Biocompatibility and Corrosion of Microplasma-Sprayed Titanium and Tantalum Coatings versus Titanium Alloy
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Surface Treatment of Ti6Al4V
2.2.1. Gas Abrasive Surface Treatment
2.2.2. Microplasma Spraying of Ti and Ta Coatings
2.3. Coating Microstructure Characterization
2.4. Adhesion Strength Test
2.5. Surface Roughness Assessment
2.6. Corrosion Test
2.7. Cell Viability
2.8. Cell Proliferation
2.9. Alkaline Phosphatase Activity
2.10. Alizarin Red S Staining
2.11. Scanning Electron Microscopy (SEM) Sample Preparation
2.12. Statistical Analysis
3. Results and Discussion
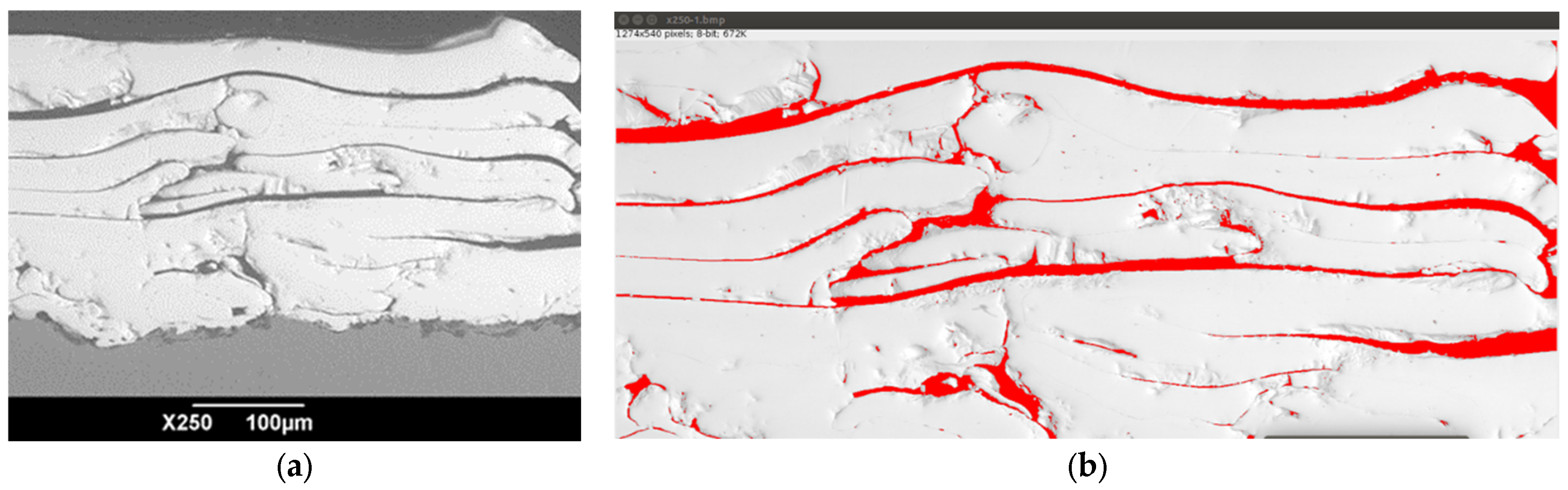
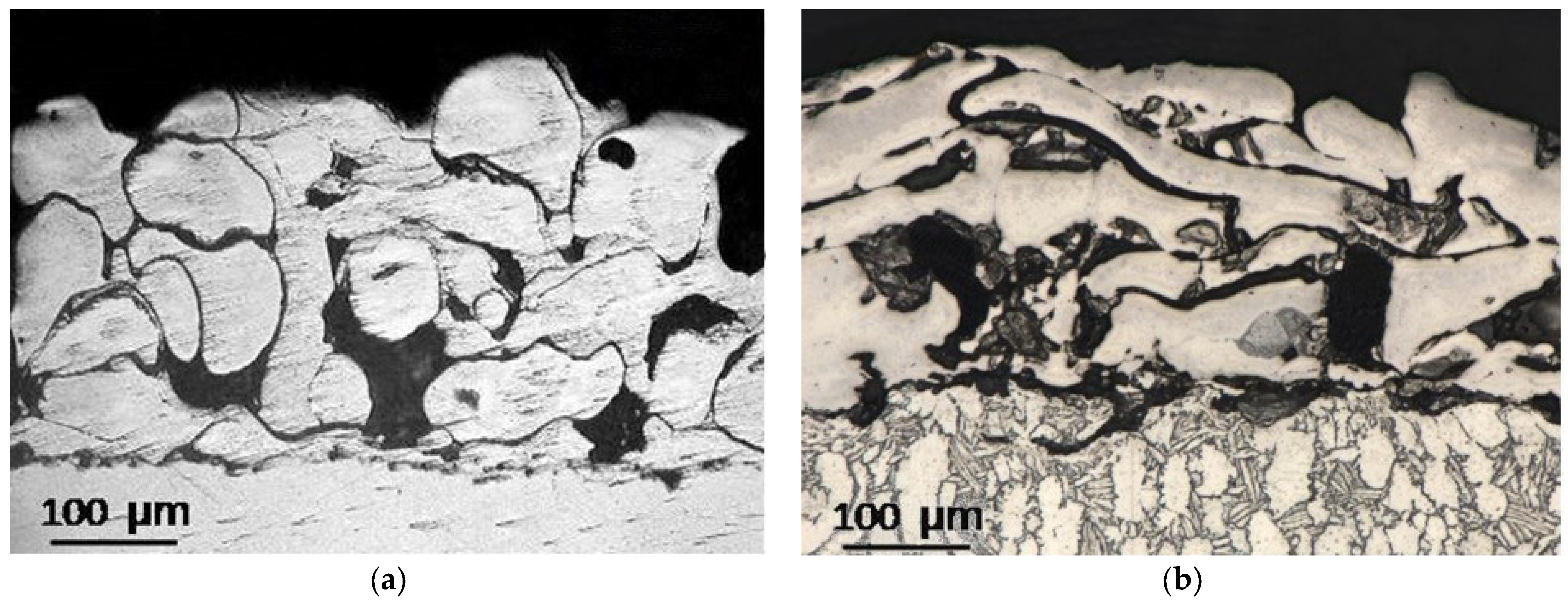
3.1. Coating Microstructure Characterization
3.2. Adhesion Strength
3.3. Surface Roughness
- Group 1 involves scenarios where particles are fully melted before reaching the substrate. This results in the formation of dense coatings with an average porosity below 4%.
- Group 2 describes situations where particles are partially solidified as they approach the substrate, along with fully molten particles. The resulting coating structure is typically porous, with an average porosity of around 8%.
- Group 3 encompasses coatings created from particles that have begun to solidify and are moving at a slower speed upon reaching the substrate. These conditions lead to the formation of coatings with the highest average porosity, approximately 20%, and feature large pores ranging from 20 μm to 200 μm in size. Coatings with such pore sizes in endoprostheses can facilitate the in-growth of blood vessels into the coating’s pores. This process is beneficial for bone tissue formation and nourishment, thereby improving the fixation and osseointegration of the endoprosthesis within the human body.
3.4. Corrosion Analysis
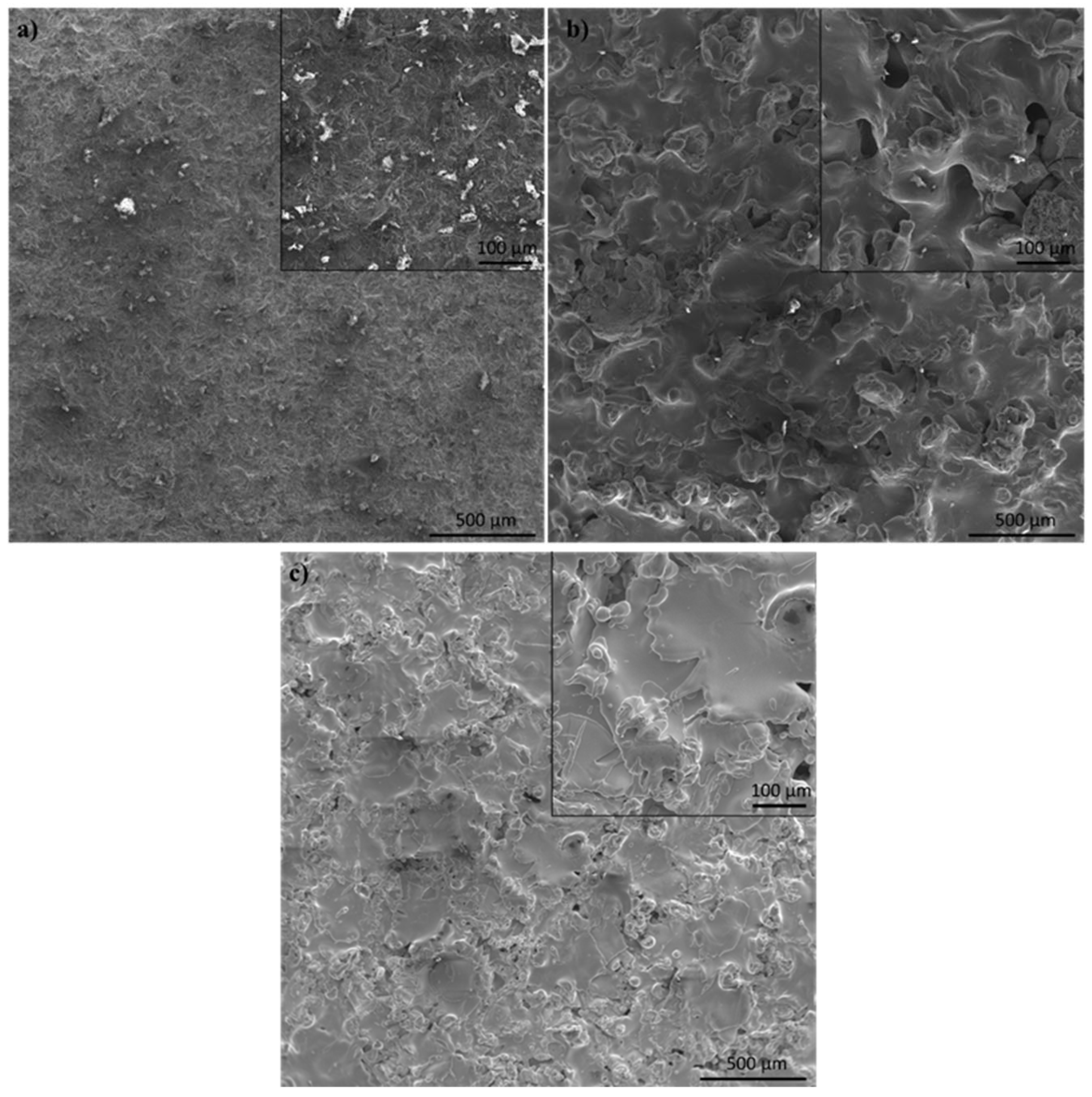
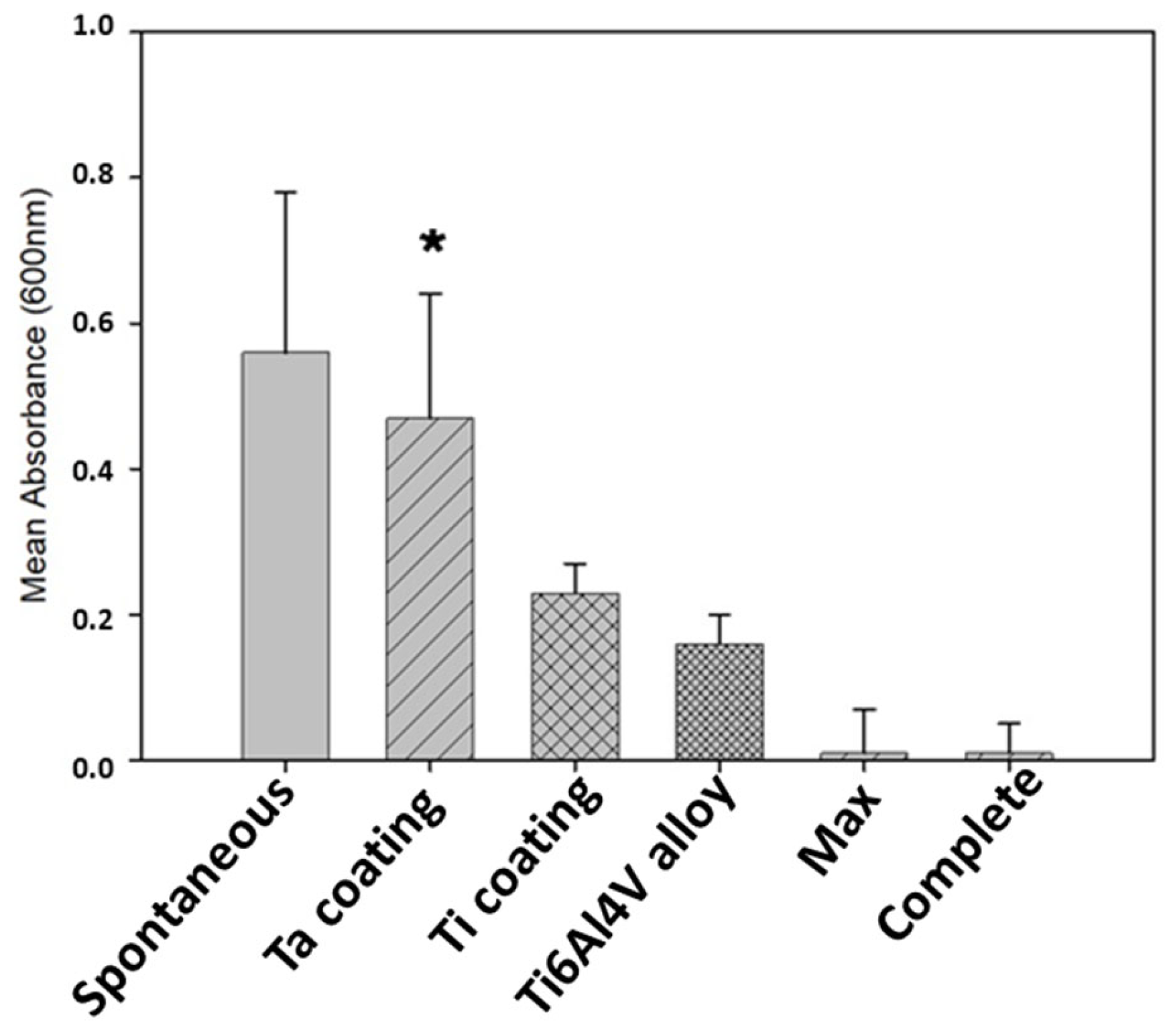
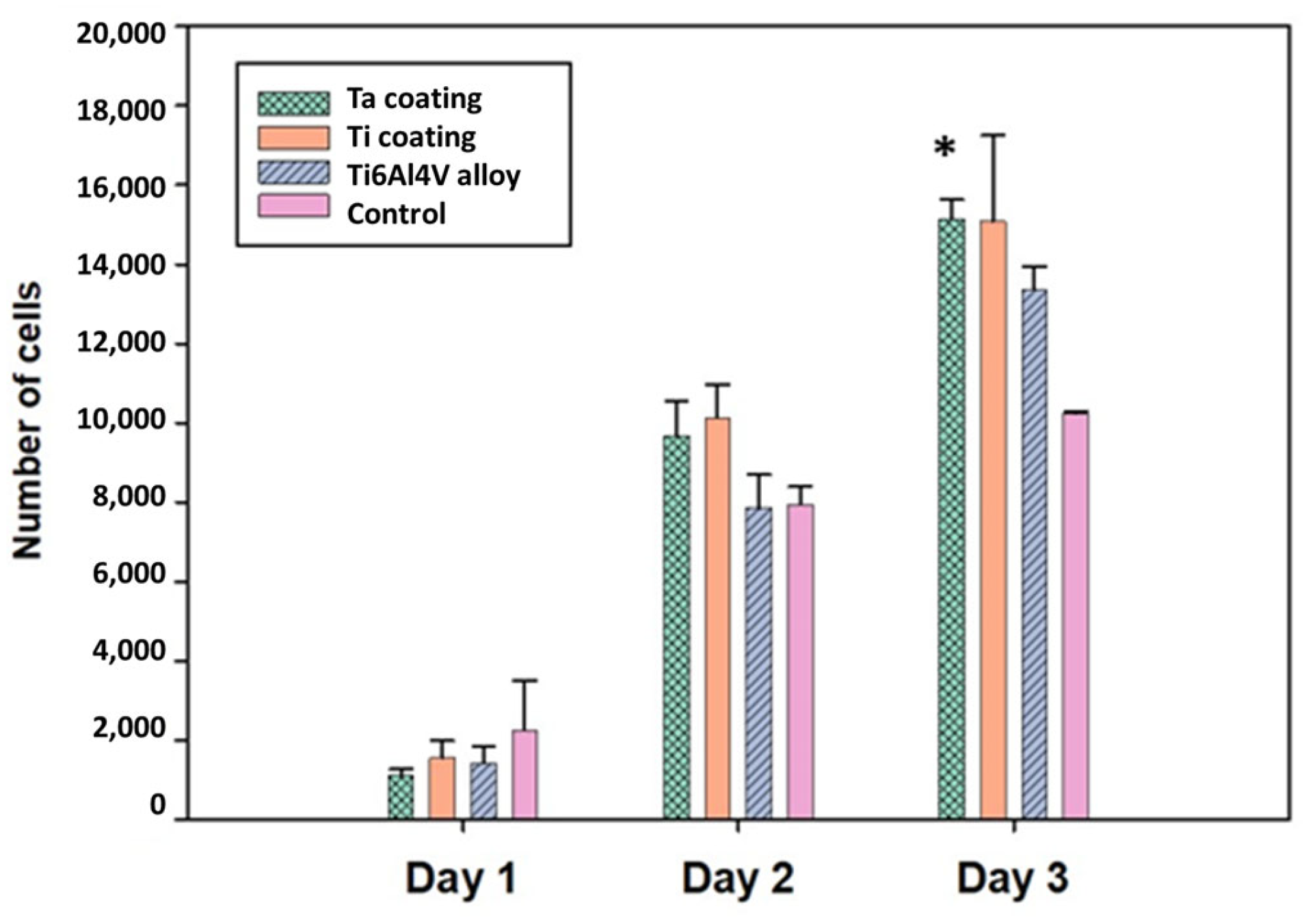
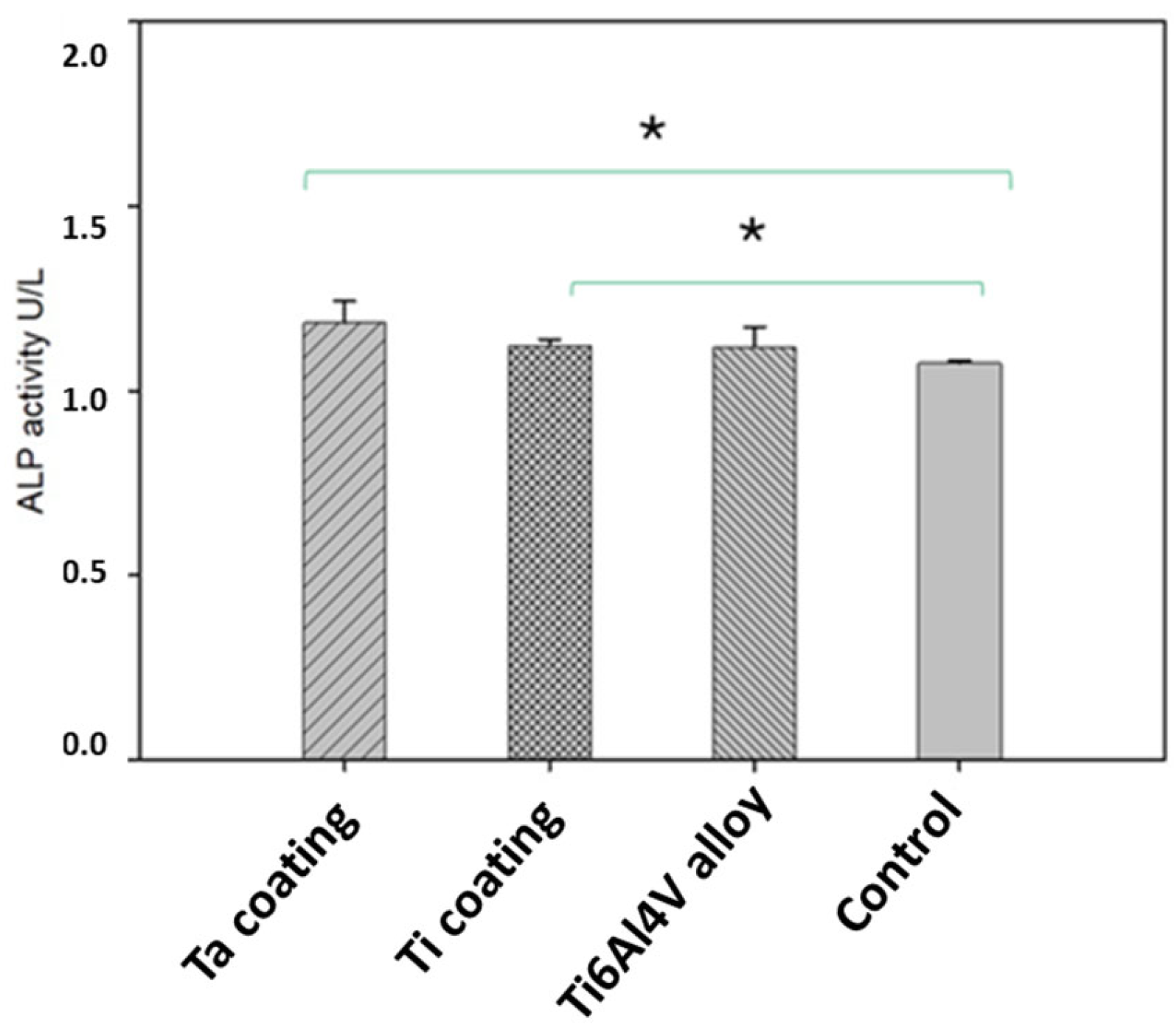
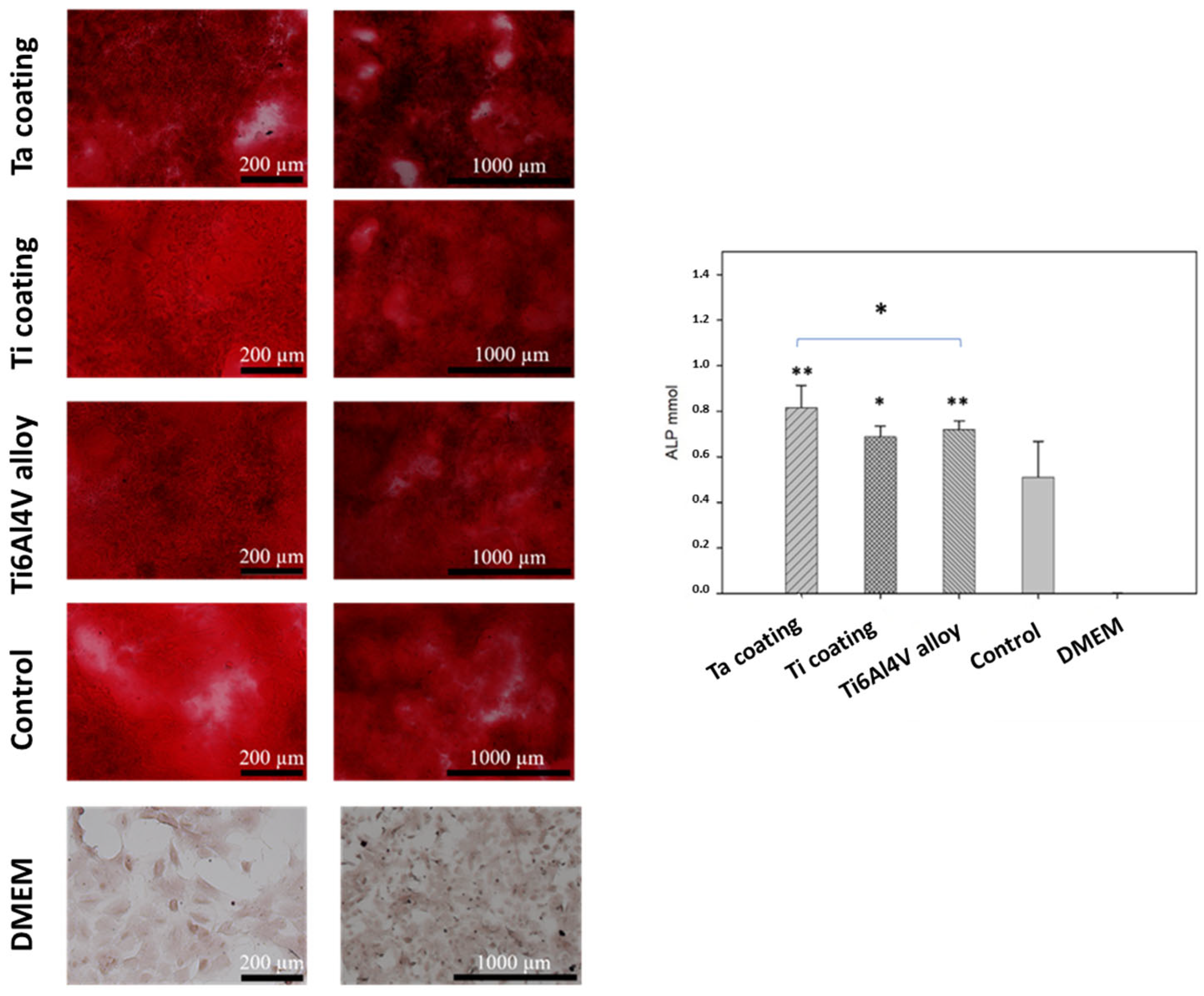
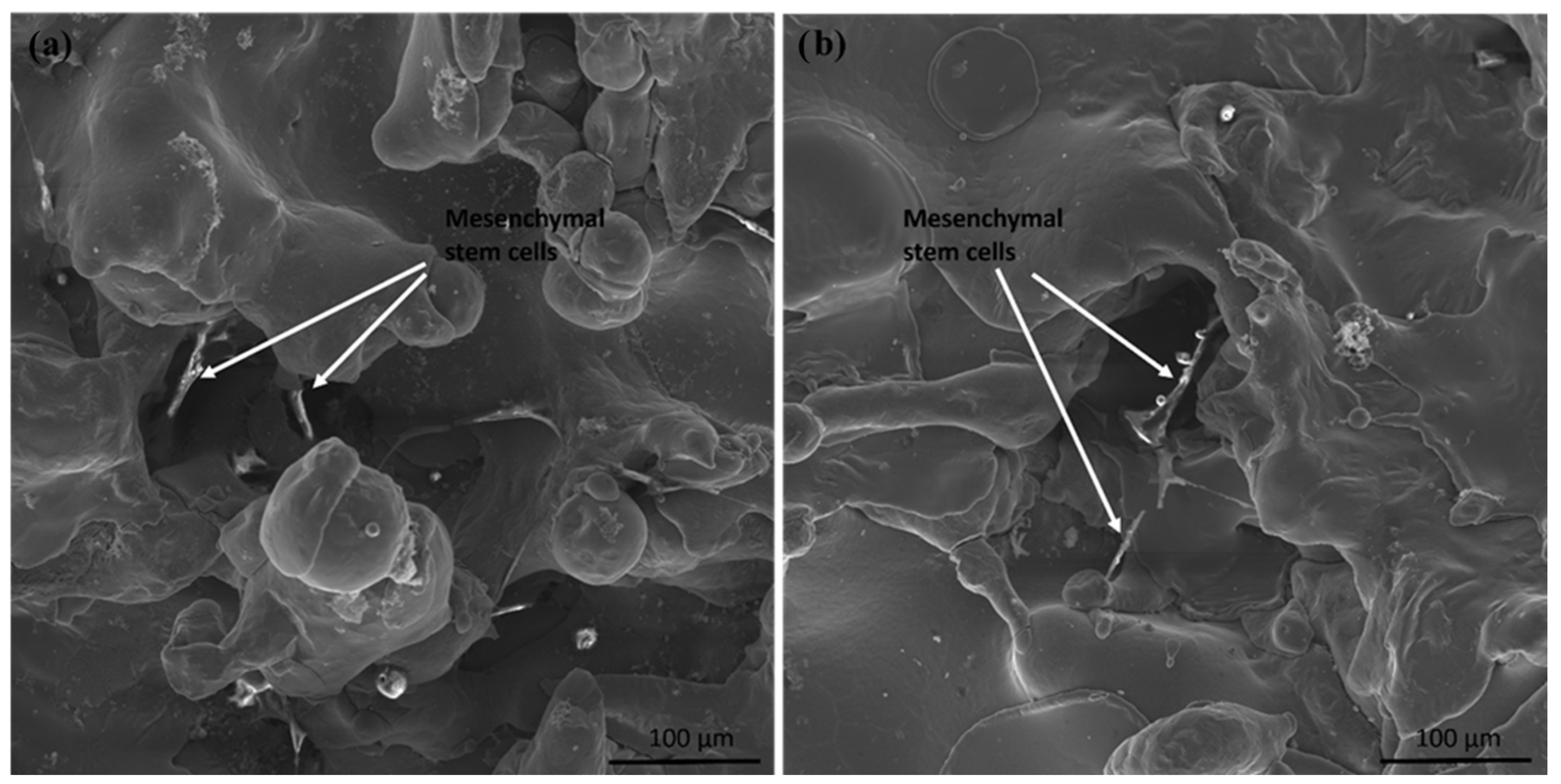
3.5. In Vitro Tests
4. Further Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stich, T.; Alagboso, F.; Křenek, T.; Kovářík, T.; Alt, V.; Docheva, D. Implant-bone-interface: Reviewing the impact of titanium surface modifications on osteogenic processes in vitro and in vivo. Bioeng. Transl. Med. 2022, 7, e10239. [Google Scholar] [CrossRef]
- Pandey, A.; Awasthi, A.; Saxena, K.K. Metallic implants with properties and latest production techniques: A review. Adv. Mater. Process. Technol. 2020, 6, 405–440. [Google Scholar] [CrossRef]
- Vishwakarma, V.; Kaliaraj, G.S.; Amirtharaj Mosas, K.K. Multifunctional Coatings on Implant Materials—A Systematic Review of the Current Scenario. Coatings 2023, 13, 69. [Google Scholar] [CrossRef]
- Alontseva, D.; Azamatov, B.; Safarova, Y.; Voinarovych, S.; Nazenova, G. A Brief Review of Current Trends in the Additive Manufacturing of Orthopedic Implants with Thermal Plasma-Sprayed Coatings to Improve the Implant Surface Biocompatibility. Coatings 2023, 13, 1175. [Google Scholar] [CrossRef]
- Saini, M.; Singh, Y.; Arora, P.; Arora, V.; Jain, K. Implant biomaterials: A comprehensive review. World J. Clin. Cases 2015, 3, 52–57. [Google Scholar] [CrossRef]
- Wilson, J. 1—Metallic biomaterials: State of the art and new challenges. In Fundamental Biomaterials: Metals; Balakrishnan, P., Sreekala, M.S., Thomas, S., Eds.; Woodhead Publishing: Cambridge, UK, 2018; pp. 1–33. [Google Scholar] [CrossRef]
- Eliaz, N. Corrosion of Metallic Biomaterials: A Review. Materials 2019, 12, 407. [Google Scholar] [CrossRef]
- Yavuz, H.I.; Yamanoglu, R. β tipi Ti alaşımlarının özellikleri üzerine bir derleme: Mikroyapı, mekanik, korozyon özellikleri ve üretim yöntemleri. Politek. Derg. 2023, 2023, 1. [Google Scholar] [CrossRef]
- Ali, S.; Abdul Rani, A.M.; Baig, Z.; Ahmed, S.W.; Hussain, G.; Subramaniam, K.; Rao, T.V. Biocompatibility and corrosion resistance of metallic biomaterials. Corros. Rev. 2020, 38, 381–402. [Google Scholar] [CrossRef]
- Nicholson, W.; Titanium, J. Alloys for Dental Implants: A Review. Prosthesis 2020, 2, 100–116. [Google Scholar] [CrossRef]
- Tang, Z.; Xie, Y.; Yang, F.; Huang, Y.; Wang, C.; Dai, K.; Zheng, X.; Zhang, X. Porous tantalum coatings prepared by vacuum plasma spraying enhance bmscs osteogenic differentiation and bone regeneration in vitro and in vivo. PLoS ONE 2013, 8, e66263. [Google Scholar] [CrossRef]
- Cui, J.; Zhang, S.; Huang, M.; Mu, X.; Hei, J.; Yau, V.; He, H. Micro-nano porous structured tantalum-coated dental implants promote osteogenic activity in vitro and enhance osseointegration in vivo. J. Biomed. Mater. Res. A 2023, 111, 1358–1371. [Google Scholar] [CrossRef]
- Ren, B.; Wan, Y.; Liu, C.; Wang, H.; Yu, M.; Zhang, X.; Huang, Y. Improved osseointegration of 3D printed Ti-6Al-4V implant with a hierarchical micro/nano surface topography: An in vitro and in vivo study. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 118, 111505. [Google Scholar] [CrossRef]
- Lu, R.-J.; Wang, X.; He, H.-X.; E, L.-L.; Li, Y.; Zhang, G.-L.; Li, C.-J.; Ning, C.-Y.; Liu, H.-C. Tantalum-incorporated hydroxyapatite coating on titanium implants: Its mechanical and in vitro osteogenic properties. J. Mater. Sci. Mater. Med. 2019, 30, 111. [Google Scholar] [CrossRef]
- Tantalum 73Ta: The Essentials. Physical Properties. Crystal Structures. WebElements. Available online: https://www.webelements.com/tantalum/ (accessed on 1 December 2023).
- Titanium: 22Ti: The Essentials. Physical Properties. Crystal Structures. WebElements. Available online: https://www.webelements.com/titanium/ (accessed on 1 December 2023).
- Fan, H.; Deng, S.; Tang, W.; Muheremu, A.; Wu, X.; He, P.; Tan, C.; Wang, G.; Tang, J.; Guo, K.; et al. Highly Porous 3D Printed Tantalum Scaffolds Have Better Biomechanical and Microstructural Properties than Titanium Scaffolds. Hindawi BioMed Res. Int. 2021, 2021, 2899043. [Google Scholar] [CrossRef]
- Khoshnaw, F.; Yamanoglu, R.; Basci, U.G.; Muratal, O. Pressure assisted bonding process of stainless steel on titanium alloy using powder metallurgy. Mater. Chem. Phys. 2021, 259, 124015. [Google Scholar] [CrossRef]
- Yamanoglu, R.; Khoshnaw, F.; Daoud, I.; Efendi, E. Effect of silver content on the wear and mechanical properties of powder metallurgical Ti-5Al-2.5Fe-xAg alloys. J. Min. Metall. Sect. B Metall. 2020, 56, 119–125. [Google Scholar] [CrossRef]
- Abir, M.M.M.; Otsuka, Y.; Ohnuma, K.; Miyashita, Y. Effects of composition of hydroxyapatite/gray titania coating fabricated by suspension plasma spraying on mechanical and antibacterial properties. J. Mech. Behav. Biomed. Mater. 2022, 125, 104888. [Google Scholar] [CrossRef]
- Łatka, L.; Pawlowski, L.; Chicot, D.; Pierlot, C.; Petit, F. Mechanical properties of suspension plasma sprayed hydroxyapatite coatings submitted to simulated body fluid. Surf. Coat. Technol. 2010, 205, 954–960. [Google Scholar] [CrossRef]
- Alontseva, D.L.; Ghassemieh, E.; Voinarovych, S.; Russakova, A.; Kyslytsia, O.; Polovetskyi, Y.; Toxanbayeva, A. Characterisation of the microplasma spraying of biocompatible coating of titanium. J. Microsc. 2020, 279, 148–157. [Google Scholar] [CrossRef]
- Alontseva, D.; Ghassemieh, E.; Voinarovych, S.; Kyslytsia, O.; Polovetskyi, Y.; Prokhorenkova, N.; Kadyroldina, A. Manufacturing and Characterisation of Robot Assisted Microplasma Multilayer Coating of Titanium Implants Biocompatible coatings for medical implants with improved density and crystallinity. Johns. Matthey Technol. Rev. 2020, 64, 180–191. [Google Scholar] [CrossRef]
- Alontseva, D.L.; Khozhanov, A.R.; Gert, S.S.; Krasavin, A.L.; Prokhorenkova, N.V.; Kalyuzhny, S. Manufacturing and Characterization of Tantalum Microplasma Coatings for Biomedical Application. In Proceedings of the 2020 7th International Congress on Energy Fluxes and Radiation Effects (EFRE), Tomsk, Russia, 14–26 September 2020; pp. 813–816. [Google Scholar]
- Wang, B.J.X. The Effects of Surface Roughness on the Functionality of Ti13Nb13Zr Orthopedic Implants. Biomed. J. Sci. Tech. Res. 2021, 38, 9. [Google Scholar] [CrossRef]
- Lewallen, E.A.; Trousdale, W.H.; Thaler, R.; Yao, J.J.; Xu, W.; Denbeigh, J.M.; Nair, A.; Kocher, J.P.; Dudakovic, A.; Berry, D.J.; et al. Surface Roughness of Titanium Orthopedic Implants Alters the Biological Phenotype of Human Mesenchymal Stromal Cells. Tissue Eng. Part A 2021, 27, 1503–1516. [Google Scholar] [CrossRef] [PubMed]
- Pligovka, A.; Lazavenka, A.; Turavets, U.; Hoha, A.; Salerno, M. Two-Level 3D Column-like Nanofilms with Hexagonally–Packed Tantalum Fabricated via Anodizing of Al/Nb and Al/Ta Layers—A Potential Nano-Optical Biosensor. Materials 2023, 16, 993. [Google Scholar] [CrossRef] [PubMed]
- Pligovka, A. Reflectant photonic crystals produced via porous-alumina-assisted-anodizing ofAl/Nb and Al/Ta systems. Surf. Rev. Lett. 2021, 28, 2150055. [Google Scholar] [CrossRef]
- Kalita, V.I.; Malanin, D.A.; Mamaev, A.I.; Mamaeva, V.A.; Novochadov, V.V.; Komlev, D.I.; Komlev, V.S.; Radyuk, A.A. 3D bioactive coatings with a new type of porous ridge/cavity structure. Materialia 2021, 15, 101018. [Google Scholar] [CrossRef]
- Civantos, A.; Beltrán, A.M.; Domínguez-Trujillo, C.; Garvi, M.D.; Lebrato, J.; Rodríguez-Ortiz, J.A.; García-Moreno, F.; Cauich-Rodriguez, J.V.; Guzman, J.J.; Torres, Y. Balancing Porosity and Mechanical Properties of Titanium Samples to Favor Cellular Growth against Bacteria. Metals 2019, 9, 1039. [Google Scholar] [CrossRef]
- Fotovvati, B.; Namdari, N.; Dehghanghadikolaei, A. On Coating Techniques for Surface Protection: A Review. J. Manuf. Mater. Process. 2019, 3, 28. [Google Scholar] [CrossRef]
- Cizek, J.; Matejicek, J. Medicine Meets Thermal Spray Technology: A Review of Patents. J. Therm. Spray Technol. 2018, 27, 1251–1279. [Google Scholar] [CrossRef]
- Alontseva, D.; Krasavin, A.; Abilev, M.; Zhilkashinova, A. Microplasma Deposition of Biocompatible Coatings Using an Intelligent Robotic System for Plasma Processing. Acta Phys. Pol. A 2019, 136, 3. [Google Scholar] [CrossRef]
- Kadyroldina, A.; Alontseva, D.; Voinarovych, S.; Łatka, L.; Kyslytsia, O.; Azamatov, B.; Khozhanov, A.; Prokhorenkova, N.; Zhilkashinova, A.; Burburska, S. Microplasma spraying of hydroxyapatite coatings on additive manufacturing titanium implants with trabecular structures. Mater. Sci. Pol. 2022, 40, 28–42. [Google Scholar] [CrossRef]
- Sun, K.; Fu, R.; Liu, X.; Xu, L.; Wang, G.; Chen, S.; Zhai, Q.; Pauly, S. Osteogenesis and angiogenesis of a bulk metallic glass for biomedical implants. Bioact. Mater. 2021, 8, 253–266. [Google Scholar] [CrossRef] [PubMed]
- ASTM F560-17; Standard Specification for Unalloyed Tantalum for Surgical Implant Applications (UNS R05200, UNS R05400). ASTM: West Conshohocken, PA, USA, 2022. [CrossRef]
- No. 1 2012; Interstate Standard—Group B51: Wrought Titanium and Titanium Alloys—Grades. GOST R: Moscow, Russia, 1992.
- ISO 5832-3:2016; Implants for Surgery—Metallic Materials—Part 3: Wrought Titanium 6-Aluminium 4-Vanadium Alloy. International Organization for Standardization: Geneva, Switzerland, 2016.
- Mellali, M.; Grimaud, A.; Leger, A.C.; Fauchais, P.; Lu, J. Alumina grit blasting parameters for surface preparation in the plasma spraying operation. J. Therm. Spray Technol. 1997, 6, 217–227. [Google Scholar] [CrossRef]
- Yushenko, K.; Borisov, Y.; Voynarovych, S.; Fomakin, O. Plasmatron for Spraying of Coatings. WO2004010747A1, 29 January 2004. [Google Scholar]
- ASTM E2109-01; Standard Test Methods for Determining Area Percentage Porosity in Thermal Sprayed Coatings. ASTM International: West Conshohocken, PA, USA, 2014.
- ASTM F1147-05(2017)e1; Standard Test Method for Tension Testing of Calcium Phosphate and Metallic Coatings. ASTM: West Conshohocken, PA, USA, 2017. Available online: https://cdn.standards.iteh.ai/samples/97515/da85e07083004bf3abdeba46c7755740/ASTM-F1147-05-2017-e1.pdf (accessed on 1 December 2023).
- ISO 25178-600:2019(E); Geometrical Product Specifications (GPS)—Surface Texture: Areal—Part 600: Metrological Characteristics for Areal Topography Measuring Methods. International Organization for Standardization: Geneva, Switzerland, 2019.
- Geekiyanage, N.M.; Balanant, M.A.; Sauret, E.; Saha, S.; Flower, R.; Lim, C.T.; Gu, Y. A coarse-grained red blood cell membrane model to study stomatocyte-discocyte-echinocyte morphologies. PLoS ONE 2019, 14, e0215447. [Google Scholar] [CrossRef] [PubMed]
- ISO 13179-1:2021; Implants for Surgery. Coatings on Metallic Surgical Implants. Part 1: Plasma-Sprayed Coatings Derived from Titanium or Titanium-6 Aluminum-4 Vanadium Alloy Powders. International Organization for Standardization: Geneva, Switzerland, 2021.
- Griffiths, B.J.; Gawne, D.T.; Dong, G. The erosion of steel surfaces by grit-blasting as a preparation for plasma spraying. Wear 1996, 194, 95–102. [Google Scholar] [CrossRef]
- Borsari, V.; Giavaresi, G.; Fini, M.; Torricelli, P.; Salito, A.; Chiesa, R.; Chiusoli, L.; Volpert, A.; Rimondini, L.; Giardino, R. Physical characterization of different-roughness titanium surfaces, with and without hydroxyapatite coating, and their effect on human osteoblast-like cells. J. Biomed. Mater. Res. Part B Appl. Biomater. 2005, 75, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, V.; Szymczyk-Ziółkowska, P.; Rusińska, M.; Poradowski, D.; Janeczek, M.; Ziółkowski, G.; Dybała, B. Study of cytotoxic activity of Ti–13Nb–13Zr medical alloy with different surface finishing techniques. J. Mater. Sci. 2021, 56, 17747–17767. [Google Scholar] [CrossRef]
- Gupta, V.B.; Anitha, S.; Hegde, M.L.; Zecca, L.; Garruto, R.M.; Ravid, R.; Shankar, S.K.; Stein, R.; Shanmugavelu, P.; Rao, K.S.J. Aluminium in Alzheimer’s disease: Are we still at a crossroad? CMLS Cell. Mol. Life Sci. 2005, 62, 143–158. [Google Scholar] [CrossRef]
- Le Guéhennec, L.; Soueidan, A.; Layrolle, P.; Amouriq, Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent. Mater. 2007, 23, 844–854. [Google Scholar] [CrossRef]
- Kuo, T.Y.; Chin, W.H.; Chien, C.S.; Hsieh, Y.H. Mechanical and biological properties of graded porous tantalum coatings deposited on titanium alloy implants by vacuum plasma spraying. Surf. Coat. Technol. 2019, 372, 399–409. [Google Scholar] [CrossRef]
- Eliaz, N.; Gileadi, E. Physical Electrochemistry: Fundamentals, Techniques, and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Mani, G.; Porter, D.; Grove, K.; Collins, S.; Ornberg, A.; Shulfer, R. A comprehensive review of biological and materials properties of Tantalum and its alloys. J. Biomed. Mater. Res. Part A 2022, 110, 1291–1306. [Google Scholar] [CrossRef]
- Park, C.; Park, S.; Kim, J.; Han, A.; Ahn, S.; Min, S.-K.; Jae, H.J.; Chung, J.W.; Lee, J.-H.; Jung, H.-D.; et al. Enhanced endothelial cell activity induced by incorporation of nano-thick tantalum layer in artificial vascular grafts. Appl. Surf. Sci. 2020, 508, 144801. [Google Scholar] [CrossRef]
- Maccauro, G.; Iommetti, P.R.; Muratori, F.; Raffaelli, L.; Manicone, P.F.; Fabbriciani, C. An overview about biomedical applications of micron and nano size tantalum. Recent Pat. Biotechnol. 2009, 3, 157–165. [Google Scholar] [CrossRef]
- Zitter, H.; Plenk, H., Jr. The electrochemical behavior of metallic implant materials as an indicator of their biocompatibility. J. Biomed. Mater. Res. 1987, 21, 881–896. [Google Scholar] [CrossRef]
- Hee, A.C.; Jamali, S.S.; Bendavid, A.; Martin, P.J.; Kong, C.; Zhao, Y. Corrosion behaviour and adhesion properties of sputtered tantalum coating on Ti6Al4V substrate. Surf. Coat. Technol. 2016, 307, 666–675. [Google Scholar] [CrossRef]
- Li, H.; Yao, Z.; Zhang, J.; Cai, X.; Li, L.; Liu, G.; Liu, J.; Cui, L.; Huang, J. The progress on physicochemical properties and biocompatibility of tantalum-based metal bone implants. SN Appl. Sci. 2020, 2, 671. [Google Scholar] [CrossRef]
- Ding, Z.; Tang, Y.; Liu, L.; Ding, Z.; Tan, Y.; He, Q. Improving the adhesive, mechanical, tribological properties and corrosion resistance of reactive sputtered tantalum oxide coating on Ti6Al4V alloy via introducing multiple interlayers. Ceram. Int. 2022, 48, 5983–5994. [Google Scholar] [CrossRef]
- Zhou, Y.L.; Niinomi, M.; Akahori, T.; Fukui, H.; Toda, H. Corrosion resistance and biocompatibility of Ti–Ta alloys for biomedical applications. Mater. Sci. Eng. A 2005, 398, 28–36. [Google Scholar] [CrossRef]
- Yao, Z.; Lin, T.H.; Pajarinen, J.; Sato, T.; Goodman, S. Chapter 12—Host Response to Orthopedic Implants (Metals and Plastics). In Host Response to Biomaterials; Badylak, S.F., Ed.; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar]
- Ding, D.; Xie, Y.; Li, K.; Huang, L.; Zheng, X. Micro/Nano Structural Tantalum Coating for Enhanced Osteogenic Differentiation of Human Bone Marrow Stem Cells. Materials 2018, 11, 546. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.S.; Huber, S.; Ferguson, S.J. Comprehensive in vitro comparison of cellular and osteogenic response to alternative biomaterials for spinal implants. Mater. Sci. Eng. C 2021, 127, 112251. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.C.S.; Agrelli, A.; Andrade, A.N.; Mendes-Marques, C.L.; Arruda, I.R.S.; Santos, L.R.L.; Vasconcelos, N.F.; Machado, G. Titanium Dental Implants: An Overview of Applied Nanobiotechnology to Improve Biocompatibility and Prevent Infections. Materials 2022, 15, 3150. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Wu, Y.; Diao, X.; Shi, W.; Feng, F.; Qian, F.; Umeda, J.; Kondoh, K.; Xin, H.; Shen, J. Mechanical properties and biocompatibility of titanium with a high oxygen concentration for dental implants. Mater. Sci. Eng. C 2020, 117, 111306. [Google Scholar] [CrossRef] [PubMed]
- Lagonegro, P.; Trevisi, G.; Nasi, L.; Parisi, L.; Manfredi, E.; Lumetti, S.; Rossi, F.; Macaluso, G.M.; Salviati, G.; Galli, C. Osteoblasts preferentially adhere to peaks on micro-structured titanium. Dent. Mater. J. 2018, 37, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Parisi, L.; Toffoli, A.; Cutrera, M.; Bianchi, M.; Lumetti, S.; Bussolati, O.; Macaluso, G. Plasma Proteins at the Interface of Dental Implants Modulate Osteoblasts Focal Adhesions Expression and Cytoskeleton Organization. Nanomaterials 2019, 9, 1407. [Google Scholar] [CrossRef] [PubMed]
- Parisi, L.; Toffoli, A.; Ghezzi, B.; Lagonegro, P.; Trevisi, G.; Macaluso, G.M. Preparation of hybrid samples for scanning electron microscopy (SEM) coupled to focused ion beam (FIB) analysis: A new way to study cell adhesion to titanium implant surfaces. PLoS ONE 2022, 17, e0272486. [Google Scholar] [CrossRef]
- Galli, C.; Passeri, G.; Ravanetti, F.; Elezi, E.; Pedrazzoni, M.; Macaluso, G.M. Rough surface topography enhances the activation of Wnt/beta-catenin signaling in mesenchymal cells. J. Biomed. Mater. Res. A 2010, 95, 682–690. [Google Scholar] [CrossRef]




| Standard | Fe | N | O | Nb | C | Ti | W | Mo | H | Ni | Ta |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ASTM F560-17 [36] | ≤0.010 | ≤0.010 | ≤0.015 | ≤0.10 | ≤0.010 | ≤0.010 | ≤0.050 | ≤0.020 | ≤0.0015 | ≤0.010 | Balance |
| Standard | Al | Fe | Si | C | N | H | O | Ti |
|---|---|---|---|---|---|---|---|---|
| Interstate Standard Group B51 [37] | <0.3 | <0.15 | <0.08 | <0.05 | <0.03 | <0.003 | <0.12 | Balance |
| Standard | Fe | N | O | Al | C | V | H | Ti |
|---|---|---|---|---|---|---|---|---|
| ISO 5832-3 [38] | <0.3 | <0.05 | <0.2 | 5.5–6.75 | <0.08 | 3.5–4.5 | <0.015 | Balance |
| Parameters | Settings |
|---|---|
| Fraction of abrasive, mm | 0.6 |
| The pressure of compressed air, MPa | 0.6 |
| Distance from the nozzle cut to the treated surface, mm | 100 |
| The incident angle of an abrasive jet on the surface to be treated, degrees | 90 |
| Linear speed of pistol movement, mm/min | 250 |
| Coating Material | Current I (A) | Plasma Gas Flow Rate Gp (slpm) | Spraying Distance H (mm) | Wire Feed Rate Vw (m/min) |
|---|---|---|---|---|
| Ta | 31.0 | 4.0 | 30.0 | 3.2 |
| Ti | 16.0 | 2.3 | 40.0 | 3.0 |
| Material/Characteristics | Ta Coating | Ti Coating | Ti6Al4V Alloy after Gas Abrasive Treatment |
|---|---|---|---|
| Porosity | up to 20 ± 2% pore sizes from 20 μm to 200 μm | up to 22 ± 2.5% pore sizes from 20 μm to 200 μm From this point of view, it is feasible to | N/A |
| Mean surface roughness (Sa), μm | 16.4 ± 0.5 | 27.6 ± 2.6 | 4.6 ± 0.1 |
| Root mean square roughness (Sq) | 21.0 ± 1.1 | 34.4 ± 3.5 | 5.8 ± 0.1 |
| Mean static tensile strength of the coating, MPa | 28.0 ± 4.9 | 27.6 ± 0.9 | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alontseva, D.; Safarova, Y.; Voinarovych, S.; Obrosov, A.; Yamanoglu, R.; Khoshnaw, F.; Yavuz, H.I.; Nessipbekova, A.; Syzdykova, A.; Azamatov, B.; et al. Biocompatibility and Corrosion of Microplasma-Sprayed Titanium and Tantalum Coatings versus Titanium Alloy. Coatings 2024, 14, 206. https://doi.org/10.3390/coatings14020206
Alontseva D, Safarova Y, Voinarovych S, Obrosov A, Yamanoglu R, Khoshnaw F, Yavuz HI, Nessipbekova A, Syzdykova A, Azamatov B, et al. Biocompatibility and Corrosion of Microplasma-Sprayed Titanium and Tantalum Coatings versus Titanium Alloy. Coatings. 2024; 14(2):206. https://doi.org/10.3390/coatings14020206
Chicago/Turabian StyleAlontseva, Darya, Yuliya Safarova (Yantsen), Sergii Voinarovych, Aleksei Obrosov, Ridvan Yamanoglu, Fuad Khoshnaw, Hasan Ismail Yavuz, Assem Nessipbekova, Aizhan Syzdykova, Bagdat Azamatov, and et al. 2024. "Biocompatibility and Corrosion of Microplasma-Sprayed Titanium and Tantalum Coatings versus Titanium Alloy" Coatings 14, no. 2: 206. https://doi.org/10.3390/coatings14020206
APA StyleAlontseva, D., Safarova, Y., Voinarovych, S., Obrosov, A., Yamanoglu, R., Khoshnaw, F., Yavuz, H. I., Nessipbekova, A., Syzdykova, A., Azamatov, B., Khozhanov, A., & Weiß, S. (2024). Biocompatibility and Corrosion of Microplasma-Sprayed Titanium and Tantalum Coatings versus Titanium Alloy. Coatings, 14(2), 206. https://doi.org/10.3390/coatings14020206








.JPG)


