Design of an Azopolymer for Photo-Switchable Adhesive Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Azopolymer Synthesis
2.2. Azopolymer Coating
2.3. Atomic Force Microscopy (AFM)
2.4. Ellipsometry
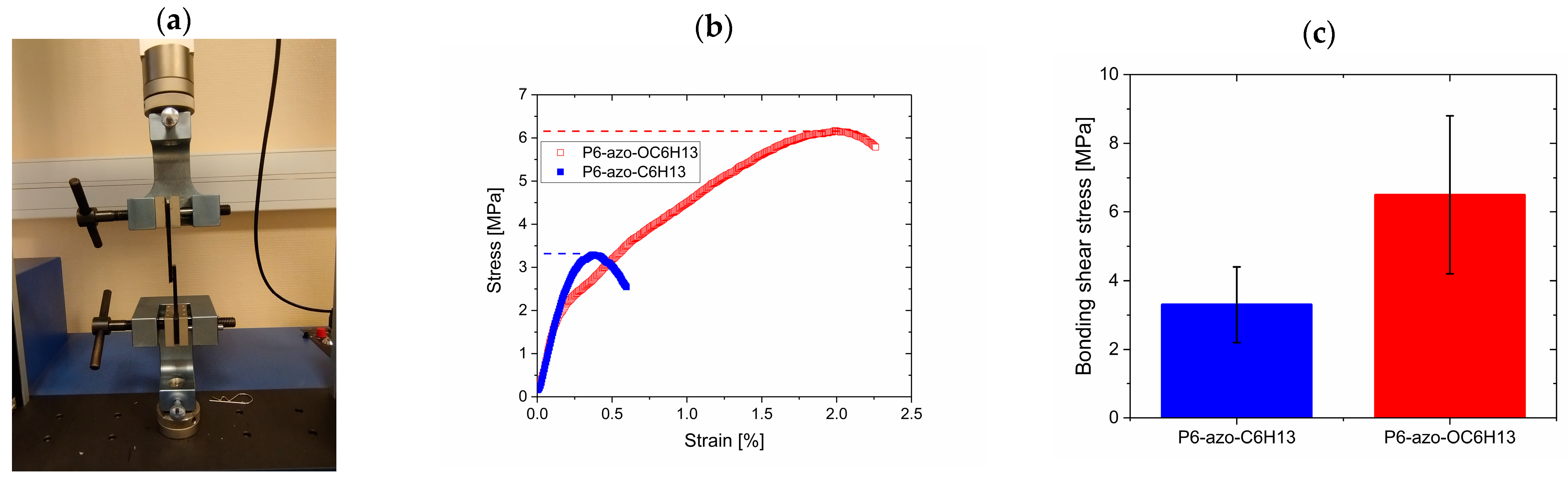
2.5. Adhesion Strength Measurement
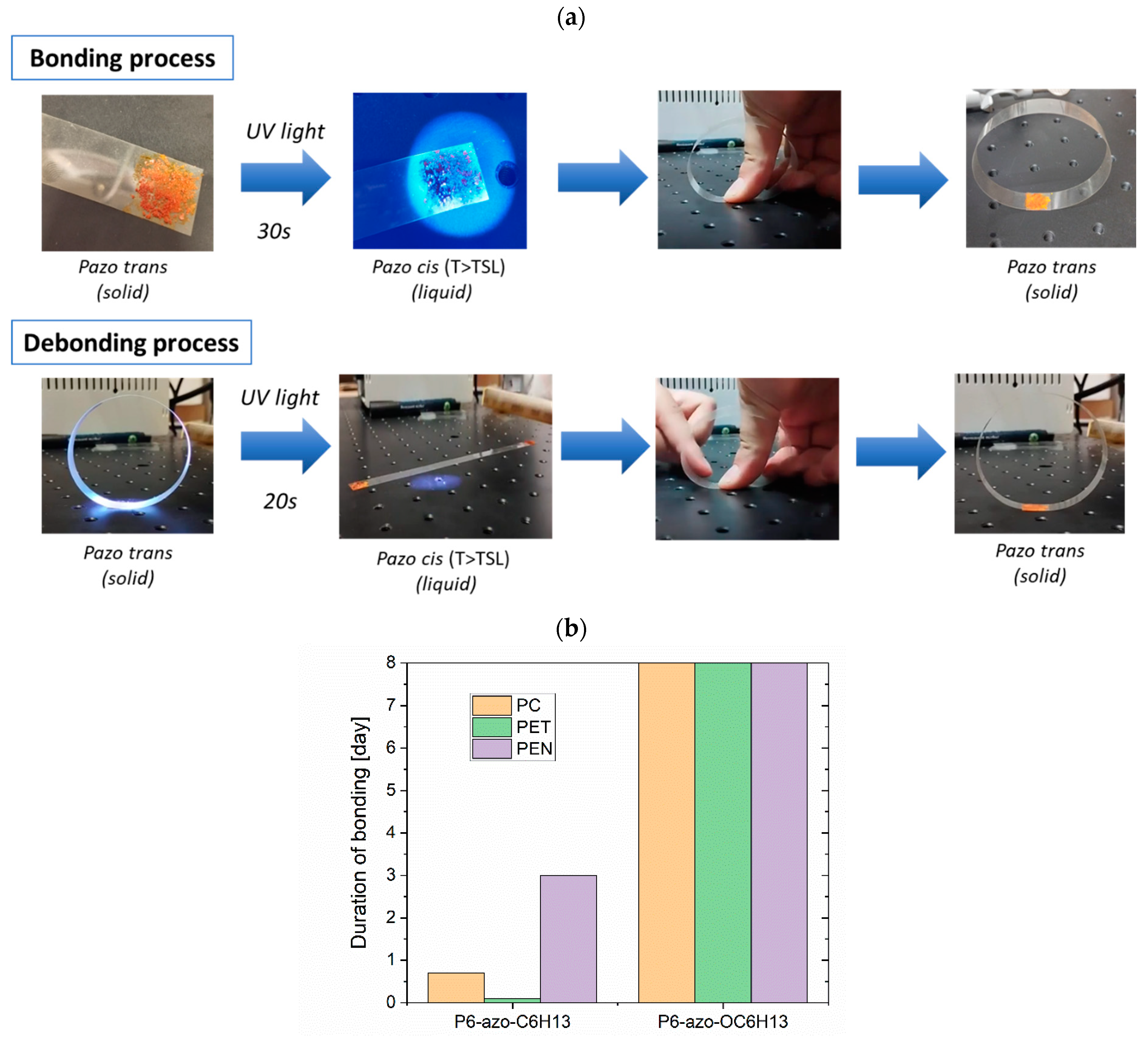
2.6. Reversible Bonding–Debonding Experiment
3. Results
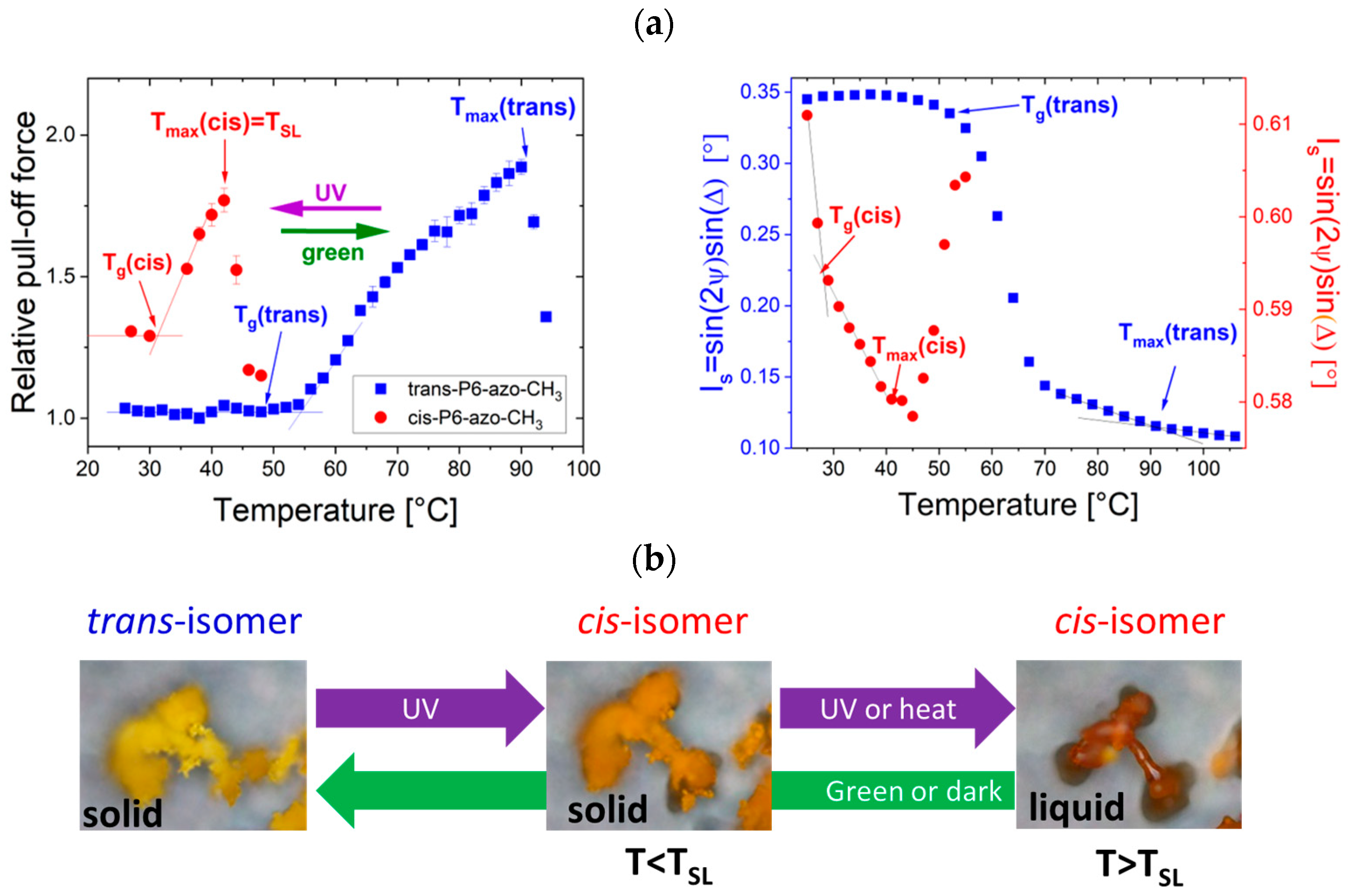
3.1. Measurement of Tg and TSL
3.2. Influence of Azopolymer Chain Length
3.3. Influence of the Alkyl Length Ligand
3.4. Influence of the Substituent Nature
3.5. Photo-Switchable Adhesive
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Z.; Yan, F. Switchable Adhesion: On-Demand Bonding and Debonding. Adv. Sci. 2022, 9, 2200264. [Google Scholar] [CrossRef] [PubMed]
- Benedek, I. Pressure-Sensitive Adhesives and Applications; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar] [CrossRef]
- Zhou, H.; Xue, C.; Weis, P.; Suzuki, Y.; Huang, S.; Koynov, K.; Auernhammer, G.K.; Berger, R.; Butt, H.-J. Photoswitching of glass transition temperatures of azobenzene-containing polymers induces reversible solid-to-liquid transitions. Nat. Chem. 2017, 9, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Yamashita, A.; Akiyama, H.; Kihara, H.; Yoshida, M. Azobenzene-Based (Meth)acrylates: Controlled Radical Polymerization, Photoresponsive Solid–Liquid Phase Transition Behavior, and Application to Reworkable Adhesives. Macromolecules 2018, 51, 3243–3253. [Google Scholar] [CrossRef]
- Akiyama, H.; Fukata, T.; Yamashita, A.; Yoshida, M.; Kihara, H. Reworkable adhesives composed of photoresponsive azobenzene polymer for glass substrates. J. Adhes. 2017, 93, 823–830. [Google Scholar] [CrossRef]
- Ohzono, T.; Saed, M.O.; Terentjev, E.M. Enhanced Dynamic Adhesion in Nematic Liquid Crystal Elastomers. Adv. Mater. 2019, 31, 1902642. [Google Scholar] [CrossRef]
- Koike, M.; Aizawa, M.; Minamikawa, H.; Shishido, A.; Yamamoto, T. Photohardenable Pressure-Sensitive Adhesives using Poly(methyl methacrylate) containing Liquid Crystal Plasticizers. ACS Appl. Mater. Interfaces 2021, 13, 39949–39956. [Google Scholar] [CrossRef]
- Lee, T.-H.; Han, G.-Y.; Yi, M.-B.; Kim, H.-J.; Lee, J.-H.; Kim, S. Rapid Photoresponsive Switchable Pressure-Sensitive Adhesive Containing Azobenzene for the Mini-Light Emitting Diode Transfer Process. ACS Appl. Mater. Interfaces 2021, 13, 43364–43373. [Google Scholar] [CrossRef]
- Zhang, P.; Cai, F.; Wang, W.; Wang, G.; Yu, H. Light-Switchable Adhesion of Azobenzene-Containing Siloxane-Based Tough Adhesive. ACS Appl. Polym. Mater. 2021, 3, 2325–2329. [Google Scholar] [CrossRef]
- Imato, K.; Momota, K.; Kaneda, N.; Imae, I.; Ooyama, Y. Photoswitchable Adhesives of Spiropyran Polymers. Chem. Mater. 2022, 34, 8289–8296. [Google Scholar] [CrossRef]
- Zhao, R.; Mu, J.; Bai, J.; Zhao, W.; Gong, P.; Chen, L.; Zhang, N.; Shang, X.; Liu, F.; Yan, S. Smart Responsive Azo-Copolymer with Photoliquefaction for Switchable Adhesive Application. ACS Appl. Mater. Interfaces 2022, 14, 16678–16686. [Google Scholar] [CrossRef]
- Hu, J.; Song, T.; Yu, M.-M.; Yu, H. Optically Controlled Solid-to-Liquid Phase Transition Materials Based on Azo Compounds. Chem. Mater. 2023, 35, 4621–4648. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Q.-Y.; Lu, X.-B. Azopolyesters with Intrinsic Crystallinity and Photoswitchable Reversible Solid-to-Liquid Transitions. Angew. Chem. Int. Ed. 2023, 62, e202311158. [Google Scholar] [CrossRef] [PubMed]
- Shang, C.; Xiong, Z.; Liu, S.; Yu, W. Molecular Dynamics of Azobenzene Polymer with Photoreversible Glass Transition. Macromolecules 2022, 55, 3711–3722. [Google Scholar] [CrossRef]
- Yang, B.; Cai, F.; Huang, S.; Yu, H. Athermal and Soft Multi-Nanopatterning of Azopolymers: Phototunable Mechanical Properties. Angew. Chem. Int. Ed. 2020, 59, 4035–4042. [Google Scholar] [CrossRef] [PubMed]
- Pessoni, L.; Siniscalco, D.; Boussonnière, A.; Castanet, A.-S.; Billon, L.; Delorme, N. Photo-reversible solid to liquid transition of azobenzene containing polymers: Impact of the chemical structure and chain length. Eur. Polym. J. 2022, 174, 111297. [Google Scholar] [CrossRef]
- Siniscalco, D.; Pessoni, L.; Billon, L.; Boussonnière, A.; Castanet, A.-S.; Bardeau, J.-F.; Nickmilder, P.; Leclère, P.; Delorme, N. Measurement of the Transition Temperature Governing the Photoinduced Reversible Solid-to-Liquid Transition of Azobenzene-Containing Polymers. ACS Appl. Polym. Mater. 2023, 5, 7358–7363. [Google Scholar] [CrossRef]
- Hermanowicz, P.; Sarna, M.; Burda, K.; Gabryś, H. AtomicJ: An open source software for analysis of force curves. Rev. Sci. Instrum. 2014, 85, 063703. [Google Scholar] [CrossRef]
- El Ouakili, A.; Vignaud, G.; Balnois, E.; Bardeau, J.-F.; Grohens, Y. Multiple glass transition temperatures of polymer thin films as probed by multi-wavelength ellipsometry. Thin Solid Film 2011, 519, 2031–2036. [Google Scholar] [CrossRef]
- Delorme, N.; Chebil, M.S.; Vignaud, G.; Le Houerou, V.; Bardeau, J.F.; Busselez, R.; Gibaud, A.; Grohens, Y. Experimental evidence of ultrathin polymer film stratification by AFM force spectroscopy. Eur. Phys. J. E 2015, 38, 56. [Google Scholar] [CrossRef]
- Chen, M.; Yao, B.; Kappl, M.; Liu, S.; Yuan, J.; Berger, R.; Zhang, F.; Butt, H.-J.; Liu, Y.; Wu, S. Entangled Azobenzene-Containing Polymers with Photoinduced Reversible Solid-to-Liquid Transitions for Healable and Reprocessable Photoactuators. Adv. Funct. Mater. 2020, 30, 1906752. [Google Scholar] [CrossRef]
- Fox, T.G.; Loshaek, S. Influence of molecular weight and degree of crosslinking on the specific volume and glass temperature of polymers. J. Polym. Sci. 1955, 15, 371–390. [Google Scholar] [CrossRef]
- Weis, P.; Hess, A.; Kircher, G.; Huang, S.; Auernhammer, G.K.; Koynov, K.; Butt, H.-J.; Wu, S. Effects of Spacers on Photoinduced Reversible Solid-to-Liquid Transitions of Azobenzene-Containing Polymers. Chem. Eur. J. 2019, 25, 10946–10953. [Google Scholar] [CrossRef]
- Liang, S.; Li, S.; Yuan, C.; Zhang, D.; Chen, J.; Wu, S. Polyacrylate Backbone Promotes Photoinduced Reversible Solid-To-Liquid Transitions of Azobenzene-Containing Polymers. Macromolecules 2023, 56, 448–456. [Google Scholar] [CrossRef]
- Li, T.; Li, H.; Wang, H.; Lu, W.; Osa, M.; Wang, Y.; Mays, J.; Hong, K. Chain flexibility and glass transition temperatures of poly(n-alkyl (meth)acrylate)s: Implications of tacticity and chain dynamics. Polymer 2021, 213, 123207. [Google Scholar] [CrossRef]
- Velde, C.V.; Bultinck, E.; Tersago, K.; Alsenoy, C.V.; Blockhuys, F. From anisole to 1,2,4,5-tetramethoxybenzene: Theoretical study of the factors that determine the conformation of methoxy groups on a benzene ring. Int. J. Quantum Chem. 2007, 107, 670–679. [Google Scholar] [CrossRef]
- Arakawa, Y.; Ishida, Y.; Shiba, T.; Igawa, K.; Sasaki, S.; Tsuji, H. Effects of alkylthio groups on phase transitions of organic molecules and liquid crystals: A comparative study with alkyl and alkoxy groups. CrystEngComm 2022, 24, 1877–1890. [Google Scholar] [CrossRef]
- Li, W.; Bouzidi, L.; Narine, S.S. Current Research and Development Status and Prospect of Hot-Melt Adhesives: A Review. Ind. Eng. Chem. Res. 2008, 47, 7524–7532. [Google Scholar] [CrossRef]
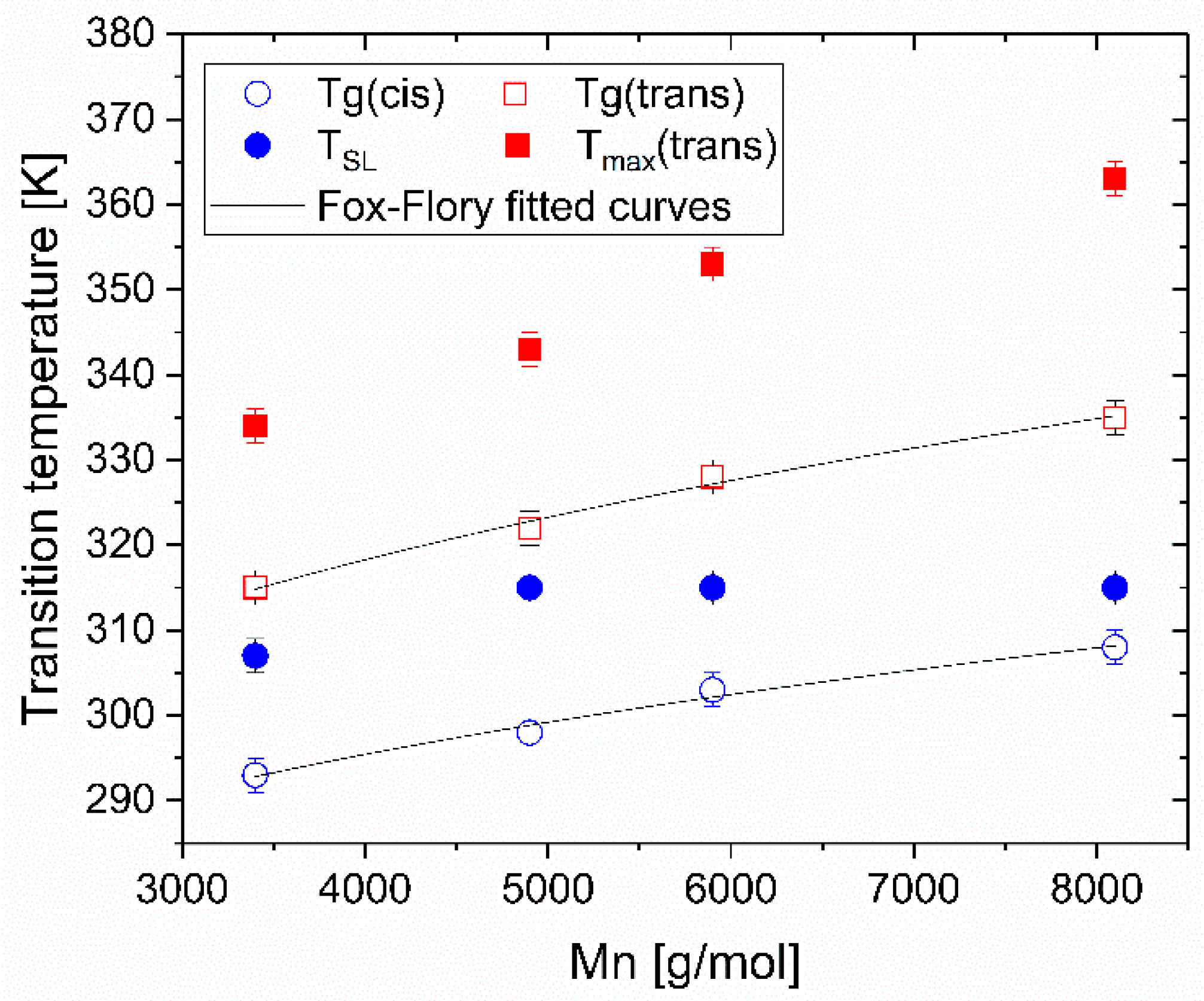
| Formula | R1 | R2 | Name | Mn (g/mol) | DP(1) | Conversion (%) |
|---|---|---|---|---|---|---|
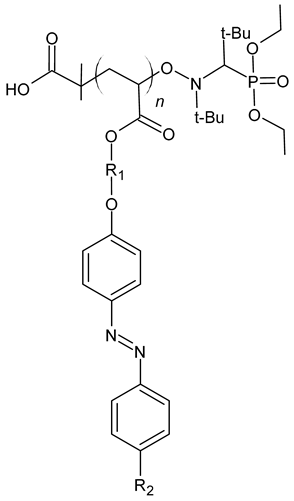 | C6H12 | CH3 | P6-azo-CH3 | 3400 | 7 | 93 |
| 4900 | 10 | 96 | ||||
| 5900 | 16 | 96 | ||||
| 8100 | 27 | 95 | ||||
| C10H20 | CH3 | P10-azo-CH3 | 6000 | 10 | 92 | |
| 11,400 | 27 | 97 | ||||
| C6H12 | C6H13 | P6-azo-C6H13 | 7100 | 10 | 100 | |
| C6H12 | O-C6H13 | P6-azo-OC6H13 | 7400 | 10 | 100 |
| Tg (°C) | Tmax (°C) | ||
|---|---|---|---|
| P6-azo-CH3 Mn=5900 g/mol | Trans | 52 | 80 |
| Cis | 30 | 42 (TSL) | |
| P10-azo-CH3 Mn=6000 g/mol | Trans | 52 | 75 |
| Cis | 0 | 15 (TSL) | |
| P6-azo-CH3 Mn=8100 g/mol | Trans | 54 | 90 |
| Cis | 32 | 42 (TSL) | |
| P10-azo-CH3 Mn=11400 g/mol | Trans | 60 | 85 |
| Cis | 47 | 60 (TSL) | |
| Tg (°C) | Tmax (°C) | ||
|---|---|---|---|
| P6-azo-CH3 Mn=8100 g/mol | Trans | 54 | 90 |
| Cis | 32 | 42 (TSL) | |
| P6-azo-C6H13 Mn=7100 g/mol | Trans | 33 | 57 |
| Cis | <−10 | 0 (TSL) | |
| P6-azo-OC6H13 Mn=7400 g/mol | Trans | 45 | 87 |
| Cis | <20 | 30 (TSL) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siniscalco, D.; Pessoni, L.; Boussonnière, A.; Castanet, A.-S.; Billon, L.; Vignaud, G.; Delorme, N. Design of an Azopolymer for Photo-Switchable Adhesive Applications. Coatings 2024, 14, 275. https://doi.org/10.3390/coatings14030275
Siniscalco D, Pessoni L, Boussonnière A, Castanet A-S, Billon L, Vignaud G, Delorme N. Design of an Azopolymer for Photo-Switchable Adhesive Applications. Coatings. 2024; 14(3):275. https://doi.org/10.3390/coatings14030275
Chicago/Turabian StyleSiniscalco, David, Laurence Pessoni, Anne Boussonnière, Anne-Sophie Castanet, Laurent Billon, Guillaume Vignaud, and Nicolas Delorme. 2024. "Design of an Azopolymer for Photo-Switchable Adhesive Applications" Coatings 14, no. 3: 275. https://doi.org/10.3390/coatings14030275
APA StyleSiniscalco, D., Pessoni, L., Boussonnière, A., Castanet, A.-S., Billon, L., Vignaud, G., & Delorme, N. (2024). Design of an Azopolymer for Photo-Switchable Adhesive Applications. Coatings, 14(3), 275. https://doi.org/10.3390/coatings14030275








