl. Prior to the corrosion process, the prefabricated Ti6Al4V samples underwent microstructure analysis, hardness assessment, and wear evaluation. The microstructure characterization revealed that the microstructure of the Ti6Al4V alloy is composed of α and modified β phases. The Ti6Al4V sample’s hardness value was determined to be 334.23 HB. The Ti6Al4V sample’s wear rate was determined to be 0.0033 g/Nm, while the friction coefficient was determined to be 0.0326. Corrosion testing was conducted at intervals of 24, 48, 72, 168, and 336 h. Based on the corrosion rate measurements, the sample exhibited the minimum corrosion rate of 1.928519 mg/(dm2·day) in a 9% NaCl environment. The sample with a combination of 9% NaCl and 25% HCl had the maximum corrosion rate, measured at 6.493048 mg per square decimetre per day. The formation of a larger oxide layer in the Ti6Al4V corrosion sample immersed in a 9% NaCl solution serves as a protective barrier on the surface and enhances its resistance to corrosion.
1. Introduction
Since its original appearance in the early 1950s, Ti6Al4V has continually shown its superiority as the optimal selection among titanium alloys. Presently, it is widely used in many sectors, such as automotive, aerospace, chemical, marine, and biomedical, owing to its outstanding combination of mechanical properties. The Ti6Al4V alloy consists of 90% titanium, 6% aluminium, and 4% vanadium [1]. This alloy demonstrates superior mechanical properties in comparison to commercially pure titanium, including a high strength-to-weight ratio, excellent biocompatibility, increased resistance to corrosion [2,3,4,5,6,7,8,9], minimal thermal expansion qualities [10,11,12,13], and high fracture toughness [3,4,5,6,7,8,14]. Additionally, it has favourable biocompatibility, rendering it appropriate for medical implants and dental applications [12,15]. Additionally, the alloy is used in microstructural composite materials because of its favourable mechanical characteristics and broad range of applications in many sectors [16]. The exceptional characteristics of the Ti6Al4V alloy make it the preferred choice for use in aviation compressors, helicopter rotor blades, valves, pipelines, and centrifugal pumps used in industrial wastewater systems [17].
Ti6Al4V alloy may be fabricated by several manufacturing methods including laser powder bed fusion [18], laser solid forming [19], and laser additive manufacturing [20]. In general, the Ti6Al4V alloy is a very adaptable material that has a distinctive blend of characteristics, making it appropriate for a diverse range of uses.
The corrosion characteristics of the Ti6Al4V alloy have been thoroughly investigated in several research projects. The corrosion resistance of Ti6Al4V alloy is influenced by several factors including surface treatments, coatings, microstructure, and alloy composition. Previous research investigations have examined the impact of thermally oxidised surfaces on the corrosion characteristics of the Ti6Al4V alloy [21]. Research has also examined the impact of surface coatings, such as graphene and niobium pentoxide, on the ability of the Ti6Al4V alloy to resist corrosion [22]. In addition, the study investigated the synthesis of TiO2 nanoparticles and their corrosion characteristics on the Ti6Al4V alloy [23]. Furthermore, the researchers also investigated the corrosion characteristics of the Ti6Al4V alloy in Hank’s solution and physiological saline [9,21,24,25]. In their study, Wang et al. [26] conducted electrochemical corrosion experiments on dual-phase TiAl-Ti3Al alloys, comparing single-crystal and polycrystalline samples. They found that the dual-phase single crystals had a lower corrosion rate and a greater pitting potential of around 280 mV. The lack of beta phase led to an enhancement in corrosion resistance. Comparative corrosion studies have been carried out on the Ti6Al4V alloy, stainless steel, and other alloys, including ZrTi-based cast metallic glasses [5,27]. These research investigations have mostly examined the corrosion behaviour of the Ti6Al4V alloy across several applications, with a particular focus on its use in the biomedical area. It emphasises the significant interest in comprehending and enhancing. Nevertheless, the literature has similarities with our research in the automotive, aerospace, chemical, and marine sectors for the Ti6Al4V alloy, as shown by references [3,4,5,6,7,8,9]. Despite its purported convenience, there is a lack of research on the issue presented in [14].
The wear characteristics of the Ti6Al4V alloy have been thoroughly Investigated In several research articles. The wear resistance of the Ti6Al4V alloy is affected by many elements, such as cutting pressures, chip shape, tool wear, sliding speed, surface improvement, and surface modifications [28,29,30]. The Ti6Al4V alloy may experience wear via many processes, such as abrasive wear, adhesive wear, corrosive wear, and oxidative wear [19,31]. The wear characteristics of the Ti6Al4V alloy may be influenced by several elements, including the friction coefficient, cutting temperature, surface roughness, and wear scar width [30,32,33]. Wear resistance is of utmost importance in biomedical applications, and researchers have examined the wear characteristics of the Ti6Al4V alloy in simulated bodily fluids [34]. Overall, the wear characteristics of the Ti6Al4V alloy are influenced by several aspects, making it a complicated matter. Extensive study has been carried out to comprehend and enhance its resistance to wear in diverse applications. This research aims to analyse the wear behaviour of the Ti6Al4V alloy under certain stress conditions and for a defined duration, with the objective of contributing to the existing literature on its suitability for mechanical applications.
While there have been several studies on the utilisation of titanium (Ti) and its alloys in the biomedical area, there is a scarcity of research investigating the performance of pure titanium (grade 2–grade 16) [35] and the Ti6Al4V alloy [36] in harsh conditions. The research [2,37,38,39] also used the surface processing approach. Nevertheless, despite the Ti6Al4V alloy’s commendable strength-to-weight ratio and exceptional resistance to corrosion, there is a noticeable scarcity of research on the utilisation of Ti alloys in mechanical applications and chemically hostile corrosive settings without any surface treatments. Hence, the objective of this study was to investigate the mechanical characteristics and corrosion resistance of the Ti6Al4V alloy when exposed to solutions containing 9% NaCl, 25% HCl, and a mixture of 9% NaCl and 25% HCl. The corrosion tests were conducted at the specific time intervals of 24, 48, 72, 168, and 336 h.
2. Materials and Methods
2.1. Material
The investigation used Ti6Al4V materials provided by Birçelik Paslanmaz Çelik Tic. Inc. An analysis was conducted on the microstructure, hardness, wear, and corrosion characteristics of the Ti6Al4V material.
2.2. Microstructure Analysis
The chemical composition of the Ti6Al4V material was analysed using the XRF (X-ray fluorescence) technique using the Rigaku ZSX Primus II equipment, USA. The weight percentage ratios of the alloying elements were identified in this approach by using secondary radiations with distinct wavelengths that are distinctive to each element, known as fluorescence. X-ray diffraction (XRD) analyses were conducted on the Ti6Al4V sample using a Rigaku device. The examinations included a scanning angle range of 10°–90° and used a scanning speed of 3°/min. The purpose of these analyses was to identify the phases present in the structure of the sample.
To examine the microstructure of the Ti6Al4V alloy, first, samples measuring 10 × 10 mm were cut. The specimens, which were trimmed to suitable dimensions, underwent sanding using SiC paper with grit sizes of 400, 600, 800, 1000, 1200, 2000, and 2500 on a Mikrotest sanding and polishing apparatus. Subsequently, they were polished on a polishing felt using 1 μm alumina paste. The etching method used an etchant solution composed of 1 mL of hydrofluoric acid, 4 mL of nitric acid, and 5 mL of ethanol. The microstructural analysis was performed via a Carl Zeiss optical microscope. In order to carry out a comprehensive examination, we used a Carl Zeiss Ultra Plus Gemini Fesem, Germany, apparatus to take scanning electron microscope (SEM) images at the Research Laboratory of the Iron and Steel Institute at Karabük University.
2.3. Hardness Test
The hardness of Ti6Al4V was measured using a BMS 3000-HB BRINELL, Turkey, brand hardness tester. The Brinell hardness test used a ball diameter of 5 mm, a force of 750 N, and a loading duration of 10 s. The diameters of each sample were measured by generating three traces, and the hardness values were determined by computing their mean.
2.4. Wear Test
The reciprocating wear tester seen in Figure 1 was used to conduct wear tests on the samples, maintaining a consistent load, speed, and distance throughout the experiments. The sample was trimmed to accommodate the sample bed in the device, and its surfaces were abraded, cleansed, and readied for the wear test. Wear experiments were conducted with a load of 20 N, a sliding speed of 0.1 m/s, and a sliding distance of 1000 m. The load cell attached to the tribometer arm accurately measured the friction force experienced throughout the wear process and instantly recorded this data into the computer. The friction coefficient was calculated by proportioning the recorded friction force data in Newton to the applied constant 20 N load. The penetrating tip material utilized is a high-hardness steel ball of AISI 52100 grade. The wear mechanisms were discovered after conducting a thorough investigation of the worn surfaces using SEM images.
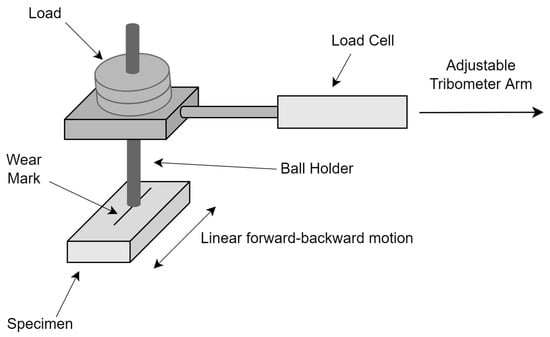
Figure 1.
Back-and-forth wear tester.
2.5. Corrosion Test
An immersion test was conducted on the Ti6Al4V sample to evaluate its corrosion capabilities. The test included immersing the sample in solutions containing 9% NaCl, 25% HCl, and a mixture of 9% NaCl and 25% HCl. The samples were originally divided into about equal proportions, and then their surfaces were polished using sandpaper with a grain size of 1200. In order to perform calculations based on the immersion test, the surface areas of the samples were computed using the following formula: (2πr2) + (2πrh). Prior to immersion in water, the weights were originally measured using a high-precision balance. Afterwards, equal amounts of solution were divided into glass jars, and the samples were hung using a net. The specimens that were submerged were retrieved from the fluid at certain time intervals: 24, 48, 72, 168, and 336 h. The surfaces of the samples were cleaned and their weights were measured and recorded. The specimens were immersed in a solution of chromic acid, which was prepared by dissolving 182 grammes per litre in distilled water, for a duration of 10 min. The corrosion products that developed on the surface were eliminated on an hourly basis. Afterwards, the samples underwent ultrasonic vibration in a device containing chromic acid solution, then were cleaned with ethyl alcohol, and finally submerged in the solution again.
3. Results and Discussion
3.1. Microstructural Characterisation
The research provides the average chemical composition of the Ti6Al4V material, which is shown in Table 1. Figure 2 displays the SEM image at 1000× magnification (Figure 2a), and the SEM image at 5000× magnification (Figure 2b). The Ti6Al4V alloy’s microstructure comprises altered β phases, as seen in Figure 2b [40]. The microstructure exhibits initial coarse grains (light area) and altered β regions that extend along the deformation direction, as seen in previous studies [17,37]. Compared to the literature, grains were found to be smaller in size (2–3 µm) [41].

Table 1.
XRF analysis of Ti6Al4V sample.
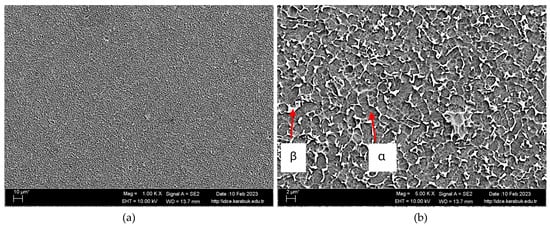
Figure 2.
Ti6Al4V sample shown as (a) SEM image with 1000× magnification and (b) SEM image with 5000× magnification.
Figure 3 is the SEM image of Ti6Al4V material at 10,000× magnification. Table 2 gives the EDX analysis results taken from this SEM image. The microstructure images were examined using the XRD spectra shown in Figure 4 and the EDX data supplied in Table 2. Upon examining the EDX study, it is evident that the alloys include α-Ti and β-Ti phases, which are also shown in the XRD analysis (Figure 4). The SEM investigation of area 1 revealed the presence of 90.42% titanium, 9.03% aluminium, and 0.55% vanadium. The grey structure in this area exhibits little white spots, which are believed to be Al-Ti binary intermetallics resulting from the low concentration of vanadium. Region number 2 has a white rod-shaped object composed of 83.39% titanium, 5.08% aluminium, and 11.53% vanadium. The composition of this area is believed to be mostly composed of binary intermetallic compounds, namely Al45V7 or Al3V.

Figure 3.
SEM image of Ti6Al4V sample with 10,000× magnification.

Table 2.
EDX analysis results from Figure 3.
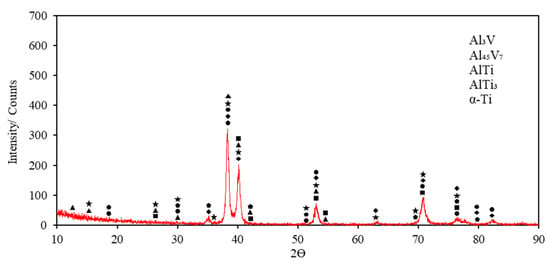
Figure 4.
XRD result of Ti6Al4V sample.
Figure 4 shows the XRD spectra of the Ti6Al4V sample. AlTi3 is the most visible phase peak. The XRD peaks of the Ti6Al4V alloy started with AlTi3 and Al45V7 phases at 15°. At the peak seen at 26° degrees, AlTi3, Al45V7, and Al3V phases were determined. AlTi3, β-Ti, AlTi, and Al45V7 were observed at 30°. β-Ti and α-Ti phases were detected at 35°; Al45V7, AlTi3, AlTi, β-Ti, and α-Ti were detected at 38°; Al3V, Al45V7, AlTi3, and α-Ti were detected at 40°; β-Ti, α-Ti, AlTi3, Al45V7, and Al3V were detected at 53°; α-Ti and AlTi3 were detected at 63°; AlTi3, α-Ti, β-Ti, and Al3V were detected at 71°; α-Ti, AlTi3, β-Ti, Al3V, and AlTi were detected at 77°; and AlTi, α-Ti, and β-Ti were detected at 80°. Finally, the XRD peaks of the AlTi and α-Ti phases ended at 82°.
3.2. Examination of Mechanical Properties
The Ti6Al4V sample’s mechanical characteristics were assessed using Brinell hardness and back-and-forth wear tests. Table 3 provides the hardness value, wear coefficient, and friction coefficient of the Ti6Al4V sample. The hardness test yielded a value of ±334.23 HB/5/750/10. The values mentioned above are as follows: 334.23 for the Brinell hardness, 5 mm for the diameter of the steel ball, 750 N for the applied load, and 10 s for the duration. The measured hardness value is consistent with the findings of Liu et al. [42]. The wear coefficient of the Ti6Al4V sample, determined using a reciprocal wear test, is 0.011 g/Nm. The calculated weight loss per unit hardness is 3.3 × 10−11 g/m3. The determined friction coefficient is 0.0326. According to Johns et al., the friction coefficient of untreated Ti6Al4V material was determined to be 0.5 [43].

Table 3.
Mechanical properties of Ti6Al4V sample.
Figure 5 displays the SEM picture of a Ti6Al4V sample that has been worn across a distance of 1000 m. The image is captured at various magnifications. The worn sample exhibits a formation of voids on its surface when seen under macro magnification. The SEM picture, magnified at 500×, shows the presence of oxides, which are visible as white areas. Furthermore, it is observed that the oxidised particles obtained from the worn surfaces by the abrasive wear mechanism remain on the surface and persistently abrade the material surface underneath the abrasive ball. According to Singh et al. [44], the wear scar of Ti-6Al-4V showed many regularly spaced deep circular grooves, which were created by the micro-cutting process. This phenomenon is often noticed in abrasive wear when soft materials are paired with a hard opposing surface. In addition, a process of adhesive wear was also found [44]. Adebiyi et al. reported the presence of grooves in the wear morphology of Ti-6Al-4V, and noted that the materials dislodging from these grooves created protrusions [42].
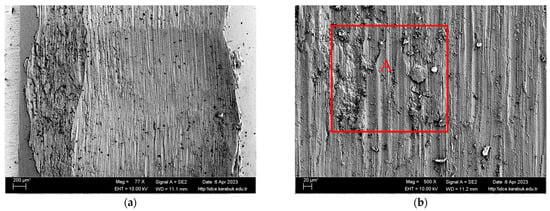
Figure 5.
SEM image of a 1000 m worn Ti6Al4V sample with (a) 77× and (b) 500× magnification.
The findings of the EDX examination conducted on the worn surface can be seen in Table 4 and Figure 6. Based on the EDX readings, the oxygen levels were elevated, indicating the presence of oxidative wear debris [45]. The SEM picture reveals that the oxide layer, which is generated during the process of wear, fractures into fragments and scatters throughout the surface [46]. The literature has shown that the occurrence of an oxide layer in titanium alloys is a typical phenomenon, serving as a diffusion barrier during the first stages of the etching process [47]. Bartolomeu et al. [48] have asserted that the hard oxide layers that develop during the wear process and detach from the surface function as abrasives, triggering the three-body effect wear mechanism and expediting the wear process. The EDX examination findings reveal a significant presence of titanium and aluminium in the pieces attached to the surface of the sample. Hence, it may be inferred that the wear debris seen on the surface of the sample originates from the sample itself. Furthermore, the presence of a minimal quantity of iron indicates that oxidation has taken place on the surface of the sample [39].

Table 4.
EDX analysis results taken from Figure 6.
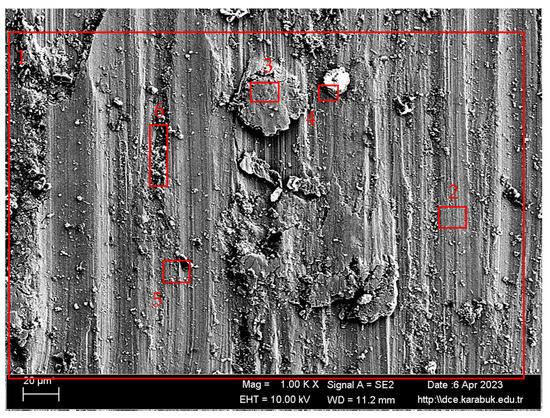
Figure 6.
SEM image at 1000× magnification of the 1000 m worn Ti6Al4V sample taken from region A in Figure 5.
3.3. Immersion Corrosion Test Results
Titanium metal has inherent reactivity. When titanium and titanium alloys with polished and lustrous surfaces come into contact with the air, a humid environment, or a reducing environment, a protective oxide layer develops on the surface of the material. This film is very stable, continuous, and strongly clings to the substance. Due to its stated ability to provide titanium and its alloys with exceptional resistance to corrosion [49,50,51,52], this layer is extensively used in many environmental circumstances, including aerospace, marine, medical, and chemical sectors. In order to assess the corrosion resistance of the Ti6Al4V sample, immersion experiments were conducted at room temperature. The experiments were carried out at the specific time intervals of 24, 48, 72, 168, and 336 h. The weight losses of the sample were measured in three different solutions: 9% NaCl, 25% HCl, and a mixture of 9% NaCl and 25% HCl. The changes in weight loss over time were evaluated and presented in Figure 7.
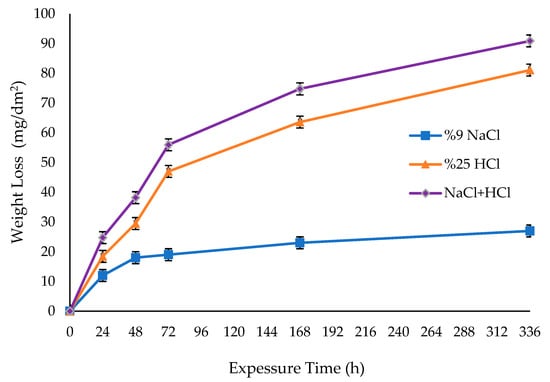
Figure 7.
Weight loss result of immersion corrosion of Ti6Al4V sample in different solutions.
Based on Figure 7, the weight reduction after 24 h was determined to be 11.99 mg/dm2 in a 9% NaCl solution, 18.43 mg/dm2 in a 25% HCl solution, and 24.74 mg/dm2 in a solution comprising 9% NaCl and 25% HCl. The weight reductions after 72 h were 19.00 mg/dm2, 47.00 mg/dm2, and 55.94 mg/dm2, respectively. Although a 72 h immersion corrosion experiment provided adequate understanding of the corrosion behaviour, the experiment was extended to 336 h due to the corrosion resistance of the Ti6Al4V sample utilised in this work. After 168 h, the Ti6Al4V sample exhibited greater resistance in a 9% NaCl solution. However, its corrosion resistance fell by a factor of 2.76 in a 25% HCl solution and by a factor of 3.25 in a mixture of 9% NaCl and 25% HCl. The cause of this phenomenon is believed to be intergranular corrosion that takes place inside the material when exposed to a corrosion solution containing 9% NaCl and 25% HCl. The weight loss at the 336th hour of the experiment was measured to be 90.90 mg/dm2 in a solution containing 9% NaCl and 25% HCl, which was the highest recorded. The lowest weight loss, on the other hand, was 27.00 mg/dm2 in an environment with 9% NaCl. In their study on the categorization of corrosion flaws in titanium alloys exposed to harsh conditions, Pran-do et al. [35] observed that the presence of the β phase at grain boundaries leads to intergranular and/or crevice corrosion. In addition, Elhasslouk et al. [53] discovered that the NaCl/HCl solution facilitated intergranular corrosion. Figure 2 displays the microstructure of the Ti6Al4V alloy, which was analysed for its corrosion properties in solutions containing 9% NaCl, 25% HCl, and a mixture of 9% NaCl and 25% HCl. The microstructure reveals the presence of grain boundary β phases.
The corrosion rate determination included calculating the Mdd (mass loss in milligrammes per day) using weight losses measured at 24, 48, 72, 168, and 336 h. The resulting Mdd values, expressed in milligrammes per square decimetre per day (mg/dm2.day), are shown in Figure 8. According to the corrosion rate data shown in Figure 8, the sample demonstrated the minimum corrosion rate of 1.93 mg/(dm2*day) in a 9% NaCl environment. The sample with a combination of 9% NaCl and 25% HCl had the maximum corrosion rate, measuring 6.493048 mg/(dm2*day). Figure 8 demonstrates that Ti6Al4V exhibited a consistently steady corrosion behaviour in all corrosive conditions after 168 h.
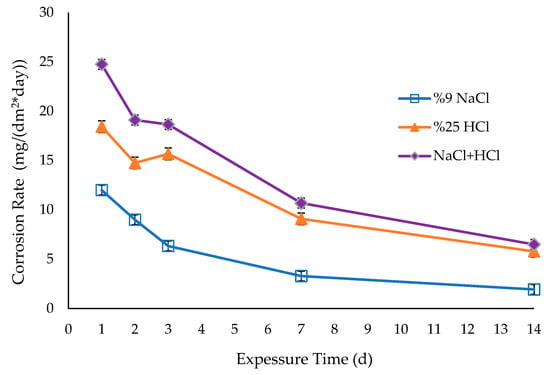
Figure 8.
Corrosion rates of Ti6Al4V sample in different solutions.
Figure 9 displays the corrosion rate data of the Ti6Al4V sample after a 14-day period. The corrosion rate of the sample was determined after 14 days in different solutions. In a 9% NaCl solution, the corrosion rate was found to be 1.93 mg/dm2*day. In a 25% HCl solution, the corrosion rate was 5.79 mg/dm2*day. In a solution containing both 9% NaCl and 25% HCl, the corrosion rate was 6.49 mg/dm2*day.
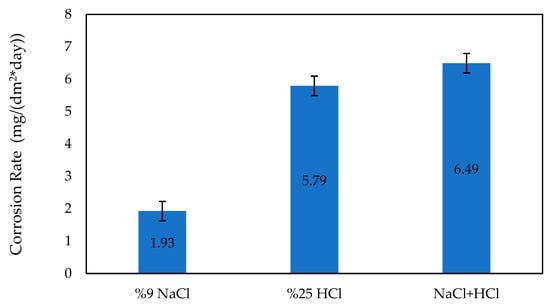
Figure 9.
Corrosion rates of Ti6Al4V sample after 14 days.
The SEM picture shown in Figure 10 illustrates the corrosion of the Ti6Al4V sample in a 9% NaCl solution, obtained at a magnification of 500×. Figure 11 depicts a SEM image of the Ti6Al4V sample that underwent corrosion in a 9% NaCl solution, seen at a magnification of 1000×. The findings of the EDX investigation performed on the data acquired from Figure 11 are shown in Table 5. Upon examination of the SEM image, it is evident that stratified corrosion is present in a specific region of the sample.

Figure 10.
SEM image at 500× magnification of the Ti6Al4V sample corroded in 9% NaCl solution.
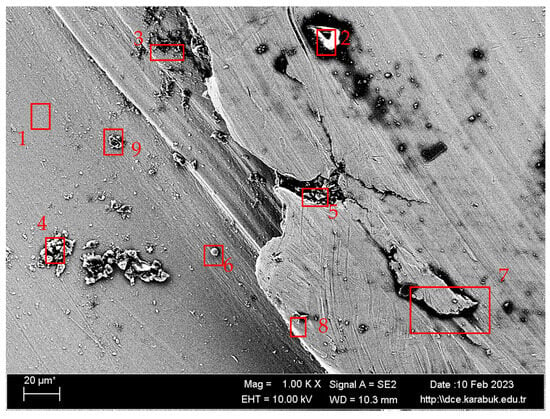
Figure 11.
SEM image at 1000× magnification taken from region A in Figure 10 of the Ti6Al4V sample corroded in 9% NaCl solution.

Table 5.
EDX analysis results taken from Figure 11.
The large quantities of titanium (Ti) and oxygen (O) elements in regions 3, 4, 5, 7, and 8, as specified in Table 5, suggest the development of a passive oxide film layer on the sample’s surface. The formation of a TiO2 oxide layer provides surface protection to titanium and its alloys, hence improving their resistance to corrosion [54,55]. Figure 11 shows that the surface of the Ti6Al4V sample with a 9% NaCl solution has the lowest corrosion rate and is coated with a layer of TiO2. The uniformity of the oxide layer is a significant factor in enhancing corrosion resistance.
Figure 12 displays the SEM picture of the Ti6Al4V sample that underwent corrosion in a 25% hydrochloric acid (HCl) solution, seen at a magnification of 500×. Figure 13 depicts a SEM image of the Ti6Al4V sample that underwent corrosion in a 25% HCl solution, seen at a magnification of 1000×. The findings of the EDX research performed in Figure 13 are shown in Table 6. Upon analysis of the SEM image, it is clear that the sample has adhesive corrosion. Figure 13 illustrates that the oxide layer remains undamaged after the corrosion process. Upon conducting a comparison between the SEM image of the NaCl-corroded sample and the HCl-corroded Ti6Al4V sample, it is apparent that the distribution of the oxide layer is not uniform. This elucidates the cause of increased corrosion. The Ti6Al4V sample immersed in HCl solution exhibited a higher level of corrosion compared to the Ti6Al4V sample in NaCl solution. This may be attributed to the fact that the rate of disintegration of the existing oxide layer on the Ti6Al4V sample is larger than its regeneration rate. Additionally, the presence of alloying elements in the Ti6Al4V sample results in distinct energy regions, further contributing to the observed difference in corrosion behaviour [56].

Figure 12.
SEM image at 500× magnification of the Ti6Al4V sample corroded in 25% HCl solution.

Figure 13.
SEM image at 1000× magnification taken from region A in Figure 12 of the Ti6Al4V sample corroded in 25% HCl solution.

Table 6.
EDX analysis results taken from Figure 13.
The SEM picture in Figure 14 depicts the corrosion of the Ti6Al4V sample in a solution containing 9% NaCl and 25% HCl, seen at a magnification of 500×. Figure 15 displays the SEM picture of the Ti6Al4V sample corroded in a 25% hydrochloric acid (HCl) solution at a magnification of 1000 times. Table 7 presents the findings of the energy-dispersive X-ray (EDX) analysis conducted on the data obtained from Figure 15. The SEM pictures of the sample containing 9% NaCl and 25% HCl revealed an increase in layered corrosion, providing further evidence of a reduction in corrosion resistance. Upon examination of the SEM picture, it is evident that adhesions and layers have developed, covering a majority of the sample’s surfaces. Comparatively, the adhesion is elevated and the oxide layer is diminished in relation to the corrosion samples exposed to 9% NaCl and 25% HCl. Cl− is an anion that can be easily adsorbed [57,58,59]. It can readily infiltrate the metal–oxide interface via the intergranular boundaries of the oxide–hydroxide scale. Therefore, it may cause local disintegration of the passive film formed on the alloy. Chlorine anions are adsorbed onto the passive film and penetrate the layer through grain boundaries and other structural defects [60]. Using NaCl and HCl together as a corrosive medium also caused increased corrosion by releasing more Cl ions in the structure [53]. Additionally, the higher corrosion potential of the 9% NaCl and 25% HCl corrosion sample may be attributed to the oxygen rate inside the structure. Increased oxygen levels result in a greater degree of oxidation. Upon examining Table 7, it becomes apparent that there is a drop in the oxygen quantity, which therefore impacts the rate of corrosion.
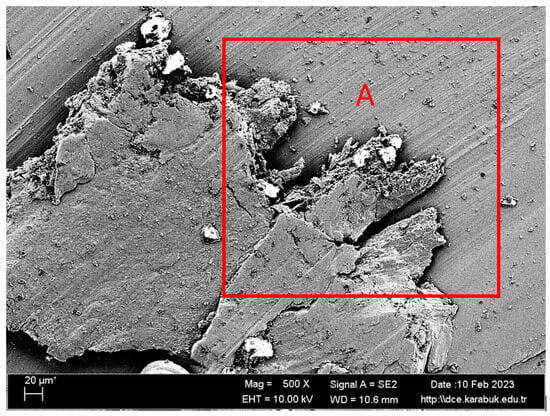
Figure 14.
SEM image at 500× magnification of the corroded Ti6Al4V sample in 9% NaCl and 25% HCl solution.
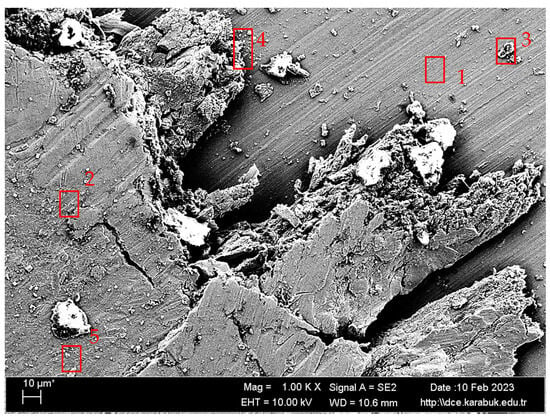
Figure 15.
SEM image at 1000× magnification of the Ti6Al4V sample corroded in 9% NaCl + 25% HCl solution, taken from region A in Figure 13.

Table 7.
EDX analysis results taken from Figure 14.
According to Yang et al. [61], halides generated during the pitting corrosion of titanium alloys migrate into the corrosion pits, leading to localized acidification in the corrosion area. Thus, as the halide concentration rises, the release of H+ ions increases, leading to a higher likelihood of pitting nucleation and development. Lu et al. [62] and Bodunrin et al. [63] found that the α phases and α–β phase interface were selectively dissolved in hydrochloric acid solutions.
4. Conclusions
Regarding the microstructure, mechanical, and corrosion characteristics of Ti6Al4V materials, the following results were made:
- The Ti6Al4V alloy’s microstructure comprises α and modified β phases. The microstructure exhibits large initial α grains and β areas that have undergone transformation, extending parallel to the direction of deformation.
- The Ti6Al4V sample had a hardness value of 334.23 HB. The acquired hardness is appropriate for the manufacturing and use characteristics of the alloy. The Ti6Al4V sample’s wear rate was determined to be 0.0033 g/Nm, while the friction coefficient was determined to be 0.0326. Elevated quantities of oxygen in the wear EDX findings suggest the presence of an oxidative wear process.
- Based on the immersion corrosion findings, the Ti6Al4V sample exhibited greater resistance in a 9% NaCl solution. However, its corrosion resistance fell by a factor of 2.76 in a 25% HCl solution and by a factor of 3.25 in a mixture of 9% NaCl and 25% HCl. The cause of this phenomenon is believed to be intergranular corrosion that takes place in the material when exposed to a corrosion solution containing 9% NaCl and 25% HCl.
- The accelerated corrosion of the Ti6Al4V sample in a solution containing 9% NaCl and 25% HCl is attributed to the rise in Cl− ions and the acidification of the solution caused by an increase in H+ ions. The preferential dissolution of α phases and α plus β phases increased as the corrosion solution became more acidic, leading to the creation of galvanic cells.
Author Contributions
Conceptualization, I.E., M.M.M.G. and H.A.; methodology, I.E. and M.M.M.G.; investigation, M.M.M.G.; writing—original draft preparation, B.A.; writing—review and editing, I.E., M.M.M.G. and H.A.; supervision, I.E. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Haydar, H.J.; Al-Deen, J.; AbidAli, A.K.; Mahmoud, A.A. Improved Performance of Ti6Al4V Alloy in Biomedical Applications—Review. J. Phys. Conf. Ser. 2021, 1973, 012146. [Google Scholar] [CrossRef]
- Atar, E.; Kayali, E.S.; Cimenoglu, H. Characteristics and wear performance of borided Ti6Al4V alloy. Surf. Coatings Technol. 2008, 202, 4583–4590. [Google Scholar] [CrossRef]
- Han, J.; Yang, J.; Yu, H.; Yin, J.; Gao, M.; Wang, Z.; Zeng, X. Microstructure and Mechanical Property of Selective Laser Melted Ti6Al4V Dependence on Laser Energy Density. Rapid Prototyp. J. 2017, 23, 217–226. [Google Scholar] [CrossRef]
- Markhoff, J.; Krogull, M.; Schulze, C.H.; Rotsch, C.; Hunger, S.; Bader, R. Biocompatibility and Inflammatory Potential of Titanium Alloys Cultivated With Human Osteoblasts, Fibroblasts and Macrophages. Materials 2017, 10, 52. [Google Scholar] [CrossRef] [PubMed]
- Staehlke, S.; Rebl, H.; Finke, B.; Mueller, P.; Gruening, M.; Nebe, B. Enhanced Calcium Ion Mobilization in Osteoblasts on Amino Group Containing Plasma Polymer Nanolayer. Cell Biosci. 2018, 8, 22. [Google Scholar] [CrossRef]
- Chen, J.; Li, J.; Hu, F.-C.; Zou, Q.; Mei, Q.; Li, S.; Hao, Y.; Hou, W.; Li, J.; Li, Y.; et al. Effect of Microarc Oxidation-Treated Ti6Al4V Scaffold Following Low-Intensity Pulsed Ultrasound Stimulation on Osteogenic Cells in Vitro. Acs Biomater. Sci. Eng. 2019, 5, 572–581. [Google Scholar] [CrossRef]
- Naghavi, S.H.R.; Wang, H.; Varma, S.N.; Tamaddon, M.; Marghoub, A.; Galbraith, R.; Galbraith, J.; Moazen, M.; Hua, J.; Xu, W.; et al. On the Morphological Deviation in Additive Manufacturing of Porous Ti6Al4V Scaffold: A Design Consideration. Materials 2022, 15, 4729. [Google Scholar] [CrossRef] [PubMed]
- Abbass, M.K.; Khadhim, M.J.; Jasim, A.N.; Issa, M.J.; Khashan, K.S. Fabrication and Optimization of Electrophoretic Deposition Parameters Using Alternating Current by Taguchi Design. Al-Nahrain J. Eng. Sci. 2021, 24, 8–15. [Google Scholar] [CrossRef]
- Benea, L.; Simionescu, N.; de Jos, D. Evaluation of Corrosion Resistance of Implant-Use Ti6Al4V Alloy in Hank Biological Solution in the Presence of Microorganism’s Metabolic Product Lactic Acid. Ann. “Dunarea Jos” Univ. Galati Fascicle Ix Metall. Mater. Sci. 2020, 43, 31–38. [Google Scholar] [CrossRef]
- Wang, Q.; Bruschi, S.; Ghiotti, A.; Mu, Y. Modelling of Fracture Occurrence in Ti6Al4V Sheets at Elevated Temperature Accounting for Anisotropic Behaviour. Int. J. Mech. Sci. 2019, 150, 471–483. [Google Scholar] [CrossRef]
- Zhong, C.; Liu, J.; Zhao, T.; Schopphoven, T.; Fu, J.; Gasser, A.; Schleifenbaum, J.H. Laser Metal Deposition of Ti6Al4V—A Brief Review. Appl. Sci. 2020, 10, 764. [Google Scholar] [CrossRef]
- Delikanli, Y.E.; Kayacan, M.C. Design, Manufacture, and Fatigue Analysis of Lightweight Hip Implants. J. Appl. Biomater. Funct. Mater. 2019, 17, 2280800019836830. [Google Scholar] [CrossRef]
- Rajadurai, M.; Muthuchamy, A.; Annamalai, A.R.; Agrawal, D.K.; Jen, C.-P. Effect of Molybdenum (Mo) Addition on Phase Composition, Microstructure, and Mechanical Properties of Pre-Alloyed Ti6Al4V Using Spark Plasma Sintering Technique. Molecules 2021, 26, 2894. [Google Scholar] [CrossRef] [PubMed]
- Khalaf, M.K.; Al-Taay, H.F.; Ali, D.S. Effect of Radio Frequency Magnetron Sputtering Power on Structural and Optical Properties of Ti6Al4V Thin Films. Photonic Sens. 2017, 7, 163–170. [Google Scholar] [CrossRef]
- Wei’an, Z.; Zhang, S.; Zhang, C.; Ren, L.; Wang, Q. Design and Characterization of Selective Laser-melted Ti6Al4V–5Cu Alloy for Dental Implants. Mater. Corros. 2020, 71, 1697–1710. [Google Scholar] [CrossRef]
- Han, Y.; Huang, G.; Lv, Y.; Shao, X.; Lu, W.; Zhang, D. Effect of ECAP Numbers on Microstructure and Properties of Titanium Matrix Composite. Mater. Des. 2015, 75, 113–119. [Google Scholar] [CrossRef]
- Fidan, S.; Avcu, E.; Karakulak, E.; Yamanoglu, R.; Zeren, M.; Sinmazcelik, T. Effect of heat treatment on erosive wear behaviour of Ti6Al4V alloy. Mater. Sci. Technol. 2013, 29, 1088–1094. [Google Scholar] [CrossRef]
- Dzogbewu, T.C. Laser Powder Bed Fusion of Ti6Al4V-xCu: Process Parameters. J. Met. Mater. Miner. 2021, 31, 62–70. [Google Scholar] [CrossRef]
- Pan, S. Enhanced Strength and High-Temperature Wear Resistance of Ti6Al4V Alloy Fabricated by Laser Solid Forming. J. Manuf. Sci. Eng. 2022, 144, 111011. [Google Scholar] [CrossRef]
- Wang, C.; Shang, C.; Xu, G.; Zhicheng, J.; Liu, J.; Yunhai, S. Microstructure and Mechanical Property Improvement of Laser Additive Manufacturing Ti–6Al–4V via the Niobium Addition. Mater. Trans. 2020, 61, 723–728. [Google Scholar] [CrossRef]
- Cao, L.; Wan, Y.; Yang, S.; Pu, J. The Tribocorrosion and Corrosion Properties of Thermally Oxidized Ti6Al4V Alloy in 0.9 Wt.\% NaCl Physiological Saline. Coatings 2018, 8, 285. [Google Scholar] [CrossRef]
- Zafar, M.S.; Amin, F.; Ghabbani, H.M.; Riaz, S.; Khurshid, Z.; Kumar, N. Biomimetic Aspects of Restorative Dentistry Biomaterials. Biomimetics 2020, 5, 34. [Google Scholar] [CrossRef]
- Karpagavalli, R.; Zhou, A.; Chellamuthu, P.; Nguyen, K. Corrosion behavior and biocompatibility of nanostructured TiO2 film on Ti6Al4V. J. Biomed. Mater. Res. Part A 2007, 83, 1087–1095. [Google Scholar] [CrossRef]
- Woźniak, A.; Walke, W.; Jakóbik-Kolon, A.; Ziębowicz, B.; Brytan, Z.; Adamiak, M. The Influence of ZnO Oxide Layer on the Physicochemical Behavior of Ti6Al4V Titanium Alloy. Materials 2021, 14, 230. [Google Scholar] [CrossRef]
- Elshamy, I.H.; Ibrahim, M.A.M.; Rehim, S.S.A.; El Boraei, N.F. Electrochemical Characteristics of a Biomedical Ti70Zr20Nb7.5Ta2.5 Refractory High Entropy Alloy in an Artificial Saliva Solution. J. Bio- Tribo-Corrosion 2022, 9, 10. [Google Scholar] [CrossRef]
- Wang, D.; Chen, G.; Wang, A.; Wang, Y.; Qiao, Y.; Liu, Z.; Qi, Z.; Liu, C.T. Corrosion behavior of single- and poly-crystalline dual-phase TiAl-Ti3Al alloy in NaCl solution. Int. J. Miner. Met. Mater. 2023, 30, 689–696. [Google Scholar] [CrossRef]
- Sobieszczańska, B.; Sobieszczańska, B.; Biały, M.; Chęcmanowski, J.; Chęcmanowski, J.; Bożemska, E.; Wawrzyńska, M.; Wawrzyńska, M. Production, Mechanical Properties and Biomedical Characterization of ZrTi-Based Bulk Metallic Glasses in Comparison With 316L Stainless Steel and Ti6Al4V Alloy. Materials 2021, 15, 252. [Google Scholar] [CrossRef]
- Arrazola, P.J.; Garay, A.; Iriarte, L.-M.; Armendia, M.; Marya, S.; Maître, F. Le Machinability of Titanium Alloys (Ti6Al4V and Ti555.3). J. Am. Acad. Dermatol. 2009, 209, 2223–2230. [Google Scholar] [CrossRef]
- Avcu, Y.Y.; Yetik, O.; Guney, M.; Iakovakis, E.; Sinmazçelik, T.; Boccaccini, A.R. Surface, Subsurface and Tribological Properties of Ti6Al4V Alloy Shot Peened Under Different Parameters. Materials 2020, 13, 4363. [Google Scholar] [CrossRef] [PubMed]
- Soori, M.; Arezoo, B. The Effects of Coolant on the Cutting Temperature, Surface Roughness and Tool Wear in Turning Operations of Ti6Al4V Alloy. Mech. Based Des. Struct. Mach. 2023. [Google Scholar] [CrossRef]
- Pu, J.; Wu, D.; Zhang, Y.; Zhang, X.; Jin, Z. An Experimental Study on the Fretting Corrosion Behaviours of Three Material Pairs at Modular Interfaces for Hip Joint Implants. Lubricants 2021, 9, 12. [Google Scholar] [CrossRef]
- Khramenkov, M.; Jersák, J. Effect of the Dressing Process on the Surface Roughness in Cylindrical Grinding of Ti6Al4V Alloy Using Stationary Diamond Dressing Tools. Manuf. Technol. 2021, 21, 640–646. [Google Scholar] [CrossRef]
- Ersoy, E.; Doğan, C.; Doğan, C. Investigation of the Surface Properties of TiN-Coated Ti6Al4V Alloy. Appl. Eng. Lett. J. Eng. Appl. Sci. 2021, 6, 175–183. [Google Scholar] [CrossRef]
- Burkov, A.A.; Chigrin, P.G.; Sazonova, E.N.; Ilchenko, P.A. Biocompatibility of Ti—Al Intermetallic Coatings on Titanium Alloy Ti6Al4V. Perspekt. Mater. 2023, 3. [Google Scholar] [CrossRef]
- Prando, D.; Brenna, A.; Diamanti, M.V.; Beretta, S.; Bolzoni, F.; Ormellese, M.; Pedeferri, M.P. Corrosion of titanium: Part 1: Aggressive environments and main forms of degradation. J. Appl. Biomater. Funct. Mater. 2017, 15, e291–e302. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Wang, Z.; Cai, J.; Xu, X.; Luo, K.; Wu, L.; Lu, J. Effects of laser shock peening on the hot corrosion behaviour of the selective laser melted Ti6Al4V titanium alloy. Corros. Sci. 2021, 188, 109558. [Google Scholar] [CrossRef]
- Balla, V.K.; Soderlind, J.; Bose, S.; Bandyopadhyay, A. Microstructure, mechanical and wear properties of laser surface melted Ti6Al4V alloy. J. Mech. Behav. Biomed. Mater. 2014, 32, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Molinari, A.; Straffelini, G.; Tesi, B.; Bacci, T. Dry sliding wear mechanisms of the Ti6A14V alloy. Wear 1997, 208, 105–112. [Google Scholar] [CrossRef]
- Gül, O. Plazma Nitrürlenmiş Ti-6Al-4V Alaşımının Adhezif Aşınma Özelliklerinin İncelenmesi. Master’s Thesis, Kocaeli Üniversitesi, İzmit, Türkiye, 2013. [Google Scholar]
- Memu, F. Katmanlı İmalat Yöntemiyle Üretilmiş Ti-6Al-4V Alaşımının Mekanik Özelliklerinin İncelenmesi. Master’s Thesis, TOBB University of Economics and Technology, Ankara, Türkiye, 2019. [Google Scholar]
- Bertolini, R.; Lizzul, L.; Pezzato, L.; Ghiotti, A.; Bruschi, S. Improving surface integrity and corrosion resistance of additive manufactured Ti6Al4V alloy by cryogenic machining. Int. J. Adv. Manuf. Technol. 2019, 104, 2839–2850. [Google Scholar] [CrossRef]
- Adebiyi, D.I.; Popoola, A.P.I. Mitigation of abrasive wear damage of Ti-6Al-4V by laser surface alloying. Mater. Des. 2015, 74, 67–75. [Google Scholar] [CrossRef]
- Johns, S.M.; Bell, T.; Samandi, M.; Collins, G.A. Wear resistance of plasma immersion ion implanted Ti6A14V. Surf. Coatings Technol. 1996, 85, 7–14. [Google Scholar] [CrossRef]
- Singh, R.; Kurella, A.; Dahotre, N.B. Laser surface modification of Ti-6Al-4V: Wear and corrosion characterization in simulated biofluid. J. Biomater. Appl. 2006, 21, 49–73. [Google Scholar] [CrossRef] [PubMed]
- Şahin, F. Akımsız Nikel- Fosfor Kaplanan Ti-6Al-4V Alaşımının Aşınma Davranışının İncelenmesi. Master’s Thesis, Eskişehir Osmangazi Üniversitesi, Eskişehir, Turkey, 2016. [Google Scholar]
- Güneşsu, E. Makine Mühendisliği Anabilim Dalı Seçici Lazer Ergitme Yöntemi İLE Üretilen Ti-6Al-4V Malzemelerinin Sürüklenerek Yüzeylerinin İyileştirilmesi VE aşınma Karakteristiklerinin İncelenmesi. Master’s Thesis, Marmara Üniversitesi Fen Bilimleri Enstitüsü, Istanbul, Türkiye, 2020. [Google Scholar]
- Zivic, F.; Babic, M.; Mitrovic, S.; Vencl, A. Continuous control as alternative route for wear monitoring by measuring penetration depth during linear reciprocating sliding of Ti6Al4V alloy. J. Alloys Compd. 2011, 509, 5748–5754. [Google Scholar] [CrossRef]
- BARTOLOMEU, F.; BUCIUMEANU, M.; PINTO, E.; ALVES, N.; SILVA, F.S.; CARVALHO, O.; MIRANDA, G. Wear behavior of Ti6Al4V biomedical alloys processed by selective laser melting, hot pressing and conventional casting. Trans. Nonferrous Met. Soc. China 2017, 27, 829–838. [Google Scholar] [CrossRef]
- Gündüz, S. Titanyum Ve Alaşımında (Tİ6AL4V) Oluşturulan Oksitlerin Yüzey Gerilimi Ve Korozyon Davranışlarının Değişimi. Master’s Thesis, Çukurova Üniversitesi, Adana, Turkey, 2017. [Google Scholar]
- Elements of Metallurgy and Engineering Alloys; Campbell, F.C., Ed.; ASM International: Almere, The Netherlands, 2008; ISBN 978-1-62708-251-8. [Google Scholar]
- Erhaıma, M.E.E. An Investıgatıon of The Effect of Mn And Zr Addıtıon on the Mıcrostructure, Mechanıcal and Corrosıon Propertıes of TI6AL4V Tıtanıum Alloy. Master’s Thesis, Karabuk University, Karabük, Turkey, 2020. [Google Scholar]
- Cheng, X.Y.; Li, S.J.; Murr, L.E.; Zhang, Z.B.; Hao, Y.L.; Yang, R.; Medina, F.; Wicker, R.B. Compression deformation behavior of Ti-6Al-4V alloy with cellular structures fabricated by electron beam melting. J. Mech. Behav. Biomed. Mater. 2012, 16, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Elhasslouk, M.M.M.; Esen, İ.; Ahlatcı, H.; Akın, B. Effect of a 3.5% NaCl−10% HCl Corrosive Environment on the Fatigue Behavior of Hot Rolled Aluminum 5083-H111. Materials 2023, 16, 4996. [Google Scholar] [CrossRef]
- Şimşek, İ. Toz Metaluürjisi Ile Üretilen Titanyum Alaşımı Biyomalzemelerin Korozyon Ve Aşınma Davranışlarının Incelenmesi. Doctoral Thesis, Karabük Üniversitesi Fen Bilim, Enstitüsü İmalat Mühendisliği Anabilim Dalı, Karabük, Turkey, 2017. [Google Scholar]
- Bocchetta, P.; Chen, L.Y.; Tardelli, J.D.C.; Dos Reis, A.C.; Almeraya-Calderón, F.; Leo, P. Passive layers and corrosion resistance of biomedical ti-6al-4v and β-ti alloys. Coatings 2021, 11, 487. [Google Scholar] [CrossRef]
- Yılmaz, Y. Biyomalzeme Olarak Kullanılan Titanyum Alaşımlarının Mekanik Davranışları ve Korozyon Özellikleri Üzerine Isıl İşlemin Etkilerinin İncelenmesi. Doctoral Dissertation, Fırat Üniversitesi, Elazığ, Turkey, 2019. [Google Scholar]
- Gerhátová, Ž.; Babincová, P.; Drienovský, M.; Pašák, M.; Černičková, I.; Ďuriška, L.; Havlík, R.; Palcut, M. Microstructure and Corrosion Behavior of Sn–Zn Alloys. Materials 2022, 15, 7210. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Z.; Xie, J.; Ma, J.; Zhang, G.; Suganuma, K. Understanding corrosion mechanism of Sn—Zn alloys in NaCl solution via corrosion products characterization. Mater. Corros. 2016, 67, 522–530. [Google Scholar] [CrossRef]
- Liu, J.; Park, S.; Nagao, S.; Nogi, M.; Koga, H.; Ma, J. The role of Zn precipitates and Cl À anions in pitting corrosion of Sn—Zn solder alloys. Corros. Sci. 2014, 92, 263–271. [Google Scholar] [CrossRef]
- Fromhold, A.T., Jr.; Noh, S.J. The transport of ions and electrons through microscopically inhomogeneous passive films: Breakdown implications. Corros. Sci. 1989, 29, 237–255. [Google Scholar] [CrossRef]
- Yang, J.; Song, Y.; Dong, K.; Han, E. Research progress on the corrosion behavior of titanium alloys. Corros. Rev. 2023, 41, 5–20. [Google Scholar] [CrossRef]
- Lu, J.; Ge, P.; Li, Q.; Zhang, W.; Huo, W.; Hu, J.; Zhang, Y.; Zhao, Y. Effect of microstructure characteristic on mechanical properties and corrosion behavior of new high strength Ti-1300 beta titanium alloy. J. Alloys Compd. 2017, 727, 1126–1135. [Google Scholar] [CrossRef]
- Bodunrin, M.O.; Chown, L.H.; van der Merwe, J.W.; Alaneme, K.K.; Oganbule, C.; Klenam, D.E.P.; Mphasha, N.P. Corrosion behavior of titanium alloys in acidic and saline media: Role of alloy design, passivation integrity, and electrolyte modification. Corros. Rev. 2020, 38, 25–47. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).